Abstract
Chronic wounds are a major cause of morbidity and mortality. Approximately 20–23% of non-healing wounds that are refractory to vascular intervention have other etiologies including vasculitis, pyoderma gangrenosum and other autoimmune diseases. The purpose of this article is to review the autoimmune and coagulopathic diseases that are commonly associated with leg ulceration.
OBJECTIVES
To review the literature across medical and surgical specialties with regards to refractory chronic wounds associated with vasculitis and autoimmune diseases and to delineate clinical outcomes of these wounds in response to vascular and other interventions.
METHODS
An electronic search encompassing MEDLINE®, PubMed®, Cochrane Library and Scopus ® was completed using the following search terms: rheumatoid arthritis; systemic sclerosis; systemic lupus erythematosus; ANCA associated vasculitis; mixed connective tissue disease; antiphospholipid syndrome; pyoderma gangrenosum; thromboangiitis obliterans; cryoglobulinemia; hydroxyurea; sickle-cell; atrophie blanche; livedoid vasculitis; cholesterol emboli; calciphylaxis; antiphospholipid antibodies; prothrombotic; combined with the terms: chronic wound and leg ulcer.
Full text articles published in English up to March 1, 2016 that investigated the clinical outcomes of chronic wounds associated with autoimmune diseases were included. Review articles and evaluations of management of chronic wounds were also reviewed. Primary outcomes included in the review were amputation, ulcer healing, reduction in wound size, overall survival and freedom from reintervention. Owing to the heterogeneity of data reporting among articles, qualitative analysis is also reported.
CONCLUSIONS
Autoimmune and vasculitic etiologies should be considered in patients with chronic wounds who do not respond to appropriate vascular intervention and standard local wound care. A multidisciplinary approach with the involvement of rheumatology allows investigation for underlying systemic disease and improves clinical outcomes for many of these challenging patients.
Keywords: Chronic Wound, Vasculitis, Pyoderma Gangrenosum, Buergers disease, Scleroderma, Raynauds
Introduction
Chronic wounds that are non-responsive to 3 months of appropriate wound care affect approximately 6.5 million people in the US with a prevalence of 1% and costs estimated at $25 billion per year (1). In addition to the financial costs, these wounds significantly impact mortality (2) and cause considerable pain, affecting patient reported psychosocial wellbeing and quality of life (3).
Normal wound healing involves four overlapping phases (Figure 1) that progress sequentially regardless of wound etiology to achieve restoration of the skin barrier function(4). Chronic wounds are arrested in the inflammatory phase (4) and are unable to transition to the proliferation phase with concurrent upregulation of angiogenesis and matrix deposition. Interactions between the many pathways contributing to the inflammatory state in chronic wounds are poorly understood; however, clinically vasculitic and autoimmune etiologies along with coagulopathies should be considered in patients presenting with refractory chronic wounds. While large epidemiologic studies show that up to 79.7% of leg ulcers have a vascular etiology(5) (venous, peripheral arterial disease or mixed), 20 – 23% of patients have wounds from other more complex etiologies including vasculitis, pyoderma gangrenosum and other autoimmune diseases(5, 6). It is essential that vascular surgeons recognize the contribution that immune dysfunction plays in delayed wound healing.
Figure 1.
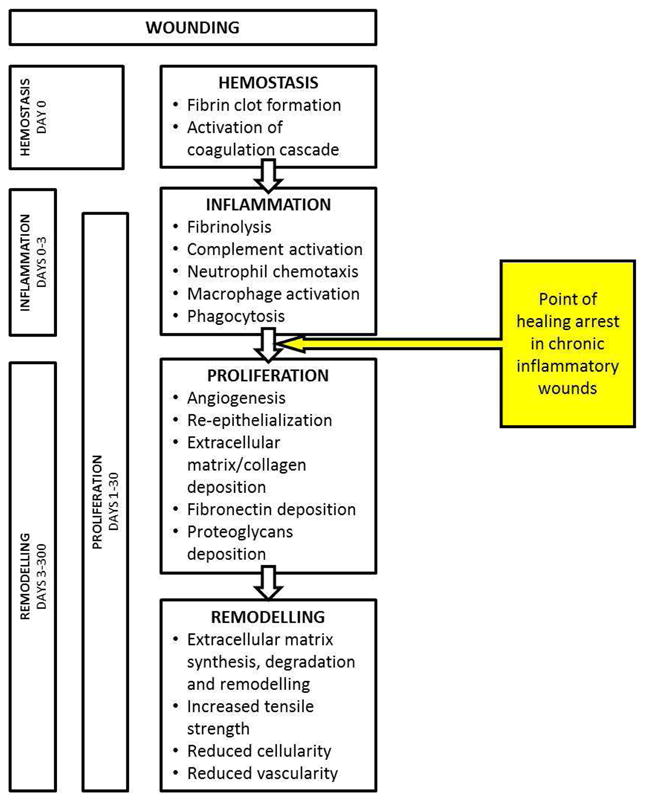
Four phases of normal wound healing.
The purpose of this manuscript is to systematically review the data regarding autoimmune diseases commonly associated with leg ulceration, particularly focusing on the prevalence of these wounds, and the evidence to support vascular interventions and targeted biologic therapy for these patients.
Methods
An electronic search encompassing MEDLINE®, PubMed®, Cochrane Library and Scopus ® was completed using the following search terms: rheumatoid arthritis; systemic sclerosis; systemic lupus erythematosus; ANCA associated vasculitis; mixed connective tissue disease; antiphospholipid syndrome; pyoderma gangrenosum; thromboangiitis obliterans; cryoglobulinemia; hydroxyurea; sickle-cell; atrophie blanche; livedoid vasculitis; cholesterol emboli; calciphylaxis; antiphospholipid antibodies; prothrombotic; combined with the terms: chronic wound and leg ulcer.
Full text articles published in English up to March 1, 2016 that investigated the clinical outcomes of chronic wounds associated with autoimmune diseases were included. Review articles and evaluations of management of chronic wounds were also reviewed. Primary outcomes included in the review were amputation, ulcer healing, reduction in wound size, overall survival and freedom from re-intervention. Owing to the heterogeneity of data reporting among articles, qualitative analysis is also reported.
Results
Prevalence of Autoimmune and vasculitic wounds
While it is clinically recognized that patients with autoimmune diseases can develop leg ulceration secondary to vasculitis of the small or medium-sized cutaneous blood vessels, prevalence studies investigating how common this issue is in patients with chronic wounds are relatively limited. Two important studies have been completed. The first was a questionnaire study conducted in Germany(5). In this study, 100 German wound care professionals were asked to complete a survey regarding diagnosis and underlying etiology in their leg ulcer patients. Data from 31619 patients was reported. As would be expected, venous and arterial insufficiency were common, and reported as the dominant causative factor in 47.6% and 14.5% of cases, with 17.6% of cases reported to be due to combined venous and arterial insufficiency. Vasculitis and other inflammatory diseases including pyoderma gangrenosum, calciphylaxis and drug induced etiologies accounted for 20.3% of cases. This study was limited owing to the reporting bias, there was no confirmatory testing or consistency in diagnostic methods across the clinicians under study. A retrospective study investigating more than 500 consecutive patients scheduled for evaluation at a single wound center over a 3-month period found a prevalence of autoimmune disease in 23% of cases(6), suggesting a similar prevalence of immune disease in the US population. In this study, the wounds in patients with autoimmune disease at presentation had significantly larger mean surface area (33.4cm2 compared to 22.5 cm2, p=0.02). This study also demonstrated that the patients who had co-existent immune disease had a much higher rate of split thickness skin graft (STSG) failure (50% compared to 97% in the patients without immune disease, p=0.0002). This suggests that presenting wound surface area, and failure of STSG might be predictors of immune related pathologies that warrant further evaluation.
LEG ULCERS ASSOCIATED WITH RHEUMATOID ARTHRITIS
Prevalence of rheumatoid arthritis-associated leg ulcers
One of the most common autoimmune diseases known to be associated with leg ulceration is RA. A retrospective review of data from Olmsted County, Minnesota residents who fulfilled criteria for RA between the dates of 1980–2007 and were followed until death or migration was analyzed in April 2012. Of the 813 patients, with 9771 person-years of follow up, 125 developed leg ulcers with a cumulative incidence at 5 years after RA diagnosis of 4.8% increasing to 26.2% by 25 years after the RA diagnosis. In this cohort, 6% of ulcers led to amputation and ulcers were associated with increased mortality adjusted for age (HR 2.42; 95% CI 1.7, 3.42). Risk factors for leg ulcers in this cohort included age (HR 1.73 per 10-year increase), rheumatoid factor positivity (HR 1.63), presence of rheumatoid nodules (HR 2.14) and venous thromboembolism (HR 2.16). Other studies investigating the prevalence of leg ulcers in the RA population have found similar prevalence data. In a study of 366 RA patients, the period-prevalence of leg ulcers was 4.37% over 3-years of follow-up (7). Similar to the Mayo Clinic study, in this study patients with ulcers had prolonged duration of rheumatoid arthritis (mean disease duration at ulcer development 25.9 years). All patients with ulcers had erosive disease and 63% were seropositive. In this study, the use of anti-tumor necrosis factor-α therapy was associated with significantly higher likelihood of healing (RR 3.27, 95% CI 0.59–18.29, p=0.039).
Role of the biopsy in RA-associated leg ulcers
Confirmation of vasculitis on tissue biopsy of rheumatoid-associated leg ulcers is notoriously challenging. Data from biopsy studies shows that vasculitis is present histologically in approximately 50–55% of patients with RA-associated leg ulcers(7–9). Some biopsies in RA patients will show non-specific findings such as fibrosis, scar tissue and changes of a chronic ulcer(9). However, the main value of biopsy in RA-associated leg ulcers is to exclude atypical infections such as chronic mycobacterial infections, and malignancies which can develop in longstanding chronic wounds.
Review of data regarding management of RA-associated leg ulcers
There are no randomized controlled trials to investigate specific therapeutic interventions for RA-associated leg ulcers. Data is drawn from review of case-series reports. Surgical revascularization has been investigated in a case series study that included both patients with RA and other connective tissue diseases(10). In this study 10 patients received endovascular treatment (EVT) for critical limb ischemia (CLI) related ulcers on 11 limbs. EVT was successful in all the cases. No procedure related morbidity or mortality was reported and during mean follow-up of 26 months, patients had no major amputations. Of the 11 limbs, 8 demonstrated ulcer healing and reduction in Rutherford category. The overall 1-year rates of amputation-free survival and freedom from re-intervention were 89% and 91% respectively.
In the absence of CLI, RA-associated leg ulcers are generally managed medically. While clinicians are often concerned about escalating immunotherapy in patients with RA-associated leg ulcers due to risks of infection and delayed wound healing, this concern is not supported by evidence. Studies show that while glucocorticoids may predispose to skin fragility and thus serve as a risk factor for ulceration in patients with RA, more aggressive therapy for the underlying RA, using disease modifying antirheumatic drugs (DMARD) and biologic agents such as TNF-α inhibtors and rituximab is beneficial (Figure 2) (7, 11–13). Furthermore, in a large series investigating postoperative wound dehiscence in surgical patients, prior use of steroids or immunosuppressive drugs in the 100 days prior to the index surgery was not a risk factor for post-operative wound dehiscence(14) suggesting that steroid therapy may not be a direct risk factor for wound development. It is quite likely that the association between steroid use and ulceration in RA patients may rather be a manifestation of the known relationship between RA severity and leg ulceration(15).
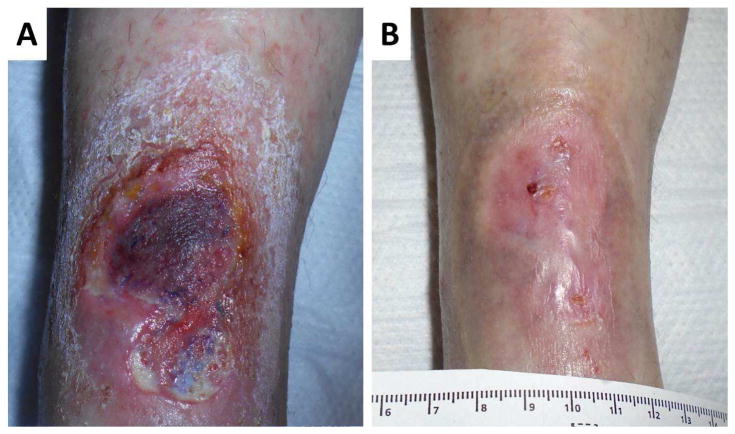
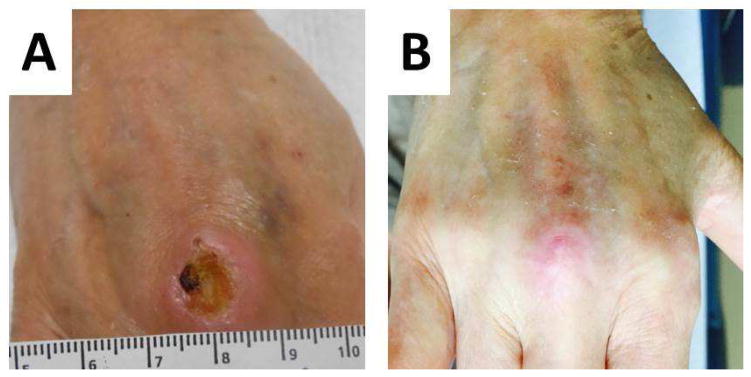
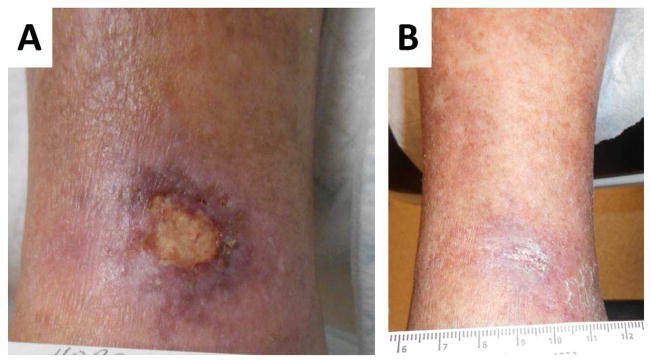
Figure 2.
Example of a rheumatoid leg ulcer caused by small vessel vasculitis A. Prior to rituximab therapy, B. complete healing after rituximab therapy.
In patients with leg ulcers and underlying RA, in whom limb vascularization has been adequately addressed, we strongly recommend referral to rheumatology to evaluate whether escalation of RA therapy could be beneficial.
LEG ULCERS ASSOCIATED WITH SYSTEMIC LUPUS ERYTHEMATOSUS
Prevalence of Systemic Lupus Erythematosus associated leg ulcers
Leg ulceration is a rare but well recognized complication of Systemic Lupus Erythematosus (SLE)(9). In a study of 340 patients with chronic wounds evaluated in a tertiary wound center, 2.6% had underlying SLE (compared to a reported prevalence of 0.05–0.15% in the general population, p<0.001) (6).
Pathology of SLE-associated ulcers
Similar to RA-associated leg ulcers, ulceration in lupus patients may be secondary to immune complex mediated vasculitis. However, coexistent prothrombotic states such as antiphospholipid syndrome may also play a role in some patients (Figure 3) (16, 17). Typically, histologic examination of wounds in SLE shows a leukocytoclastic vasculitis with fibrinoid necrosis of the vessel wall and prominent polymorphonuclear cell infiltration. Thromboocclusive histologic findings can be associated with the presence of antiphospholipid antibodies(18). Venous insufficiency may also play a role in delayed healing in lupus patients(19).
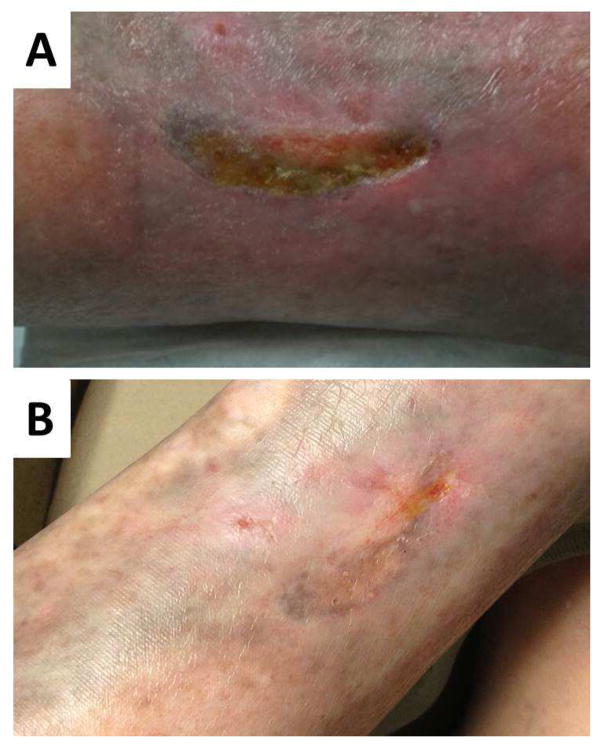
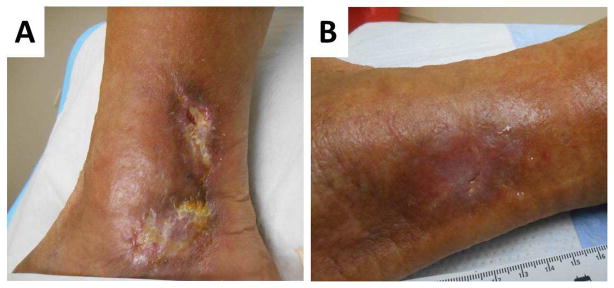
Figure 3.
A: Vasculitic leg ulcer in a patient with longstanding SLE and antiphospholipid syndrome that had been refractory to prednisone, mycophenolate mofetil, methotrexate, plaquenil and anticoagulation. B: After commencing belimumab and continuing prednisone, enoxaparin and hydroxychloroquine the wounds have completely healed.
Management of SLE-associated leg ulcers
Treatment of these wounds should focus on the management of the underlying autoimmune and inflammatory disease. There are no randomized controlled trials investigating this complication, however in clinical practice it is well recognized that patients with active vasculitis frequently require management with steroids in conjunction with disease modifying therapies. Those with co-existent prothrombotic states often also require anticoagulant therapy. Belimumab (Benlysta®, GSK, US) is a monoclonal antibody that inhibits the B-cell survival factor BLyS which is thought to play a critical role in some patients with SLE. Studies of Belimumab have shown benefit in musculoskeletal and cutaneous manifestations of SLE(20).
SCLERODERMA AND MIXED CONNECTIVE TISSUE DISEASE ASSOCIATED ULCERS
Prevalence of ulcers in Scleroderma and Mixed Connective Tissue Disease
Scleroderma (SSc) is a rare autoimmune disease characterized by immune activation, vasculopathy, fibroblast stimulation, and connective tissue fibrosis. Mixed connective tissue disease (MCTD) is a heterogeneous group of disorders which may present with overlapping features of several autoimmune disease (often scleroderma with features of SLE or RA) and is characterized by the presence of anti-RNP antibodies. In both SSc and MCTD, end-organ damage occurs due to progressive tissue fibrosis and vasculopathy. Lower extremity ulcers are a known complication of longstanding scleroderma, affecting approximately 4% of patients and causing significant morbidity(21). In the previously described epidemiologic study of patients with chronic wounds, the prevalence of scleroderma in patients with chronic wounds was 2.35% compared to 0.02% in the general population (p<0.001)(6).
Pathology of SSc-associated ulcers
The etiology of lower extremity ulcers in scleroderma is often multifactorial(22). Scleroderma ulcers are bilateral in 70% of cases, and the most common histologic finding is fibrin occlusive vasculopathy with intimal thickening and some inflammation(21). Coexistent prothrombotic states often contribute to refractory ulcers in scleroderma and in practice we recommend screening patients with refractory ulcers for antiphospholipid antibodies and genetic prothrombotic states. Arterial and venous disease may also contribute to healing delay in scleroderma and can be seen in as many as 50% of patients. Therefore, vascular evaluation is recommended in all patients (23).
Management of SSc and MCTD-associated leg ulcers
Limb salvage in patients with SSc and MCTD requires a multidisciplinary approach. While vascular interventions play a role, the long-term effectiveness of bypass surgery is limited due to the associated distal vasculopathy. A study evaluating bypass surgery outcomes in scleroderma patients with critical limb ischemia due to tibial artery occlusion (n=8) showed high rates of graft failure and limb loss, despite early pain relief and wound healing (24). In addition, coexisting prothrombotic states in patients with SSc often contribute to graft failure (Figure 4). Endovascular treatment has been shown to be an acceptable treatment option(10). In a study of 10 patients with coexistent connective tissue disease and critical limb ischemia-related leg ulcers (in 11 limbs), 3 of whom had SSc, endovascular treatment was technically successful as evidenced by attainment of straight-line flow of blood to the foot, absence of flow-limiting dissection, and residual stenosis of less than 30% of the vessel diameter. After a mean follow-up period of 26 months, there were no major amputations and reduction in Rutherford category was observed in 8 out of 11 limbs(10). Lower limb amputation is a treatment option when patients have severe, non-healing ulcers(24).
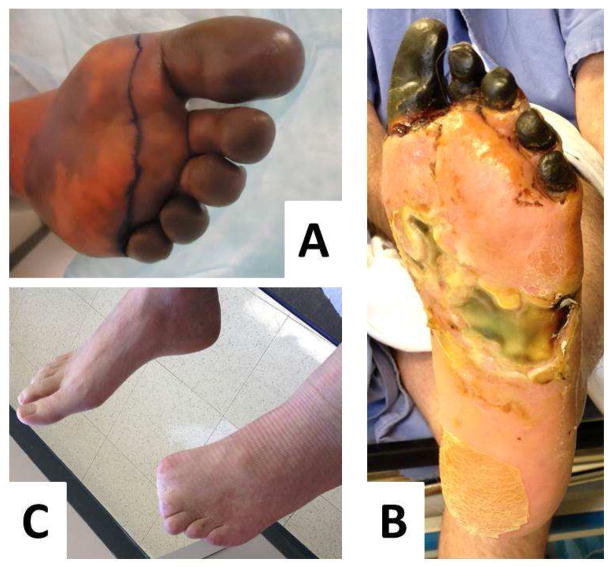
Figure 4.
A: Digital ischemia and B: forefoot gangrene in a patient with longstanding scleroderma and antiphospholipid syndrome. C: A multidisciplinary approach with vascular bypass, anticoagulation, vasodilator therapy and hyperbaric oxygen allowed limb salvage although the distal toes had to be amputated.
Medical interventions proposed to address the vasculopathic etiology of SSc-associated digital and other ulcers include vasodilators such as calcium channel blockers, prostanoids such as iloprost and endothelin receptor antagonists such as Bosentan. Topical and systemic opioids are often used to address the severe pain patients experience with these vasculopathic ulcers but it should be noted that retrospective data on post-operative wound dehiscence (14) and longitudinal data from the Wound Healing and Etiology (WE-HEAL) Study has shown that in a more general population with wound issues, higher opioid exposure is associated with slower wound healing.
LEG ULCERS DUE TO PYODERMA GANGRENOSUM
Pyoderma gangrenosum (PG) is a neutrophilic dermatosis resulting in cutaneous ulceration(25). Lesions typically develop as a pustule or bulla that subsequently ulcerates with purulent drainage. The lesions have rapid onset and progression with necrotic borders and surrounding inflammation and erythema. Classically, PG lesions exhibit pathergy manifested by worsening in response to surgical debridement or biopsy. Often there is an underlying systemic immune disease such as inflammatory bowel disease, psoriasis, or ankylosing spondylitis (Figure 5). PG has also been described in association with hematologic diseases including acute and chronic myelogenous leukemia, hairy cell leukemia, myelodysplasia and IgA monoclonal gammopathy. While there are no specific diagnostic tests, the diagnosis hinges on the finding of neutrophilic infiltration in the dermis, with negative cultures and a typical history.
Figure 5.
A: Pydoerma gangrenosum associated with psoriatic arthropathy; B: Treatment of the underlying cytokine disturbance using the IL-12/IL-23 inhibitor ustekinumab resulted in complete healing of the wounds
Classically, PG lesions exhibit pathergy, manifested by worsening in response to surgical debridement or biopsy, and in the absence of clear cut pathologic findings this characteristic in the history can help diagnostically. Increasingly, cytokine drivers of PG have been recognized and treatment targeted at the underlying immune dysfunction in specific cases has been successful (26, 27)(Figure 5).
LEG ULCERS DUE TO ANCA-ASSOCIATED VASCULITIS
The Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitides include Granulomatosis with polyangiitis (GPA, formerly known as Wegener’s Granulomatosis), Microscopic Polyangiitis (MPA), and the Churg-Strauss Syndrome (CSS). All three of these forms of systemic vasculitis, as well as other forms of systemic vasculitis, have been associated with vasculitic leg ulcers(28, 29); however, no prevalence studies have been performed.
Pathologic findings in ANCA-associated vasculitic leg ulcers
In cases of systemic vasculitis but negative ANCA-serology, tissue biopsy is often helpful to confirm the diagnosis(28). Biopsy should include the subcuticular tissue and biopsy of early lesions is often most informative(30). Typically, leukocytoclastic vasculitis is seen with infiltration of arterioles and postcapillary venules by neutrophils undergoing degranulation and fragmentation. There is often fibrinoid necrosis of the inflamed vessels with vasculitis sometimes seen in medium and small arteries of the reticular dermis and fat. Direct immunofluorescence can be helpful to confirm immunoglobulin and complement deposits.
Management of ANCA-associated vasculitis
As with ulcers caused by other autoimmune etiologies, aggressive treatment of the underlying autoimmune disease often results in healing of the wound (Figure 6) (29, 31). With the advent of B-cell depletion therapy for ANCA associated vasculitis, several authors have reported successful treatment of vasculitic leg ulcers with rituximab (Rituxan®, Genentech) (31, 32).
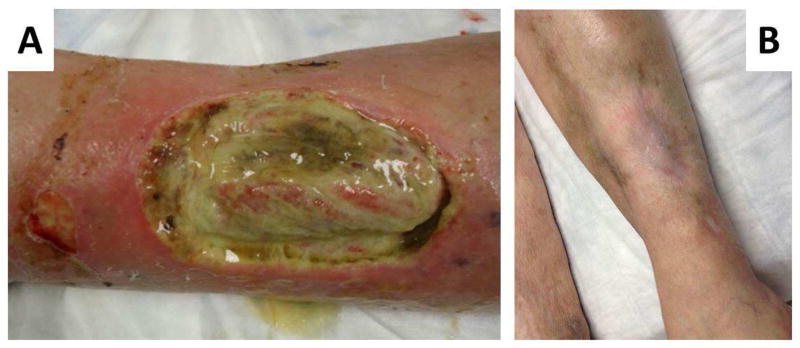
Figure 6.
A: Vasculitic ulcer associated with Granulomatosis with polyangiitis. B: Complete healing following rituximab therapy.
LEG ULCERS DUE TO THROMBOANGIITIS OBLITERANS (Buerger’s Disease)
Features of Thromboangiitis Obliterans
Thromboangiitis obliterans (TO, previously known as Buerger’s Disease) is a non-atherosclerotic, segmental inflammatory occlusion of small to medium sized arteries and veins. Most commonly, the disease affects arteries and veins of the arms, hands, legs, and feet(33). Ischemia of distal arteries and veins occur early on, with eventual progression of the disease to ischemia of more proximal arteries. Patients typically present with complaints of claudication of the extremities, but rest pain and ischemic ulceration can develop(33). Other presenting symptoms include splinter hemorrhages, Raynaud’s phenomenon, or livedo reticularis.
Prevalence of TO
TO affects men more commonly than women and is commonly associated with cigarette smoking. The prevalence of TO among patients with peripheral arterial disease ranges from as low as 0.5–5.6% in Western Europe as high as 45–63% in India, and up to 80% in the Jewish population of Ashkenazi ancestry living in Israel. TO should be considered in any patient with critical limb ischemia under the age of 50.
Pathologic findings in TO
In contrast to other forms of vasculitis, pathologic specimens in TO show highly cellular inflammatory occlusive thrombosis, but relative sparing of the blood vessel wall and internal elastic lamina. Acute phase reactants including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are usually normal and serologic markers and autoantibodies are typically negative(33).
Imaging findings in TO
Angiographic features of TO include distal segmental occlusive lesions interspersed with normal appearing arteries, with areas of collateralization around the occlusions termed “corkscrew collaterals”. Typically the disease is infrapopliteral in the lower extremities and distal to the brachial artery in the upper extremities. Proximal atherosclerotic lesions are usually absent. It should be noted that the segmental occlusive disease seen in TO can also be seen in the autoimmune and vasculitic diseases described above, including RA, Scleroderma and SLE and so these diseases should be ruled out serologically before attributing lesions to Buerger’s disease(33).
Management of TO
Although smoking appears to be a major inciting factor in TO, the pathologic mechanisms underlying this condition remain unclear. It has been postulated that smoking may cause delayed hypersensitivity or toxic angiitis. An association between periodontitis and smoking has been found, and bacterial DNA has been detected in the thrombotic samples. Others have postulated a role for endothelial cell antibodies in the pathogenesis of this disease and impaired endothelium-dependent vasodilation in the peripheral vasculature(33).
The mainstay of treatment for TO is smoking cessation. Smoking cessation reduces the risk of limb amputations and slows progression of the disease. Nicotine replacement therapy in these patients is not recommended and some patients continue to have claudication symptoms even after smoking cessation. While other therapeutic interventions proposed for TO include vasodilators, prostacyclin analogues, antiplatelet drugs, autologous whole bone marrow stem cell transplantation, and in some cases endovascular treatment, the only truly effective therapy is smoking cessation (Figure 7).
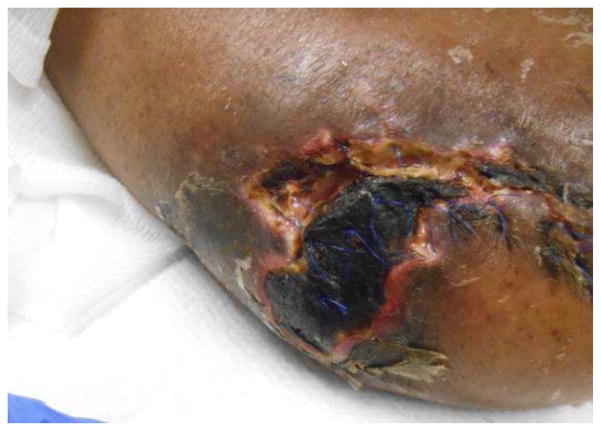
Figure 7.
Ulceration overlying below knee amputation site associated with Buerger’s disease in a patient who was unable to stop smoking
DIFFERENTIAL DIAGNOSIS OF VASCULITIC WOUNDS
Infections associated with vasculitic and chronic wounds
Several chronic infections are associated with cutaneous ulcerations and should be included in the differential when evaluating a patient who is not responding to usual therapy.
Hepatitis C can cause essential mixed cryoglobulinemia (Type II cryoglobulinemia), which can result in small to medium vessel vasculitis and ulceration (Figure 8). As with ANCA associated vasculitides B-cell directed therapy with rituximab has shown to be an effective treatment for these patients(34). With introduction of novel antiviral agents providing potential clearance of the hepatitis C it is likely that this complication will be seen less frequently(35).
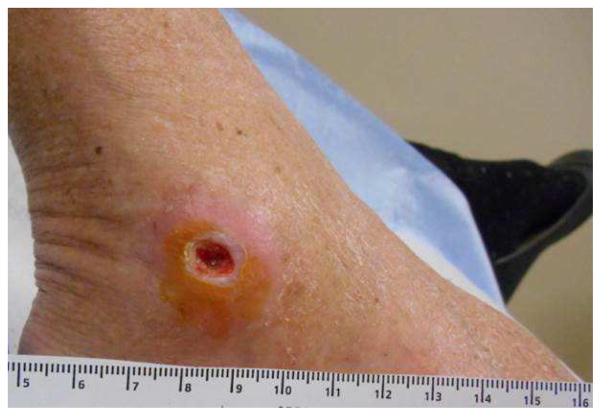
Figure 8.
Chronic non healing malleolar ulcer secondary to hepatitis C.
Hepatitis B is associated with polyarteritis nodosa (PAN) which can sometimes present with an isolated cutaneous vasculitis(36). While idiopathic PAN requires systemic immunosuppression the mainstay of management of hepatitis B associated PAN involves antiviral therapy.
Non-tuberculous mycobacteria should also be considered when a patient with a chronic wound is not responding to therapy. While first described as the Buruli ulcer in Africa these lesions can be seen in Australia and the US. Since atypical mycobacteria are slow growing and grow only under very specific culture conditions so it is imperative to request tissue biopsy with appropriate handling by the microbiology laboratory in order to ensure that the diagnosis can be made(37).
Erythema Nodosum and Panniculitis
Panniculitis is a disorder characterized by inflammation, induration and ulceration of subcutaneous adipose tissue. Panniculitis of the lower extremities may occur with nodular vasculitis, erythema nodosum, erythema induratum, or may be due to pancreatic disease or α-1 antitrypsin deficiency(38).
Erythema nodosum and erythema induratum are more common in young and middle aged women and classically presents with tender nodules and plaques on the anterior or posterior calf that ulcerate and drain(38). Biopsy shows mixed septal and lobular inflammatory infiltrate with vasculitis. The differential diagnosis includes TB, Sarcoidosis and certain medications (e.g. sulfonamides and oral contraceptives).
Pancreatic panniculitis is a rare suppurative panniculitis which may develop in benign or malignant pancreatic disease. Classically, patients present with painful subcutaneous nodules on the lower extremities and trunk which ulcerate with oily drainage. Patients may have systemic symptoms including fever, abdominal pain, arthritis, ascites and pleural effusions. Histologic examination reveals septal and lobular inflammation along with characteristic “ghost cells” which are pathognomic for saponification of the adipose tissue seen in pancreatic panniculitis(38).
In α-1 Antitrypsin deficiency patients develop a neutrophilic panniculitis with tender erythematous and purpuric nodules on the trunk and lower extremities which ulcerate and drain oily discharge before healing with scarring. Pathology shows neutrophilic infiltrate of the adipose tissue with necrosis and destruction of the fat lobules. Diagnosis is confirmed with serum α-1 antitrypsin activity and genotyping(38).
Sickle Cell Associated Leg Ulcers
Sickle Cell Disease (SCD) is an inherited hemoglobinopathy in which patients that are homozygous for hemoglobin S develop recurrent painful vaso-occlusive and hemolytic crises. Leg ulcers are a frequent complication of SCD and other hemoglobinopathies(39) affecting 2.5% of patients over 10 years old in the Cooperative Study of Sickle Cell Disease. Ulcer development is correlated with parameters of ongoing hemolysis such as low steady-state hemoglobin and high lactate dehydrogenase (LDH). Presence of HbF appears to be protective. This combined with perfusion studies suggests that ulceration in sickle cell disease may not be purely a vaso-occlusive process but more likely vasculopathy and inflammatory state related to chronic hemolysis (Figure 9).
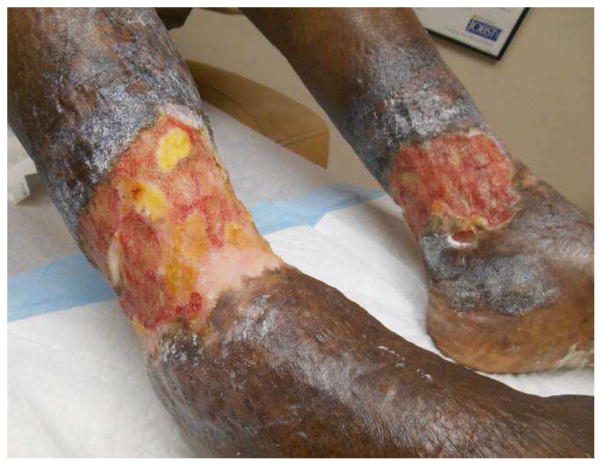
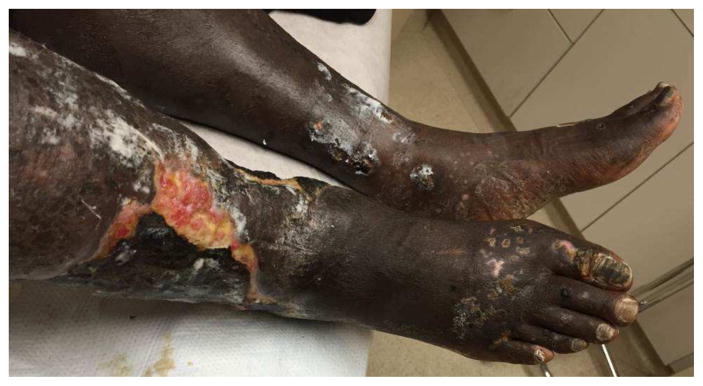
Figure 9.
Bilateral non-healing ulcers secondary to sickle cell disease
Hydroxyurea-associated leg ulcers
One of the most commonly used treatments for sickle cell disease is Hydroxyurea. Hydroxyurea is an antimetabolite that inhibits DNA repair by inhibiting ribonucleotide reductase and it is used in sickle cell disease to increase HbF production, thereby reducing the proportion of sickle hemoglobin. While Hydroxyurea is generally well tolerated it is known to be associated with dermatologic side effects including alopecia, diffuse hyperpigmentation, erythema, skin atrophy, an amyopathic dermatomyositis, nail changes, poikilodermatous dermatitis, and resistant leg ulcers. Leg ulceration is seen in approximately 9% of patients receiving hydroxyurea in the setting of myeloproliferative syndromes and in approximately 29% of patients taking hydroxyurea for management of sickle cell anemia(40). This complication seems to be dose dependent, with studies reporting an association with a mean cumulative exposure to hydroxyurea of 3.2 (range 1.4 to 5.5) kg and mean duration of hydroxyurea treatment of 6.1 (range 2 to 15) years. Biopsy specimens have shown nonspecific changes, with 1 series reporting epidermal atrophy, dermal fibrosis, and occasional fibrin occlusive vasculopathy similar to that seen in livedoid vasculitis(41). Although it has been postulated that the pathogenesis of leg ulcers relates in part to the underlying prothrombotic state, to date, the only effective therapy for these ulcers has been withdrawal of hydroxyurea, (Figure 10)(41, 42). In many cases, the underlying hematologic disease precludes this. However the introduction of oral janus kinase inhibitors for treatment of polycythemia has been successful in some patients(43).
Figure 10.
A: Hydroxyurea-associated ulcer in a patient with polycythemia. B: The ulcer healed after hydroxyurea was discontinued and the patient was changed to the JAK inhibitor ruxolitinib
Occlusive Diseases Resulting in Ulcers
Several diseases can be associated with occlusion of dermal vessels with thrombi or other occlusive material (such as cholesterol emboli or immune complexes). These ulcers are usually acute in onset and exquisitely painful yet the patient has intact pulses. Careful work up is needed to identify the appropriate underlying etiology through blood work including autoimmune and prothrombotic work-up, (Table I) and histopathology as outlined below.
Table I.
Laboratory testing to investigate autoimmune and prothrombotic states in patients with chronic non-healing wounds.
| Test | Disease detected |
|---|---|
| Anti-nuclear antibody | Systemic Lupus Erythematosus and other autoimmune diseases |
| Anti-Smith antibody | Systemic Lupus Erythematosus |
| Anti-dsDNA antibody | Systemic Lupus Erythematosus |
|
Complement C3 Complement C4 |
Systemic Lupus Erythematosus (low in active disease) |
| Anti-Centromere antibody | Scleroderma |
| Anti-Scl70 antibody | Scleroderma |
| Anti-ribonuclear protein (RNP) | Mixed connective tissue disease |
| SSA and SSB antibodies | Sjogrens Syndrome |
| Rheumatoid Factor | Rheumatoid Arthritis |
| Anti-Cyclic Citrullinated Peptide | Rheumatoid Arthritis |
| Anti-neutrophil cytoplasmic antibodies | Granulomatosis with polyangiitis, Microscopic Polyangiitis, Eosinophilic granulomatosis with polyangiitis, Cocaine and Levamisole associated vasculitis |
| Anti-β2-glycoprotein I antibodies | Antiphospholipid syndrome |
| Anti-cardiolipin antibodies | Antiphospholipid syndrome |
| Lupus Anticoagulant | Antiphospholipid syndrome |
| Prothrombin gene mutation | Genetic prothrombotic state |
| Factor-V Leiden mutation | Genetic prothrombotic state |
| Plasminogen Activator Inhibitor | Genetic prothrombotic state |
| MTHFR mutation | Genetic prothrombotic state |
| Quantiferon gold | Tuberculosis exposure |
| HIV test | HIV |
| Hepatitis B and C | Hepatitis B and C |
Atrophie Blanche or Livedoid Vasculopathy
Atrophie blanche or Livedoid Vasculopathy (LV) is a chronic small vessel vasculopathy which causes recurrent leg ulcers that heal with the formation of stellate porcelain white scars (Figure 11). It is often bilateral, involving the skin of the gaiter area around the dorsal ankle and foot. It is more common in women(44). Reported etiologies for LV include autoimmune diseases (45–53), hypercoagulable states and situations with impaired fibrinolysis(54). However, approximately one third of cases are idiopathic. On biopsy characteristic pathologic findings include the presence of hyaline thrombi in the mid and upper dermal vessels with fibrinoid changes.
Figure 11.
A: Livedoid vasculopathy leg ulceration B: Healing with stellate porcelain white scar formation which is classic for this vasculopathic disease
Cholesterol Emboli
In patients with vascular disease or those undergoing vascular procedures, cholesterol emboli should be considered as a potential explanation for leg ulceration. Typically patients present acutely with painful livedo reticularis and gangrene of a digit or limb – a syndrome commonly referred to as “blue toe syndrome”(55, 56). This syndrome is more common in men over 50 with atherosclerosis and often there is a history of recent (hours to days) arterial manipulation. Sometimes the syndrome is described as being precipitated by initiation of anticoagulation; however, this relationship has not been well delineated. Histopathology of affected skin and tissue will demonstrate elongated cholesterol-clefts in the deep dermal arterioles. It is important to recognize this etiology for leg ulceration since it has a high mortality. Treatment includes removal or stenting of unstable atheromatous plaques as well as initiation of statins(16).
Calciphylaxis
Calciphylaxis is a calcific uremic arteriolopathy seen in patients with renal failure. It results in progressive occlusion of dermal vessels and manifests with acutely painful, indurated plaques that develop necrosis and ulceration (Figure 12). It carries a very poor prognosis with less than 50% 1 year survival(57). Diagnosis is histopathologic but requires full thickness biopsy of an involved region – calcium deposition in the media of adipose vasculature is required to make the diagnosis. Peri-eccrine calcium deposition is a specific but not sensitive finding. Treatments focused on surgical wound debridement and lowering the calcium-phosphate product have some benefit, but parathyroidectomy is generally not effective. Numerous other treatments including sodium thiosulphate have been tried for this condition but with conflicting results (58, 59).
Figure 12.
Leg ulceration due to calciphylaxis with characteristic gangrenous tissue necrosis.
Cryoglobulin
Cryoglobulins are immune complexes that precipitate at temperatures below 37°C. There are several types.
Type I cryoglobulins are usually IgM class and are associated with hematologic malignancies such as Waldenstroms macroglobulinemia and multiple myeloma. Patients with this type of cryoglobulinemia develop symptoms of hyperviscosity and acral purpura, especially in cold weather. Histopathologic examination shows bland hyaline thrombi or red cell occlusion of superficial dermal vessels.
Types II and III are mixed cryoglobulins, i.e. they are composed of monoclonal or polyclonal immunoglobulins, often with rheumatoid factor activity (recognizing the Fc portion of IgG). These types of cryoglobulins are associated with chronic viral infections (such as Hepatitis B, C and HIV) and with autoimmune diseases (lupus, RA, Sjogrens Syndrome). Both types II and III mixed cryoglobulinemia can present with cutaneous ulceration and a prodrome of arthralgias, myalgias and fatigue. Cryoglobulins are easily missed unless the laboratory is instructed to draw blood into syringes and collection tubes that have been pre-warmed to 37°C without anticoagulants. After clotting at 37°C for one hour, the serum is then separated by centrifugation at 37°C and placed in a graduated (Wintrobe) tube at 4°C for three to five days to allow precipitation of the cryoglobulin. The cryocrit is measured by comparing the centrifuged volume of the precipitate as a percentage of the original serum volume at 4°C.
Prothrombotic wounds
Antiphospholipid syndrome
Leg ulceration is a recognized complication in patients with antiphospholipid antibody syndrome (APLS). Antiphospholipid antibodies react with the phospholipid portion of the prothrombin activator complex and can cause occlusion of the small dermal vessels. Typically these patients present with painful livedo reticularis, splinter hemorrhages, leg ulcers, superficial thrombophlebitis and focal ischemia. Livedo reticularis can be associated with arterial lesions and multiple thromboses in APLS but tends to be less common in patients who only experience venous thrombosis(60). Treatment with anticoagulants, particularly those with an anti-fibrinolytic action, is beneficial for healing.
Genetic prothrombotic states
Several genetic prothrombotic states have been reported in patients with chronic wounds, and coagulopathies are a recognized comorbidity in young patients with leg ulcers(61) and patients with livedoid vasculopathy. Correlating with this clinical observation we have been able to demonstrate the impact of prothrombotic states on human skin graft failure using a correlative model from the Wound Healing and Etiology (WE-HEAL) study (62). In patients with chronic wounds that are refractory to standard wound care genetic prothrombotic work-up is recommended as listed in Table I. Evidence from small studies supports use of fibrinolytic agents and anticoagulation in patients with livedoid vasculopathy or other wounds with an associated prothrombotic state(63, 64).
RECOMMENDATIONS FOR EVALUATION AND MANAGEMENT OF RECALCITRANT LEG ULCERS
In a limb salvage program, autoimmune and vasculitic etiologies should be considered in patients with chronic wounds who do not respond to appropriate vascular intervention and standard local wound care. A multidisciplinary approach with collaboration with rheumatology allows investigation for underlying systemic disease contributing to delayed wound healing in these cases. When vasculitis is in the differential diagnosis and a biopsy is planned, the team should be careful to include reticular dermis and subcutaneous tissue. A methodical approach with meticulous history and physical exam along with laboratory work-up (as outlined in Table I) improves clinical outcomes for many of these challenging patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escandon J, Vivas AC, Tang J, Rowland KJ, Kirsner RS. High mortality in patients with chronic wounds. Wound Repair and Regeneration. 2011;19(4):526–8. doi: 10.1111/j.1524-475X.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 3.Price P, Harding K. The impact of foot complications on health-related quality of life in patients with diabetes. J Cutan Med Surg. 2000;4(1):45–50. doi: 10.1177/120347540000400112. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clinics in Dermatology. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Körber A, Klode J, Al-Benna S, Wax C, Schadendorf D, Steinstraesser L, et al. Etiology of chronic leg ulcers in 31, 619 patients in Germany analyzed by an expert survey. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2011;9(2):116–21. doi: 10.1111/j.1610-0387.2010.07535.x. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugam VK, Schilling A, Germinario A, Mete M, Kim P, Steinberg J, et al. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre. International Wound Journal. 2011:1–9. doi: 10.1111/j.1742-481X.2011.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanmugam V, DeMaria D, Attinger C. Lower extremity ulcers in rheumatoid arthritis: features and response to immunosuppression. Clinical Rheumatology. 2011;30(6):849–53. doi: 10.1007/s10067-011-1710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oien RF, Hakansson A, Hansen BU. Leg ulcers in patients with rheumatoid arthritis--a prospective study of aetiology, wound healing and pain reduction after pinch grafting. Rheumatology. 2001;40(7):816–20. doi: 10.1093/rheumatology/40.7.816. [DOI] [PubMed] [Google Scholar]
- 9.Chia HY, Tang MB. Chronic leg ulcers in adult patients with rheumatological diseases - a 7-year retrospective review. Int Wound J. 2014;11(6):601–4. doi: 10.1111/iwj.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obara H, Matsubara K, Fujimura N, Sekimoto Y, Kitagawa Y. Preliminary Report of Endovascular Treatment for Critical Limb Ischemia Patients with Connective Tissue Disease: Cases Series and Review of the Literature. Int J Angiol. 2015;24(2):137–42. doi: 10.1055/s-0035-1547516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Current Opinion in Rheumatology. 2015;27(1):63–70. doi: 10.1097/BOR.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 12.Assmann G, Pfreundschuh J, Voswinkel J. Rituximab in patients with rheumatoid arthritis and vasculitis-associated cutaneous ulcers. Clin Exp Rheumatol. 2010;28(1 Suppl 57):81–3. [PubMed] [Google Scholar]
- 13.Sayah A, English JC., III Rheumatoid arthritis: A review of the cutaneous manifestations. Journal of the American Academy of Dermatology. 2005;53(2):191–209. doi: 10.1016/j.jaad.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam VK, Fernandez S, Evans KK, McNish S, Banerjee A, Couch K, et al. Postoperative wound dehiscence: predictors and associations. Wound Repair and Regeneration. 2015 doi: 10.1111/wrr.12268. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jebakumar AJ, Udayakumar PD, Crowson CS, Gabriel SE, Matteson EL. Occurrence and Effect of Lower Extremity Ulcer in Rheumatoid Arthritis — A Population-based Study. The Journal of Rheumatology. 2014;41(3):437–43. doi: 10.3899/jrheum.130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabiri G, Falanga V. Connective tissue ulcers. Journal of Tissue Viability. 2013;22(4):92–102. doi: 10.1016/j.jtv.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakakibara K, Matsumoto M, Motohashi S, Suzuki S. Recurrent refractory arterial thromboembolism in a patient with anti-phospholipid antibody syndrome. Intern Med. 2013;52(10):1145–6. doi: 10.2169/internalmedicine.52.0185. [DOI] [PubMed] [Google Scholar]
- 18.Goslen JB. Autoimmune ulceration of the leg. Clin Dermatol. 1990;8(3–4):92–117. doi: 10.1016/0738-081x(90)90050-b. [DOI] [PubMed] [Google Scholar]
- 19.Fink AM, Kottas-Heldenberg A, Bayer PM, Bednar R, Steiner A. Lupus anticoagulant in patients with chronic venous insufficiency. Acta Derm Venereol. 2003;83(4):287–9. doi: 10.1080/00015550310016553. [DOI] [PubMed] [Google Scholar]
- 20.Manzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Annals of the Rheumatic Diseases. 2012;71(11):1833–8. doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanmugam V, Price P, Attinger C, Steen V. Lower Extremity Ulcers in Systemic Sclerosis: Features and Response to Therapy. Int J Rheumatol. 2010 doi: 10.1155/2010/747946. pii:747946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagojevic J, Piemonte G, Benelli L, Braschi F, Fiori G, Bartoli F, et al. Assessment, Definition, and Classification of Lower Limb Ulcers in Systemic Sclerosis: A Challenge for the Rheumatologist. The Journal of Rheumatology. 2016;43(3):592–8. doi: 10.3899/jrheum.150035. [DOI] [PubMed] [Google Scholar]
- 23.Hafner J, Schneider E, Burg G, Cassina PC. Management of leg ulcers in patients with rheumatoid arthritis or systemic sclerosis: the importance of concomitant arterial and venous disease. J Vasc Surg. 2001;32(2):322–9. doi: 10.1067/mva.2000.106942. [DOI] [PubMed] [Google Scholar]
- 24.Deguchi J, Shigematsu K, Ota S, Kimura H, Fukayama M, Miyata T. Surgical result of critical limb ischemia due to tibial arterial occlusion in patients with systemic scleroderma. J Vasc Surg. 2009;49(4):918–23. doi: 10.1016/j.jvs.2008.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): An updated review. Journal of the American Academy of Dermatology. 2015;73(4):691–8. doi: 10.1016/j.jaad.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Kaur MR, Lewis HM. Severe recalcitrant pyoderma gangrenosum treated with infliximab. British Journal of Dermatology. 2005;153(3):689–91. doi: 10.1111/j.1365-2133.2005.06812.x. [DOI] [PubMed] [Google Scholar]
- 27.Guenova E, Teske A, Fehrenbacher B, Hoerber S, Adamczyk A, Schaller M, et al. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Archives of Dermatology. 2011;147(10):1203–5. doi: 10.1001/archdermatol.2011.168. [DOI] [PubMed] [Google Scholar]
- 28.Perniciaro CV, Winkelmann RK, Hunder GG. Cutaneous manifestations of Takayasu’s arteritis. A clinicopathologic correlation. J Am Acad Dermatol. 1987;17(6):998–1005. doi: 10.1016/s0190-9622(87)70289-8. [DOI] [PubMed] [Google Scholar]
- 29.Boudny C, Nievergelt H, Braathen LR, Simon D. Wegener’s granulomatosis presenting as pyoderma gangrenosum. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2008;6(6):477–9. doi: 10.1111/j.1610-0387.2007.06497.x. [DOI] [PubMed] [Google Scholar]
- 30.Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 56(1):3–23. doi: 10.1111/j.1365-2559.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami T, Okano T, Soma Y. Rituximab therapy for deep toe ulcer with microscopic polyangiitis refractory to corticosteroids and cyclophosphamide. The Journal of Dermatology. 2014;41(2):191–2. doi: 10.1111/1346-8138.12396. [DOI] [PubMed] [Google Scholar]
- 32.Patel FB, Couch KS, McNish S, Miller JD, Siegel R, Easley S, et al. A 66 year-old woman with hemoptysis. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olin JW. Thromboangiitis Obliterans (Buerger’s Disease) New England Journal of Medicine. 2000;343(12):864–9. doi: 10.1056/NEJM200009213431207. [DOI] [PubMed] [Google Scholar]
- 34.Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64(3):835–42. doi: 10.1002/art.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dammacco F, Sansonno D. Therapy for Hepatitis C Virus–Related Cryoglobulinemic Vasculitis. New England Journal of Medicine. 2013;369(11):1035–45. doi: 10.1056/NEJMra1208642. [DOI] [PubMed] [Google Scholar]
- 36.De Virgilio A, Greco A, Magliulo G, Gallo A, Ruoppolo G, Conte M, et al. Polyarteritis nodosa: A contemporary overview. Autoimmunity Reviews. doi: 10.1016/j.autrev.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Raju RM, Raju SM, Zhao Y, Rubin EJ. Leveraging Advances in Tuberculosis Diagnosis and Treatment to Address Nontuberculous Mycobacterial Disease. Emerg Infect Dis. 2016;22(3):365–9. doi: 10.3201/eid2203.151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan MP. Neutrophilic Panniculitis: Algorithmic Approach to a Heterogeneous Group of Disorders. Archives of Pathology & Laboratory Medicine. 2014;138(10):1337–43. doi: 10.5858/arpa.2014-0270-CC. [DOI] [PubMed] [Google Scholar]
- 39.Koshy M, Entsuah R, Koranda A, Kraus A, Johnson R, Bellvue R, et al. Leg ulcers in patients with sickle cell disease [see comments] Blood. 1989;74(4):1403–8. [PubMed] [Google Scholar]
- 40.Chaine B, Neonato M, Girot R, Aractingi S. Cutaneous adverse reactions to hydroxyurea in patients with sickle cell disease. Archives of Dermatology. 2001;137(4):467–70. [PubMed] [Google Scholar]
- 41.Sirieix M, Debure C, Baudot N, Dubertret L, Roux M, Morel P, et al. Leg ulcers and hydroxyurea: Forty-one cases. Archives of Dermatology. 1999;135(7):818–20. doi: 10.1001/archderm.135.7.818. [DOI] [PubMed] [Google Scholar]
- 42.Best PJ, Daoud MS, Pittelkow MR, Petitt RM. Hydroxyurea-Induced Leg Ulceration in 14 Patients. Annals of Internal Medicine. 1998;128(1):29–32. doi: 10.7326/0003-4819-128-1-199801010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Shanmugam VK, McNish S, Shara N, Hubley KJ, Kallakury B, Dunning DM, et al. Chronic Leg Ulceration Associated with Polycythemia Vera Responding to Ruxolitinib (Jakafi®) The Journal of Foot and Ankle Surgery. doi: 10.1053/j.jfas.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alavi A, Hafner J, Dutz JP, Mayer D, Sibbald RG, Criado PR, et al. Atrophie Blanche: Is It Associated with Venous Disease or Livedoid Vasculopathy? Advances in Skin & Wound Care. 2014;27(11):518–24. doi: 10.1097/01.ASW.0000455098.98684.95. [DOI] [PubMed] [Google Scholar]
- 45.Acland KM, Darvay A, Wakelin SH, Russell-Jones R. Livedoid vasculitis: a manifestation of the antiphospholipid syndrome? British Journal of Dermatology. 1999;140(1):131–5. doi: 10.1046/j.1365-2133.1999.02622.x. [DOI] [PubMed] [Google Scholar]
- 46.Callen JP. Livedoid Vasculopathy: What It Is and How the Patient Should Be Evaluated and Treated. Arch Dermatol. 2006;142(11):1481–2. doi: 10.1001/archderm.142.11.1481. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso R, Gonçalo M, Tellechea O, Maia R, Borges C, Silva JAP, et al. Livedoid vasculopathy and hypercoagulability in a patient with primary Sjögren’s syndrome. International Journal of Dermatology. 2007;46(4):431–4. doi: 10.1111/j.1365-4632.2007.03229.x. [DOI] [PubMed] [Google Scholar]
- 48.Frances C, Barete S. Difficult Management of Livedoid Vasculopathy. Arch Dermatol. 2004;140(8):1011. doi: 10.1001/archderm.140.8.1011. [DOI] [PubMed] [Google Scholar]
- 49.Jorizzo JL. Livedoid Vasculopathy: What Is It? Arch Dermatol. 1998;134(4):491–3. doi: 10.1001/archderm.134.4.491. [DOI] [PubMed] [Google Scholar]
- 50.Mimouni D, Ng PP, Rencic A, Nikolskaia OV, Bernstein BD, Nousari HC. Cutaneous polyarteritis nodosa in patients presenting with atrophie blanche. British Journal of Dermatology. 2003;148(4):789–94. doi: 10.1046/j.1365-2133.2003.05176.x. [DOI] [PubMed] [Google Scholar]
- 51.Tran M, Becherel P, Cordel N, Piette JC, Frances C. “Idiopathic” livedoid vasculitis. Ann Dermatol Venerol. 2001;128:1003–7. [PubMed] [Google Scholar]
- 52.Wakelin SH, Ellis JP, Black MM. Livedoid vasculitis with anticardiolipin antibodies: improvement with danazol. Br J Dermatol. 1998;139(5):935–7. doi: 10.1046/j.1365-2133.1998.02541.x. [DOI] [PubMed] [Google Scholar]
- 53.Winkelmann R, Schroeter A, Kierland R, Ryan T. Clinical studies of livedoid vasculitis: (segmental hyalinizing vasculitis) Mayo Clinic Proceedings. 1974;49(10):746–50. [PubMed] [Google Scholar]
- 54.Hairston BR, Davis M, Pittelkow MR, Ahmed I. Livedoid Vasculopathy: Further Evidence for Procoagulant Pathogenesis. Arch Dermatol. 2006;142(11):1413–8. doi: 10.1001/archderm.142.11.1413. [DOI] [PubMed] [Google Scholar]
- 55.Charabaty S, Shanmugam V. A 65-year-old man with longstanding seropositive rheumatoid arthritis and lower extremity ulceration. Arthritis Care & Research. 2009;61(9):1275–80. doi: 10.1002/art.24700. [DOI] [PubMed] [Google Scholar]
- 56.Falanga V, Fine MJ, Kapoor WN. THe cutaneous manifestations of cholesterol crystal embolization. Archives of Dermatology. 1986;122(10):1194–8. [PubMed] [Google Scholar]
- 57.Weenig RH, Sewell LD, Davis MDP, McCarthy JT, Pittelkow MR. Calciphylaxis: Natural history, risk factor analysis, and outcome. Journal of the American Academy of Dermatology. 2007;56(4):569–79. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 58.Malbos S, Ureña-Torres P, Bardin T, Ea H-K. Sodium thiosulfate is effective in calcific uremic arteriolopathy complicating chronic hemodialysis. Joint Bone Spine. 2016;83(1):89–92. doi: 10.1016/j.jbspin.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Lonowski S, Martin S, Worswick S. Widespread calciphylaxis and normal renal function: no improvement with sodium thiosulfate. Dermatology online journal. 2015;21(5) [PubMed] [Google Scholar]
- 60.Francès C, Niang S, Laffitte E, Pelletier Fl, Costedoat N, Piette JC. Dermatologic manifestations of the antiphospholipid syndrome: Two hundred consecutive cases. Arthritis & Rheumatism. 2005;52(6):1785–93. doi: 10.1002/art.21041. [DOI] [PubMed] [Google Scholar]
- 61.Wiszniewski A, Bykowska K, Bilski R, Jaśkowiak W, Proniewski J. Prevalence rate for inherited thrombophilia in patients with chronic and recurrent venous leg ulceration. Wound Repair and Regeneration. 2011;19(5):552–8. doi: 10.1111/j.1524-475X.2011.00716.x. [DOI] [PubMed] [Google Scholar]
- 62.Shanmugam VK, McNish S, Duncan J, Root B, Tassi E, Wellstein A, et al. Late failure of a split-thickness skin graft in the setting of homozygous factor V Leiden mutation: a case report and correlative animal model from the Wound Etiology and Healing (WE-HEAL) study. International Wound Journal. 2013 doi: 10.1111/iwj.12156. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sams W. Livedo vasculitis. Therapy with pentoxifylline. Arch Dermatol. 1988;124(5):684–7. [PubMed] [Google Scholar]
- 64.Weishaupt C, Strölin A, Kahle B, Kreuter A, Schneider SW, Gerss J, et al. Anticoagulation with rivaroxaban for livedoid vasculopathy (RILIVA): a multicentre, single-arm, open-label, phase 2a, proof-of-concept trial. The Lancet Haematology. 3(2):e72–e9. doi: 10.1016/S2352-3026(15)00251-3. [DOI] [PubMed] [Google Scholar]