Abstract
One of the characteristics of the neurons that distinguishes them from other cells is their complex and polarized structure consisting of dendrites, cell body, and axon. The complexity and diversity of dendrites are particularly well recognized, and accumulating evidences suggest that the alterations in the dendrite structure are associated with many neurodegenerative diseases. Given the importance of the proper dendritic structures for neuronal functions, the dendrite pathology appears to have crucial contribution to the pathogenesis of neurodegenerative diseases. Nonetheless, the cellular and molecular basis of dendritic changes in the neurodegenerative diseases remains largely elusive. Previous studies in normal condition have revealed that several cellular components, such as local cytoskeletal structures and organelles located locally in dendrites, play crucial roles in dendrite growth. By reviewing what has been unveiled to date regarding dendrite growth in terms of these local cellular components, we aim to provide an insight to categorize the potential cellular basis that can be applied to the dendrite pathology manifested in many neurodegenerative diseases.
Keywords: Cytoskeleton, Dendrite pathology, Golgi outposts, Mitochondria, Neurodegenerative diseases
INTRODUCTION
Neurodegenerative diseases include a range of neuronal disabilities such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and Lou Gehrig’s disease/Amyotrophic lateral sclerosis (ALS) (1). Although the manifestation of the symptoms and the progress of these diseases differ, there exist certain features which are common to many diseases. One such feature is the accumulation of toxic proteins (1). The other common feature is the massive neuronal loss in the brain of the late-onset patients, which renders their name “neurodegenerative diseases”(2), although the affected brain regions vary depending on the types of the diseases. Notably, this massive neuronal cell death is associated primarily with the late stages of the diseases, and may not account for the disease symptoms at the early stages. For example, the brains of polyglutamine (polyQ) disease patients typically show eventual neuronal loss 10–20 years after onset of symptoms (3). Consistent with this, it has been observed that the animal models of neurodegenerative diseases, such as HD (4) and PD (5, 6), displayed behavioral symptoms even without severe neuronal loss. In other words, these findings indicate that certain neuronal alterations in function and/or morphology preceding cell death contribute crucially to the initiation of symptoms at the early stages of neurodegenerative diseases.
As a plausible candidate of these neuronal alterations preceding cell death, dendrite pathology in AD (7), PD (8), polyQ diseases (9, 10), and ALS (11) have been well recognized in both animal models and human patients. However, the exact nature of the dendrite pathology in these neurodegenerative diseases still remains largely elusive. From extensive studies done so far, we are now aware that the different diseases are associated with different patterns of dendritic spine and dendrite destabilization (12). It is notable that the dendrite pathology at the early stages of the diseases is phenotypically distinguishable from the dendrite degeneration of dying neurons. Dying neurons display dendrite blebbing/beading, followed by extensive cleavages of dendrites involving rapid disruption of dendritic cytoskeletons shortly after the turning-on of the death signal [e.g. under hypoxic condition (13)]. On the other hand, the dendrites affected by neurodegenerative diseases at the early stage tend to undergo relatively slow and progressive alterations, or maintain the changed shape for a while [e.g. polyQ diseases, (9)].
The molecular control of dendrite growth seems very complex and involves a large number of regulator molecules performing diverse molecular functions such as transcriptional control, RNA metabolism, signal transduction, and so on (14). Since numerous molecules are either directly or indirectly associated with the pathogenesis of neurodegenerative diseases, when we consider a possible molecular mechanism underlying each case of dendrite defects in various neurodegenerative diseases, there are numerous combinations of these molecules regulating the dendrite growth. On the other hand, only a small number of cellular components are closely associated with dendrite growth. These cellular components, such as cytoskeletal structures, are supposed to serve as a final effector of dendrite growth at the downstream of the complex signaling from the aforementioned numerous molecules associated with the dendrite growth. Importantly, emerging evidence suggests that local cellular components located within dendrites play very crucial roles in the control of their growth (14). These local cellular components include dendritic cytoskeletal structures consisting of F-actin and microtubules, Golgi outposts (GOPs, local Golgi apparatus in dendrites), and dendritic mitochondria (Fig. 1). To our knowledge, the dendrite defects appear to be the outcome of combinatorial alterations in these local cellular components. Although the defects in the same type of cellular components (e.g. defects in dendritic F-actin) can result in variable dendrite phenotypes (e.g. dendrite branching defect harboring much less number of branches or terminal dendrite shortening), the altered cellular component from these known or predictable phenotypic variations in dendrites can be easily predicted. This article reviews how local cellular players affect the dendrite growth in both normal and pathological conditions, and we hope to establish clear criteria for categorizing types of dendrite pathology manifested in a number of neurodegenerative diseases. In the following section, we will focus on three local cellular components [dendritic cytoskeleton as a structural backbone, GOPs as a local supplier of plasma membrane (PM), and dendritic mitochondria as a local supplier of Ca2+ and ATP] (Fig. 2) closely associated with dendrite growth.
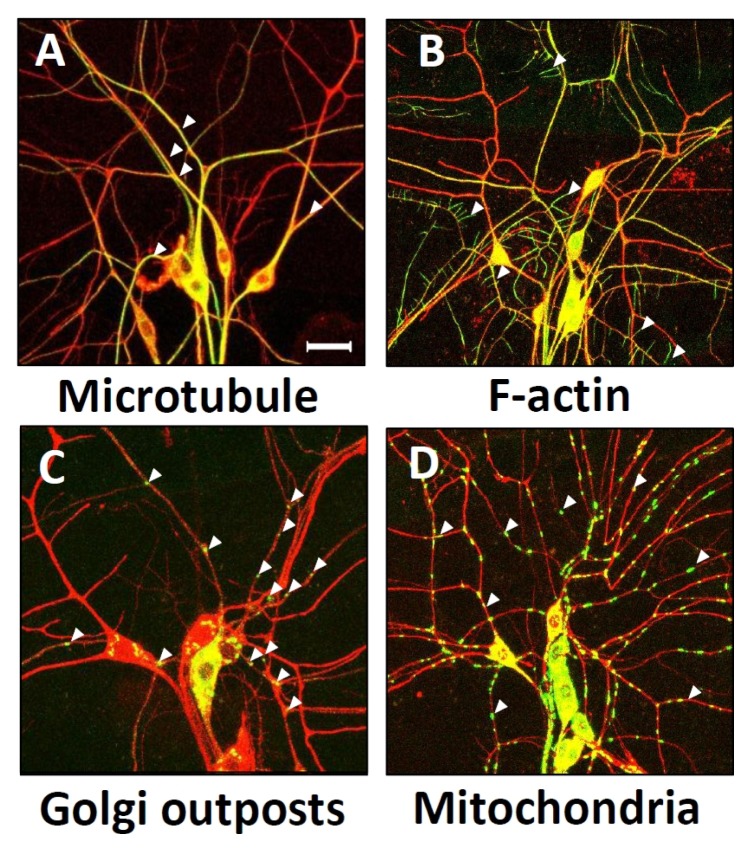
Fig. 1.
Localization of dendritic cellular components in Drosophila melanogaster dorsal cluster dendritic arborization (da) neurons. Merged images of local cellular components (green, marked by arrowheads) with membrane marker protein, CD4-tdTOM (red). Dendritic distribution of microtubules labeled by Tau-GFP (A), F-actin labeled by GMA (B), GOPs labeled by galT-eGFP (C), and mitochondria labeled by Mito-GFP (D), was examined in dorsal cluster da neurons by using 109(2)80-Gal4 driver. Dendrite images of da neurons of Drosophila 3rd instar larva located in abdominal segments A2 to A4 were captured using confocal microscopy. Scale bar indicates 50 μm.
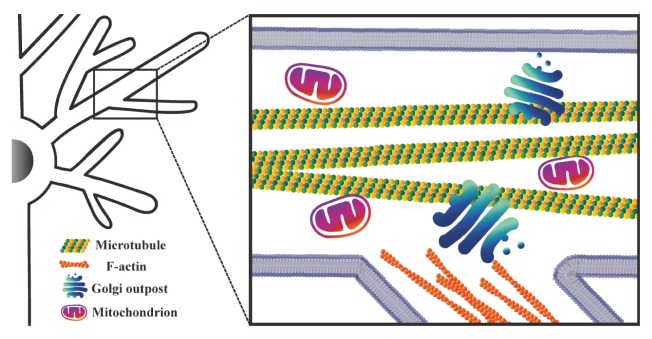
Fig. 2.
A schematic diagram showing local cellular components crucial for dendrite growth. Microtubules are located mostly in the primary branches, and F-actin is located in both primary branches and terminal dendrites (the diagram depicts F-actin at terminal dendrites only). Mitochondria and GOPs are distributed throughout the dendrites.
DISTURBANCE OF LOCAL CELLULAR COMPONENTS TO BE RESPONSIBLE FOR THE DENDRITE PATHOLOGY IN NEURODEGENERATIVE DISEASES
Dendritic changes resulted from cytoskeletal alterations in dendrites
There are two major cytoskeletal components within cells. They are filamentous actin (F-actin) and microtubules (15). To perform diverse biological activities, cells constantly undergo dynamic assembly and disassembly of these cytoskeletons under tight control of upstream regulators. The cytoskeletons serve as a backbone of cells that primarily supports the cellular structures, and are also involved in the transport of intracellular cargoes. In highly polarized neuron cells, the relative composition of these two components in dendrites is a crucial factor determining the dendrite shapes (12). In general, the main dendrite branches are known to be primarily supported by a packed network of microtubules, whereas the terminal dendrites (such as spines and filopodia) are mainly supported by F-actin structures (12). A previous study intriguingly proposed the exception to this general rule that microtubules are also present in spines, and may play an important role in the control of spine development (16). After complete establishment of the dendritic field, the main dendrite branch (supported primarily by microtubules) remains relatively static at some homeostatic ‘set point’ in dendrite size (12), while the terminal dendrite (mainly consisting of F-actin) continuously undergoes dynamic changes (17–20).
Given the role of dendritic cytoskeletons as a structural backbone of highly polarized neurons, it is easy to conceive that alterations in these dendritic cytoskeleton result in dendritic changes. The best example of microtubule-mediated dendritic changes during normal development, comes from studies on the dendrite remodeling using a Drosophila sensory neuronal system (21). These studies successfully identified the severing of microtubule structures by local caspases as a key step in the remodeling process of dendrites known as dendrite pruning (21, 22). Another example potentially links microtubules to dendrite growth in normal conditions; a recent study reported that the microtubule array near a dendritic branch site serves as a docking site for a GOP, another crucial regulator of dendrite growth, which potentially links the microtubule organization to the regulation of dendrite growth (23). The question remains whether there is any evidence linking alterations in microtubules to dendrite pathology in neurodegenerative diseases. Firstly, the changes in microtubule dynamics have been reported in many neurodegenerative diseases (24). A representative example is the change in the phosphorylation status of tau, a microtubule-binding protein, leading to the formation of neurofibrillary tangle in AD (25). In addition, microtubule depolymerization has been proposed as crucial for PD pathogenesis (26). Other direct evidences link microtubules to dendrite pathology. For example, LRRK2/Park8 mutation is known to induce dendrite degeneration involving microtubule fragmentation together with tau (27). Moreover, the alterations in dendritic microtubule dynamics have been reported to be linked to the dendrite pathology of polyQ diseases (28).
As for F-actin, several key players in the actin regulatory machinery such as Rac1, RhoA, and their associated signaling pathways, have been extensively studied for their roles in dynamic control of terminal dendrite growth (29). For tight control of F-actin formation, numerous regulator molecules of F-actin act on multiple processes related to F-actin, such as capping, severing, nucleation, and crosslinking/bundling, and so on (30). According to the previous studies, over 80% of the dendritic F-actin undergoes turn-over every minute, whereas 75% of dendritic microtubules undergo turn-over within tens of minutes (12). This means that F-actin is dynamically better controlled than microtubules, and thus neurons may be more sensitive to temporal or chronic changes in the regulator activity of F-actin in shaping terminal dendrites after the full establishment of dendritic arbors. In line with this, the pathological implication of F-actin defect was investigated in the context of many neurodegenerative diseases, including a subset of polyQ diseases, PD, and AD. Our previous study characterized that the toxicity from a subset of nuclear polyQ proteins, such as pathogenic forms of SCA3/MJD1 and SCA1, cause almost complete ablation of F-actin structures in dendrites in Drosophila sensory neurons (9). Interestingly, these F-actin alterations lead to cell type-specific terminal dendritic defects due to different compositions of cytoskeletal make-ups of dendrites in the different types of neurons. In addition, the LRRK2/Park8 function has been linked to the regulation of actin dynamics (31). Moreover, abnormal bundling and accumulation of F-actin were reported in the tau-induced AD model (32). In AD brain, aggregates of ADF/Cofilin have also been described in association with both amyloid plaques and neurofibrillary tangles (33, 34). Despite these extensive studies, further studies are required to clearly link F-actin defects to specific dendrite pathology in neurodegenerative diseases.
Dendritic changes derived from alterations of GOPs
Neurons have a vast PM area due to their complex structure. For example, it has been estimated that neurites may have about 30 times greater surface area than the surface area of a soma in cultured hippocampal neurons (35). This implies that highly polarized neurons should have an active machinery performing PM supply and recycling, compared to other cells. Especially, the terminal structures of neurons, such as dendrite terminals, need a constant and very active membrane supply due to their nature of undergoing dynamic changes in morphology (36). Indeed, neurons are known to have their unique local systems for membrane supply and recycling to fulfill this local need (37).
In general, Golgi apparatus receives materials from ER, and plays a key role in the secretory pathway that sends vesicles intra-cellularly and supplies PM through exocytosis, regardless of cell types. In neurons, a specialized local Golgi structure, GOP, is present in dendrites (38, 39). According to a recent study, GOP ablation inhibits the dendritic growth (40). In spite of these seminal works, details about GOPs remain largely elusive. For example, the exact differences in their nature between somatic Golgi and GOPs is unknown. Also, the source of GOPs remain controversial, whether somatic Golgi fragment is transported to distal dendrites to form GOPs or local ER elements generate the GOPs (42).
To date, GOPs have been implicated with a few neurodegenerative diseases. Interestingly, a recent study showed that Lrrk2 mutation associated with PD is able to induce GOP-mediated dendrite reduction (43). In addition, TDP43 mutation associated with ALS induces fragmentation of somatic Golgi (44), but the impact of somatic Golgi fragmentation on Golgi outposts is unidentified. However, a lot of unsolved questions still exist such as to how GOPs are affected by these disease conditions.
Alteration in mitochondrial physiology affecting dendrites
Mitochondria are critical for supplying cellular energy and buffering Ca2+. In the neuronal system, these functions of mitochondria are more crucial than in other cells because of the following reasons. First, neurons have elongated and complex structures, therefore the simple diffusion of ATP produced from somatic mitochondria is difficult and not sufficient to fulfill the local need (45). Second, neuronal firing is accompanied by massive influx of Ca2+, hence the ability of mitochondria to buffer Ca2+ is particularly important in neurons (46).
These functions of mitochondria are crucial for the dendrite growth. For example, adequate supply of ATP by mitochondria is very essential for F-actin elongation associated with dendrite growth (47). In addition, Ca2+ signaling has been implicated in the regulation of dendritic development (48). High amount of Ca2+ is known to result in excitotoxicity, inducing dendritic degeneration (49). Notably, there are a couple of papers regarding changes in mitochondrial physiology linked to dendrite phenotypes: mitochondrial fusion/fission status has been linked to dendrite phenotypes (50), and a mutation in a specific mitochondrial protein, preli-like, is known to induce dendritic changes (51). However, the exact mechanism of how mitochondria affect dendrite morphology still remains unknown.
Notably, mitochondrial dysfunction has been reported in many neurodegenerative diseases (52). The changes in mitochondrial quality control system has been proposed as a key pathogenic mechanism of familial cases of PD (53). Thus, it will be interesting to see whether these mitochondrial changes are indeed linked to the dendrite pathology in these diseases.
Drugs that have potential for amelioration of dendrite pathology
In this section, we discuss the possible therapeutic strategies targeting the cytoskeleton in neurodegenerative diseases, and future targets. Microtubule-stabilizing compounds have been investigated and used as cancer therapeutics. Brain penetrant epothilone D (EpoD), which stabilizes microtubule similar to Taxol, has been demonstrated to rescue microtubule organization, cognitive defect, and tau pathology in a couple of mouse models (54, 55). Based on these results, a phase I clinical trial by Bristol-Myers Squibb was carried out from 2012 to 2013, but the results have not been announced so far (ClinicalTrials. gov identifier NCT01492374). In addition, EpoD rescued microtubule defects in PD mice model (56). Given that the balance of microtubule dynamics is important to maintain dendrite and axon integrity, the blood-brain barrier (BBB)-permeable agents that can stabilize microtubule dynamics remain promising therapeutics for neurodegenerative diseases.
Microtubule dynamics is mainly regulated by microtubule-associated proteins (MAPs). Tau and collapsin response mediator protein 2 (CRMP2), which belong to MAPs, are hyperphosphorylated in AD. GSK3β and CDK5 are the kinases responsible for the hyperphosphorylation (57, 58). The phosphorylation of tau and CRMP2 by CDK5 is a prerequisite for the subsequent phosphorylation by GSK3β, which is called ‘priming’. Both kinases are involved in neurodegenerative disorders (58, 59). Various compounds that block the activity of GSK3β have been investigated in neurodegeneration mice model and clinical trials. Tideglusib, a selective GSK3β inhibitor, showed not only decreased tau phosphorylation and improved behavioral functions, but also decreased Aβ pathology and neuronal death in AD models (60, 61). However, a phase II trial using Tideglusib in AD patients showed the drug safety in 26 weeks without clinical benefit (62). A recent work published this year demonstrated that DYRK1, a kinase overexpressed in Down’s syndrome patients, can phosphorylate tubulin per se and inhibit their polymerization, leading to the defective dendrite patterning (63). It would be intriguing to see whether selective DYRK1 inhibitors modulate the disease symptoms of AD, PD, and ALS.
RhoA/ROCK pathway regulates the polymerization of actin cytoskeleton and has been implicated in AD, PD, HD and ALS (64). Although it is unclear whether the inhibition of this signaling is beneficial for the treatment of neurodegeneration, several evidences reveal that ROCK inhibitors may have potential to cure the diseases. For example, the knockdown of ROCK2 (an isoform strongly expressed in the brain) reduces the loss of dopaminergic neurons and improves motor behavior in 6-OHDA mice model (65). Several compounds selective for ROCK2 have been recently developed to treat autoimmune diseases. It would be of much interest to test the compounds in neurodegeneration mouse models. However, there is a concern to use ROCK inhibitors as a therapeutic for the neurodegenerative diseases. Recent work unveiled that RhoA/ROCK pathway is required for GOP formation (66), hence the study of these inhibitors should be carefully interpreted.
Besides studying the specific targets for the diseases, several compounds that restore the ability of dendritic spine formation in neurons have been screened. One of the efforts led to the discovery of benzothiazole amphiphiles, which promote the formation of dendritic spines (67). These chemicals may facilitate spinogenesis through the activation of Ras pathway, although they are a bit toxic to the cells at their active concentration.
The drugs intervening cytoskeleton functions seem to have a huge potential in improving the symptoms in neurodegenerative diseases in mice models, but many obstacles still remain to be overcome. Target-related toxicity due to non-specific inhibition of cytoskeleton or kinases will be a great concern. In addition, the delivery of compounds to the central nervous system is always a tough question in drug discovery. Despite these circumstances, it is essential to make an effort to seek more selective and brain-penetrant therapeutics.
CONCLUSION
In this article, we reviewed alterations in the local structural supports by dendritic cytoskeletons, local supply of PM components by GOPs, and local supply of Ca2+ and ATP by dendritic mitochondria to be potentially associated with dendrite pathology in neurodegenerative diseases. As reviewed here, we begin to recognize the key players for decoding the mystery of the dendrite pathology, but many questions still remain to be addressed. We do not know the exact contribution of the dendrite pathology to the pathogenesis of the diseases and to the disease symptoms. Also, in particular, not much is known about the nature of GOPs. Thus, further studies are required to clearly address these questions. With the tremendous efforts of the related research fields, we hope to fully understand the cellular basis of the dendrite pathology manifested in many neurodegenerative diseases someday in the future, which will greatly help to develop effective treatments specifically targeting early stages of the diseases.
ACKNOWLEDGEMENTS
This work was supported by the DGIST R&D and MIREBraiN program, Basic Science Research Program through the ministry of science, ICT & future planning of Korea (16-BD-0402, 2013R1A1A1004978), and the Development of Platform Technology for Innovative Medical Measurements Program from the Korea Research Institute of Standards and Science (KRISS-2016-16011064) (S.B.L.).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting financial interests.
REFERENCES
- 1.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 2.Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 3.Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- 4.Li X-J. The early cellular pathology of Huntington’s disease. Mol Neurobiol. 1999;20:111–124. doi: 10.1007/BF02742437. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg MS, Fleming SM, Palacino JJ, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 6.Gispert S, Ricciardi F, Kurz A, et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baloyannis SJ. Dendritic pathology in Alzheimer’s disease. J Neurol Sci. 2009;283:153–157. doi: 10.1016/j.jns.2009.02.370. [DOI] [PubMed] [Google Scholar]
- 8.Villalba RM, Smith Y. Striatal spine plasticity in Parkinson’s disease. Front Neuroanat. 2010;4:133. doi: 10.3389/fnana.2010.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SB, Bagley JA, Lee HY, Jan LY, Jan YN. Pathogenic polyglutamine proteins cause dendrite defects associated with specific actin cytoskeletal alterations in Drosophila. Proc Natl Acad Sci U S A. 2011;108:16795–16800. doi: 10.1073/pnas.1113573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiFiglia M, Sapp E, Chase KO, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 11.Nakano I, Hirano A. Atrophic cell processes of large motor neurons in the anterior horn in amyotrophic lateral sclerosis: observation with silver impregnation method. J Neuropathol Exp Neurol. 1987;46:40–49. doi: 10.1097/00005072-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3:215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 14.Jan Y-N, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer RS, Fowler VM. Thematic Minireview Series: The State of the Cytoskeleton in 2015. J Biol Chem. 2015;290:17133–17136. doi: 10.1074/jbc.R115.663716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J, Firestein BL, Zheng JQ. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–12124. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 18.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 19.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 20.Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/S0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 21.Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132:3631–3642. doi: 10.1242/dev.01928. [DOI] [PubMed] [Google Scholar]
- 22.Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubey J, Ratnakaran N, Koushika SP. Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci. 2015;9:343. doi: 10.3389/fncel.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zempel H, Mandelkow E. Lost after translation: missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014;37:721–732. doi: 10.1016/j.tins.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Feng J. Microtubule: a common target for parkin and Parkinson’s disease toxins. Neuroscientist. 2006;12:469–476. doi: 10.1177/1073858406293853. [DOI] [PubMed] [Google Scholar]
- 27.Lin CH, Tsai PI, Wu RM, Chien CT. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ss. J Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Stone MC, Tao J, Rolls MM. Axon injury and stress trigger a microtubule-based neuroprotective pathway. Proc Natl Acad Sci U S A. 2012;109:11842–11847. doi: 10.1073/pnas.1121180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch MD, Mullins RD. Cellular control of actin nucleation. Annu Rev Cell Dev Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- 31.Parisiadou L, Cai H. LRRK2 function on actin and microtubule dynamics in Parkinson disease. Commun Integr Biol. 2010;3:396–400. doi: 10.4161/cib.3.5.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulga TA, Elson-Schwab I, Khurana V, et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 33.Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2:628–636. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- 34.Heredia L, Helguera P, de Olmos S, et al. Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: a potential mechanism of neuronal dystrophy in Alzheimer’s disease. J Neurosci. 2006;26:6533–6542. doi: 10.1523/JNEUROSCI.5567-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye B, Zhang YW, Jan LY, Jan YN. The secretory pathway and neuron polarization. J Neurosci. 2006;26:10631–10632. doi: 10.1523/JNEUROSCI.3271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 37.Hanus C, Ehlers MD. Secretory Outposts for the Local Processing of Membrane Cargo in Neuronal Dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardiol A, Racca C, Triller A. Dendritic and postsynaptic protein synthetic machinery. J Neurosci. 1999;19:168–179. doi: 10.1523/JNEUROSCI.19-01-00168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Sengupta D, Linstedt AD. Control of organelle size: the Golgi complex. Annu Rev Cell Dev Biol. 2011;27:57–77. doi: 10.1146/annurev-cellbio-100109-104003. [DOI] [PubMed] [Google Scholar]
- 43.Lin CH, Li H, Lee YN, Cheng YJ, Wu RM, Chien CT. Lrrk regulates the dynamic profile of dendritic Golgi outposts through the golgin Lava lamp. J Cell Biol. 2015;210:471–483. doi: 10.1083/jcb.201411033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita Y, Mizuno Y, Takatama M, Okamoto K. Anterior horn cells with abnormal TDP-43 immunoreactivities show fragmentation of the Golgi apparatus in ALS. J Neurol Sci. 2008;269:30–34. doi: 10.1016/j.jns.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Hubley MJ, Locke BR, Moerland TS. The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochim Biophys Acta. 1996;1291:115–121. doi: 10.1016/0304-4165(96)00053-0. [DOI] [PubMed] [Google Scholar]
- 46.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 47.KoRN ED, Carlier M-F, Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987;238:638–644. doi: 10.1126/science.3672117. [DOI] [PubMed] [Google Scholar]
- 48.Redmond L, Ghosh A. Regulation of dendritic development by calcium signaling. Cell Calcium. 2005;37:411–416. doi: 10.1016/j.ceca.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Greenwood SM, Connolly CN. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology. 2007;53:891–898. doi: 10.1016/j.neuropharm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Okamoto K-I, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Tsubouchi A, Tsuyama T, Fujioka M, et al. Mitochondrial protein Preli-like is required for development of dendritic arbors and prevents their regression in the Drosophila sensory nervous system. Development. 2009;136:3757–3766. doi: 10.1242/dev.042135. [DOI] [PubMed] [Google Scholar]
- 52.Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 53.Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway: a mitochondrial quality control system? J Bioenerg Biomembr. 2009;41:499–503. doi: 10.1007/s10863-009-9253-3. [DOI] [PubMed] [Google Scholar]
- 54.Barten DM, Fanara P, Andorfer C, et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B, Carroll J, Trojanowski JQ, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cartelli D, Casagrande F, Busceti CL, et al. Microtubule alterations occur early in experimental parkinsonism and the microtubule stabilizer epothilone D is neuroprotective. Sci Rep. 2013;3:1837. doi: 10.1038/srep01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng HC, Zhou Y, Shen Y, Tsai LH. A survey of Cdk5 activator p35 and p25 levels in Alzheimer’s disease brains. FEBS Lett. 2002;523:58–62. doi: 10.1016/S0014-5793(02)02934-4. [DOI] [PubMed] [Google Scholar]
- 58.Lei P, Ayton S, Bush AI, Adlard PA. GSK-3 in Neurodegenerative Diseases. Int J Alzheimers Dis. 2011;2011;189246 doi: 10.4061/2011/189246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheung ZH, Ip NY. Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012;22:169–175. doi: 10.1016/j.tcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Noh MY, Chun K, Kang BY, et al. Newly developed glycogen synthase kinase-3 (GSK-3) inhibitors protect neuronal cells death in amyloid-beta induced cell model and in a transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2013;435:274–281. doi: 10.1016/j.bbrc.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 61.Sereno L, Coma M, Rodriguez M, et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 62.Lovestone S, Boada M, Dubois B, et al. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimers Dis. 2015;45:75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 63.Ori-McKenney KM, McKenney RJ, Huang HH, et al. Phosphorylation of beta-Tubulin by the Down Syndrome Kinase, Minibrain/DYRK1a, Regulates Microtubule Dynamics and Dendrite Morphogenesis. Neuron. 2016;90:551–563. doi: 10.1016/j.neuron.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eira J, Silva CS, Sousa MM, Liz MA. The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog Neurobiol. 2016;141:61–82. doi: 10.1016/j.pneurobio.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Saal KA, Koch JC, Tatenhorst L, et al. AAV.shRNA-mediated downregulation of ROCK2 attenuates degeneration of dopaminergic neurons in toxin-induced models of Parkinson’s disease in vitro and in vivo. Neurobiol Dis. 2015;73:150–162. doi: 10.1016/j.nbd.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Quassollo G, Wojnacki J, Salas DA, et al. A RhoA Signaling Pathway Regulates Dendritic Golgi Outpost Formation. Curr Biol. 2015;25:971–982. doi: 10.1016/j.cub.2015.01.075. [DOI] [PubMed] [Google Scholar]
- 67.Cifelli JL, Dozier L, Chung T, Patrick GN, Yang J. Benzothiazole Amphiphiles Promote the Formation of Dendritic Spines in Primary Hippocampal Neurons. J Biol Chem. 2016;291:11981–11992. doi: 10.1074/jbc.M115.701482. [DOI] [PMC free article] [PubMed] [Google Scholar]