Abstract
Rationale:
Plasmablastic lymphoma (PBL) is a rare subtype of human immunodeficiency virus (HIV)-related non-Hodgkin's lymphoma that predominantly manifests in the oral cavity.
Patient concerns:
Three cases of HIV-negative PBL were reported.
Diagnoses:
HIV-negative PBL
Interventions:
The patient had undergone chemotherapy.
Outcomes:
Clinical outcomes were very poor in Cases 1 and 3; Case 2, whose diagnosis suggested no bone marrow involvement, is still alive.
Lessons subsections:
These cases served to broaden the reported clinical spectrum of HIV-negative PBL. Clinicians and pathologists need to be familiar with lymphoma in the identified extra-oral PBL variation and there levant differential diagnosis procedures for this particular disease.
Keywords: case report, diverse clinical manifestation and treatment, HIV negative, immunosuppressive state, non-Hodgkin lymphoma, plasmablastic lymphoma
1. Introduction
Plasmablastic lymphoma (PBL) is a rare and aggressive variant of diffuse large B-cell lymphoma that predominantly occurs in the oral cavity of individuals infected with human immunodeficiency virus (HIV).[1] However, there has been a recent increase in the number of reported PBL cases in immunocompetent patients. First described by Delecluse et al as occurring in HIV patients,[2] PBL has now been labeled as a new disease entity in the 4th WHO classification and remains a diagnostic and therapeutic challenge because of the expression of plasma cell-associated markers.[3,4] Here, we present 3 cases of PBL in HIV-negative patients. We also review the current literature concerning HIV-negative PBL, its clinicopathological features, immunophenotype, and current modes of treatment. Informed consent was obtained from each patient presented in this report, and the study was approved by the Institute Research Ethics Committee at Zhengzhou University.
1.1. Case 1
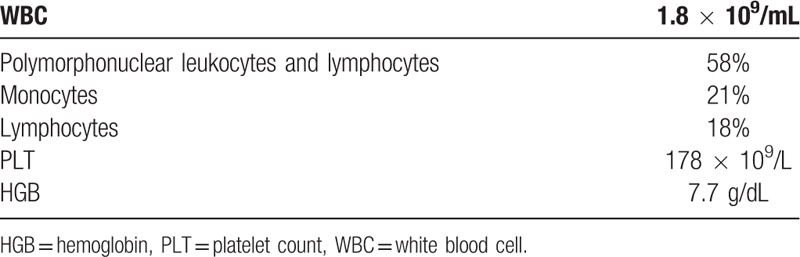
A 52-year-old male was admitted to the Department of Respiratory Medicine in our hospital owing to dyspnea and durative dorsal pain on the left side, the pain got worse in activity while relief when rest. The patient had previously undergone removal of his left testis at a local hospital because of swelling. Upon physical examination, he was not suffering from weight loss, fever, or night sweats. The patient's laboratory data are summarized in Table 1.
Table 1.
Case 1 serum levels.

The level of hepatitis B antigens were normal and serum antibodies for hepatitis C, HIV, and syphilis were negative. A bone marrow biopsy revealed a 72.4% plasma cell level (Fig. 1A). The patient then underwent a whole body positron emission tomography–computed tomography (PET-CT) scan, which revealed hypermetabolic masses in most organs. Immunohistochemistry of biopsy showed that tumor cells exhibited a diffuse growth pattern (Fig. 2A and B) and were strongly positive for CD38 (Fig. 2C), CD138 (Fig. 2D) and MUM-1, weakly positive for CD43, IgG kappa (Fig. 2E) and IgG lambda (Fig. 2F), and negative for creatine kinase (CK), CD20 (Fig. 2H), CD79a, CD3 (Fig. 2G), CD10, Bcl-6, CD30, and CD56. In situ hybridization demonstrated the sample was negative for EBV-encoded RNA (EBER). The proliferation index, as indicated by Ki-67 immunohistochemistry, was over 80% (Fig. 2I).
Figure 1.
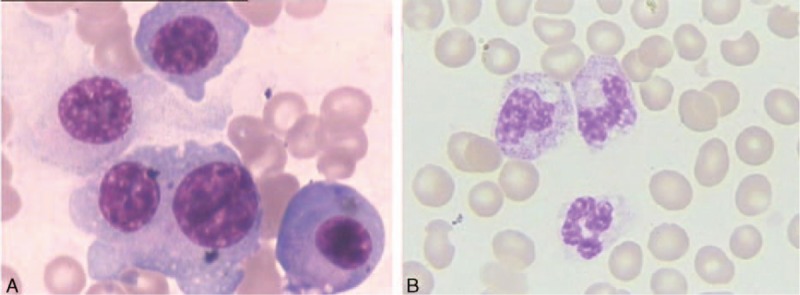
The bone marrow aspirate contained numerous lymphoplasmacytic forms (A). The bone marrow aspirate demonstrated a normal morphology (B).
Figure 2.
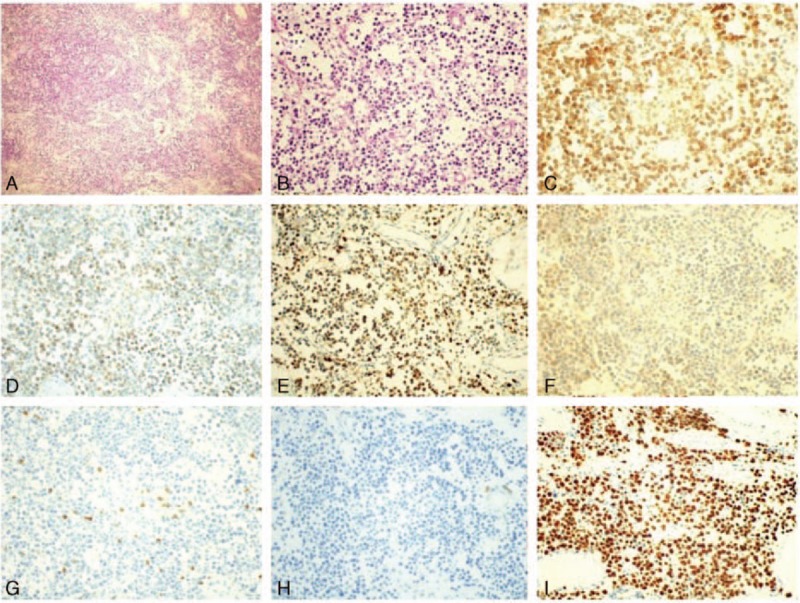
Diffuse plasmablast infiltrates, and to a lesser extent mature plasma cells (H&E) (A), higher-magnification (H&E) (B), immunohistochemical examination of CD38 (C), CD138 (D), IgG kappa (E), and IgG lambda (F), which were all intensively positive in neoplastic cells (×40). Immunohistochemical examination of CD3 (G) and CD20 (H), which were intensively positive in neoplastic cells (×40). Immunohistochemistry showed Ki-67 (I) expression in the nuclei of 80% of neoplastic cells (×40). H&E = hematoxylin and eosin.
The patient was treated with the combination of hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and high-dose cytarabine, also known as Hyper-CVAD. He completed 5 cycles uneventfully, and clinical remission was achieved after 4 cycles (Fig. 1B). However, this was still considered to be an aggressive case because of ongoing abdominal distension, and a CT scan revealed a mass in the abdomen. Further treatment options were discussed, but after consideration only palliative care was pursued. The patient died approximately 6 months after receiving chemotherapy.
1.2. Case 2
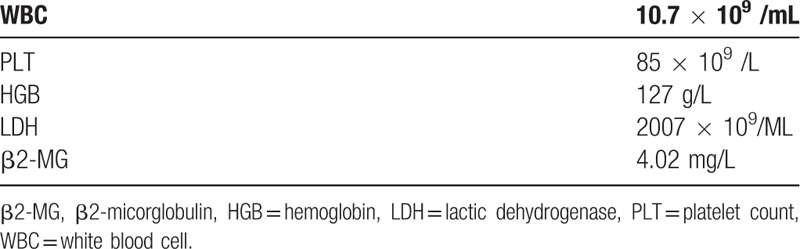
A 20-year-old male arrived at our hospital with complaints of fever and abdominal distension. Physical examination revealed a 38.4°C temperature, severe splenopathy, and no enlargement of superficial lymph nodes. Laboratory results are shown in Table 2. Biochemical analyses of plasma demonstrated that hepatic and renal functions were both within normal limits, whereas lactic dehydrogenase (LDH) and β2-micorglobulin (β2-MG) levels were above normal limits (565 U/L and 3.85 mg/L, respectively). Serology results were negative for syphilis, hepatitis B and C, and HIV. Serum tumor markers were normal. An abdominal mass (maximum: 59 mm × 50 mm), splenomegaly, and left pleural effusion were observed on chest and abdominal CT scans. A bone marrow biopsy showed no abnormalities. Laparoscopic biopsy of retroperitoneal lymph nodes was preliminarily diagnosed as B-cell non-Hodgkin's lymphoma of uncertain classification.
Table 2.
Case 2 serum levels.
Considering the patient's rapid disease development, we administered 1 cycle of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) without antiretroviral therapy. Meanwhile, biopsy samples were sent to the Beijing Friendship Hospital for consultation and diagnosis. Immunohistochemical analysis indicated that tumor cells were positive for CD38, MUM-1, PAX-5, CD30, and IgG lambda, weakly positive for IgG kappa, and negative for CD20, CD3, and ALK. The proliferation index, as indicated by Ki-67 immunohistochemistry, approached 90%. The large neoplastic cells were diffusely positive for EBER, as shown through in situ hybridization. Finally, a diagnosis was made of stage IV PBL (retroperitoneal primary, left pleural effusion); thus, another cycle of CHOP followed. At the end of the second cycle of therapy, the patient's performance status improved and CT reexamination demonstrated that the left pleural effusion disappeared and masses in the retroperitoneum were significantly reduced. Evaluation of response to therapy was partial remission; however, a CT scan after 6 cycles of CHOP revealed stable disease. We then altered the chemotherapy regimen to Hyper-CVAD for further treatment. To our disappointment, the primary mass increased after 1 course of Hyper-CVAD. Subsequently, a multiple-agent chemotherapy regimen consisting of hydroxycamptothecin, oxaliplatin, methylprednisolone, and dacarbazine, widely used for the treatment of relapsed or refractory B-cell lymphoma, was chosen to control the disease. The patient obtained complete remission after receiving 2 courses this regime. To date, the patient has undergone thalidomide maintenance therapy for 4 months and is still alive.
1.3. Case 3
A 60-year-old male was admitted with the chief complaint being swelling on the left side of the jaw. The patient had been diagnosed with hypertension in 2003, which had since been well-controlled. Additionally, the patient underwent successful treatment for scrofula and malaria several years prior.
The patient underwent an operation, and the initial laboratory data are shown in Table 3. Both serum and urinary immunofixation electrophoresis were negative, except IgG (24.10 g/L). Hepatitis B antigen levels were normal, and the serum was negative for antibodies against hepatitis C, HIV, and syphilis. A bone marrow biopsy revealed a 66.8% plasma cell level, and a CT scan showed bilateral masses in lymph nodes of neck. Immunohistochemistry showed the atypical cells were positive for CD38, CD138, and MUM-1, and negative for CD20, PAX5, CD79a, CD3, CD43, CK, CD10, Bcl-6, and CD23. The proliferation index, as indicated by Ki-67 labeling, was over 90%.
Table 3.
Case 3 serum levels.
The patient received 4 cycles of etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) chemotherapy and 1 cycle of etoposide, cytarabine, cisplatin, and dexamethasone but continued to clinically deteriorate, ultimately developing multiorgan failure. The patient died approximately 6 months later. A comparison of the clinical and pathological features in our 3 cases is summarized in Table 4.
Table 4.
Case study summary of demographic, clinical presentation, treatment, and outcome of the HIV-negative PBL.

2. Discussion
PBL is a rare disease that mostly involves the oral cavity of HIV-positive individuals. However, an increasing number of cases have been reported in HIV-negative individuals, as well as occurrences in extra-oral regions, including the nasal cavity and sinus, gastrointestinal tract, skin, and lymph nodes. Using the keywords “plasmablastic lymphoma and human immunodeficiency virus-negative or immunocompetent,” we found a vast number of published reports on PubMed. Only cases with a definitive pathologic diagnosis of PBL and description of no HIV infection were selected. A total of 128 cases (including our own) of HIV-negative PBL were described in case reports or in small sample case analyses between February 1997 and July 2015.[1–3,5–63]
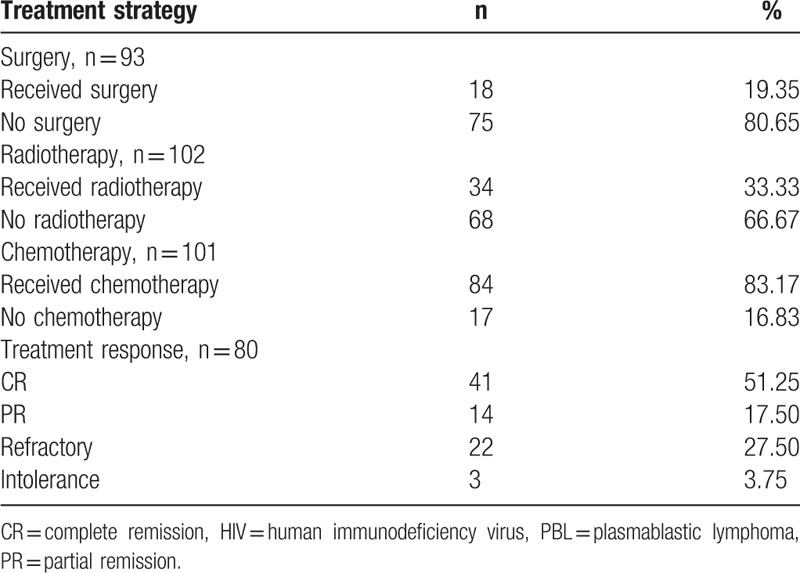
HIV-negative PBL occurred in a wide spectrum of patients between the ages of 2 and 86 years, with a mean age at diagnosis of 58.41 years. Not surprisingly, HIV-negative PBL mostly occurred in older patients. As shown in Table 5, patients over 60 years old accounted for 55.47% of all cases. There was a 2:1 ratio of male-to-female patients with HIV-negative PBL. With respect to clinical staging, stage IV was the most common, and stage I was the second-most common. Although stage IV occurred in 44.35% of the cases, bone marrow involvement was noted in only 16.49% of cases. However, bone marrow involvement was detected in some cases after relapse. The majority of primary sites were extra-nodal; 24.04% occurred in the oral cavity, 19.23% in the nasal and sinus cavities, 21.15% in the gastrointestinal tract, and 6.73% on the skin.
Table 5.
Clinical features of the HIV-negative PBL cases.
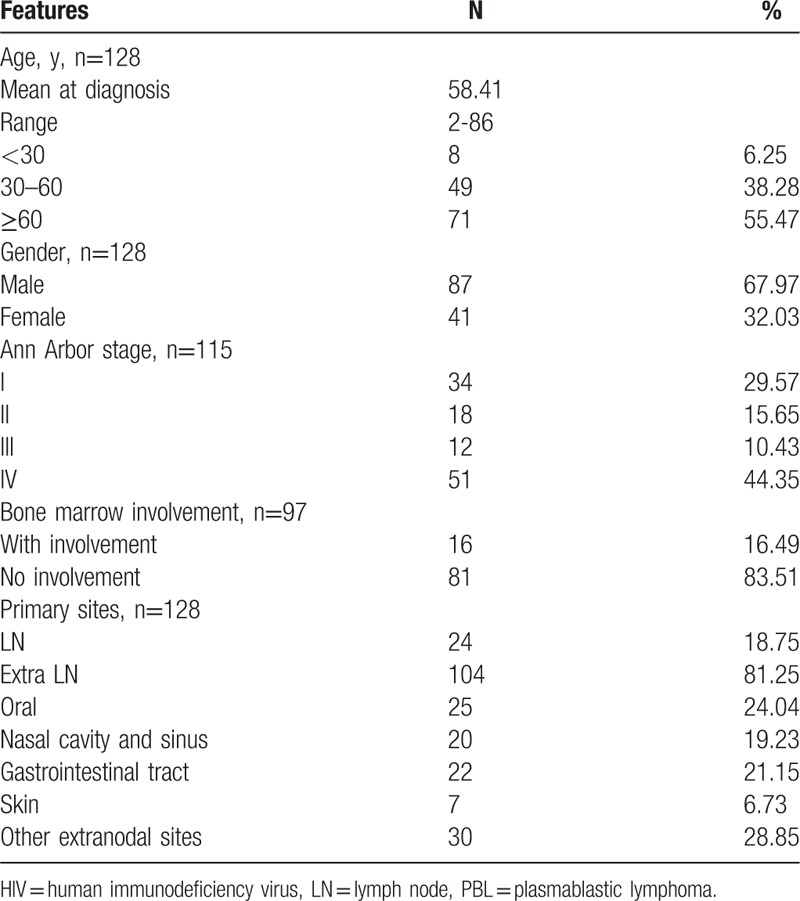
As shown in Table 6, the plasma cell markers CD38, VS38c, CD138, and MUM1 were universally expressed in HIV-negative PBL cases. A majority of patients (54.84%) expressed epithelial membrane antigen, and CD45 was variably expressed, positive in 44.23% of patients. A total of 41.43% of cases had CD79a expression, and only a few cases expressed the B-cell marker CD20 (only 1 case +, 7 cases ± ). Notably, a minority of patients expressed the T-cell markers CD3 (12.07%) and CD5 (12%). Furthermore, 25% of patients expressed the natural killer-cell marker CD56. EBV infection was common in HIV-negative PBL, involved in 58.42% of the patients. Ki-67 expression, which indicates an aggressive phenotype, was universally high, with a mean value of 83%.
Table 6.
Pathological findings of the HIV-negative PBL cases.
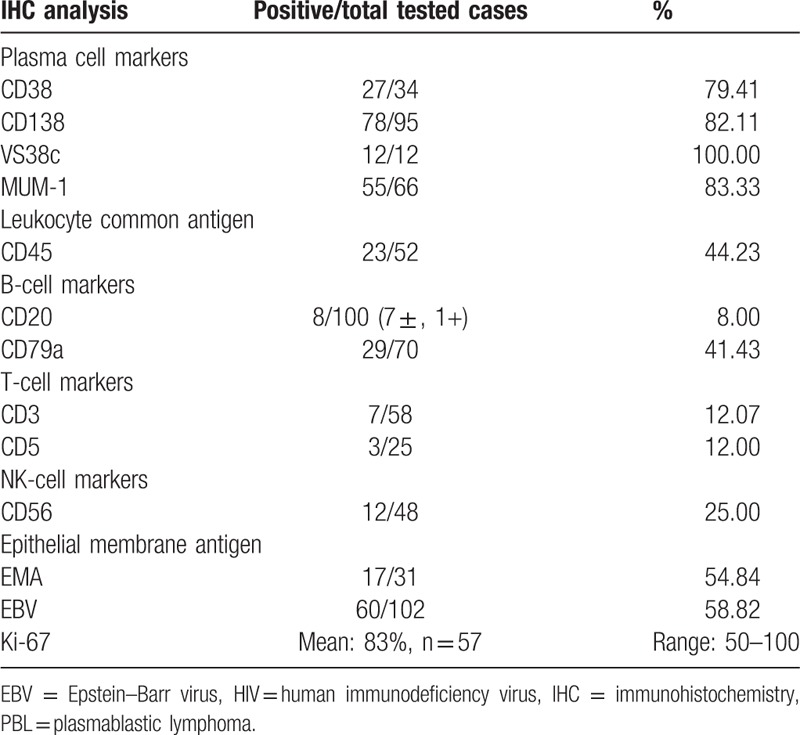
As Table 7 shows, 19.35% of the patients received surgery, 33.33% underwent radiotherapy, whereas a majority (83.17%) received chemotherapy. The complete remission, partial remission, and refractory rates were 51.25%, 17.50% and 27.50%, respectively. The median overall survival was approximately 19 months. The 1- and 2-year survival rates were 50.92% and 42.34%, respectively. There is no standard chemotherapeutic regimen for HIV-negative PBL, and the reported chemotherapeutic regimens included CHOP, hyper-CVAD, EPOCH, and CODOX-M/IVAC.[64] Aggressive chemotherapeutic treatment regimens were not found to produce statistically significant improvements in outcome. Further study of MYC translocations, which are usually associated with Burkitt's lymphoma, may help us better understand the disease mechanism and guide future pharmacological research and chemotherapeutic regimens.[65] There were several features associated with better survival, including use of antiretroviral therapy, clinical stage, and autologous stem cell transplantation. Autologous stem cell transplantation could be of benefit for short-term disease-free survival. Additionally, the proteasome inhibitor Bortezomib may become a new therapeutic option for PBL given the dramatic responses from previous results.
Table 7.
Treatment strategies, response, and prognosis of the HIV-negative PBL cases.
Although PBL cells morphologically resemble B-cell immunoblasts, they show the typical immunophenotype of plasma cells, expressing CD38, CD138 and MUM1, and variably expressing CD79a, CD56, CD45, CD10, CD30, and EBER. CD20 and PAX5 are not expressed in PBL cells.[4] It is difficult to distinguish PBL from plasma cell myeloma (PCM), especially in cases of extra-oral sites without HIV infection. In many PBL cases the clinical manifestation of the disease include markers that are also important diagnostic indicators for PCM, such as osteolytic lesions, diffuse bone marrow involvement, and the presence of M protein. Similarly, the frequent association with HIV and Epstein–Barr virus infections and common manifestation in the oral cavity are useful for diagnosing PBL. However, with atypical clinical features, both PCM and PBL can pose significant diagnostic challenges.[66]
In a recent case report and literature review of HIV-negative PBL patients, no previous cases of primary testis involvement were reported; to our knowledge, Case 1 is the first reported case. To date there are only 3 patients with a longer OS than Case 2. After reviewing 128 PBL cases, we conclude that in cases where a male patient presents with an atypical immunophenotype and the previously described clinical features a definitive pathological diagnosis is the cornerstone of proper management and should precede any therapeutic approach. Since PBL is usually disseminated at the time of the initial diagnosis, it is unknown whether the primary site affects disease outcomes.[56]
In conclusion, these cases served to broaden the reported clinical spectrum of HIV-negative PBL, which mostly arises in the oral cavity of HIV-positive patients. Extra-oral PBL, often found in HIV-negative patients, was highly invasive with poor prognosis and clinical manifestations and treatment varied in the 3 cases. Clinicians and pathologists need to be familiar with lymphoma in the identified extra-oral PBL variation and the relevant differential diagnosis procedures for this particular disease. Patients with immune suppression should also be suspected of having PBL; only in this way will there be an appropriate therapeutic approach and improved outcomes.
Footnotes
Abbreviations: β2-MG = β2-micorglobulin, CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone, CK = creatine kinase, EBER = EBV-encoded RNA, EPOCH = etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, HIV = human immunodeficiency virus, LDH = lactic dehydrogenase, PBL = plasmablastic lymphoma, PCM = plasma cell myeloma, PET-CT = positron emission tomography–computed tomography.
LL and XZ authors contributed equally to this work.
Funding: This study was supported by grants from General Program of the National Natural Science Foundation of China (81470364), the Key Science and Technology Research Project of Henan province (14B320033 and 201302011).
The authors have no conflicts of interest to disclose.
References
- [1].Choi SY, Cho YA, Hong SD, et al. Plasmablastic lymphoma of the oral cavity in a human immunodeficiency virus-negative patient: a case report with literature review. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:e115–120. [DOI] [PubMed] [Google Scholar]
- [2].Hatanaka K, Nakamura N, Kishimoto K, et al. Plasmablastic lymphoma of the cecum: report of a case with cytologic findings. Diagn Cytopathol 2011;39:297–300. [DOI] [PubMed] [Google Scholar]
- [3].Cao C, Liu T, Zhu H, et al. Bortezomib-contained chemotherapy and thalidomide combined with CHOP (Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) play promising roles in plasmablastic lymphoma: a case report and literature review. Clin Lymphoma Myeloma Leuk 2014;14:e145–150. [DOI] [PubMed] [Google Scholar]
- [4].Stein H, Harris N, Campo E. Swerdlow S, Harris N, Sunder-Plassmann G, et al. Plasmablastic lymphoma. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. 256–7. [Google Scholar]
- [5].Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 1997;89:1413–20. [PubMed] [Google Scholar]
- [6].Tille JC, Pelte MF, Schwartz J, et al. Plasmablastic lymphoma clinically presenting in the urinary tract. Ann Diagn Pathol 2012;16:219–23. [DOI] [PubMed] [Google Scholar]
- [7].Takeuchi M, Ogawa F, Onishi T, et al. Plasmablastic lymphoma in an elderly immunocompetent patient. Pathol Int 2012;62:347–50. [DOI] [PubMed] [Google Scholar]
- [8].Mihaljevic BS, Todorovic MR, Andjelic BM, et al. Unusual presentation of gastric plasmablastic lymphoma in HIV-negative patient. Med Oncol 2012;29:1186–9. [DOI] [PubMed] [Google Scholar]
- [9].Zhang LY, Lin HY, Gao LX, et al. Primary central nervous system plasmablastic lymphoma presenting in human immunodeficiency virus-negative but Epstein–Barr virus-positive patient: a case report. Diagn Pathol 2012;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lipstein M, O’Connor O, Montanari F, et al. Bortezomib-induced tumor lysis syndrome in a patient with HIV-negative plasmablastic lymphoma. Clin Lymphoma Myeloma Leuk 2010;10:E43–46. [DOI] [PubMed] [Google Scholar]
- [11].Khurana A, Jalpota Y. Plasmablastic lymphoma in a human immunodeficiency virus negative patient. Indian J Pathol Microbiol 2010;53:368–9. [DOI] [PubMed] [Google Scholar]
- [12].Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol 2012;67:e202–5. [DOI] [PubMed] [Google Scholar]
- [13].Wang HW, Yang W, Sun JZ, et al. Plasmablastic lymphoma of the small intestine: case report and literature review. World J Gastroenterol 2012;18:6677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tani J, Miyoshi H, Nomura T, et al. A case of plasmablastic lymphoma of the liver without human immunodeficiency virus infection. World J Gastroenterol 2013;19:6299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brahmania M, Sylwesterowic T, Leitch H. Plasmablastic lymphoma in the ano-rectal junction presenting in an immunocompetent man: a case report. J Med Case Rep 2011;5:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin F, Zhang K, Quiery AT, et al. Plasmablastic lymphoma of the cervical lymph nodes in a human immunodeficiency virus-negative patient: a case report and review of the literature. Arch Pathol Lab Med 2004;128:581–4. [DOI] [PubMed] [Google Scholar]
- [17].Tzankov A, Brunhuber T, Gschwendtner A, et al. Incidental oral plasmablastic lymphoma with aberrant expression of CD4 in an elderly HIV-negative patient: how a gingival polyp can cause confusion. Histopathology 2005;46:348–50. [DOI] [PubMed] [Google Scholar]
- [18].Scheper MA, Nikitakis NG, Fernandes R, et al. Oral plasmablastic lymphoma in an HIV-negative patient: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:198–206. [DOI] [PubMed] [Google Scholar]
- [19].Masgala A, Christopoulos C, Giannakou N, et al. Plasmablastic lymphoma of visceral cranium, cervix and thorax in an HIV-negative woman. Ann Hematol 2007;86:615–8. [DOI] [PubMed] [Google Scholar]
- [20].Guan B, Zhang X, Hu W, et al. Plasmablastic lymphoma of the oral cavity in an HIV-negative patient. Ann Diagn Pathol 2011;15:436–40. [DOI] [PubMed] [Google Scholar]
- [21].Mansoor M, Alani FS, Aslam MB, et al. A case report of cecal plasmablastic lymphoma in a HIV-negative patient. Eur J Gastroenterol Hepatol 2012;24:332–5. [DOI] [PubMed] [Google Scholar]
- [22].Matsuki E, Miyakawa Y, Asakawa S, et al. Identification of loss of p16 expression and upregulation of MDR-1 as genetic events resulting from two novel chromosomal translocations found in a plasmablastic lymphoma of the uterus. Clin Cancer Res 2011;17:2101–9. [DOI] [PubMed] [Google Scholar]
- [23].Saraceni C, Agostino N, Cornfield DB, et al. Plasmablastic lymphoma of the maxillary sinus in an HIV-negative patient: a case report and literature review. Springerplus 2013;2:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marques I, Lagos A, Costa-Neves B. Gastric plasmablastic lymphoma in HIV-negative patient. Rev Esp Enferm Dig 2013;105:166–7. [DOI] [PubMed] [Google Scholar]
- [25].Liu JJ, Zhang L, Ayala E, et al. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res 2011;35:1571–7. [DOI] [PubMed] [Google Scholar]
- [26].Kim JE, Kim YA, Kim WY, et al. Human immunodeficiency virus-negative plasmablastic lymphoma in Korea. Leuk Lymphoma 2009;50:582–7. [DOI] [PubMed] [Google Scholar]
- [27].Qing X, Sun N, Chang E, et al. Plasmablastic lymphoma may occur as a high-grade transformation from plasmacytoma. Exp Mol Pathol 2011;90:85–90. [DOI] [PubMed] [Google Scholar]
- [28].Suzuki Y, Yoshida T, Nakamura N, et al. CD3- and CD4-positive plasmablastic lymphoma: a literature review of Japanese plasmablastic lymphoma cases. Intern Med 2010;49:1801–5. [DOI] [PubMed] [Google Scholar]
- [29].Tiong IS, Strauss M, Lau MB, et al. Cutaneous plasmablastic lymphoma in an immunocompetent patient with long-term pyrimethamine use for essential thrombocythemia: a case report and literature review. Case Rep Hematol 2013;2013:541783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gogia A, Bakhshi S. Plasmablastic lymphoma of oral cavity in a HIV-negative child. Pediatr Blood Cancer 2010;55:390–1. [DOI] [PubMed] [Google Scholar]
- [31].McGlaughlin KL, Bajel A, Mow CD. A case of plasmablastic lymphoma harbouring an IgH/MYC translocation in a HIV negative individual. Pathology 2010;42:697–9. [DOI] [PubMed] [Google Scholar]
- [32].Teruya-Feldstein J, Chiao E, Filippa DA, et al. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol 2004;15:1673–9. [DOI] [PubMed] [Google Scholar]
- [33].Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol 2004;28:736–47. [DOI] [PubMed] [Google Scholar]
- [34].Borenstein J, Pezzella F, Gatter KC. Plasmablastic lymphomas may occur as post-transplant lymphoproliferative disorders. Histopathology 2007;51:774–7. [DOI] [PubMed] [Google Scholar]
- [35].Takahashi Y, Saiga I, Fukushima J, et al. Plasmablastic lymphoma of the retroperitoneum in an HIV-negative patient. Pathol Int 2009;59:868–73. [DOI] [PubMed] [Google Scholar]
- [36].Liu F, Asano N, Tatematsu A, et al. Plasmablastic lymphoma of the elderly: a clinicopathological comparison with age-related Epstein–Barr virus-associated B cell lymphoproliferative disorder. Histopathology 2012;61:1183–97. [DOI] [PubMed] [Google Scholar]
- [37].Verma S, Nuovo GJ, Porcu P, et al. Epstein–Barr virus- and human herpesvirus 8-associated primary cutaneous plasmablastic lymphoma in the setting of renal transplantation. J Cutan Pathol 2005;32:35–9. [DOI] [PubMed] [Google Scholar]
- [38].Rao DD, Aggarwal N, Anehosur V, et al. Plasmablastic lymphoma of the oral cavity in immunocompetent patients: report of two cases. Int J Oral Maxillofac Surg 2010;39:1036–9. [DOI] [PubMed] [Google Scholar]
- [39].Nicol I, Boye T, Carsuzaa F, et al. Post-transplant plasmablastic lymphoma of the skin. Br J Dermatol 2003;149:889–91. [DOI] [PubMed] [Google Scholar]
- [40].Ustun C, Reid-Nicholson M, Nayak-Kapoor A, et al. Plasmablastic lymphoma: CNS involvement, coexistence of other malignancies, possible viral etiology, and dismal outcome. Ann Hematol 2009;88:351–8. [DOI] [PubMed] [Google Scholar]
- [41].Pruneri G, Graziadei G, Ermellino L, et al. Plasmablastic lymphoma of the stomach. A case report. Haematologica 1998;83:87–9. [PubMed] [Google Scholar]
- [42].Kravetz JD, Rose MG, Payne-Blackman S, et al. Plasmablastic lymphoma presenting as an arm mass in an individual negative for human immunodeficiency virus: a case report. Clin Lymphoma Myeloma 2006;6:493–5. [DOI] [PubMed] [Google Scholar]
- [43].Dasanu CA, Bauer F, Codreanu I, et al. Plasmablastic haemato-lymphoid neoplasm with a complex genetic signature of Burkitt lymphoma responding to bortezomib. Hematol Oncol 2013;31:164–6. [DOI] [PubMed] [Google Scholar]
- [44].Miller DV, Mookadam F, Mookadam M, et al. Primary cardiac plasmablastic (diffuse large B-cell) lymphoma mimicking left ventricular aneurysm with mural thrombus. Cardiovasc Pathol 2007;16:111–4. [DOI] [PubMed] [Google Scholar]
- [45].Mondal SK, Bera H, Biswas PK, et al. High-grade plasmablastic neoplasm of humerus in an HIV-negative patient, which was indeterminate between plasmablastic lymphoma and plasmablastic myeloma. J Cancer Res Ther 2011;7:214–6. [DOI] [PubMed] [Google Scholar]
- [46].Black CL, Foster-Smith E, Lewis ID, et al. Post-transplant plasmablastic lymphoma of the skin. Australas J Dermatol 2013;54:277–82. [DOI] [PubMed] [Google Scholar]
- [47].Ojanguren J, Collazos J, Martínez C, et al. Epstein–Barr virus-related plasmablastic lymphomas arising from long-standing sacrococcygeal cysts in immunosuppressed patients. AIDS 2003;17:1582–4. [DOI] [PubMed] [Google Scholar]
- [48].Montes-Moreno S, Gonzalez-Medina AR, Rodriguez-Pinilla SM, et al. Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica 2010;95:1342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saba NS, Dang D, Saba J, et al. Bortezomib in plasmablastic lymphoma: a case report and review of the literature. Onkologie 2013;36:287–91. [DOI] [PubMed] [Google Scholar]
- [50].Robak T, Urbańska-Ryś H, Strzelecka B, et al. Plasmablastic lymphoma in a patient with chronic lymphocytic leukemia heavily pretreated with cladribine (2-CdA): an unusual variant of Richter's syndrome. Eur J Haematol 2001;67:322–7. [DOI] [PubMed] [Google Scholar]
- [51].Liu M, Liu B, Wang Q, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases. Oncol Rep 2015;33:1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee OJ, Kim KW, Lee GK. Epstein–Barr virus and human immunodeficiency virus-negative oral plasmablastic lymphoma. J Oral Pathol Med 2006;35:382–4. [DOI] [PubMed] [Google Scholar]
- [53].Jiang P, Liu M, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma in the neck: a rare case report and literature review. Eur J Med Res 2014;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Luria L, Nguyen J, Zhou J, et al. Manifestations of gastrointestinal plasmablastic lymphoma: a case series with literature review. World J Gastroenterol 2014;20:11894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Samoon Z, Idrees R, Masood N, et al. Plasmablastic lymphoma of the oral cavity with breast recurrence: a case report. BMC Res Notes 2015;8:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Matikas A, Kanellis G, Papadimitriou C, et al. Plasmablastic lymphoma of the breast in an immunocompetent patient: long-lasting complete response induced by chemotherapy and autologous stem cell trasplantation. Anticancer Res 2014;34:5111–5. [PubMed] [Google Scholar]
- [57].Haramura T, Haraguchi M, Irie J, et al. Case of plasmablastic lymphoma of the sigmoid colon and literature review. World J Gastroenterol 2015;21:7598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vourexakis Z, Dulguerov P. Extensive maxillofacial plasmablastic lymphoma in an immunocompetent patient. BMJ Case Rep 2014;2014. pii: bcr2014204042. doi: 10.1136/bcr-2014-204042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jain BB, Majumdar A, Chowdhary KM. Plasmablastic lymphoma versus anaplastic myeloma in a human immunodeficiency virus negative male: a diagnostic quandary. Arch Iran Med 2013;16:608–10. [PubMed] [Google Scholar]
- [60].Dorwal P, Sachdev R, Mishra P, et al. Extraoral plasmablastic lymphoma detected using ascitic fluid cytology and flow cytometry: a case report with a review of the literature. Acta Cytol 2014;58:309–17. [DOI] [PubMed] [Google Scholar]
- [61].Nguyen DD, Loo BW, Tillman G, et al. Plasmablastic lymphoma presenting in a human immunodeficiency virus-negative patient: a case report. Ann Hematol 2003;82:521–5. [DOI] [PubMed] [Google Scholar]
- [62].Cha JM, Lee JI, Joo KR, et al. A case report with plasmablastic lymphoma of the jejunum. J Korean Med Sci 2010;25:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Redmond M, Quinn J, Murphy P, et al. Plasmablastic lymphoma presenting as a paravertebral mass in a patient with Crohn's disease after immunosuppressive therapy. J Clin Pathol 2007;60:80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Castillo JJ, Reagan JL. Plasmablastic lymphoma: a systematic review. Sci World J 2011;11:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Valera A, Balagué O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol 2010;34:1686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lorsbach RB, Hsi ED, Dogan A, et al. Plasma cell myeloma and related neoplasms. Am J Clin Pathol 2011;136:168–82. [DOI] [PubMed] [Google Scholar]


