Abstract
GLUT4 in muscle and adipose tissue is important in maintaining glucose homeostasis. However, the role of insulin-responsive GLUT4 in the central nervous system has not been well characterized. To assess its importance, a selective knockout of brain GLUT4 (BG4KO) was generated by crossing Nestin-Cre mice with GLUT4-floxed mice. BG4KO mice had a 99% reduction in GLUT4 protein expression throughout the brain. Despite normal feeding and fasting glycemia, BG4KO mice were glucose intolerant, demonstrated hepatic insulin resistance, and had reduced glucose uptake in the brain. In response to hypoglycemia, BG4KO mice had impaired glucose sensing, noted by impaired epinephrine and glucagon responses and impaired c-fos activation in the hypothalamic paraventricular nucleus. Moreover, in vitro glucose sensing of glucose-inhibitory neurons from the ventromedial hypothalamus was impaired in BG4KO mice. In summary, BG4KO mice are glucose intolerant, insulin resistant, and have impaired glucose sensing, indicating a critical role for brain GLUT4 in sensing and responding to changes in blood glucose.
Introduction
The facilitative GLUT4 is the major GLUT in skeletal muscle, heart, and adipose tissue. In response to insulin, GLUT4 is translocated to the plasma membrane to facilitate glucose entry into the cell (1,2). Disruption of GLUT4 in skeletal muscle or adipose tissue leads to impaired glucose tolerance and insulin resistance, two prominent features associated with the pathogenesis of diabetes (3,4). GLUT4 is also expressed in the brain (5–7), although its role there has yet to be elucidated. In neuronal cells, insulin and leptin stimulate GLUT4 translocation to the plasma membrane (8). The level of GLUT4 protein in the brain may be dependent on insulin levels; mice with reduced insulin signaling in the brain (neuronal insulin receptor knockout [NIRKO]) have a 68% reduction in hypothalamic GLUT4 protein (9). In addition, the insulin-deficient streptozotocin diabetic rodent has reduced GLUT4, whereas the diabetic hyperinsulinemic db/db mouse has increased GLUT4 in the cerebellum (10). Interestingly, GLUT4 is coexpressed with the insulin receptor in glucose-sensing neurons (11). Together, these findings suggest a potential important role of insulin-responsive GLUT4 in the brain.
In addition to the hippocampus, cortex, and cerebellum, GLUT4 is also expressed in the hypothalamus, an area important in the regulation of whole-body glucose and energy homeostasis (5). Whether dependent on or independent of brain insulin action, GLUT4 is therefore strategically located to play an important role in sensing neuronal glucose and modulating whole-body glucose homeostasis. To determine the impact of brain GLUT4 deletion on glucose tolerance, insulin sensitivity, and detection of hypoglycemia under hyperinsulinemic conditions, GLUT4 was selectively knocked out in the brain, and the effects on whole-body glucose homeostasis were examined. It was hypothesized that brain GLUT4 is required for regulating whole-body glucose homeostasis as well as for sensing and responding to decreases in glucose.
Research Design and Methods
Animals
Brain-specific GLUT4 knockout (BG4KO) mice were created by crossing mice expressing Cre driven by the neuron-specific promoter Nestin with mice with exon 10 of GLUT4 flanked by loxP (FVB strain) (3,4,12–15). Unless otherwise indicated, 8- to 16-week-old male mice were studied. BG4KO mice were GLUT4(lox+/+);Nestin-Cre(+/−). The study included three control groups: wild type (WT; GLUT4(lox−/−)Nestin-Cre(−/−)); GLUT4 Lox (GLUT4(lox+/+);Nestin-Cre(−/−)); and Nestin-Cre (GLUT4(lox−/−);Nestin-Cre(+/−)). Mice were genotyped by PCR analysis of DNA from the tail using established primers and PCR conditions (3,4,12,14).
Animals were housed in temperature- and light-controlled (12 h light/12 h dark) environments and fed standard chow ad libitum. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Animal Studies Committee of Washington University in St. Louis. In all studies, animals were awake and moving freely.
Western Blotting
Animals were sacrificed using isoflurane, and tissues were excised and frozen. Brain, skeletal muscle, heart, and white adipose tissue lysates were separated by SDS-PAGE and immunoblotted with antibodies against GLUT1 (1:1,000), GLUT3 (1:2,000), GLUT4 (1:1,000) (all from Chemicon, Temecula, CA), or β-actin (1:6,000; Sigma-Aldrich, St. Louis, MO) (7). Hypothalamus and liver lysates were immunoblotted with antibodies against phospho-AKT (1:500; Cell Signaling Technology, Danvers, MA), total AKT (1:500; Cell Signaling Technology), insulin receptor β (anti-CD220, 1:500; BD Biosciences, San Jose, CA), and β-actin from mice fasted 5 h; the immunoblotting took place 30 min (hypothalamus) or 15 min (liver) after intraperitoneal injection of insulin (3 units/kg; Humulin-R; Eli Lilly, Indianapolis, IN) or saline.
Glucose Tolerance Tests and Insulin Tolerance Tests
Intraperitoneal glucose tolerance tests (GTTs) (2 g/kg) and insulin tolerance tests (ITTs) (Humulin-R; 1 units/kg) were performed in mice fasted 5 h. Plasma glucose and insulin were measured from the tail vein. First-phase insulin secretion was assessed during the GTT.
High-Fat Diet
BG4KO and control mice were fed a high-fat diet (HFD) (60% kcal from fat; Research Diets Inc., New Brunswick, NJ) starting at 5 weeks of age. Body weight was measured weekly, and blood glucose was measured in fed mice before and after 12 weeks of the HFD. GTTs and ITTs were performed as described above.
Hyperinsulinemic-Euglycemic Clamp
Mice underwent surgery for jugular vein and femoral artery cannulation (9). Hyperinsulinemic (4 mU · kg−1 · min−1)–euglycemic (∼110 mg/dL) clamps were performed in awake, unrestrained mice, as previously described (16). Whole-body glucose flux was determined using primed (5 μCi) continuous infusion (0.05 μCi/min) of [3-3H] glucose (PerkinElmer, Boston, MA) (16). Plasma insulin and [3H]-glucose were obtained in basal and steady states.
Tissue-specific insulin-stimulated glucose uptake was measured with a bolus (5 μCi) of 2-deoxy-D-[1-14C]-glucose (2-[14C]DG; Amersham) administered 45 min before the end of the clamp. Mice were euthanized and skeletal muscle, adipose tissue, and brain harvested. Plasma [3-3H] glucose and 2-[14C]DG concentrations were determined by a dual-channel scintillation counter (Tri-Carb 2800TR; PerkinElmer) after deproteinization and drying. Tissue 2-[14C]DG and 2-[14C]DG-6-phosphate were separated by ion-exchange chromatography.
Whole-body glucose turnover, endogenous glucose production (EGP), and glucose disposal (Rd) were determined using 3-3H-glucose tracer dilution methodology during the steady state, as previously described (16). Glucose uptake by skeletal muscle and adipose tissue was calculated from tissue 2-[14C]DG-6-phosphate content normalized against the area under the plasma 2-[14C]DG decay curve (17).
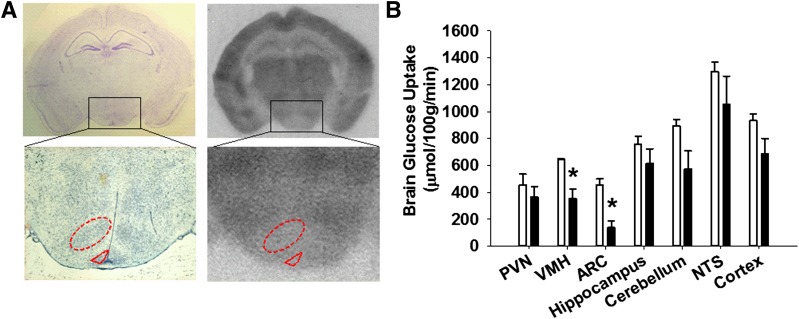
To determine glucose uptake in the brain, isotope concentrations in regions of interest were measured from 20-μm coronal sections after exposure to autoradiograph film via optical densitometry. Areas of interest were identified by Nissl staining (Fig. 4). Rates of glucose uptake were calculated using the Sokoloff equation (18,19).
Figure 4.
Reduced glucose uptake in the brains of BG4KO mice. Glucose uptake in the brain was measured using 2-[14C]DG autoradiography in mice that underwent a hyperinsulinemic-euglycemic clamp. A: The areas of interest were precisely identified by Nissl staining (left panels), identifying the desired areas (i.e., the VMH; red dashed oval outline) and arcuate nucleus (ARC; red dashed triangular outline) and measuring the density of the outlined areas of interest on the exposed autoradiographic films (right panels). B: Quantification of glucose uptake in the brain during euglycemia using the Sokoloff equation in BG4KO mice (n = 5; black bars) and Nestin-Cre control mice (n = 5; white bars). Glucose uptake in the brain was significantly reduced in BG4KO mice compared with control mice in the VMH and ARC (*P < 0.05, t test). Data are expressed as the mean ± SEM. NTS, nucleus tractus solitarus.
Hyperinsulinemic-Hypoglycemic Clamp
Mice underwent hyperinsulinemic (20 mU · kg−1 · min−1)–hypoglycemic clamps and c-fos immunostaining, as previously described (9). Four anatomically matched sections per animal were used to quantify c-fos immunostaining in the paraventricular nucleus (PVN).
Pharmacological Inhibition of Brain GLUT4
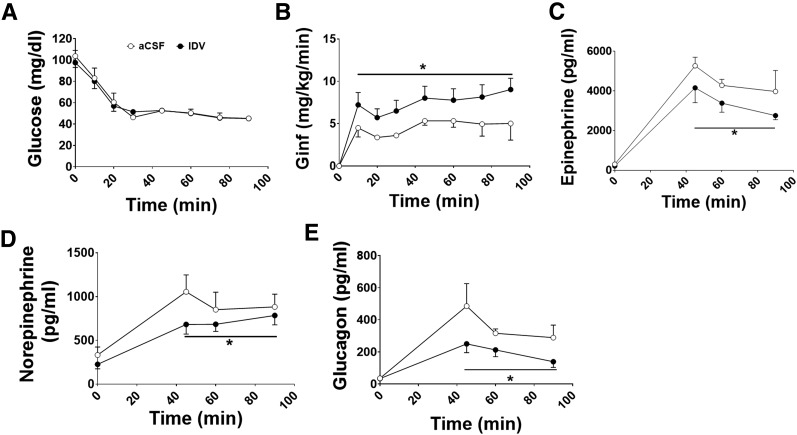
For brain infusion experiments, 9-week-old male Sprague-Dawley rats (Charles River Laboratories International) were chosen because of their larger size, better dual-surgical survival, and no need for genetic manipulation. Following surgical recovery, rats underwent a hyperinsulinemic (20 mU · kg−1 · min−1)–hypoglycemic (∼45 mg/dL) clamp, as previously described (20). Three hours before and throughout the clamp, indinavir (IDV) (10 μg/min; Merck, Whitehouse Station, NJ; n = 9) or vehicle (artificial cerebrospinal fluid [aCSF]; n = 6) was infused into the third ventricle (−2.8 mm from the bregma; Plastics One Inc., Roanoke, VA). IDV has been shown to inhibit GLUT4 activity and transport in cell cultures and hippocampal brain slices (21–23). Arterial blood samples were taken for clamp hormone determinations.
Electrophysiological Studies
BG4KO and Nestin-Cre+ mice (28–42 days old) were anesthetized and the brains sectioned, as previously described (9). Glucose-inhibited (GI) neurons from the ventromedial hypothalamus (VMH) of control and BG4KO mice were identified as reversibly depolarized, and their action potential frequency and input resistance increased in response to glucose decreases from 2.5 to 0.1 mmol/L. Input resistance was calculated by dividing the change in membrane voltage (millivolts) by the current (picoamperes). The effects of glucose and insulin (5 nmol/L) on action potential frequency, membrane potential, and input resistance were evaluated in current-clamp recordings (standard whole-cell recording configuration) of neurons in the VMH, as previously described (9,24,25). Current–voltage relationships were determined in the voltage-clamp recording mode using 10-mV steps in voltage (from −50 to −150 mV) from a holding potential of −60 mV.
Analytical Measurements
Blood glucose was measured using Ascensia Contour blood glucose monitors (Bayer HealthCare, Mishawaka, IN) and plasma glucose was assayed by glucose oxidase (BioTek Instruments, Inc., Winooski, VT). Insulin levels were measured by ELISA (Crystal Chem Inc., Chicago, IL), catecholamines by a single isotope-derived (radioenzymatic) method (26), and glucagon (Linco Research Inc., St. Charles, MO) and corticosterone (MP Biomedicals, Orangeburg, NY) by radioimmunoassay.
Statistical Analyses
All data are presented as the mean ± SEM. Statistical significance was set at P < 0.05 and was determined by either the Student t test, one-way ANOVA, or repeated-measures ANOVA, as indicated.
Results
Verification of GLUT4 Deletion
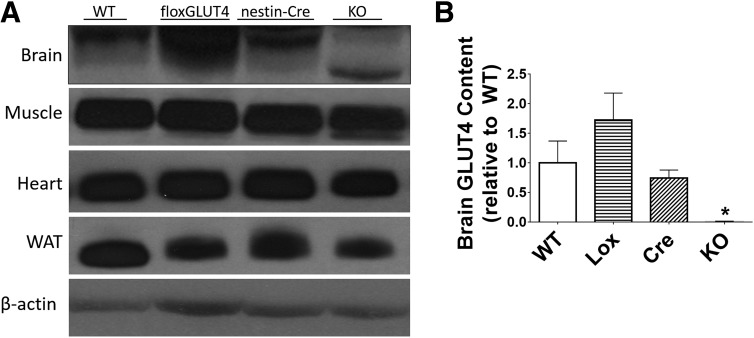
BG4KO mice had a >99% reduction in GLUT4 protein levels in the brain relative to WT, GLUT4 Lox-expressing (Lox), and Nestin-Cre–expressing (Cre) mice (P = 0.0009, ANOVA; F = 6.8) (Fig. 1). A lower-molecular-weight band in brains of BG4KO mice represents the 7.3-kDa removal of exon 10, as previously shown (4). Importantly, GLUT4 content in the heart, muscle, and adipose tissue of BG4KO mice was expressed at levels similar to those in controls (Fig. 1). No significant difference in whole-brain GLUT1 and GLUT3 protein levels were observed between BG4KO and controls (Supplementary Fig. 1A and B). Furthermore, there were no significant differences in hypothalamic insulin receptor or insulin-stimulated AKT phosphorylation between the groups, confirming that brain insulin signaling was unaltered by brain GLUT4 deletion (Supplementary Fig. 1C).
Figure 1.
Brain-specific deletion of GLUT4. A: Whole-brain tissue, gastrocnemius muscle, heart muscle, and white adipose tissue (WAT) were harvested and homogenized for Western blotting of GLUT4. GLUT4 was markedly reduced in the brains of BG4KO mice (KO) compared with WT, Lox, and Cre mice. GLUT4 protein concentrations in muscle, heart, and WAT were similar between BG4KO and control mice. It should be noted that 50 times more protein was loaded for the brain compared with muscle, heart, and WAT in order to detect GLUT4 protein levels because of their relatively lower expression compared with that in muscle, heart, and WAT. B: Quantification of brain GLUT4 protein content. BG4KO mice (n = 11; black bar) had a >99% reduction in brain GLUT4 levels compared with WT (n = 6; white bar), Lox (n = 6; horizontal lines), and Cre (n = 6; slanted lines) mice. *P = 0.0009 (ANOVA) vs. WT, Lox, and Cre mice. Data are expressed as mean ± SEM.
Effect of BG4KO on Glucose Homeostasis
Nestin-Cre and BG4KO mice had significantly reduced body weight and crown-to-rump body length (at 12 weeks of age) compared with WT and Lox mice, with no difference in fat mass (P = 0.0001, ANOVA; F = 16) (Supplementary Fig. 2A and B). Thus Nestin-Cre expression reduced body weight, consistent with a previous report (27). Control mice therefore included Nestin-Cre, WT, and Lox mice.
BG4KO Mice Are Glucose Intolerant and Insulin Resistant
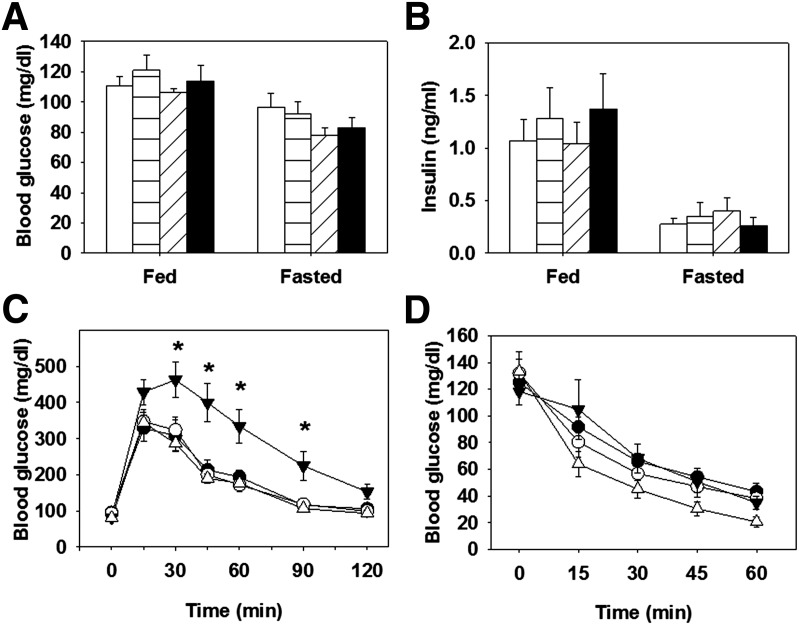
Fasting and fed glucose and insulin concentrations were similar in all groups on normal chow (Fig. 2A and B). GTTs and ITTs were performed to determine the role of brain GLUT4 in glucose tolerance and insulin sensitivity. BG4KO mice had significantly higher glucose excursions during the GTT than control mice (P = 0.006, repeated-measures ANOVA; F = 14.6) (Fig. 2C). To determine whether the impaired glucose tolerance in BG4KO mice was attributed to altered glucose-stimulated insulin secretion, insulin concentrations were measured during a GTT. No differences in plasma insulin concentrations or in first-phase insulin secretion were observed between BG4KO mice and controls (Supplementary Fig. 3A and B). Despite the impaired glucose tolerance, BG4KO mice had a similar reduction in blood glucose in response to insulin during an ITT compared with controls (Fig. 2D).
Figure 2.
Normal blood glucose but impaired glucose tolerance in BG4KO mice. A: BG4KO mice (black bars) have fed and fasting blood glucose concentrations and fed and fasting plasma insulin concentrations similar to those of WT (white bar), Lox (horizontal lines), and Cre (slanted lines) mice. B: BG4KO mice had fed and fasting blood insulin concentrations similar to those of the control groups. C: Intraperitoneal GTTs (2 mg/kg) were performed in 12-week-old male mice fed normal chow. BG4KO mice (black triangles) had significantly higher excursions in blood glucose compared with WT (white circles), Lox (black circles), and Cre (white triangles) mice, where indicated. D: No difference in blood glucose was observed between BG4KO and the control mice during an insulin tolerance test. *P = 0.006 (repeated-measures ANOVA) vs. WT, Lox, and Cre mice. Data are expressed as the mean ± SEM (n = 6–17 mice per group).
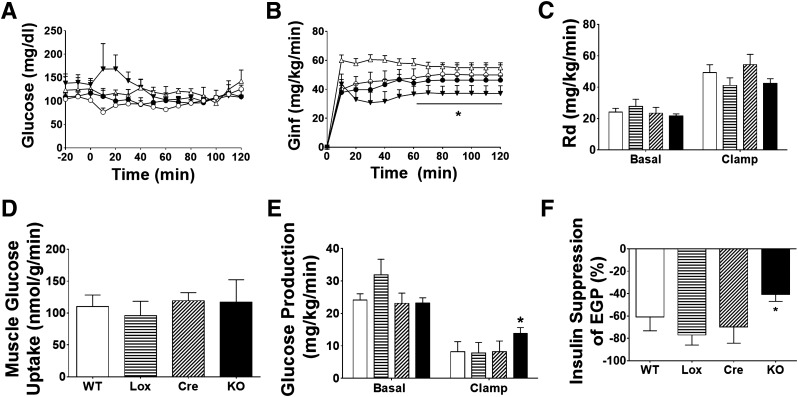
The effect of BG4KO on whole-body insulin sensitivity was directly assessed by hyperinsulinemic-euglycemic clamps. Blood glucose concentrations were evenly matched between BG4KO mice and controls (WT, Lox, Cre) (Fig. 3A). Basal and clamp insulin were not different among the groups (data not shown). Interestingly, BG4KO mice required an ∼26% lower glucose infusion rate (37 ± 5 vs. 50 ± 7, 46 ± 3, and 55 ± 3 mg · kg−1 · min−1 in WT, Lox, and Cre mice, respectively; P = 0.002 vs. WT, Lox, and Cre, repeated-measures ANOVA; F = 6.3) to maintain euglycemia compared with controls, demonstrating whole-body insulin resistance (Fig. 3B). Since BG4KO mice had similar basal Rd values and a similar insulin-stimulated increase in Rd compared with controls, the defect in whole-body insulin sensitivity was not due to changes in glucose disposal (Fig. 3C); this was verified by similar tissue-specific glucose uptake in muscle (Fig. 3D) and adipose tissue (data not shown) in BG4KO and control mice. However, while basal EGP was not different among the groups, the ability of insulin to suppress EGP was only 41% in BG4KO compared with 61%, 77%, and 70% in WT, Lox, and Cre mice, respectively (P = 0.05, ANOVA; F = 2.96) (Fig. 3E and F). To test whether insulin resistance was the result of impaired hepatic insulin signaling, insulin-induced AKT phosphorylation was analyzed; it increased similarly in BG4KO and control mice (Supplementary Fig. 3C and D).
Figure 3.
Reduced insulin sensitivity and hepatic insulin resistance in BG4KO mice. A: Blood glucose during a hyperinsulinemic-euglycemic clamp. No difference in blood glucose before or during the last hour of the clamp was observed between BG4KO (black triangles) and WT (white circles), Lox (black circles), and Cre (white triangles) mice. B: Despite matched blood glucose concentrations, BG4KO mice required a significantly lower glucose infusion rate (Ginf) to maintain euglycemia during the final hour of the clamp compared with controls, indicating insulin resistance (*P = 0.002, repeated-measures ANOVA, BG4KO vs. WT, Lox, and Cre). C: Whole-body Rd values at baseline and during the last 30 min of the hyperinsulinemic clamp were not significantly different between BG4KO (black bar) and WT (white bar), Lox (horizontal lines), and Cre (diagonal lines) mice. D: 2-[14C]DG–determined glucose uptake specifically in muscle was similar between BG4KO (KO; n = 7) and WT (n = 6), Lox (n = 6), and Cre (n = 5) mice during the hyperinsulinemic clamp. E: EGP was not different at baseline among the groups, but EGP during the clamp was significantly higher in BG4KO mice compared with the three groups of control mice. F: The percent suppression of EGP from baseline to during the hyperinsulinemic clamp was impaired in BG4KO mice compared with WT, Lox, and Cre mice (BG4KO 41 ± 7 vs. WT 61 ± 36, Lox 77 ± 10, and Cre 70 ± 17% suppression; *P = 0.05, ANOVA) during the last hour of the hyperinsulinemic clamp. All data are expressed as mean ± SEM (n = 6–11 mice per group).
To determine whether deletion of GLUT4 in the brain results in altered glucose uptake, glucose uptake in the brain during the euglycemic clamp was measured and found to be lower in BG4KO compared with control mice in the VMH (P = 0.041, t test; t = 2.7; df = 5) and arcuate nucleus (P = 0.01, t test; t = 4.1; df = 5), with a trend toward decreased uptake in the PVN, hippocampus, cerebellum, nucleus tractus solitarus, and cortex (Fig. 4A and B).
BG4KO Does Not Affect Susceptibility to Diet-Induced Obesity
To test whether BG4KO mice are prone to diet-induced obesity, mice were fed an HFD. No difference in body weight change or random glucose after feeding was observed between BG4KO and control mice (Supplementary Fig. 4A and B). Further, after 12 weeks on the HFD, GTTs and ITTs demonstrated impaired glucose tolerance and insulin resistance compared with mice fed chow (Fig. 2C and D), but there were no differences between BG4KO and control mice eating the HFD (Supplementary Fig. 4C and D).
Role of Central GLUT4 in the Counterregulatory Response to Hypoglycemia
Previous research in our laboratory revealed that brain insulin receptors are important in regulating hypoglycemic counterregulation. Knockout of insulin receptors in the brain (NIRKO) diminishes hypothalamic GLUT4 expression (9), suggesting a role of brain GLUT4 in regulating hypoglycemic counterregulation.
Pharmacological and Genetic Inhibition of Central GLUT4 Attenuates the Counterregulatory Response to Hypoglycemia
To test whether brain GLUT4 is important in the counterregulatory response to hypoglycemia, complementary genetic studies (in BG4KO mice) and pharmacological studies (in rats) were performed. In rats, either aCSF or IDV, a GLUT4 activity inhibitor (21–23), was infused into the third ventricle during a hyperinsulinemic-hypoglycemic clamp. Blood glucose was not different between the groups (Fig. 5A). Interestingly, the glucose infusion rate required to maintain glucose at ∼45 mg/dL was 44% higher in the IDV-treated rats compared with controls, indicating an impaired counterregulatory response (P < 0.0001, repeated-measures ANOVA; F = 6.0) (Fig. 5B). The higher glucose infusion rate in IDV-treated rats was associated with diminished epinephrine (24% decrease; P = 0.05, t test; t = 1.9), norepinephrine (22% decrease; P = 0.03, t test; t = 2.3), and glucagon (45% decrease; P = 0.004, t test; t = 3.1) responses to hypoglycemia compared with controls (Fig. 5C–E).
Figure 5.
Intracerebroventricular (ICV) infusion of IDV, an inhibitor of GLUT4 transport, reduced the counterregulatory response to hypoglycemia. IDV (n = 9; black circles) or aCSF (n = 6; white circles) was infused for 90 min before and for the duration of a 90-min hyperinsulinemic-hypoglycemic (∼45 mg/dL) clamp in Sprague-Dawley rats. A: Blood glucose was precisely matched between rats treated with ICV infusions of either IDV or aCSF. B: Despite matched blood glucose concentrations, IDV-treated rats (black circles) required a significantly higher glucose infusion rate (Ginf) than control rats (white circles) (*P < 0.0001, repeated-measures ANOVA). The higher glucose infusion rate was attributed to the attenuated counterregulatory response to hypoglycemia caused by the reduced GLUT4-mediated glucose sensing. Epinephrine (C), norepinephrine (D), and glucagon (E) responses to hypoglycemia were significantly reduced in IDV-treated rats vs. controls (*P < 0.05, t test, for all hypoglycemic time points for epinephrine, norepinephrine, and glucagon and area under the curve). Data are expressed as mean ± SEM.
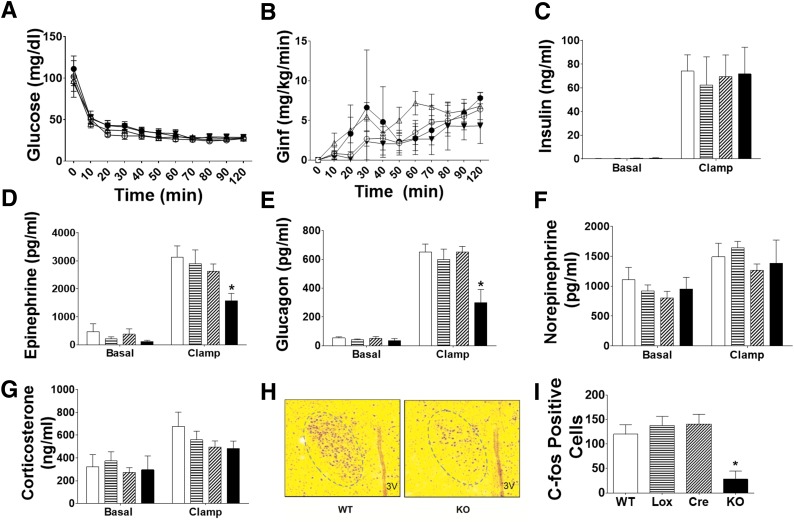
To complement these pharmacological studies, the role of brain GLUT4 in mediating hypoglycemic counterregulation was also studied in BG4KO mice. During a hyperinsulinemic-hypoglycemic (∼30 mg/dL) clamp, glucose and insulin concentrations were similar among BG4KO mice and controls (Fig. 6A and C). Glucose infusion rates were also similar among the groups (Fig. 6B). During hypoglycemia, peak epinephrine concentrations increased similarly in the WT, Lox, and Cre mice (3133 ± 600, 2902 ± 560, and 2633 ± 355 pg/mL, respectively) (Fig. 6D), but were blunted in BG4KO mice (1575 ± 101 pg/mL; P = 0.03, ANOVA; F = 3.2). Furthermore, the glucagon response to hypoglycemia was significantly attenuated in BG4KO mice (BG4KO 299 ± 92; WT 650 ± 57; Lox 597 ± 74; Cre 651 ± 39 pg/mL; P = 0.0005, ANOVA; F = 6.9) (Fig. 6E). Norepinephrine and corticosterone responses to hypoglycemia were similar among the groups (Fig. 6F and G). The blunted epinephrine response was unique to hypoglycemia: heat stress tests (42°C for 90 min [9]) in BG4KO mice resulted in epinephrine and norepinephrine concentrations similar to those in controls (Supplementary Fig. 5A and B).
Figure 6.
BG4KO mice have an impaired counterregulatory response to hypoglycemia. BG4KO and control mice were subjected to hyperinsulinemic-hypoglycemic (∼30 mg/dL) clamps. A: Blood glucose was not different before or during the clamp between BG4KO (n = 5; black triangles), WT (n = 5; white circles), Lox (n = 6; black circles), and Cre (n = 11; white triangles) mice. B: Glucose infusion rates (Ginf) during the hypoglycemic clamp were not different among the groups. C: Insulin concentrations in the basal state and during the hypoglycemic clamp were not different between the groups: WT (white bars), Lox (horizontal lines), Cre (slanted lines), and BG4KO (black bars). Epinephrine (D) and glucagon (E) responses to hypoglycemia were significantly reduced in BG4KO (black bars) mice compared with WT (white bars), Lox (horizontal lines), and Cre (slanted lines) mice. Norepinephrine (F) and corticosterone (G) concentrations during the hypoglycemic clamp were similar in all groups. H: Representative c-fos immunostaining of the hypothalamic PVN in WT and BG4KO (KO) mice after 2 h of hypoglycemia. I: Quantification of c-fos immunostaining. The number of c-fos–positive cells in the PVN was greatly reduced in BG4KO mice (black bar) compared with WT (white bar), Lox (horizontal lines), and Cre (slanted lines) mice. *P < 0.03 (ANOVA) vs. WT, Lox, and Cre. Data are expressed as mean ± SEM (n = 5–11 mice per group).
To determine whether the impaired counterregulatory response in the BG4KO mice was associated with impaired hypothalamic neuronal activation, c-fos activation was measured in response to hypoglycemia. BG4KO mice had an ∼80% reduction in c-fos–positive cells in the PVN compared with controls during the hypoglycemic clamp (BG4KO 28 ± 17 vs. WT 120 ± 20, Lox 138 ± 19, Cre 141 ± 20 c-fos–positive cells; P = 0.003, ANOVA; F = 6.7) (Fig. 6H and I).
BG4KO Causes Impaired Neuronal Glucose Sensing
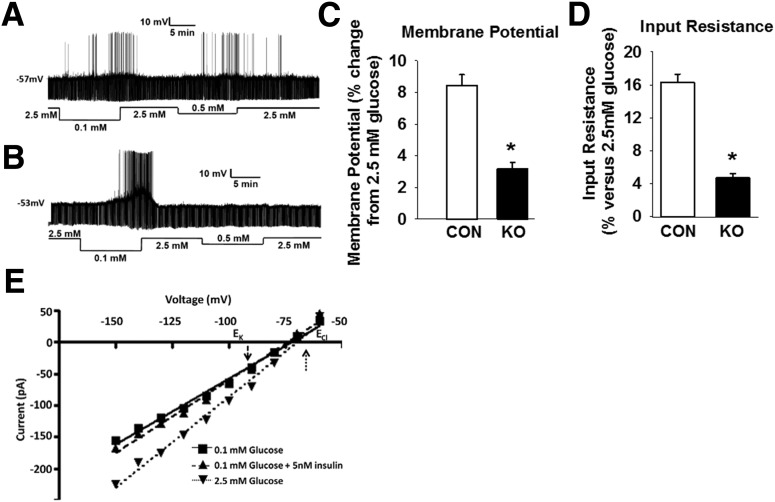
To test whether the observed impaired counterregulatory response to insulin-induced hypoglycemia in BG4KO mice is a result of impaired brain glucose sensing or impaired insulin action, changes in membrane potential and input resistance of individual GI neurons of the VMH were measured using whole-cell current-clamp recordings in the presence and absence of insulin. As expected, for GI neurons of the VMH bathed in 2.5 mmol/L glucose, action potential frequency was low in control mice (0.43 ± 0.12 Hz; n = 14). Baseline action potential frequency tended to be higher in GI neurons of the VMH from BG4KO mice (0.75 ± 0.22; n = 16; P = 0.2, t test; t = 1.2; df = 28). In addition, GI neurons of the VMH from BG4KO mice exhibited higher input resistance at baseline (BG4KO 1465 ± 90 MΩ [n = 16]; control 1042 ± 77 MΩ [n = 14]; P = 0.002, t test; t = 3.5; df = 28) and a depolarized membrane potential compared with GI neurons from control mice (BG4KO −56 ± 1 mV; control −60 ± 1 mV; P = 0.002, t test; t = 3.4; df = 28). Increased excitability of GI neurons of the VMH in the absence of GLUT4 is consistent with reduced glucose uptake in the VMH.
The glucose sensitivity of GI neurons of the VMHs from control and BG4KO mice is illustrated in Fig. 7A and B. GI neurons from BG4KO mice demonstrated characteristic increased action potential firing in response to a glucose decrease from 2.5 to 0.1 mmol/L. However, GI neurons from BG4KO mice had significantly impaired changes in membrane potential and input resistance (62% [P < 0.001, t test; t = 6.9; df = 14] and 71% impairment [P < 0.0001, t test; t = 10.6; df = 14], respectively) in response to a 2.5–0.5 mmol/L decrease in glucose (Fig. 7A–D), indicating that BG4KO mice have impaired glucose sensing in the VMH.
Figure 7.
Impaired glucose sensing in BG4KO mice. Whole-cell current-clamp traces of GI neurons in slices of the VMH from Nestin-Cre control (A) and BG4KO (B) mice. The upward deflections represent action potentials, the resting membrane potential is given to the left of each trace, and the downward deflections are the voltage response to a constant-current pulse. Compared with GI neurons from controls (CON; white bars), GI neurons from BG4KO mice (KO; black bars) had markedly impaired changes in membrane potential (C) and input resistance (D) when glucose concentrations were changed from 2.5 to 0.5 mmol/L. E: Current–voltage relationships for the effects of glucose or insulin on a GI neuron from the VMH of control mice. The reversal potential for the effect of glucose (intersection of 2.5 vs. 0.1 mmol/L glucose; upward dotted arrow) is close to the theoretical Cl− equilibrium potential (ECl ∼ −60 mV), suggesting that glucose activates a chloride channel. In contrast, the reversal potential for the effect of insulin (intersection of 0.1 mmol/L glucose vs. 0.1 mmol/L glucose + 5 nmol/L insulin; downward dashed arrow) is near the theoretical K+ equilibrium potential (EK ∼ −99 mV), suggesting that insulin activates a potassium channel. *P < 0.002 (t test) vs. control. Data are expressed as mean ± SEM (n = 14–16 mice per group).
To determine whether the effects of insulin on GI neurons in the VMH are mediated by GLUT4, we evaluated the effects of insulin on the electrical properties of GI neurons in the VMHs from BG4KO and control mice. Interestingly, insulin reversed the excitatory effects of 0.1 mmol/L glucose by decreasing input resistance and hyperpolarizing the membrane potential of these neurons to a similar extent in both genotypes (BG4KO: membrane potential −8 ± 0.4% change, input resistance −14 ± 0.7 [n = 7]; control: membrane potential −9 ± 0.6, input resistance −16 ± 1 [n = 8]; P = not significant, t test), indicating that the actions of insulin on these neurons is independent of GLUT4. In addition, the current–voltage relationships obtained in voltage-clamp mode indicate that the reversal potential for the effects of insulin and glucose were significantly different (P < 0.05). As expected, for GI neurons from the VMH, the effect of glucose reversed near the Cl− equilibrium potential (−71 ± 6 mV [n = 5]; ECl −60 mV) (Fig. 7E). By contrast, the effect of insulin reversed near the K+ equilibrium potential (−94 ± 4 mV [n = 4]; EK −99 mV). This suggests that the inhibitory effect of insulin was mediated via a K+ channel rather than a Cl− channel, as seen for glucose.
Discussion
On the basis of immunohistochemical studies that identified GLUT4 in discrete areas of the brain, including the hypothalamus (5), brain GLUT4 has been suggested to have a role in neuronal glucose sensing and whole-body energy homeostasis. However, the potential role(s) of central GLUT4 remains unknown. This study demonstrated a novel role of GLUT4 in the modulation of glucose sensing in the brain, the counterregulatory response to hypoglycemia, and whole-body glucose homeostasis.
Using Cre-Lox technology, knockout of brain GLUT4 was virtually complete, whereas GLUT4 protein remained normal in peripheral tissues. Further evidence for unaltered peripheral expression of GLUT4 was noted by similar rates of whole-body glucose disposal between BG4KO and control mice during the hyperinsulinemic-euglycemic clamp.
The expression of Cre driven by the Nestin promoter resulted in a slight reduction in body weight and crown-to-rump length, similar to previous studies (27,28); this is potentially attributable to mild hypopituitarism (29). Since both BG4KO and Nestin-Cre mice express Cre+, the lack of difference in body weight between these two groups indicate that the deletion of brain GLUT4 did not have a further effect on body weight. Notably, other metabolic parameters assessed, such as glucose, insulin, glucose tolerance, insulin sensitivity, and counterregulatory responses, were similar among WT, Lox, and Cre mice. Importantly, a pharmacological approach using IDV to inhibit brain GLUT4 during hypoglycemia produced results similar to those of the genetic approach, indicating that GLUT4 is important in glucose sensing in the brain. Consistent with a critical role for GLUT4 in mediating glucose sensing in the brain, in recent work whole-body GLUT4 knockout mice showed impaired neuronal activation during hypoglycemia (B.B.K., personal communication).
Fasting and fed glucose concentrations were similar in BG4KO and control mice, consistent with similar baseline rates of EGP and glucose disposal. However, when challenged with a glucose load during a GTT, BG4KO mice had higher blood glucose excursions than control mice, indicating glucose intolerance. These findings are consistent with the glucose intolerance recently reported in a mouse model in which GLUT4 neurons were ablated (30). Furthermore, glucose-stimulated insulin secretion was not different between BG4KO and control mice, indicating that pancreatic β-cell responses to glucose remained unaffected by GLUT4 deletion in the brain. During the ITT, BG4KO mice had blood glucose concentrations similar to those of control mice, which could be explained by the opposing effects of insulin resistance and impaired hypoglycemic counterregulation.
During the euglycemic clamp, BG4KO mice required a lower glucose infusion rate to maintain euglycemia than controls, indicating insulin resistance, which can be attributed to hepatic insulin resistance, as noted by the impaired ability of insulin to suppress EGP. Since insulin-stimulated AKT phosphorylation in the liver was normal in BG4KO mice, hepatic insulin resistance in BG4KO mice seems to be independent of direct insulin signaling in the liver and is likely mediated via indirect mechanisms. Central insulin signaling may suppress EGP indirectly, possibly via efferent vagal and sympathetic actions (31,32). The absence of brain GLUT4 may have limited the central actions of insulin to indirectly suppress EGP in BG4KO mice, although this is not a result of proximal defects in hypothalamic insulin signaling, which is preserved in BG4KO mice, as shown both in vivo, with normal hypothalamic insulin-induced AKT phosphorylation, and in vitro, as shown by a normal response to insulin by GI neurons in the VMH. Chronic adaptations in BG4KO mice may have led to altered neuronal development and plasticity, altering the neuronal circuitry involved in glucose homeostasis and thus diminishing EGP (33). Alternatively, reduced centrally mediated inhibition of glucagon secretion by insulin may lead to a failure to suppress EGP (34). Regardless of the mechanism, the results indicate that insulin suppresses EGP in part through brain GLUT4.
Several studies have suggested a relationship between central insulin action and GLUT4 (5,11,30,35). Under hyperinsulinemic conditions, glucose uptake into brain regions that normally express high levels of GLUT4 was reduced in BG4KO mice. The reduced glucose uptake in brains of BG4KO mice was expected to be small because GLUT4 is normally expressed at low levels (11). GLUT4 and insulin receptors are coexpressed in up to 75% of glucose-sensing neurons in the VMH (11), indicating that brain GLUT4 may be important in the ability of neurons to sense and respond to changes in glucose. Both pharmacological inhibition (IDV) and genetic deletion of central GLUT4 (BG4KO mice) resulted in impaired epinephrine and glucagon responses to hypoglycemia. IDV is an HIV protease inhibitor that is known to cause insulin resistance, which has been attributed to its selective inhibition of GLUT4-mediated glucose transport (21–23,36). Pharmacological inhibition of brain GLUT4 was associated with an expected elevated glucose infusion rate, compensating for hypoglycemia-associated counterregulatory failure. However, the paradoxically equivalent glucose infusion rates during hypoglycemia in BG4KO (vs. control) mice in the setting of documented counterregulatory failure were likely a result of their hepatic insulin resistance induced by chronic GLUT4 deficiency in the brain. The adrenomedullary responses to heat stress were identical in BG4KO and control mice, indicating that the impaired counterregulatory response is specific to hypoglycemia.
The impaired counterregulatory response to hypoglycemia in the BG4KO mice was associated with impaired hypothalamic neuronal activation, which has been independently confirmed in another whole-body GLUT4 knockout model (unpublished data), and is consistent with other models of impaired glucose sensing and impaired counterregulation (9,37,38). Electrophysiological studies were performed to identify the more specific role of brain GLUT4 in glucose sensing. GI neurons from the VMHs of BG4KO mice have an impaired response to decreasing glucose concentrations, thus supporting the findings that BG4KO mice have impaired hypothalamic glucose sensing.
The simultaneous attainment of hyperinsulinemia and hypoglycemia with the clamp studies does not allow us to determine whether reduced glucose sensing/uptake in the brains of BG4KO mice is due to insulin-dependent or insulin-independent glucose uptake; however, our electrophysiology data support the latter. First, in the absence of insulin, enhanced excitability of GI neurons in the VMH of BG4KO mice at baseline is consistent with reduced intracellular glucose. Second, insulin equally inhibited GI neurons from the VMHs of control and BG4KO mice. Finally, the current–voltage relationships indicate that insulin did not lead to activation of a Cl− channel, as would be expected if insulin increased glucose uptake (39). Interestingly, while insulin has been shown to inhibit hypothalamic AMPK (40), this is unlikely to explain insulin’s effects on GI neurons because AMPK inhibition stimulates GI neurons by closing a Cl− channel (41), and the inhibitory effect of insulin on GI neurons resulted from the activation of a K+ channel, potentially the ATP-sensitive K+ channel, since insulin activates this channel in other hypothalamic glucose-sensing neurons (42,43). These data suggest that insulin inhibits GI neurons through GLUT4/glucose-independent activation of a K+ channel.
Previous research revealed that NIRKO mice have small amounts of GLUT4 in the brain and also demonstrate 1) glucose intolerance, 2) insulin resistance, 3) impaired epinephrine responses to hypoglycemia, 4) impaired neuronal activation in response to hypoglycemia, and 5) impaired glucose sensing in GI neurons of the VMH (9,13,14). The similarities in the phenotypes of NIRKO and BG4KO mice suggest that insulin action in the brain is partly mediated by GLUT4.
In summary, BG4KO results in insulin resistance, glucose intolerance, impaired hypoglycemic counterregulation, and impaired glucose sensing in the VMH, supporting our hypothesis. Because GLUT4 is expressed in diverse regions of the brain, BG4KO mice will be a useful tool in elucidating potential roles of brain GLUT4 in other central nervous system functions. In conclusion, brain GLUT4 plays an important role in the modulation of glucose sensing in the brain, the counterregulatory response to hypoglycemia, and whole-body glucose homeostasis.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. P. Cryer and his laboratory (Washington University in St. Louis) for performing the catecholamine assay. Dr. C.R. Kahn (Joslin Diabetes Center, Harvard University) supplied the Nestin-Cre mice. The authors thank Ron Perez for his technical expertise and Yasuko Suzuki and Ioanna Georgopoulos for their help with the experiments (Washington University in St. Louis).
Funding. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK073683 and NS070235 [to S.J.F.], DK55619 and DK081358 [to V.H.R.], and DK43051 [to B.B.K.]), JDRF (S.J.F. and V.H.R.), the JPB Foundation (B.B.K.), and core grant support from Washington University’s Diabetes Research and Training Center (DK020579 [to S.J.F.]) and Nutrition Obesity Research Center (P30DK056341 [to S.J.F.]).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.R. and E.C.P. designed and performed the experiments and wrote the manuscript. Z.S. and V.H.R. performed experiments and wrote part of the manuscript. D.D.-I. and A.J.B. performed experiments. B.B.K. designed experiments, interpreted data, and provided mice. S.J.F. designed experiments and edited the manuscript. S.J.F. is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Part of this research was presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 5–9 June 2009; and at the 70th Scientific Sessions of the American Diabetes Association, Orlando, FL, 25–29 June 2010.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0917/-/DC1.
References
- 1.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 2007;5:237–252 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A 1980;77:2542–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001;409:729–733 [DOI] [PubMed] [Google Scholar]
- 4.Zisman A, Peroni OD, Abel ED, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 2000;6:924–928 [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol 2004;490:13–24 [DOI] [PubMed] [Google Scholar]
- 6.Rayner DV, Thomas ME, Trayhurn P. Glucose transporters (GLUTs 1–4) and their mRNAs in regions of the rat brain: insulin-sensitive transporter expression in the cerebellum. Can J Physiol Pharmacol 1994;72:476–479 [DOI] [PubMed] [Google Scholar]
- 7.Leloup C, Arluison M, Kassis N, et al. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res Mol Brain Res 1996;38:45–53 [DOI] [PubMed] [Google Scholar]
- 8.Benomar Y, Naour N, Aubourg A, et al. Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase-dependent mechanism. Endocrinology 2006;147:2550–2556 [DOI] [PubMed] [Google Scholar]
- 9.Diggs-Andrews KA, Zhang X, Song Z, Daphna-Iken D, Routh VH, Fisher SJ. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes 2010;59:2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res 1998;797:1–11 [DOI] [PubMed] [Google Scholar]
- 11.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 2004;53:549–559 [DOI] [PubMed] [Google Scholar]
- 12.Abel ED, Kaulbach HC, Tian R, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 1999;104:1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 14.Fisher SJ, Brüning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes 2005;54:1447–1451 [DOI] [PubMed] [Google Scholar]
- 15.Tronche F, Kellendonk C, Kretz O, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999;23:99–103 [DOI] [PubMed] [Google Scholar]
- 16.Fisher SJ, Kahn CR. Insulin signaling is required for insulin’s direct and indirect action on hepatic glucose production. J Clin Invest 2003;111:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARgamma-deficeint mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 2003;112:608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977;28:897–916 [DOI] [PubMed] [Google Scholar]
- 19.Toyama H, Ichise M, Liow JS, et al. Absolute quantification of regional cerebral glucose utilization in mice by 18F-FDG small animal PET scanning and 2-14C-DG autoradiography. J Nucl Med 2004;45:1398–1405. [PubMed] [Google Scholar]
- 20.Ishihara KK, Haywood SC, Daphna-Iken D, Puente EC, Fisher SJ. Brain insulin infusion does not augment the counterregulatory response to hypoglycemia or glucoprivation. Metabolism 2009;58:812–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando RN, Albiston AL, Chai SY. The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus–potential role in modulation of glucose uptake in neurones? Eur J Neurosci 2008;28:588–598 [DOI] [PubMed] [Google Scholar]
- 22.Hertel J, Struthers H, Horj CB, Hruz PW. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem 2004;279:55147–55152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata H, Hruz PW, Mueckler M. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS 2002;16:859–863 [DOI] [PubMed] [Google Scholar]
- 24.Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 2005;54:15–22 [DOI] [PubMed] [Google Scholar]
- 25.Song Z, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol 2006;291:R1283–R1287 [DOI] [PubMed] [Google Scholar]
- 26.Shah SD, Clutter WE, Cryer PE. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med 1985;106:624–629 [PubMed] [Google Scholar]
- 27.Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 2010;59:3074–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 2006;12:917–924 [DOI] [PubMed] [Google Scholar]
- 29.Galichet C, Lovell-Badge R, Rizzoti K. Nestin-Cre mice are affected by hypopituitarism, which is not due to significant activity of the transgene in the pituitary gland. PLoS One 2010;5:e11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren H, Lu TY, McGraw TE, Accili D. Anorexia and impaired glucose metabolism in mice with hypothalamic ablation of Glut4 neurons. Diabetes 2015;64:405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61 [DOI] [PubMed] [Google Scholar]
- 32.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 33.Levin BE, Sherwin RS. Peripheral glucose homeostasis: does brain insulin matter? J Clin Invest 2011;121:3392–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paranjape SA, Chan O, Zhu W, et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes 2010;59:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin HV, Ren H, Samuel VT, et al. Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes 2011;60:700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarasheski KE, Tebas P, Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr 1999;21:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kale AY, Paranjape SA, Briski KP. I.c.v. administration of the nonsteroidal glucocorticoid receptor antagonist, CP-472555, prevents exacerbated hypoglycemia during repeated insulin administration. Neuroscience 2006;140:555–565 [DOI] [PubMed] [Google Scholar]
- 38.Niimi M, Sato M, Tamaki M, Wada Y, Takahara J, Kawanishi K. Induction of Fos protein in the rat hypothalamus elicited by insulin-induced hypoglycemia. Neurosci Res 1995;23:361–364 [DOI] [PubMed] [Google Scholar]
- 39.Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 2007;56:1219–1227 [DOI] [PubMed] [Google Scholar]
- 40.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569–574 [DOI] [PubMed] [Google Scholar]
- 41.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol 2009;297:C750–C758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 2000;3:757–758 [DOI] [PubMed] [Google Scholar]
- 43.Cotero VE, Routh VH. Insulin blunts the response of glucose-excited neurons in the ventrolateral-ventromedial hypothalamic nucleus to decreased glucose. Am J Physiol Endocrinol Metab 2009;296:E1101–E1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.