Abstract
HRPT2 and parafibromin studies improved the diagnostic accuracy in two patients with primary hyperparathyroidism (PHPT) referred to us after surgery, in whom the clinical data were at variance with the pathological diagnosis of adenoma and carcinoma, respectively. Patients were referred to us after parathyroidectomy. Patient #1 had had a 1.5-cm tumor easily removed with a histological diagnosis of parathyroid carcinoma and normocalcemia for 2 years. Re-examination of the histology showed no cardinal signs of parathyroid cancer. Patient #2, with severe PHPT, had had the removal of a 3.5-cm tumor described histologically as adenoma. Ten years later PHPT recurred and persisted despite removal of two mildly enlarged parathyroid glands that were histologically normal. Re-review of the initial histology showed a trabecular pattern, fibrous bands, and atypical mitoses, suggesting an atypical adenoma. Because of the suspicion that case #1 could be an atypical adenoma and case #2 a carcinoma further molecular studies were performed. No HRPT2 and parafibromin abnormalities were identified in patient #1, strongly indicating a benign lesion. In patient #2, an HRPT2 germline mutation was found (E115X in exon 4) and associated with no parafibromin staining. These data, together with the clinical features, supported the suspicion of a parathyroid carcinoma that was confirmed by histological examination of further slides of the tumor, showing capsular and vascular invasion. A lung 1.5-cm nodule detected by computed tomography was excised. Histology showed a metastasis of parathyroid carcinoma. HRPT2 gene studies improved the diagnostic accuracy in 2 parathyroid tumors that are of uncertain type.
Keywords: Atypical parathyroid adenoma, HRPT2, parafibromin, parathyroid carcinoma, primary hyperparathyroidism
INTRODUCTION
Parathyroid carcinoma has a rather indolent course and the diagnosis of malignancy is often made in retrospect when the disease recurs (1). Indeed, at the time of initial surgery, the diagnosis of malignancy can be established with certainty only when there is evidence of vascular invasion, perineural space invasion, growth into adjacent tissues or distant metastases (2). Other histological features, such as trabecular pattern, mitoses, thick fibrous bands and capsular invasion, cannot be considered diagnostic since they may also be found in atypical adenomas (3–5). Additional histological techniques have been proposed to improve the diagnostic accuracy of parathyroid tumors, but none of them has gained wide acceptance in clinical practice (2, 6–9).
HRPT2 gene abnormalities and loss of immunoreactivity for parafibromin, its gene product, have been demonstrated in the majority of parathyroid carcinomas (10–16). In a recent study we found that immunohistochemistry for parafibromin has the highest diagnostic sensitivity (100%) for parathyroid carcinoma, with a specificity of 86% (15). We describe 2 men with sporadic primary hyperparathyroidism (PHPT) in whom the histological diagnoses of carcinoma and adenoma, respectively, were challenged by the clinical course, re-review of the routine histology, and, ultimately, by the application of modern molecular probes.
MATERIALS AND METHODS
Initial presentation
Patient #1
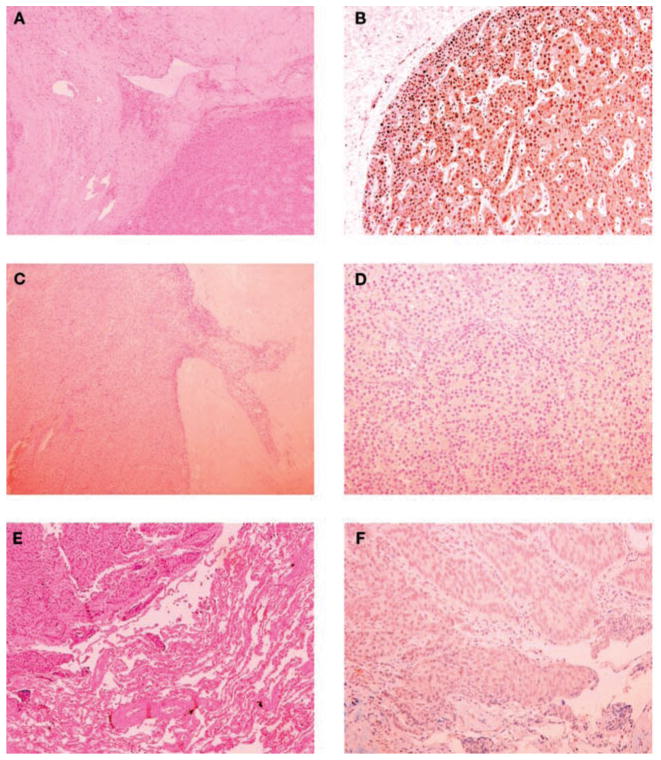
A 49-yr-old man was referred to our Department in March 2006 for further advice after the diagnosis of parathyroid cancer was made. The diagnosis of PHPT was made in June 2005, on the basis of hypercalcemia (3.1 mmol/l; normal range 2.05–2.55 mmol/l) and an elevated serum PTH concentration (206 ng/l, normal range 10–65 ng/l). The history was positive for bilateral kidney stones. Pre-operative localization by planar 99mTc-ses-tamibi imaging showed an enlarged parathyroid gland in the lower pole of the right thyroid lobe. At surgery, in October 2005, a 1.5-cm right lower pole gland was removed. There was no mention of local invasion in the surgical report. No other abnormal parathyroid glands were found. Histology by frozen section showed features of malignancy, but it was not clear whether the tissue was parathyroid or thyroid. As a result, total thyroidectomy was carried out. The final histological report showed a trabecular pattern with fibrous bands, mild atypical nuclei, and was scored as parathyroid carcinoma. Transient symptomatic hypocalcemia after surgery was treated with appropriate amounts of calcium and calcitriol. Levo-T4 therapy was also started. When seen in our Department, 5 months later, the patient was well but concerned about the diagnosis of parathyroid cancer. There was no family history of hypercalcemia. Ultrasound of the neck and computerized tomography (CT) of the total body were negative for metastatic disease. The re-examination of the slides of tissue removed at the time of surgery confirmed the trabecular pattern and fibrous bands, but no cardinal signs of parathyroid cancer, namely grossly vascular and capsular invasion were present (Fig. 1). No cystic features were evident. Thus, the diagnosis appeared to be most consistent with atypical parathyroid adenoma. Up to November 2007, the patient remained normocalcemic with normal PTH levels.
Fig. 1.
Pathology (left panels) and parafibromin immunohistochemistry (right panels) of the parathyroid tumors and lung metastasis. Upper panels: Parathyroid tumor of patient #1. A) Trabecular pattern with fibrous vascular pseudo invasion (hematoxylin and eosin, ×100); B) diffuse nuclear immunoreactivity of parathyroid cells (×200). Middle panels: Parathyroid tumor of patient #2. C) Oncocytic cells arranged in trabeculae, fibrous bands, and capsular invasion (hematoxylin and eosin, x100); D) the neoplastic cells were completely negative for parafibromin (×200). Lower panels: Lung metastasis of patient #2. E) A solid trabecular neoplastic lesion and adjacent normal lung parenchyma (hematoxylin and eosin, ×100); F) the tumor cells showed a diffuse loss of parafibromin staining (×200).
Patient #2
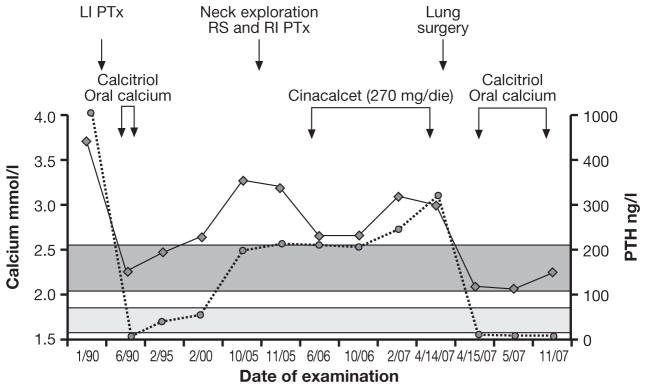
A 53-yr-old man was referred to our Department in July 2006 for recurrent PHPT (Fig. 2). The clinical history was notable for recurrent bilateral nephrolithiasis since the age of 20 yr. In April 1990, at the age of 38 yr, severe PHPT was diagnosed by marked hypercalcemia (serum calcium 3.75 mmol/l) and levels of PTH that were above the accurate measurement range of the assay (>1000 ng/l). Osteitis fibrosa cystica was also present on the skeletal X-rays. At surgery, 1 month later, a markedly enlarged left parathyroid gland (~3.5 cm) was removed. There was no mention of local invasion in the surgical report. The histological diagnosis was chief-cell parathyroid adenoma. Post-operative hungry bone syndrome required calcium and calcitriol, but only for a few months. In 2000, 10 years later, recurrent PHPT was apparent and over the next 5 years, serum calcium and PTH levels increased progressively up to 3.1 mmol/l and 198 ng/l, respectively. At the time of the second neck operation, in November 2005, no parathyroid glands were visualized on the left; both right parathyroid glands appeared to be mildly enlarged and were excised. A fragment of the right inferior gland was implanted in the right sternocleidomastoid muscle. The histology showed mild chief cell hyperplasia. Post-operative serum calcium and PTH levels remained elevated (3.3 mmol/l and 227 ng/l, respectively) and treatment with cinacalcet was started (90 mg tid). At the time of our evaluation, the patient, in good health, showed elevated serum calcium and PTH levels of 2.85 mmol/l and 251 ng/l, respectively as well as elevated markers of bone turnover and 24-h urinary calcium (135 mmol). Bilateral nephrocalcinosis was seen by ultrasound of the abdomen. 99mTc-sestamibi scintigraphy and neck ultrasound did not show any residual parathyroid tissue. A chest CT, however, showed 3 lung micronodules. Orthopantography of the jaw was negative. There was no evidence of other endocrinopathies. When considered together, the following clinical features raised the possibility of parathyroid carcinoma: male gender, relatively young age, markedly elevated serum calcium and PTH, bone and renal involvement, and the size of the parathyroid lesion at initial surgery (1). The negative cervical exploration at the time of the second operation also raised doubts about the initial benign diagnosis. Upon re-review of the original slides of the left parathyroid gland removed at the time of the initial neck operation, oncocytic cells arranged in trabeculae, the presence of fibrous bands, atypical frequent mitoses were observed. These features were at least consistent with the diagnosis of atypical parathyroid adenoma (Fig. 1). No cystic lesions were observed. Both right parathyroid glands that were removed at the time of the second neck operation were read as normal.
Fig. 2.
Evolution of serum calcium and PTH concentrations in patient #2. The dark grey and light grey areas represent the normal ranges of serum calcium and PTH, respectively. The arrows indicate the date of surgery and medical treatments. LI: left inferior; PTx: parathyroidectomy; RS: right superior; RI: right inferior.
The two patients and their family members gave their informed written consent for both serological and genetic studies. The study was approved by our Institutional Review Board.
Tissue samples
Serial sections from paraffin-embedded blocks were visualized after H&E staining. Lung tissue was obtained at the time of surgery, immediately snap frozen in liquid nitrogen and stored at −80 C until use.
Genetic analyses
Genomic DNA was isolated from peripheral blood leukocytes, paraffin-embedded parathyroid specimens, and fresh frozen lung tissue by standard methods. Allelic deletions and direct sequencing of HRPT2 gene were assessed as described previously (9). Nucleotide sequences were determined on double strands at least twice. The intragenic polymorphisms at introns 10 and 14 were used. The region of interest was also amplified using constitutional DNA of all family members.
Immunohistochemistry
Parafibromin immunostaining was carried out as previously described (15) using a parafibromin antibody (kindly donated by Bin Tean Teh) (13). The positive control was normal parathyroid; negative controls included experiments omitting primary antibody or using primary antibody pre-absorbed with a 20-fold excess of the immunizing peptide. For each sample 6 different sections were analyzed.
RESULTS
Genetic analyses
All tissue specimens were heterozygous for the microsatellite markers. In case #1, retained heterozygosity and no HRPT2 mutation were found in the parathyroid tissue, in agreement with the revised diagnosis of a benign parathyroid tumor.
In case #2, loss of heterozygosity of both intragenic markers was identified in the tissue removed at the time of the initial neck operation. Sequence analysis of PCR-amplified tumor DNAs revealed a nonsense mutation in exon 4 at codon 115 of HRPT2 gene causing a premature stop codon predicting a truncated protein (E115X). Peripheral DNA analysis showed that the E115X was a germline mutation.
Immunohistochemistry
The parathyroid specimen of patient #1 showed strong and diffuse parafibromin staining (Fig. 1). Complete loss of staining was found in the primary tumor and, as described below, in the lung metastasis of patient #2 (Fig. 1).
Screening of family members of patient #2
There was no family history of PHPT. The proband’s two sons, aged 20 and 18 yr, and his mother and brother were asymptomatic, with normal serum calcium and PTH levels. The proband’s father died at age 63 yr of a carcinoma in his liver. The genetic analysis performed in all detected the germline E115X mutation in the younger son and in the paraffin-embedded liver tissue from the father.
Clinical surveillance of patient #2
Follow-up evaluation of case #2 confirmed a mild hypercalcemia [10.6 mg/dl (2.65 mmol/l)] and elevated PTH levels (191 pg/ml) on cinacalcet treatment (Fig. 2). Because of the clinical suspicion of parathyroid carcinoma, further slides were prepared from the paraffin-embedded parathyroid tumor and examined by our pathologist. In addition to the features already shown in the original slides (see above, patient #2), minimal signs of capsular and vascular invasion were present, suggesting the diagnosis of parathyroid carcinoma. In February 2007, a chest CT was performed. The 3 previously detected lung micronodules were unchanged but a “new” 1.5-cm nodule, close to the inferior pulmonary vein, was detected in the inferior right pulmonary lobe. A more detailed CT study suggested a metastasis. Re-examination of the chest CT performed in July 2006 confirmed that this “new” lesion was already present, but somewhat smaller. In April 2007, the lung lesion was excised and the histology showed a metastasis of parathyroid carcinoma. Parafibromin immunohistochemistry showed no staining. Following surgery, PTH become undetectable (<15 ng/l), hypocalcemia developed (1.75 mmol/l) and treatment with calcium and calcitriol was started. At the most recent evaluation (November 2007), serum calcium remained normal and PTH was still undetectable (Fig. 2). The mutation-positive son was found to be in good health. Total and ionized serum calcium and PTH were normal. Neck ultrasound was negative for parathyroid lesion. Jaw pantomograms and renal ultrasound were negative for stones, tumors or cysts. Twice yearly monitoring of serum calcium and PTH and yearly neck ultrasound were advised.
DISCUSSION
The clinical features of parathyroid cancer are primarily due to hypercalcemia rather than to tumor mass or to distant metastases and therefore the challenge to the clinician is to distinguish this rare variant of PHPT from its much more common benign counterpart. Several clinical manifestations may suggest a malignant lesion: male gender, young age, marked hypercalcemia, severe and concomitant bone disease and renal involvement (1). In addition, intraoperative findings can be helpful (large and stony-hard mass, adherence to the adjacent tissues, and most important, gross local infiltration), but they may be absent; frozen section is of little value (1).
As in many endocrine neoplasms, the histopathological distinction between benign and malignant parathyroid tumors is difficult and, in the absence of local invasion or metastases at initial surgery, a definite diagnosis cannot be established with certainty (2–5).
Taken together, these considerations may account for diagnostic “underreading” (as in patient #2) or “overreading” (as in patient #1) of the parathyroid pathology. Mis-diagnosis may have important psychological and clinical consequences. Indeed, as exemplified in patient #2, the incorrect diagnosis of benign adenoma suggested possible multiglandular disease at the time of recurrence, providing justification for a second neck operation, and delaying more appropriate investigation and management. On the other hand, in patient #1 the correct initial diagnosis would have spared the patient unnecessary anxiety of a malignancy that he did not have.
Several attempts have been made in recent years to identify diagnostic markers which could be of potential clinical utility in parathyroid tumors of uncertain pathology (6–9). A relevant step in this direction is the demonstration of an HRPT2 gene inactivating mutation and loss of parafibromin immunostaining in the large majority of sporadic parathyroid carcinomas (10–16). However, because of the rarity of parathyroid carcinomas, staining for parafibromin and HRPT2 gene analysis cannot be recommended for all parathyroid tumors. These combined diagnostic approaches, nevertheless, could be of great utility in parathyroid tumors with equivocal histological features. Indeed, with the exception of hyperparathyroidism-jaw tumor (HPT-JT)-associated tumors, in which benign lesions also show HRPT2 mutations and loss of parafibromin staining, and, exceptionally, of sporadic benign adenomas, the simultaneous finding of both abnormalities, even if not absolute criteria, is suggestive of parathyroid malignancy. Of course, the diagnostic usefulness of HRPT2 mutations and parafibromin immuhistochemistry should be evaluated in a large series of parathyroid tumors with equivocal histological diagnosis. Nonetheless, this approach proved to be of great utility in the two patients described herein.
In patient #1, beside the original pathology, no clinical features or surgical findings suggested a malignant parathyroid tumor. Histological re-examination had already suggested a benign rather than malignant tumor. This conclusion was strengthened greatly by molecular studies of HRPT2 gene showing lack of mutations in the coding regions of the gene and retained expression of parafibromin, by immunohistochemical analysis. In a previous study, we showed that the combined finding of parafibromin expression and wild-type HRPT2 gene sequence was invariably associated with a benign lesion while all parathyroid cancer specimens did not show positive staining for parafibromin (15). It may be argued that a 2-yr follow up without evidence of recurrent disease cannot definitely prove the benign nature of the parathyroid lesion since the average time between surgery and recurrence of parathyroid cancer is approximately 3 yr and a disease-free interval as long as 20 yr has been reported (1). Hence, in patient #1, these additional tests were of great clinical value.
Several presenting features of patient #2 could have suggested a malignant etiology of PHPT, but the course after surgery did not appear to counter the diagnosis of a benign parathyroid tumor. On the other hand, it is well known that parathyroid cancer is an indolent neoplasm with a relatively low malignant potential and both local recurrence and metastases may occur late in the course of the disease (1). Indeed our patient had a disease-free interval of 10 yr between initial surgery and recurrence. This case illustrates the dilemma that even experienced endocrine pathologists may have when clinical and pathological features are at variance. Perhaps in such situations, the tissue should be examined further for the HRPT2 mutation and the tissue stained for parafibromin. In addition, in selected patients with recurrent PHPT and clinical features suggestive of malignancy, it may be valuable to have the slides reviewed again and perhaps also subject the tissue to molecular analysis for HRPT2 and stained for parafibromin.
Although there was no history of familial PHPT, the HRTP2 mutation in patient #2 was unexpectedly germline and detected in the liver specimen of the father and in one of the available family members. In the latter, an extensive evaluation did not show parathyroid abnormalities, nor HPT-JT-related features. Germline HRPT2 mutations have previously been reported in apparently sporadic parathyroid carcinoma (11, 12). It is worth noting that the recognition of the carrier status in kindreds carrying a germline HRPT2 mutation enabled the early detection of a parathyroid cancer (17–18). Thus, a regular surveillance of subjects carrying a germline HRPT2 mutation should be performed using serum calcium and PTH assays and neck ultrasound for early detection of affected individuals (17–18).
In conclusion, parafibromin immunostaining and HRPT2 mutation analyses improved the diagnostic accuracy in these 2 patients in whom the clinical presentation and course were at variance with the pathological diagnosis. Studies on large series of parathyroid tumors with uncertain histological diagnosis are needed to establish the role of HRPT2 mutations and parafibromin immunohistochemistry status in this setting. Moreover, the identification of germline HRPT2 carrier status may allow an earlier detection and cure of potentially malignant tumors.
Acknowledgments
We would like to thank Dr. Bin Tean Teh, Van Andel Research Institute, Grand Rapids, Michigan, USA, for the generous gift of monoclonal parafibromin antibody and Prof. Alfredo Mussi, Division of Thoracic Surgery, Cardiac and Thoracic Department of the University of Pisa, for helpful discussion and lung surgery of patient #2. We also thank Pasquale Cofelice for technical assistance and the patients who graciously agreed to participate in the study.
This work was supported in part by the University of Pisa (to C.M.), the Ministero dell’Università e della Ricerca Scientifica e Tecnologica (to C.M.), and the Ministero della Sanità, Ricerca Oncologica 2006 (RF06ED01) (to C.M.).
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- 1.Shane E. Clinical Review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;2:485–93. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 2.DeLellis RA. Parathyroid carcinoma: an overview. Adv Anat Pathol. 2005;12:53–61. doi: 10.1097/01.pap.0000151319.42376.d4. [DOI] [PubMed] [Google Scholar]
- 3.Bondenson L, Grimelius L, DeLellis RA, et al. Parathyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and genetics of tumours of endocrine organs. Lyon, FR: IARC Press; 2004. pp. 124–7. WHO Classification of Tumour. [Google Scholar]
- 4.Grimelius L, DeLellis RA, Bondenson L, et al. Parathyroid adenoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and genetics of tumours of endocrine organs. Lyon, FR: IARC Press; 2004. pp. 128–32. WHO Classification of Tumour. [Google Scholar]
- 5.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–5. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Cryns VL, Thor A, Xu HJ, et al. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. N Engl J Med. 1994;330:757–61. doi: 10.1056/NEJM199403173301105. [DOI] [PubMed] [Google Scholar]
- 7.Naccarato AG, Marcocci C, Miccoli P, et al. Bcl-2, p53 and MIB-1 expression in normal and neoplastic parathyroid tissues. J Endocrinol Invest. 1998;213:136–41. doi: 10.1007/BF03347291. [DOI] [PubMed] [Google Scholar]
- 8.Farnebo F, Auer G, Farnebo LO, et al. Evaluation of retinoblastoma and Ki-67 immunostaining as diagnostic markers of benign and malignant parathyroid disease. World J Surg. 1999;23:68–74. doi: 10.1007/s002689900567. [DOI] [PubMed] [Google Scholar]
- 9.Cetani F, Pardi E, Viacava P, et al. A reappraisal of the Rb1 gene abnormalities in the diagnosis of parathyroid cancer. Clin Endocrinol (Oxf) 2004;60:99–106. doi: 10.1111/j.1365-2265.2004.01954.x. [DOI] [PubMed] [Google Scholar]
- 10.Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657–63. doi: 10.1136/jmg.40.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shattuck TM, Välimäki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–9. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 12.Cetani F, Pardi E, Borsari S, et al. Genetic analyses of the HRPT2 gene in primary hyperparathyroidism: germline and somatic mutations in familial and sporadic parathyroid tumors. J Clin Endocrinol Metab. 2004;89:5583–91. doi: 10.1210/jc.2004-0294. [DOI] [PubMed] [Google Scholar]
- 13.Tan MH, Morrison C, Wang P, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res. 2004;10:6629–37. doi: 10.1158/1078-0432.CCR-04-0493. [DOI] [PubMed] [Google Scholar]
- 14.Gill AJ, Clarkson A, Gimm O, et al. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT–JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol. 2006;30:1140–9. doi: 10.1097/01.pas.0000209827.39477.4f. [DOI] [PubMed] [Google Scholar]
- 15.Cetani F, Ambrogini E, Viacava P, et al. Should parafibromin staining replace HRPT2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? Eur J Endocrinol. 2007;156:547–54. doi: 10.1530/EJE-06-0720. [DOI] [PubMed] [Google Scholar]
- 16.Juhlin CC, Villablanca A, Sandelin K, et al. Parafibromin immunoreactivity: its use as an additional diagnostic marker for parathyroid tumor classification. Endocr Relat Cancer. 2007;14:501–12. doi: 10.1677/ERC-07-0021. [DOI] [PubMed] [Google Scholar]
- 17.Guarnieri V, Scillitani A, Muscarella LA, et al. Diagnosis of parathyroid tumors in familial isolated hyperparathyroidism with HRPT2 mutation: implications for cancer surveillance. J Clin Endocrinol Metab. 2006;91:2827–32. doi: 10.1210/jc.2005-1239. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TG, Shattuck TM, Reyes-Mugica M, et al. Surveillance for early detection of aggressive parathyroid disease: carcinoma and atypical adenoma in familial isolated hyperparathyroidism associated with a germline HRPT2 mutation. J Bone Miner Res. 2006;21:1666–71. doi: 10.1359/jbmr.060702. [DOI] [PubMed] [Google Scholar]