Abstract
Sustained dysregulation of blood glucose (hyper- or hypoglycemia) associated with type 1 diabetes (T1D) has been linked to cognitive deficits and altered brain anatomy and connectivity. However, a significant gap remains with respect to how T1D affects spontaneous at-rest connectivity in young developing brains. Here, using a large multisite study, resting-state functional MRI data were examined in young children with T1D (n = 57; mean age = 7.88 years; 27 females) as compared with age-matched control subjects without diabetes (n = 26; mean age = 7.43 years; 14 females). Using both model-driven seed-based analysis and model-free independent component analysis and controlling for age, data acquisition site, and sex, converging results were obtained, suggesting increased connectivity in young children with T1D as compared with control subjects without diabetes. Further, increased connectivity in children with T1D was observed to be positively associated with cognitive functioning. The observed positive association of connectivity with cognitive functioning in T1D, without overall group differences in cognitive function, suggests a putative compensatory role of hyperintrinsic connectivity in the brain in children with this condition. Altogether, our study attempts to fill a critical gap in knowledge regarding how dysglycemia in T1D might affect the brain’s intrinsic connectivity at very young ages.
Introduction
Young children with type 1 diabetes (T1D) are particularly susceptible to extreme swings of hyper- and hypoglycemia (dysglycemia) due to their underlying sensitivity to insulin, unpredictable eating and exercise patterns, and inability to reliably communicate signs or symptoms of hypoglycemia. As the brain is reliant on glucose for metabolism, the effect of dysglycemia on brain development and functioning is an area of physiological and clinical interest (1). Several previous studies have found that individuals diagnosed with T1D at a younger age (∼4 years) may be at a greater risk for developing neuropsychological deficits at later ages (2–4). Accordingly, better understanding of how dysglycemia can affect brain anatomy and connectivity in very young children might allow clinicians to better identify patients at greatest risk for developing cognitive alterations in the future.
Successfully maintaining control of blood glucose levels requires an effective alliance between interoceptive sensing of changing glycemic levels as well as complex behavioral management strategies by the individual that include awareness of dysglycemic symptoms, motivation, food/fluid intake, etc. (1). Thus, different brain functional units must coordinate as networks to mediate the cognitive and behavioral aspects associated with dysglycemia (1). However, very little is known about the deleterious effects of T1D on this network-level coordinated functioning across brain regions in young children.
Two studies have previously used resting-state functional MRI (rsfMRI) to examine differences in intrinsic (or at-rest) connectivity among brain regions in adults with T1D as compared with control subjects without diabetes (1,5). Bolo et al. (1) used a sequential euglycemic-hypoglycemic clamp to examine changes in resting-state functional connectivity (RSFC) associated with experimentally induced hypoglycemia in adults with T1D and control subjects without diabetes. The authors found that during hypoglycemia, adults with T1D showed increased within-group RSFC in the right prefrontal and insular brain regions. Further, the increased connectivity was positively associated with concurrent higher glycosylated hemoglobin levels. The authors concluded that the increased RSFC in prefrontal and insular regions could reflect the brain’s adaptive response to chronic hyperglycemia in adults with T1D (1).
In another study, van Duinkerken et al. (5) examined the alterations in RSFC in adults with T1D as compared with adults without diabetes and how these alterations vary with disease progression (i.e., degree of microangiopathy). As compared with control subjects, individuals with T1D without microangiopathy showed increased connectivity in networks involved in the motor and visual processes, whereas individuals with T1D with microangiopathy demonstrated decreased connectivity in similar networks. Further, increased connectivity in the group with T1D was associated with better information processing speed and general cognitive ability (5). Taken together, in adults with T1D, in the early stages of disease progression (i.e., prior to microangiopathy), increased RSFC was observed. But in the later stages of disease progression (i.e., after microangiopathy), reduced RSFC was observed. Whether alterations in connectivity would be observed in children with T1D has not been previously studied; thus, a significant knowledge gap remains with respect to how T1D affects functional connectivity in young developing brains.
Here, we examined how early-onset T1D in young children (ages 4–11 years) affects RSFC relative to age- and sex-matched individuals without diabetes. Group differences in RSFC were examined using both model-driven seed-based analysis and model-free independent component analysis (ICA). For the seed-based analysis, seed locations were selected a priori based on recent work from our group showing that gray matter volumes in the right lingual gyrus and left prefrontal cortex best differentiated young children with T1D and individuals without diabetes (6). Given the correspondence between structural topology and network dynamics in the brain (7), we hypothesized that the previously observed gray matter morphometric changes would be associated with functional connectivity differences in young children with T1D. For the ICA, group differences in connectivity within and between large-scale brain networks were assessed. To better understand the effects of T1D on RSFC in young children relative to adults, we examined RSFC within large-scale resting-state networks (RSNs) previously investigated in adults with T1D (1,5). In addition to this primary analysis, we examined alterations in between-network connectivity associated with T1D in children. To our knowledge, alterations in between-network connectivity have not been previously investigated in T1D. Overall, we predicted that dysglycemia in young children with T1D would be associated with aberrant within- and between-network connectivity.
Research Design and Methods
Participants and Recruitment
Children were recruited for this study at five clinical centers in the Diabetes Research in Children Network (DirecNet) consortium (Nemours Children’s Clinic, Stanford University, University of Iowa, Washington University in St. Louis, and Yale University). The institutional review board at each participating center approved the study protocol. Informed written consent was obtained from the parent or legal guardian of all participants, and verbal assent was obtained from study participants as per local guidelines. Eligibility and exclusion criteria are provided in the Supplementary Data.
Cognitive Testing and Blood Glucose Measurement
Trained examiners collected data on intelligence quotients (IQs) using the age-appropriate Wechsler Preschool and Primary Scale of Intelligence, the Wechsler Intelligence Scale for Children IV, or the Wechsler Adult Intelligence Scale test for parents (8–10). Cognitive results for full-scale IQ and composite neuropsychological assessments were converted to normalized z scores (Table 1). Within the group with T1D, before or near the time of magnetic resonance (MR) scan, a blood sample was collected to measure HbA1c values using the DCA Vantage (Siemans Medical Solutions) or similar local point of care device. Details regarding the assessment of cognitive function (including IQ) and measurement of blood glucose levels (HbA1c) have been described previously (11,12). For participants with T1D, blood glucose concentrations were required to be between 70 and 300 mg/dL at the time of both imaging and cognitive assessments (four blood glucose levels were tested: 2 h, 1 h, and just before and right after assessment using a finger stick on a home glucose meter). All participants were monitored for symptoms of hypoglycemia throughout the assessments. Insulin or food was given to titrate blood glucose levels as needed. Every 3 months, during clinic visits, parents completed a survey regarding severe hypoglycemia events (see Mazaika et al. [12] and Cato et al. [13] for more details).
Table 1.
Descriptive statistics of the study cohort
| T1D | Control subjects without diabetes | P value | |
|---|---|---|---|
| n = 83 | 57 (27 females) | 26 (14 females) | — |
| Age (years) | 7.88 (1.79) | 7.43 (1.86) | 0.298 |
| Cognitive functioning (z scores) | |||
| IQ | −0.14 (0.97) | 0.15 (0.92) | 0.203 |
| Executive functioning | −0.04 (0.62) | 0.06 (0.68) | 0.535 |
| Learning and memory | −0.05 (0.78) | −0.21 (0.79) | 0.399 |
| Processing speed | 0.01 (1.06) | 0.27 (1.08) | 0.307 |
| Clinical measures | |||
| HbA1c (mmol/mol) | 63 (9.9) | 33 (2.1) | <0.0001 |
| HbA1c (%) | 7.9 (0.91) | 5.1 (0.19) | <0.0001 |
| T1D onset age (years) | 4.24 (1.80) | NA | NA |
| Duration of clean rsfMRI data (min) | 4.42 (0.27) | 4.51 (0.30) | 0.160 |
| Number of scrubbed rsfMRI frames | 29.39 (8.0) | 26.58 (9.1) | 0.162 |
| FD in rsfMRI data (post-scrubbing) (mm) | 0.13 (0.04) | 0.13 (0.03) | 0.908 |
Mean (SD) is reported for each variable. P values from an independent-sample Student t test are reported to assess any group differences.
MR Data Acquisition
Unsedated MRI was performed on a Siemens 3T Tim Trio using a standard 12-channel head coil. All six imaging sites had the same scanner hardware, and an identical imaging protocol was uploaded to every scanner. Sagittal T1 images of the brain were acquired using an MP-RAGE pulse sequence with the following parameters: repetition time (TR) = 2,300 ms, echo time (TE) = 2.98 ms, inversion time (TI) = 900 ms, flip angle = 9°, slice thickness = 1 mm, field of view (FOV) = 25.6 cm × 24 cm, 160 slices, matrix = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm, and duration = 4:54 min. A gradient echo imaging pulse sequence was used to acquire 5 min 50 s of rsfMRI data (T2*-weighted images) with the following parameters: TR = 2,000 ms, TE = 27 ms, flip angle = 80°, FOV = 22 cm × 22 cm, 33 slices, matrix = 74 × 74, voxel size = 3 × 3 × 4 mm, and duration = 5:50 min.
Data Preprocessing
Standard rsfMRI preprocessing was performed using the Configurable Pipeline for the Analysis of Connectomes (C-PAC version 0.3.4; http://fcp-indi.github.io/docs/user/index.html). Preprocessing included discarding the first 10 volumes (or TRs) of data for signal stabilization, slice timing correction, motion correction (FSL MCFLIRT), skull stripping (FSL BET), grand mean scaling, spatial smoothing (FWHM 4 mm), and temporal band-pass filter (0.01 Hz < f < 0.1 Hz). Additionally, nuisance signal correction was done on the data by regressing out 1) linear and quadratic trends, 2) mean time series from the white matter (WM) and the cerebrospinal fluid, 3) 24 motion parameters obtained by motion correction (the 6 motion parameters of the current volume and the preceding volume plus each of these values squared), and 4) signals extracted using the CompCor algorithm (14).
To ensure that group differences in RSFC are not influenced by spurious motion-related noise, scrubbing (“censuring”) was performed (15–17). Frame-wise displacement (FD) and DVARS (D referring to temporal derivative of time course, VARS referring to RMS variance over voxels) were used in union to censure data points. In addition, to accommodate temporal smoothing of blood oxygen level–dependent data, we also marked one back and two forward frames from any marked frames where the FD/DVARS threshold (determined using fsl_motion_outliers command) was crossed. No group differences were observed for any of the following metrics: 1) total number of outlier frames, 2) mean FD after scrubbing, and 3) duration of “clean” (after scrubbing) resting-state data (see Table 1).
Seed-Based Connectivity Analysis
Extracted time series from the two seed locations were modeled with the general linear model analysis using the FMRI Expert Analysis Tool (FEAT). For the group-level comparisons, age, sex, and data acquisition site location were used as covariates of no interest. Group-level maps were cluster corrected using a standard value of z = 2.3 and family-wise error P < 0.05. For details, see Supplementary Data.
ICA-Based Within- and Between-Network Connectivity Analysis
Using group ICA and dual regression methodology (18), we examined short-range (or within network) RSFC differences associated with T1D within large-scale brain networks. This analysis involves three main steps. First, data-driven spatial maps were created by running group ICA (18) on temporally concatenated data from an equal number of participants from both groups. To reduce the bias from the fact that the number of participants in the group with T1D was twice that in the control group without diabetes, we randomly selected 26 participants from the group with T1D to match the number of participants in the control group without diabetes. Second, the group ICA components were then dual regressed into the subject space for all 83 subjects (18). Third, we examined within-network connectivity differences in 10 large-brain brain networks (including the dorsal attention network [DAN] and ventral attention network [VAN]) associated with T1D. Group-level analysis was performed by contrasting subject-specific spatial maps for the 10 large-scale networks, while controlling for age, sex, and data acquisition site location. To determine significant group differences, FSL’s randomize permutation tool was used; it uses a threshold-free cluster enhancement (TFCE) procedure at a family-wise error (P < 0.05) with 10,000 iterations (19). For details, see Supplementary Data.
As an exploratory analysis, we also examined differences in between-network connectivity across all the networks identified by the probabilistic group ICA (using FSLNets version 0.6). Out of the 66 components derived from the probabilistic group ICA, 18 were deemed bad based on their topographic location (e.g., in WM or outside of the brain) and power spectra (e.g., flat power spectra depicting white noise). On the basis of previous work (20), partial correlation (regularized with ρ = 0.01) was used to estimate the 48 × 48 connectivity matrix. After estimating this matrix for each participant, group-wise differences were estimated (while controlling for age, sex, and data acquisition site location) using FSL’s randomize tool with TFCE. The false discovery rate (FDR) was used to correct for 1,128 [(48 × 47)/2] tests. For details, see Supplementary Data.
Correlation With Behavioral Performance and Blood Glucose Variables
Correlation analyses were conducted for each group using Spearman ρ. Variance associated with age and sex was removed from performance scores using regression analysis before correlating with the neuroimaging variables.
Results
Seed-Based Connectivity Analysis
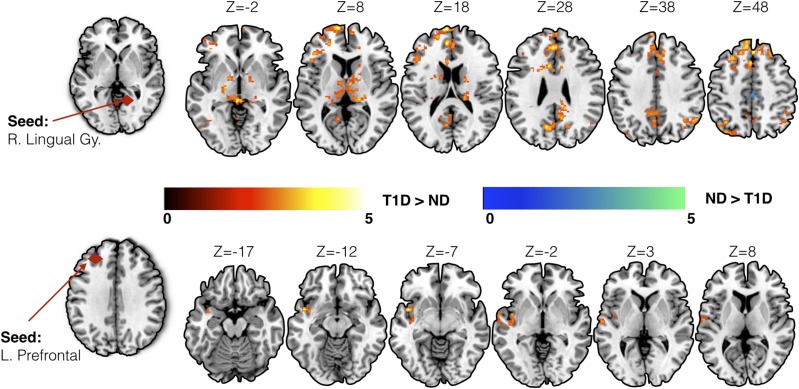
For seed-based analysis, we chose brain regions previously shown to be associated with glycemic dysregulation in T1D. Specifically, a priori seed locations were chosen in the left prefrontal cortex (Montreal Neurological Institute [MNI] coordinates: −28, 40, 33) and right lingual gyrus (MNI coordinates: 14, −53, 0) (6). While controlling for age, sex, and data acquisition site location, converging evidence of increased connectivity in individuals with T1D was observed across both seed locations (Fig. 1 and Table 2). Increased seed-based connectivity was observed in young children with T1D (as compared with control subjects) between the right lingual gyrus seed and left precuneus, bilateral thalamus, left lateral occipital cortex, and right angular gyrus. Similarly, in children with T1D, increased connectivity was observed between the left prefrontal seed and left planum polare, insular cortex, and temporal pole.
Figure 1.
Results from the seed-based connectivity analysis using seeds in the right lingual gyrus (R. Lingual Gy.) and left prefrontal (L. Prefrontal) cortex. Increased connectivity in children with T1D as compared with control subjects without diabetes (ND) was observed using both seeds. Group differences in connectivity are overlaid on the MNI-152 average brain. Color bars represent standard cluster-corrected z stats (using threshold of z = 2.3) at family-wise error P < 0.05. Warm color scale (red to yellow) indicates T1D > ND, whereas cool color scale (blue to green) represents ND > T1D.
Table 2.
Seed-based analysis
| Seed location | Direction of effect | Cluster index | Cluster size (in voxels) | z max | P value | MNI coordinates (in mm) for the peak location |
Peak region | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Right lingual gyrus | T1D > ND | 9 | 3,009 | 4.67 | <0.00001 | −6 | 64 | 18 | Left frontal pole |
| T1D > ND | 8 | 912 | 3.8 | <0.00001 | −8 | −62 | 34 | Left precuneus | |
| T1D > ND | 7 | 738 | 4.31 | <0.00001 | 10 | −68 | −32 | Right cerebellum | |
| T1D > ND | 6 | 510 | 4.04 | <0.00001 | −8 | −8 | 6 | Left thalamus | |
| T1D > ND | 5 | 393 | 3.86 | 0.000123 | −38 | −56 | −30 | Left cerebellum | |
| T1D > ND | 4 | 311 | 3.82 | 0.000993 | −20 | −16 | −4 | Left pallidum | |
| T1D > ND | 3 | 263 | 4.28 | 0.00367 | −38 | −72 | 52 | Left lateral occipital cortex | |
| T1D > ND | 2 | 258 | 3.44 | 0.00422 | 54 | −56 | 38 | Right angular gyrus | |
| T1D > ND | 1 | 208 | 4.01 | 0.0181 | 18 | −26 | 16 | Right thalamus | |
| ND > T1D | 2 | 287 | 4.34 | 0.00189 | 14 | −18 | 72 | Right precentral gyrus | |
| ND > T1D | 1 | 272 | 3.79 | 0.00286 | −8 | −18 | 56 | Left precentral gyrus | |
| Left prefrontal | T1D > ND | 1 | 387 | 4.22 | 0.000317 | −44 | 4 | −10 | Left planum polare |
ND, subjects without diabetes.
Within-Network Connectivity Analysis
Using group ICA and dual regression, we examined RSFC differences associated with T1D within large-scale brain networks. On the basis of previous work in adults with T1D (1,5), we specifically examined within-network (or short-range) connectivity in 10 RSNs, namely, the DAN and VAN, default mode network, salience network, auditory network, primary and secondary visual networks, sensory motor network, and bilateral fronto-parietal networks.
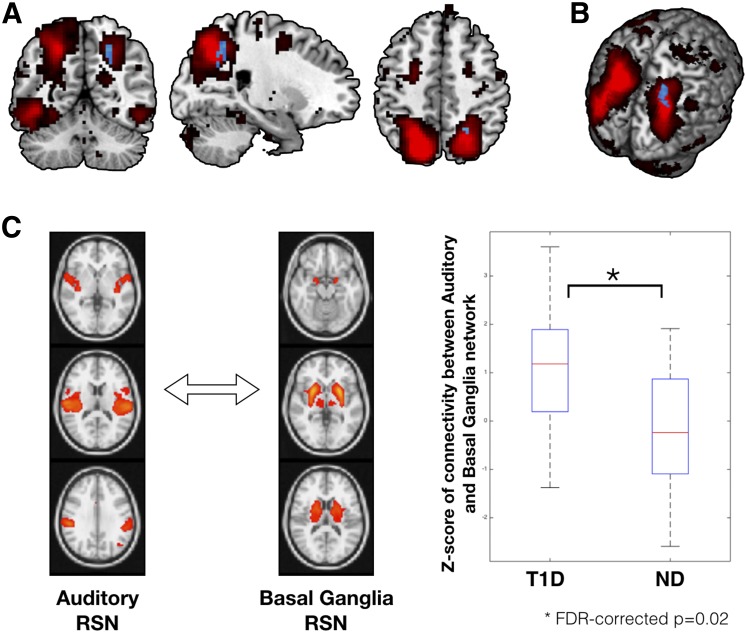
In line with the seed-based connectivity analysis, increased within-network connectivity was also observed in children with T1D as compared with control subjects without diabetes while controlling for age, sex, and data acquisition site location in one of the 10 networks. Specifically, within-network connectivity within the DAN was significantly higher in children with T1D in the region of right superior parietal lobule (peak MNI coordinates: 21, −54, 48; P = 0.024, Student t test value = 3.45) (Fig. 2). No other network was observed to be significantly different between the two groups.
Figure 2.
Results from spatial ICA based on within-network (A and B) and between-network (C) connectivity analysis. A: The DAN, extracted using group ICA, is overlaid on the average MNI-152 brain in red color. Within DAN, significant group differences were observed for a cluster encompassing the region of right superior parietal lobule (peak MNI coordinates: 21, −54, 48; P = 0.024, Student t test value = 3.45) in the direction of T1D > ND (shown in blue color). No group differences were observed for within-DAN connectivity in the reverse direction (i.e., ND > T1D). B: Within-DAN group differences on a three-dimensional rendering of the MNI-152 brain. C: Group differences for the between-network (or long-range) connectivity. Group differences in between-network connectivity were examined for all combinations of RSNs extracted using group ICA (corrected for multiple comparisons; see research design and methods). Out of all between-network connectivity examinations, two networks (auditory and basal ganglia) were found to be significantly different between groups (FDR-corrected P = 0.02). Participants with T1D had significantly higher between-network connectivity than control subjects without diabetes for the auditory and basal ganglia resting-state networks. ND, subjects without diabetes.
Between-Network Connectivity Analysis
As none of the previous studies have examined such between-network long-range connectivity in individuals with T1D, as an exploratory analysis, we examined differences across 48 networks (see research design and methods). Increased between-network connectivity between the basal ganglia and auditory large-scale networks was observed in young children with T1D as compared with control subjects without diabetes (FDR-corrected P = 0.023; uncorrected P < 0.0002) (Fig. 2), while controlling for age, sex, and data acquisition site location as nuisance variables.
Correlation Between Connectivity Differences and Cognitive and Glycemic Metrics
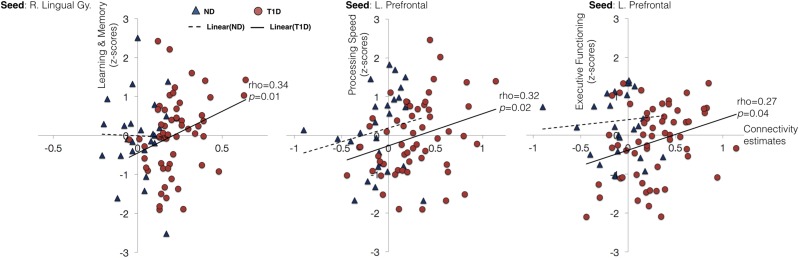
As an exploratory post hoc analysis, we examined whether the observed increase in connectivity in children with T1D was associated with cognitive functioning and glycemic variables. We examined the association between individual connectivity estimates from seed-based and ICA-based analyses with cognitive function scores and HbA1c levels. Figure 3 shows scatter plots of these associations.
Figure 3.
Correlation plots between connectivity estimates and cognitive functioning for both groups. Within the group with T1D, significant positive associations were observed between seed-based connectivity estimates and cognitive functioning. Red circles denote participants with T1D, and blue triangles denote control subjects without diabetes (ND). Solid line depicts linear trend within the group with T1D, and dashed line depicts linear trend within the control group without diabetes. No significant correlations were observed in the control group. L. Prefrontal, left prefrontal; R. Lingual Gy., right lingual gyrus.
To reduce the number of overall correlations (and possible impact of multiple comparisons), for each seed location, mean connectivity was estimated across all brain regions where the group differences were observed. Thus, for seed-based analysis, we had two connectivity estimates for each of the seeds per participant. These connectivity estimates were examined for correlations with HbA1c levels and performance on four cognitive domains, including intelligence (IQ), executive functioning, learning and memory, and processing speed (13).
Within group, increased seed-based connectivity in children with T1D for a seed in the left prefrontal cortex was positively associated with performance on executive functioning (ρ = 0.27, P = 0.04) and processing speed domains (ρ = 0.32, P = 0.015). Similarly, increased seed-based connectivity in children with T1D for a seed in the right lingual gyrus was positively associated with performance on the cognitive domain of learning and memory (ρ = 0.34, P = 0.01). No significant associations between connectivity estimates and cognitive performance were observed in the control group. To estimate the significance of between-group differences in correlation coefficients, Fisher r-to-z transformation was performed. The between-group differences in correlation coefficients were not significant for any of the four associations found within the group with T1D (all P > 0.05). Further, no significant associations between connectivity estimates and HbA1c values were found in the group with T1D.
Altogether, increased connectivity was observed in children with T1D (as compared with control subjects without diabetes) using both seed- and ICA-based analysis. The observed increase in connectivity is correlated with behavioral performance within the group with T1D.
Discussion
This large multisite study examined, for the first time, the differences in RSFC in young children with T1D as compared with age-matched control subjects without diabetes. Across different analytic approaches for assessing RSFC, converging evidence indicated hyper- (or increased) connectivity in children with T1D. The increase in connectivity was observed across several cortical and subcortical regions, including the prefrontal cortex, insula, cingulate gyrus, thalamus, and DAN. Additionally, the increase in connectivity estimates was found to be positively associated with cognitive functioning in children with T1D.
Several plausible mechanisms may account for the increased intrinsic connectivity observed in young children with T1D. For example, increased connectivity in young children with T1D could be due to the antagonistic nature of RSNs. Evidence suggests that the task-positive (i.e., DAN) and task-negative (i.e., default mode network) RSNs are inversely correlated (21). Thus, an increase in connectivity in one RSN could be a reaction to loss in connectivity elsewhere. In our data, we mostly observed increases in intrinsic connectivity in children with T1D, with the exception of reduced seed-based connectivity between a right lingual gyrus seed and bilateral precentral gyrus. To our knowledge, there is no known inverse correlation between intrinsic activity in the precentral gyrus and other regions of the brain. Thus, it is unlikely that the observed significant increases in connectivity in children with T1D were associated with loss in connectivity elsewhere.
Another, and most likely, explanation could be that increased connectivity is a sign of functional reorganization of the brain (5,22). Such functional reorganization has previously been thought of as a compensatory mechanism in response to aberrations in brain structure. Such reorganization theoretically occurs by means of increased activation and/or synchronization of specific brain regions or networks (22). Previous work in examining resting-state networks in other clinical populations has shown a similar increase in RSFC in the early stages of brain disorders (23,24). Interestingly, the functional reorganization and resulting changes in RSFC are considered an early and finite phenomenon, as the compensatory changes are eventually lost with disease progression (23). We have previously reported significant early aberrations in brain anatomy (both gray matter volume and WM connectivity) in young children with T1D (6,25). Further, we also assessed differences in cognitive functioning in children with T1D as compared with control subjects without diabetes (13). As previously reported, no significant differences in cognitive functioning were observed between the groups across domains of executive functioning, learning, and memory as well as processing speed (13).
In the current study, which uses a subset of participants from the same cohort as previous publications, we also report no significant cognitive functioning differences between the groups (Table 1). However, within the group with T1D, increased connectivity between the prefrontal seed region and insular-temporal regions was positively associated with cognitive performance in executive functioning and processing speed. Further, increased connectivity associated with seed regions from the lingual gyrus was positively related to performance in the cognitive domain of learning and memory in the group with T1D. Lack of decline in cognitive functioning in young children with T1D and positive associations between hyperconnectivity and cognitive performance in different domains suggest that increased connectivity could be compensatory and due to functional reorganization. It will be of great interest and clinical importance to follow both the cognitive and imaging parameters of our cohort over time.
A hypothesis of functional reorganization also potentially explains previous results on differences in RSFC (1,5) as well as in task-related activation (26) in adults with T1D. van Duinkerken et al. (5) concluded that reduction in RSFC with advanced disease progression could be a consequence of failing functional reorganization. Bolo et al. (1) experimentally induced hypoglycemia in adults with T1D and control subjects without diabetes and showed a temporary increase in connectivity in the right prefrontal and insular regions within the group with T1D. Although their study used a small sample size (n = 16) and wide age range (19–46 years) of participants with T1D, the data suggest that increased RSFC in prefrontal and insular regions could reflect the brain’s adaptive reorganization to offset the effects of chronic glycemic dysregulation in individuals with this condition (1).
The examination of differences in within-network connectivity associated with T1D in adults by van Duinkerken et al. (5) showed reduced within-network connectivity for the VAN in patients with microangiopathy as compared with control subjects without diabetes. We did not observe T1D-related differences in the VAN in young children with T1D. This contrast is, however, in accordance with the van Duinkerken study, because the VAN-related differences were seen mostly in the advanced stages of the disease. With regard to the DAN, it is unclear why the T1D-associated differences observed in our young cohort were not observed in adults with T1D (5). Previous work has suggested developmentally related enhanced within-network connectivity in young children’s DAN as compared with adults (27). Thus, it is plausible that the differences observed in the DAN in young children with T1D could be partially due to their developmental stage. Future research is needed to parse these developmentally related differences with that of functional reorganization due to T1D.
Although increased seed-based connectivity in the group with T1D was significantly correlated with cognitive functioning, no significant associations were observed between cognition and increased connectivity observed using ICA. It is possible that the increase in within-network connectivity (i.e., within DAN) is also associated with functional reorganization of the brain and that the absence of a significant association with cognition is due to a lack of statistical power. Another plausible explanation for the lack of such an association could be that as multiple brain networks interact to facilitate complex cognitive behavior, connectivity differences in one network alone may not directly relate to cognitive outcomes.
As an exploratory analysis, we also examined differences in between-network connectivity in the two groups. Using a rigorous multiple comparison correction, significant between-group differences were observed for internetwork connectivity between the basal ganglia and auditory resting-state networks. The direction of connectivity differences is in line with other results in this article, i.e., increased connectivity associated with T1D. Previous research has separately reported the effects of diabetes on basal ganglia activation (26) as well as auditory functioning (28,29). However, to our knowledge, none of the previous studies reported diabetes-related differences in connectivity between the basal ganglia and auditory resting-state networks. Emerging evidence from human neuroimaging suggests that the basal ganglia facilitate auditory perceptual processing through an auditory-cortico-striatal loop (30). Lasagni et al. (31) recently reported subclinical abnormalities in qualitative auditory perception, despite normal hearing, in young adults with T1D. Thus, it is plausible to speculate that the observed increase in internetwork connectivity between auditory and basal ganglia networks in young children could also be related to functional reorganization, where the enhanced connectivity with basal ganglia could be facilitating compensation toward diabetes-related alterations in auditory functioning.
A few limitations of our work should be noted. First, although our results are based on a relatively large sample size for each group, unequal group sizes could impact ICA-based connectivity results by biasing group ICA maps. We addressed this limitation by including an equal number of participants from both groups for creating group ICA maps. Second, several factors are known to confound connectivity results, namely, age, sex, intersite differences, IQ, cognitive functioning, blood pressure, depressive symptoms, etc. Our groups either were matched for these confounding factors (e.g., for age and sex) or were not statistically different (e.g., for IQ and cognitive functioning). Additionally, we included factors of age, sex, and MR data acquisition location as a covariate of no interest in analyses. Third, the connectivity estimates are known to be affected by head movements during data collection, especially in children (15). We used state-of-the-art motion correction approaches, as explained in detail in research design and methods, to reduce the effect of head movement in estimating connectivity. Finally, the between-group differences for correlation coefficients between connectivity estimates and cognitive markers were not significant. One potential cause for the lack of such between-group differences could be a relatively small group size of participants without diabetes. To better mine potential relations between the blood glucose–related variables (and other sensory data) and rsfMRI connectivity in the future, we plan to use advanced machine learning methods like canonical correlation analysis (32).
In conclusion, we present differences in RSFC in very young children with T1D compared with age- and sex-matched control subjects without diabetes. Our results provide preliminary evidence for functional brain reorganization in young children with T1D. The observed hyperconnectivity might play a compensatory role in provisionally offsetting the adverse effects of T1D in the brain at a young age. As no differences in cognitive functioning have been observed in our cohort with T1D relative to control subjects without diabetes, it is possible that functional connectivity can be used as a biomarker of adaptation to disease progression. Ongoing, long-term longitudinal studies are underway to better understand the role of changes in RSFC across development and life span in children with T1D.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants and their families as well as the clinical and imaging staff at all of the investigator sites. The authors also thank their external collaborators for the use of their imaging facilities, including the University of California at San Francisco (San Francisco, CA), El Camino Hospital (Mountain View, CA), and the University of Florida and Shands Jacksonville Medical Center (Jacksonville, FL). The authors are also grateful to Karen Winer (Eunice Kennedy Shriver National Institute of Child Health and Human Development) for advice and support.
Funding. This research was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10 HD041906, U10 HD041908, U10 HD041915, U10 HD041918, U10 HD056526, and R01 HD078463-01A1). Additional support was provided by a Career Development Award from the National Institute of Mental Health to M.S. (K99-MH104605).
Duality of Interest. N.M. has received payment to her institution from a Medtronic grant and device research supply agreement from Medtronic. N.H.W. has received payment for consultancy from Novo Nordisk and Daiichi Sankyo and payments to his institution from Bristol-Myers Squibb for a research grant. S.W. has received payment to his institution from a Medtronic grant, and he has received payment from Animas, Insulet, Medtronic, and Tandem for consultancy. B.B. has received sensors at a research discount from Medtronic and payment for serving on the membership board for Medtronic and Sanofi-Aventis, and he reports money paid to his institution for a pending Medtronic grant and National Institutes of Health grant HD41908. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S. designed the data analysis pipeline, performed data analysis, and wrote the manuscript. E.T. contributed to study design and data collection and wrote the manuscript. N.M., P.M., N.H.W., S.W., B.B., T.H., and A.L.R. contributed to study design, data collection, and discussion and edited and reviewed the manuscript. A.L.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
The DirecNet Study Group: (personnel are listed as PI for principal investigator, I for co-investigator, C for coordinator, and PM for psychometrician). Clinical Centers: Department of Pediatrics, University of Iowa Carver College of Medicine: Eva Tsalikian, MD (PI), Michael J. Tansey, MD (I), Julie Coffey, ARNP, MSN (C), Joanne Cabbage (C), Sara Salamati (C), Amy Conrad, PhD (PM); Nemours Children’s Health System: Nelly Mauras, MD (PI), Larry A. Fox, MD (I), Allison Cato, PhD (I), Kim Englert, RN, BSN, CDE (project manager), Kaitlin Sikes, ARNP, MSN (C), Tina Ewen (C); Division of Endocrinology and Diabetes, Department of Pediatrics, Stanford University School of Medicine: Bruce Buckingham, MD (PI), Darrell M. Wilson, MD (I), Tandy Aye, MD (I), Kimberly Caswell, ARNP (C), Kristin Schleifer (PM), Christian Ambler (PM); Department of Pediatrics, Yale University School of Medicine: Stuart Weinzimer, MD (PI), William V. Tamborlane, MD (I), Amy Steffen, BS (C), Kate Weyman, MSN (C), Melinda Zgorski, BSN (C), Jodie Ambrosino, PhD (I); Washington University in St. Louis: Neil H. White, MD, CDE (PI), Ana Maria Arbelaez, MD (I), Lucy Levandoski, PA-C (C), Angie Starnes, RN, BSN, CDE (C), Tamara Hershey, PhD (I); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD, Katrina J. Ruedy, MSPH, Craig Kollman, PhD, Peiyao Cheng, MPH, Beth Stevens, Nelly Njeru, Ryan Chapman; TJ Mouse Image and Data Coordinating Center: Allan L. Reiss, MD, Paul Mazaika, PhD, Daniel X. Peng, BS; Cognitive Core: Tamara Hershey, PhD, Allison Cato, PhD, Emily Bihun, MA, Amal Al-Lozi, BA, Allison Bischoff, BA, Michaela Cuneo, BA, Aiden Bondurant, BA; Data and Safety Monitoring Board: Mark Sperling, MD, Dorothy M. Becker, MBBCh, Patricia Cleary, MS, Carla Greenbaum, MD, Antoinette Moran, MD.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0414/-/DC1.
A complete list of the Diabetes Research in Children Network (DirecNet) Study Group can be found in the appendix.
See accompanying article, p. 574.
References
- 1.Bolo NR, Musen G, Simonson DC, et al. . Functional connectivity of insula, basal ganglia, and prefrontal executive control networks during hypoglycemia in type 1 diabetes. J Neurosci 2015;35:11012–11023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman FR, Epport K, Engilman R, Halvorson M. Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications 1999;13:31–38 [DOI] [PubMed] [Google Scholar]
- 3.McCarthy AM, Lindgren S, Mengeling MA, Tsalikian E, Engvall JC. Effects of diabetes on learning in children. Pediatrics 2002;109:E9. [DOI] [PubMed] [Google Scholar]
- 4.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 1985;75:921–927 [PubMed] [Google Scholar]
- 5.van Duinkerken E, Schoonheim MM, Sanz-Arigita EJ, et al. . Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes 2012;61:1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al.; Diabetes Research in Children Network (DirecNet) . Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes 2014;63:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honey CJ, Thivierge J-P, Sporns O. Can structure predict function in the human brain? Neuroimage 2010;52:766–776 [DOI] [PubMed] [Google Scholar]
- 8.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, The Psychological Corporation, 1999. 25462692 [Google Scholar]
- 9.Wechsler D. Wechsler Preschool Primary Scale of Intelligence-Third Edition. San Antonio, The Psychological Corporation, 2002. 25462692 [Google Scholar]
- 10.Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition. San Antonio, The Psychological Corporation, 2003. 25462692 [Google Scholar]
- 11.Mauras N, Mazaika P, Buckingham B, et al.; Diabetes Research in Children Network (DirecNet) . Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes 2015;64:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazaika PK, Weinzimer SA, Mauras N, et al.; Diabetes Research in Children Network (DirecNet) . Variations in brain volume and growth in young children with type 1 diabetes. Diabetes 2016;65:476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cato MA, Mauras N, Ambrosino J, et al.; Diabetes Research in Children Network (DirecNet) . Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soc 2014;20:238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 2015;105:536–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan C-G, Cheung B, Kelly C, et al. . A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 2013;76:183–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippini N, MacIntosh BJ, Hough MG, et al. . Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 2009;106:7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, Miller KL, Salimi-Khorshidi G, et al. . Network modelling methods for FMRI. Neuroimage 2011;54:875–891 [DOI] [PubMed] [Google Scholar]
- 21.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoonheim MM, Geurts JJG, Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology 2010;74:1246–1247 [DOI] [PubMed] [Google Scholar]
- 23.Roosendaal SD, Schoonheim MM, Hulst HE, et al. Resting state networks change in clinically isolated syndrome. Brain 2010;133:1612–1621 [DOI] [PubMed]
- 24.Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin 2015;7:732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnea-Goraly N, Raman M, Mazaika P, et al.; Diabetes Research in Children Network (DirecNet) . Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care 2014;37:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallardo-Moreno GB, González-Garrido AA, Gudayol-Ferré E, Guàrdia-Olmos J. Type 1 diabetes modifies brain activation in young patients while performing visuospatial working memory tasks. J Diabetes Res 2015;2015:703512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrant K, Uddin LQ. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci 2015;12:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rance G, Chisari D, O’Hare F, et al. . Auditory neuropathy in individuals with type 1 diabetes. J Neurol 2014;261:1531–1536 [DOI] [PubMed] [Google Scholar]
- 29.Abd El Dayem SM, Abd El Ghany SM, Beshr AE, Hassan AG, Attaya MS. Assessment of hearing in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2014;27:393–402 [DOI] [PubMed] [Google Scholar]
- 30.Geiser E, Notter M, Gabrieli JDE. A corticostriatal neural system enhances auditory perception through temporal context processing. J Neurosci 2012;32:6177–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasagni A, Giordano P, Lacilla M, et al. Cochlear, auditory brainstem responses in type 1 diabetes: relationship with metabolic variables and diabetic complications. Diabet Med 2016;33:1260–1267 [DOI] [PubMed]
- 32.Smith SM, Nichols TE, Vidaurre D, et al. . A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci 2015;18:1565–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.