Abstract
Background
Ventricular tachycardia (VT) and premature ventricular complexes (PVC) most frequently occur in the context of structural heart disease. However, the burden of idiopathic ventricular arrhythmias (VA) in the general population is unknown.
Methods and Results
We identified incident cases of idiopathic VA between 2005and 2013 from Olmsted County, MN using the Rochester Epidemiology Project database. For PVC cohorts, we included those with frequent (defined as ≥100 PVC/24 hours) symptomatic PVCs. We defined idiopathic VA-associated cardiomyopathy as an EF drop of ≥10% from baseline. Between 2005 and 2013, we identified 614 individuals with incident idiopathic VA. (229 [37.3%] were male, average age was 52.1 ± 17.2 years). Of these, 177 (28.8%) had idiopathic VT, 408 (66.5%) had symptomatic PVCs and 29 (4.7%) had idiopathic VA-associated cardiomyopathy. The age- and sex- adjusted incidence rates in 2005–2007, 2008–2010 and 2011–2013 were 44.9 per 100,000 (95% CI 38.0–51.8), 47.6 per 100,000 (95% CI 40.8–54.5) and 62.0 per 100,000 (95% CI 54.4–69.6), respectively. In idiopathic VT, there was an increase in incidence rate with age s (P<0.001) but not between sexes (P=0.12). The age-adjusted incidence of symptomatic PVC was higher in females than males (46.2 per 100,000 [95% CI 40.9–51.6] versus 20.5 per 100,000 [95% CI 16.8–24.3], p<0.001). The small number of individuals with idiopathic VA-associated cardiomyopathy precluded any formal testing.
Conclusions
The incidence of idiopathic VA is increasing. Furthermore, overall incidence increases with age. While the rate of idiopathic VT is similar across genders, women have a higher incidence of symptomatic PVC.
Keywords: epidemiology, ventricular arrhythmia, premature ventricular contraction arrhythmia, ventricular tachycardia, idiopathic VT
Introduction
Ventricular tachycardia (VT) and premature ventricular complexes (PVC) most frequently occur in the context of a structurally abnormal heart, e.g. coronary artery disease, severe valvular heart disease or low ejection fraction (EF). However, ventricular arrhythmias (VAs) may also occur in individuals with no apparent structural heart disease. These so called ‘idiopathic VT’ cases are said to account for 10% of all VT diagnoses1. Although the prognosis is typically favorable compared to structural heart disease-associated VT, debilitating symptoms and even death have been reported2. Symptoms include fatigue, palpitations, dyspnea, presyncope, syncope (although rare in PVCs unless in the context of severely depressed cardiac function) and heart failure 3–5. Yet, while studies have tried to define the prevalence of idiopathic VA, data has predominantly originated from referral centers performing ablation procedures6. As such, the burden of idiopathic VAs is unknown and population-based data are scarce. Thus, the purpose of this study was to determine the incidence of idiopathic VA from a stable community-based population in South-East Minnesota. We postulated that, although relatively infrequent, incident cases of idiopathic VA are increasing in frequency given the increasing use of monitoring devices and device interrogation.
Methods
Study setting and cohort
This population-based cohort study was conducted within Olmsted County, Minnesota utilizing the Rochester Epidemiology Project (REP)7–10. The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. Even with racial and socioeconomic disparities in Olmsted County compared to the general US population, biological phenomena are generalizable to population at large. The geographic characteristics of this region enable epidemiological studies because Olmsted County is relatively isolated from other urban centers. Furthermore, care to the majority of Olmsted Country residents is provided by Mayo Clinic, Olmsted Medical Center and a few private care physicians each of whom use a comprehensive medical record system. All medical records of county residents are indexed, thus enabling retrieval of data whilst ensuring complete capture of all healthcare-related events occurring in the county for county residents. These sources provide information concerning virtually all the care delivered for Olmsted County residents. The medical records linkage system of the REP now encompasses 6,239,353 person-years of follow-up among 502,820 unique individuals attended heath care providers at least once between 1966 and 2010 (counting both current and previous residents)‥
The study period was 2005–2013. This period was chosen to reflect the current clinical practice and outcomes associated with idiopathic VT taking into considering the advancements in mapping and ablation that have occurred over the last 2 decades. The cohort consisted of patients aged 18 years and older with incident (first-ever) idiopathic VA (idiopathic VT, idiopathic PVC and idiopathic VA-associated cardiomyopathy – see below for definitions). Patients with VAs documented prior to 2005 (i.e., prevalent cases) were excluded. Asymptomatic patients with frequent PVCs and low EF at presentation during the study period were considered as prevalent cases and were excluded.
Development of the cohort
Given that our aim was to determine incidence of idiopathic VA, Olmsted County residents were reviewed for patients who had received a diagnosis of VA using International Classification of Diseases, Ninth Revision (ICD-9) codes for VT (427.1) or PVC (427.69). Individuals with a coexisting diagnosis of coronary artery disease (ICD-9 codes 410–415) were excluded from the study.
Trained abstractors reviewed the medical records and collected information on symptoms11, triggers12 and treatment strategies13‥ Palpitations, dizziness, fatigue, presyncope, syncope and heart failure symptoms attributable to PVCs at a frequency >100/24 hours were considered significant. In clinical practice symptoms that correlate with ectopy are often reported among patients with fairly low burden (<500/ 24hours). Taking day to day variability of IVA burden into consideration a threshold of >100/ 24 hours was chosen for the study. Patient reported triggers for palpitations were abstracted. Medical therapy for VA was grouped according to Vaughan-Williams classification. Baseline demographic and clinical characteristics, including hypertension, hyperlipidemia, chronic renal disease, diabetes mellitus, thyroid disease, and liver disease were obtained electronically from the medical record using diagnostic codes. Information regarding coronary artery disease (CAD) including angina presumed to be from CAD, prior myocardial infarction, physiologically significant epicardial coronary artery disease (lesion>70%) were abstracted from the medical records. Patients with underlying structural heart disease resulting from coronary artery disease, prior myocarditis, adult congenital heart disease, arrhythmogenic right ventricular cardiomyopathy, ion channelopathy, valvular heart disease (≥moderate valvular lesion) and cardiomyopathies (dilated with ejection fraction<50%, infiltrative or hypertrophic) were excluded after chart review. Data regarding changes in structural function (EF) or size (left ventricular end-diastolic diameter, LVEDD) were obtained by review of M-mode and 2D echocardiography when available.
Electrocardiographic information based on 12- lead electrocardiography (ECG) tracings, and ambulatory recordings (Holters and event monitors) was obtained. Premature ventricular contractions (PVCs) were defined as wide complex beats (QRS >120ms) without any preceding atrial activity14. Among patients with frequent supraventricular ectopic beats or atrial fibrillation, aberrancy was determined by typical QRS morphology and preceding long short sequence15. PVC burden was determined by dividing the number of ectopic beats by the number of total QRS complexes in 24 hours.
Patients with idiopathic VA were divided into three cohorts. The definitions of each are as follows:
Idiopathic Ventricular Tachycardia (VT): 3 or more sequential wide complex beats arising from the ventricles, rate ≥100bpm16.
Symptomatic PVCs: Frequent wide complex beats (≥100 beats/24 hours) occurring singly or in couplets arising from the ventricles associated with above described symptoms.
Idiopathic VA associated with cardiomyopathy (IVA-CM): Drop in EF ≥10% from baseline in the absence of other causes associated with cardiomyopathy.
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). Categorical variables were expressed as percentages; whereas continuous variables were expressed as mean ± standard deviation (SD) Comparison of categorical variables was performed using the chi-square test, whereas comparison of continuous variables was performed with the Analysis of Variance. All p-values were 2-sided, and p-values <0.05 were considered significant. The overall incidence was estimated using the age and sex- specific population figures in Olmsted County. Yearly incidence rates for each sex was determined by dividing the number of cases within that group by the estimated total Olmsted County resident population of the group for that given year. Population figures for 2000 and 2010 came from the US census data; figures for inter-census years were estimated by using linear interpolation. Rates were adjusted to age and/or sex distribution of the US white population from 2010. Poisson regression models were used to test for trends over time, and across age and sex.
Results
Using billing codes, we identified 2931 individuals satisfying our definition of idiopathic VA between 2005 and 2013. Of these, 355 patients were excluded either due to duplicate entries (182 patients) or prevalent diagnoses of VT/PVC i.e., prior to 2005 (173 patients). A further 1962 patients were excluded due to identification of structural heart disease or evidence of a supraventricular rhythm with aberrancy. Therefore, 614 patients remained that were suitable for inclusion between 2005–2013(Figure 1)
Figure 1.
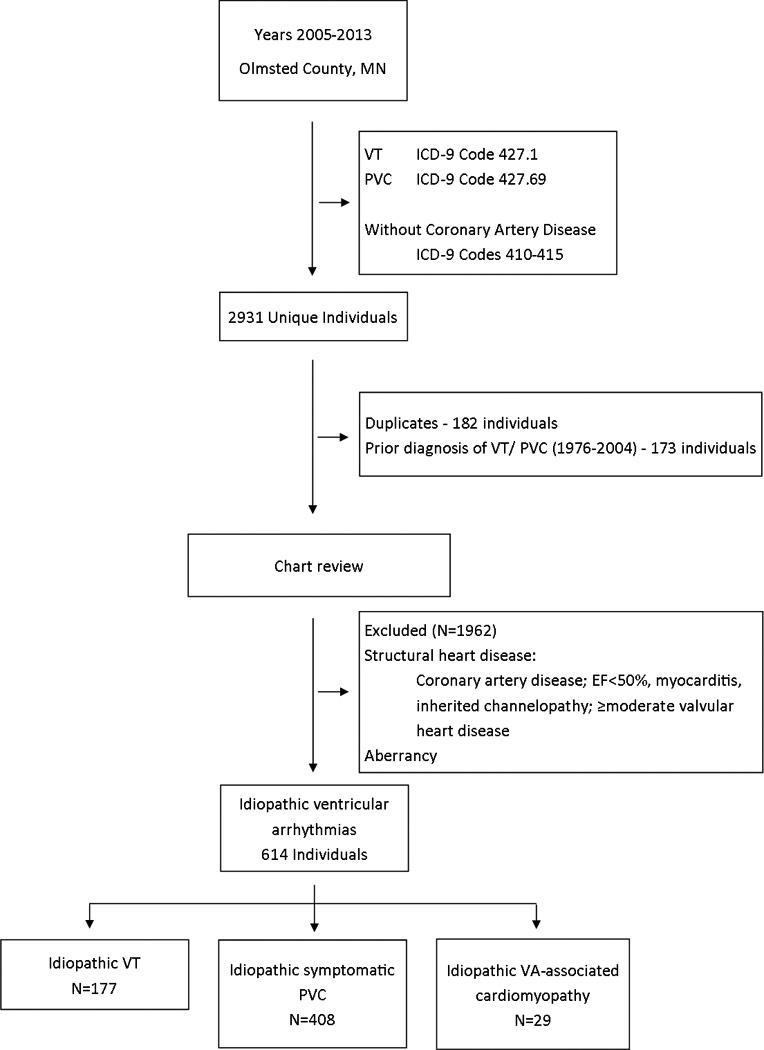
Consort diagram showing derivation of the idiopathic ventricular arrhythmia cohort. PVC, premature ventricular complex; VT, ventricular tachycardia.
Baseline characteristics
Of 614 patients, 177 (28.8%) had idiopathic VT, 408 (66.4%) had symptomatic PVCs and 29 (4.7%) had IVA-CM. The average age was 52.1 ± 17.2 years (range 18–93) and 229 (37.3%) were male. Of the overall cohort, 47% had dyslipidemia, 37% had hypertension, 16% had thyroid disease and 10% had diabetes mellitus. Only 8% had chronic kidney disease or liver disease (Table 1). Significant intergroup differences were seen for age and hypertension. Patients were older and had more comorbidities in the IVA-CM group. As expected, beta-blockers and calcium channel blockers were the most frequent medications used (45% of patients). Class I and III antiarrhythmic drugs were used in 5%; however, their use was less frequent in symptomatic/ frequent idiopathic PVC. Ablation was performed in 7.3% of patients (11.9% in idiopathic VT; 2.9% in symptomatic PVC; 37.9% in IVA-CM).
Table 1.
Clinical characteristics according to ventricular arrhythmia type.
| Total (614) |
Idiopathic VT (N=177) |
Symptomatic PVC (N= 408) |
IVA-CM (N=29) |
P-value | |
|---|---|---|---|---|---|
| Age | 52.1 ± 17.2 | 59.2 ± 17.6 | 48.5 ± 15.8 | 59.2 ± 19.0 | <0.001 |
| EF, % | 62.1 (4.9) | 61.7 (4.8) | 62.7 (4.8) | 59.8 (9.1) | 0.004 |
| LVEDD, mm | 48.7 (5.2) | 49.5 (6.3) | 48.0 (4.3) | 50.5 (4.9) | 0.03 |
| DM | 62 (10%) | 21 (12%) | 37 (9%) | 4 (14%) | 0.468 |
| CKD | 28 (4%) | 10 (6%) | 11 (3%) | 2 (7%) | 0.148 |
| HTN | 243 (37%) | 84 (47%) | 121 (30%) | 15 (52%) | <0.001 |
| Hyperlipidemia | 312 (47%) | 90 (51%) | 178 (44%) | 17 (59%) | 0.110 |
| Hyperthyroid | 19 (3%) | 5 (3%) | 12 (3%) | 1 (3%) | 0.983 |
| Hypothyroid | 88 (13%) | 26 (15%) | 50 (12%) | 2 (7%) | 0.453 |
| Liver disease | 23 (4%) | 8 (5%) | 12 (3%) | 2 (7%) | 0.395 |
| Vaughan-Williams class* | |||||
| I | 22 (4%) | 8 (5%) | 6 (1%) | 8 (28%) | <0.001 |
| II | 254 (41%) | 83 (47%) | 147 (36%) | 24 (83%) | <0.001 |
| III | 20 (3%) | 11 (6%) | 2 (1%) | 7 (24%) | <0.001 |
| IV | 85 (14%) | 34 (19%) | 40 (10%) | 11 (38%) | <0.001 |
| PVC burden † | 5.3 ± 8.1 | 7.5 ± 9.9 | 3.8 ± 6 | 9.1 ± 11 | <0.001 |
| Stimulation as trigger ‡ | 14 (7%) | 13 (7%) | 1 (1%) | <0.001 | |
EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; PVC, premature ventricular complex; DM, Diabetes Mellitus; HTN, Hypertension; CKD, chronic kidney disease; IVA-CM, idiopathic ventricular arrhythmia-associated cardiomyopathy; VT, ventricular tachycardia.
Vaughn- Williams Classification is based on the action mechanism of drugs. Note that the numbers of patients treated with medications do not add up to 100%.
PVC Burden= PVC count/total number of beats expressed as %.
Stimulation includes exercise, pain and caffeine.
Average EF and LVEDD were 62.1 ± 4.9% and 48.7 ± 5.2 mm (normal range 40–54mm), respectively. Both EF and LVEDD were significantly different between groups (p=0.004 and p=0.03 respectively) although the actual differences are small in magnitude. Baseline characteristics are summarized in table 1.
Overall incidence
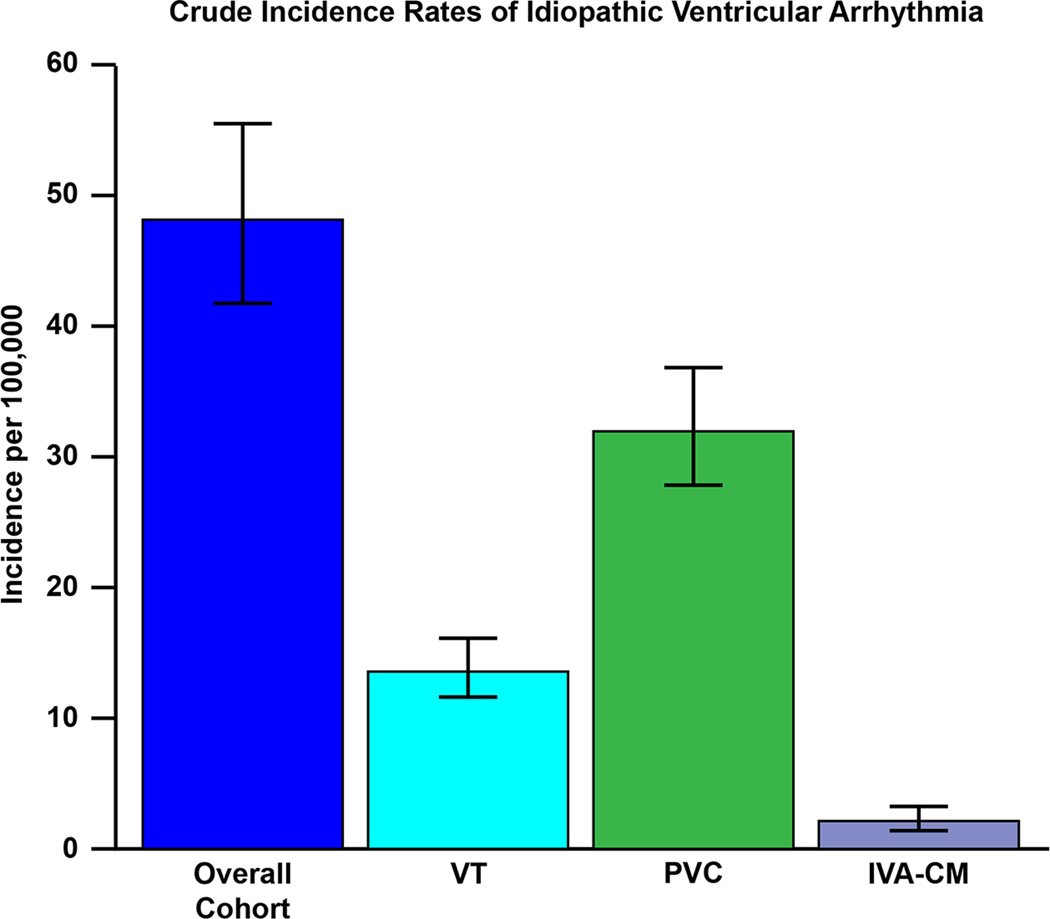
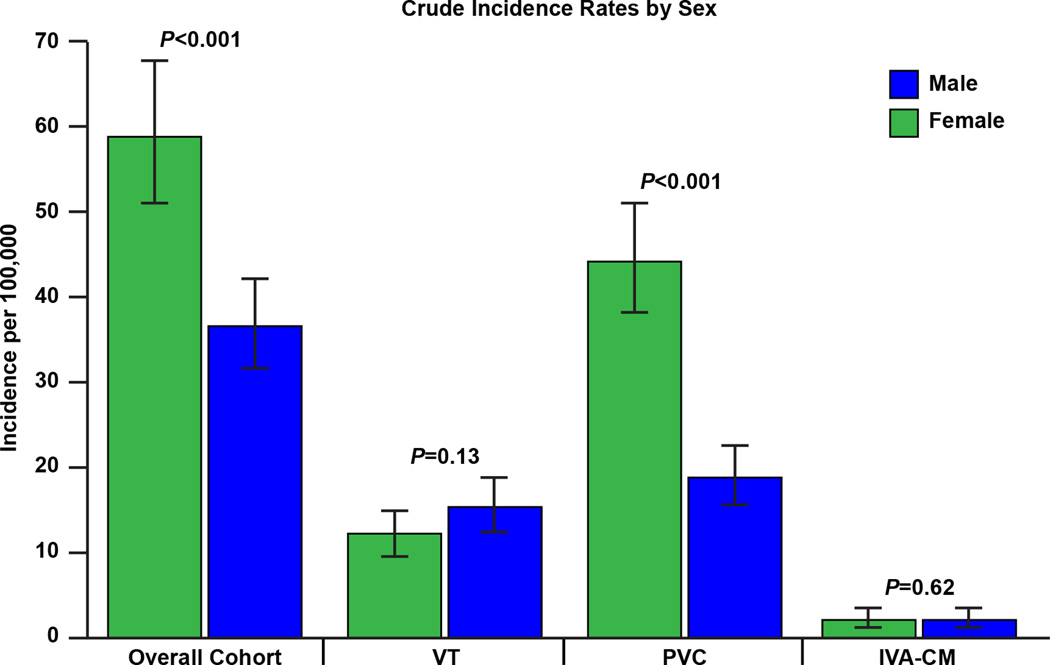
The crude incidence rates of idiopathic VA, idiopathic VT symptomatic PVC, and IVA-CM are shown in table 2 and figure 2. The overall age and sex-adjusted incidence of idiopathic VA was 51.9 per 100,000 (95% CI 47.7–56.0). This predominantly consisted of symptomatic PVCs, with an age and sex-adjusted incidence rate of 33.5 (30.2–36.8) per 100,000. Of note, the age-adjusted incidence of VA overall was greater in females than males (62.0 per 100,000 [95% CI 55.7–68.2] versus 42.4 per 100,000 [95% CI 36.8–48.0], p<0.001) (figure 3). Interestingly, there appeared to be a significant difference in crude incidence rates of idiopathic VA between males and females, both across age groups and over time (p<0.001 for all) (figures 4 & 5A).
Table 2.
Table demonstrating the incidence rate of idiopathic ventricular arrhythmias.
| Overall cohort (N=614) |
Idiopathic VT (N=177) |
Symptomatic PVC (N=408) |
IVA-CM (N=29) |
|
|---|---|---|---|---|
| Overall crude incidence rate | 48.10 (41.75–55.41) | 13.85 (11.92–16.09) | 31.93 (27.72–36.78) | 2.27 (1.52–3.27) |
| ○ Males | 36.65 (31.81–42.22) | 15.52 (12.59–18.93) | 18.88 (15.69–22.69) | 2.24 (1.22–3.76) |
| ○ Females | 58.97 (51.19–67.93) | 12.25 (9.71–15.19) | 44.42 (38.56–51.17) | 2.30 (1.29–3.80) |
| ○ 2005–2007 | 41.34 (35.43–48.20) | 12.71 (9.49–16.65) | 26.42 (22.25–32.02) | |
| ○ 2008–2010 | 44.05 (38.06–50.92) | 12.42 (9.30–16.27) | 29.52 (24.68–35.28) | |
| ○ 2011–2013 | 58.13 (50.46–66.97) | 16.29 (12.74–20.53) | 39.36 (33.81–45.78) | |
| Age-adjusted incidence rate | 52.05 (47.90–56.01) | 15.79 (13.45–18.13) | 33.72 (30.42–37.02) | 2.55 (1.61–3.48) |
| ○ Males | 42.43 (36.83–48.03) | 19.18 (15.29–23.06) | 20.54 (16.77–24.31) | 2.72 (1.27–4.17) |
| ○ Females | 61.97 (55.74–68.20) | 13.31 (10.37–16.24) | 46.22 (40.87–51.57) | 2.44 (1.20–3.68) |
| Age- and sex-adjusted | 51.86 (47.72–56.01) | 15.80 (13.46–18.15) | 33.51 (30.23–36.80) | 2.55 (1.61–3.48) |
| ○ 2005–2007 | 44.91 (38.04–51.78) | 14.90 (10.80–18.99) | 27.52 (22.26–32.78) | |
| ○ 2008–2010 | 47.62 (40.76–54.49) | 13.91 (10.15–17.68) | 31.32 (25.80–36.84) | |
| ○ 2011–2013 | 62.01 (54.37–69.65) | 18.41 (14.14–22.69) | 40.84 (34.73–46.95) | |
Rates per 100,000 (95% CIs).
PVC, premature ventricular complex; IVA-CM, idiopathic ventricular arrhythmia-associated cardiomyopathy; VT, ventricular tachycardia.
Figure 2.
Crude incidence rates of idiopathic ventricular arrhythmia overall, and according to subgroups. VT, ventricular tachycardia; PVC, premature ventricular complex; IVA-CM, idiopathic ventricular arrhythmia-associated cardiomyopathy.
Figure 3.
Crude incidence rates of idiopathic ventricular arrhythmia overall and according to subgroups according to gender. VT, ventricular tachycardia; PVC, premature ventricular complex; IVA-CM, asymptomatic premature ventricular complex-associated cardiomyopathy.
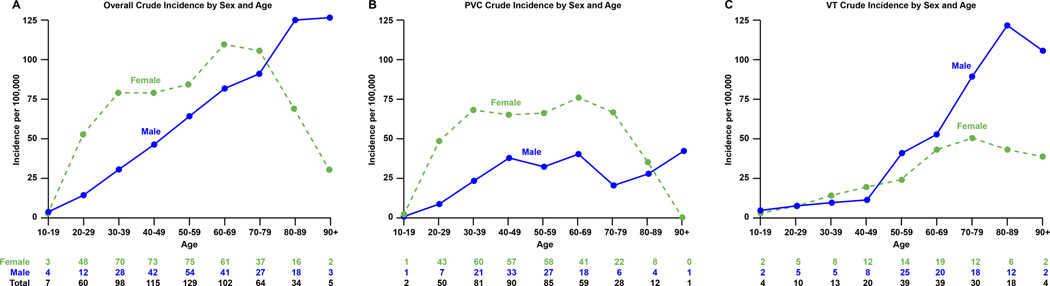
Figure 4.
Panel A, Crude incidence rate of idiopathic ventricular arrhythmia according to age and gender. Panel B, crude incidence rate of idiopathic PVC according to age and gender. Panel C, crude incidence rate of idiopathic VT according to age and gender.
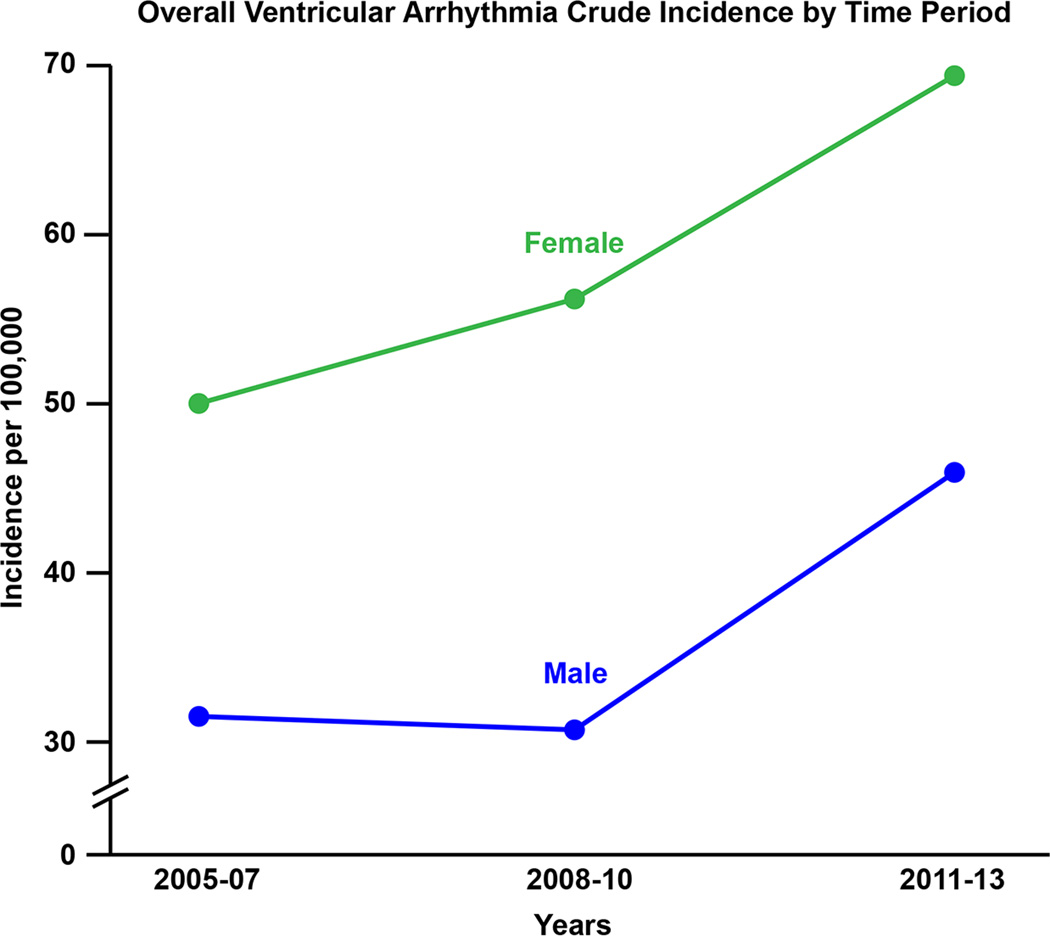
Figure 5.
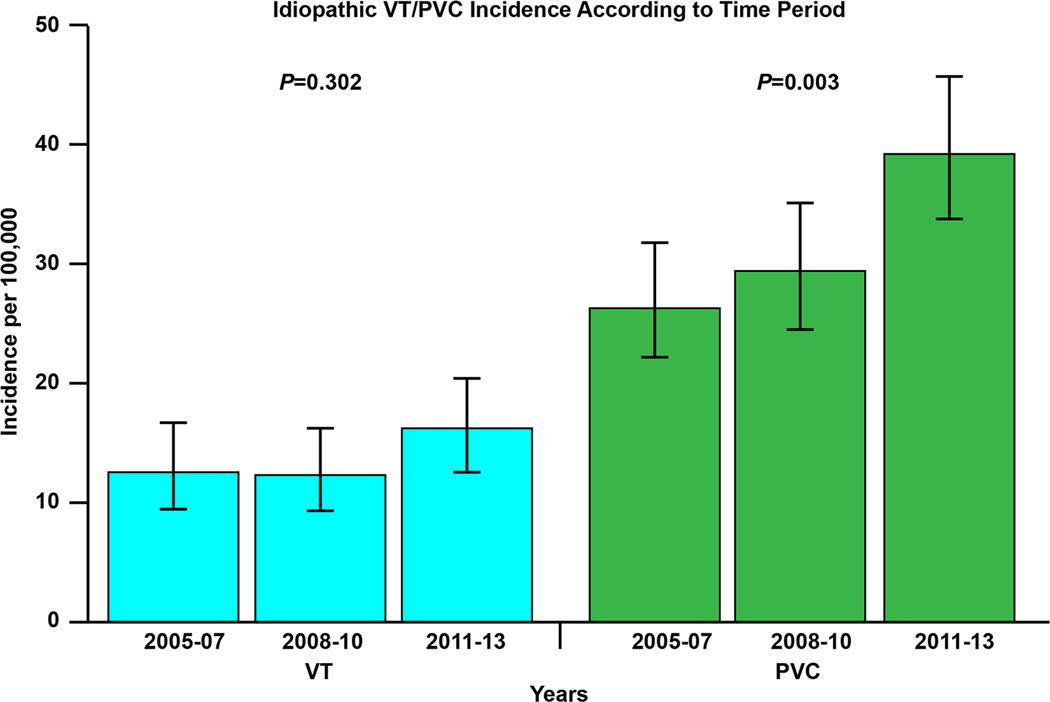
A: Incidence rate of idiopathic ventricular arrhythmia according to specific study time periods. B. Incidence rate of idiopathic ventricular tachycardia (VT) and premature ventricular complex (PVC) according to specific study time periods.
There was a clear increase in the documented incidence rate of VA from 2005 to 2013. The age and sex-adjusted incidence rates in 2005–2007, 2008–2010 and 2011–2013 were 44.9 per 100,000 (95% CI 38.0–51.8), 47.6 per 100,000 (95% CI 40.8–54.5) and 62.0 per 100,000 (95% CI 54.4–69.6), respectively, which was driven by an increase in symptomatic PVC (figure 5B).
Idiopathic VT
Of 177 patients in this category, 97 (55%) were male and 80 (45%) were female. The average age was 59.2 ± 17.6 years. The overall age and sex-adjusted incidence of idiopathic VT was 15.8 per 100,000 (95% CI 13.5–18.1). The age-adjusted rate was not significantly different between sexes (19.2 per 100,000 [95% CI 15.3–23.1] in males versus 13.3 per 100,000 [95% CI 10.4–16.2] in males, p=0.11). There was, however, an increase in incidence rate across age groups (p<0.001). There was no increase in the age and sex-adjusted incidence over time (p=0.30): the age and sex-adjusted incidence rates in 2005–2007, 2008–2010 and 2011–2013 were 14.9 per 100,000 (95% CI 10.8–19.0), 13.9 per 100,000 (95% CI 10.2–17.7) and 18.4 per 100,000 (95% CI 14.1–22.7).
Idiopathic PVC
Of 408 patients with symptomatic PVC, 290 (71.1%) were female and 118 (28.9%) were male with an average age of 48.4 ± 15.8 years. The age and sex-adjusted incidence rate increased throughout the study period. Specifically, the age and sex-adjusted incidence rates between 2005–2007, 2008–2010 and 2011–2013 were 27.5 per 100,000 (95% CI 22.3–32.8), 31.3 per 100,000 (95% CI 25.8–36.8) and 40.8 per 100,000 (95% CI 34.7–46.9), respectively (p=0.003). Furthermore, there was a significant increase with age (p<0.001). The average PVC burden in the group was 3.8±6%
Idiopathic VA-associated cardiomyopathy
Of 29 patients with IVA-CM, 15 (51.7%) were female and 14 (48.3%) were male with an average age of 59.2 ± 19.2 years. The overall age and sex-adjusted incidence was 2.6 per 100,000 (95% CI 1.6–3.5). The age- adjusted incidence between sexes appeared similar (2.4 per 100,000 [95% CI 1.2–3.7] versus 2.7 per 100,000 [95% CI 1.3–4.2] in females and males, respectively); however the small number of individuals precluded any formal testing. The average PVC burden in the IVA-CM group was 9.1 ± 11%.
Ventricular arrhythmia burden and development of cardiomyopathy
The mean burden of VA (supplemental figure) during a 24- hour period in the entire idiopathic VA cohort was 4.2 ± 7% (Range 0–39). During a 24 hour period, 88 (14.3 %) patients had burden above 10% (table 3). The mean EF was 46±11 % and the median time to low EF was 5.1 years among the IVA-CM group. The incidence of cardiomyopathy is similar among patients with less than 10% burden when compared to >10% burden (p value = 0.07) in the entire cohort.
Table 3.
Arrhythmia burden among patients with idiopathic VA.
| PVC Burden* |
Total n (%) | Idiopathic VT (N=177) |
Symptomatic PVC (N= 408) |
IVA-CM |
|---|---|---|---|---|
| <10% | 382 (81.3) | 110 (70.9) | 254 (88.2) | 18 (66.7%) |
| 11–20% | 55 (11.7) | 26 (16.8) | 26 (9.0) | 3 (11.1%) |
| 21–30% | 22 (4.7) | 13 (8.4) | 5 (1.7) | 4 (14.8%) |
| 31–40% | 10 (2.1) | 5 (3.2) | 3 (1.0) | 2 (7.4%) |
| >41% | 1 (0.2) | 1 (0.6) | 0 | 0 |
PVC Burden= PVC count/ Total number of beats over a 24 hour period expressed as %. PVC burden reported here are at the time of diagnosis.
Discussion
This population based study of a non-referral based cohort of Olmsted County residents demonstrates that the overall age and sex-adjusted incidence of idiopathic ventricular arrhythmias (VA) amongst individuals 18 years or older is 51.9 per 100,000. This is lower than the incidence of atrial fibrillation17 and other common cardiovascular disorders including myocardial infarction18, and heart failure19. In addition, the incidence of idiopathic VA appears to be rising (in contrast to myocardial infarction and heart failure) and varies according to age group and gender.
Rising incidence
The rising incidence of idiopathic VA is driven by an increase in idiopathic PVC incidence which is likely due to the increasing awareness and recognition of their occurrence. For example, smart phone applications, portable single-lead electrocardiogram recorders as well as an expanding menu of ambulatory recording devices more readily permit identification of arrhythmias20. Notably our cohort excluded patients with cardiac implantable electronic devices. Our data provide important insights by highlighting the incidence of VT and PVC by age group and gender in those typically deemed to be at low risk for cardiovascular disease given that recent studies have associated even a low burden of PVCs with a decrease in LVEF, an increase in incident heart failure, and increased mortality21–23. Furthermore, the contribution of PVC burden to heart failure was comparable to conventional risk factors23. However, it is difficult to determine whether early detection and treatment of idiopathic VA, particularly PVCs, alters the natural history of patients Certainly, studies assessing the impact of idiopathic VA therapy on outcomes are warranted.
Age difference
We found an increase in incidence with age for VA – this was driven by an increase in idiopathic VT and symptomatic PVCs as patients got older. It is plausible that increased medical contact for non-specific complaints with age leads to more testing and thus identification of arrhythmias that would have otherwise been unnoticed24. Although the emerging technology available to the public may lead to an increased detection rate in general, the low usage rate of such technology in the older population argues against this being a factor in that group25. Rather, the increased incidence in ageing individuals suggests that subclinical structural changes, e.g., myocardial fibrosis, may be contributing26. Therefore, the increased sensitivity of cardiac tests in the future will likely reclassify many ‘idiopathic’ VA. In one study of VT patients with no structural abnormalities detected on usual cardiac investigations, cardiac magnetic resonance imaging (MRI) demonstrated structural abnormalities in approximately 5% of patients with idiopathic VA of right ventricular origin and 40% of left ventricular origin27. Given the current prohibitive cost of widespread MRI, in our opinion the definition of idiopathic VA should ideally consist of the following: absence of clinically significant coronary artery and structural heart disease, EF ≥50% (unless low EF is secondary to idiopathic VA), absence of ECG evidence of scar (for example, a fractionated QRS28) and a normal signal averaged ECG29, Certainly, the ability to detect myocardial fibrosis is important as this has been associated with an increased vulnerability for arrhythmias30, 31.
Gender difference
We found that the incidence of symptomatic PVC was greater in women while that of idiopathic VT was similar among sexes. In keeping with other studies gender differences in arrhythmias, including idiopathic VT, have been well reported32–34. Studies have suggested that the gender-related variation in the incidence of idiopathic VA may in part be secondary to hormonal difference between men and women12. Additionally, sex steroids may alter K+ channel activity thus altering the action potential duration and susceptibility to reentrant VA35. However, although action potential duration may be longer in women, they have less transmural dispersion of refractoriness which may actually protect against reentrant arrhythmias33. Of course, the variability in the incidence of idiopathic VA may be related to difference in symptom perception and tendency to seek medical attention36. For example, in addition to hormonal differences, women may be more sensitive to PVCs such that they seek medical attention sooner, while males wait for more severe symptoms, which might explain our observed lack of difference with idiopathic VT. This is supported by studies demonstrating that males are more likely to develop IVA-CM4. However, it is not possible to determine this from our study.
Limitations
An incorrect estimation of the true incidence of idiopathic ventricular arrhythmia by this study may have occurred. The standard definition for idiopathic VT may miss individuals with subclinical coronary artery disease or scar; more sensitive imaging techniques may detect these abnormalities and thus reclassify these patients lowering true incidence rates. Current evidence suggests that IVA-CM is seen in patients with >10% burden. It is possible that some of the patients with burden <10% had non ischemic cardiomyopathy and PVCs were secondary to underlying disease process. Sensitive imaging tests to detect myocardial scar, longitudinal population studies evaluating the relationship are needed to clarify the relationship between PVC burden and development of cardiomyopathy. Further, not every resident of Olmsted County had some form of ECG recorded during the study period, thus our incidence rate may be an underestimate. Thus the trends may reflect ascertainment bias with time. Given that the majority of residents in Olmsted County are from a homogeneous ethnic group (Caucasians), incidence estimates may not be applicable to other ethnicities. Treatment rates with medical therapy are low in our cohort. It is plausible that incidence rates of IVA may be lower with higher rates of medical therapy. Small number of patients in the IVA-CM group precluded meaningful descriptive or discriminative analysis. Finally, location or type of idiopathic VA is not reported – further data detailing this may provide important insights.
Conclusion
The incidence of idiopathic VA appears to be increasing and is mainly driven by increasing PVC incidence rates. The overall incidence of VA increases with age and women have a higher incidence of symptomatic PVC. The relationship between PVC burden and development of cardiomyopathy is less clear in our population-based study however, the rate of IVA-CM appears to be extremely low in the general population.
Supplementary Material
WHAT IS KNOWN
Idiopathic ventricular arrhythmias (VA) occur in patients with structurally normal hearts
The burden of idiopathic VA in the general population is unknown
WHAT THE STUDY ADDS
The age and sex adjusted incidence of idiopathic VA is 51.86 per 100,000 (95% CI 47.72–56.01).
The incidence increases with age. The incidence of ventricular tachycardia is similar across sexes while PVC’s are more common among women.
Acknowledgments
Sources of Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Siva K Mulpuru received research support from Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN. Alanna M. Chamberlain is a co-investigator of the Rochester Epidemiology Project (R01 AG034676).
Footnotes
Disclosures: All other authors have no conflicts of interest in relationship to the article.
References
- 1.Brooks R, Burgess JH. Idiopathic ventricular tachycardia. A review. Medicine (Baltimore) 1988;67:271–294. doi: 10.1097/00005792-198809000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Viskin S, Rosso R, Rogowski O, Belhassen B. The "short-coupled" variant of right ventricular outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005;16:912–916. doi: 10.1111/j.1540-8167.2005.50040.x. [DOI] [PubMed] [Google Scholar]
- 3.Brugada P, Gursoy S, Brugada J, Andries E. Investigation of palpitations. Lancet. 1993;341:1254–1258. doi: 10.1016/0140-6736(93)91155-f. [DOI] [PubMed] [Google Scholar]
- 4.Latchamsetty R, Bogun F. Premature Ventricular Complexes and Premature Ventricular Complex Induced Cardiomyopathy. Curr Probl Cardiol. 2015;40:379–422. doi: 10.1016/j.cpcardiol.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Singh SM, Kadmon E, Suszko A, Chauhan VS. Syncope triggered by a premature ventricular complex: a case of atrial fibrillation and paroxysmal atrioventricular block. Heart Rhythm. 2012;9:1650–1651. doi: 10.1016/j.hrthm.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Tada H, Ito S, Naito S, Higuchi K, Kumagai K, Hachiya H, Hirao K, Oshima S, Taniguchi K, Aonuma K, Isobe M. Gender and age differences in candidates for radiofrequency catheter ablation of idiopathic ventricular arrhythmias. Circ J. 2011;75:1585–1591. doi: 10.1253/circj.cj-10-0941. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmayer KS, Gerstenfeld EP. Diagnosis and management of idiopathic ventricular tachycardia. Curr Probl Cardiol. 2013;38:131–158. doi: 10.1016/j.cpcardiol.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J. 2000;139:1009–1013. doi: 10.1067/mhj.2000.106164. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, Dorian P, Huikuri H, Kim YH, Knight B, Marchlinski F, Ross D, Sacher F, Sapp J, Shivkumar K, Soejima K, Tada H, Alexander ME, Triedman JK, Yamada T, Kirchhof P, Lip GY, Kuck KH, Mont L, Haines D, Indik J, Dimarco J, Exner D, Iesaka Y, Savelieva I. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm. 2014;11:e166–e196. doi: 10.1016/j.hrthm.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, Dorian P, Huikuri H, Kim YH, Knight B, Marchlinski F, Ross D, Sacher F, Sapp J, Shivkumar K, Soejima K, Tada H, Alexander ME, Triedman JK, Yamada T, Kirchhof P, Lip GY, Kuck KH, Mont L, Haines D, Indik J, Dimarco J, Exner D, Iesaka Y, Savelieva I, Ep-Europace UK. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm. 2014;11:e166–e196. doi: 10.1016/j.hrthm.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Wellens HJ. Electrophysiology: Ventricular tachycardia: diagnosis of broad QRS complex tachycardia. Heart. 2001;86:579–585. doi: 10.1136/heart.86.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology/American Heart Association Task F, European Society of Cardiology Committee for Practice G, European Heart Rhythm A and Heart Rhythm S. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 17.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 18.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh JA, 3rd, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation. 2014;130:573–581. doi: 10.1161/CIRCULATIONAHA.114.009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med. 1992;117:990–996. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal SK, Simpson RJ, Jr, Rautaharju P, Alonso A, Shahar E, Massing M, Saba S, Heiss G. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk In Communities [ARIC] Study) Am J Cardiol. 2012;109:105–109. doi: 10.1016/j.amjcard.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular Ectopy as a Predictor of Heart Failure and Death. J Am Coll Cardiol. 2015;66:101–109. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perissinotto CM, Ritchie C. Atypical Presentations of Illness in Older Adults. In: Williams BA, Chang A, Ahalt C, Chen H, Conant R, Landefeld CS, Ritchie C, Yukawa M, editors. Current Diagnosis & Treatment: Geriatrics. 2. New York, NY: McGraw-Hill Education; 2014. [Google Scholar]
- 25.Fox CS, Hwang SJ, Nieto K, Valentino M, Mutalik K, Massaro JM, Benjamin EJ, Murabito JM. Digital Connectedness in the Framingham Heart Study. J Am Heart Assoc. 2016;5:e003193. doi: 10.1161/JAHA.116.003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogami A. Gender differences in idiopathic ventricular arrhythmias. Circ J. 2011;75:1569–1570. doi: 10.1253/circj.cj-11-0442. [DOI] [PubMed] [Google Scholar]
- 27.Nucifora G, Muser D, Masci PG, Barison A, Rebellato L, Piccoli G, Daleffe E, Toniolo M, Zanuttini D, Facchin D, Lombardi M, Proclemer A. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: a magnetic resonance imaging study. Circ Arrhythm Electrophysiol. 2014;7:456–462. doi: 10.1161/CIRCEP.113.001172. [DOI] [PubMed] [Google Scholar]
- 28.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 29.Gomes JA, Cain ME, Buxton AE, Josephson ME, Lee KL, Hafley GE. Prediction of long-term outcomes by signal-averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation. 2001;104:436–441. doi: 10.1161/hc2901.093197. [DOI] [PubMed] [Google Scholar]
- 30.de Jong S, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol. 2011;57:630–638. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 31.Aljaroudi WA, Flamm SD, Saliba W, Wilkoff BL, Kwon D. Role of CMR imaging in risk stratification for sudden cardiac death. JACC Cardiovasc Imaging. 2013;6:392–406. doi: 10.1016/j.jcmg.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa M, Takahashi N, Nobe S, Ichinose M, Ooie T, Yufu F, Shigematsu S, Hara M, Yonemochi H, Saikawa T. Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:633–638. doi: 10.1046/j.1540-8167.2002.00633.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa M, Takahashi N, Watanabe M, Ichinose M, Nobe S, Yonemochi H, Ito M, Saikawa T. Gender differences in ventricular repolarization: terminal T wave interval was shorter in women than in men. Pacing Clin Electrophysiol. 2003;26:59–64. doi: 10.1046/j.1460-9592.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamada T, Maddox WR, McElderry HT, Doppalapudi H, Plumb VJ, Kay GN. Radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from intramural foci in the left ventricular outflow tract: efficacy of sequential versus simultaneous unipolar catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:344–352. doi: 10.1161/CIRCEP.114.002259. [DOI] [PubMed] [Google Scholar]
- 35.Pham TV, Sosunov EA, Gainullin RZ, Danilo P, Jr, Rosen MR. Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by I(K)-blocking drugs. Circulation. 2001;103:2207–2012. doi: 10.1161/01.cir.103.17.2207. [DOI] [PubMed] [Google Scholar]
- 36.Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.