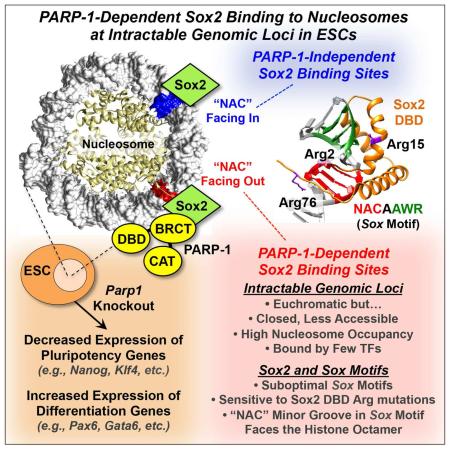
Summary
Pioneer transcription factors (TFs) function as genomic ‘first responders,’ binding to inaccessible regions of chromatin to promote enhancer formation. The mechanism by which pioneer TFs gain access to chromatin remains an important unanswered question. Here we show that PARP-1, a nucleosome-binding protein, cooperates with intrinsic properties of the pioneer TF Sox2 to facilitate its binding to ‘intractable’ genomic loci in embryonic stem cells. These actions of PARP-1 occur independent of its poly(ADP-ribosyl) transferase activity. PARP-1-dependent Sox2 binding sites reside in euchromatic regions of the genome with relatively high nucleosome occupancy and low co-occupancy by other transcription factors. PARP-1 stabilizes Sox2 binding to nucleosomes at suboptimal sites through cooperative interactions on DNA. Our results define intrinsic and extrinsic features that determine Sox2 pioneer activity. The ‘conditional’ pioneer activity observed with Sox2 at a subset of binding sites may be a key feature of other pioneer TFs operating at intractable genomic loci.
Keywords: PARP-1, Sox2, nucleosome, embryonic stem cells, pioneer transcription factor, transcription, chromatin, nucleosome rotational positioning
Graphical abstract
Introduction
The HMG box-containing transcription factor (TF) Sox2 plays a fundamental role in regulating embryonic stem cell identity and differentiation (Feng and Wen, 2015). It functions with Oct4, Nanog, and Klf4, which serve as ‘master regulator’ TFs to control the pluripotency of embryonic stem cells (Young, 2011). Sox2 binds to a conserved DNA sequence, the Sox motif, and regulates the transcription of key pluripotency target genes (Zhang and Cui, 2014). Thus, the regulation of Sox2 binding to chromatin is critical for the proper control of the fate of ESCs. Although Sox2 is thought to function as a ‘pioneer’ transcription factor (Iwafuchi-Doi and Zaret, 2014), it frequently co-binds with other transcription factors, such as Oct4 and Brn2, in a cell type-specific manner, facilitating access of Sox2 to the genome (Lodato et al., 2013; Young, 2011; Zhang and Cui, 2014). In this regard, the manner, mechanisms, and extent to which Sox2 and other TFs (e.g., FoxA1) function as pioneer TFs for genome accessibility has been debated in the recent literature (Franco et al., 2015; Iwafuchi-Doi et al., 2016; Soufi et al., 2015; Swinstead et al., 2016; Zaret et al., 2016).
During the early stages of somatic cell reprogramming, Sox2, Oct4, and Klf4 bind to genomic loci that have a closed chromatin conformation (Soufi et al., 2012). Both Sox2 and Oct4 are able to bind to nucleosomes with an affinity comparable to naked DNA (Soufi et al., 2012). However, unlike Oct4, Sox2 binding to nucleosomes is a mixture of sequence-specific and non-specific interactions, which can be efficiently competed by non-specific DNA (Soufi et al., 2015). Whether Sox2’s pioneer factor activity is sufficient to allow the binding of Sox2 to all ‘closed’ genomic loci in the absence of other co-binding transcription factors, and how such binding might occur, is unknown. Recent studies have suggested that the chromatin-associated proteins poly(ADP-ribose) polymerase-1 (PARP-1) may interact physically and functionally with Sox2 to control its activity (Doege et al., 2012; Gao et al., 2009; Hemberger et al., 2003; Lai et al., 2012; Roper et al., 2014).
PARP-1 is an abundant nuclear protein that controls a variety of nuclear processes, including transcription and DNA repair. PARP-1 has a DNA binding domain, which allows it to bind to nucleosomes and modulate chromatin structure, as well as a catalytic domain, which can use NAD+ as a substrate to poly(ADP-ribosyl)ate (PARylate) target proteins (Hottiger, 2015; Krishnakumar and Kraus, 2010a). As a nucleosome-binding protein, PARP-1 acts to block the binding of the linker histone H1 to facilitate the maintenance of an open chromatin conformation at gene promoters (Kim et al., 2004; Krishnakumar et al., 2008). As an enzyme, PARP-1 PARylates key chromatin- and transcription-related proteins to regulate gene expression outcomes (Hottiger, 2015; Krishnakumar and Kraus, 2010a). For example, PARP-1 PARylates the histone demethylase KDM5B to block its binding to chromatin and inhibit its catalytic activity (Krishnakumar and Kraus, 2010b). Likewise, PARP-1 PARylates the negative transcription elongation factor NELF to inhibit promoter-proximal pausing by RNA polymerase II, thus allowing transcription elongation to proceed (Gibson et al., 2016). PARP-1 has also been shown to regulate the activities of sequence-specific DNA-binding transcription factors, such as NF-κB (Martin-Oliva et al., 2004) and E2F-1 (Simbulan-Rosenthal et al., 2003). Thus, PARP-1 has distinct catalytic-dependent and catalytic-independent functions in gene regulation (Hottiger, 2015; Krishnakumar and Kraus, 2010a).
Recent studies have begun to elucidate the various roles of PARP-1 protein and its enzymatic activity in the biology of embryonic stem cells (ESCs), including the maintenance of pluripotency, lineage-specific differentiation, and the reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) (Fig. 1A). For example, PARP-1 promotes the maintenance of ground state pluripotency and inhibits the differentiation of ESCs into trophoblast derivatives (Hemberger et al., 2003; Roper et al., 2014). PARP-1 and Sox2 interact in ESCs and regulate each other’s activities (Lai et al., 2012). During ESC differentiation, PARP-1 PARylates Sox2 to reduce Sox2 protein levels and allow activation of an enhancer that promotes Fgf4 gene expression (Gao et al., 2009; Weber et al., 2013). PARP-1 also collaborates with the methylcytosine dioxygenase Tet2 to regulate an epigenetic program that controls the transcription of pluripotency genes during somatic cell reprogramming (Doege et al., 2012). Although these studies have defined important and distinct roles for PARP-1 and its catalytic activity in various aspects of ESC biology, which are directed primarily at the establishment or maintenance of pluripotency, the molecular mechanisms by which PARP-1 controls the pluripotency gene expression program have not been elucidated.
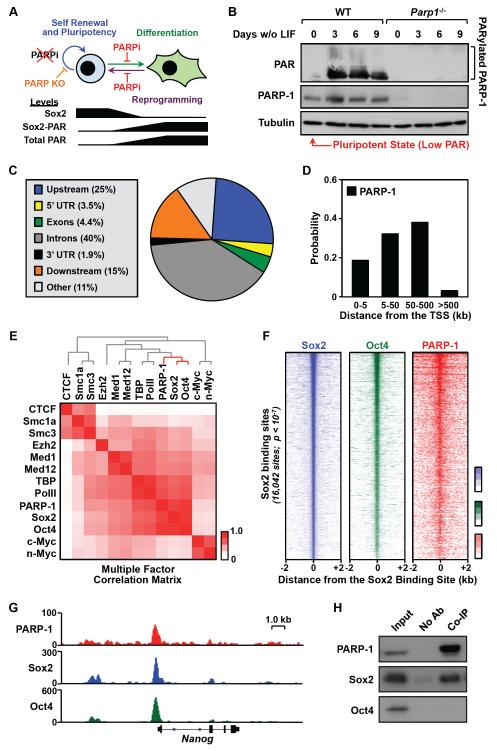
Figure 1. PARP-1 colocalizes with Sox2 genome-wide.
A) Top, Summary of the known roles of PARP-1 protein and PARylation activity in embryonic stem cells. Bottom, Summary of known changes in Sox2 protein and PARylation levels, total cellular PARylation levels during embryonic stem cell differentiation.
B) The level of PARP-1-mediated PARylation is very low in undifferentiated mESCs and increases upon differentiation. Western blots of poly(ADP-ribose) (PAR) and PARP-1 showing their relative levels in mESCs during a 9 day time course of differentiation upon LIF removal.
C) Distribution of significant peaks of PARP-1 binding from ChIP-seq across genomic features in mESCs.
D) Distribution of significant peaks of PARP-1 binding relative to the TSSs of all RefSeq genes.
E) PARP-1 colocalizes with Sox2 genome-wide. Correlation matrix of genome-wide enrichment for chromatin- and transcription-related factors from ChIP-seq data in mESCs, organized and ordered using hierarchical clustering.
F) Left, Heatmap of high confidence Sox2 peaks in mESCs from ChIP-seq data (n = 16,042; p-value < 10−7) centered on the Sox2 binding sites (± 2 kb) and ordered top to bottom by signal intensity. Middle and right, Oct4 and PARP-1 ChIP-seq signals associated with the corresponding Sox2 binding sites.
G) Genome browser tracks of PARP-1, Sox2 and Oct4 ChIP-seq data around the Nanog gene in WT mESCs.
H) PARP-1 binds to Sox2, but not Oct4. Flag-tagged PARP-1 was expressed in HEK293T cells with Sox2 and Oct4, and immunoprecipitated using a Flag antibody. Western blots showing the relative levels of PARP-1, Sox2, and Oct4 in the input and the co-immunoprecipitated (co-IP) material.
See also Fig. S1.
Here, we show that PARP-1 facilitates the binding of Sox2 to nucleosomes in ‘intractable’ regions of chromatin, ultimately promoting the Sox2-dependent transcriptional program that helps to maintain pluripotency. In addition, we defined the molecular determinants for PARP-1-dependent Sox2 binding to nucleosomes. Collectively, our results define intrinsic and extrinsic features that determine Sox2 pioneer activity.
Results
To explore the molecular mechanisms underlying the PARP-1-dependent transcriptional program that controls ESC biology, as described in a series of recent papers (Doege et al., 2012; Gao et al., 2009; Hemberger et al., 2003; Roper et al., 2014) (Fig. 1A), we used mouse ESCs (mESCs) with genetic deletion of the Parp1 gene (Gao et al., 2009). In the pluripotent state, prior to differentiation, we find that mESCs have nearly undetectable levels of PAR, as assessed by Western blotting, indicating very low levels of PARP activity (Fig. 1B). This observation is in agreement with Yoo et al. (Yoo et al., 2011) and is supported by results using another mESC line (ES-E14TG2a; Fig. S1, A and B), but differs from Gao et al. (Gao et al., 2009). Upon differentiation in response to removal of leukemia inhibitory factor (LIF), PAR levels in mESCs rise dramatically, primarily as automodified PARP-1 (Fig. 1B). Thus, mESCs in the pluripotent state represent an excellent biological model for studying possible enzyme-independent functions of PARP-1. In this regard, we performed a series of genomic analyses in mESCs that led to specific mechanistic conclusions, which we then tested in detail in a series of biochemical analyses with reconstituted nucleosomes and recombinant proteins, as described below.
PARP-1 Controls a Pluripotency Gene Expression Program in mESCs Independent of Its Catalytic Activity
To determine how PARP-1 might regulate gene expression in mESCs, we performed a series of genomic analyses, including chromatin immunoprecipitation-sequencing (ChIP-seq) for PARP-1. We found that PARP-1 is enriched in both genic and intergenic regions of the genome (Fig. 1, C and D). In genic regions, PARP-1 localizes to the promoters of actively expressed genes (Fig. S1, C and D), as we have shown previously (Krishnakumar et al., 2008; Krishnakumar and Kraus, 2010b). Genome-wide clustering analyses using ChIP-seq data for a panel of chromatin- and transcription-related factors revealed a striking correlation between the localization of PARP-1, Sox2, and Oct4 in mESCs (Fig. 1E), which is clearly evident in heatmaps (Fig. 1F) and browser track representations (Fig. 1G; note the colocalization of PARP-1, Sox2, and Oct4 upstream of the Nanog gene). In this regard, co-immunoprecipitation assays from mESCs revealed an interaction between PARP-1 and Sox2, as suggested previously (Gao et al., 2009), but not between PARP-1 and Oct4 (Fig. 1H). These results suggest that PARP-1 and Sox2 physically associate and colocalize across the genome in mESCs.
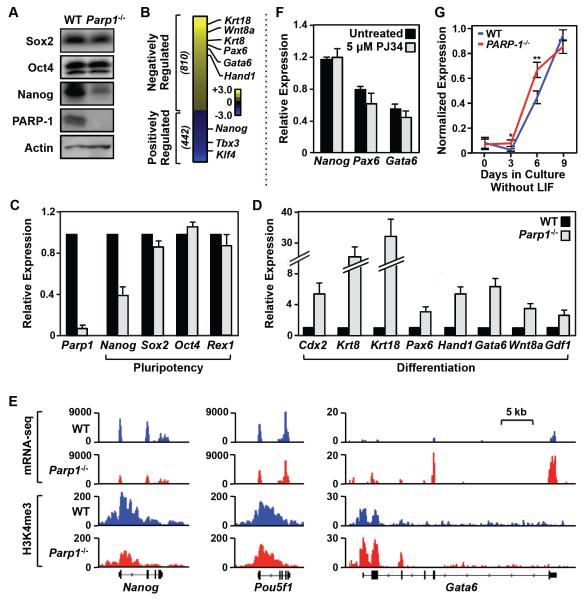
Next, we sought to explore how PARP-1 genomic localization might control gene expression outcomes. Parp1 knockout caused a dramatic reduction in Nanog protein levels (Fig. 2A). More generally, Parp1 knockout caused a marked decrease in the expression of pluripotency genes and increase in the expression of differentiation genes, as determined by global and gene-specific assays (Fig. 2, B-E; Fig. S2, A-C), without a gross alteration in the morphology of the undifferentiated mESCs. In this regard, the expression of Rex1, a sensitive marker of pluripotency found in undifferentiated embryonic stem cells (Rogers et al., 1991), was unaffected by Parp1 knockout (Fig. 2C), while the expression of Nanog was reduced by 60% (Fig. 2C). We defined genes whose expression decreased upon Parp1 knockout as ‘positively regulated’ by PARP-1, and genes whose expression increased upon Parp1 knockout as ‘negatively regulated’ by PARP-1 (Fig. 2B; Fig. S2, A and B). Treatment with the PARP inhibitor PJ34 had no effect on the expression of the pluripotency gene Nanog, or the differentiation genes Pax6 and Gata6 (Fig. 2F), as expected based on the lack of detectable PARylation activity in mESCs (Fig. 1B). Interestingly, the kinetics of differentiation upon LIF removal, as assessed by the expression of a set of differentiation genes, was significantly enhanced by Parp1 knockout (Fig. 2G; Fig. S2D). Together, these results indicate that PARP-1 controls a pluripotency gene expression program in mESCs independent of its catalytic activity.
Figure 2. PARP-1 is required for maintaining transcriptional program in embryonic stem cells.
A) Western blots showing the relative levels of three pluripotency factors (Sox2, Oct4, and Nanog) and PARP-1 in WT and Parp1−/− mESCs. Actin is used as an internal loading control.
B) Effect of Parp1 knockout on gene expression in mESCs as determined by RNA-seq. The heatmap shows the relative expression levels of genes whose expression significantly (FDR < 5%) increased upon Parp1 knockout (“Negatively Regulated” by PARP-1) or decreased upon Parp1 knockout (“Positively Regulated” by PARP-1). The data are log2(Parp1−/− RPKM/WT RPKM; RPKM = Reads per kilobase of transcript per million mapped reads).
C) Effect of Parp1 knockout on the expression of pluripotency-associated genes in mESCs, as determined by RT-qPCR. The data for Parp1−/− mESCs are expressed relative to WT ESCs. Each bar represents the mean plus the SEM, n ≥ 3. The differences observed for Nanog and Parp1 are significant (Student’s t test, p-value < 0.05).
D) Effect of Parp1 knockout on the expression of differentiation-associated genes in mESCs, as determined by RT-qPCR. The data for Parp1−/− mESCs are expressed relative to WT ESCs. Each bar represents the mean plus the SEM, n ≥ 3. The differences observed for all of the genes shown are significant (Student’s t test, p-value < 0.05).
E) Genome browser tracks of mRNA-seq data (top) and H3K4me3 ChIP-seq data (bottom) around the Nanog, Oct4, and Gata6 genes in WT and Parp1−/− mESCs.
F) The expression of Nanog (a pluripotency-associated gene), as well as Pax6 and Gata6 (differentiation-associated genes), in undifferentiated (‘Day 0”) mESCs is not affected by treatment with the PARP inhibitor PJ34. RT-qPCR was performed using total RNA isolated from mESCs treated with 5 M PJ34 for 24 hrs. The expression levels we standardized to the expression of the Gapdh gene. Each bar represents the mean plus the SEM, n ≥ 3. The small differences are not significant.
G) Analysis of mRNA expression for a panel of 19 differentiation-associated genes in mESCs during a 9 day time course of differentiation upon LIF removal. The expression levels of each mRNA are normalized to Gapdh mRNA levels and scaled. The individual genes are listed in Fig. S2D. * p = 0.01, ** p = 0.0002.
See also Fig. S2.
PARP-1 Is Required for the Binding of Sox2 to a Subset of Its Genomic Binding Sites: Links to Gene Expression Outcomes
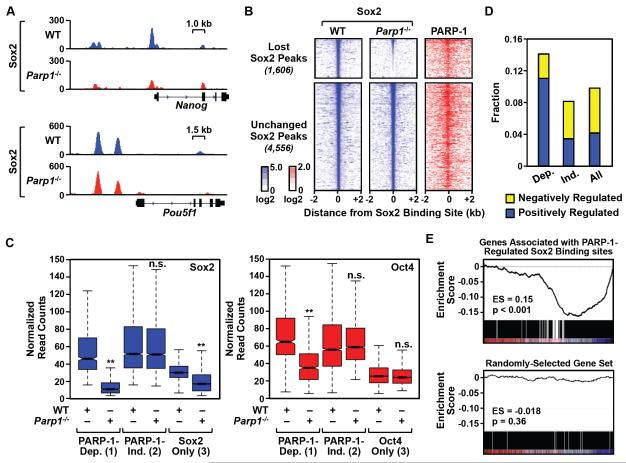
To explore the functional link between Sox2 and PARP-1 in more detail, we determined the effect of genetic depletion of PARP-1 in mESCs on Sox2 binding genome-wide. Parp1 knockout caused a significant reduction in Sox2 binding across the genome. Out of 16,042 Sox2 peaks that we detected, we identified 1,606 sites (10% of the total) that were definitively lost (‘PARP-1-dependent’ Sox2 binding sites) and 4,556 sites that were definitively unchanged (‘PARP-1-independent’ Sox2 binding sites) upon Parp1 knockout (Fig. 3, A and B; Fig. S3A). This includes ‘PARP-1-dependent’ Sox2 binding sites located in the promoter region of the Nanog gene (Fig. 3A). The binding of Sox2 to PARP-dependent sites was unaffected by treatment with PJ34, again indicating the catalytic-independent functions of PARP-1 in this system (Fig. S3B). Unexpectedly, both the PARP-1-dependent and PARP-1-independent Sox2 binding sites were co-occupied by PARP-1 (Fig. 3B). As we show below, however, PARP-1 is only functionally required at the PARP-1-dependent sites. Importantly, although some PARP-1-dependent Sox2 binding sites were co-occupied by Oct4 (e.g., Fig. 1G), the effects of PARP-1 on Sox2 binding were not mediated through Oct4. This is illustrated by the observation that Sox2 binding sites without Oct4, but not Oct4 binding sites without Sox2, exhibit PARP-1-dependent binding (Fig. 3C).
Figure 3. PARP-1 is required for the binding of Sox2 to a subset of its genomic sites in mESCs.
A) Genome browser tracks of Sox2 ChIP-seq data around the Nanog and Pou5f1 (encoding Oct4) genes in WT and Parp1−/− mESCs.
B) Left and middle, Heatmaps of Sox2 ChIP-seq signals in WT and Parp1−/− mESCs centered on the Sox2 binding sites (± 2 kb) and ordered top to bottom by signal intensity. Right, PARP-1 ChIP-seq signals in WT mESCs associated with the corresponding Sox2 binding sites. PARP-1-dependent Sox2 binding sites are defined as those sites whose ChIP-seq signals are significantly decreased upon PARP-1 knockout (p-value < 0.01; n = 1,606,). PARP-1-independent Sox2 binding sites show no change upon PARP-1 knockout (n = 4,556).
C) Normalized ChIP-seq read counts for Sox2 (left) and Oct4 (right) at (1) PARP-1-dependent (Dep.) sites where Sox2 and Oct4 binding overlap, (2) PARP-1-independent (Ind.) sites where Sox2 and Oct4 binding overlap, and (3) sites with Sox2 or Oct4 only. Asterisks indicate significant differences (Student’s t-test, p-value < 2.2 × 10−16).
D) Fraction of genes associated with different categories of Sox2 binding sites. Dep. = PARP-1-dependent Sox2 binding sites; Ind. = PARP-1 independent Sox2 binding sites; All = All Sox2 binding sites. Yellow, Genes whose expression significantly increases upon Parp1 knockout (“Negatively Regulated” by PARP-1). Blue, Genes whose expression significantly decreases upon Parp1 knockout (“Positively Regulated” by PARP-1).
E) GSEA analysis showing the relationship between PARP-1-regulated Sox2 binding sites (n = 1,606) and gene expression changes upon Parp1 knockout in mESCs. Top, The expression of genes associated with PARP-1-regulated Sox2 binding sites is significantly decreased in Parp1−/− mESCs compared to WT mESCs (p-value < 0.001) based on RNA-seq. Bottom, A randomly selected and equally sized set of genes (n = 1,606) shown as a control (p-value = 0.36).
See also Fig. S3.
Reduced Sox2 binding upon Parp1 knockout near genes encoding key pluripotency factors and stem cell markers (e.g., Nanog, Klf4, Kit) correlated with reduced expression of the genes (Figs. 2E and 3A; Fig. S3A). Globally, genes positively regulated by PARP-1 were enriched for PARP-1-dependent Sox2 binding sites near their promoters (Fig. 3D). Gene set enrichment analysis (GSEA) revealed that the expression of genes associated with PARP-1-dependent Sox2 binding sites was significantly decreased in Parp1−/− mESCs compared to WT mESCs based on RNA-seq (Fig. 3E). Some of the genes that are upregulated upon Parp1 knockout are likely to be driven by the PARP-1-dependent Sox2 binding site in the Nanog gene promoter. In this regard, GSEA analyses revealed that the expression of a previously-defined set of Nanog-repressed genes (Loh et al., 2006), including differentiation genes, is upregulated in Parp1−/− mESCs compared to WT mESCs based on RNA-seq (Fig. S3C). Furthermore, rescue of Nanog expression using the MEK inhibitor PD184352 (Silva et al., 2009) restored the repressed expression of differentiation genes (e.g., Gata6 and Sox17) in Parp1−/− mESCs (Fig. S3, D and E). Together, these results suggest that altered gene expression upon Parp1 knockout in mESCs is likely to be a direct result of the loss of a subset of Sox2 binding upon PARP-1 depletion.
Defining the Genomic Determinants of PARP-1-Dependent Sox2 Binding
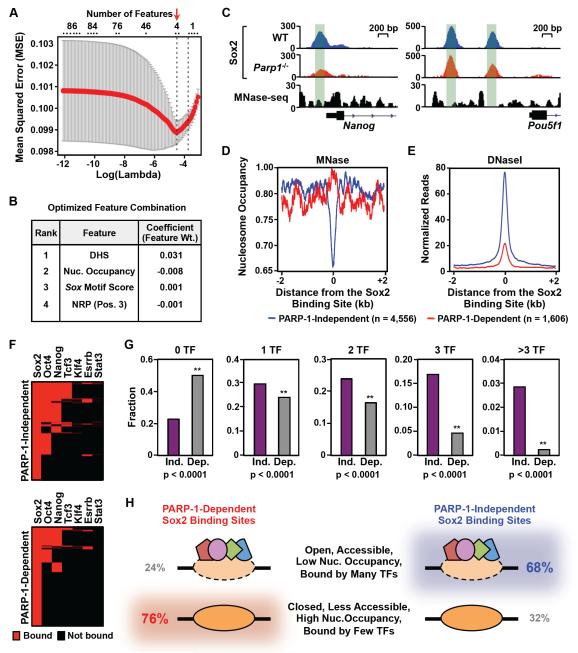
We hypothesized that a subset of genomic features might distinguish PARP-1-dependent and PARP-1-independent Sox2 binding sites. A chromatin state analysis using the chromHMM analytical tool (Ernst and Kellis, 2012) showed that PARP-1-dependent Sox2 binding sites are enriched in regions of chromatin with features indicative of weak active enhancers, poised enhancers, and basal chromatin, which overlaps the distribution of PARP-1-independent Sox2 binding (Fig. S4A). Importantly, PARP-1-dependent Sox2 binding sites do not reside in regions of repressed heterochromatin (Fig. S4A). To determine which genomic features might distinguish PARP-1-dependent and PARP-1-independent Sox2 binding sites, we used a machine learning approach with the LASSO algorithm (Tibshirani, 1996), which allowed us to select the feature combination producing the optimal prediction accuracy, as evaluated by the mean square error (MSE). Eighty-six genomic features (from available genomic data sets or derived from DNA sequence) that might influence the binding of transcription factors to chromatin were used as the initial feature list. A combination of four features (DNase hypersensitivity, nucleosome occupancy, Sox motif score, and nucleosome rotational positioning) best predicted the p-value of the difference in the strength of Sox2 binding between wild-type and Parp1−/− mESCs, as determined from ChIP-seq (Fig. 4, A and B).
Figure 4. Genomic determinants of PARP-1-dependent Sox2 binding.
A) Identification of a minimal set of genomic features that accurately predict the PARP-1 dependence of Sox2 binding sites using the machine learning algorithm, LASSO (Tibshirani, 1996). The features included nucleosome occupancy, transcription factor co-occupancy, DNaseseq signal strength, Sox motif sequence, rotational orientation of the Sox motif in the nucleosome, and predicted structural features of the DNA at Sox2 binding sites (e.g., nucleosome rotational positioning, minor groove width, roll, x-displacement, slide). Prediction accuracy was evaluated by 10-fold cross validation. The combination of features that produced the best prediction accuracy with the smallest mean squared error (MSE; Y-axis) was selected (red arrow). X-axis, Log10 values of the penalty score lambda. Top, Number of features corresponding to the respective lambda values.
B) List of the optimized feature combination from the LASSO algorithm producing the best prediction accuracy. The complete list of 86 features tested in this analysis is listed in the Supplemental Materials.
C) Genome browser tracks of Sox2 ChIP-seq data and MNase-seq data around the Nanog gene in WT and Parp1−/− mESCs. The green shading highlights the relationship between Sox2 binding and nucleosome occupancy.
D) Average MNase-seq signals surrounding PARP-1-dependent (red) and PARP-1-independent (blue) Sox2 binding sites in mES cells. The data are centered on the Sox2 binding sites determined by ChIP-seq (± 2 kb).
E) Average DNase-seq signals surrounding PARP-1-dependent (red) and PARP-1-independent (blue) Sox2 binding sites in mES cells. The data are centered on the Sox2 binding sites determined by ChIP-seq (± 2 kb).
F) Heatmaps showing the binding of other transcription factors at Sox2 binding sites. Top, Results for PARP-1-independent Sox2 binding sites. Bottom, Results for PARP-1-dependent Sox2 binding sites. Red, significant binding. Black, no significant binding.
G) Fraction of PARP-1-independent (Ind.) and PARP-1-dependent (Dep.) Sox2 binding sites associated with the specified number transcription factors (TFs; 0, 1, 2, 3, >3) based on ChIP-seq in mESCs. Asterisks indicate significant differences (Fisher’s exact test, p-value < 0.0001).
H) Summary of genomic features for PARP-1-independent and PARP-1-dependent Sox2 binding sites based on Fig. 4 and Fig. S4. 76% of PARP-1-dependent Sox2 binding sites have the features shown in the bottom row, while 68% of PARP-1-independent Sox2 binding sites have the features shown in the top row.
See also Fig. S4.
Further characterization of the PARP-1-dependent Sox2 binding sites in mESCs confirmed the outcomes from the LASSO analysis. Notably, PARP-1-dependent Sox2 binding sites, in comparison to PARP-1-independent Sox2 binding sites: (1) tend to be occupied by nucleosomes, as assessed by MNase-seq (Fig. 4, C and D), (2) reside in less accessible regions of chromatin, as assessed by DNase-seq (Fig. 4E), (3) have less optimal Sox motifs (Fig. S4, B-D) and fewer composite Sox/Pou motifs (Fig. S4E), and (4) are co-bound by fewer additional transcription factors (Fig. 4, F and G; Fig. S4, F-H). Collectively, our genomic and computational analyses revealed a class of Sox2 binding sites that require PARP-1 for binding and have a specific set of features that distinguish them from PARP-1-independent Sox2 binding sites (Fig. 4H).
Reconstitution of PARP-1-Dependent Sox2 Binding in Biochemical Assays
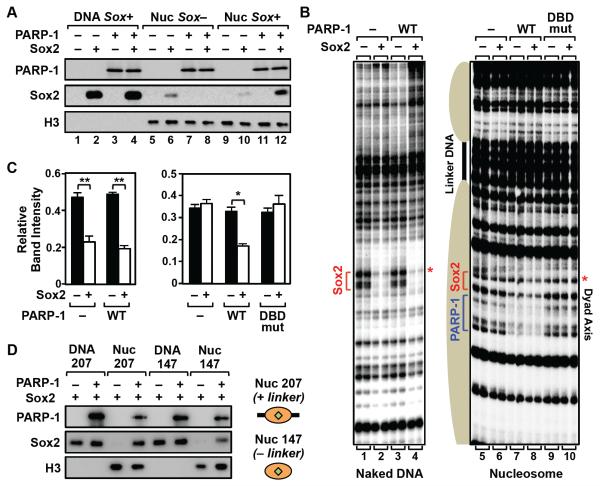
Based on our genomic analyses in mESCs, we established in vitro assays with reconstituted nucleosomes containing the 601 nucleosome positioning element (NPE) (Fig. S5) to explore the biochemical and molecular basis by which PARP-1 facilitates Sox2 binding to chromatin. We observed PARP-1-dependent binding of Sox2 to mononucleosomes containing a Sox motif near the nucleosomal dyad axis (Fig. 5A, lane 10 versus lane 12), but not to naked DNA containing a Sox motif (Fig. 5A, lane 2 versus lane 4) or mononucleosomes lacking a Sox motif (Fig. 5A, lane 6 versus lane 8). We also performed DNaseI footprinting assays using a trinucleosome template lacking accessible DNA ends (Clark et al., 2012) and containing a Sox motif located near the dyad axis of the middle nucleosome (Fig. 5B). These assays showed PARP-1-dependent binding of Sox2 to the middle nucleosome, indicated by enhanced protection of the DNA (Fig. 5B, compare lanes 7 and 8; note the red asterisk), but not to the same site in naked DNA (Fig. 5, B and C). We also observed some PARP-1-dependent protection near the Sox motif in the absence of Sox2, likely due to preferential binding of PARP-1 at the nucleosomal dyad axis (Kim et al., 2004) (Fig. 5B, compare lanes 5 and 7). Interestingly, although PARP-1 is also known to contact the linker DNA when binding at the dyad axis (Clark et al., 2012; Kim et al., 2004), binding assays using mononucleosomes without linker DNA showed that linker DNA is not required for PARP-1 to enhance the binding of Sox2 (Fig. 5D). In fact, interactions with Sox2 on nucleosomes may be able to redirect PARP-1 from the linker DNA to the adjacent sites of Sox2 binding on the nucleosome (Fig. S6, A and B).
Figure 5. PARP-1 stabilizes Sox2 binding to nucleosome in vitro.
A) In vitro nucleosome binding assays show PARP-1-dependent binding of Sox2 to nucleosomes, but not naked DNA. Ten nM of biotin-labeled 601 NPE DNA (207 bp) with or without a Sox motif (Sox+ or Sox−, respectively), or the same DNA assembled into a mononucleosome (Nuc), was immobilized on streptavidin beads and incubated ± recombinant Sox2 (10 nM) and PARP-1 (10 nM), as indicated. After washing, the bound proteins were analyzed by Western blotting.
B) In vitro DNase I footprinting assays show PARP-1-dependent binding of Sox2 to nucleosomes, but not naked DNA. Left, Footprinting assay with naked 3x 601 NPE DNA containing a Sox motif. Right, Footprinting assay with a trinucleosome containing a Sox motif located at the dyad axis of the middle nucleosome. Addition of 10 nM WT PARP-1, but not a DNA binding domain mutant PARP-1 (DBDmut), enhances Sox2 (25 nM) binding, as indicated. PARP-1 binding to the nucleosome is also evident.
C) Quantification of the DNase I footprinting assays shown in panel C. The bands marked with a red asterisk in panel B were quantified using a phosphorimager and ImageJ software. Each bar represents the mean plus the SEM, n = 3. Asterisks indicate significant differences versus the control (−Sox2) (Student’s t test, ** p < 0.01 and * p < 0.05).
D) PARP-1 stabilizes Sox2 binding to nucleosomes with or without linker DNA. In vitro nucleosome binding assay, as in panel A, using 601 NPE mononucleosomes with (Nuc 207; 207 bp) or without (Nuc 147; 147 bp) linker DNA, or the corresponding naked DNA (“DNA”).
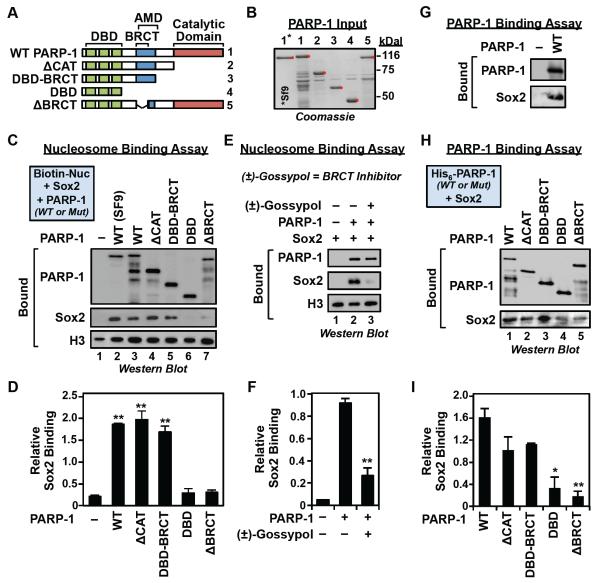
Similar nucleosome binding and footprinting assays using point (Kim et al., 2004) or deletion mutants of PARP-1 (Fig. 6, A and B) demonstrated a requirement for both the PARP-1 DNA binding domain (DBD) (Fig. 5, B and C) and the PARP-1 BRCA1 C-terminus (BRCT) domain for enhanced binding of Sox2 to nucleosomes (Fig. 6, C and D). Neither the PARP-1 DBD nor BRCT domain alone, however, is sufficient to enhance Sox2 binding to nucleosomes (Fig. 6, C and D). The requirement for the PARP-1 BRCT domain was further confirmed using the competitive BRCT domain inhibitor (±)-gossypol (Na et al., 2015), which inhibited PARP-1-dependent Sox2 binding to nucleosomes (Fig. 6, E and F). The PARP-1 BRCT domain was also required for the direct binding of PARP-1 to Sox2 (Fig. 6, G - I). We did not, however, define which domain of Sox2 (i.e., the N-terminal HMG domain or the C-terminal transactivation domain) is required for the direct binding of Sox2 to PARP-1; interactions with either are possible. Nonetheless, these results demonstrate the importance of specific interactions between PARP-1 and Sox2 that underlie the PARP-1-dependent binding of Sox2 to Sox motifs located in nucleosomes.
Figure 6. The BRCT domain of PARP-1 is required for stabilizing Sox2 binding to nucleosomes.
A) Schematics of the PARP-1 deletion mutants used in the nucleosome binding assays.
B) Coomassie blue staining of purified bacterially-expressed wild-type and deletion mutant PARP-1 proteins used in the nucleosome binding assays (lanes 1-5). Purified wild-type PARP-1 protein expressed in Sf9 insect cells is shown for comparison (lane 1*).
C) The PARP-1 BRCT domain is required for PARP-1-dependent binding of Sox2 to nucleosomes. In vitro nucleosome binding assays using various PARP-1 deletion mutants, with biotin-labeled 601 NPE DNA (207 bp) as in Fig. 5A.
D) Quantification of the nucleosome binding assays in panel C. The bands were quantified by densitometry. Each bar represents the mean plus the SEM, n = 3. Asterisks indicate significant differences versus the control (−PARP-1) (Student’s t test, ** p ≤ 0.01 and * p < 0.02).
E) The competitive BRCT domain inhibitor (±)-gossypol blocks PARP-1-dependent Sox2 binding to nucleosomes. In vitro nucleosome binding assays ± 60 M of (±)-gossypol, as in panel C, but using the Position 32 nucleosome shown in Fig. 7B.
F) Quantification of the nucleosome binding assays in panel E. The bands were quantified by densitometry. Each bar represents the mean plus the SEM, n = 3. Asterisks indicate significant differences versus the control (−gossypol) (Student’s t test, ** p ≤ 0.01).
G) PARP-1 interacts with Sox2 protein in vitro. Sox2 was incubated with immobilized PARP-1. Bound material was analyzed by Western blotting for PARP-1 and Sox2, as indicated.
H) The PARP-1 BRCT domain mediates interactions between PARP-1 and Sox2. Sox2 was incubated with immobilized wild-type or deletion mutant PARP-1 proteins, as described in panel A. Bound material was analyzed by Western blotting for PARP-1 and Sox2, as indicated.
I) Quantification of the binding assays in panel H. The Sox2 bands were quantified by densitometry and the signals were normalized to the molarity of the PARP-1 protein immobilized on the beads. Each bar represents the mean plus the SEM, n = 3. Asterisks indicate significant differences versus the control (WT PARP-1) (Student’s t-test, * p = 0.018; ** p = 0.005).
Rotational Phasing of the Sox Motif in the Nucleosome and the Sox2 DNA Binding Domain Determine the PARP-1-Dependence of Sox2 Binding to Nucleosomes
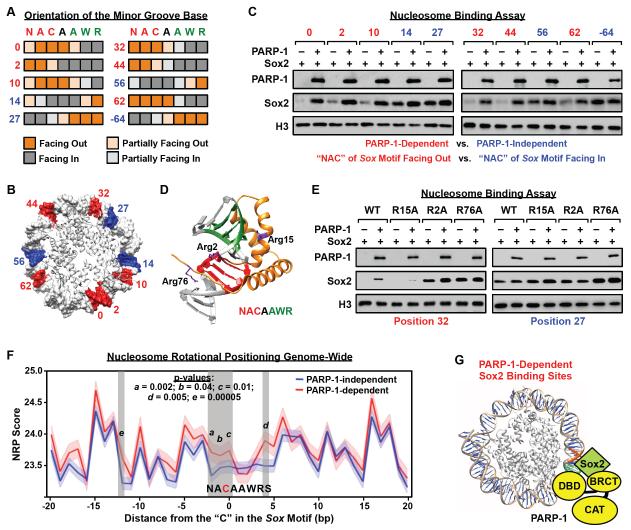
The binding affinity of a transcription factor to its motif in nucleosomal DNA is governed by the rotational phasing of the motif sequence relative to the histone octamer (Cui and Zhurkin, 2010, 2014; Sekiya et al., 2009; Zaret and Carroll, 2011). In this regard, we asked whether the translational position of the Sox motif along the length of the nucleosomal DNA might affect the dependency of Sox2 on PARP-1 for binding to the nucleosome. To answer this question, we created a set of ten 601 NPE-based nucleosomal templates, each with the Sox motif located at a different position in the DNA, leading to different orientations of the minor groove base relative to the histone octamer for each base pair in the motif (i.e., facing in or facing out) (Fig. 7, A and B; Fig S7, A and B). These templates allowed us to examine the relationship between Sox motif rotational positioning in the nucleosome and the PARP-1 dependency of Sox2 binding.
Figure 7. Cooperative interactions with PARP-1 facilitate Sox2 binding to suboptimal sites in nucleosomes.
A) Orientation of the minor groove base relative to the histone octamer for each base pair in the Sox motif in the 601 NPE nucleosome, as determined from high resolution X-ray crystal structures (PBD: 3LZ0, 3LZ1, 3MVD). Heatmaps showing the orientation of each position in the Sox motif (orange shading, minor groove facing away from the histone octamer; gray shading, minor groove facing toward the histone octamer). Letters indicate the nucleotides at each position of the Sox motif (N = A, C, G, or T; W = A or T; R = A or G) and numbers indicate the position of the Sox motif on the nucleosome, as shown in panel B.
B) Position of the “NAC” in the Sox motif mapped on the 601 NPE nucleosome for a series of different constructs where the Sox motif has been moved along the length of the DNA. Red, PARP-1-dependent Sox2 binding site. Blue: PARP-1-independent Sox2 binding site. Only half of the nucleosome is shown. The numbering is based on the distance of the “C” in the first half of the Sox motif to the dyad axis of the nucleosome (the −64 position is not shown).
C) The rotational orientation of the Sox motif determines the PARP-1-dependence of Sox2 binding to nucleosomes. In vitro nucleosome binding assays, with biotin-labeled 601 NPE DNA (207 bp) as in Fig 5A, using a set of nucleosome templates with a Sox motif placed in different translational positions across the nucleosome, as shown in panel B.
D) X-ray crystal structure of Sox2 HMG domain binding to DNA (1GT0) (Remenyi et al., 2003) with the locations of three key arginine residues (Arg2, Arg15, Arg76) indicated (side chains shown in purple).
E) Arginine residues in the HMG domain mediate PARP-1-dependent binding of Sox2 to nucleosomes. In vitro nucleosome binding assays, as in panel C, using a set of Sox2 Arg → Ala mutant proteins (R2A, R15A, R76A). The Sox2 mutants were assayed with two different nucleosome constructs: “position 32” (PARP-1-dependent) and “position 27” (PARP-1-independent).
F) NRP scores associated with PARP-1-dependent and PARP-1-independent Sox2 binding sites in mESCs. See Fig. S7H for an outline of the methods. The x-axis represents the position in nucleotides of the Sox motif associated with the Sox2 binding site, as determined by ChIP-seq, with the “C” in the first half of the Sox motif set to 0. The y-axis represents average NRP score associated with each nucleotide position (dark lines), with the SEM range indicated by lighter shading. Vertical grey shading highlights regions exhibiting a significant difference in NRP score between PARP-1-dependent and PARP-1-independent Sox2 binding sites. Higher NRP scores indicate a greater tendency for the sequence to face away from the histone octamer (see Fig. S7B).
G) Model for Sox2 binding to nucleosomes at PARP-1-dependent sites. PARP-1 interacts with nucleosomal DNA through its DNA binding domain (DBD) and Sox2 through its BRCT motif. PARP-1 stabilizes Sox2 interaction with nucleosomal DNA when the Sox motif sequence is positioned unfavorably along the nucleosome for Sox2 to bind.
See also Fig. S7.
Using the nucleosome binding assay described above with the set of ten nucleosomal templates (Fig. S7C), we observed a striking effect of the position of the Sox motif on the PARP-1-dependence of Sox2 binding. When the first three base pairs of the Sox motif were positioned with their minor groove bases facing towards the histone octamer, Sox2 bound in a PARP-1-independent manner (Fig. 7C, constructs 14, 27, 56, and 64). In contrast, when the first three base pairs of the Sox motif were positioned with their minor groove bases facing away from the histone octamer, Sox2 bound in a PARP-1-dependent manner (Fig. 7C, constructs 0, 2, 10, 32, 44, and 62). A similar effect of PARP-1 on the binding of Oct4 was not observed (Fig. S7D). These results clearly show that the rotational phasing of Sox2 motif in the nucleosome determines the dependency of Sox2 on PARP-1 for binding to nucleosomes. Many of the structural features of nucleosomal DNA are determined by the rotational phasing of the DNA relative to the histone octamer (Cui and Zhurkin, 2010). In fact, many features of the DNA vary in a quantitative way as the DNA wraps around the histone octamer (Fig. S7A). At present, we cannot determine for certain which specific feature (e.g., minor groove width, roll, x-displacement, etc.) is driving the effect.
We also considered the possibility that structural features of the Sox2 DNA binding domain (DBD) might influence the PARP-1 dependence of Sox2 binding to nucleosomes. Previous studies have shown that the binding of arginine residues in the DBD to the minor grooves in DNA is an important mode of protein-DNA recognition for many transcription factors (Abe et al., 2015; Rohs et al., 2009). In this regard, we used the molecular structure of the Sox2 DBD bound to DNA to design Sox2 proteins with mutations at key arginine residues (Arg2, Arg15, and Arg76) (Fig. 7D). We expressed and purified these Sox2 mutants (Fig. S7E) and showed that all three were able to bind to a Sox motif in naked 601 NPE DNA (Fig. S7, F and G), as well as PARP-1 in an in vitro pull-down assay (Fig. S7H) similarly to wild-type Sox2. We then tested the ability of the Sox2 mutants to bind in vitro to nucleosomes with a Sox motif located at a PARP-1-dependent site (Position 32) or a PARP-1-independent site (Position 27). Interestingly, the R15A mutant lost binding at the PARP-1-dependent site, but was unaffected at the PARP-1-independent site (Fig. 7E). In contrast, the R2A and R76A mutants lost PARP-1 dependence at the PARP-1-dependent site, but were unaffected at the PARP-1-independent site (Fig. 7E). These results indicate that specific molecular features of the Sox2 DBD determine PARP-1 dependence of Sox2 binding to nucleosomes.
Our results indicate that PARP-1 facilitates the binding of Sox2 to Sox motifs that are positioned suboptimally in the nucleosome. Interestingly, a similar effect can be discerned for native nucleosomes examined across the genome of mESCs. We identified Sox motifs under Sox2 ChIP-seq peaks located within nucleosomes defined by MNase-seq. We then calculated the nucleosome rotational positioning (NRP) score (Cui and Zhurkin, 2010), which indicates the orientation of DNA with respect to the histone octamer, around each nucleosomal Sox motif identified (Fig. S7I). A higher NRP score indicates a greater tendency for the DNA sequence to face away from the histone octamer (Cui and Zhurkin, 2010) (Fig. S7B). Native nucleosomal PARP-1-dependent Sox2 binding sites exhibited significantly higher NRP scores than PARP-1-independent Sox2 binding sites, most noticeably at the third position of the Sox motif (Fig. 7F; see also Fig. 4, A and B). These results provide a strong link between our observations about the effects of PARP-1 on Sox2 binding to nucleosomes in vitro and with our observations in cells.
Discussion
Recent studies have defined a role for PARP-1 in regulating the biology of ESCs (Doege et al., 2012; Gao et al., 2009; Hemberger et al., 2003; Roper et al., 2014) (Fig. 1A), but the molecular mechanisms of this regulation has been poorly defined. Our genomic, computational, and biochemical studies described herein provide a mechanistic basis for the regulation. Specifically, our studies demonstrate a potent effect of PARP-1 on the binding of Sox2 to a subset of its genomic binding sites, which are suboptimal with respect to the chromatin state, and the sequence and location of the underlying Sox motif in the genomic DNA. Although the PARP-1-dependent Sox2 binding sites represent only 10% of the total, they drive the transcription of key genes (e.g., Nanog), whose expression helps to maintain the pluripotent phenotype of mESCs. Collectively, our results indicate that the previously described pioneer activity of Sox2 is insufficient for binding to a subset of intractable genomic loci.
Structural Features of the Nucleosome, PARP-1, and Sox2 that Determine the PARP-1 Dependence of Sox2 Binding to Nucleosomes
Our genomic studies showed that PARP-1 promotes the binding of Sox2 to inaccessible nucleosomes located in closed regions of chromatin co-occupied by few, if any, partner transcription factors (Fig. 4H). In addition, our biochemical studies showed that PARP-1 helps Sox2 overcome the barrier to binding to Sox motifs that are positioned unfavorably in the nucleosomal DNA (Fig. 7). Using nucleosome binding assays, DNase I footprinting, and protein-protein interaction assays with wild-type and mutant PARP-1 and Sox2 proteins, we determined the structural features of the nucleosome, PARP-1, and Sox2 that determine the PARP-1 dependence of Sox2 binding to nucleosomes. These include the rotational phasing of the Sox motif in the nucleosome, the width of the minor groove, and key arginine residues in the Sox2 DNA binding domain (Arg2, Arg15, and Arg76). PARP-1-dependent binding of Sox2 to the nucleosomes also requires the PARP-1 DBD, which allows PARP-1 to bind to nucleosomal DNA, and the PARP-1 BRCT domain, which promotes interactions between PARP-1 and Sox2 (Fig. 7G).
Our nucleosome binding assays show that: (1) the first half of the Sox motif in PARP-1-dependent Sox2 binding sites preferentially faces out (i.e., away from the histone octamer) (Fig. 7A); (2) PARP-1-dependent Sox2 binding sites have wider minor grooves than PARP-1-independent Sox2 binding sites (Fig. S7A); and (3) selected point mutations in the Sox2 DBD can alter the PARP-1 dependence of Sox2 at PARP-1-dependent Sox2 binding sites (Fig. 7E). Together, these results suggest that PARP-1 may help Sox2 overcome the barrier to binding caused by the widening of the minor groove at outward-facing Sox motifs as the DNA wraps around the nucleosome. DNA binding by PARP-1 may act to reduce the effective minor groove width or stabilize the complex to allow Sox2 binding. Further analysis will require a molecular structure. Our results illustrate how PARP-1 can act at the level of the nucleosome, in concert with structural features of the nucleosomes and a transcription factor DNA binding domain, to produce global effects on transcription factor binding that drive biologically important gene expression outcomes.
Role of PARP-1 PARylation in regulating chromatin association of Sox2
Previous studies exploring the role of PARP-1 in gene regulation have defined both catalytic-dependent and catalytic -independent functions for PARP-1 (Hottiger, 2015; Krishnakumar and Kraus, 2010a). In this study, we showed that PARP-1 can function in undifferentiated mESCs independent of its ADP-ribosyltransferase activity to promote the binding of Sox2 to nucleosomes. Three lines of evidence support the conclusion that PARP-1 enzymatic activity is not required for these effects: (1) PAR levels are nearly undetectable in undifferentiated mESCs, (2) inhibition of PARP-1 activity using a chemical inhibitor fails to elicit the same gene regulatory effects as PARP-1 depletion, (3) withholding NAD+ or deleting PARP-1’s catalytic domain has no affect on PARP-1’s ability to promote Sox2 binding to a nucleosome. Interestingly, PARP-1 PARylation activity dramatically increases when mESCs undergo differentiation (Fig 1B), which may be an important feature of differentiation in certain lineages. Enhanced PARylation of protein targets by PARP-1 during differentiation may use a different mechanism to regulate Sox2. In this regard, PARylation of Sox2 has been reported to promote Sox2 degradation in differentiating ES cells (Gao et al., 2009). In addition, increased PARylation by PARP-1 during differentiation, likely through an automodification reaction, decreases the affinity of PARP-1 for chromatin, while increasing the affinity of PARP-1 for Sox2, which may inhibit the interaction of Sox2 with chromatin (Lai et al., 2012). Therefore, under different cell states, variable PARP-1 catalytic activity can have different effects on the same target protein. This adds an additional layer to the regulatory functions of PARP-1 in response to different cellular cues. Since PARP-1 acts as a critical component or target of many cellular signaling pathways (Hottiger, 2015; Krishnakumar and Kraus, 2010a), PARP-1 may connect environmental signals to the Sox2-dependent gene regulatory program to control cell fates.
Implications for Sox2 Pioneer Activity
The manner, mechanisms, and extent to which Sox2 and other TFs (e.g., FoxA1) function as pioneer TFs for genome accessibility has been debated in the recent literature (Franco et al., 2015; Iwafuchi-Doi et al., 2016; Soufi et al., 2015; Swinstead et al., 2016; Zaret et al., 2016). The arguments have focused on (1) an inability of pioneer TFs to access some sites in the genome, (2) cooperative binding with other TFs at other sites, and the (3) the fraction of ‘pioneer-dependent’ TF binding sites that actually require the pioneer TF (e.g., nuclear receptors with FoxA1) (Zaret et al., 2016). Our results provide a demonstration of the range of determinants that can impact how and when Sox2 binds to a nucleosome (i.e., key residues in the Sox2 DNA binding domain; rotational position of the Sox motif in nucleosome; accessory proteins, such as PARP-1). Our results indicate that Sox2 pioneer activity may be ‘conditional’ and not equivalent at all genomic loci. Related effects have been observed with FoxA1, which gains access to previously inaccessible sites in the genome in response to TNFα signaling (Franco et al., 2015). Collectively, our results provide insight on the molecular and genomic mechanisms by which Sox2 and related TFs function as pioneer TFs to control physiologically-relevant gene expression programs.
STAR★Methods
Detailed methods are provided in the online version of this paper. [See the separate files containing the Key Resources Table and Table S1].
CONTACT FOR REAGENT AND RESOURCE SHARING
EXPERIMENTAL MODEL AND SUBJECT DETAILS
- METHOD DETAILS
-
○Culture and Differentiation of Mouse Embryonic Stem Cells (mESCs)
-
○Antibodies and Other Reagents
-
○Analysis of Protein and PARylation Levels by Western Blotting
-
○In Vitro PARylation Assays
-
○Rescuing Nanog Expression Using a MEK Inhibitor
-
○RNA Extraction and RT-qPCR
-
○RNA-seq Library Preparation
-
○Chromatin Immunoprecipitation and ChIP-qPCR
-
○ChIP-seq Library Preparation
-
○Protein Co-immunoprecipitation Assays
-
○Expression and Purification of Recombinant PARP-1 and Sox2
-
-Purification of proteins expressed in insect cells
-
-Purification of proteins expressed in bacteria
-
-
-
○PARP-1-Sox2 Interaction Assays
-
○Nucleosome Assembly
-
-Assembly of trinucleosomes
-
-Assembly of mononucleosome
-
-
-
○DNase I Footprinting Assays
-
-Footprinting with trinucleosomes
-
-Footprinting with mononucleosome
-
-
-
○Sox2 Nucleosome Binding Assays
-
○Sox2 Electrophoretic Mobility Shift Assays (EMSAs)
-
○Oligonucleotide Sequences for PCR, RT-qPCR, and ChIP-qPCR
-
○
- QUANTIFICATION AND STATISTICAL ANALYSIS
-
○Analysis of mRNA-seq Data
-
○ChIP-seq Data Analysis
-
-Read alignment and peak calling
-
-Generating a correlation matrix of transcription and chromatin factor enrichment
-
-Heatmaps
-
-Box plots
-
-Determining changes in Sox2 binding
-
-
-
○Additional Genomic Data Analyses
-
-Relationship between Sox2 binding and gene expression
-
-Motif analyses
-
-Analysis of transcription factors located near Sox2 binding sites
-
-MNase-seq and DNase-seq data analyses
-
-Predicting nucleosome phasing of Sox motifs by calculating nucleosome rotational positioning (NRP) scores
-
-
-
○Annotation of Chromatin States using ChromHMM
-
○Feature Selection for Predicting the PARP-1 Dependence of Sox2 Genomic Occupancy
-
○Determining DNA Shape Parameters
-
○
- DATA AND SOFTWARE AVAILABILITY
-
○Genomic Data Sets
-
○Custom Scripts
-
○
CONTACT FOR REAGENT AND RESOURCE SHARING
As Lead Contact, W. Lee Kraus is responsible for all reagent and resource requests. Please contact W. Lee Kraus at Lee.Kraus@utsouthwestern.edu with requests and inquiries. Raw and processed genomic data have been made publicly available through the NCBI’s GEO portal, as described below. Computing scripts and the initial output from computational analyses are available on request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Parp1+/+ (wild-type; WT) and Parp1−/− (PARP-1 KO) mESCs were kindly provided by Dr. Zhao-Qi Wang (Leibniz Institute on Aging, Fritz Lipmann Institute, Jena, Germany) (Gao et al., 2009; Yang et al., 2004).
METHOD DETAILS
Culture and Differentiation of Mouse Embryonic Stem Cells (mESCs)
Parp1+/+ (wild-type; WT) and Parp1−/− (PARP-1 KO) mESCs (Gao et al., 2009; Yang et al., 2004) were maintained on a feeder layer in mESC growth medium: Dulbecco’s modified Eagle’s medium (Gibco; 11965) containing 2 mM L-glutamine (Sigma-Aldrich), supplemented with 15% (v/v) FBS (Atlanta Biologicals; S12450), 0.1 mM nonessential amino acids (Sigma-Aldrich; M7145), 1 mM sodium pyruvate (Invitrogen; 11360070), 1000 units/mL mLIF (Millipore; ESG1106), penicillin and streptomycin (Invitrogen; 15140), and 0.1 mM β-mercaptoethanol (Sigma-Aldrich). The cells were passaged twice to eliminate feeder cells before being used in experiments. To differentiate the mESCs into embryoid bodies, the cells were separated from the feeder layer before culturing in 10 cm diameter ultra low attachment plates at a density of 4 × 106 cells per plate in mESC growth medium containing 15% (v/v) FBS without mLIF. The medium was changed every other day prior to collection of the cells for experiments.
Antibodies and Other Reagents
The antibodies used for Western blotting, co-IP, and/or ChIP were as follows: PARP-1 (previously characterized custom rabbit polyclonal antibody (Kim et al., 2004); now available from Active Motif; 39561), PAR (Trevigen; 4335-AMC), Sox2 (Santa Cruz; sc-17320), Oct4 (Santa Cruz; sc-8628), Nanog (Abcam; ab8092), H3K4me3 (Active Motif; 3915), H3K27me3 (Millipore; 170622), β-actin (Sigma-Aldrich; A5316), Flag (Sigma-Aldrich; F3165), histone H3 (Abcam; ab1791).
Purified human Sox2 protein was purchased from Abcam (ab169843). The competitive BRCT domain inhibitor, (±)-gossypol (Na et al., 2015), was purchased from Sigma (G8761).
Analysis of Protein and PARylation Levels by Western Blotting
Whole cell extracts (WCEs) were prepared from mESCs using cell lysis buffer [50 mM Tris-HCl pH 7.5, 0.5 M NaCl, 1.0 mM EDTA, 1% NP-40, and 10% glycerol, 1x protease inhibitor cocktail (Roche)] containing 1 μM PJ34 (to inhibits PARP activity) and 100 μM tannic acid (to inhibit PARG activity). After clarification of the WCEs by centrifugation, aliquots containing equal amounts of total protein, as determined by a BCA assay (Pierce), were run on 8 to 10% polyacrylamide-SDS gels, transferred to nitrocellulose membrane, and subjected to Western blotting using the antibodies listed in the Key Resources Table and a chemilumenescent detection system (Thermo scientific).
In Vitro PARylation Assays
The in vitro PARylation assays in Fig.S1B were performed using of nuclear extract from E14Tg2a mESCs subjected to PARP-1 knockdown. Five g of nuclear extract were incubated with 1 g of recombinant PARP-1 protein in a 25 L reactions containing 50 mM Tris-HCl, pH 8.0, 4 mM MgCl2, 250 M DTT, 1x protease inhibitor cocktail, and 250 nM 32P-NAD+ for 30 min at 30 °C. The reactions were then separated by SDS-PAGE. The extent of PARP-1 auto(ADP-ribosyl)ation was analyzed using a phosphorimager (Bio-Rad). Input PARP-1 protein was assessed by staining with SDS-PAGE followed by staining using Coomassie Blue.
Rescuing Nanog Expression Using a MEK Inhibitor
To rescue Nanog expression, Parp1−/− mES cells were treated with 3 M of the MEK inhibitor PD184352 for 72 hours (Silva et al., 2009). The cells were then collected and subjected to Western blotting (for Nanog and β-actin protein levels) or RT-qPCR (for Nanog, Pou5f1, Gata6, and Sox17 mRNA expression levels).
RNA Extraction and RT-qPCR
RNA isolation and RT-qPCR were performed using a standard protocol, as previously described (Hah et al., 2011). Briefly, total RNA was extracted from mESCs using Trizol reagent (Life Technologies) following the manufacturer’s instructions, reverse transcribed, and subjected to qPCR using the gene-specific primers listed in Table S1. Unless specified, all target gene expression levels were normalized to β-actin mRNA. All RT-qPCR assays were performed a minimum of three times to ensure reproducibility.
RNA-seq Library Preparation
Total RNA was isolated as described above. The integrity of the RNA was assessed and verified using an Experion Automated Electrophoresis System (Bio-Rad) before mRNA-seq libraries were prepared using methods described previously (Zhong et al., 2011). Briefly, polyA+ RNA was enriched using Dynabeads oligo(dT)25 (Invitrogen), heat fragmented, and reverse transcribed using random hexamers in the presence of dNTPs. Second strand cDNA synthesis was performed with dNTPs, but replacing dTTP with dUTP. After end-repair, dA-tailing, ligation to adaptors containing barcode sequences, and size selection using AMPure beads (Agencourt), the synthesized second-strand was digested using uracil DNA glycosylase (Enzymatics). A final PCR reaction was performed using Phusion high-fidelity DNA polymerase (NEB). After library quality control assessment using a Bioanalyzer (Agilent), the samples were subjected to 50 bp sequencing using an Illumina HiSeq 2000 Sequencing System.
Chromatin Immunoprecipitation and ChIP-qPCR
mESCs were passaged twice without a feeder layer and then grown in gelatin-coated plates to ~70 to 80% confluence. The cells were cross-linked using 1% paraformaldehyde at room temperature for 10 min., followed by quenching in 125 mM glycine for 5 min. at 4°C. The crosslinked cells were collected, and the nuclei were released by gentle pipetting three times in lysis buffer [10 mM Tris-HCl pH 7.5, 2 mM MgCl2, 3 mM CaCl2, 0.5% NP-40, 10% glycerol, 1 mM DTT, 1x protease inhibitor cocktail (Roche)]. The nuclei were collected by gentle centrifugation and resuspended in sonication buffer [1x PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1x protease inhibitor cocktail (Roche)]. The nuclei were then incubated in sonication buffer on ice for 10 min., and sonicated at 4°C using a Biorupter (Diagenode) on the high setting, three cycles of 5 min. sonication (30 seconds on/30 seconds off) with 5 min. intervals) to generate genomic DNA fragments of 200 - 500 bp in length. The sonicated chromatin was clarified by centrifugation and pre-cleared with agarose beads.
Aliquots of the pre-cleared chromatin were immunoprecipitated with various antibodies at 4°C overnight, followed by collection of the immunoprecipitates using protein A (Millipore) or G (Invitrogen) agarose beads (protein A beads for H3K4me3, H3K27me3, and PARP-1 antibodies; protein G beads for Sox2 and Oct4 antibodies). The beads were collected by gentle centrifugation and washed in low salt wash buffer (20 mM Tris-HCl pH 7.9, 2 mM EDTA, 125 mM NaCl, 0.05% SDS, 1% Triton X-100, 1x protease inhibitor cocktail), high salt wash buffer (low salt wash buffer containing 500 mM NaCl), and LiCl wash buffer (10 mM Tris-HCl pH 7.9, 1 mM EDTA, 250 mM LiCl, 1% NP-40, 1% sodium deoxycholate, 1x protease inhibitor cocktail). The immunoprecipitated genomic DNA was eluted and the crosslinks were reversed by incubation in elution buffer (100 mM NaHCO3, 1% SDS) at 65°C overnight. The genomic DNA was then deproteinized by digestion with proteinase K and extraction with phenol:chloroform:isoamyl alcohol, followed by precipitation with ethanol. The ChIPed DNA was then subjected to qPCR using the locus-specific primers listed in Table S1. All ChIP-qPCR assays were performed a minimum of three times to ensure reproducibility.
ChIP-seq Library Preparation
Approximately 50 ng of ChIPed DNA (quantified using a NanoDrop) was used to prepare each ChIP-seq library using methods described previously (Franco et al., 2015). Briefly, the genomic DNA fragments were end-polished, dA-tailed, and ligated to Y-adaptors containing barcode sequences. After agarose gel-based size selection and purification, the DNA was amplified for 13 - 15 cycles by PCR using Phusion high-fidelity DNA polymerase (NEB). The final ChIP-seq libraries were subjected to quality control assessment using a Bioanalyzer (Agilent), followed by 50 bp sequencing using an Illumina HiSeq 2000 Sequencing System.
Protein Co-immunoprecipitation Assays
To explore potential interactions of PARP-1 with Sox2 and Oct4, HEK293T cells were transiently transfected with a plasmid for expressing Flag-tagged PARP-1 together with a plasmid for expressing either Sox2 or Oct4. The cells were collected and lysed by incubating on ice for 30 min in lysis buffer [25 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 10% glycerol, 1x protease inhibitor cocktail (Roche)] with intermittent gentle inversion. After clarification of the lysates by centrifugation, aliquots containing equal amounts of protein were subjected to PARP-1 co-immunoprecipitation in the same buffer by adding 5 μl Flag monoclonal antibody (Sigma-Aldrich) and incubating in 4°C overnight. The immunoprecipitates were collected by incubation with protein A/G agarose beads for 2 hours at 4°C. The beads were collected by gentle centrifugation, washed three times using wash buffer [25 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5% NP-40, 10% glycerol, 1x protease inhibitor cocktail (Roche)], and boiled in SDS-PAGE loading solution. The eluted immunoprecipitates were run on 8% polyacrylamide-SDS gels, transferred to nitrocellulose, and subjected to Western blotting using antibodies to PARP-1, Sox2, and Oct4 and a chemilumenescent detection system (Thermo Scientific).
Expression and Purification of Recombinant PARP-1 and Sox2
Purification of proteins expressed in insect cells
Flag-tagged human PARP-1 proteins (wild-type and DBD point mutant) were expressed in insect cells using a baculovirus expression system and purified by using Flag-affinity purification, as described previously (Kim et al., 2004). A similar approach was used for mouse Sox2 proteins (wild-type and DBD point mutants). Briefly, Sf9 cells infected with a baculovirus vector for expression of recombinant PARP-1 were collected, resuspended in lysis buffer (20 mM Tris-HCl pH 7.5, 0.5 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20% glycerol, 2 mM DTT, 1 mM PMSF, 20 μg/ml leupeptin, 20 μg/ml aprotinin), and homogenized by douncing 15 times on ice using a tight pestle. After clarifying the lysate by centrifugation, the supernatant was collected and mixed with an equal volume of dilution buffer (20 mM Tris-HCl pH 7.5, 1.5 mM MgCl2, 0.2 mM EDTA, 10% glycerol, 2 mM DTT, 1 mM PMSF, 20 μg/ml leupeptin, 20 μg/ml aprotinin). The diluted supernatant was mixed with α-Flag M2 affinity resin (Sigma-Aldrich) and incubated for 3 hours at 4°C. The resin was then collected by gentle centrifugation and washed four times in wash buffer (20 mM Tris-HCl pH 7.5, 1 M NaCl, 10% glycerol, 0.2 mM EDTA, 1.5 mM MgCl2, 0.2% IGEPAL, 2mM DTT, 1 mM PMSF; the high salt concentration removes nucleic acids that are bound by the PARP-1 protein. Purified PARP-1 was eluted using elution buffer (20 mM Tris-HCl, 100 mM NaCl, 15% glycerol, 0.2 mM EDTA, 2 mM DTT, 0.2 mg/ml Flag peptide, 0.5 mg/ml recombinant human insulin), quantified using a BCA assay (Pierce), and stored in aliquots at −80°C. Flag-tagged human Sox2 was expressed in Sf9 cells and purified using the same method as described above for PARP-1.
Purification of proteins expressed in bacteria
His-tagged human PARP-1 proteins (wild-type and deletion mutants) were expressed in bacteria (BL21 cells) using a plasmid for IPTG-inducible expression (Wacker et al., 2007). Bacteria transformed with the expression plasmid were cultured to an OD of 0.4 and induced with 1 mM IPTG for 2 hours at 37°C. The cell pellets were collected by centrifugation and the cells were lysed using lysis buffer (10 mM Tris-HCl pH 7.5, 0.5 M NaCl, 0.5 mM EDTA, 0.1% NP-40, 10% glycerol, 10 mM imidazole; 1 mM PMSF, 2 mM DTT, 20 μg/ml leupeptin, 20 μg/ml aprotinin) with sonication. After clarification by centrifugation, the cell lysates were incubated with Ni-NTA agarose beads (Qiagen) at 4°C for 2 hours, followed by multiple washes with wash buffer (10 mM Tris-HCl pH 7.5, 1 M NaCl, 0.2% NP-40, 10% glycerol, 10 mM imidazole, 1 mM PMSF). The purified proteins were eluted using elution buffer (10 mM Tris-HCl pH 7.5, 200 mM NaCl, 0.1% NP-40, 10% glycerol, 500 mM imidazole, 1 mM PMSF, 2 mM DTT), dialyzed into elution buffer without imidazole, and concentrated using a centrifugal concentrator (Millipore). The purified proteins were quantified using a BCA assay (Pierce) and stored in aliquots at −80°C.
PARP-1-Sox2 Interaction Assays
Three μg of 6xHis-tagged human PARP-1 protein purified from bacteria was incubated with 1 μg of mouse Sox2 protein (purified from SF9 cells) in 1 mL of binding buffer (20 mM HEPES pH 7.9, 150 mM NaCl, 0.2 mM EDTA, 20% glycerol, 0.1% NP-40, 0.1 ng/μL sonicated salmon sperm DNA, 1 mM DTT, and 1x complete protease inhibitor cocktail) for 2 h at 4°C in the presence of 30 μl of Ni-NTA agarose beads (Qiagen). After 3 washes each with 1 mL of binding buffer, the beads were collected and boiled in 2x SDS loading dye. The eluted material was analyzed by Western blotting.
Nucleosome Assembly
Assembly of trinucleosomes
Core histones were prepared from HeLa cells as described previously (Kim et al., 2004). To prepare a DNA template for the assembly of trinucleosomes, similar to a template described previously (Clark et al., 2012; Muthurajan et al., 2014), a pGEM1-based plasmid carrying three tandem copies of the 601 nucleosome positioning element (NPE) (Lowary and Widom, 1998) with the middle NPE containing a natural composite Pou/Sox motif from the Nanog gene (sequence 5′-TTTTGCATTACAATG-3′; red, Pou; blue, Sox), was amplified in E. coli and purified using the PureLink HiPure plasmid filter maxiprep kit (Invitrogen). The purified plasmid DNA was digested using the EcoRV restriction enzyme to release the three NPE cassette with blunt ends, followed by selective precipitation of the plasmid backbone using 5% PEG and 10 mM MgCl2. The three NPE cassette was then precipitated using 10% PEG and 10 mM MgCl2, collected by centrifugation, and dissolved in 1x TE. To prepare a DNA template for the assembly of mononucleosomes, a DNA fragment containing the 601 NPE with or without a Sox2 binding site plus 30 bp of flanking linker DNA sequence was amplified by PCR. The Amplified DNA was subjected to phenol-chloroform extraction, followed by ethanol precipitation. Tri-nucleosomes and mono nucleosomes were assembled by salt gradient dialysis as described (Utley et al., 1997). The efficiency of assembly and the quality of assembled nucleosomes were checked by running aliquots of the assembled product on native 4% PAGE gels run in 0.25x TBE buffer, followed by staining with SYBR Gold (Life Technologies).
Assembly of mononucleosomes
Mononucleosomes with 5′ biotinylated DNA were assembled by salt gradient dialysis as described above using 601 NPE DNA (with or without a composite Pou/Sox motif) that was amplified by PCR with 5′ biotinylated primers (Sigma-Aldrich).
DNase I Footprinting Assays
Footprinting with trinucleosomes
Sox2 binding to naked DNA or the reconstituted trinucleosome templates described above was assayed by DNase I footprinting. For these assays (see Fig. 5B), 46 ng of the three NPE cassette as naked DNA or reconstituted into trinucleosomes was incubated with 25 nM of Sox2 protein (purchased from Abcam; ab169843) in the presence or absence of 10 nM of purified PARP-1 protein in a 20 μL reaction for 1 hour at 30°C under the following buffer conditions: 15 mM Tris-HCl pH 7.5, 0.3 mM EDTA, 0.2 mM DTT, 2% glycerol, 50 mM NaCl, 150 ng/μL BSA, 1x protease inhibitor cocktail (Roche). The DNA was digested by the addition of 0.1 unit (for naked DNA) or 1 unit (for nucleosomal DNA) of amplification grade DNase I (Invitrogen) for 5 min. at 25°C. Digestion was stopped by addition of an equal volume of DNaseI stop solution containing 20 mM Tris-HCl pH 7.5, 50 mM EDTA, 2% SDS, 0.2 mg/ml proteinase K, 300 ng/μL glycogen with incubation for 1 hour at 55°C. The digested DNA was extracted with phenol:chloroform:isoamyl alcohol, precipitated with ethanol, dissolved in 1x TE, and subjected to 10 cycles of primer extension using a 32P end-labeled primer that anneals to the linker region (5′-CCATGGAAGCTTCAGGTCACAGTGCTCGAG-3′). The resulting DNA fragments were run on an 8% PAGE-urea buffer-gradient gel in TBE. The gel was dried, exposed to a phosphorimager screen, and visualized using PharosFX system (Bio-Rad).
Footprinting with mononucleosomes
Sox2 binding to the reconstituted mononucleosome templates described above was assayed by DNase I footprinting. For these assays (see Fig. S6), mononucleosomes corresponding to 375 ng of DNA were incubated with 300 ng of Sox2 protein with or without 600 ng of His-tagged human PARP-1 in 50 μL of binding buffer (25 mM HEPES pH 7.5, 100 mM KCl, 20% glycerol, 0.1% NP-40, 10 μM ZnSO4, 1 mM DTT, 1x complete protease inhibitor cocktail) for 1 hour at 30°C, followed by the addition of 5 U of DNaseI (Worthington; DPFF grade, diluted in 50 μL of digestion buffer containing 10 mM MgCl2 and 5 mM CaCl2) and incubated at 25°C for 5 min. The digestion reactions were stopped by the addition of 100 μL of DNaseI stop solution (20 mM Tris-HCl pH 7.5, 50 mM EDTA, 2% SDS, 0.2 mg/ml proteinase K, 300 ng/μL glycogen) with incubation for 1 hour at 55°C. The digested DNA was then extracted with phenol:chloroform:isoamyl alcohol, precipitated with ethanol, dissolved in 1x TE, and subjected to 9 cycles of primer extension using an Alexa 488 fluorescent end-labeled primer that anneals near the edge of the nucleosome (5′-CTGGAGAATCCCGGTGCCGAGGCC-3′). The resulting DNA fragments were run on an 8% PAGE denaturing buffer-gradient gel in TBE. The gel was dried, exposed to a phosphorimager screen, and visualized using PharosFX system (Bio-Rad).
Sox2 Nucleosome Binding Assays
Nucleosome binding “pulldown” assays were performed as previously described (Bartke et al., 2010), with slight modification. Briefly, 10 nm of biotinylated mononucleosomes were immobilized on 30 μl of Dynabeads Streptavidin MyOne T1 (Invitrogen) in reconstitution buffer (10 mM Tris-HCl pH 7.5, 250 mM KCl, 1 mM EDTA, 0.1 % NP-40, 1 mM DTT) and incubated with 10 nM of Sox2 protein purchased from Abcam (Abcam; Ab169843) or purified from Sf9 cells as describe above with or without 10 nM of His-tagged human PARP-1 in 1 ml of binding buffer (20 mM HEPES pH 7.9, 150 mM NaCl, 0.2 mM EDTA, 20% glycerol, 0.1% NP-40, 1 mM DTT, and 1x complete protease inhibitor cocktail) for 2 h at room temperature. After 3 washes each with 1 ml of binding buffer, the beads were boiled in 2x SDS loading dye and the proteins were analyzed by Western blotting. In some cases, the Sox2 nucleosome binding assays were performed in the presence of 60 M of the BRCT inhibitor (±)-gossypol from Sigma-Aldrich (Na et al., 2015).
Sox2 Electrophoretic Mobility Shift Assays (EMSAs)
EMSAs were performed using an Alexa 488 fluorophore-labeled DNA probe containing a Sox motif, which were generated by PCR using fluorescently-labeled primers (Sigma-Aldrich). For each binding reaction, 50 nM of labeled probe were incubated with purified recombinant Sox2 protein in the following reaction conditions: 50 mM Tris-HCl, pH 7.5, 100 mM KCl, 50 mM NaCl, 10 mM MgCl2, 0.1% NP40, 1 mg/ml BSA, 5% Glycerol, 5 mM DTT, and 1 mg/ml poly-dIdC at room temperature for 30 min. Free DNA and Sox2-bound DNA were separated on 5% non-denaturing PAGE gels and visualized using a phosphorimager (Bio-Rad).
Oligonucleotide Sequences for PCR, RT-qPCR, and ChIP-qPCR
Please refer to Table S1 for oligonucleotide sequences.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis of mRNA-seq Data
mRNA-seq reads were subjected to quality-control using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), trimmed to remove adapter sequences, and aligned to the mouse reference genome (mm9) using Tophat (Trapnell et al., 2009) before transcript assembly using Cufflinks (Trapnell et al., 2010). The data were then converted to wiggle (WIG) file format using PeakRanger (Feng et al., 2011) for visualization using the Integrative Genomics Viewer (IGV2.3) (Robinson et al., 2011). Cuffdiff (Trapnell et al., 2010) was used to identify genes that showed significant differential regulation upon PARP-1 depletion, with a false discovery rate (FDR) cutoff of 5%. The expression data were visualized in heatmaps using Java TreeView (Saldanha, 2004), ranked in order based on the log2 of the PARP-1-1 KO to WT RPKM ratio.
We used the DAVID bioinformatics tool (Huang et al., 2007) for gene ontology analysis, with the PARP-1 positively-regulated and negatively-regulated gene sets as input. Enriched GO terms were ranked by p-value and the top 10% of enriched terms are listed in Fig. S2C. The GO terms were arranged based on germ layer categories.
We used gene set enrichment analysis (GSEA) tools developed by the Broad Institute for the GSEA analyses (http://www.broadinstitute.org/gsea/index.jsp).
ChIP-seq Data Analysis
Read alignment and peak calling
ChIP-seq reads were subjected to quality-control using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), trimmed to remove adapter sequences, and aligned to the mouse reference genome (mm9) using Bowtie 0.12.7 (Langmead et al., 2009), allowing for one mismatch during alignment. Significant peaks of PARP-1 enrichment in WT mESCs were called using MACS 1.4.2 (Zhang et al., 2008), with a p-value cutoff of 0.001. Significant peaks of Sox2 and Oct4 enrichment in WT mESCs were called using PeakRanger 1.1 (Feng et al., 2011) with a p-value cutoff of 1 × 10−7. Genomic DNA isolated from sonicated chromatin without immunoprecipitation was used as an input control for identifying regions of enrichment. The data were then converted to wiggle (WIG) file format using PeakRanger (Feng et al., 2011) for visualization using the Integrative Genomics Viewer (IGV2.3) (Robinson et al., 2011).
Generating a correlation matrix of transcription and chromatin factor enrichment
For each of the 13 transcription- and chromatin-related factors included in this analysis (see Fig. 1E), significantly enriched regions were called using MACS and merged to generate a total number of 87,487 genomic loci showing enrichment for at least one factor. Read counts for each factor at the 87,487 loci were determined and normalized to (1) the total number of aligned reads for that factor and (2) the length of each locus. Pearson correlation coefficients for all possible pairwise comparisons of factors were calculated based on the normalized reads, which were further converted to distance values. A larger distance value represents a lower correlation. Hierarchical clustering based on the distance values was performed using the “hclust” function in R, using the complete linkage method. The factors in the correlation matrix were ordered based on hierarchical clustering and the correlation matrix was visualized using Java TreeView (Saldanha, 2004).
Heatmaps
ChIP-seq read densities were visualized in heatmaps using Java TreeView (Saldanha, 2004). For the heatmaps of H3K4me3 and H3K27me3, we determined the read densities in a 10 kb window (± 5 kb) around the transcription start sites (TSSs) of the PARP-1 regulated genes, using a 20 bp moving window. For the heatmaps of Sox2 and PARP-1, we determined the read densities for Sox2 in a 4 kb window (± 2 kb) around the Sox2 peak summit using a 20 bp moving window. Corresponding PARP-1 read densities in the corresponding regions were determined for the same windows and plotted in the same order as the Sox2 data.
Box plots
Read intensities for H3K4me3 and H3K27me3 in a 1 kb window (± 0.5 kb) around the TSSs of RefSeq genes were determined and plotted using the box plot function in R.
Determining changes in Sox2 binding
Sox2 binding sites showing altered enrichment upon PARP-1 depletion were identified by mining the Sox2 peaks identified using PeakRanger in WT mESCs. The read counts across those peaks in both replicates of the Sox2 ChIP-seq data from the WT and PARP-1 KO samples were determined for a 400 bp region (± 200 bp) around the peak summit. Read counts were further normalized to the total reads in sample. Sox2 and Oct4 binding sites significantly changed upon PARP-1 depletion were identified using edgeR (Robinson et al., 2010) with a p-value cutoff of 0.01. To define PARP-1-independent Sox2 binding sites, Rc (Reads change) values of all Sox2 peaks were calculated using the following formula:
where Tp and Tw represent the binding intensity in the PARP-1 KO and WT samples, respectively (Franco et al., 2015). Peaks with the highest tercile of Rc values were defined as PARP-1-independent binding sites.
Additional Genomic Data Analyses
Relationship between Sox2 binding and gene expression
To identify genes possibly regulated by adjacent Sox2 binding sites, each Sox2 binding site was assigned to the nearest RefSeq gene TSS within 10 kb (“associated genes”). For the GSEA analysis (Mootha et al., 2003; Subramanian et al., 2005), we used genes associated with PARP-1-dependent Sox2 binding sites and their associated expression profiles from both replicates of WT and PARP-1 KO RNA-seq data. An equal number of randomly selected genes were used as control. Gene set enrichment analysis (GSEA) tools developed by broad institute were used for the GSEA analyses (http://www.broadinstitute.org/gsea/index.jsp).
Motif analyses
To identify the occurrence of sox motifs under Sox2 ChIP-seq peaks, we used the DNA sequences in 100 bp (± 50 bp) regions around the Sox2 peak summits as input in a directed search for the consensus sox motif ACAAWRS using FIMO in the MEME suite (Bailey et al., 2009) with a p-value cutoff of 0.01. When multiple ACAAWRS motifs were present in one peak, the motif with the lowest p-value and least number of mismatches was assigned to the peak. When multiple motifs with the same p-value and number of mismatches were found in one peak, the motif nearest to the peak summit was chosen.
To identify Sox2 peaks associated with the Sox/Pou motif, we performed a directed search for the pou motif ATGCWRA in regions 30 bp upstream and downstream of the sox motifs assigned to each Sox2 peak, with a p-value cutoff of 0.01 used to determine the association of a pou motif with the respective peak. The location of the Sox/Pou motif center within the same peak was then calculated. Optimal Sox/Pou motifs were defined as those sox and pou motifs are on opposite strands and are separated by 1 bp.
Analysis of transcription factors located near Sox2 binding sites
Nanog, Tcf3, Klf4, ERRβ, and Stat3 ChIP-seq data sets from mESCs were obtained from public data repositories (see below). The data were aligned and peaks called as described above for Sox2 and Oct4. Those transcription factors with peak summit distances <100 bp for a Sox2 peak summit were defined as “colocalized” with Sox2 at that locus. For the heatmap showing the extent of colocalization with Sox2, we generated a binary matrix, where “0” indicates that there was no colocalization of the transcription factor with Sox2, while “1” indicates that there was colocalization. The Sox2 binding sites were ranked by hierarchical clustering using Cluster (Eisen et al., 1998), with an uncentered correlation similarity metric using the single linkage method. Java TreeView (Saldanha, 2004) was used for visualization.
MNase-seq and DNase-seq data analyses
MNase-seq data (Li et al., 2012) and DNaseseq data (2012) from mESCs were obtained from public data repositories (see below). In both cases, the sequencing reads were aligned to the mouse reference genome (mm9) using Bowtie 0.12.7 (Langmead et al., 2009),. For the MNase-seq data, uniquely aligned reads were used to call nucleosome occupancy using DANPOS-2.1.2 (Chen et al., 2013). Metaplots of nucleosome occupancy were generated by calculating the average nucleosome density for a 4 kb (± 2 kb) region around all Sox2 peak summits using a moving window of 20 bp. For the DNase-seq data, the metaplots were generated by calculating the average read counts for a 4 kb (± 2 kb) region around all Sox2 peak summits using a moving window of 20 bp.
Predicting nucleosome phasing of Sox motifs by calculating nucleosome rotational positioning (NRP) scores
To calculate NRP scores based on DNA sequences, Sox motifs associated with Sox2 binding sites from ChIP-seq data (within 20 bp of the Sox2 peak) were searched as described above. The Sox motifs located within a nucleosome, as determined from MNase-seq data, were then selected for further analysis. To calculate the NRP score of a particular nucleotide at position n, we took the DNA sequence from n−73 to n+73 into consideration. We then defined 14 minor-groove bending sites (n±4~n±7, n±14~n±17, n±24~n±27, n±34~n±37, n±45~n±48, n±56~n±59, n±66~n±69) and 12 major-groove bending sites (n±9~n±12, n±19~n±22, n±29~n±32, n±39~n±42, n±50~n±53, n±61~n±64), as described previously (Cui and Zhurkin, 2014). The NRP score of a certain sequence motif x was calculated by summing the counts Ci for the motifs occurring at all minor groove or major groove bending sites:
where wi is the weight of bending site i. NRP scores of sequence motifs WW, WWW, SS, SSS, YR, YYRR, RYRY were calculated and summed to determine the NRP score at position n (Cui and Zhurkin, 2010). The higher the NRP score of a particular nucleotide, the more likely it is to bend into major groove (Cui and Zhurkin, 2010).
Annotation of Chromatin States using ChromHMM
To better understand the chromatin states associated with PARP-1-dependent Sox2 binding sites across the genome, we used the chromHMM analytical tool (Ernst and Kellis, 2012). We evaluated the overlap between Sox2 binding sites and each chromatin state in the mES cells. The following chromatin features were used for the functional annotation:
-
1)
DNA methylation: 5hmC, 5mC
-
2)
Histone variants: H3.2, H3.3, H2Az
-
3)
Histone modifications: H3K4me1, H3K27ac, H3K4me3, H3K27ac, H3K27me3, H3K9me3, H4K20me3.
Based on these chromatin features, the genome was segregated into the following states: strong promoter, weak promoter, bivalent promoter, gene body/transcription elongation, strong active enhancer, weak active enhancer, poised enhancer, basal chromatin, as well as heterochromatin. We then determined the association between Sox2 binding sites and the different chromatin states by counting the numbers of Sox2 peaks from ChIP-seq data that overlapped with the respective chromatin state regions. A heatmap showing the emission scores, the total number of Sox2 sites, and the number of Sox2 binding sites associated with each chromatin state was generated using Java TreeView (Saldanha, 2004).
Feature Selection for Predicting the PARP-1 Dependence of Sox2 Genomic Occupancy
To gain insight into the potential factors that might affect the PARP-1 dependence of Sox2 binding to chromatin, we used the LASSO algorithm (Tibshirani, 1996), which allowed us to select the feature combination producing the optimal prediction accuracy, as evaluated by the mean square error (MSE). Eight-six genomic features (from available genomic data sets or derived from DNA sequence) that might influence the binding of transcription factors to chromatin were used as the initial feature list. These features include nucleosome occupancy, transcription factor co-occupancy, DNase-seq signal strength, Sox motif sequence, rotational orientation of the Sox motif in the nucleosome, and predicted structural features of the DNA at Sox2 binding sites. The LASSO algorithm was used to determine which combination of features best predict the p-value of the difference in the strength of Sox2 binding between wild-type and Parp1−/− mESCs, as determined from ChIP-seq data using edgeR (Robinson et al., 2010). Feature selection using the LASSO algorithm was executed using the R package glmnet 2.0-3. The penalty score lambda was scanned through the logarithmic space from −12 to −3. A 10-fold cross-validation was used to assess the model performance on the test data set.
Determining DNA Shape Parameters
The 3DNA web-based tool (Lu and Olson, 2003) was used to determine the DNA shape parameters associated with the Sox motif located at different positions in the 601 nucleosome (see Figs. 7B and S7A) from high resolution X-ray crystal structures. Minor groove widths (MGWs) of three 601 nucleosome structures (PBD: 3LZ0, 3LZ1, 3MVD (Makde et al., 2010; Vasudevan et al., 2010)), including two different orientations (3LZ0 and 3LZ1), were measured and compared. The shape parameters determined from all three nucleosome structures were similar. The data in Fig. S7A were plotted based on the 3MVD structure.
DATA AND SOFTWARE AVAILABILITY
Genomic Data Sets
The new RNA-seq and ChIP-seq data sets generated specifically for this study can be accessed from the NCBI’s Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo/) using the superseries accession number GSE81168 (subseries GSE74111 and GSE74112), as well as the individual accession numbers listed in the Key Resources Table. In addition, all of the published RNA-seq and ChIP-seq data sets from mESCs used in this study can be accessed through the GEO repository or the ENCODE Project repository (https://www.encodeproject.org) using the accession numbers listed in the Key Resources Table.
Custom Scripts
Custom R scripts for genomic data analyses are available from the Lead Contact on request.
Additional references for data sets and software in the Key Resources Table
(Bailey et al., 2009; Chen et al., 2013; Chen et al., 2008; Cui and Zhurkin, 2014; Eisen et al., 1998; Ernst and Kellis, 2012; Feng et al., 2011; Gao et al., 2009; Huang et al., 2007; Kagey et al., 2010; Kim et al., 2004; Ku et al., 2008; Langmead et al., 2009; Li et al., 2012; Liu et al., 2011; Marson et al., 2008; Mootha et al., 2003; Robinson et al., 2011; Robinson et al., 2010; Saldanha, 2004; Tibshirani, 1996; Trapnell et al., 2010; Wacker et al., 2007; Yang et al., 2004; Zhang et al., 2008)
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Zhao-Qi Wang (Leibniz Institute for Age Research, Fritz Lipmann Institute) for providing the Parp1 knockout mESCs. The authors would like to thank Drs. Bryan Gibson, Shrikanth Gadad, Rebecca Gupte, and Laura Banaszynski for providing critical feedback on this work and the manuscript. This work was supported by a grant from the NIH/NIDDK (DK069710) and the Cecil H. and Ida Green Center for Reproductive Biology Sciences Endowment to W.L.K.
Footnotes
Supplemental information includes seven figures and one table, and can be found with this article online. [See the Supplemental Information file.]
Author Contributions
Z.L. conceived the genomic aspects of the project and W.L.K. conceived the biochemical aspects of the project. Z.L. developed both aspects of the work with input from W.L.K. Z.L. performed all of the experiments and computational analyses. W.L.K. secured funding and provided intellectual support. Z.L. and W.L.K. prepared the figures and wrote the paper.
Disclosure
W.L.K. is a founder and consultant for Ribon Therapeutics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Dror I, Yang L, Slattery M, Zhou T, Bussemaker HJ, Rohs R, Mann RS. Deconvolving the recognition of DNA shape from sequence. Cell. 2015;161:307–318. doi: 10.1016/j.cell.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010;143:470–484. doi: 10.1016/j.cell.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xi Y, Pan X, Li Z, Kaestner K, Tyler J, Dent S, He X, Li W. DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 2013;23:341–351. doi: 10.1101/gr.142067.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Clark NJ, Kramer M, Muthurajan UM, Luger K. Alternative modes of binding of poly(ADP-ribose) polymerase 1 to free DNA and nucleosomes. J Biol Chem. 2012;287:32430–32439. doi: 10.1074/jbc.M112.397067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TEP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Zhurkin VB. Structure-based analysis of DNA sequence patterns guiding nucleosome positioning in vitro. J Biomol Struct Dyn. 2010;27:821–841. doi: 10.1080/073911010010524947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Zhurkin VB. Rotational positioning of nucleosomes facilitates selective binding of p53 to response elements associated with cell cycle arrest. Nucleic Acids Res. 2014;42:836–847. doi: 10.1093/nar/gkt943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, Guarnieri P, Bhagat G, Vanti WB, Shih A, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods. 2012;9:215–216. doi: 10.1038/nmeth.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Wen J. Overview of the roles of Sox2 in stem cell and development. Biol Chem. 2015;396:883–891. doi: 10.1515/hsz-2014-0317. [DOI] [PubMed] [Google Scholar]
- Feng X, Grossman R, Stein L. PeakRanger: a cloud-enabled peak caller for ChIP-seq data. BMC Bioinformatics. 2011;12:139. doi: 10.1186/1471-2105-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Nagari A, Kraus WL. TNFalpha signaling exposes latent estrogen receptor binding sites to alter the breast cancer cell transcriptome. Mol Cell. 2015;58:21–34. doi: 10.1016/j.molcel.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Kwon SW, Zhao Y, Jin Y. PARP1 poly(ADP-ribosyl)ates Sox2 to control Sox2 protein levels and FGF4 expression during embryonic stem cell differentiation. J Biol Chem. 2009;284:22263–22273. doi: 10.1074/jbc.M109.033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BA, Zhang Y, Jiang H, Hussey KM, Shrimp JH, Lin H, Schwede F, Yu Y, Kraus WL. Chemical genetic discovery of PARP targets reveals a role for PARP-1 in transcription elongation. Science. 2016;353:45–50. doi: 10.1126/science.aaf7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, Kraus WL. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145:622–634. doi: 10.1016/j.cell.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemberger M, Nozaki T, Winterhager E, Yamamoto H, Nakagama H, Kamada N, Suzuki H, Ohta T, Ohki M, Masutani M, et al. Parp1-deficiency induces differentiation of ES cells into trophoblast derivatives. Dev Biol. 2003;257:371–381. doi: 10.1016/s0012-1606(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Hottiger MO. Nuclear ADP-ribosylation and its role in chromatin plasticity, cell differentiation, and epigenetics. Annu Rev Biochem. 2015;84:227–263. doi: 10.1146/annurev-biochem-060614-034506. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M, Donahue G, Kakumanu A, Watts JA, Mahony S, Pugh BF, Lee D, Kaestner KH, Zaret KS. The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol Cell. 2016;62:79–91. doi: 10.1016/j.molcel.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M, Zaret KS. Pioneer transcription factors in cell reprogramming. Genes Dev. 2014;28:2679–2692. doi: 10.1101/gad.253443.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Mauro S, Gevry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Krishnakumar R, Gamble MJ, Frizzell KM, Berrocal JG, Kininis M, Kraus WL. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–821. doi: 10.1126/science.1149250. [DOI] [PubMed] [Google Scholar]
- Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010a;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar R, Kraus WL. PARP-1 regulates chromatin structure and transcription through a KDM5B-dependent pathway. Mol Cell. 2010b;39:736–749. doi: 10.1016/j.molcel.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, Presser A, Nusbaum C, Xie X, Chi AS, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YS, Chang CW, Pawlik KM, Zhou D, Renfrow MB, Townes TM. SRY (sex determining region Y)-box2 (Sox2)/poly ADP-ribose polymerase 1 (Parp1) complexes regulate pluripotency. Proc Natl Acad Sci U S A. 2012;109:3772–3777. doi: 10.1073/pnas.1108595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Gadue P, Chen K, Jiao Y, Tuteja G, Schug J, Li W, Kaestner KH. Foxa2 and H2A.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell. 2012;151:1608–1616. doi: 10.1016/j.cell.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Scannell DR, Eisen MB, Tjian R. Control of embryonic stem cell lineage commitment by core promoter factor, TAF3. Cell. 2011;146:720–731. doi: 10.1016/j.cell.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato MA, Ng CW, Wamstad JA, Cheng AW, Thai KK, Fraenkel E, Jaenisch R, Boyer LA. SOX2 co-occupies distal enhancer elements with distinct POU factors in ESCs and NPCs to specify cell state. PLoS Genet. 2013;9:e1003288. doi: 10.1371/journal.pgen.1003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- Lu XJ, Olson WK. 3DNA: a software package for the analysis, rebuilding and visualization of three-dimensional nucleic acid structures. Nucleic Acids Res. 2003;31:5108–5121. doi: 10.1093/nar/gkg680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makde RD, England JR, Yennawar HP, Tan S. Structure of RCC1 chromatin factor bound to the nucleosome core particle. Nature. 2010;467:562–566. doi: 10.1038/nature09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Oliva D, O'Valle F, Munoz-Gamez JA, Valenzuela MT, Nunez MI, Aguilar M, Ruiz de Almodovar JM, Garcia del Moral R, Oliver FJ. Crosstalk between PARP-1 and NF-kappaB modulates the promotion of skin neoplasia. Oncogene. 2004;23:5275–5283. doi: 10.1038/sj.onc.1207696. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Muthurajan UM, Hepler MR, Hieb AR, Clark NJ, Kramer M, Yao T, Luger K. Automodification switches PARP-1 function from chromatin architectural protein to histone chaperone. Proc Natl Acad Sci U S A. 2014;111:12752–12757. doi: 10.1073/pnas.1405005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na Z, Peng B, Ng S, Pan S, Lee JS, Shen HM, Yao SQ. A small-molecule protein-protein interaction inhibitor of PARP1 that targets its BRCT domain. Angew Chem Int Ed Engl. 2015;54:2515–2519. doi: 10.1002/anie.201410678. [DOI] [PubMed] [Google Scholar]
- Remenyi A, Lins K, Nissen LJ, Reinbold R, Scholer HR, Wilmanns M. Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 2003;17:2048–2059. doi: 10.1101/gad.269303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MB, Hosler BA, Gudas LJ. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- Rohs R, West SM, Sosinsky A, Liu P, Mann RS, Honig B. The role of DNA shape in protein-DNA recognition. Nature. 2009;461:1248–1253. doi: 10.1038/nature08473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SJ, Chrysanthou S, Senner CE, Sienerth A, Gnan S, Murray A, Masutani M, Latos P, Hemberger M. ADP-ribosyltransferases Parp1 and Parp7 safeguard pluripotency of ES cells. Nucleic Acids Res. 2014;42:8914–8927. doi: 10.1093/nar/gku591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes Dev. 2009;23:804–809. doi: 10.1101/gad.1775509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbulan-Rosenthal CM, Rosenthal DS, Luo R, Samara R, Espinoza LA, Hassa PO, Hottiger MO, Smulson ME. PARP-1 binds E2F-1 independently of its DNA binding and catalytic domains, and acts as a novel coactivator of E2F-1-mediated transcription during re-entry of quiescent cells into S phase. Oncogene. 2003;22:8460–8471. doi: 10.1038/sj.onc.1206897. [DOI] [PubMed] [Google Scholar]
- Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–568. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinstead EE, Miranda TB, Paakinaho V, Baek S, Goldstein I, Hawkins M, Karpova TS, Ball D, Mazza D, Lavis LD, et al. Steroid receptors reprogram FoxA1 occupancy through dynamic chromatin transitions. Cell. 2016;165:593–605. doi: 10.1016/j.cell.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society. Series B (Methodological) 1996;58:267–288. [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utley RT, Cote J, Owen-Hughes T, Workman JL. SWI/SNF stimulates the formation of disparate activator-nucleosome complexes but is partially redundant with cooperative binding. J Biol Chem. 1997;272:12642–12649. doi: 10.1074/jbc.272.19.12642. [DOI] [PubMed] [Google Scholar]
- Vasudevan D, Chua EY, Davey CA. Crystal structures of nucleosome core particles containing the '601' strong positioning sequence. J Mol Biol. 2010;403:1–10. doi: 10.1016/j.jmb.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Wacker DA, Ruhl DD, Balagamwala EH, Hope KM, Zhang T, Kraus WL. The DNA binding and catalytic domains of poly(ADP-ribose) polymerase 1 cooperate in the regulation of chromatin structure and transcription. Mol Cell Biol. 2007;27:7475–7485. doi: 10.1128/MCB.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber FA, Bartolomei G, Hottiger MO, Cinelli P. Artd1/Parp1 regulates reprogramming by transcriptional regulation of Fgf4 via Sox2 ADP-ribosylation. Stem Cells. 2013;31:2364–2373. doi: 10.1002/stem.1507. [DOI] [PubMed] [Google Scholar]
- Yang YG, Cortes U, Patnaik S, Jasin M, Wang ZQ. Ablation of PARP-1 does not interfere with the repair of DNA double-strand breaks, but compromises the reactivation of stalled replication forks. Oncogene. 2004;23:3872–3882. doi: 10.1038/sj.onc.1207491. [DOI] [PubMed] [Google Scholar]
- Yoo YD, Huang CT, Zhang X, Lavaute TM, Zhang SC. Fibroblast growth factor regulates human neuroectoderm specification through ERK1/2-PARP-1 pathway. Stem Cells. 2011;29:1975–1982. doi: 10.1002/stem.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Lerner J, Iwafuchi-Doi M. Chromatin scanning by dynamic binding of pioneer factors. Mol Cell. 2016;62:665–667. doi: 10.1016/j.molcel.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cui W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells. 2014;6:305–311. doi: 10.4252/wjsc.v6.i3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Joung JG, Zheng Y, Chen YR, Liu B, Shao Y, Xiang JZ, Fei Z, Giovannoni JJ. High-throughput illumina strand-specific RNA sequencing library preparation. Cold Spring Harb Protoc. 2011;2011:940–949. doi: 10.1101/pdb.prot5652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.