Abstract
BACKGROUND
Limiting the duration of antimicrobial treatment constitutes a potential strategy to reduce the risk of antimicrobial resistance among children with acute otitis media.
METHODS
We assigned 520 children, 6 to 23 months of age, with acute otitis media to receive amoxicillin–clavulanate either for a standard duration of 10 days or for a reduced duration of 5 days followed by placebo for 5 days. We measured rates of clinical response (in a systematic fashion, on the basis of signs and symptomatic response), recurrence, and nasopharyngeal colonization, and we analyzed episode outcomes using a noninferiority approach. Symptom scores ranged from 0 to 14, with higher numbers indicating more severe symptoms.
RESULTS
Children who were treated with amoxicillin–clavulanate for 5 days were more likely than those who were treated for 10 days to have clinical failure (77 of 229 children [34%] vs. 39 of 238 [16%]; difference, 17 percentage points [based on unrounded data]; 95% confidence interval, 9 to 25). The mean symptom scores over the period from day 6 to day 14 were 1.61 in the 5-day group and 1.34 in the 10-day group (P = 0.07); the mean scores at the day-12-to-14 assessment were 1.89 versus 1.20 (P = 0.001). The percentage of children whose symptom scores decreased more than 50% (indicating less severe symptoms) from baseline to the end of treatment was lower in the 5-day group than in the 10-day group (181 of 227 children [80%] vs. 211 of 233 [91%], P=0.003). We found no significant between-group differences in rates of recurrence, adverse events, or nasopharyngeal colonization with penicillin-nonsusceptible pathogens. Clinical-failure rates were greater among children who had been exposed to three or more children for 10 or more hours per week than among those with less exposure (P=0.02) and were also greater among children with infection in both ears than among those with infection in one ear (P<0.001).
CONCLUSIONS
Among children 6 to 23 months of age with acute otitis media, reduced-duration antimicrobial treatment resulted in less favorable outcomes than standard-duration treatment; in addition, neither the rate of adverse events nor the rate of emergence of antimicrobial resistance was lower with the shorter regimen. (Funded by the National Institute of Allergy and Infectious Diseases and the National Center for Research Resources; ClinicalTrials.gov number, NCT01511107.)
Next to the common cold, acute otitis media is the most frequently diagnosed illness in children in the United States1 and the most commonly cited indication for antimicrobial treatment.2 Concerns about the possible encouragement of antimicrobial resistance have led to recommendations by some clinicians that antimicrobial agents be withheld in large subgroups of children with acute otitis media, unless symptoms persist or worsen.3 However, two trials lend support for routine antimicrobial treatment in young children, because affected participants younger than 3 years of age who received antimicrobial treatment for 7 or 10 days had more favorable outcomes than those who received placebo.4,5
A potential strategy for reducing the risk of antimicrobial resistance is to limit the duration of antimicrobial treatment.3,6 Clinical trials that have compared reduced-duration treatment with standard-duration treatment in children with acute otitis media have shown either no difference in outcome or modest differences that favor standard-duration treatment (composite number needed to treat to prevent clinical failure, 28).6 Given methodologic limitations in available publications,7 we undertook the current trial involving children 6 to 23 months of age to determine whether limiting antimicrobial treatment to 5 days rather than using the standard 10-day regimen would afford equivalent outcomes and whether doing so also for subsequent episodes would lead to a reduction in the overall use of antimicrobial treatment, with a resulting reduction in the development of antimicrobial resistance.
METHODS
ELIGIBILITY AND ENROLLMENT
We conducted this trial from January 2012 through September 2015 at Children’s Hospital of Pittsburgh, at affiliated pediatric practices, and at Kentucky Pediatric and Adult Research in Bardstown, Kentucky. The protocol, available with the full text of this article at NEJM.org, was approved by the institutional review board at each site. Written informed consent was obtained from a parent of each enrolled child. The authors attest that the study was performed in accordance with the protocol, including its statistical analysis plan. There was no commercial support for this study.
Eligible children, 6 to 23 months of age, were required to have received at least two doses of pneumococcal conjugate vaccine and to have acute otitis media that was diagnosed on the basis of three criteria — onset of symptoms within the preceding 48 hours that parents rated with a total score of 3 or more on the seven-item semiquantitative Acute Otitis Media–Severity of Symptoms (AOM-SOS) scale,8,9 the presence of middle-ear effusion, and moderate or marked bulging of the tympanic membrane or slight bulging accompanied by apparent otalgia or marked tympanic-membrane erythema. The AOM-SOS scale consists of seven discrete items — tugging of ears, crying, irritability, difficulty sleeping, diminished activity, diminished appetite, and fever. Parents are asked to rate these symptoms, as compared with the child’s usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2. Thus, total scores range from 0 to 14, with higher scores indicating greater severity of symptoms. Children who had tympanic-membrane perforation or another illness, who were allergic to amoxicillin, or who had received more than one dose of an antimicrobial agent within the previous 96 hours were excluded.
RANDOMIZATION
We stratified children according to age (6 to 11 months, 12 to 17 months, and 18 to 23 months) and according to their exposure or nonexposure to three or more children for 10 or more hours per week. At each study site, within each stratum, we randomly assigned children in blocks of four to receive either a 10-day course of amoxicillin–clavulanate at a total daily dose of 90 mg of amoxicillin and 6.4 mg of clavulanate per kilogram of body weight, provided in two bottles (for days 1 to 5 and days 6 to 10), or a 5-day course of amoxicillin–clavulanate (provided in one bottle) followed by a 5-day course of placebo (provided in a second bottle). The placebo was prepared by the University of Pittsburgh Medical Center Investigational Drug Service and was similar to the active product in color, texture, odor, and taste. All the parents, research personnel, and health care providers were unaware of the children’s group assignments. We suggested the administration of appropriate doses of acetaminophen for participating children who had fever or who appeared to have pain.
FOLLOW-UP
We assessed children on day 4, 5, or 6 by means of telephone conversations with the parents and at an end-of-treatment office visit, usually on day 12, 13, or 14. We also asked parents to record their child’s AOM-SOS scores daily. Thereafter, we scheduled in-office assessments every 6 weeks through the end of the respiratory-infection season (defined as October 1 through May 31), at interim visits for illness, and at an end-of-study visit, which usually occurred during the following September. For children who had clinical failure with the index episode and received rescue treatment (see below), we scheduled an additional, post-treatment assessment.
EXAMINATION, ASSESSMENT, AND TREATMENT
All the study clinicians successfully completed an otoscopic validation program before the start of the study.10,11 Whenever practicable, we also obtained otoendoscopic photographs of children’s tympanic membranes (Figs. S1, S2, and S3 in the Supplementary Appendix, available at NEJM.org). For each episode of acute otitis media, we categorized children as having had either clinical success or clinical failure, without regard to persistence or resolution of middle-ear effusion. Children were considered to have clinical failure if they had worsening of symptoms or of otoscopic signs of infection (primarily tympanic-membrane bulging) or if they did not have complete or nearly complete resolution of symptoms and signs attributable to acute otitis media by the end of treatment. We treated children who had clinical failure with a rescue regimen that consisted preferentially of amoxicillin–clavulanate or, as the alternative, ceftriaxone or cefdinir.
For recurrence of acute otitis media, which was defined as an episode occurring any time after day 16 of the index episode, we treated children with their originally assigned regimen and followed them as for the index episode. However, any child who had two recurrences received, for any subsequent recurrence, rescue treatment as described above. At most study visits we obtained a nasopharyngeal specimen for culturing.
OUTCOME MEASURES
All the outcome measures were prespecified. The primary measure was the percentage of children who had clinical failure after treatment of the index infection. Secondary measures included symptom burden over the period from day 6 (when placebo use in the 5-day group began) to day 14 of the index episode, rates of recurrence of acute otitis media, outcomes in treating recurrences of acute otitis media, total days of antimicrobial treatment during the respiratory-infection season, rates of nasopharyngeal colonization, use of other health care services, rates of missed work or special childcare arrangements necessitated by children’s illness, and parental satisfaction as assessed on a 5-point scale (with higher numbers indicating greater satisfaction with the study treatment).
STATISTICAL ANALYSIS
Analyses were based on a noninferiority study design and on the intention-to-treat principle. We also conducted per-protocol analyses. All the analyses were performed with the use of two-sided tests and, when appropriate, included adjustment for study stratification variables and duration of follow-up. Significance levels were set at an alpha level of 0.05. We estimated that among children receiving 10-day antimicrobial treatment, 15% would have clinical failure,4 and we specified that the 5-day treatment would be considered to be noninferior (and accordingly, clinically acceptable) if the upper boundary of the 95% confidence interval of the between-group difference in the percentage of children with clinical failure (percentage in the 5-day group minus the percentage in the 10-day group) would be no greater than 10 percentage points. Assuming clinical-failure rates of 15% in the 10-day group and 25% in the 5-day group, statistical power of 0.95, and a 10% rate of loss to follow-up or withdrawal from the study, we calculated that the study would need to include a sample of 300 children per group. Assuming a between-group difference in clinical-failure rates of 10 percentage points, we calculated that actual rates in the 10-day group ranging from 10 to 20% would provide power values ranging from 0.82 to 0.97.
Subgroup analyses, with the use of logistic-regression models, were patterned after those performed in a previous trial.4 We compared the mean rates of the recurrence of acute otitis media in the two study groups by assuming a Poisson distribution and applying a generalized linear model. For symptoms, we compared average AOM-SOS scores over specified study periods using generalized estimated equations. We also compared, using logistic regression, the percentages of children whose symptomatic response to treatment we considered to be satisfactory, which we defined as a decrease of more than 50% in the AOM-SOS score from entry to the end-of-treatment assessment.12 For analyses of nasopharyngeal colonization and antimicrobial resistance, we used logistic-regression models, and for resistance analyses we also calculated simple and conditional odds ratios.13
RESULTS
STUDY POPULATION
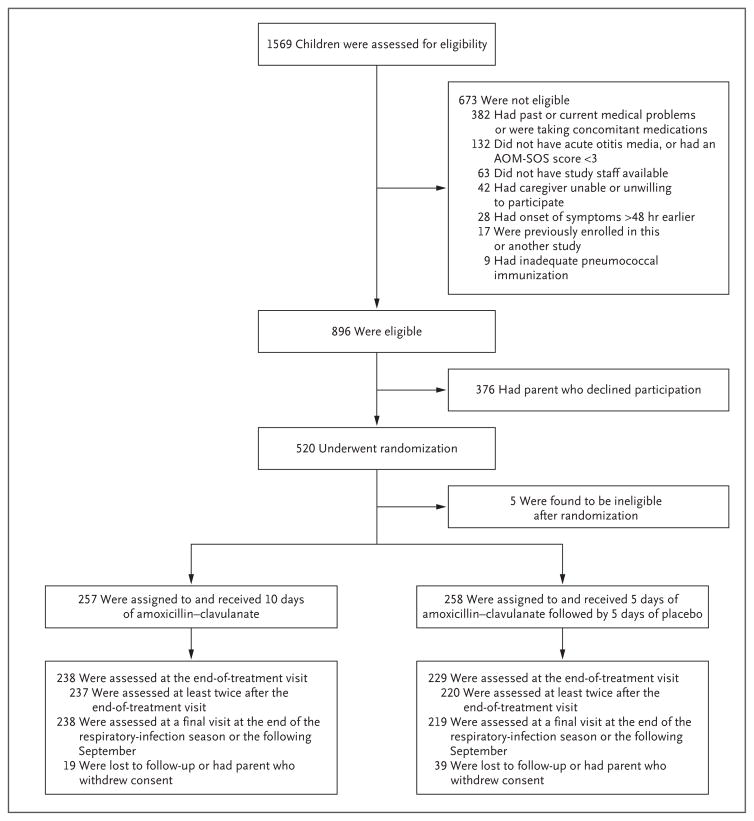
We screened 1569 children, of whom 896 were eligible for the clinical trial and 520 underwent randomization (Fig. 1). Enrollment was then discontinued, because the external data and safety monitoring board for the study considered that the primary objective of the study had been realized. Selected demographic and clinical characteristics of the children are shown in Table 1 and in Table S1 in the Supplementary Appendix. There were no significant differences between enrolled children and children whose parents withheld consent, except with respect to race and ethnic group (Table S1 in the Supplementary Appendix).
Figure 1. Enrollment, Randomization, and Follow-up of Children in the Trial.
The Acute Otitis Media–Severity of Symptoms (AOM-SOS) scale consists of seven discrete items — tugging of ears, crying, irritability, difficulty sleeping, diminished activity, diminished appetite, and fever. Parents are asked to rate these symptoms, as compared with the child’s usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2. Total scores range from 0 to 14, with higher scores indicating greater severity of symptoms. The respiratory-infection season was defined as October 1 through May 31.
Table 1.
Selected Demographic and Clinical Characteristics of the Children, According to Treatment Group.*
| Characteristic | 10-Day Group (N = 257) | 5-Day Group (N = 258) | All Children (N = 515) |
|---|---|---|---|
| Age at entry — no. (%) | |||
| 6–11 mo | 129 (50) | 132 (51) | 261 (51) |
| 12–17 mo | 80 (31) | 77 (30) | 157 (30) |
| 18–23 mo | 48 (19) | 49 (19) | 97 (19) |
| Sex — no. (%) | |||
| Female | 115 (45) | 123 (48) | 238 (46) |
| Male | 142 (55) | 135 (52) | 277 (54) |
| Exposure to other children — no. (%)† | |||
| No | 109 (42) | 105 (41) | 214 (42) |
| Yes | 148 (58) | 153 (59) | 301 (58) |
| AOM-SOS score at entry‡ | |||
| Mean score | 8.6±3 | 8.2±3 | 8.4±3 |
| Distribution — no. (%) | |||
| 3–5 | 43 (17) | 59 (23) | 102 (20) |
| 6–8 | 75 (29) | 70 (27) | 145 (28) |
| 9–11 | 98 (38) | 95 (37) | 193 (37) |
| 12–14 | 41 (16) | 34 (13) | 75 (15) |
| Estimated severity of illness on the basis of pain and fever history only — no. (%)§ | |||
| Probably nonsevere | 111 (43) | 121 (47) | 232 (45) |
| Probably severe | 146 (57) | 137 (53) | 283 (55) |
| No. of ears affected by acute otitis media — no. (%) | |||
| One ear | 136 (53) | 126 (49) | 262 (51) |
| Two ears | 121 (47) | 132 (51) | 253 (49) |
| Degree of tympanic-membrane bulging in worse ear — no. (%) | |||
| Slight | 35 (14) | 45 (17) | 80 (16) |
| Moderate | 137 (53) | 135 (52) | 272 (53) |
| Marked | 85 (33) | 78 (30) | 163 (32) |
Plus–minus values are means ±SD. There were no significant between-group differences at baseline in the characteristics listed here. Percentages may not total 100 because of rounding.
Exposure to other children was defined as exposure to three or more children for 10 or more hours per week.
The Acute Otitis Media–Severity of Symptoms (AOM-SOS) scale consists of seven discrete items — tugging of ears, crying, irritability, difficulty sleeping, diminished activity, diminished appetite, and fever. Parents are asked to rate these symptoms, as compared with the child’s usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2. Total scores range from 0 to 14, with higher scores indicating greater severity of symptoms.
The current American Academy of Pediatrics clinical practice guideline concerning the management of acute otitis media refers to children with “severe signs or symptoms” as those with “moderate or severe otalgia or otalgia for at least 48 hours or temperature 39°C (102.2°F) or higher.”3 In an effort to simulate that definition with the use of scores on only two of the AOM-SOS items, we categorized the episode of acute otitis media as “probably severe” if the parent described the child as having had “a lot” of ear tugging or “a lot” of fever during the preceding 24 hours.
End-of-treatment assessments for the index episode were completed for 238 of 257 children (93%) in the 10-day group and for 229 of 258 (89%) in the 5-day group (P = 0.18). The mean (±SD) duration of follow-up was 4.4±2.0 months in the 10-day group and 3.9±2.2 months in the 5-day group (P = 0.007). Of 470 children for whom medication data for the index episode were available, 435 (93%) reportedly received all scheduled medication doses during the first 3 days of treatment, and 418 (89%) also received at least 80% of doses overall.
CLINICAL FAILURE AND PROGNOSTIC FACTORS IN INDEX EPISODE
Table 2 summarizes the results for the index episode of acute otitis media. Clinical failure was observed in a greater percentage of children treated with amoxicillin–clavulanate for 5 days than of those treated for 10 days (77 of 229 children [34%] vs. 39 of 238 [16%]; difference, 17 percentage points [based on unrounded data]; 95% confidence interval, 9 to 25; number needed to treat to prevent clinical failure, 6). Because the upper boundary of this confidence interval — namely 25 percentage points — was greater than 10 percentage points, the prespecified established criterion for the noninferiority of 5-day treatment was not met.
Table 2.
Clinical-Failure Rates for the Index Episode of Acute Otitis Media at or before the End-of-Treatment Visit, According to Selected Characteristics at Entry.*
| Characteristic | 10-Day Group (N = 257) | 5-Day Group (N = 258) | All Children (N = 515) | Odds Ratio (95% CI)† | P Value |
|---|---|---|---|---|---|
| no. of children with clinical failure/total no. (%) | |||||
| All children | 39/238 (16) | 77/229 (34) | 116/467 (25) | NA | — |
|
| |||||
| Age at entry | 0.94 | ||||
| 12–23 mo | 15/116 (13) | 41/111 (37) | 56/227 (25) | Reference | |
| 6–11 mo | 24/122 (20) | 36/118 (31) | 60/240 (25) | 1.0 (0.7–1.6) | |
|
| |||||
| Exposure to other children | 0.02 | ||||
| No | 13/101 (13) | 24/96 (25) | 37/197 (19) | Reference | |
| Yes | 26/137 (19) | 53/133 (40) | 79/270 (29) | 1.7 (1.1–2.7) | |
|
| |||||
| AOM-SOS score at entry | 0.19 | ||||
| ≤8 | 20/108 (19) | 43/117 (37) | 63/225 (28) | Reference | |
| >8 | 19/130 (15) | 34/112 (30) | 53/242 (22) | 0.8 (0.5–1.2) | |
|
| |||||
| Ears affected by otitis media | <0.001 | ||||
| One | 10/124 (8) | 26/113 (23) | 36/237 (15) | Reference | |
| Both | 29/114 (25) | 51/116 (44) | 80/230 (35) | 2.9 (1.9–4.7) | |
|
| |||||
| Degree of tympanic-membrane bulging in worse ear | 0.12 | ||||
| Slight or moderate | 23/158 (15) | 49/157 (31) | 72/315 (23) | Reference | |
| Marked | 16/80 (20) | 28/72 (39) | 44/152 (29) | 1.4 (0.9–2.2) | |
|
| |||||
| Estimated severity of illness on the basis of pain and fever history only | 0.38 | ||||
| Probably nonsevere | 21/104 (20) | 28/107 (26) | 49/211 (23) | Reference | |
| Probably severe | 18/134 (13) | 49/122 (40) | 67/256 (26) | 1.2 (0.8–1.9) | |
|
| |||||
| No. of significantly unfavorable characteristics | <0.001‡ | ||||
| 0 | 4/52 (8) | 12/55 (22) | 16/107 (15) | Reference | |
| 1 | 15/121 (12) | 26/99 (26) | 41/220 (19) | 1.4 (0.7–2.6) | |
| 2 | 20/65 (31) | 39/75 (52) | 59/140 (42) | 4.3 (2.3–8.2) | |
Data were not available for some children because of missed visits. NA denotes not applicable.
The odds ratio (with 95% confidence interval [CI]) for clinical failure was calculated for the comparison of the favorable characteristic category (reference) versus the unfavorable characteristic category, with study groups combined. The category that was considered to be clinically favorable on the basis of clinical experience, earlier studies,4,13 or both is listed first, and the unfavorable characteristic second. The odds ratios and P values were adjusted for treatment group; in the case of the two characteristics showing significant differences (i.e., exposure to other children and one versus both ears affected by acute otitis media), the odds ratios and P values were also adjusted for the other such characteristic. Of 14 tests for significant interactions between treatment and the individual characteristics, all were nonsignificant except in the case of the estimated severity of acute otitis media at entry. In the 10-day group, the clinical-failure rate was higher among children with probably nonsevere illness than among those with probably severe illness, and in the 5-day group, the rate was higher among children with probably severe illness than among those with probably nonsevere illness (P = 0.01 for interaction).
The P value was derived from a test for linear trend from zero to two.
The results in subgroups consistently favored the group of children assigned to receive the 10-day treatment. We found an interaction between treatment and estimates of episode severity that were based on history of pain and fever; the magnitude of the difference between the 10-day group and the 5-day group in the percentage of children with clinical failure was greater among those with probably severe illness than among those with probably nonsevere illness (Table 2). In contrast, we did not find interactions between treatment and other indexes of severity, namely AOM-SOS scores, infection in both ears versus one ear, and degrees of tympanic-membrane bulging. At the times of clinical failure, AOM-SOS scores greater than 8 were noted only in the 5-day group, although the between-group difference did not reach significance (Table S2 in the Supplementary Appendix).
Overall, in the two groups combined, clinical-failure rates were greater among children with exposure to three or more children for 10 or more hours per week than among those with less exposure (P = 0.02) and greater among children with bilateral acute otitis media than in those with unilateral acute otitis media (P<0.001). Within the two groups, clinical-failure rates were higher according to the number of children’s characteristics (none, one, or two) that were considered to be unfavorable on the basis of clinical experience or findings in earlier studies (Table 2)4,14 or that were found in the current study to be associated with significant odds ratios. Results in the per-protocol analysis were similar to those in the intention-to-treat analysis.
SYMPTOMATIC RESPONSE IN INDEX EPISODE
Table 3 summarizes, for the index episode, key measures of symptomatic response according to parent-recorded AOM-SOS scores. The mean symptom scores over the period from day 6 to day 14 were 1.61 in the 5-day group and 1.34 in the 10-day group (P = 0.07); the mean scores at the day-12-to-14 assessment were 1.89 versus 1.20 (P = 0.001). The percentage of children whose symptom scores decreased more than 50% (indicating less severe symptoms) from baseline to the end of treatment was lower in the 5-day group than in the 10-day group (181 of 227 children [80%] vs. 211 of 233 [91%], P=0.003). Within each treatment group, scores were higher (i.e., less favorable) in children who had been categorized on the basis of otoscopic findings as having clinical failure than in those who had been categorized as having clinical success. Over the period from day 6 to day 14, the mean scores were higher in children whose scores at entry had been greater than 8 than in children whose scores had been 8 or less (mean scores in the 10-day group, 1.6±2.0 vs. 1.0±1.3; P = 0.02; mean scores in the 5-day group, 1.9±2.2 vs. 1.3±1.6; P = 0.02).
Table 3.
Symptomatic Response in Index Episode of Acute Otitis Media as Measured by AOM-SOS Score.*
| Measure of Symptomatic Response | 10-Day Group (N = 242) | 5-Day Group (N = 238) | All Children (N = 480) | P Value |
|---|---|---|---|---|
| AOM-SOS score over period from day 6 to 14† | ||||
|
| ||||
| All children | ||||
| No. of children | 232 | 228 | 460 | |
| Mean score | 1.34 ±1.76 | 1.61±1.96 | 1.47±1.86 | 0.07‡ |
|
| ||||
| Children with clinical success§ | ||||
| No. of children | 193 | 145 | 338 | |
| Mean score | 1.32 ±1.81 | 1.34±1.69 | 1.33±1.76 | 0.02¶ |
|
| ||||
| Children with clinical failure | ||||
| No. of children | 33 | 73 | 106 | |
| Mean score | 1.63±1.53 | 1.99±2.20 | 1.88±2.02 | |
|
| ||||
| AOM-SOS score at the day-12-to-14 assessment | ||||
|
| ||||
| All children | ||||
| No. of children | 233 | 227 | 460 | |
| Mean score | 1.20±2.06 | 1.89±2.73 | 1.54±2.44 | 0.001‡ |
|
| ||||
| Children with clinical success§ | ||||
| No. of children | 199 | 151 | 350 | |
| Mean score | 1.04±2.02 | 1.27±2.02 | 1.14±2.02 | <0.001¶ |
|
| ||||
| Children with clinical failure | ||||
| No. of children | 34 | 76 | 110 | |
| Mean score | 2.15±2.06 | 3.13±3.45 | 2.83±3.11 | |
|
| ||||
| Decrease of >50% in AOM-SOS score from base-line to the day-12-to-14 assessment — no./total no. (%)|| | ||||
|
| ||||
| All children | 211/233 (91) | 181/227 (80) | 392/460 (85) | 0.003‡ |
| Children with clinical success§ | 182/199 (91) | 133/151 (88) | 315/350 (90) | <0.001¶ |
| Children with clinical failure | 29/34 (85) | 48/76 (63) | 77/110 (70) | |
Data were missing for some children for some analyses. Analyses included adjustments for study site, age group, exposure or nonexposure to three or more other children for 10 or more hours per week, AOM-SOS score at entry, day of diary notation, and treatment, singly or in combination as appropriate.
Day 6 marked the beginning of placebo use in place of amoxicillin–clavulanate in the 5-day group. Over the period from day 1 to day 5, during which treatment in the 10-day group and the 5-day group was uniform, the mean AOM-SOS scores in the 10-day group and the 5-day group, with adjustment for scores at entry, did not differ significantly. In children with clinical failure, the period that was considered for this analysis was from day 6 to the day preceding the day on which clinical failure was determined.
The P value is for the comparison between the 10-day group and the 5-day group.
There was no significant interaction between treatment and AOM-SOS scores in relation to clinical outcome (clinical success vs. clinical failure).
The P value is for the comparison, in the study groups combined, between children who had clinical success and those who had clinical failure.
The cutoff of 50% or less versus more than 50% was based on data from a study of minimal clinically important difference in AOM-SOS scores.12
RESIDUAL MIDDLE-EAR EFFUSION AND RECURRENCE
To compare the two study groups with regard to the prevalence of residual middle-ear effusion after the treatment of the index episode, we used the end-of-treatment assessment in children who had clinical success and the assessment after rescue treatment in children who had clinical failure. We found effusion in 139 of 226 children (62%) in the 10-day group (61 children with unilateral effusion and 78 with bilateral effusion) and in 141 of 216 (65%) in the 5-day group (49 with unilateral effusion and 92 with bilateral effusion) (P=0.35).
Overall, the percentage of children who had one or more recurrences of acute otitis media was greater among children with residual effusion than among those without residual effusion (48% vs. 29%, P<0.001); this was the case both among children who had clinical success (45% vs. 29%, P = 0.01) and among those who had clinical failure (59% vs. 32%, P = 0.008). Patterns were similar in the 10-day group and the 5-day group (Table S3 in the Supplementary Appendix). The groups did not differ significantly in the mean monthly rate of recurrence (0.14±0.18 episodes in the 10-day group and 0.12±0.19 in the 5-day group, P = 0.22) or in the frequency distributions of the recurrences (P = 0.56).
Overall, among children with recurrent episodes, the rate of clinical failure was consistently higher in the 5-day group than in the 10-day group (28% vs. 19%), and the criterion for non-inferiority of the 5-day treatment was again not met. The rates of residual middle-ear effusion after treatment of recurrences were similar in the two groups.
CUMULATIVE DURATION OF ANTIMICROBIAL TREATMENT
Cumulatively over the course of the 243-day respiratory-infection season, the mean numbers of days on which children received systemic antimicrobial treatment were 21±13 in the 10-day group and 15±12 in the 5-day group (P<0.001). Amoxicillin–clavulanate administered for the initial treatment of index and recurrent episodes of acute otitis media combined accounted for 70% of the days in the 10-day group and for 46% of the days in the 5-day group. The mean number of days on which an antimicrobial agent was administered for other reasons (mainly as rescue medication in children with clinical failure) was 6±9 in the 10-day group and 8±11 in the 5-day group (P=0.05).
NASOPHARYNGEAL COLONIZATION
Table 4 summarizes findings regarding children’s nasopharyngeal colonization with major pathogens for acute otitis media. After antimicrobial treatment for the index episode, the level of colonization with penicillin-susceptible strains of Streptococcus pneumoniae decreased in both the 10-day group and the 5-day group, and nonsusceptible strains now accounted for larger percentages of the remaining pneumococcal isolates. Over time, colonization with both susceptible strains and nonsusceptible strains returned to levels that were similar to those found at study entry. Colonization with susceptible strains and nonsusceptible strains of Haemophilus inf luenzae remained relatively unchanged throughout. These findings were also reflected in both the simple and conditional odds ratios — the latter of which may serve as a measure of community-wide effects of antimicrobial treatment on resistance13 — which did not differ significantly between the two treatment groups.
Table 4.
Distribution of Acute Otitis Media Pathogens in Nasopharyngeal Cultures, According to Type of Visit.*
| Variable | Index Episode | End-of-Treatment Assessment for Index Episode | Recurrent Episode† | Routine Nonillness† | Final | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10-Day Group | 5-Day Group | 10-Day Group | 5-Day Group | 10-Day Group | 5-Day Group | 10-Day Group | 5-Day Group | 10-Day Group | 5-Day Group | |
| Visits | ||||||||||
|
| ||||||||||
| No. of visits with culture obtained/total no. of visits | 256/257 | 258/258 | 233/236 | 222/224 | 148/162 | 119/125 | 415/421 | 359/361 | 225/225 | 195/195 |
|
| ||||||||||
| S. pneumoniae | ||||||||||
|
| ||||||||||
| No. of positive cultures (%)‡ | 125 (49) | 138 (53) | 28 (12) | 36 (16) | 72 (49) | 57 (48) | 146 (35) | 144 (40) | 88 (39)§ | 70 (36) |
| Susceptible | 84 (33) | 94 (36) | 5 (2) | 10 (5) | 41 (28) | 24 (20) | 82 (20) | 89 (25) | 54 (24) | 48 (25) |
| Intermediate | 20 (8) | 28 (11) | 10 (4) | 12 (5) | 14 (9) | 13 (11) | 33 (8) | 31 (9) | 24 (11) | 11 (6) |
| Resistant | 21 (8) | 16 (6) | 13 (6) | 14 (6) | 17 (11) | 20 (17) | 31 (7) | 24 (7) | 9 (4) | 11 (6) |
|
| ||||||||||
| Simple odds ratio (95% CI)¶ | NA | NA | 0.6 (0.3–1.0) | 0.6 (0.4–1.1) | 1.4 (0.8–2.3) | 1.9 (1.1–3.1) | 1.0 (0.6–1.5) | 0.9 (0.6–1.4) | 0.9 (0.6–1.5) | 0.6 (0.4–1.1) |
| P value | NA | 0.76 | 0.43 | 0.79 | 0.31 | |||||
|
| ||||||||||
| Conditional odds ratio (95% CI)|| | NA | NA | 9.4 (3.3–26.6) | 5.6 (2.5–12.5) | 1.6 (0.8–2.8) | 2.9 (1.6–5.6) | 1.6 (1.0–2.6) | 1.3 (0.8–2.2) | 1.2 (0.7–2.2) | 1.0 (0.5–1.8) |
| P value | NA | 0.43 | 0.15 | 0.59 | 0.57 | |||||
|
| ||||||||||
| H. influenzae | ||||||||||
|
| ||||||||||
| No. of positive cultures (%)** | 84 (33) | 61 (24) | 77 (33) | 65 (29) | 39 (26) | 41 (34) | 49 (12) | 42 (12) | 21 (9) | 25 (13) |
| Susceptible | 53 (21) | 37 (14) | 39 (17) | 37 (17) | 28 (19) | 27 (23) | 33 (8) | 30 (8) | 14 (6) | 16 (8) |
| Nonsusceptible | 31 (12) | 24 (9) | 38 (16) | 28 (13) | 11 (7) | 14 (12) | 16 (4) | 12 (3) | 7 (3) | 9 (5) |
|
| ||||||||||
| Simple odds ratio (95% CI)¶ | NA | NA | 1.4 (0.8–2.4) | 1.4 (0.8–2.5) | 0.6 (0.3–1.2) | 1.3 (0.6–2.6) | 0.3 (0.2–0.5) | 0.3 (0.2–0.7) | 0.2 (0.1–0.5) | 0.5 (0.2–1.0) |
| P value | NA | 0.99 | 0.12 | 0.76 | 0.23 | |||||
|
| ||||||||||
| Conditional odds ratio (95% CI)|| | NA | NA | 1.7 (0.9–3.1) | 1.2 (0.6–2.4) | 0.7 (0.3–1.5) | 0.8 (0.4–1.8) | 0.8 (0.4–1.7) | 0.6 (0.3–1.4) | 0.8 (0.3–2.4) | 0.9 (0.3–2.3) |
| P value | NA | 0.46 | 0.77 | 0.61 | 0.98 | |||||
In the analyses for Streptococcus pneumoniae and Haemophilus influenzae, the denominator for the percentage calculations is the number of visits with culture obtained, unless otherwise noted. P values are for the comparison of the 10-day group with the 5-day group.
Data shown are for all such visits combined.
Susceptibility to penicillin was determined as follows: when the minimum inhibitory concentration (MIC) was available, susceptibility was defined as an MIC of less than 0.1 μg per milliliter, intermediate as an MIC of 0.1 to 1 μg per milliliter, and resistant as an MIC of more than 1 μg per milliliter; when the MIC was not available, susceptibility was defined as an oxacillin-disk zone size of more than 20 mm, intermediate as a zone size of 9 to 20 mm, and resistant as a zone size of 8 mm or less.
Sensitivity was not available for one isolate, so the denominator to calculate the percentages in the sensitivity distribution is 224 rather than 225.
The simple odds ratio constitutes the odds for a specific treatment group, as compared with the status of that group at the index episode, of being colonized at a later time with a nonsusceptible organism.13 Results are presented without regard to multiple observations in individual children.
The conditional odds ratio constitutes the odds for a specific treatment group, as compared with the status of that group at the index episode, of being colonized at a later time with a nonsusceptible organism conditional on overall carriage of that organism (i.e., both susceptible and nonsusceptible strains) at that time.13 Results are presented without regard to multiple observations in individual children.
Susceptibility was defined as a beta-lactamase–negative result and an ampicillin E test MIC of 1 μg per milliliter or less; nonsusceptibility was defined as either a beta-lactamase–positive result or a beta-lactamase–negative result and an ampicillin E test MIC of more than 1 μg per milliliter.
Among children whose nasopharyngeal isolates that were recovered during the index episode were either nonpathogens or penicillin-susceptible pathogens and for whom at least one subsequent culture was performed, 85 of 181 children (47%) in the 10-day group and 78 of 177 (44%) in the 5-day group were eventually found to be colonized with a penicillin-nonsusceptible pathogen (P=0.58).
ADVERSE EVENTS AND OTHER MEASURES
For all episodes within the first 16 days of follow-up, protocol-defined diarrhea (occurrence of three or more watery stools in 1 day or two watery stools daily for 2 consecutive days) occurred in 78 of 257 children (30%) in the 10-day group and in 75 of 258 (29%) in the 5-day group; dermatitis in the diaper area that required a prescription of a topical antifungal agent occurred in 85 of 257 (33%) and in 87 of 258 (34%), respectively. Diarrhea and dermatitis frequently occurred together. There were no significant between-group differences in the rates of use of other health care services, of missed work, or of special childcare arrangements because of children’s illness or in the levels of parental satisfaction.
DISCUSSION
In this noninferiority trial involving children 6 to 23 months of age with acute otitis media, reduced-duration treatment with amoxicillin–clavulanate for 5 days was less effective than standard-duration treatment for 10 days, with a magnitude of difference that exceeded the prespecified non-inferiority margin. The difference in outcome between the two treatment groups also was large enough to show that standard-duration treatment was superior in efficacy to reduced-duration treatment.15 The outcome differences we found were larger than the differences that have been reported previously,6 mainly because the rates of clinical failure among children who received reduced-duration treatment were higher in our trial than in previous trials. The single interaction we found between treatment and estimated severity of the index episode was inconsistent with findings concerning other indicators of severity — AOM-SOS scores, bilateral versus unilateral infection, and degrees of tympanic-membrane bulging — that have been used more often in other studies.
We observed no significant differences between the standard-duration treatment group and the reduced-duration treatment group in the rates of adverse events, recurrence of acute otitis media, or antimicrobial resistance. In the two groups, children with residual middle-ear effusion after treatment of the index episode were more likely than those without effusion to have recurrent episodes.
The strengths of our study include the limitation of enrollment to children younger than 2 years of age, which is the age group that is most prone to having treatment failure and recurrence of acute otitis media; the reliance on validated otoscopists applying stringent diagnostic criteria; the use of a validated scale for the rating of severity of symptoms; the use of amoxicillin–clavulanate, which is currently the most efficacious oral anti-microbial agent for the treatment of acute otitis media; the administration of a placebo during the latter 5 days in the reduced-duration treatment group; and a consistent strategy for the treatment of recurrent episodes. These same attributes may also be considered to be limitations, because they limit the generalizability of our findings. In particular, our findings cannot be generalized to children who are older than the children included in the study.
In conclusion, in the current study involving children 6 to 23 months of age, the treatment of acute otitis media with amoxicillin–clavulanate for 5 days afforded less-favorable short-term outcomes than treatment for 10 days; in addition neither the rate of adverse events nor the rate of emergence of antimicrobial resistance was lower with the shorter regimen.
Supplementary Material
Acknowledgments
Supported by a contract (HHSN272201000047C) from the National Institute of Allergy and Infectious Diseases and by University of Pittsburgh Clinical and Translational Science Awards (UL1RR024153 and UL1TR000005) from the National Center for Research Resources, now at the National Center for Advancing Translational Sciences, National Institutes of Health.
We thank the many house officers at Children’s Hospital of Pittsburgh; the faculty and nurses of General Academic Pediatrics; Kris Daw, R.N., of Pediatric PittNet, for referring children to the study; Marian G. Michaels, M.D., M.P.H., for serving as the independent safety monitor; Ellen R. Wald, M.D., Aino Ruohola, M.D., Ph.D., Stephen I. Pelton, M.D., Stephen J. Gange, Ph.D., and Louis Vernacchio, M.D., for serving as members of the external data and safety monitoring board; Linda Lambert, M.D., Robin Mason, M.H.A., M.B.A., Valerie Riddle, M.D., and Wendy Buchanan, M.S., B.S.N., of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, for administrative support; and the children and their families for their generosity and cooperation in participating in this trial.
Footnotes
The views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Drs. Hoberman and Martin report receiving consulting fees from Genocea Biosciences; and Dr. Hoberman, receiving grant support from Ricoh Innovations and holding pending patents related to the development of a reduced clavulanate concentration version of amoxicillin–clavulanate potassium (U.S. patent application serial number, 14/371,731) and the development of a method and apparatus for aiding in the diagnosis of otitis media by classifying tympanic-membrane images (U.S. patent application serial number, 14/418,509). No other potential conflict of interest relevant to this article was reported.
References
- 1.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 2011;13:1–38. [PubMed] [Google Scholar]
- 2.Finkelstein JA, Metlay JP, Davis RL, Rifas-Shiman SL, Dowell SF, Platt R. Antimicrobial use in defined populations of infants and young children. Arch Pediatr Adolesc Med. 2000;154:395–400. doi: 10.1001/archpedi.154.4.395. [DOI] [PubMed] [Google Scholar]
- 3.Lieberthal AS, Carroll AE, Chonmai-tree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–99. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 4.Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364:105–15. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tähtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364:116–26. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 6.Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short-course antibiotics for acute otitis media. Cochrane Database Syst Rev. 2010;9:CD001095. doi: 10.1002/14651858.CD001095.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan R, McCracken GH., Jr Flaws in design and conduct of clinical trials in acute otitis media. Pediatr Infect Dis J. 2002;21:894–902. doi: 10.1097/00006454-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh N, Hoberman A, Paradise JL, et al. Responsiveness and construct validity of a symptom scale for acute otitis media. Pediatr Infect Dis J. 2009;28:9–12. doi: 10.1097/INF.0b013e318185a3a0. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh N, Hoberman A, Paradise JL, et al. Development and preliminary evaluation of a parent-reported outcome instrument for clinical trials in acute otitis media. Pediatr Infect Dis J. 2009;28:5–8. doi: 10.1097/INF.0b013e318185a387. [DOI] [PubMed] [Google Scholar]
- 10.Kaleida PH, Stool SE. Assessment of otoscopists’ accuracy regarding middle-ear effusion: otoscopic validation. Am J Dis Child. 1992;146:433–5. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 11.Kaleida PH, Ploof DL, Kurs-Lasky M, et al. Mastering diagnostic skills: Enhancing Proficiency in Otitis Media, a model for diagnostic skills training. Pediatrics. 2009;124(4):e714–20. doi: 10.1542/peds.2008-2838. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Rockette HE, Hoberman A, Kurs-Lasky M, Paradise JL. Determination of the minimal important difference for the Acute Otitis Media Severity of Symptom scale. Pediatr Infect Dis J. 2015;34(3):e41–3. doi: 10.1097/INF.0000000000000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipsitch M. Measuring and interpreting associations between antibiotic use and penicillin resistance in Streptococcus pneumoniae. Clin Infect Dis. 2001;32:1044–54. doi: 10.1086/319604. [DOI] [PubMed] [Google Scholar]
- 14.Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 15.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. doi: 10.1186/1745-6215-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.