Abstract
GUIDED-HF is a multi-center randomized trial of a patient-centered transitional care intervention in patients with acute heart failure (AHF) who are discharged either directly from the Emergency Department (ED) or after a brief period of ED-based observation. To optimize care and reduce ED and hospital revisits there has been significant emphasis on improving transitions at the time of hospital discharge for HF patients. Such efforts have been almost exclusively directed at hospitalized patients; individuals with AHF who are discharged from the ED or ED-based observation are not included in these transitional care initiatives. Patients with AHF discharged directly from the ED or after a brief period of ED-based observation are randomly assigned to our transition GUIDED-HF strategy or standard ED discharge. Patients in the GUIDED arm receive a tailored discharge plan via the study team, based on their identified barriers to outpatient management and associated guideline-based interventions. This plan includes: conducting a home visit soon after ED discharge combined with close outpatient follow-up and subsequent coaching calls to improve post-discharge care and avoid subsequent ED revisits and inpatient admissions. Up to 700 patients at 11 sites will be enrolled over three years of the study. GUIDED-HF will test a novel approach to AHF management strategy that includes tailored transitional care for patients discharged from the ED or ED-based observation. If successful, this program may significantly alter the current paradigm of AHF patient care.
Subject Terms: Acute Heart Failure, Quality, Readmission, Transitional Care, Emergency Medicine
Despite a relative reduction in the hospitalization rate of heart failure (HF), the actual number of HF hospitalizations remains over one million annually. This figure is expected to significantly worsen with the aging United States population and the growing HF prevalence. Over 80% of patients who are hospitalized are initially seen in the emergency department (ED).1 However, not all those seen in the ED for HF are admitted; a small proportion are discharged without hospitalization, either directly from the ED or after a brief period (typically < 24 hours) of ED-based observation. As disposition decisions for those who present with acute heart failure (AHF) rest largely with ED providers, the ED has a central role in avoiding unnecessary hospitalizations. However, because HF patients are older, with significant co-morbidities and polypharmacy, the high rate of ED admission may be driven by factors other than acuity – a circumstance compounded in settings were socioeconomic challenges exist.
To optimize care and reduce ED and hospital revisits, there has been significant emphasis on improving transitions at the time of hospital discharge for HF patients. Such efforts have been almost exclusively directed at hospitalized patients; individuals with AHF who are discharged from the ED are generally not included in these transitional care initiatives. Ensuring optimal care transitions for discharged ED patients with AHF is a critical unmet need. Data show patients with AHF discharged from the ED receive suboptimal guideline directed medical therapy, suggesting interventions to improve AHF transitions are needed in the ED setting.2 This is particularly true for patients in resource limited settings, many of whom have vulnerable characteristics. While the uninsured are more likely to be discharged, when admitted to the hospital they consume a disproportionately larger amount of hospital resources.1 Limited outpatient resources may place a burden on inpatient providers to ensure a comprehensive work-up is performed prior to discharge. Admission for this specific reason may not be ideal. However, when HF patients are discharged from the ED with a poor or absent transitional care plan, emergency physicians may be the sole or primary providers delivering treatment, precluding initiation of a more holistic management plan.
Transition of Care Initiatives
There have been mixed results with existing transition of care initiatives designed to shift hospitalized patients to the outpatient setting with the goals of reducing mortality and 30-day return visits, while improving quality of life (QOL) indicators.3, 4 Many have been developed within broader quality improvement portfolios by sponsoring professional organizations, widely adopted, and resulted in end-user insight into what is effective what does and does not work.5 For instance, 599 hospitals participating in the American College of Cardiology sponsored Hospital to Home Program were surveyed to evaluate the programmatic strategies most effective at reducing hospital risk-standardized 30-day readmission rates for HF. Despite presumed effectiveness, factors such as arranging follow-up visits before discharge were found to contribute only modest reductions of the risk adjusted standardized 30-day readmission rates, with the average reduction of less than half a percentage point.6
Get with the Guidelines Heart Failure (GWTG:HF), an American Heart Association (AHA) sponsored program designed to measure and implement evidence-based HF care, is one of the most successful transition of care endeavors. As of July 2016, over 600 hospitals and 1.3 million patients have participated in GWTG:HF. One goal of the program is to reduce sex and race/ethnicity related disparities in health care. Hospitals enrolled in GWTG:HF have demonstrated better processes of care, reductions in quality of care and outcome disparities, and lower 30-day readmission rates when compared with non-participating hospitals.7–9 Because these barriers disproportionally affect vulnerable populations, including those with low health literacy, low socioeconomic status, the uninsured, and minorities, these interventions may have greater impact in such patients and thereby reduce disparities in ED revisits and hospitalizations.10,11 Unfortunately, the GWTG:HF program is currently designed for hospitalized patients and focuses on optimizing inpatient post-discharge care. Tailoring this evidence-based intervention to target patients who are missed in current GWTG:HF programs, namely the 15–20% of AHF patients who are discharged directly from the ED or after ED-based observation would address a critical unmet need.
Self-Care in Patients with Acute Heart Failure
A key component of transition initiatives is facilitating self-care management. Self-care has been defined as a naturalistic decision-making process related to patient behavioral choices that maintain physiologic stability and the response to symptoms when they occur.12 In HF, self-care maintenance involves adherence to medications, a low sodium diet and exercise. Self-care management requires patients to monitor their symptoms (e.g. edema), recognize a change, and respond with appropriate interventions (e.g. take additional dose of diuretic and call their caregiver).12 Much of the experience in self-management comes from subjects with chronic HF. A recent comprehensive review of self-care in patients with HF concluded that self-management reduced the time to HF-related hospitalization, the combined endpoint of HF-related hospitalization or all-cause mortality, and improved 12-month HF-related QOL.13 In patients less than 65 years old, self-care management was associated with a reduction of HF related hospital days. However, in a subset of patients with moderate to severe depression, a self-care strategy was associated with reduced survival.13 Additionally, self-care strategies did not impact all cause hospitalization, QOL, and mortality. ED patients with AHF who are discharged after a brief period of observation have similar or even greater needs. However, self-care initiatives for AHF have not been well studied in this cohort.
Safe Transition to the Outpatient Setting from the ED: An Unmet Need
Patients who follow-up with their provider soon after discharge are much less likely to be readmitted or experience an adverse event.14, 15 However, follow-up may occur in as few as 50% of patients within 30-days of ED discharge.15 Inadequate or poorly understood discharge instructions and systems issues are responsible for a large proportion of these missed follow-up opportunities.16 Vulnerable AHF populations, including those with low health literacy, low socioeconomic status, the uninsured and minorities are disproportionally affected by an ineffective transition strategy. Because vulnerable patients are highly represented in ED AHF populations, we hypothesize interventions in this cohort may have even greater impact in reducing disparities in ED revisits and hospitalizations.10,11 A carefully designed randomized trial comparing usual care with our GUIDED-HF transition strategy (Figure 1) in AHF patients discharged from the ED would bridge this critical evidence gap. This study will provide prospective, randomized trial data to support how EDs should manage lower risk AHF patients. It will also evaluate the effectiveness of interventions to reduce health disparities in cardiovascular disease.
Figure 1.
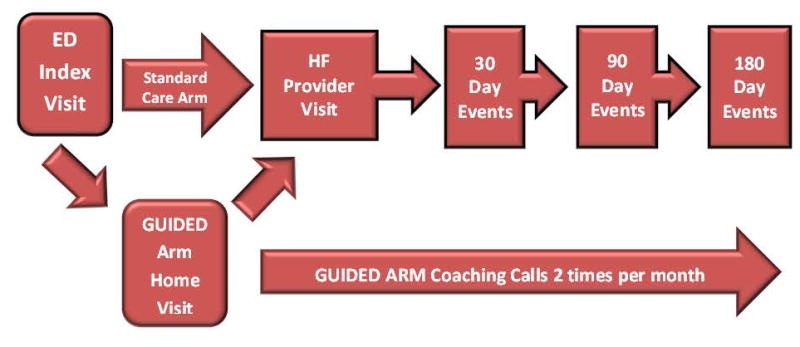
Guided Patient Flow: Flow of Patients through the study in the GUIDED and standard care arms.
Design of the GUIDED-HF Study
GUIDED-HF (Clinical Trials.gov, NCT02519283) is a multi-center randomized trial of a transition intervention compared with standard ED discharge in patients with AHF who are discharged either directly from the ED or after a brief period of ED-based observation. The conceptual model (Figure 2) for the study was based on overcoming barriers to self-care and improving transitions from the ED to outpatient setting to improve our proposed outcomes. This conceptual model aims to improve upon these outcomes by utilizing: 1) services, such as emergency medicine and heart failure providers and guideline recommended HF therapy, and 2) mediators, such as utilizing the communication skills and medical knowledge of the study team and providers.
Figure 2.
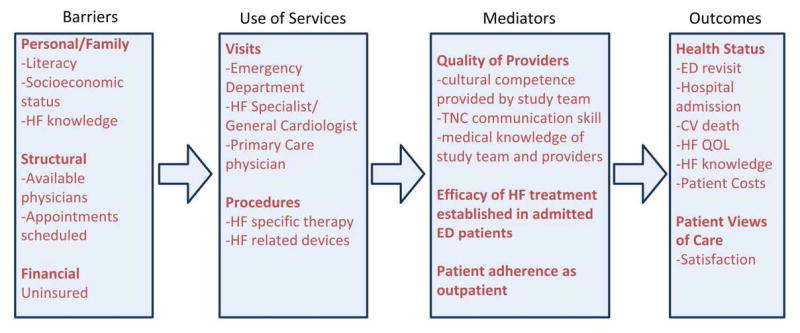
Conceptual model for the GUIDED HF study proposal.
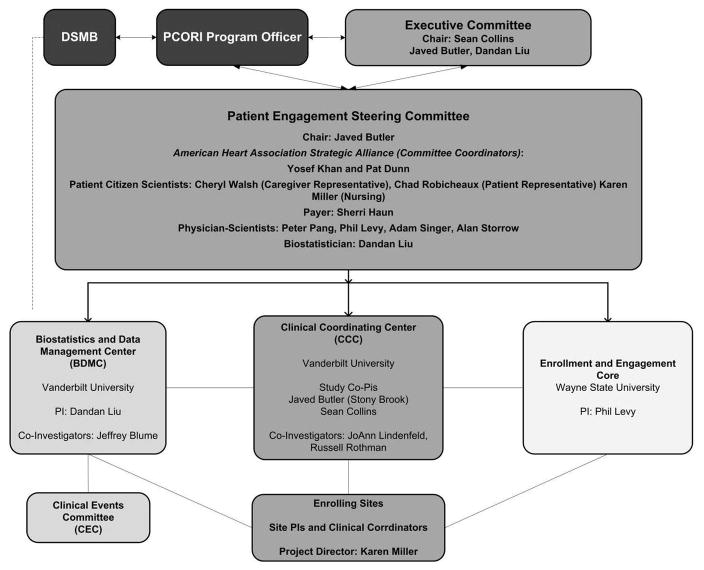
The overall study design is described in Figure 1. A total of up to 700 patients at 11 sites will be enrolled over three years. All patients in GUIDED-HF will provide written informed consent. The study was designed and led by a steering committee consisting of physician scientists, patients, caregivers, and AHA representatives. GUIDED-HF is funded by the Patient Centered Outcome Research Institute (PCORI) and, as such, a cornerstone of protocol development involved stakeholder engagement. In GUIDED-HF this included direct, face to face solicitation of input from chronic HF patients and their caregivers using an established Community Engagement Studio at Vanderbilt University and, outreach to the broader HF population through the AHA’s Virtual Online Community of Patients, Caretakers, and Stakeholders.17 For the latter, digital channels on Facebook and Twitter were used to host a “Highlight on HF” week, where more than 1,400 HF patients provided feedback informing the study design, plan for implementation, and ultimately dissemination of study findings. This feedback was collated and had significant influence on our primary and secondary aims and the structure of the study and its committees and coordinating centers. (Figure 3) In addition, HF patients are active participant in monthly study calls and manuscript preparation.
Figure 3.
Study team structure of the GUIDED HF proposal.
Study Populations
Patients who present to the ED with signs or symptoms of AHF will be screened. Eligibility criteria are: 1) ED physician AHF diagnoses, 2) plan is discharge either directly or after a brief period of ED-based observation, and 3) patients who will not have difficulties complying with the protocol due to psychiatric disease, dementia, or distance from the enrolling institution, making the home visit problematic. Specific inclusion and exclusion criteria (Table 1) were chosen to identify a low-risk patient cohort able to engage in our comprehensive outpatient intervention, including a home visit shortly after discharge.
Table 1.
GUIDED-HF Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Age ≥21 years 2. Prior history of HF 3. Patients deemed by emergency physician to have AHF 4. Discharge from ED or ED based, 23-hour observation unit |
1. Systolic BP <100 mmHg 2. Evidence of acute coronary syndrome based on typical symptoms and ischemia on ECG or Troponin elevation 3. Outpatient inotrope infusion 4. Unable to comply with protocol-due to psychiatric disease or distance from the hospital |
While the ED diagnosis may disagree with an inpatient diagnosis in 10–15% of cases,18 our design is pragmatic. We are interested in enrolling patients diagnosed with and treated for AHF in the ED, not a cohort of patients where AHF was confirmed after hospital admission. We have excluded complex and high-risk patients, such as those with a systolic BP < 100 mmHg, acute coronary syndrome based on typical signs and symptoms and ECG changes or cardiac troponin elevations, as well as those receiving an inotrope infusion as an outpatient. These would be atypical features among patients discharged from the ED or ED-based observation. We also excluded patients with no prior history of HF as these patients require extensive evaluations outside the context of the current proposal. In essence we aim to enroll a patient cohort with established HF where important aspects of our intervention, such as initiation or titration of guideline directed medical therapy, can be quantified and implemented.
Study Treatment
Patients are block randomized to either the GUIDED-HF strategy or standard discharge by site. Patients in the GUIDED arm receive a tailored discharge plan via the study team, based on their identified barriers to outpatient management and associated guideline-based interventions. As with all PCORI funded studies, GUIDED-HF is inherently geared towards patient preferences, i.e. working with participants to define the care components of most importance to them, devise an intelligible, acceptable, and achievable care plan, and assess the impact of treatment on patient-centered outcomes (defined below). The study team at each site is afforded flexibility to execute this tailored discharge plan based on the patient’s needs and their available study personnel. All staff carrying out the home visits have been trained by study personnel with HF expertise. The investigators felt this was an important pragmatic feature of the trial and will afford rapid dissemination after study completion. These study personnel focus on four main areas as part of the intervention.
Disease Education: We have previously used the short literacy screen to identify literacy barriers to understanding discharge and medication instructions in ED patients with AHF.19 For those patients with either self-reported difficulty or a short literacy screen score less than 12, the study team will utilize our medication regimen visual aid and teach back to confirm comprehension of all instructions.19, 20 Those patients without literacy deficiencies will receive standard HF disease education utilizing an accepted HF disease module.21
Lifestyle Interventions: To maintain consistency with GWTG:HF, smokers receive smoking cessation information. The study team also provides dietary education based on the HF disease education module, emphasizing low-sodium intake. Patients are instructed to track their daily weights with provision of a scale as needed. Patients are instructed to contact their physician when HF symptoms arise such as weight gain, swelling, and dyspnea.
Guideline Recommendations for Medications and Device Referral: Patients’ HF characteristics (ejection fraction, renal function, blood pressure, heart rate) and GWTG:HF recommendations determine the need for prescriptions for guideline-directed medical therapy including angiotensin converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, aldosterone antagonists, anticoagulants and referral for pacemaker/defibrillator consideration. The study team works with the patient to ensure affordable and accessible prescriptions (using generic substitutions and nearby pharmacies where low-cost monthly prescriptions are available when possible). For patients without access to a pharmacy, the study team may pick up the prescribed medications for the patient prior to the study-specific home visit, arrange for a caregiver to assist, have the medications mailed to the patient, or arrange for hospital supplied bridge dosing.
Outpatient Follow-Up Appointment: The study team works with the patient’s provider, or arranges for a new provider, to ensure a follow-up appointment within 7–10 days of ED discharge. For patients without a physician, this will be with a HF provider at each of the study sites. The study team also visits the patient within 48 hours of discharge to assess their home environment and further tailor the patient’s plan as needed. Potential areas of discussion and intervention include healthy eating habits (e.g. avoiding salt, frozen vegetables), setting up a scale for recording daily weights, showing the patient and/or caregiver on how to utilize a weekly medication organizer, and reinforcing early signs and symptoms of worsening HF. After the home visit, the study team performs coaching calls twice per month to answer any questions and assist with further needs and outpatient follow-up.
Patients assigned to the standard discharge arm receive ED or ED-based observation discharge instructions as per routine institutional practice. Similar to the GUIDED-HF arm, the study team also assists with facilitating a follow-up appointment within 7–10 days of discharge. However, these patients do not receive a home visit or coaching calls. While the discharge instructions delivered in the standard arm are determined by local practice, we have provided the sites with an outline of what structured standard practice should include. The completeness of this type of instruction and the degree of knowledge retention is followed as part of the GUIDED data collection, and is a subject of ongoing scrutiny.22 While we provide recommendations for structuring ED discharge instructions and follow-up in the standard care arm, the rigorous ED evaluation and discharge instructions, home visit and coaching calls only occur in the GUIDED arm. While there could be a small amount of contamination in the standard care arm, our structured discharge process aims to minimize this.
Endpoints
The primary outcome is a 90-day composite endpoint of: 1) time to ED revisit or hospital admission for AHF, 2) a clinic visit for AHF resulting in intravenous diuretic administration, or 3) cardiovascular death.(Table 2) The 90-day endpoint was chosen predominantly to ensure sustainability of our interventions and include both safety and efficacy. However, an important secondary endpoint will be the impact of our intervention at 30 days, which is more temporally related to the ED visit. Data on the number, timing, and reasons for admissions, ED visits, hospital procedures and study-related home visits are abstracted from patients’ records and by telephone follow-up, with recording on standardized data collection forms. Death is determined by documentation in the medical record, caregiver reports on follow-up, and by query of the social security death index six months after the index ED visit. A clinical events committee is available to review the medical records in patients who have experienced events to determine if the event qualifies as a primary endpoint. Information which could unblind the clinical events committee to the treatment group is redacted from the medical record. Secondary outcomes captured by phone follow-up at 30- and 90-days includes the Kansas City Cardiomyopathy Questionnaire [(KCCQ), measuring QOL; ranged 0–100], the Dutch HF knowledge scale,23 and estimates of out-of-pocket expenses due to hospitalizations, ED/office visits, diagnostic and laboratory testing, and medications. We also evaluate medication adherence using the Adherence to Refills and Medications Scale (ARMS-7),24 and reasons for inability to adhere, including medication costs. Finally, patient satisfaction at the time of ED discharge is measured via a Likert scale, and NIH Patient-Reported Outcomes Measurement Information System (PROMIS) tools are utilized to capture potential effect modification by depression and anxiety. To minimize bias by study personnel who are ascertaining follow-up, we require each site to conduct phone follow-ups using standardized interviews, and conducted by members of the study team blinded to treatment assignment. This key characteristic of the study will be monitored and corrective action plans will be instituted if any sites do not adhere to enrollment criteria, exhibit bias in selection of patients for enrollment, or do not adhere to the study protocol. We acknowledge that information may be transmitted during the follow-up phone calls that may suggest to the blinded site personnel that a subject is in either the standard or GUIDED arm.
Table 2.
Subject time sequence through GUIDED-HF data collection time points.
| Time | Measure | Role in Analysis | Source | Time |
|---|---|---|---|---|
| ED Presentation | Demographics, medical history, income, insurance, current living condition (alone, homeless, with caregiver/family) | Aim 1/2 predictors | Patient, records | 10 min |
| Prior HF hospitalizations in past 6 months | Aim 1/2 predictor | Patient, records | 1 min | |
| NYHA Class | Aim 1/2 predictor | Patient | 1 min | |
| Medication management (adherence) – ARMS-7 | Aim 1/2 predictors | Patient | 2 min | |
| ED tests and treatments | Aim 1/2 predictors | Records | -- | |
| Dutch HF Knowledge Scale | Aim 1/2 predictors | Patient | 5 min | |
| Numeracy (SNS) and Literacy (BHLS) Scale, PROMIS | Aim 1/2 predictors | Patient | 15 min | |
| HF health-related QOL – KCCQ-short version | Aim 1/2 predictors | Patient | 10 min | |
| 48 hours | Transition Nurse Coordinator home visit (yes/no) | Patient | -- | |
| 30, 90, 180 days | HF health-related QOL – KCCQ-short version, PROMIS | Aim 2 outcome | Patient | 15 min |
| ARMS-7, Dutch HF Knowledge Scale, and out of pocket costs | Aim 2 outcome | Patient | 15 min | |
| All-cause and HF-related ED revisits and hospitalizations | Aim 1 and safety outcome | Patient, records | 5 min | |
| All-cause and CV Mortality | Aim 1 and safety outcome | Records, SSDI | -- |
In addition, to determine sustainability of our intervention, we collect 180-day data on our primary and select secondary outcomes. A key challenge of our design is ensuring adherence to the GUIDED-HF interventions and follow-up for those patients enrolled. All sites have extensive prior experience following patients from ED enrollment up to 180 days of follow-up and beyond. Our sites formal follow-up process includes a medical record review, at least three calls to the patient and the alternative phone number provided, and sending a certified letter. In our prior ED-based studies we have completed follow-up in greater than 95% of enrolled subjects.25, 26
Safety Measures
Our safety endpoints include all-cause 90-day events: 1) ED revisits, 2) hospital admissions, and 3) death. An independent data safety and monitoring board oversees the safety of patients throughout the trial. During the planning phase of GUIDED-HF, the data safety and monitoring board met to approve the study protocol and approve the specific monitoring plan, including the monitoring frequency, data to be reviewed, and statistical procedures.
Statistical Analysis and Power Calculations
We are utilizing a time-to-event (i.e., survival) analysis to characterize the observed patterns while properly accounting for loss to follow-up, dropout and other types of censoring events. Non-CV death will be treated as a censoring event for the primary outcome, but will be followed as part of our safety endpoint. Since the impact of our tailored intervention will depend on the participant’s clinical profile, the model will adjust for the following covariates: age, sex, race, prior ejection fraction, baseline KCCQ scores, AHF therapy during the ED stay, and ED testing results including blood urea nitrogen and creatinine, systolic blood pressure, natriuretic peptide and troponin concentrations. This is standard ED data collected in over 95% of patients in our prior studies.25–27
The secondary outcomes are longitudinally measured patient-reported measures including KCCQ and the Dutch HF score which will be collected at the time of discharge (baseline), and again at 30- and 90-days after discharge. We will track total out-of-pocket charges related to hospitalizations and ED/office visits, diagnostic and laboratory testing and medications as reported by patient at their 30-day and 90-day follow-up.
The planned sample size of 700 subjects, equally distributed across the two intervention arms stratified by site and vulnerable and non-vulnerable populations, will provide excellent precision to address our primary and secondary outcomes with a two-sided type I error rate of 5%. The power calculation is based on standard methods for a proportional hazards model.28 Based on our prior ED cohort studies and existing literature,26, 29–31 we expect, in the usual care arm, event rates of 26% and 62% for our primary outcome at 30- and 90-days, and censoring rates of 2% at 30- and 4% at 90-days. For testing the intervention effects in reducing disparities, we assume 70% of the discharged patients will have a vulnerable characteristic (low socioeconomic status, minority, uninsured, and low health literacy). Using the 90-day information, the proposed sample size will offer 80% power to detect a hazard ratio of 0.55=1/1.82 for the interaction term, which corresponds to 45% reduction of disparity in relative risk between vulnerable and non-vulnerable population.
Conclusions
Transitions initiatives in patients with AHF have historically not included patients discharged from the ED or ED-based observation. Self-care is a cornerstone of successful chronic disease management. Addressing self-care may be even more important in patients who are not hospitalized. ED patients have a high proportion of vulnerable characteristics and known disparities in outcomes. GUIDED-HF will test a novel approach to transitional care, utilizing an early home visit combined with close outpatient follow-up and subsequent coaching calls to avoid unnecessary ED and hospital admissions. Further, the flexibility in conducting home visits and coaching calls facilitates the local implementation of the GUIDED strategy using a variety of healthcare providers. Ultimately, if GUIDED-HF proves to be successful, its impact will hinge on successful integration of the GUIDED-HF strategy into clinical practice guidelines and quality improvement initiatives.
Clinical Perspective.
Ensuring optimal care transitions for patients with AHF who are discharged from the ED is a critical unmet need. These patients often receive suboptimal guideline directed medical therapy and early follow-up, suggesting interventions to improve AHF transitions are needed in the ED setting. The GUIDED-HF study seeks to optimize this transition by using practical and relevant protocols to reinforce self-care strategies.
Translational Outlook.
Successful integration of the GUIDED-HF strategy into clinical practice guidelines and quality improvement initiatives will be a measure of success for this program. We hypothesize that utilizing an early home visit combined with close outpatient follow-up and subsequent coaching calls will avoid unnecessary ED and hospital admissions. To translate the GUIDED-HF strategy to the care of individual patients beyond the study period, GUIDED-HF allows for flexibility in conducting home visits and coaching calls. This flexibility facilities the local implementation of the GUIDED strategy using a variety of healthcare providers, enhances the sustainability of this initiative after the study has been completed.
Acknowledgments
Funding Sources: This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (AD-1409-21656).
Footnotes
Disclaimer: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Disclosures: Gregory J. Fermann, MD – Grant/Research Support: PCORI, Cardiorentis, Novartis, Cardioxyl, Siemens, Radiometer, Portola, Pfizer, and Nanodetection; Consultant Support/Other: Speakers’ Bureau for Janssen. Phillip D. Levy, MD, MPH – Grant/Research Support: PCORI Contract FC14-1409-21656; NIH Grants R01MD005849-05 and R01HL127215-01A; Consultant Support/Other: Novartis Pharmaceuticals, Cardiorentis, Trevena, Roche Diagnostics, and Siemens. Peter Pang, MD – Grant/Research Support: Roche, Novartis, PCORI, IUSM; Consultant Support/Other: BMS, Medtronic, the Medicines Company, Novartis, Trevena, scPharmaceuticals, Cardioxyl, Roche Diagnostics, Relypsa. Javed Butler, MD – Grant/Research Support: NIH European Union; Consultant Support/Other: Novartis, Bayer, Amgen, Janssen, Merck, Relypsa, Trevena, Stealth Peptide, CardioCell, Boehringer Ingelheim, and ZS Pharma. JoAnn Lindenfeld, MD – Grant Research Support: Novartis; Consultant Support/Other: St. Jude, Abbott, Relypsa, RESMED, Cardiokinetix, CVRx. W. Frank Peacock, MD – Grant Research Support: Abbott, Alere, Banyan, Cardiorentis, Janssen, Portola, Pfizer, Roche, ZS Pharma; Consultant Support: Alere, Beckman, Boehringer-Ingelheim, Cardiorentis, Instrument Labs, Janssen, Phillips, Portola, Prevencio, Singulex, The Medicine’s Company, ZS Pharma; Other: Ownership Interests-Comprehensive Research Associates LLC, Emergencies in Medicine LLC. Russell L. Rothman, MD, MPP – Consultant Support/Other: edLogics, Boehringer-Ingelheim. Adam Singer, MD – Speakers’ Bureau: Janssen, Abbott; Consulting: Janssen, Abbott, ZS Pharma, Daiichi Sankyo. Alan B. Storrow, MD – Grant Research Support: Centers for Medicaid and Medicare Services, NIH/NHLBI, Abbott Diagnostics, Roche Diagnostics, Beckman-Coulter, and Novartis Pharmaceuticals; Consultant Support/Other: Roche Diagnostics, Beckman-Coulter, Novartis Pharmaceuticals, and Trevena.Cheryl Walsh – Grant Research Support: PCORI. Sean P. Collins, MD, MSc – Grant Research Support: NIH/NHLBI, PCORI, Cardiorentis, Novartis, and Cardioxyl; Consultant Support/Other: Novartis, Trevena, Cardiorentis, Cardioxyl, and Siemens.
References
- 1.Storrow AB, Jenkins CA, Self WH, Alexander PT, Barrett TW, Han JH, McNaughton CD, Heavrin BS, Gheorghiade M, Collins SP. The burden of acute heart failure on u.S. Emergency departments. JACC Heart Fail. 2014;2:269–277. doi: 10.1016/j.jchf.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DS, Stukel TA, Austin PC, Alter DA, Schull MJ, You JJ, Chong A, Henry D, Tu JV. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122:1806–1814. doi: 10.1161/CIRCULATIONAHA.110.940262. [DOI] [PubMed] [Google Scholar]
- 3.Kansagara D, Chiovaro JC, Kagen D, Jencks S, Rhyne K, O’Neil M, Kondo K, Relevo R, Motu’apuaka M, Freeman M, Englander H. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11:221–230. doi: 10.1002/jhm.2502. [DOI] [PubMed] [Google Scholar]
- 4.Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: See you in 7. JACC Heart Fail. 2015;3:765–773. doi: 10.1016/j.jchf.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, Paul S, Ryan CJ, White-Williams C American Heart Association Complex Cardiovascular P, Family Care Committee of the Council on C, Stroke Nursing CoCC, Council on Quality of C, Outcomes R. Transitions of care in heart failure: A scientific statement from the american heart association. Circ Heart Fail. 2015;8:384–409. doi: 10.1161/HHF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 6.Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN, Goldmann D, White N, Pina IL, Krumholz HM. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444–450. doi: 10.1161/CIRCOUTCOMES.111.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellrodt AG, Fonarow GC, Schwamm LH, Albert N, Bhatt DL, Cannon CP, Hernandez AF, Hlatky MA, Luepker RV, Peterson PN, Reeves M, Smith EE. Synthesizing lessons learned from get with the guidelines: The value of disease-based registries in improving quality and outcomes. Circulation. 2013;128:2447–2460. doi: 10.1161/01.cir.0000435779.48007.5c. [DOI] [PubMed] [Google Scholar]
- 8.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: Have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas KL, Hernandez AF, Dai D, Heidenreich P, Fonarow GC, Peterson ED, Yancy CW. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–754. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Wu JR, Holmes GM, DeWalt DA, Macabasco-O’Connell A, Bibbins-Domingo K, Ruo B, Baker DW, Schillinger D, Weinberger M, Broucksou KA, Erman B, Jones CD, Cene CW, Pignone M. Low literacy is associated with increased risk of hospitalization and death among individuals with heart failure. J Gen Intern Med. 2013;28:1174–1180. doi: 10.1007/s11606-013-2394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: Accumulating evidence of inequality. Eur J Heart Fail. 2012;14:138–146. doi: 10.1093/eurjhf/hfr168. [DOI] [PubMed] [Google Scholar]
- 12.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, Peterson PN, Pressler SJ, Schocken DD, Whellan DJ American Heart Association Council on Cardiovascular N, American Heart Association Council on Cardiovascular N, American Heart Association Council on Clinical C, American Heart Association Council on Nutrition PA Metabolism, American Heart Association Interdisciplinary Council on Quality of C, Outcomes R. State of the science: Promoting self-care in persons with heart failure: A scientific statement from the american heart association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 13.Jonkman NH, Westland H, Groenwold RH, Agren S, Atienza F, Blue L, Bruggink-Andre de la Porte PW, DeWalt DA, Hebert PL, Heisler M, Jaarsma T, Kempen GI, Leventhal ME, Lok DJ, Martensson J, Muniz J, Otsu H, Peters-Klimm F, Rich MW, Riegel B, Stromberg A, Tsuyuki RT, van Veldhuisen DJ, Trappenburg JC, Schuurmans MJ, Hoes AW. Do self-management interventions work in patients with heart failure? An individual patient data meta-analysis. Circulation. 2016;133:1189–1198. doi: 10.1161/CIRCULATIONAHA.115.018006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 15.Feldman DE, Huynh T, Lauriers JD, Giannetti N, Frenette M, Grondin F, Michel C, Sheppard R, Montigny M, Lepage S, Nguyen V, Behlouli H, Pilote L. Access to heart failure care post emergency department visit: Do we meet established benchmarks and does it matter? Am Heart J. 2013;165:725–732. doi: 10.1016/j.ahj.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SM, Vergel de Dios J, Hanneman K. Noncompletion of referrals to outpatient specialty clinics among patients discharged from the emergency department: A prospective cohort study. Cjem. 2010;12:325–330. doi: 10.1017/s1481803500012410. [DOI] [PubMed] [Google Scholar]
- 17.Joosten YA, Israel TL, Williams NA, Boone LR, Schlundt DG, Mouton CP, Dittus RS, Bernard GR, Wilkins CH. Community engagement studios: A structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646–1650. doi: 10.1097/ACM.0000000000000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins SP, Lindsell CJ, Peacock WF, Eckert DC, Askew J, Storrow AB. Clinical characteristics of emergency department heart failure patients initially diagnosed as non-heart failure. BMC Emerg Med. 2006;6:11. doi: 10.1186/1471-227X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNaughton CD, Collins SP, Kripalani S, Rothman R, Self WH, Jenkins C, Miller K, Arbogast P, Naftilan A, Dittus RS, Storrow AB. Low numeracy is associated with increased odds of 30-day emergency department or hospital recidivism for patients with acute heart failure. Circ Heart Fail. 2013;6:40–46. doi: 10.1161/CIRCHEARTFAILURE.112.969477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong-Grotz K, Castro C, Bindman AB. Closing the loop: Physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163:83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 21.DeWalt DA, Pignone M, Malone R, Rawls C, Kosnar MC, George G, Bryant B, Rothman RL, Angel B. Development and pilot testing of a disease management program for low literacy patients with heart failure. Patient Educ Couns. 2004;55:78–86. doi: 10.1016/j.pec.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Engel KG, Buckley BA, Forth VE, McCarthy DM, Ellison EP, Schmidt MJ, Adams JG. Patient understanding of emergency department discharge instructions: Where are knowledge deficits greatest? Acad Emerg Med. 2012;19:E1035–1044. doi: 10.1111/j.1553-2712.2012.01425.x. [DOI] [PubMed] [Google Scholar]
- 23.van der Wal MH, Jaarsma T, Moser DK, van Veldhuisen DJ. Development and testing of the dutch heart failure knowledge scale. Eur J Cardiovasc Nurs. 2005;4:273–277. doi: 10.1016/j.ejcnurse.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the adherence to refills and medications scale (arms) among low-literacy patients with chronic disease. Value Health. 2009;12:118–123. doi: 10.1111/j.1524-4733.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- 25.Collins SP, Lindsell CJ, Jenkins CA, Harrell FE, Fermann GJ, Miller KF, Roll SN, Sperling MI, Maron DJ, Naftilan AJ, McPherson JA, Weintraub NL, Sawyer DB, Storrow AB. Risk stratification in acute heart failure: Rationale and design of the stratify and decide studies. Am Heart J. 2012;164:825–834. doi: 10.1016/j.ahj.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins SP, Peacock WF, Lindsell CJ, Clopton P, Diercks DB, Hiestand B, Hogan C, Kontos MC, Mueller C, Nowak R, Chen WJ, Huang CH, Abraham WT, Amsterdam E, Breidthardt T, Daniels L, Hasan A, Hudson M, McCord J, Naz T, Wagoner LE, Maisel A. S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Ann Emerg Med. 2009;53:748–757. doi: 10.1016/j.annemergmed.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian-Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M. Acute heart failure syndromes: Emergency department presentation, treatment, and disposition: Current approaches and future aims: A scientific statement from the american heart association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 28.Schmoor C, Sauerbrei W, Schumacher M. Sample size considerations for the evaluation of prognostic factors in survival analysis. Stat Med. 2000;19:441–452. doi: 10.1002/(sici)1097-0258(20000229)19:4<441::aid-sim349>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Brar S, McAlister FA, Youngson E, Rowe BH. Do outcomes for patients with heart failure vary by emergency department volume? Circ Heart Fail. 2013;6:1147–1154. doi: 10.1161/CIRCHEARTFAILURE.113.000415. [DOI] [PubMed] [Google Scholar]
- 30.Maisel A, Hollander JE, Guss D, McCullough P, Nowak R, Green G, Saltzberg M, Ellison SR, Bhalla MA, Bhalla V, Clopton P, Jesse R. Primary results of the rapid emergency department heart failure outpatient trial (redhot). A multicenter study of b-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol. 2004;44:1328–1333. doi: 10.1016/j.jacc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Rame JE, Sheffield MA, Dries DL, Gardner EB, Toto KH, Yancy CW, Drazner MH. Outcomes after emergency department discharge with a primary diagnosis of heart failure. Am Heart J. 2001;142:714–719. doi: 10.1067/mhj.2001.118473. [DOI] [PubMed] [Google Scholar]