Abstract
Type 1 diabetes development in the NOD mouse model is widely reported to be dependent on high-level production by autoreactive CD4+ and CD8+ T cells of interferon-γ (IFN-γ), generally considered a proinflammatory cytokine. However, IFN-γ can also participate in tolerance-induction pathways, indicating it is not solely proinflammatory. This study addresses how IFN-γ can suppress activation of diabetogenic CD8+ T cells. CD8+ T cells transgenically expressing the diabetogenic AI4 T-cell receptor adoptively transferred disease to otherwise unmanipulated NOD.IFN-γnull, but not standard NOD, mice. AI4 T cells only underwent vigorous intrasplenic proliferation in NOD.IFN-γnull recipients. Disease-protective IFN-γ could be derived from any lymphocyte source and suppressed diabetogenic CD8+ T-cell responses both directly and through an intermediary nonlymphoid cell population. Suppression was not dependent on regulatory T cells, but was associated with increased inhibitory STAT1 to STAT4 expression levels in pathogenic AI4 T cells. Importantly, IFN-γ exposure during activation reduced the cytotoxicity of human-origin type 1 diabetes–relevant autoreactive CD8+ T cells. Collectively, these results indicate that rather than marking the most proinflammatory lymphocytes in diabetes development, IFN-γ production could represent an attempted limitation of pathogenic CD8+ T-cell activation. Thus, great care should be taken when designing possible diabetic intervention approaches modulating IFN-γ production.
Introduction
Interferon-γ (IFN-γ) is a crucial cytokine in various immune responses produced by multiple cell types (1,2) and has long been considered a contributor to autoimmune type 1 diabetes (T1D). This paradigm is partly based on reports IFN-γ expression correlates with disease progression in BB rats (3), NOD mice (4), and humans (5), and pharmacologically blocking this cytokine can inhibit diabetes (6,7). Numerous immunomodulatory protocols also reportedly inhibit diabetes development in NOD mice by skewing cytokine production by pathogenic T cells from a Th1 (including IFN-γ) to Th2 profile (reviewed in Ref. 8). However, other evidence indicates IFN-γ can exert nonredundant immunoregulatory roles suppressing at least some components of diabetes development. This includes a report diabetes is inhibited in IFN-γ–treated NOD mice (9). Treatment of NOD mice with syngeneic antigen-presenting dendritic cells (DCs) matured ex vivo with IFN-γ also reportedly inhibits diabetes development (10). Furthermore, the ability of some nonspecific immunostimulatory agents, including complete Freund’s adjuvant and Bacillus Calmette-Guérin vaccine, to inhibit diabetes onset in NOD mice requires IFN-γ production (11–13). Immunological tolerance-induction mechanisms, such as indoleamine 2,3-dioxygenase (IDO) production by DCs as well as eliciting activation-induced cell death (AICD) responses by autoreactive T cells, also require IFN-γ (14–16). Genetic ablation of IFN-γ or its receptor also has little effect on diabetes development in NOD mice (11,17–19). Such contradictory effects complicate determination of the overall contribution of IFN-γ to diabetes development.
One potential explanation for the above collective findings might be that IFN-γ exerts supportive, suppressive, or neutral effects on diabetes development in a manner both under temporal control and influenced by tissue microenvironment differences. If this theory is correct, then measuring IFN-γ expression by T cells as a surrogate for their diabetogenic activity is an oversimplification not accounting for the diverse, possibly including disease-protective, effects of this cytokine. Indeed, our current work indicates diabetic interventions focused on suppressing IFN-γ production could, in some circumstances, actually promote pathogenic CD8+ T-cell responses.
Research Design and Methods
Mice
NOD/ShiLtDvs mice were maintained in a specific pathogen-free research colony. NOD mice lacking IFN-γ (NOD.IFN-γnull), its receptor (NOD.IFN-γRnull), all T cells (NOD.TCRαnull), CD8+ T cells (NOD.CD8null), B cells (NOD.IgHnull), and T and B lymphocytes (NOD.Rag1null, NOD.scid), as well as a stock transgenically expressing the T-cell receptor (TCR) from the diabetogenic AI4 CD8+ T-cell clone plus carrying an inactivated Rag1 gene (NOD.Rag1null.AI4), have been described (11,18,20–25).
AI4 T-Cell Transfer of Diabetes
Recipient mice that did or did not receive a preconditioning 600-cGy irradiation dose were injected i.v. with 1 × 107 NOD.Rag1null.AI4 splenocytes or 1 × 106 magnetic bead–purified (Miltenyi Biotec) AI4 T cells to induce diabetes. One experiment analyzed NOD.scid recipients also receiving 3 × 106 CD4+ purified NOD or NOD.IFN-γnull splenic T cells. Other experiments analyzed NOD.Rag1null and NOD.Rag1null.IFN-γRnull recipients receiving AI4 T cells and purified splenic CD4+ T cells from NOD or NOD.IFN-γRnull donors. In other studies, NOD.Rag1null.AI4 splenocytes were infused into NOD.IFN-γnull recipients combined with 2 × 107 splenocytes from indicated donors or NOD splenocytes depleted of T and/or B cells by magnetic beads. Another study used NOD mice receiving three biweekly i.p. injections from 6 weeks of age of 250 μg regulatory T cell (Treg)–depleting CD25 specific antibody (PC61) or an irrelevant rat IgG. One week after treatment initiation, recipients were injected i.v. with 1 × 107 NOD.Rag1null.AI4 splenocytes. Diabetes development was assessed by monitoring of glycosuria onset with Ames Diastix (Bayer Diagnostics Division, Elkhart, IN). To assess AI4 T-cell activation, NOD.Rag1null.AI4 splenocytes were prelabeled with 2.5 mmol carboxyfluorescein succinimidyl ester (CFSE). After 4 or 8 days, viable AI4 T cells from spleens and pancreatic lymph nodes (PLNs) were identified by flow cytometry using a combination of CFSE, antibodies against CD8 (53-6.7) and CD3 (145-2C11), and a previously described clonotypic tetramer (26) (Supplementary Fig. 1). Expression of T-cell surface markers was assessed using CD44- (IM7.8.1) and CD62L-specific (MEL-14) antibodies.
PCR Analysis of IFN-γ mRNA Expression
Splenocytes from unmanipulated NOD mice or those injected i.v. 3 days previously with 2 × 107 NOD.Rag1null.AI4 splenocytes were stained and sorted by an FACSAria instrument (BD Biosciences, San Jose, CA) using the following gating: CD4+ T cells (CD8−CD4+), B cells (B220+ CD19+CD3−), and host-type CD8+ T cells (CD8+CD4−TCRVa8−). cDNA was generated using an RNeasy kit (74004; Qiagen). Primer sequences are as follows: IFN-γ forward (5′-ACTGGCAAAAGGATGGTGAC-3′) and reverse (5′-TGAGCTCATTGAATGCTT-3′); and 18sRNA forward (5′-CCGCAGCTAGGAA-3′) and reverse (5′-CGAACCTCCGACT-3′). Samples and primers were combined with Power SYBR Green PCRMaster Mix (4367659; Applied Biosystems) and acquired on an Applied Biosystems ViiA7 Real Time PCR System (Life Technologies).
Treg Activity and STAT Expression Analyses
Viable stained cells were detected using LSR II or FACSCalibur flow cytometers (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Palo Alto, CA). A previously described flow cytometry assay (27) assessed the ability of NOD and NOD.IFN-γnull Tregs to inhibit CD4+CD25− T-cell proliferation in vitro. To assess intracellular STAT expression, extracellular antigens were stained prior to fixation with BD Cytofix/Cytoperm (BD Biosciences) for 10 min at 37°C and then incubated for 30 min at 4°C with BD Perm Buffer III (BD Biosciences). Previously validated STAT antibodies (28) were purchased from BD Biosciences: STAT1 (AF647-conjugated 1/STAT1) and STAT4 (unconjugated 8/STAT4 followed by allophycocyanin-conjugated Anti-Mouse A85-1). Transferred AI4 T cells were identified using FITC-CD8α (53-6.72) antibody and phycoerythrin-labeled YAIENYLEL/H-2Db tetramer (NIH Tetramer Core Facility, Atlanta, GA).
Human CD8+ T-Cell Transduction and Cell-Mediated Lysis Assays
CD8+ T cells were enriched from whole blood of five healthy human subjects using the RosetteSep Enrichment kit (STEMCELL Technologies, Vancouver, British Columbia, Canada). Cells were stained with CD8 (clone SK1; BioLegend), CD45RA (clone HI100; BioLegend), and CD45RO (clone UCHL1; BioLegend) and sorted for naive CD8+ T cells (CD8+CD45RA+CD45RO−) using an FACSAria III (BD Biosciences). CD8+ T cells were plated at 2.5 × 105/well in 1 mL complete RPMI 1640 (10% heat-inactivated FCS, GE Healthcare; 2 mmol l-glutamine, Thermo Fisher Scientific; 5 mmol sodium pyruvate, Cellgro; 5 mmol nonessential amino acids, Life Technologies; 5 mmol HEPES, Cellgro; 50 mg/mL penicillin/streptomycin, Gemini Bio Products; and 50 μmol 2-mercaptoethanol, Sigma-Aldrich), activated with Human T-Activator CD3/CD28 Dynabeads (Thermo Fisher Scientific), and cultured with or without human recombinant IFN-γ (1,000 U/mL; R&D Systems). At 48 h, cells were transduced with lentivirus produced as previously described (29) encoding either HLA-A*02-01–restricted islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)–autoreactive or irrelevant MART-1–specific TCRs (pCCL.IGRPopt.eGFP or LV.Mart1.TCR.RK, respectively) (30–32). Transductions were conducted in the presence of protamine sulfate (8 µg/mL; Sigma-Aldrich) and spinnoculated for 30 min at 32°C and 1,000 × g. Complete media and interleukin (IL)-2 (PeproTech) were added and cells incubated at 37°C in 5% CO2 ± IFN-γ and IL-2 (100 U/mL) every other day until day 9. Cells were then washed and cryopreserved in 90% FCS/10% DMSO prior to use.
IGRP- or MART-1–specific CD8+ T cells were added, in triplicate, at a series of effector-to-target (E:T) ratios to wells containing 10,000 HLA-A*0201+ class I–positive human BetaLox5 (BL5) target cells. To enhance their sensitivity to CD8+ T cell–mediated lysis, BL5 target cells were pre-exposed to 1,000 U/mL IFN-γ for 24 h and washed before use in the cytotoxicity assay. Target cells were preloaded with 51Cr as previously described (33). Target cell lysis was assessed after overnight incubation by measuring 51Cr release using a Wallac Wizard γ counter (PerkinElmer). Percentage specific lysis was calculated as follows:
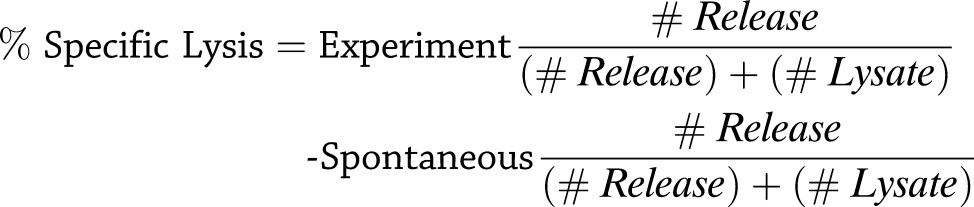 |
Results
Nonirradiated IFN-γ–Deficient, but Not Intact, NOD Mice Are Susceptible to AI4 T Cell–Induced Diabetes
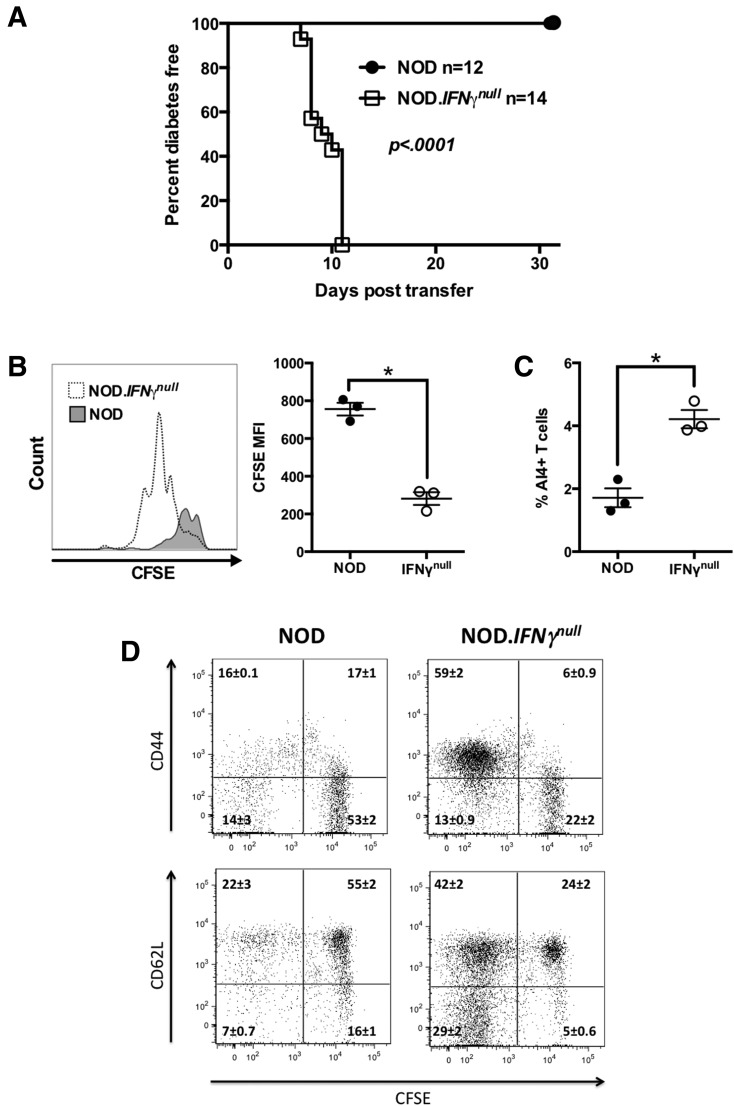
NOD mice preconditioned by sublethal irradiation rapidly develop diabetes after infusion with splenocytes from a NOD stock transgenically expressing a TCR derived from the pathogenic AI4 CD8+ T-cell clone and also homozygous for the Rag1null mutation (NOD.Rag1null.AI4) (34). Without irradiation, standard NOD mice are entirely resistant to AI4 T cell–induced diabetes. However, nonirradiated NOD mice genetically deficient for IFN-γ (NOD.IFN-γnull) are fully susceptible to AI4-mediated diabetes (Fig. 1A). Analysis before overt disease development revealed significantly greater accumulation of proliferating AI4 T cells in the PLNs of otherwise unmanipulated NOD.IFN-γnull than NOD mice (Fig. 1B and C). Activated AI4 T cells also accumulated in the spleens of mutant, but not wild-type, recipients by 8 days posttransfer (Fig. 1D). Thus, IFN-γ reduces the frequency of activated AI4 T cells in vivo.
Figure 1.
IFN-γ–deficient but not standard NOD mice develop AI4 T cell–induced T1D. A: Diabetes development in female NOD and NOD.IFN-γnull mice injected i.v. at 6 weeks of age with 1 × 107 NOD.Rag1null.AI4 splenocytes. Survival curves compared by log-rank test. B and C: In vivo proliferation and activation of CFSE-labeled NOD.Rag1null.AI4 T cells in PLNs of NOD and NOD.IFN-γnull mice. B: CFSE dilution of AI4 T cells in PLNs of NOD and NOD.IFN-γnull mice at 3 days posttransfer. Representative histograms are shown in the left panel, and mean fluorescence intensity (MFI) of CFSE staining of AI4 T cells is shown in the right panel. C: The frequency of AI4 CD8+ T cells among live PLN cells at 3 days posttransfer. D: CFSE dilution and activation of AI4 T cells in spleens of NOD and NOD.IFN-γnull mice at 8 days posttransfer. Results for each quadrant represent the mean ± SE of three mice per treatment. B–D represent results from a single experiment. *P < 0.05 determined by one-way ANOVA.
IFN-γ Production by Any Lymphocytes Inhibits AI4 T-Cell Activation
We tested what host cell types produce IFN-γ–suppressing activity of diabetogenic AI4 T cells transferred into otherwise unmanipulated standard NOD recipients. Initially, we compared IFN-γ mRNA transcript levels in different splenocyte populations in standard NOD mice injected or not 3 days previously with AI4 T cells. Transferred AI4 T cells increased IFN-γ mRNA expression in host-type CD4+ T cells (Fig. 2A). Host-type B cells and CD8+ T cells in unmanipulated NOD mice expressed baseline levels of IFN-γ mRNA transcripts remaining unchanged following AI4 T-cell infusion (Fig. 2A).
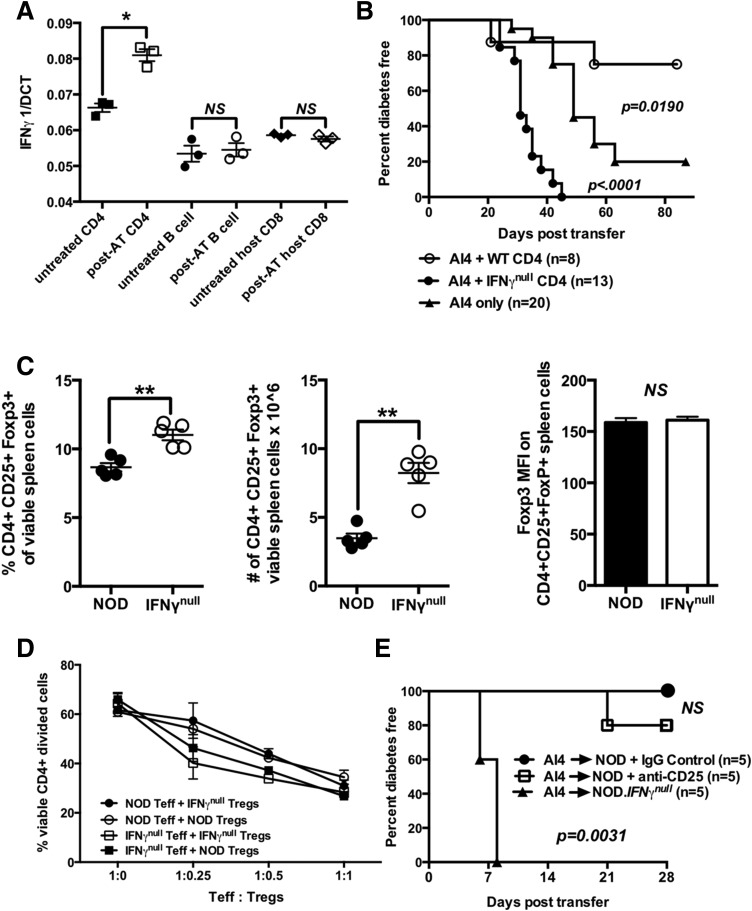
Figure 2.
IFN-γ–producing CD4+ T cells suppress diabetogenic CD8+ T cells through mechanisms that do not involve quantitative or functional variations in Tregs. A: Quantitative PCR analysis of IFN-γ mRNA expression by host-type CD4+ and CD8+ (Vα8−) T cells and B cells purified from spleens of NOD mice 3 days postadoptive transfer with 2 × 107 NOD.Rag1null.AI4 splenocytes (post-AT) or untreated NOD mice (untreated). Results represent the mean ± SE of three samples per treatment. B: Diabetes incidence for female NOD.scid mice injected at 6–8 weeks of age with 1 × 107 NOD.Rag1null.AI4 splenocytes in the presence or absence of 3 × 106 CD4+ T cells purified from NOD or NOD.IFN-γnull donors. C: Frequencies, numbers, and mean fluorescence intensity (MFI) of FoxP3 antibody staining of splenic CD4+CD25+FoxP3+ Tregs in NOD and NOD.IFN-γnull mice. Results represent the mean ± SE of five mice per treatment. D: Crisscross cultures were established to assess the ability of CD4+CD25+ Tregs from NOD and NOD.IFN-γnull mice to suppress the anti-CD3–stimulated proliferation of CD4+CD25− effectors from both strains (assessed by flow cytometic detection of CFSE dilution). E: Beginning at 6 weeks of age, NOD female mice received three biweekly i.p. injections with the Treg-depleting CD25-specific PC61 antibody or a rat IgG1 isotype control. One week after the first treatment, mice in both groups were injected i.v. with 1 × 107 NOD.Rag1null.AI4 splenocytes and subsequently monitored for diabetes. Survival curves compared by log-rank test. *P < 0.05 determined by one-way ANOVA, **P < 0.01 determined by unpaired t test. Teff, effector T cell; WT, wild type.
We next tested whether IFN-γ–intact or –deficient CD4+ T cells differentially affected the ability of cotransferred AI4 T cells to induce diabetes in lymphopenic NOD.scid recipients. Compared with those infused with AI4 T cells alone, diabetes development was, respectively, accelerated and suppressed in NOD.scid recipients also receiving IFN-γ–deficient or –intact CD4+ T cells (Fig. 2B). AI4 T cell–induced diabetes was slower in NOD.scid recipients coengrafted with IFN-γ–deficient CD4+ T cells than NOD.IFN-γnull hosts receiving these pathogenic effectors (Fig. 1A). This is likely because of comparatively smaller numbers of IFN-γ–deficient CD4+ T cells in NOD.scid recipients.
IFN-γ converts CD4+CD25− effector T cells to immunosuppressive FoxP3+ Tregs in several settings (35,36). Thus, we hypothesized Tregs may suppress the activity of AI4 T cells infused into standard, but not IFN-γ–deficient, NOD mice. Both the frequency and total numbers of CD4+CD25+FoxP3+ Tregs were unexpectedly greater in IFN-γ–deficient than standard NOD mice, although with no differences in FoxP3 expression levels (Fig. 2C). On a per-cell basis, Tregs from NOD and NOD.IFN-γnull mice equally suppressed activation of conventional or IFN-γ–deficient NOD CD4+CD25− T cells in vitro (Fig. 2D). Finally, NOD.Rag1null.AI4 splenocytes were transferred into NOD mice treated biweekly with a Treg-depleting anti-CD25 or rat IgG control antibody. Despite efficiently depleting CD4+CD25+ T cells, anti-CD25 treatment failed to break the resistance of nonirradiated NOD mice to AI4 T-cell transfer (Fig. 2E). These collective results indicated Tregs are not the CD4+ T-cell population mediating IFN-γ–dependent inhibition of diabetogenic AI4 T cells.
Subsequent studies assessed what immunological cell populations other than Tregs mediate IFN-γ–dependent suppression of diabetogenic CD8+ T cells. AI4 T cells were infused into NOD.IFN-γnull recipients along with NOD splenocytes lacking various lymphocyte populations (Supplementary Fig. 2 and Table 1). As controls, NOD.IFN-γnull recipients injected with AI4 T cells and either total NOD.IFN-γnull or standard NOD splenocytes were, respectively, entirely susceptible and resistant to diabetes development (Table 1). AI4 recipients coinfused with NOD splenocytes lacking either CD4+ or CD8+ T cells or B cells were diabetes resistant. However, NOD splenocytes lacking all three lymphocyte subsets failed to inhibit AI4 T cell–induced diabetes. These results indicated IFN-γ produced by any lymphocyte population blocks diabetogenic AI4 T-cell activity. Subsequent experiments supporting this conclusion found AI4 T cells induce diabetes when infused into B and T cell–deficient NOD.Rag1null mice, but not NOD stocks capable of producing at least one lymphocyte population (Table 2). As described previously, IFN-γ mRNA transcripts were detected in B cells and CD8+ T cells in nonirradiated NOD mice, but did not further increase in those infused with AI4 T cells. Hence, baseline levels of IFN-γ produced by B cells and CD8+ T cells in nonirradiated NOD mice appear sufficient to suppress the diabetogenic activity of subsequently introduced AI4 T cells. It should be noted the frequency of IL-17A– and IL-17F–positive T cells capable of counteracting IFN-γ effects did not differ in NOD and NOD.IFN-γnull mice (Supplementary Fig. 3).
Table 1.
AI4 T cell–induced diabetes in NOD.IFN-γnull mice is blocked by coinfusing recipients with splenocytes from standard NOD mice individually lacking T or B cells, but not both lymphocyte subtypes
| Source of splenocytes coinjected with 1 × 107 NOD.Rag1null.AI4 splenocytes into NOD.IFN-γnull recipients* | Fraction diabetic | Percent diabetic | χ2 P value† |
|---|---|---|---|
| NOD.IFN-γnull | 25/25 | 100 | — |
| NOD | 0/10 | 0 | <0.001 |
| NOD.IgHnull | 0/5 | 0 | <0.001 |
| NOD.TCRαnull | 1/5 | 20 | <0.001 |
| NOD.CD8null | 0/5 | 0 | <0.001 |
| NOD CD8-depleted | 0/4 | 0 | <0.001 |
| NOD CD4-depleted | 0/4 | 0 | <0.001 |
| NOD CD8- and CD4-depleted | 0/4 | 0 | <0.001 |
| NOD CD8-, CD4-, and B cell–depleted | 8/10 | 80 | 0.05 > P > 0.02 |
| NOD.Rag1null | 5/5 | 100 | NS |
| NOD.scid | 9/10 | 90 | NS |
*Recipients were monitored for diabetes development for 3 weeks after adoptive transfer of splenocytes.
†P values from χ2 analyses comparing the frequency of diabetes between AI4 splenocyte–infused NOD.IFN-γnull mice coinjected with NOD.IFN-γnull splenocytes and splenocytes from other sources.
Table 2.
Total of 1 × 107 NOD.Rag1null.AI4 splenocytes fail to transfer T1D into unmanipulated genetically modified stocks of NOD mice that lack T cells or B cells, but they efficiently induce disease in NOD.Rag1null mice lacking both cell types
| Recipient* | Fraction diabetic | Percent diabetic | χ2 P value† |
|---|---|---|---|
| NOD.IFN-γnull | 5/5 | 100 | — |
| NOD | 0/5 | 0 | <0.01 |
| NOD.IgHnull | 0/5 | 0 | <0.01 |
| NOD.TCRαnull | 0/5 | 0 | <0.01 |
| NOD.CD8null | 0/6 | 0 | <0.001 |
| NOD.Rag1null | 7/7 | 100 | NS |
*Recipients were monitored for diabetes development for 10 weeks after adoptive transfer of AI4 splenocytes.
†P values from χ2 analyses comparing the frequency of AI4 T cell–induced T1D between NOD.IFN-γnull mice and NOD, NOD.IgHnull, NOD.TCRαnull, NOD.CD8null, and NOD.Rag1null stocks.
IFN-γ Directly and Indirectly Inhibits Diabetogenic AI4 T Cells
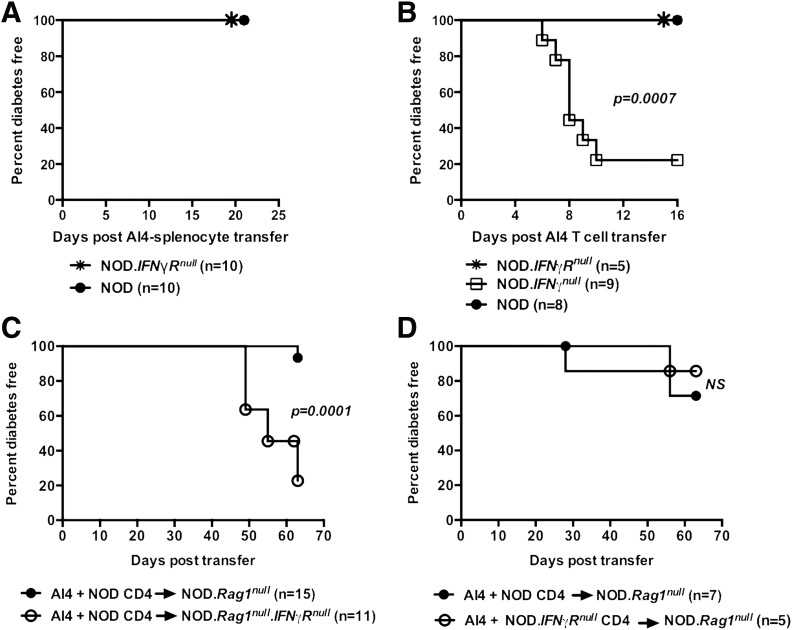
We tested whether IFN-γ directly suppresses AI4 T-cell activity. NOD.Rag1null.AI4 splenocytes, containing both AI4 T cells and myeloid lineage-derived cells, were transferred into either nonirradiated standard NOD mice or a stock lacking the IFN-γ receptor (NOD.IFN-γRnull). Although NOD.IFN-γRnull recipients produce IFN-γ, only donor splenocytes can respond to this cytokine. Like standard NOD mice, the NOD.IFN-γRnull stock was resistant to AI4 splenocyte-induced diabetes (Fig. 3A). These results indicated IFN-γ production by host or donor lymphocytes might either directly suppress diabetogenic AI4 T cells or may do so in a nonmutually exclusive indirect fashion by altering activities of a cotransferred myeloid population(s). To distinguish between these possibilities, 1 × 106 purified (>90%) AI4 T cells were injected into NOD, NOD.IFN-γnull, and NOD.IFN-γRnull recipients subsequently monitored for diabetes. Purified AI4 T cells only induced diabetes in NOD.IFN-γnull recipients (Fig. 3B). These results indicated IFN-γ could directly suppress AI4 T-cell diabetogenic activity.
Figure 3.
IFN-γ produced by T or B cells suppresses AI4 T cells through both direct and indirect mechanisms. A: Diabetes incidence in female NOD and NOD.IFN-γRnull recipient mice injected i.v. at 6 weeks of age with 1 × 107 NOD.Rag1null.AI4 splenocytes. B: Diabetes incidence in female NOD, NOD.IFN-γRnull, and NOD.IFN-γnull recipient mice injected i.v. at 6 weeks of age with 1 × 106 purified AI4 T cells. C: Diabetes incidence in female NOD.Rag1null and NOD.Rag1null.IFN-γRnull recipient mice injected i.v. with 1 × 106 purified AI4 T cells and 2 × 106 purified NOD splenic CD4+ T cells. D: Diabetes incidence in female NOD.Rag1null recipient mice injected i.v. with 1 × 107 NOD.Rag1null.AI4 splenocytes and 2 × 106 purified CD4+ splenic T cells from NOD or NOD.IFN-γRnull donors. Survival curves compared by log-rank test.
The above results did not preclude the possibility IFN-γ also partially suppresses AI4 T cells by functionally altering a host-type intermediary cell population. To test this possibility, purified AI4 T cells were cotransferred with purified NOD CD4+ T cells as a source of IFN-γ into NOD.Rag1null recipients with an intact or ablated IFN-γ receptor (latter stock designated NOD.Rag1null.IFN-γRnull). Recipients were monitored for diabetes development over 70 days instead of 2 to 3 weeks because adoptive transfer of disease takes longer into lymphopenic NOD.Rag1null mice than lymphocyte-sufficient hosts. Diabetes developed at a significantly lower level in IFN-γR intact than deficient NOD.Rag1null recipients (Fig. 3C). These results demonstrated IFN-γ also effects a host-origin nonlymphocyte population that indirectly suppresses diabetogenic CD8+ T cells. Finally, we tested whether IFN-γ suppresses AI4 T cells through effects on CD4+ T cells. AI4 T cells were injected into NOD.Rag1null recipients with purified splenic CD4+ T cells from NOD or NOD.IFN-γRnull donors. Diabetes developed at the same low rate in both groups of recipients (Fig. 3D), indicating that although they can produce AI4 T cell–inhibiting IFN-γ, CD4+ T cells do not respond to this cytokine in ways allowing them to suppress such pathogenic effectors. These collective results indicate IFN-γ suppresses diabetogenic CD8+ T cells both directly and through effects on nonlymphoid cells.
AI4 T Cells in Diabetes-Resistant Hosts Upregulate STAT1
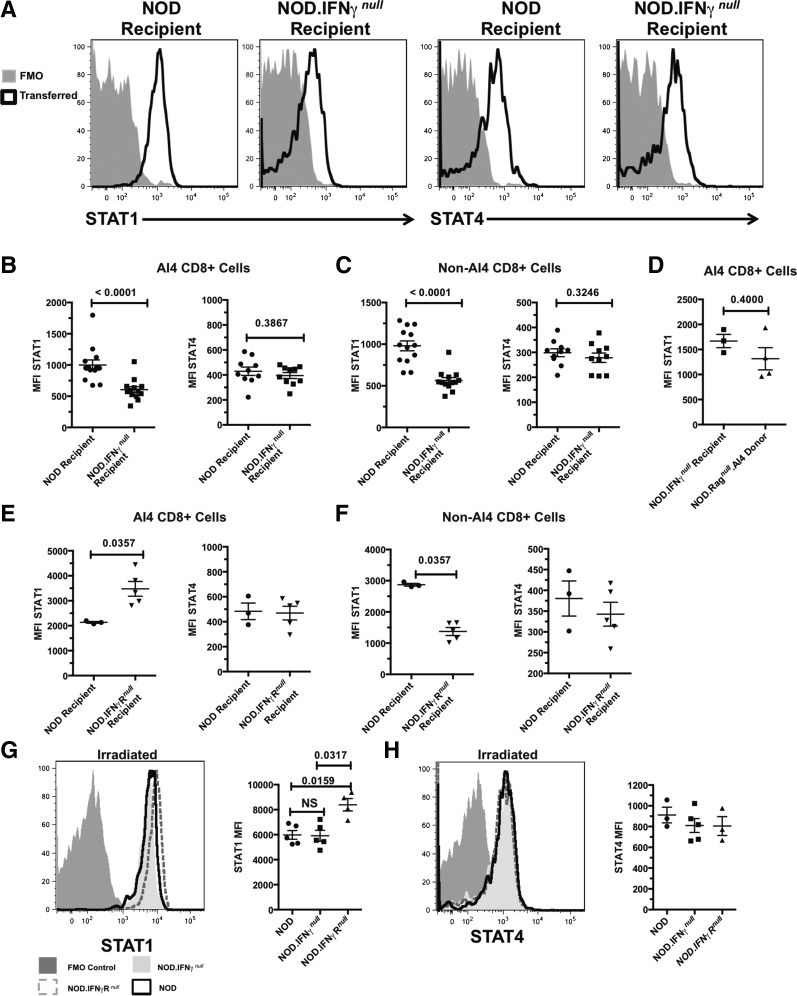
After eliminating possible contributions from multiple components of IFN-γ–triggered signaling pathways (complete list noted in the Discussion), we tested if inducing differing STAT1 and STAT4 expression levels in transferred AI4 T cells could contribute to their variable diabetogenic activity in the analyzed host types. This was done based on reports that elevated STAT1 to STAT4 expression levels suppresses T cell–proliferative capacity (28,37). AI4 T cells taken directly from the different recipients had no detectable differences in phosphorylated (p-)STAT1 or p-STAT4 (data not shown). However, total STAT1 expression was significantly higher in posttransferred AI4 T cells within standard NOD than NOD.IFN-γnull recipients (Fig. 4A and B). STAT4 levels were similar in AI4 T cells posttransfer into both NOD than NOD.IFN-γnull recipients (Fig. 4A and B). In addition to the transferred AI4 T cells, STAT1, but not STAT4, expression was also higher in host-type NOD than NOD.IFN-γnull CD8+ T cells (Fig. 4C). STAT1 levels remained similar in AI4 T cells prior to and after transfer into NOD.IFN-γnull recipients (Fig. 4D). Although not inducing diabetes in either otherwise unmanipulated NOD or NOD.IFN-γRnull recipients, infused AI4 T cells acquired even higher levels of STAT1, but not STAT4, expression in the latter environment (Fig. 4E). Such increased STAT1 expression by donor AI4 T cells was specifically induced by IFN-γ signaling, as this did not occur among host-type NOD.IFN-γRnull CD8+ T cells (Fig. 4F). Thus, an IFN-γ–dependent increase in STAT1 but not STAT4 expression by AI4 T cells transferred into nonirradiated NOD and NOD.IFN-γRnull, but not NOD.IFN-γnull, recipients could explain why such effectors only elicit diabetes in the latter host type.
Figure 4.
Higher STAT1 but not STAT4 expression by transferred AI4 T cells is associated with the lesser ability of such effectors to induce diabetes in NOD and NOD.IFN-γRnull than NOD.IFN-γnull recipients. A–C: Otherwise unmanipulated NOD or NOD.IFN-γnull recipients were injected with 1 × 106 AI4 T cells and analyzed for total STAT1 or STAT4 levels 2–7 days after transfer. A: Representative pattern (from day 4) of transferred CD8+ tetramer+ AI4 T cells from the indicated recipients showing STAT1 (left) or STAT4 (right) compared with fluorescence minus one (FMO) control stains. B: Quantification of STAT1 (left) or STAT4 (right) staining of splenic AI4 CD8+ T cells after transfer into the indicated recipients. C: Endogenous CD8+ tetramer− T cells from the indicated recipients were analyzed for mean fluorescence intensity (MFI) of STAT1 (left) or STAT4 (right) staining after transfer of AI4 CD8+ T cells. B and C display combined data for days 2–7 posttransfer of AI4 T cells. D: Comparison of STAT1 expression by tetramer+ AI4 donor T cells before and 2 days after transfer into otherwise unmanipulated NOD.IFN-γnull recipients. E: Otherwise unmanipulated NOD or NOD.IFN-γRnull recipients were injected with 1 × 106 AI4 T cells and analyzed for STAT1 or STAT4 levels 2 days after transfer. Quantification of STAT1 (left) or STAT4 (right) staining of splenic AI4 CD8+ T cells 2 days after transfer into the indicated recipient. F: Endogenous CD8+ tetramer− T cells from the indicated recipients were analyzed for MFI of STAT1 (left) or STAT4 (right) staining 2 days after transfer of AI4 CD8+ T cells. D–F display data from a single experiment. G and H: NOD, NOD.IFN-γnull, or NOD.IFN-γRnull mice were irradiated (600 cGy) and injected with 1 × 106 AI4 T cells. Two days posttransfer, AI4 T cells were analyzed for STAT1 or STAT4 levels. Left panels: Histograms showing STAT1 (G) or STAT4 (H) expression of AI4 T cells from the indicated recipients compared with an FMO control. Right panels: Quantification of MFI of STAT1 (G) or STAT4 (H) staining from one of two experiments showing n ≥ 3 per group. P values calculated using Mann–Whitney analysis.
AI4 T cells transfer diabetes to preirradiated NOD mice (34). Thus, we hypothesized if a posttransfer elevation in STAT1 expression by AI4 T cells explains why these effectors fail to elicit diabetes development in otherwise unmanipulated NOD recipients, this should not occur in preirradiated hosts. Two days after transfer into 600-cGy–irradiated recipients, AI4 T cells expressed STAT1 at similar levels in NOD and NOD.IFN-γnull hosts (Fig. 4G). In contrast, AI4 T cells transferred into irradiated NOD.IFN-γRnull mice expressed higher total STAT1 levels than in NOD or NOD.IFN-γnull recipients (Fig. 4G), with no differences observed among the three groups for STAT4 (Fig. 4H). Hence, we tested whether NOD.IFN-γRnull recipients, after irradiation preconditioning, were also more resistant to AI4 T cell–mediated diabetes. AI4 T cells transferred diabetes to preirradiated NOD and NOD.IFN-γnull but not NOD.IFN-γRnull mice (Table 3). Collectively, these data indicate, at least under some adoptive transfer conditions, diabetogenic CD8+ T cells can be functionally suppressed in an IFN-γ–dependent fashion through induction of elevated STAT1 expression.
Table 3.
Total of 1 × 107 NOD.Rag1null.AI4 splenocytes fail to transfer diabetes into irradiated NOD.IFN-γRnull recipients, but induce disease in NOD and NOD.IFN-γnull recipients
| Recipient* | Fraction diabetic |
Percent diabetic | χ2 P value† | |
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||
| NOD | 2/4 | 4/5 | 67 | 0.2125 |
| NOD.IFN-γnull | 4/4 | 3/3 | 100 | — |
| NOD.IFN-γRnull | 0/4 | 0/4 | 0 | 0.0002 |
*Recipients were monitored for 15 days after adoptive transfer of AI4 splenocytes.
†P values from χ2 analyses comparing the frequency of AI4 splenocyte–induced T1D between NOD.IFN-γnull and NOD and NOD.IFN-γRnull.
IFN-γ Directly Inhibits Human Diabetogenic CD8+ T Cells
We also determined whether IFN-γ could directly suppress human diabetogenic CD8+ T cells in vitro. Purified CD8+ T cells from five healthy individuals were transduced with lentiviral vectors encoding a TCR recognizing an HLA-A*02-01–restricted epitope derived from the β-cell autoantigen IGRP (30). The transduction process requires activation of CD8+ T cells using anti-CD3/CD28–coated Dynabeads (33) and was performed in both the presence and absence of IFN-γ. CD8+ T cells were thoroughly washed posttransduction to ensure all excess cytokine was removed. Flow cytometric analyses of EGFP expression confirmed equivalent efficiency of TCR transduction between IFN-γ–treated and –untreated groups. CD8+ T cells were added at a series of E:T ratios to 51Cr-labeled HLA-A*0201 class I–positive BL5 human β-cells used as targets (38,39). Control effectors were CD8+ T cells transduced to express a MART-1–specific TCR recognizing an HLA-A*02-01–restricted melanoma antigen not present in BL5 cells. Pre-exposure of the IGRP-specific CD8+ T cells to IFN-γ reduced their cytotoxic capacity especially at high E:T ratios (Fig. 5A). Lysis of β-cells by IGRP-specific CD8+ T cells was antigen driven because MART-1–specific CD8+ T cells had little effect on such targets (Fig. 5B). Hence, IFN-γ can also directly suppress the activity of human IGRP-autoreactive CD8+ T cells.
Figure 5.
IFN-γ exposure during activation reduces the cytotoxicity of human β-cell–reactive CD8+ T cells. A: Specific lysis of BL5 human β-cell line target cells coincubated at different E:T ratios with HLA-A*02-01–restricted IGRP-specific CD8+ T cells transduced in the presence or absence of 1,000 U/mL IFN-γ. B: Specific lysis of BL5 target cells coincubated with nondiabetogenic MART-1–specific CD8+ T cells transduced in the presence or absence of IFN-γ. BL5 cells were pre-exposed to 1,000 U/mL IFN-γ and washed before they were used in the cell-mediated lympholysis assays. P values calculated using a paired t test.
Discussion
Reducing circulating IFN-γ has been suggested as a means to suppress diabetogenic CD8+ T cells, ultimately mediating pancreatic β-cell destruction (8). However, IFN-γ also has effects inhibiting autoimmunity, including inducing IDO expression by tolerogenic DCs (14,15). IFN-γ is also required for eliciting AICD controlling the expansion and persistence of T cells (16). These mechanisms may explain why recombinant IFN-γ (9) or DCs conditioned by this cytokine (10) can inhibit diabetes development in NOD mice. The present work demonstrates, at least under some conditions, that pathogenic CD8+ T-cell activation is one component of diabetes development directly sensitive to IFN-γ inhibition in both mice and humans. IFN-γ from any lymphocyte source blocks diabetes induced by adoptively transferred AI4 T cells through a Treg-independent process. Surprisingly, B cells, not normally associated with IFN-γ expression, were equally effective as CD8+ or CD4+ T cells at suppressing diabetes induced by adoptively transferred AI4 T cells. The subset of B cells suppressing diabetogenic CD8+ T-cell responses may resemble the previously described B effector 1 population known to express significant levels of IFN-γ (2). Although it would require multiple generations of further breeding, it would ultimately be of value to assess whether IFN-γ produced by transferred AI4 T cells themselves influences their differential ability to induce diabetes in unirradiated NOD and NOD.IFN-γnull recipients.
Lymphocyte-derived IFN-γ at least in part directly suppresses diabetogenic CD8+ T-cell activation. IFN-γ also acted on one or more intermediary nonlymphoid cell types that in turn suppress diabetogenic CD8+ T-cell responses. Although these intermediary cell types still await definitive identification, one good candidate is DCs, because when conditioned with IFN-γ ex vivo they can, after adoptive transfer, suppress spontaneous diabetes in NOD mice (10). It should be noted we tested whether IFN-γ–induced IDO production by DCs suppressed diabetogenic CD8+ T cells in our model system. AI4 splenocytes were injected into standard NOD mice treated with or without the IDO inhibitor 1-methyl tryptophan. Inhibiting IDO did not alter the resistance of otherwise unmanipulated NOD mice to AI4 T cell–induced diabetes development, indicating IFN-γ does not control the activation of such pathogenic effectors through this enzyme.
AI4 T cells transferred into nonirradiated NOD or NOD.IFN-γnull recipients also did not differ in expression of IL-7Rα, TIM-3, programmed cell death-1, GITR, CD25, or IFN-αR molecules associated with immunological activation or inhibition. Hence, pathways in which these molecules participate are unlikely to contribute to variable diabetes induction by AI4 T cells in these two host types. Fas and KLRG1 involved in apoptosis induction are also unlikely to reduce AI4 T cell–induced diabetes in NOD mice, as both molecules were upregulated equally on such effectors transferred into disease-susceptible NOD.IFN-γnull recipients. Furthermore, IFN-γ added directly to a culture of AI4 T cells, DC, and an antigenic mimotope peptide failed to induce apoptotic death of such diabetogenic effectors as well as not altering their expression of granzyme A/B or perforin. Thus, it seems unlikely IFN-γ elicits AICD-mediated elimination of transferred AI4 T cells in NOD recipients or diminishes expression of their cytolytic molecules.
The ability of AI4 T cells to induce diabetes in NOD.IFN-γnull, but not standard NOD mice, was associated with greater proliferation of such effectors in the former recipients. In early antiviral responses, the interplay between type I IFNs and STATs is an important checkpoint determining whether T-cell responses are stimulated or inhibited (28,37). IFN-α can elicit STAT1-dependent antiproliferative effects on T cells (37). However, a lymphocytic choriomeningitis infection model demonstrated STAT1 levels decrease in antigen-specific CD8+ T cells that concordantly increase STAT4 expression. This allows virus-specific T cells to overcome the initial antiproliferative effects of IFN-α (28,37). Serum IFN-α was not detected in NOD or NOD.IFN-γnull recipients either before or after AI4 transfer. However, in the nonproliferative AI4 cells transferred into IFN-γ intact NOD mice (Fig. 4A and B), we observed elevated total STAT1 expression levels by these effectors, a phenotype previously observed with Hep3B cells (40). We found no differences in p-STAT1 or p-STAT4 with or without stimulation in vitro with IFN-β. AI4 T cells exhibited an IFN-γ–dependent elevation in total STAT1 expression, but with unchanged STAT4 levels only when transferred into otherwise unmanipulated recipients in which such effectors failed to elicit diabetes development (NOD and NOD.IFN-γRnull). Interestingly, STAT1 but not STAT4 expression was increased to a greater extent in AI4 T cells transferred into otherwise unmanipulated NOD.IFN-γRnull than NOD recipients. Furthermore, unlike the case for NOD recipients, transferred AI4 T cells also failed to induce diabetes in irradiation-conditioned NOD.IFN-γRnull mice. The resistance of NOD.IFN-γRnull mice under both transfer conditions to AI4 T cell–induced diabetes is likely because of higher IFN-γ levels in this strain after immune stimulation compared with standard NOD mice (18), probably owing to lessened IFN-γR–mediated sequestration of this cytokine. Collectively, these results implicate an IFN-γ–regulated increase in STAT1 but not STAT4 expression levels as mechanistically contributing to inhibition.
Antibody blockade or IFN-γ administration studies would affect both transferred AI4 and host-type cells. Thus, we posit the future creation of NOD.Rag1null.AI4 mice also carrying inactivated IFN-γ, IFN-γR, or STAT1 genes could help to further dissect pathways through which IFN-γ modulates the activity of diabetogenic CD8+ T cells. A complete loss of STAT1 impairs clonal expansion of antigen-specific CD8+ T cells (41). Thus, there may be a narrow window of pathogenic STAT1 activity in CD8+ T cells, and deviations from this range, both positively and negatively, might alter expansion of such effectors. Cells that are either STAT1 deficient, have inactive mutant forms of STAT1α, or only express the STAT1β isoform are resistant to the antiproliferative effects of IFN-γ (42). Therefore, although difficult to create, generating NOD.Rag1null.AI4 mice expressing only a full-length α or COOH-terminal–truncated β isoform of STAT1 could further help to unravel the seemingly contradictory roles of IFN-γ and STATs in diabetogenic CD8+ T-cell responses.
Our current results might appear to contradict a previous report showing the insulin-specific CD8+ T-cell clone TGNFC8 transfers diabetes less efficiently to NOD.IFN-γnull than standard NOD mice (43). It is important to note the TGNFC8 clone is poorly suited to studying events controlling the initial in vivo activation of diabetogenic CD8+ T cells. This is because the TGNFC8 clone must be preactivated in culture to adoptively transfer disease (44,45). Because activated virus-specific T cells were resistant to IFN-α–mediated tolerance [via prior TCR stimuli-mediated upregulation of STAT4 and downregulation of STAT1 (28)], the preactivation of TGNFC8 cells likely alters their ability to respond to the in vivo IFN-γ–mediated protection we currently describe. In contrast, the current study used freshly isolated and initially naive AI4 T cells capable of activation and disease induction after adoptive transfer. Thus, they are subject to all effects controlling this process, including modulation by IFN-γ.
Our collective findings indicate that the temporal timing of IFN-γ signaling in relation to antigen-mediated activation of diabetogenic CD8+ T cells may be key to whether they are pathogenically activated or suppressed. This raises a possible cautionary note that treating humans at risk for T1D with agents limiting or skewing IFN-γ production may accelerate disease if significant numbers of autoreactive CD8+ T cells are already present.
Supplementary Material
Article Information
Funding. This work was supported by National Institutes of Health grants DK-46266 and DK-95735 (to D.V.S.), Helmsley Charitable Trust grant 2014PG-T1D048 (to D.V.S.), JDRF grant 489-JD-01 (to J.P.D.), and American Diabetes Association grant 1-14-BS-051 (to J.P.D.). Additional funding was provided by National Institutes of Health grants DK-074656 (to C.E.M.) and DK-105788 (to B.N.N.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.P.D. and D.V.S. directed the research and wrote the manuscript. J.J.R. designed, performed, and analyzed the experiments; interpreted the data; and wrote the manuscript. C.Y., B.N.N., and C.M.L. performed and analyzed the experiments and contributed to the discussion. D.J.L. and H.D.C. performed the experiments. T.M.B. and C.E.M. developed the cell-mediated lympholysis assay and contributed to the discussion. Y.-G.C. contributed to the discussion. J.P.D. and D.V.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0846/-/DC1.
References
- 1.de Weerd NA, Nguyen T. The interferons and their receptors--distribution and regulation. Immunol Cell Biol 2012;90:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol 2010;10:236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Sheikh A, Suarez-Pinzon WL, Power RF, Rabinovitch A. Both CD4(+) and CD8(+) T cells are required for IFN-gamma gene expression in pancreatic islets and autoimmune diabetes development in biobreeding rats. J Autoimmun 1999;12:109–119 [DOI] [PubMed] [Google Scholar]
- 4.Schloot NC, Hanifi-Moghaddam P, Goebel C, et al. . Serum IFN-gamma and IL-10 levels are associated with disease progression in non-obese diabetic mice. Diabetes Metab Res Rev 2002;18:64–70 [DOI] [PubMed] [Google Scholar]
- 5.Arif S, Tree TI, Astill TP, et al. . Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004;113:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicoletti F, Zaccone P, Di Marco R, et al. . The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the NOD mouse. Endocrinology 1996;137:5567–5575 [DOI] [PubMed] [Google Scholar]
- 7.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest 1991;87:739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]
- 9.Sobel DO, Han J, Williams J, Yoon JW, Jun HS, Ahvazi B. Gamma interferon paradoxically inhibits the development of diabetes in the NOD mouse. J Autoimmun 2002;19:129–137 [DOI] [PubMed] [Google Scholar]
- 10.Shinomiya M, Fazle Akbar SM, Shinomiya H, Onji M. Transfer of dendritic cells (DC) ex vivo stimulated with interferon-gamma (IFN-gamma) down-modulates autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol 1999;117:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol 2001;166:1352–1359 [DOI] [PubMed] [Google Scholar]
- 12.Mori Y, Kodaka T, Kato T, Kanagawa EM, Kanagawa O. Critical role of IFN-gamma in CFA-mediated protection of NOD mice from diabetes development. Int Immunol 2009;21:1291–1299 [DOI] [PubMed] [Google Scholar]
- 13.Qin HY, Chaturvedi P, Singh B. In vivo apoptosis of diabetogenic T cells in NOD mice by IFN-gamma/TNF-alpha. Int Immunol 2004;16:1723–1732 [DOI] [PubMed] [Google Scholar]
- 14.Pallotta MT, Orabona C, Volpi C, et al. . Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011;12:870–878 [DOI] [PubMed] [Google Scholar]
- 15.Grohmann U, Fallarino F, Bianchi R, et al. . A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J Exp Med 2003;198:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med 2002;196:999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J Immunol 2000;164:3919–3923 [DOI] [PubMed] [Google Scholar]
- 18.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes 2000;49:2007–2011 [DOI] [PubMed] [Google Scholar]
- 19.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes 1996;45:812–817 [DOI] [PubMed] [Google Scholar]
- 20.Philpott KL, Viney JL, Kay G, et al. . Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science 1992;256:1448–1452 [DOI] [PubMed] [Google Scholar]
- 21.Pearson T, Markees TG, Serreze DV, et al. . Genetic disassociation of autoimmunity and resistance to costimulation blockade-induced transplantation tolerance in nonobese diabetic mice. J Immunol 2003;171:185–195 [DOI] [PubMed] [Google Scholar]
- 22.Serreze DV, Chapman HD, Varnum DS, et al. . B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 1996;184:2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shultz LD, Lang PA, Christianson SW, et al. . NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol 2000;164:2496–2507 [DOI] [PubMed] [Google Scholar]
- 24.Prochazka M, Gaskins HR, Shultz LD, Leiter EH. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci U S A 1992;89:3290–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamont D, Mukherjee G, Kumar PR, et al. . Compensatory mechanisms allow undersized anchor-deficient class I MHC ligands to mediate pathogenic autoreactive T cell responses. J Immunol 2014;193:2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol 2004;173:6727–6734 [DOI] [PubMed] [Google Scholar]
- 27.Scheuplein F, Rissiek B, Driver JP, Chen YG, Koch-Nolte F, Serreze DV. A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes. J Autoimmun 2010;34:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gil MP, Ploquin MJ, Watford WT, et al. . Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood 2012;120:3718–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura A, Meissner A, Dillon CP, et al. . Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A 2004;101:10380–10385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger WW, Pinkse GG, Mulder-van der Kracht S, et al. . Human clonal CD8 autoreactivity to an IGRP islet epitope shared between mice and men. Ann N Y Acad Sci 2007;1103:192–195 [DOI] [PubMed] [Google Scholar]
- 31.Morgan RA, Dudley ME, Wunderlich JR, et al. . Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson LA, Morgan RA, Dudley ME, et al. . Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Grieshaber S, Mathews CE. Methods to assess beta cell death mediated by cytotoxic T lymphocytes. J Vis Exp 2011;52:2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Driver JP, Scheuplein F, Chen YG, Grier AE, Wilson SB, Serreze DV. Invariant natural killer T-cell control of type 1 diabetes: a dendritic cell genetic decision of a silver bullet or Russian roulette. Diabetes 2010;59:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng G, Gao W, Strom TB, et al. . Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol 2008;38:2512–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Hong J, Sun W, et al. . Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest 2006;116:2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 2006;107:987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lightfoot YL, Chen J, Mathews CE. Oxidative stress and beta cell dysfunction. Methods Mol Biol 2012;900:347–362 [DOI] [PubMed] [Google Scholar]
- 39.Lightfoot YL, Chen J, Mathews CE. Immune-mediated β-cell death in type 1 diabetes: lessons from human β-cell lines. Eur J Clin Invest 2012;42:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radaeva S, Jaruga B, Kim WH, Heller T, Liang TJ, Gao B. Interferon-gamma inhibits interferon-alpha signalling in hepatic cells: evidence for the involvement of STAT1 induction and hyperexpression of STAT1 in chronic hepatitis C. Biochem J 2004;379:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quigley M, Huang X, Yang Y. STAT1 signaling in CD8 T cells is required for their clonal expansion and memory formation following viral infection in vivo. J Immunol 2008;180:2158–2164 [DOI] [PubMed] [Google Scholar]
- 42.Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A 1996;93:7673–7678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savinov AY, Wong FS, Chervonsky AV. IFN-gamma affects homing of diabetogenic T cells. J Immunol 2001;167:6637–6643 [DOI] [PubMed] [Google Scholar]
- 44.Wong FS, Karttunen J, Dumont C, et al. . Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med 1999;5:1026–1031 [DOI] [PubMed] [Google Scholar]
- 45.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA Jr. CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med 1996;183:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.