Abstract
The aim of the present study was to examine whether a C-reactive protein (CRP) first approach would improve the detection rate of postoperative complications by CT.
CRP is a useful biomarker to identify major complications following surgery for colorectal cancer.
Patients with histologically confirmed colorectal cancer, who underwent elective surgery between 2008 and 2015 at a single centre were included. Exceeding the established CRP threshold of 150 mg/L on postoperative day (POD) 4 was recorded. Results of CT performed between postoperative days 4 and 14 were recorded.
Four hundred ninety-five patients were included. The majority were male (58%), over 65 (68%), with node-negative disease (66%) and underwent open surgery (70%). Those patients who underwent a CT scan (n = 93), versus those who did not (n = 402), were more likely to have a postoperative complication (84% vs 35%, P < 0.001), infective complication (67% vs 21%, P < 0.001), and anastomotic leak (17% vs 2%, P < 0.001). In patients who did not undergo a CT scan (n = 402) exceeding the CRP threshold (n = 117) on POD 4 was associated with a higher rate of postoperative complication (50% vs 29%, P < 0.001), infective complications (36% vs 15%, P < 0.001), and anastomotic leak (4% vs 0.5%, P = 0.009). In patients who did undergo a CT scan (n = 93) exceeding the CRP threshold (n = 53) on POD 4 was associated with earlier CT (median POD 6 vs 8, P = 0.001) but not postoperative complications.
A CRP first approach resulted in earlier and improved detection of complications by CT following surgery for colorectal cancer.
Keywords: colorectal cancer, C-reactive protein, CT, postoperative complications
1. Introduction
Colorectal malignancy is a leading cancer diagnosis in the Western world.[1] Surgical resection is the mainstay of treatment for colorectal cancer patients. However, surgery is associated with considerable risk of postoperative complications including anastomotic leak.[2]
Postoperative complications are associated with increased short-term morbidity and mortality. Moreover, postoperative complications, particularly infective complications, have been shown to be associated with increased local recurrence and diminished short- and long-term cancer-specific survival.[3–5] Anastomotic leak and other significant postoperative complications can present in a subtle manner and often only become clinically evident relatively late in the postoperative course which is likely to contribute to their impact on outcomes.[6]
It is now well understood that the magnitude of postoperative systemic inflammatory response, measured by C-reactive protein (CRP), is associated with postoperative complications.[7,8] Recent consensus suggests that CRP concentrations exceeding 150 mg/L on postoperative days 3 or 4 should alert clinicians to possible postoperative complications, including anastomotic leak.[9] Furthermore it has been suggested that measuring the magnitude of the postoperative systemic inflammatory response may be useful in determining safe discharge, or indeed delaying it for further investigation.[10]
Computed tomography (CT) is an important imaging technique commonly used, with or without the addition of rectal and/or oral contrast, to diagnose postoperative complications including anastomotic leak.[11,12] Studies have shown CT to be both sensitive and specific in detection of these postoperative complications.[13,14] However, in general, at present CT is used sparingly and is usually carried out upon the surgical team's suspicion. As a consequence, CT is often not requested until late in the postoperative course.[15]
Due to this strong association with the development of postoperative complications, CRP may be a useful biomarker to identify those patients who would benefit from early CT. However, at present there are no data to inform as to whether a CRP first approach would result in the earlier detection of postoperative complications. The currently recruiting PRECious trial aims to test this hypothesis prospectively by allocating patients to standard care or to a postoperative care arm in which patients will undergo contrast CT if they exceed a CRP threshold of 140 mg/L on postoperative day 3, 4, or 5.[16] The investigators plan to use a stepped wedge design and will not blind clinicians in the control arm to postoperative CRP concentrations. Given that the current evidence for the association between CRP and postoperative complications is robust this raises the possibility of selection bias and crossover of patients allocated to the control arm to early CT dependent on their CRP concentrations. Another approach would be to audit surgical practice before the introduction of a CRP first postoperative protocol.
Therefore the aim of the present study, in a prospective cohort, was to examine the relationship between the magnitude of the postoperative systemic inflammatory response, postoperative CT, and complications in patients who underwent surgery for colorectal cancer.
2. Patients and methods
2.1. Patients
Patients with histologically confirmed colorectal cancer, who underwent elective surgery, with curative intent, between February 2008 and April 2015 at a single centre were included in the study. Patients who underwent emergency surgery, palliative procedures, with metastatic disease, or who had existing inflammatory conditions were excluded.
All patients received prophylactic antibiotics and venous thromboprophylaxis before the induction of anesthesia as per hospital policy. Patients had routine preoperative blood sampling including a full blood count (FBC), serum CRP, and albumin concentration.
On each postoperative day patients were clinically assessed and had blood samples, including serum CRP, obtained routinely until discharged. Further postoperative investigation and intervention was at the discretion of the patient's surgical team who were not blinded to blood results. All CT scans performed in the postoperative period were reported by a consultant radiologist at the request of the referring surgical team. The use of rectal, oral, and intravenous contrast was at the discretion of the supervising radiologist. There was no CRP first postoperative protocol in place during the study period. This study was approved as part of surgical audit by the West of Scotland Research Ethics Committee.
2.2. Methods
Data were collected prospectively in a database, anonymized, and subsequently analyzed. Recorded information included patient demographics, clinicopathological, operative, and radiological (CT) data. As CRP was being measured on postoperative day 4, only CT scans performed between postoperative days 4 and 14 were included. Where multiple CT scans were performed during this period, only the result of the first scan in the postoperative period was included.
Serum concentrations of CRP (mg/L) were measured using an autoanalyzer (Architect; Abbot Diagnostics, Maidenhead, UK) with a lower detectable limit of 0.2 mg/L as was serum albumin (normal range 35–50 g/L). The preoperative modified Glasgow Prognostic Score (mGPS) was calculated in patients for whom serum CRP and albumin concentrations were available.[17] Breaching the established CRP threshold of 150 mg/L on postoperative day 4 was recorded.[9]
Infective complications were categorized as described previously.[6] Wound (superficial surgical site) infection was defined as the presence of pus either spontaneously discharging from the wound or requiring drainage. Deep surgical site infection was defined as surgical or image-guided drainage of intraabdominal pus. Anastomotic leak was defined as radiologically verified fistula to bowel anastomosis or diagnosed at laparotomy. Pneumonia was defined by fever above 38.5°C and consolidatory chest X-ray findings requiring antibiotic treatment. Septicemia was defined by the presence of sepsis combined with positive blood culture. Urinary tract infection was only included if complicated by septicemia and confirmed with positive urine culture. Complications were also classified by severity using the Clavien Dindo grade.[18]
2.3. Statistical analysis
Categorical data were compared using the Chi-square test and Chi-square for linear association where appropriate. Continuous data were displayed as medians and ranges. These continuous data were compared using the Mann–Whitney U test. Missing data were excluded from analysis. Statistical analyses were performed using IBM SPSS version 22 for Windows (Chicago, IL). Two-tailed P values <0.05 were considered statistically significant.
3. Results
3.1. Patients
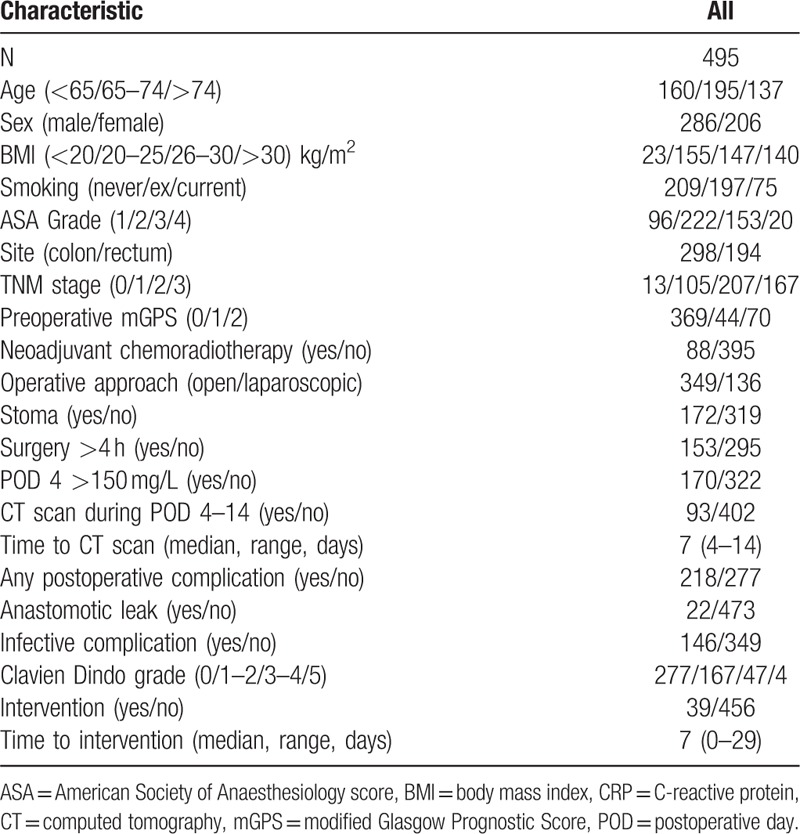
In total, 495 patients were included in the study (Fig. 1). The majority were male (286, 58%), over 65 years old (335, 68%), with node-negative disease (328, 66%) and underwent open surgery (349, 70%) (Table 1). One hundred seventy (34%) of patients exceeded the postoperative day 4 CRP threshold of 150 mg/L. Ninety-three (19%) patients underwent CT scan between postoperative days 4 and 14 following surgery, of which the majority received intravenous contrast (90, 97%) while 3 (3%) patients received additional rectal contrast. The median duration between surgery and CT scan was 7 days (range 4–14). Two hundred eighteen patients (44%) developed a postoperative complication of which 146 (29%) were infective and 51 (10%) were Clavien Dindo grade 3 to 5. There were 22 anastomotic leaks (4%).
Figure 1.
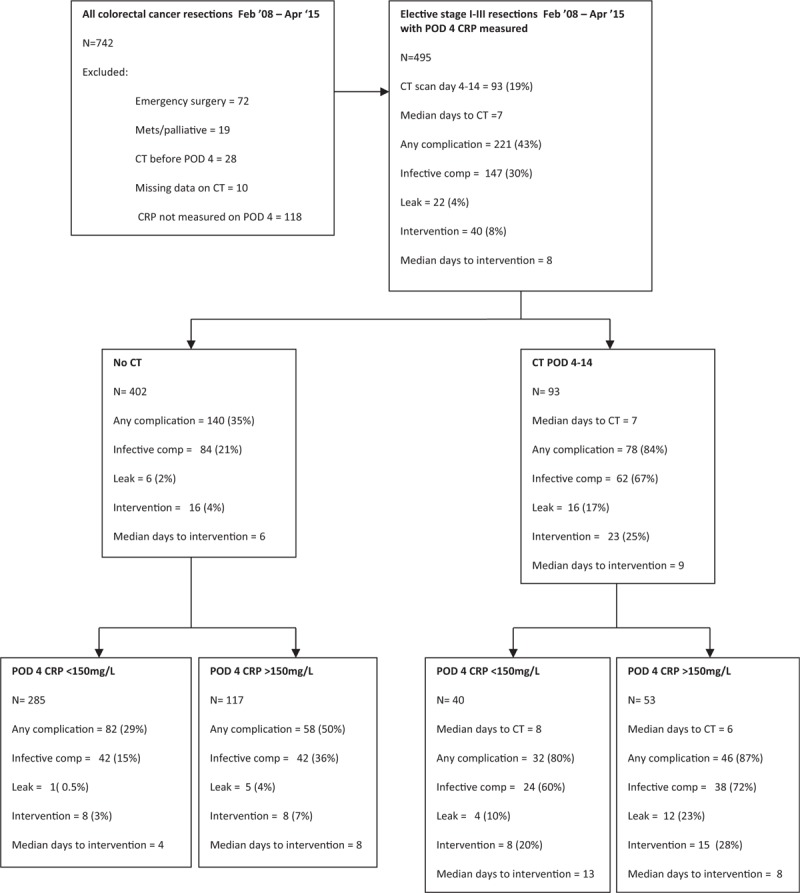
Flowchart of postoperative outcomes stratified by postoperative day (POD) 4 C-reactive protein (CRP) and CT imaging following surgery for colorectal cancer.
Table 1.
Clinicopathological and perioperative variables of patients undergoing elective surgery for colorectal cancer (N = 495).
When those patients who underwent surgery for colonic and rectal cancers were compared, there was no significant difference in the proportion of patients exceeding the established postoperative day 4 CRP threshold of 150 mg/L (P = 0.923), undergoing a postoperative CT scan (P = 0.239), having a postoperative complication (P = 0.052), anastomotic leak (P = 1.000), or the need for reoperation (P = 0.402). Therefore, the 2 groups were subsequently analyzed together.
3.2. Association between postoperative CT, CRP, and complications
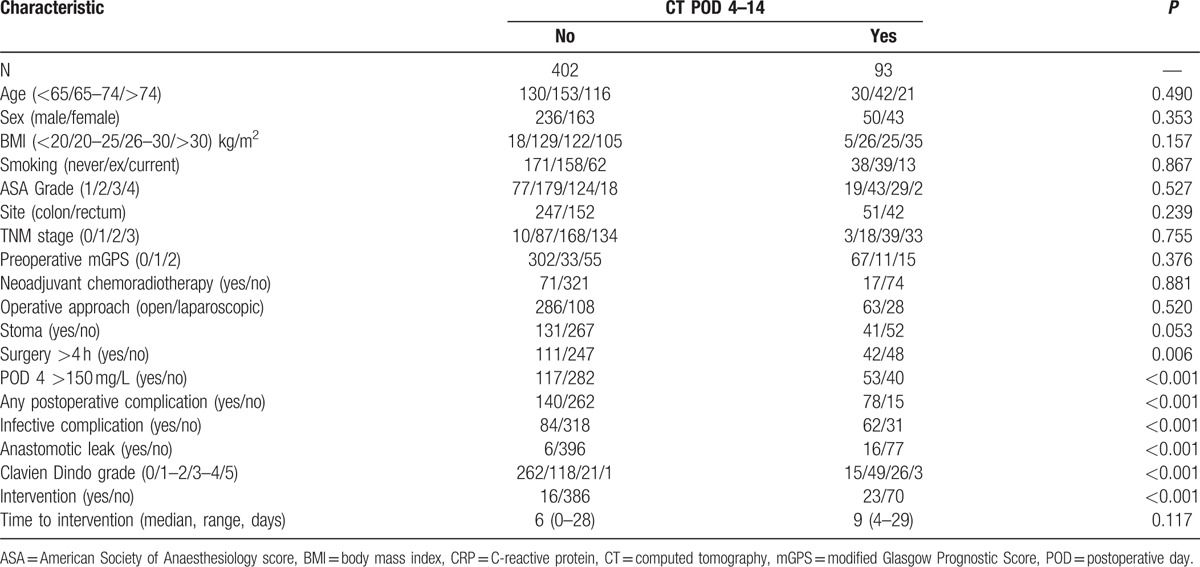
In those patients who underwent a CT scan (n = 93) compared with those who did not (n = 402, Fig. 1, Table 2) they were more likely to have a postoperative complication of any kind (84% vs 35%, P < 0.001), infective complication (67% vs 21%, P < 0.001), anastomotic leak (17% vs 2%, P < 0.001), and have a higher Clavien Dindo grade (P < 0.001). They were also significantly more likely to require postoperative percutaneous intervention or reoperation (25% vs 4%, P < 0.001), although there was no significant association with time between initial surgery and intervention.
Table 2.
Relationship between postoperative outcomes and CT between postoperative days 4 and 14 in patients undergoing elective surgery for stage I–III colorectal cancer (N = 495).
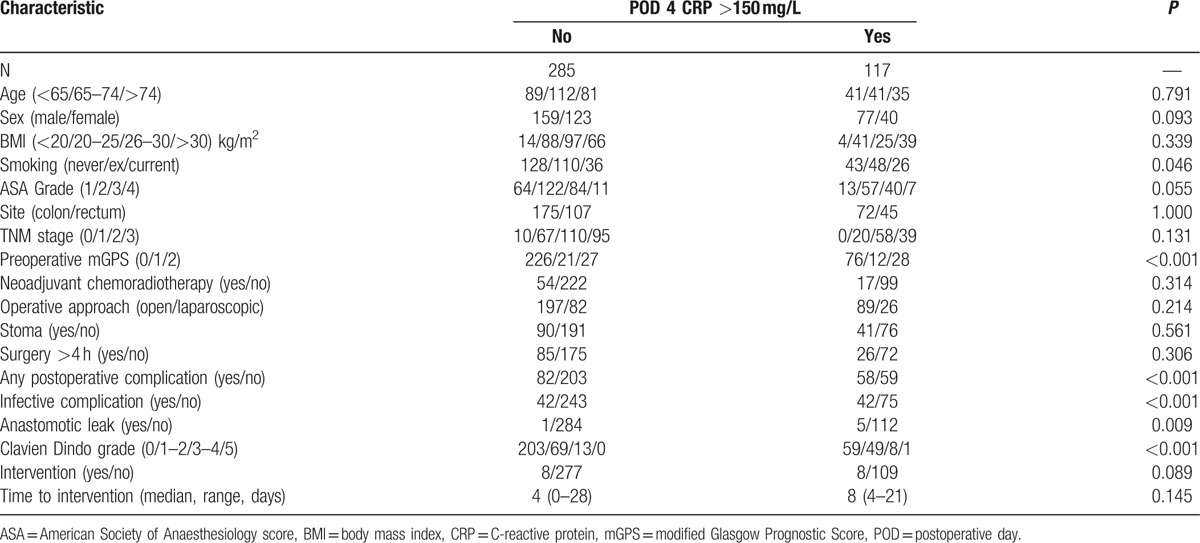
In those patients who did not undergo a CT scan (n = 402) exceeding CRP concentration of 150 mg/L (n = 117) on postoperative day 4 (Fig. 1 and Table 3) was associated with a higher rate of any kind of postoperative complication (50% vs 29%, P < 0.001), infective complications (36% vs 15%, P < 0.001), anastomotic leak (4% vs 0.5%, P = 0.009), and higher Clavien Dindo grade (P < 0.001). There was a trend toward greater need for postoperative intervention (7% vs 3%, P = 0.089). Those patients who required reoperation but did not undergo CT did so for reasons including hemorrhage, wound dehiscence, stoma complications, and discharge of enteric content from abdominal wound.
Table 3.
Relationship between postoperative outcomes and CRP on postoperative day 4 in patients undergoing elective surgery for colorectal cancer who did not undergo CT between postoperative days 4 and 14 (N = 402).
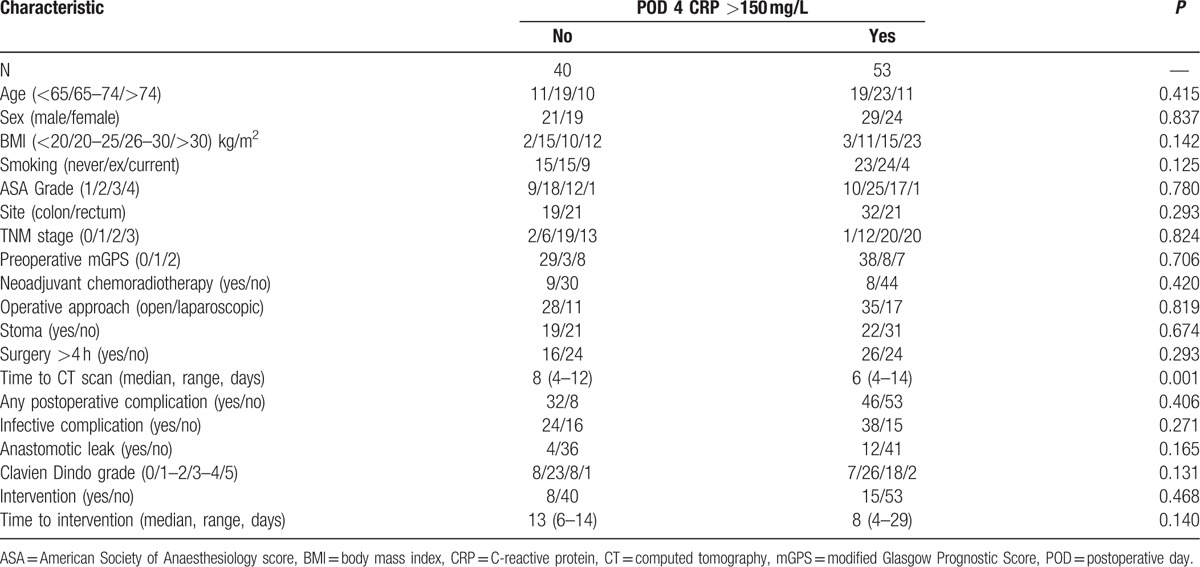
In those patients who did undergo a CT scan (n = 93) exceeding a CRP concentration of 150 mg/L (n = 53) on postoperative day 4 (Fig. 1 and Table 4) was not associated with any clinicopathological variables, or postoperative complication rates. There was a significant association with earlier CT in those patients who exceeded the established CRP threshold of 150 mg/L on postoperative day 4 (median postoperative day 6 vs 8, P = 0.001) and a trend toward earlier intervention (P = 0.140).
Table 4.
Relationship between postoperative outcomes and CRP on postoperative day 4 in patients undergoing elective surgery for colorectal cancer who underwent CT between postoperative days 4 and 14 (N = 93).
3.3. CRP before CT, and the association with complications and reoperation
In those patients who exceeded the postoperative day 4 CRP threshold of 150 mg/L (n = 170) compared with those who did not (n = 325), were more likely to undergo a CT scan (30% vs 12%, P < 0.001) and at an earlier time (median postoperative day 6 vs 8, P = 0.001). They were more likely to have any kind of postoperative complication (61% vs 36%, P < 0.001), infective complications (47% vs 21%, P < 0.001), anastomotic leak (10% vs 2%, P < 0.001), and have a higher Clavien Dindo grade (P < 0.001). They were also more likely to require postoperative percutaneous intervention or reoperation (14% vs 5%, P = 0.003). In those patients who exceeded the postoperative day 4 CRP threshold of 150 mg/L (n = 170), a subsequent CT scan (n = 53) compared to those without a CT scan was associated with a higher rate of any kind of complication (87% vs 50%, P < 0.001), infective complications (72% vs 36%, P < 0.001), anastomotic leak (23% vs 4%, P = 0.001), and a greater requirement for postoperative percutaneous intervention or reoperation (28% vs 7%, P < 0.001).
4. Discussion
The results of the present study show that the combination of a CRP first followed by CT approach appears to bring about the earlier and improved detection of postoperative complications in patients undergoing surgery for colorectal cancer.
In keeping with prior studies, the magnitude of the postoperative systemic inflammatory response was associated with complications and their severity.[8,19] Furthermore, it was of interest that there was a significant rate of clinically important (i.e., Clavien Dindo grade ≥3) morbidity and mortality in those patients who exceeded the CRP thresholds on postoperative day 4 but did not undergo CT scanning. This may represent a group of patients who were “failed to rescue.”
In contrast to the widely used measurement of CRP on postoperative day 4, postoperative CT scanning was only carried out in approximately 1 in 5 patients. In those patients who exceeded the CRP threshold on postoperative day 4, the use of CT scan increased the detection rate of all complications, infective complications, and anastomotic leak. In addition, the combination of postoperative day 4 CRP and subsequent CT scan was associated with a significantly higher rate of postoperative intervention.
A prior observational study by Straatman et al[13] reported a similar relationship between CRP and Clavien Dindo grade 3 to 5 complications, and a sensitivity and specificity of 92% and 100%, respectively, for contrast enhanced CT in the detection of these major complications in abdominal surgery. Furthermore, a recent observational study reported earlier diagnosis of postoperative complications, including by CT, and earlier intervention following surgery for colorectal cancer after the adoption of routine postoperative CRP measurement.[20] However, the accuracy of CT was not further stratified by CRP in either study.
In those patients who did not exceed the CRP threshold on postoperative day 4, the use of CT scan also increased the detection rate of complications and of anastomotic leak. Taken together with the above results it is clear that patients who underwent CT between postoperative days 4 and 14 and did not exceed the CRP thresholds on postoperative day 4 did so for reasons other than a raised CRP. Also, a small number of patients required reoperation without having undergone postoperative CT, primarily for complications which would not necessarily require a CT in their diagnosis, for example, wound and stoma complications, hemorrhage, and fistulation.
Even serious complications, such as anastomotic leak and those with a Clavien Dindo grade of 3 or more, are often not diagnosed until late in the postoperative course, in some cases as long as 12 days after surgery.[6,21] In keeping with this, half of all CT scans were performed 7 days or more after surgery in the present study. Current evidence suggests that CT imaging can accurately diagnose significant intraabdominal complications much earlier in the postoperative period.[15] Indeed, the results of the present study suggest that the use of a CRP first approach may result in CT being performed earlier in the postoperative course. However, further work is required to confirm this observation.
The present study has several limitations. Due to the observational nature of the study only a small number of patients received rectal contrast, and a small number received no contrast via any route due to renal failure, which may have reduced the diagnostic accuracy of CT. In many cases in which patients did not go on to reoperation the diagnosis of any complication relied directly on the CT scan report, although the use of Clavien Dindo grading has hopefully increased the objectivity of complication recording. Furthermore, although the present study investigated CRP thresholds on day 4, the median time to CT imaging was 7 days. Therefore the results may not reflect the accuracy of CT performed earlier in the postoperative course.
The present study suggests that a CRP first approach to the diagnosis of major complications may result in earlier and improved diagnosis of major postoperative complications by CT imaging. This approach may result in improved postoperative morbidity and mortality following surgery for colorectal cancer.
Acknowledgment
The authors thank the colorectal surgical team of the Glasgow Royal Infirmary.
Footnotes
Abbreviations: CRP = C-reactive protein, CT = computed tomography, FBC = full blood count, mGPS = modified Glasgow Prognostic Score, POD = postoperative day.
STM and BYK contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Cancer Stats, Cancer Research UK. 2014. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/commoncancers/ Accessed 24th June 2016 [Google Scholar]
- [2].Buchs NC, Gervaz P, Secic M, et al. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265–70. [DOI] [PubMed] [Google Scholar]
- [3].McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg 2005;92:1150–4. [DOI] [PubMed] [Google Scholar]
- [4].Mirzenami A, Mirzenami R, Chandrakumaran K, et al. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011;253:890–9. [DOI] [PubMed] [Google Scholar]
- [5].Artinyan A, Orcutt ST, Anaya DA, et al. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer. Ann Surg 2015;261:497–505. [DOI] [PubMed] [Google Scholar]
- [6].Platt JJ, Ramanathan ML, Crosbie RA, et al. C-reactive protein as predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 2012;19:4168–77. [DOI] [PubMed] [Google Scholar]
- [7].Singh PP, Zeng IS, Srinivasa S, et al. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 2014;101:339–46. [DOI] [PubMed] [Google Scholar]
- [8].Adamina M, Steffen T, Tarantino I, et al. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br J Surg 2015;102:590–8. [DOI] [PubMed] [Google Scholar]
- [9].McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102:462–79. [DOI] [PubMed] [Google Scholar]
- [10].Mullen JT. Identifying candidates for early discharge after gastrectomy: “It's tough to make predictions, especially about the future”. Ann Surg Oncol 2017;24:8–10. [DOI] [PubMed] [Google Scholar]
- [11].Hyman N, Manchester TL, Osler T, et al. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg 2007;245:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kauv P, Benadjaoud S, Curis E, et al. Anastomotic leakage after colorectal surgery: diagnostic accuracy of CT. Eur Radiol 2015;25:3543–51. [DOI] [PubMed] [Google Scholar]
- [13].Straatman J, Cuesta MA, Gisbertz SS, et al. Value of a step-up diagnosis plan: CRP and CT-scan to diagnose and manage postoperative complications after major abdominal surgery. Rev Esp Enferm Dig 2014;106:515–21. [PubMed] [Google Scholar]
- [14].Eckmann C, Kujath P, Schiedeck THK, et al. Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis 2004;19:128–33. [DOI] [PubMed] [Google Scholar]
- [15].Kornmann VN, van Ramshorst B, Smits AB, et al. Beware of false-negative CT scan for anastomotic leakage after colonic surgery. Int J Colorectal Dis 2014;29:445–51. [DOI] [PubMed] [Google Scholar]
- [16].Straatman J, Cuesta MA, Schreurs WH, et al. The PRECious trial PREdiction of Complications, a step-up approach, CRP first followed by CT-scan imaging to ensure quality control after major abdominal surgery: study protocol for a stepped-wedge trial. Trials 2015;16:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [18].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McSorley ST, Ramanathan ML, Horgan PG, et al. Postoperative C-reactive protein measurement predicts the severity of complications following surgery for colorectal cancer. Int J Colorectal Dis 2015;30:913–7. [DOI] [PubMed] [Google Scholar]
- [20].Mik M, Berut M, Dziki L, et al. Does C-reactive protein monitoring after colorectal resection with anastomosis give any practical benefit for patients with intra-abdominal septic complications? Colorectal Dis 2016;18:252–9. [DOI] [PubMed] [Google Scholar]
- [21].Khan AA, Wheeler JM, Cunningham C, et al. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis 2008;10:587–92. [DOI] [PubMed] [Google Scholar]


