Abstract
Group B Streptococcus (GBS) is a leading cause of morbidity and mortality in infants, and colonization of the maternal genital tract is the primary risk factor for newborn infection. Despite the importance of mucosal colonization in GBS pathogenesis, relevant host and bacterial factors are incompletely understood. We investigated the role of humoral immunity in clearance of vaginal colonization in vivo. B-cell-deficient mice or those lacking neonatal Fc-receptor, a mediator of IgG transport to the vaginal mucosa, exhibit prolonged GBS vaginal colonization compared to wild type animals. Intranasal but not intramuscular immunization induced systemic and mucosal immune responses and decreased GBS colonization duration without altering initial colonization density. Vaccine-induced clearance of GBS was serotype-specific, suggesting a role for anti-capsule antibodies in protection. Our results support a role for humoral immunity in GBS eradication from the female genital tract and suggest that mucosal vaccination may prime colonization clearance.
Keywords: Group B Streptococcus, vaccine, colonization
INTRODUCTION
Group B Streptococcus (GBS; Streptococcus agalactiae) can be transmitted to the newborn via mucosal exposure during parturition, or to the fetus via ascension of the organism from the vagina to the placenta or amniotic fluid. Colonization of the maternal genital tract is the primary risk factor for infection and occurs in 10–30% of pregnant women [1]. Clinical guidelines advocate GBS screening at 35–37 weeks gestation with intrapartum antibiotic prophylaxis (IAP) for colonized women [2]. These efforts have decreased early–onset (EO) GBS disease rates by 80% [3]. Late-onset (LO) GBS has not decreased significantly [4]. Missed opportunities for GBS prevention contribute to the ongoing burden of EO GBS disease [5]. No GBS vaccine is currently licensed, but candidate polysaccharide conjugate vaccines are in trials [6].
Immune mechanisms of GBS colonization clearance are incompletely understood. Recent data implicate IL-17 as a host response to colonization and a possible mediator of clearance [7]. Clinical studies suggest that serotype-specific immune responses may follow asymptomatic colonization [8–10] and may mediate protection against disease or colonization. We investigated the role of humoral immunity in colonization clearance. IgG predominates over IgA at the vaginal mucosa [11], and the neonatal Fc-receptor (FcRn) functions to transport IgG to the mucosal surface throughout life [12,13]. We demonstrate that mice lacking either antibody production (µMT) or FcRn have a defect in clearance of GBS from the vagina and that mucosal immunization primes clearance of colonization.
MATERIALS AND METHODS
Animals, bacterial strains and cell lines
Experiments involving animals were carried out in accordance with institutional and National Institutes of Health guidelines. Female wild-type C57BL6/J (8–12 weeks), FcRn-knockout (KO) mice (B6.129X1-Fcgrttm1Dcr/DcrJ) and µMT mice (B6.129S2-Ighmtm1Cgn/J) were from Jackson Laboratories. Streptomycin-resistant derivatives of the fully sequenced GBS strains CNCTC 10/84 (serotype V) [14] and COH1 (serotype III) [15]were grown at 37°C in trypticase soy (TS) and plated on CHROMagar™ StrepB plates. Human Endometrial Carcinoma (HEC-1A) cells (ATCC HTB-112) were propagated in McCoy’s 5a Medium with 10% heat-inactivated FBS and 1% sodium pyruvate at 37°C, 5% CO2.
Vaginal Colonization
The vaginal colonization protocol was adapted from Randis et al. [16]. Female mice at 8–12 weeks of age were estrus-synchronized (10 µg 17β-estradiol subcutaneously 48 and 24 hours prior to colonization). Washed stationary phase GBS was resuspended in a mixture of 1:1 TS broth and sterile 10% gelatin, final concentration 108 CFU/mL. Following anesthesia with 3–5% isoflurane, 50 µL GBS-gelatin mixture was administered intravaginally. Animals were housed separately for the remainder of the experiment. Vaginal swabs were collected every 2–3 days using a sterile, polyester-tipped swab agitated in 300 µL PBS, with serial dilutions plated to quantify organism recovery. Candidate GBS colonies were patched onto Columbia Naladixic Acid, 5% Sheep Blood to confirm β-hemolysis. Clearance of GBS was defined as two consecutive swabs without detectable colonization (lower limit 100 CFU/mL) 48–72 hours apart.
Immunohistochemistry
HEC-1A cells were grown to ~50% confluence in glass-bottomed chamber slides and fixed with 4% paraformaldehyde. Non-specific binding was blocked using 5% donkey serum with 0.2% triton-X 100 diluted in PBS. Primary antibody, rabbit polyclonal anti-FcRn IgG (Santa Cruz; 50 µg/mL), was added and incubated for 1 hour. Following washes with 0.2% Triton-X 100 in PBS, secondary antibody, donkey anti-rabbit IgG-AF488 (1:500), was incubated in the dark for 30 minutes. After washes, coverslips were mounted with Prolong Gold/DAPI (Life Technologies) and images acquired on a Zeiss AxioObserver Z1.
Adhesion Assay
HEC-1A cells were incubated with 50 µg/mL rabbit polyclonal anti-FcRn IgG or 50 µg/mL control rabbit polyclonal IgG for 1 hour at pH 7.4. Cells were washed with PBS and incubated in media at pH 6 for 30 min. GBS (CNCTC 10/84) liquid culture was grown to stationary phase and washed twice in PBS. GBS was then diluted in cell culture media to achieve desired MOIs and added to cells for 1 hr at 37°C/5% CO2. Wells were washed with sterile PBS, monolayers lifted via treatment with trypsin, and serial dilutions plated to enumerate CFUs.
Ethanol-killed whole-cell GBS preparation
Preparation of ethanol-killed bacteria was performed as previously described [17]. Briefly, 50 mL culture of GBS was grown overnight in TS at 37°C, centrifuged at 18,000g at 4°C, and washed twice with chilled DPBS. The pellet was resuspended in 5 mL of chilled DPBS and kept on ice. Chilled 100% EtOH was slowly added to the GBS-PBS suspension to a final concentration of 70%. The preparation was stirred at 4°C and centrifuged at 18,000×g for 10 minutes at 4°C. The pellet was washed twice with 20 mL chilled DPBS and resuspended in 2.5 mL of sterile DPBS. To confirm sterility, 25 µL of the preparation was added to 5 mL of sterile TS broth, and another 25 µL was plated on blood agar and both were incubated at 37°C overnight. The killed whole-cell GBS preparation was aliquotted and stored at −80°C.
Vaccine preparation
The whole cell GBS vaccine consisted of ethanol-killed whole cell GBS (CNCTC 10/84) at a final concentration equivalent of ~109 CFU/mL mixed with cholera toxin subunit B (Sigma) at a final concentration of 80 µg/mL (diluted in DPBS). The control vaccine preparation consisted of cholera toxin subunit B in DPBS at a final concentration of 80 µg/mL.
Immunization
For active immunization experiments, mice were anesthetized and vaginally swabbed to establish baseline antibody titers on day 1. Mice that were being immunized intranasally then received 25 µL of either GBS or control vaccination into their left naris. On day 2, mice were again anesthetized and immunized with 25 µL of their respective vaccine into their right naris. The intranasal vaccine was given over two days due to volume restraints when administering liquid via this route. Mice that were being immunized intramuscularly received 50 µL of either GBS or control vaccination in their right caudal thigh muscle on Day 1. A 50 µL dose of vaccine has 1.8 × 108 CFU equivalent of GBS. Mice received equal dosed boosters in the same manner every two weeks until GBS intravaginal inoculation on day 34 post-immunization. Vaginal swab specimens were collected weekly initially, then every 2–3 days following intravaginal GBS colonization. Blood samples were obtained biweekly throughout the entire experiment to measure plasma antibody titers (alternate weeks from boosters). Mice were anesthetized, and 100–150 µL blood obtained from the retro-orbital venous sinus or the submandibular veins.
For passive immunization, 24 hr prior to colonization, estrus-synchronized animals received an intraperitoneal injection of 100 µL of immune serum from rabbits vaccinated with ethanol-killed whole-cell GBS (Cocalico; anti-GBS antibody titers of >1:3×106 as determined by ELISA), or normal rabbit serum (anti-GBS antibody titers of 1:3×104 by ELISA).
Whole-cell GBS ELISA for plasma and vaginal titer determination
Vaginal swab specimens and plasma samples were analyzed by ELISA. GBS was grown to stationary phase, centrifuged, and resuspended in coating buffer (0.71% Na HCO3, 0.16% Na2CO3 in sterile water, pH 9.5). ELISA plates were then coated with whole cell GBS in coating buffer and incubated overnight at 4°C. Plates were then washed twice with washing buffer (0.05% Tween-20 in PBS) and blocking solution (10% FBS in PBS) added to each well for 1 hr. Samples were added to each well at five-fold dilutions (in blocking solution) for 2 hr at RT, followed by the addition of the secondary antibody, goat anti-mouse IgG-HRP (1:1000), goat anti-rabbit IgG-HRP, or HRP-goat anti-mouse IgA (1:500), incubated for 1 hour, and washed five times. Internal controls were included on each plate. Negative controls were prepared as above, with exclusion of the primary antibody. Positive controls for the IgG ELISA consisted of wells coated with recombinant vaginolysin (VLY, 10 µg/100 ml coating buffer), an immunogenic toxin produced by G. vaginalis [18] treated with monoclonal mouse anti-VLY IgG (GenScript, 1:10 dilution). Positive controls for the IgA ELISA consisted of wells coated with IgA, κ from murine myeloma clone TEPC-15 (Sigma) with addition of secondary HRP-goat anti-mouse IgA. TMB ELISA substrate was added (10 min, dark incubation), and the reaction was stopped with 2N sulfuric acid. OD450 was read on a Tecan Infinite M200. Titers were defined as the last dilution at which OD450 > 0.125.
Statistics
Colony counts and titer values were compared using Mann-Whitney-U analyses. Colonization clearance times of various groups were compared using Mantel-Cox analyses.
RESULTS
Humoral immunity contributes to clearance of GBS vaginal colonization in vivo
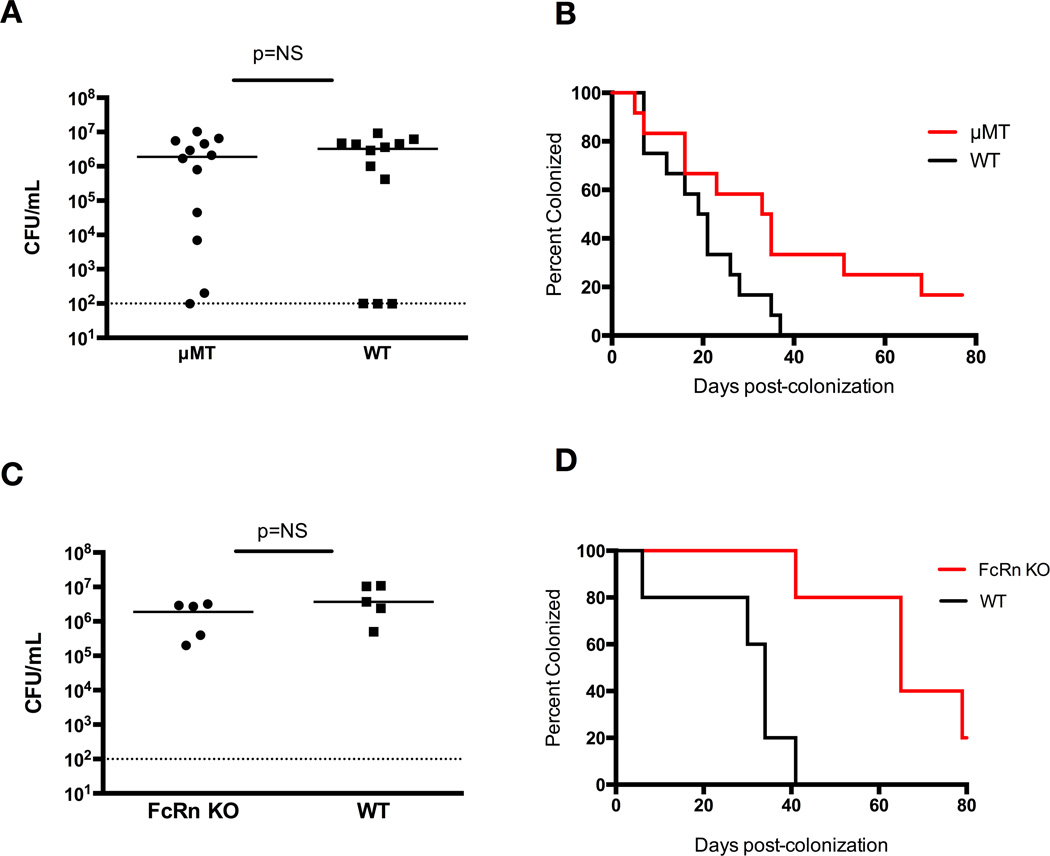
We sought to determine the role of humoral immunity in the maintenance of GBS colonization [16]. There was no difference in GBS colonization density of WT mice and µMT mice at 48 hours (Figure 1A). The µMT mice demonstrated a prolonged time to colonization clearance, with median clearance times of 34 days in the µMT vs. 20 days in the WT (P<0.05, log-rank) (Figure 1B). Similarly, FcRn-KO mice had comparable initial colonization densities to WT (Figure 1C). The FcRn-KO group demonstrated a prolonged time to colonization clearance (median clearance time of 65 days vs. 34 days in the WT group; P=0.005, log-rank) (Figure 1D).
Figure 1. The humoral immune response plays a role in host defense against GBS colonization.
(A) Vaginal CFU/mL GBS at 48 hr post-colonization of µMT mice vs. WT mice shows similar initial colonization densities. (B) Kaplan-Meier curve demonstrates significantly prolonged vaginal GBS colonization in µMT mice, 10 mice per group (P<0.05, log-rank). (C) Vaginal CFU/mL GBS at 48 hrs post-colonization of FcRn-KO mice vs. WT mice. (D) FcRn-KO mice have significantly prolonged vaginal GBS colonization, 5 mice per group (P<0.005, log-rank).
In order to evaluate the possibility that GBS could bind directly to FcRn, we performed adhesion assays using HEC-1A cells. HEC-1A cells expressed FcRn, as demonstrated by immunofluorescence (Supplemental Figure 1A), consistent with prior reports [19]. FcRn blocking antibody did not impair GBS adherence to HEC-1A cells compared to control IgG (Supplemental Figure 1B).
Intranasal immunization with ethanol-killed whole cell GBS induces a robust immune response and enhances GBS clearance
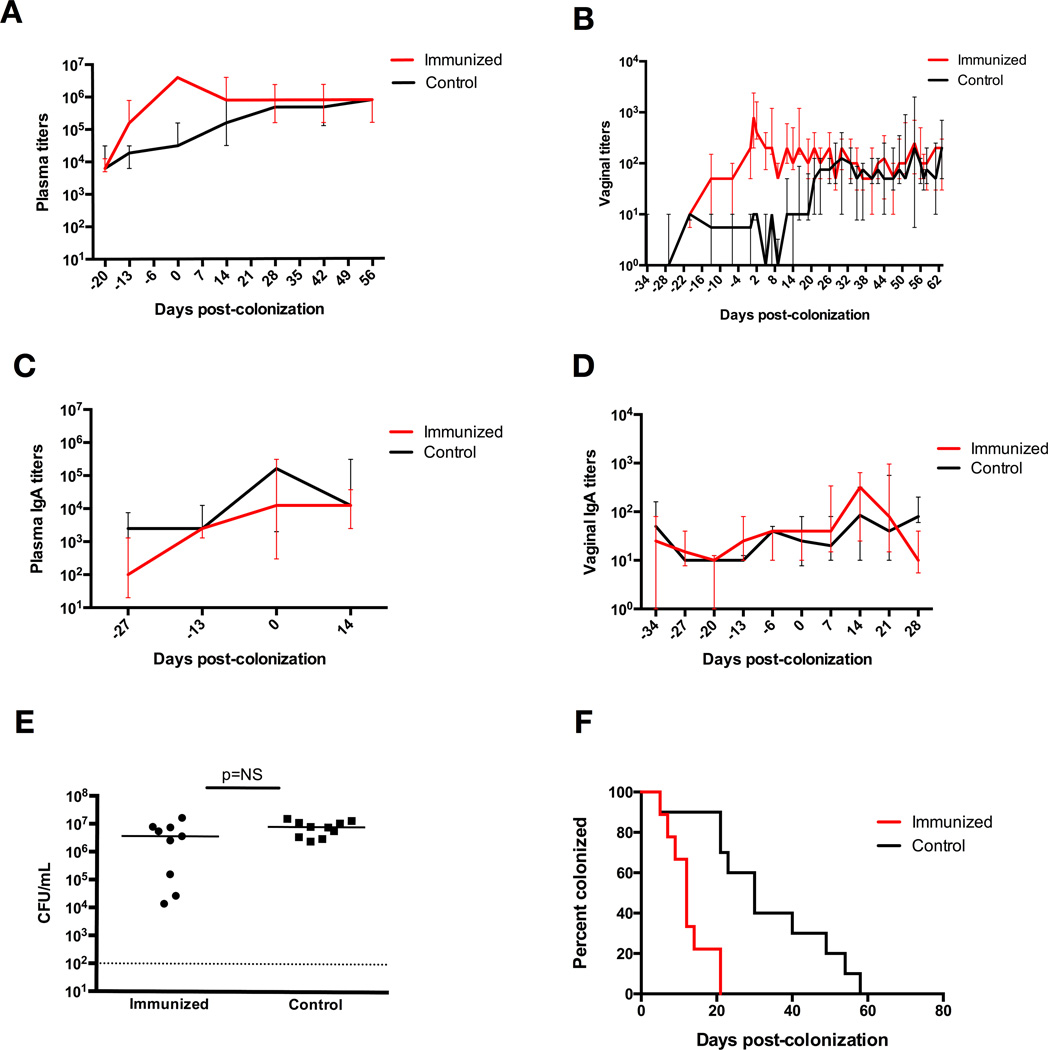
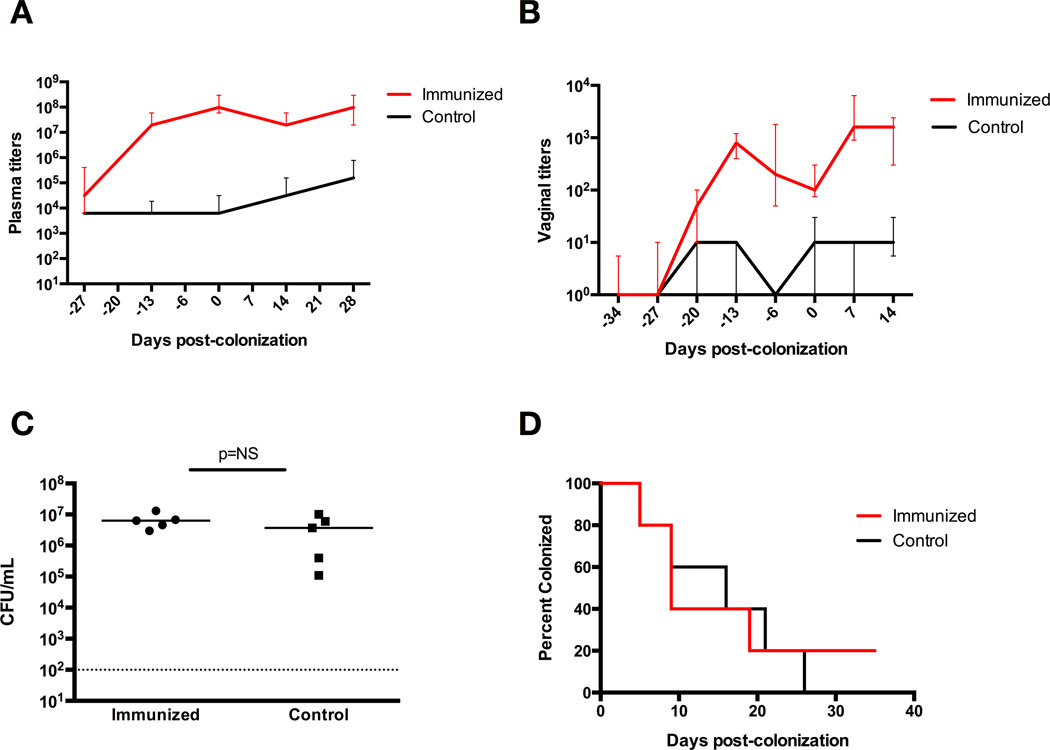
We immunized mice intranasally with ethanol-killed whole cell GBS combined with the B subunit of cholera toxin acting as an adjuvant, or a vehicle control using PBS with cholera toxin B. Systemic IgG antibody titers revealed a significant immune response in the mice receiving whole-cell GBS vaccine three weeks after immunization (P<0.0001, Mann-Whitney-U analysis), with median titers reaching 1:3×106 in the immunized group, compared to a maximum median titer of 1:3×104 in the control group (Figure 2A). We also measured vaginal mucosal IgG titers and saw a significant immune response at three weeks post-immunization (P<0.001) (Figure 2B), with peak median titer at five weeks post-immunization of 1:800 in the GBS immunized mice, compared to 1:10 in control mice. Plasma and vaginal mucosal IgA titers were similar between the two groups (Figure 2C, 2D). The mice were then vaginally inoculated with the same strain of live GBS 34 days after immunization. All mice had similar GBS burdens 48 hours after colonization (Figure 2E). Immunized mice demonstrated faster GBS colonization clearance, with a median clearance time of 12 days, compared to 30 days in the control group (P<0.001, log-rank) (Figure 2F).
Figure 2. Intranasal active immunization induces IgG immune response and enhances GBS clearance from the vaginal tract of WT mice.
(A) Plasma anti-GBS IgG titers, with significantly elevated titers in immunized mice three weeks after vaccination, at day −13 (P<0.0001, Mann-Whitney-U analysis) until four weeks after colonization, at which time the titers become statistically similar between the two groups. Data points represent median values with interquartile ranges. (B) Vaginal anti-GBS IgG titers, with significantly elevated titers in immunized mice three weeks after vaccination, at day −13 (P<0.001, Mann-Whitney-U analysis), until three weeks after colonization, at which time the titers become statistically similar between the two groups. Data points represent median values with interquartile ranges. (C) Plasma anti-GBS IgA titers with elevated titers in both groups of mice five weeks after immunization, but no significant difference between the two groups. Data points represent median values with interquartile ranges. (D) Vaginal anti-GBS IgA titers with no significant difference between the two groups. Data points represent median values with interquartile ranges. (E) Vaginal CFU/mL GBS at 48 hrs post-colonization of immunized mice vs. control mice shows similar initial colonization densities. (F) Kaplan-Meier curve demonstrates significantly enhanced vaginal GBS colonization in immunized WT mice, with median clearance times of 12 days in immunized mice and 30 days in control mice, 10 mice per group (P<0.001, log-rank).
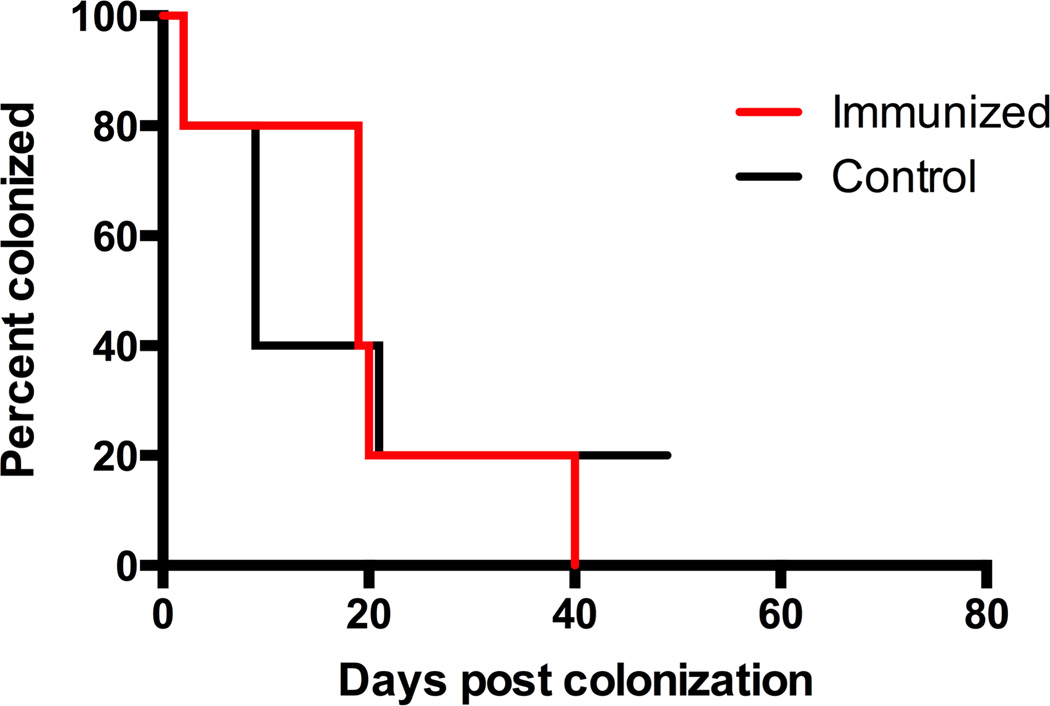
In order to determine whether prior vaccination and/or colonization conferred a benefit for subsequent colonization clearance, both groups of mice were then re-colonized with the same strain of GBS four weeks after the last control mouse had cleared the initial colonization. All mice had similar GBS burdens initially. When followed over time, the two groups were found to have statistically similar times to colonization clearance (P=0.81, log-rank), with both groups notably clearing colonization more quickly than the control group in Figure 2F (Figure 3).
Figure 3. Prior colonization with GBS enhances clearance of subsequent colonization from the vaginal tract.
Kaplan-Meier curve demonstrates enhanced GBS clearance after re-colonization of both previously immunized mice and mice that had previously received the sham vaccine, with median clearance times of 19 days in the immunized mice and 9 days in the control mice, 5 mice per group (P=0.81, log-rank).
Enhanced GBS colonization clearance with intranasal immunization with ethanol-killed whole-cell GBS is serotype-specific
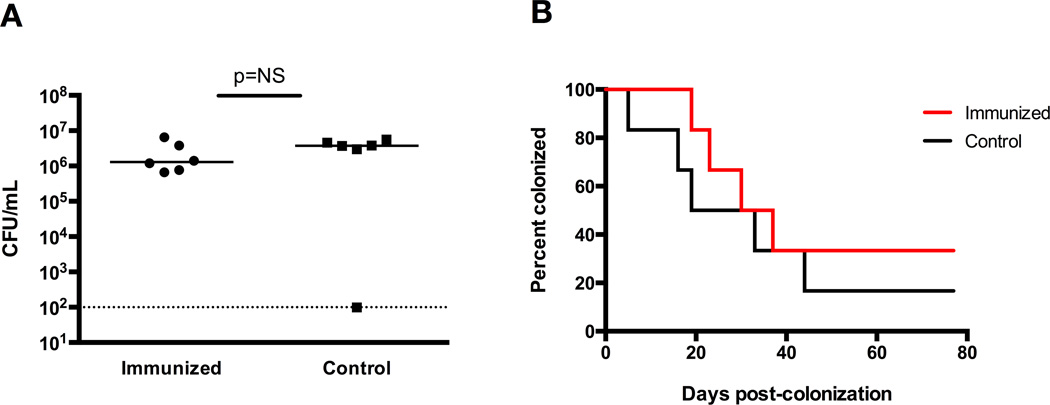
Two groups of WT mice were immunized intranasally with either the whole cell GBS or control vaccine. All mice were then colonized with the COH-1 GBS strain (serotype III) five weeks after immunization, with similar initial GBS burdens (Figure 4A). Immunized and control groups had statistically similar median times to colonization clearance, with a median clearance time of 33.5 days in the immunized mice and 26 days in the control mice (P=0.48, log-rank) (Figure 4B).
Figure 4. Intranasal immunization with killed whole-cell GBS induces serotype-specific enhancement of clearance from the female genital tract.
(A) Vaginal CFU/mL GBS at 48 hrs post-colonization of immunized mice vs. control mice shows similar initial colonization densities. (B) Kaplan-Meier curve demonstrating statistically similar GBS colonization clearance times in the female genital tract of WT mice colonized with COH-1 (serotype III) after immunization with killed whole-cell 10/84 (serotype V) GBS, with median clearance times of 33.5 days in the immunized mice and 26 days in the control mice, 6 mice per group (P=0.48, log-rank).
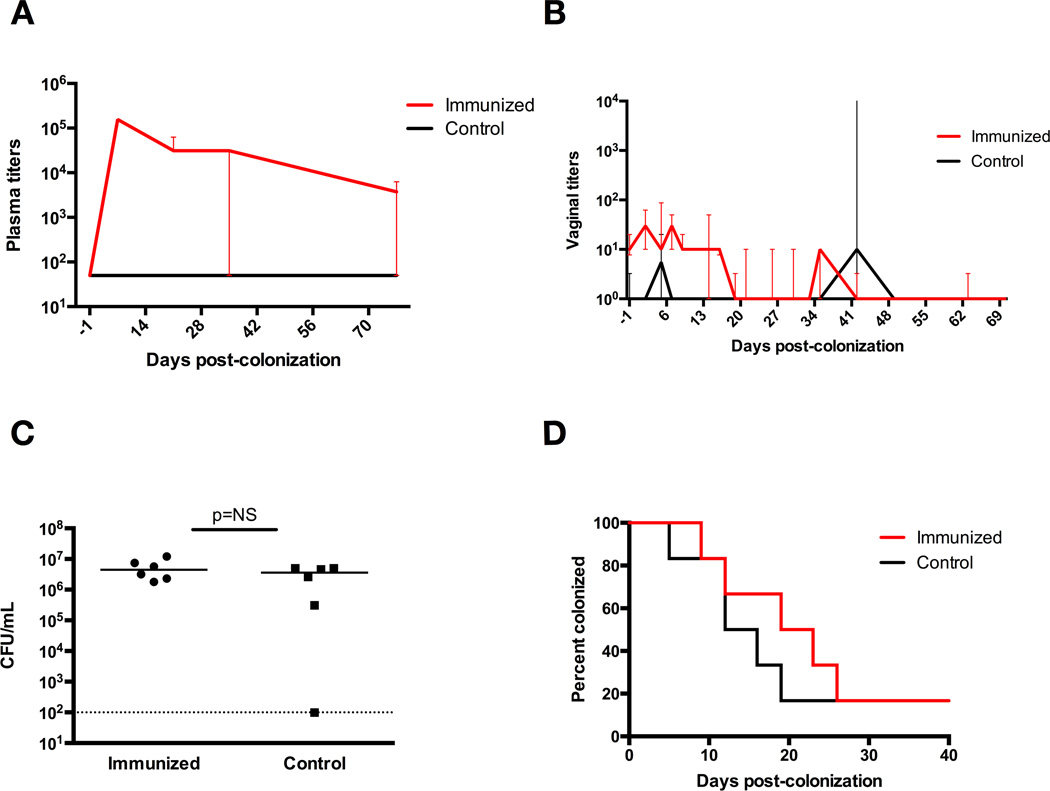
Intramuscular vaccination induces an immune response but does not enhance GBS clearance
Following intramuscular immunization, there were significant increases in anti-GBS IgG titer (both systemic and vaginal) beginning three weeks after immunization (P<0.01, Mann-Whitney-U analysis) (Figure 5A, 5B). Mice were then vaginally colonized with GBS (CNCTC 10/84) 34 days after immunization. The groups had similar GBS burden after colonization (Figure 5C). Despite the significant immune response noted in both the plasma and vaginal mucosa, the two groups of mice did not have significantly different times to colonization clearance, with median clearance times of 9 days in the immunized mice and 16 days in the control mice (P=0.87, log-rank) (Figure 5D). It is notable that both groups appeared to clear GBS colonization from the vaginal tract more quickly than typical WT clearance times noted in other experiments.
Figure 5. Intramuscular immunization induce immune response but do not enhance GBS colonization clearance from the vaginal tract of WT mice.
(A) Vaginal CFU/mL GBS at 48 hrs post-colonization of intramuscularly immunized mice and control mice, demonstrating similar initial colonization densities. (B) Serum anti-GBS IgG titers after intramuscular active immunization, with significantly elevated titers in immunized mice beginning three weeks after vaccination (P<0.01, Mann-Whitney-U analysis). Data points represent median values with interquartile ranges. (C) Vaginal anti-GBS IgG titers after intramuscular active immunization, with significantly elevated titers in immunized mice beginning three weeks after vaccination, at day −13 (P<0.01, Mann-Whitney-U analysis). Data points represent median values with interquartile ranges. (D) Kaplan-Meier curve demonstrates no significant difference in vaginal GBS clearance in intramuscularly immunized WT mice compared to control mice, with median clearance times of 9 days in immunized mice and 16 days in control mice, 5 mice per group (P=0.87, log-rank).
Passive immunization does not prime GBS colonization clearance
Similarly, we passively immunized mice with anti-whole-cell GBS rabbit immune serum. Two groups of WT mice were passively immunized with an intraperitoneal injection of 100 µL of either anti-GBS immune serum or control rabbit serum 24 hours prior to intravaginal colonization with GBS. Plasma IgG titers were measured every two weeks and vaginal IgG titers were measured weekly. We observed a significant immune response in the plasma within one week after immunization, with IgG titers of 1:1×105 compared to 1:50 in the control group, an effect that was sustained until five weeks after immunization (P<0.005, Mann-Whitney-U analysis) (Figure 6A). The immunized mice had a weak but significant vaginal immune response, with IgG titers reaching a peak of 1:75 on day 3 post-immunization, compared to 1:1 in the control group (P<0.005, Mann-Whitney-U analysis) (Figure 6B). After intravaginal colonization with the same strain of GBS (strain 10/84), all mice had a statistically similar GBS burden at 48 hours (Figure 6C). The two groups of mice had statistically similar times to colonization clearance, with a median clearance time of 21 days in immunized mice and 14 days in control mice (P=0.55, log-rank) (Figure 6D).
Figure 6. Passive immunization induces immune response but does not enhance GBS colonization clearance from the vaginal tract of WT mice.
(A) Vaginal CFU/mL GBS at 48 hrs post-colonization of immunized mice and control mice, demonstrating similar initial colonization densities. (B) Plasma anti-GBS IgG titers after intraperitoneal passive immunization, with significantly elevated titers in immunized mice noted within one week of vaccination, sustained until 5 wks post-immunization (P<0.005, Mann-Whitney-U analysis). Data points represent median values with interquartile ranges. (C) Vaginal anti-GBS IgG titers after intraperitoneal passive immunization, with mildly significant elevation of titers noted in immunized mice two days after colonization, sustained until 16 days post-colonization (P<0.05, Mann-Whitney-U analysis). Data points represent median values with interquartile ranges. (D) Kaplan-Meier curve demonstrates no significant difference in vaginal GBS clearance in immunized WT mice compared to control mice, with median clearance times of 21 days in immunized mice and 14 days in control mice, 6 mice per group (P=0.55, log-rank).
DISCUSSION
We investigated the contribution of humoral immunity to the clearance of GBS colonization and to investigate the role of immunization in colonization clearance. Other groups have studied GBS immunization strategies, typically using murine models of systemic infection [20,21] or protection against lethal neonatal disease [20,22,23]. Other reports have investigated non-vaccine mechanisms of control of GBS vaginal colonization in murine models [24–26].
Mature B cells are important in GBS colonization clearance from the female genital tract, as demonstrated by the prolonged clearance time in µMT mice. The eventual clearance of colonization in these mice suggests an additional antibody-independent mechanism of GBS clearance. FcRn-mediated transcytosis of IgG has been shown to operate bidirectionally [27], suggesting a route for both sensing and responding to infections. FcRn is expressed in uterine and vaginal tissue of mice [13]. The prolonged vaginal GBS colonization observed in the FcRn-KO mice suggests a potential role for the presence of local anti-GBS IgG in host defense against GBS colonization. We hypothesized that IgG produced from active immunization with ethanol-killed whole-cell GBS would be transported across the vaginal mucosa and contribute to local immune defense to enhance colonization clearance.
Prior data demonstrate an immune response to both colonization with GBS [28,29]and immunization against a variety of GBS proteins [30–32]. Intranasal immunization of mice with Rib, a GBS surface protein, induces systemic IgG and local IgA antibody responses and also confers protection against an intraperitoneal LD90 bacterial challenge [21]. Maione et al. immunized pregnant mice with a combination of four GBS surface proteins and found protection of pups against LD90 injections of GBS [33]. Likewise, maternal vaccination with recombinant GAPDH from GBS was protective in models of neonatal infection [23]. Santillan et al. intravaginally infected mice 46 days after immunization with C5a peptidase, and found decreased maternal colonization and protection of pups born to vaccinated dams [34,35]. The generation of GBS-specific antibodies is likely responsible for protection from GBS observed in these studies and our own. However, direct comparison of the host antibody response to various vaccination strategies is complicated by the lack of a standardized approach to titer determination.
Maternal antibody against the GBS polysaccharide capsule is associated with a reduction in neonatal disease [8,36]. Purified type-III polysaccharide from human immune serum is protective against infection with a homologous strain in neonatal rats [37]. Thus, development of GBS vaccines in humans has focused on the use of purified capsular polysaccharides with or without protein conjugates [38–44]. Our data support the potential significance of anti-capsule antibody as a mediator of colonization clearance, as the protection derived from our intranasal vaccine appears to be serotype-specific.
Vaccination route played a significant role in enhancement of colonization clearance, with intranasal (but not intramuscular) immunization producing a significant effect. Others have shown the efficacy of intranasal vaccination against viral vaginal pathogens [45–48], in addition investigations of the effects of intranasal immunization using GBS antigens [21,32]. Mucosal vaccination may provide superior protection against pathogens of the genital mucosa. Intranasal immunization with a live thymidine kinase-deficient HSV-2 induces production of effector T cells and their transport to the vaginal mucosa, whereas intraperitoneal immunization does not. These effects of mucosal immunization led to superior protection against intravaginal HSV-2 challenge. Xue et al. noted a superior immune response against GBS-specific antigens in intranasally immunized mice with significantly higher serum IgG and vaginal IgA levels than control, whereas subcutaneous immunization only resulted in elevated serum IgG levels. Of note, Xue et al. also demonstrated that the mucosal group immunized with an adjuvant elicited a greater response than the group without adjuvant. Our finding of rapid clearance of GBS from both the control and GBS-immunized mice via the intramuscular route demonstrate a potential role for systemic adjuvant in GBS colonization clearance, a non-specific effect shown by other groups [49].
Cholera toxin B is a mucosal adjuvant known to induce immune responses in the female vaginal tract [50,51]. Johansson et al. [51] investigated the vaginal immune response after administering cholera toxin or its B subunit, determining that the vaginal and intranasal routes were more efficient than intraperitoneal and perioral routes in stimulating local IgA responses, with the intranasal route inducing high levels from the vagina to the fallopian tubes.
Our results support the notions that humoral immunity enhances GBS colonization clearance from the female genital tract and that vaccination may prime colonization clearance. It is clear that additional elements of immunity play a role in colonization clearance. Patras et al. recently defined a role for IL-17 in GBS clearance [7]. Further work is needed to determine the specific roles of T cells, dendritic cells and other immune components. Understanding immune mechanisms underlying GBS clearance would facilitate the development of vaccine strategies targeting colonization, which could in turn aid population-level control of GBS disease.
Supplementary Material
(A) Immunohistochemistry demonstrating FcRn-blocking antibody binds to HEC-1A cells. Top panels show control images, with no primary Ab on left, and no secondary Ab on right. Bottom panel demonstrates binding of FcRn-blocking Ab (green) to HEC-1A cells (blue). Scale bars = 10µm. (B) Adhesion assay demonstrates equivalent CFU/mL GBS when Fc-receptor blocked vs. control (P<0.001 for FcRn-blocked cells at MOI 1 vs. 10, and p<0.001 for control). There were identical exposure times across all groups.
HIGHLIGHTS.
Asymptomatic colonization is the major risk factor for GBS disease, but relevant immune mechanisms are poorly understood.
Mice lacking antibody or the neonatal Fc receptor had prolonged vaginal GBS colonization compared to wild-type controls.
Mucosal but not systemic GBS immunization primed colonization clearance.
Acknowledgments
FUNDING INFORMATION
This work was supported by the National Institutes of Health [R01 AI092743, R33 AI098654 to A.J.R.; K23 HD065844 to T.M.R.]. The sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
All authors: no conflicts.
REFERENCES
- 1.Natarajan G, Johnson YR, Zhang F, Chen KM, Worsham MJ. Real-time polymerase chain reaction for the rapid detection of group B streptococcal colonization in neonates. Pediatrics. 2006;118:14–22. doi: 10.1542/peds.2005-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease: Revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 3.Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360:2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 4.Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, et al. Revisiting the Need for Vaccine Prevention of Late-Onset Neonatal Group B Streptococcal Disease: A Multistate, Population-Based Analysis. Pediatr Infect Dis J. 2008;27:1057–1064. doi: 10.1097/INF.0b013e318180b3b9. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine. 2016;34:2876–2879. doi: 10.1016/j.vaccine.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 7.Patras KA, Rösler B, Thoman ML, Doran KS. Characterization of host immunity during persistent vaginal colonization by Group B Streptococcus. Mucosal Immunol. 2015;8:1339–1348. doi: 10.1038/mi.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 9.Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Natural acquired humoral immunity against serotype-specific group B Streptococcus rectovaginal colonization acquisition in pregnant women. Clin Microbiol Infect. 2015;21:568.e13–568.e21. doi: 10.1016/j.cmi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Herbert J, Thomas S, Brookes C, Turner C, Turner P, Nosten F, et al. Antibody-Mediated Complement C3b/iC3b Binding to Group B Streptococcus in Paired Mother and Baby Serum Samples in a Refugee Population on the Thailand-Myanmar Border. Clin Vaccine Immunol. 2015;22:319–326. doi: 10.1128/CVI.00803-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson M, Lycke NY. Immunology of the human genital tract. Curr Opin Infect Dis. 2003;16:43. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooven TA, Randis TM, Daugherty SC, Narechania A, Planet PJ, Tettelin H, et al. Complete Genome Sequence of Streptococcus agalactiae CNCTC 10/84, a Hypervirulent Sequence Type 26 Strain. Genome Announc. 2014;2:e01338–e01314. doi: 10.1128/genomeA.01338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial "pan-genome". Proc Natl Acad Sci U S A. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randis TM, Gelber SE, Hooven TA, Abellar RG, Akabas LH, Lewis EL, et al. Group B Streptococcus β-hemolysin/Cytolysin Breaches Maternal-Fetal Barriers to Cause Preterm Birth and Intrauterine Fetal Demise in Vivo. J Infect Dis. 2014;210:265–273. doi: 10.1093/infdis/jiu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y-J, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010;17:1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelber SE, Aguilar JL, Lewis KLT, Ratner AJ. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol. 2008;190:3896–3903. doi: 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, et al. The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodeur BR, Boyer M, Charlebois I, Hamel J, Couture F, Rioux CR, et al. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect Immun. 2000;68:5610–5618. doi: 10.1128/iai.68.10.5610-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson C, Holmgren J, Lindahl G, Bergquist C. Intranasal immunization of mice with group B streptococcal protein rib and cholera toxin B subunit confers protection against lethal infection. Infect Immun. 2004;72:1184–1187. doi: 10.1128/IAI.72.2.1184-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin D, Rioux S, Gagnon E, Boyer M, Hamel J, Charland N, et al. Protection from group B streptococcal infection in neonatal mice by maternal immunization with recombinant Sip protein. Infect Immun. 2002;70:4897–4901. doi: 10.1128/IAI.70.9.4897-4901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madureira P, Andrade EB, Gama B, Oliveira L, Moreira S, Ribeiro A, et al. Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment. PLoS Pathog. 2011;7:e1002363. doi: 10.1371/journal.ppat.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavaco CK, Patras KA, Zlamal JE, Thoman ML, Morgan EL, Sanderson SD, et al. A novel C5a-derived immunobiotic peptide reduces Streptococcus agalactiae colonization through targeted bacterial killing. Antimicrob Agents Chemother. 2013;57:5492–5499. doi: 10.1128/AAC.01590-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Q, Nelson D, Zhu S, Fischetti VA. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother. 2005;49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Gregorio PR, Juárez Tomás MS, Leccese Terraf MC, Nader Macías MEF. Preventive effect of Lactobacillus reuteri CRL1324 on Group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol. 2015;118:1034–1047. doi: 10.1111/jam.12739. [DOI] [PubMed] [Google Scholar]
- 27.Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, et al. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hordnes, Tynning, Kvam, Bevanger, Brown, Jonsson, et al. Cervical Secretions in Pregnant Women Colonized Rectally with Group B Streptococci have High Levels of Antibodies to Serotype III Polysaccharide Capsular Antigen and Protein R. Scand J Immunol. 1998;47:179–188. doi: 10.1046/j.1365-3083.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 29.Hordnes K, Tynning T, Kvam AI, Jonsson R, Haneberg B. Colonization in the rectum and uterine cervix with group B streptococci may induce specific antibody responses in cervical secretions of pregnant women. Infect Immun. 1996;64:1643–1652. doi: 10.1128/iai.64.5.1643-1652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hordnes K, Tynning T, Brown TA, Haneberg B, Jonsson R. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine. 1997;15:1244–1251. doi: 10.1016/s0264-410x(97)00021-2. [DOI] [PubMed] [Google Scholar]
- 31.Hunter SK, Andracki ME, Krieg AM. Biodegradable microspheres containing group B Streptococcus vaccine: Immune response in mice. Am J Obstet Gynecol. 2001;185:1174–1179. doi: 10.1067/mob.2001.117658. [DOI] [PubMed] [Google Scholar]
- 32.Xue G, Yu L, Li S, Shen X. Intranasal immunization with GBS surface protein Sip and ScpB induces specific mucosal and systemic immune responses in mice. F FEMS Immunol Med Microbiol. 2010;58:202–210. doi: 10.1111/j.1574-695X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 33.Maione D, Margarit I, Rinaudo CD, Masignani V, Mora M, Scarselli M, et al. Identification of a Universal Group B Streptococcus Vaccine by Multiple Genome Screen. Science. 2005;309:148–150. doi: 10.1126/science.1109869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santillan DA, Andracki ME, Hunter SK. Protective immunization in mice against group B streptococci using encapsulated C5a peptidase. Am J Obstet Gynecol. 2008;198:114.e1–114.e6. doi: 10.1016/j.ajog.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Santillan DA, Rai KK, Santillan MK, Krishnamachari Y, Salem AK, Hunter SK. Efficacy of polymeric encapsulated C5a peptidase–based group B streptococcus vaccines in a murine model. Am J Obstet Gynecol. 2011;205:249.e1–249.e8. doi: 10.1016/j.ajog.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker CJ, Carey VJ, Rench MA, Edwards MS, Hillier SL, Kasper DL, et al. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis. 2014;209:781–788. doi: 10.1093/infdis/jit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Cueninck BJ, Eisenstein TK, McIntosh TS, Shockman GD, Swenson RM. Type-specific protection of neonatal rats from lethal group B streptococcal infection by immune sera obtained from human volunteers vaccinated with type III-specific polysaccharide. Infect Immun. 1982;37:961–965. doi: 10.1128/iai.37.3.961-965.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker CJ, Edwards MS, Kasper DL. Immunogenicity of polysaccharides from type III, group B Streptococcus. J Clin Invest. 1978;61:1107–1110. doi: 10.1172/JCI109011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker CJ, Kasper DL. Group B Streptococcal Vaccines. Rev Infect Dis. 1985;7:458–467. doi: 10.1093/clinids/7.4.458. [DOI] [PubMed] [Google Scholar]
- 40.Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Carey VJ, Hickman ME, et al. Use of capsular polysaccharide-tetanus toxoid conjugate vaccine for type II group B Streptococcus in healthy women. J Infect Dis. 2000;182:1129–1138. doi: 10.1086/315839. [DOI] [PubMed] [Google Scholar]
- 41.Baker CJ, Paoletti LC, Wessels MR, Guttormsen HK, Rench MA, Hickman ME, et al. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J Infect Dis. 1999;179:142–150. doi: 10.1086/314574. [DOI] [PubMed] [Google Scholar]
- 42.Baker CJ, Rench MA, Edwards MS, Carpenter RJ, Hays BM, Kasper DL. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N Engl J Med. 1988;319:1180–1185. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 43.Edwards MS, Baker CJ, Kasper DL. Opsonic Specificity of Human Antibody to the Type III Polysaccharide of Group B Streptococcus. J Infect Dis. 1979;140:1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- 44.Kasper DL, Paoletti LC, Wessels MR, Guttormsen HK, Carey VJ, Jennings HJ, et al. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallichan WS, Rosenthal KL. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 46.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–10553. doi: 10.1128/JVI.05441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato A, Suwanto A, Okabe M, Sato S, Nochi T, Imai T, et al. Vaginal memory T cells induced by intranasal vaccination are critical for protective T cell recruitment and prevention of genital HSV-2 disease. J Virol. 2014;88:13699–13708. doi: 10.1128/JVI.02279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29:158–163. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuipers K, Diavatopoulos DA, van Opzeeland F, Simonetti E, van den Kieboom CH, Kerstholt M, et al. Antigen-independent restriction of pneumococcal density by mucosal adjuvant Cholera Toxin subunit B. J Infect Dis. 2016;214:1588–1596. doi: 10.1093/infdis/jiw160. [DOI] [PubMed] [Google Scholar]
- 50.Bergquist C, Johansson EL, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansson EL, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Immunohistochemistry demonstrating FcRn-blocking antibody binds to HEC-1A cells. Top panels show control images, with no primary Ab on left, and no secondary Ab on right. Bottom panel demonstrates binding of FcRn-blocking Ab (green) to HEC-1A cells (blue). Scale bars = 10µm. (B) Adhesion assay demonstrates equivalent CFU/mL GBS when Fc-receptor blocked vs. control (P<0.001 for FcRn-blocked cells at MOI 1 vs. 10, and p<0.001 for control). There were identical exposure times across all groups.