Summary
We discuss the recent finding by Mizishima’s group regarding the role of the autophagy-related (Atg) conjugation system in mediating the closure of autophagosomes and its implication in the study of autophagy in mammalian cells. The study not only shows a novel function of the Atg conjugation system but also indicates that mammalian cells are capable of generating autophagosomes even without it.
Autophagy, a mechanism of lysosomal degradation of cytoplasmic materials and organelles, is characterized by de novo synthesis of double membrane vesicles called autophagosomes that carry cargo to lysosomes1. Degradation through autophagy is executed through multiple steps, including initial formation of isolation membranes, maturation of autophagosomes, closure of autophagosomes, fusion between autophagosomes and lysosomes, and lysosomal degradation, in a well-coordinated manner1. After the initial discovery of autophagy related (Atg) genes in yeast by Dr. Ohsumi’s group2, molecular mechanisms mediating each step of autophagosome formation have been investigated intensively. At present, one of the least well understood steps in autophagy is the closure of autophagosomes, most likely due to the lack of yeast mutants specifically deficient in autophagosome closure and the technical difficulty of continuously monitoring the closure of autophagosomes.
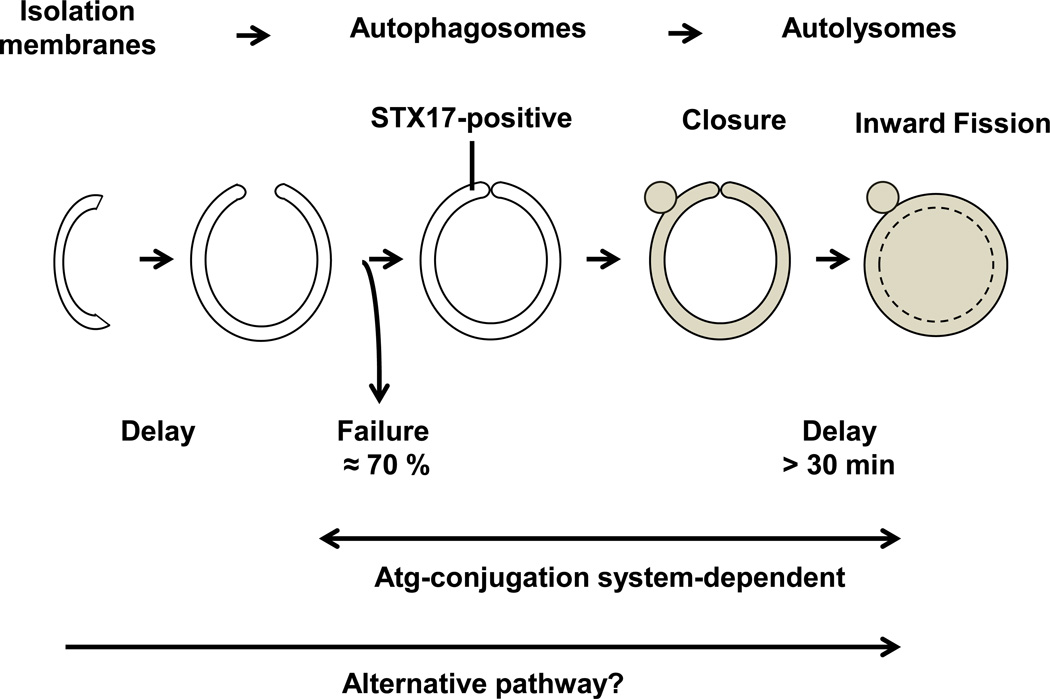
One of the unique aspects of the signaling mechanism of autophagy is the fact that a protein unrelated to ubiquitin utilizes a ubiquitination-like conjugation system. LC3 (Atg8), a ubiquitin-like protein, is conjugated to phosphatidylethanolamine (PE), a lipid, through an Atg conjugation system consisting of Atg8 and Atg12, ubiquitin-like molecules, Atg7, an E1-like enzyme, and Atg3 and Atg10, E2-like enzymes3, 4. In addition, a protein complex of Atg16L and Atg12-Atg5, recruited to the outer surface of forming autophagosomes, acts as an E3-like enzyme and promotes conjugation of Atg8 to PE (reviewed in5). Generation of LC3-PE is important for the lipid membrane to induce tethering or hemifusion, and this property of LC3-PE has been proposed to be critical for the expansion of autophagosomal membranes6. In a recent paper by Tsuboyama et al7, the authors used sophisticated live-cell imaging with syntaxin 17 (STX17), which associates with and allows visualization of the whole circumference of the ring-shape structure of autophagosomes but does not associate with isolation membranes8, to conduct time-lapse analyses in order to elucidate the molecular mechanism mediating the maturation of autophagosomes. Surprisingly, STX17-positive autophagosomes were generated in cells without the Atg conjugation system. However, both autophagosome formation and inner autophagosomal membrane (IAM) degradation were delayed more than 30 minutes compared to in wild-type cells. These results suggest that the Atg conjugation system is important for two steps during maturation of autophagosomes, namely (1) the efficient closure of autophagosomes and (2) the efficient degradation of the IAM, whereas it is dispensable for autophagosome formation (Figure).
Figure.
The Atg-conjugation system is critical for the closure and the inward membrane fission of autophagosomes. This is a schematic representation of autophagosome formation and maturation in cells lacking the Atg-conjugation system. In Atg3 KO MEF cells, the success of autophagosome formation is impaired (the success rate is 30%) and complete closure of autophagosomes and degradation of the IAM are severely delayed. Although the efficiency and the speed are compromised, autophagosomes/autolysosomes are formed in Atg3 KO MEF cells through alternative mechanisms. The brown color indicates lysosomal acid hydrolase.
Why is this study important?
We believe that the study by Tsuboyama et al is of high impact because it, for the first time, shows that the Atg conjugation system is more important for the closure of autophagosomes than for the generation of autophagosomes. In addition, the study also shows that the mechanisms mediating this process are distinct from those mediating autophagosome-lysosome fusion. This is surprising because it is believed that generation of LC3-PE through the Atg conjugation system is critical for expansion of the isolation membrane, the initial step in autophagosome formation. Since LC3-PE can tether and fuse liposomes and promote expansion of autophagosomal membranes, when the Atg conjugation system is available, it would seem that LC3-PE3 ought to be involved in autophagosome formation. In the data shown by Tsuboyama et al7, however, it appears that this process can be nearly completely overridden by another mechanism in mammalian cells, whereas, in the absence of the Atg conjugation system, further maturation/completion of autophagosome formation, namely the closure of autophagosomes and the consequent generation of autolysosomes, was severely attenuated. This suggests that the most important task of the Atg conjugation system and the generation of LC3-PE is to complete autophagosome formation by closing the loop and allowing degradation of the IAM in preparation for degradation of the cargo by lysosomal hydrolase.
These observations have several important implications. First, autophagic flux can be inhibited at the very last step of autophagosome formation, namely the closure of autophagosomes. Although accumulation of autophagosomes is often observed in the presence of lysosomal dysfunction, the blockade at the step of closure appears independent of lysosomal blockade. This reminds us of the importance of observing the presence of autolysosomes or confirming the degradation of the cargo content when investigating whether autophagy is functionally activated. Although evaluating the autophagic flux with lysosomal inhibitors is always important, the study by Tsuboyama et al clearly demonstrates that real-time imaging with appropriate markers, such as STX17, an indicator that allows visualization of the complete form of autophagosomes, and LysoTracker Red, an indicator of active lysosomes, as well as an assay for lysosomal long-lived protein degradation, is useful7.
Second, nearly complete autophagosomes can be generated and the closure of autophagosomes and inward fission between the outer autophagosomal membrane (OAM) and IAM are delayed but can still take place even in the absence of the Atg conjugation system. Although Tsuboyama et al have shown that autophagic degradation of p62/SQSTM1 and long-lived proteins is severely impaired in cells lacking the Atg conjugation system, previous studies have shown that intracellular organelles in lens epithelial cells and erythroid cells can be degraded in atg5 knock-out cells9. Degradation of intracellular organelles is mediated primarily through autophagy. Cytosolic materials and mitochondria can be degraded through an Atg5-independent alternative form of autophagy in MEF cells10, 11. Perhaps, the fact that the phenotype of mice with loss of function of the Atg conjugation system is not necessarily embryonic lethal, in contrast with those with loss of function of other genes involved in the initiation of isolation membranes, may suggest that there exist some back-up mechanisms mediating autophagosome formation and closure to allow degradation of important cellular targets. It is therefore important to further investigate the functional significance of the STX17-positive autophagosomes observed in cells lacking the Atg conjugation system under conditions of stress.
Unsolved issues
The study by Tsuboyama has raised many intriguing questions. First, what are the underlying molecular mechanisms through which autophagosomes are closed and the inward membrane fission between the outer OAM and the IAM takes place? It has been proposed that LC3-PE plays an important role in mediating the closure of the autophagosome through its ability to induce hemifusion, a docking of two membranes through a fusion of the out layer of the lipid bilayer on each membrane5. Since formation of LC3-PE critically depends upon the Atg conjugation system, the first step of closure through hemifusion may be impaired in the absence of the Atg conjugation system. After both edges of the isolation membrane fuse, the OAM from both sides of the isolation membrane is connected and forms a completely closed autophagosome, which, in turn, allows detachment of the IAM connected to the OAM through a stalk, a process termed inward fission. Although how the final detachment (fission) of the IAM from the OAM takes place remains unclear, it has been proposed that physical forces may eliminate the stalk in order for the OAM to settle in a thermodynamically stable spherical shape. At present, however, this proposal remains hypothetical and how inward fission takes place remains unclear. Since LC3 mutants, which cannot induce hemifusion, do not necessarily show incomplete closure of autophagosomes in yeast6, additional mechanisms besides LC3-PE, such as the involvement of fission proteins, cannot be excluded.
Second, how can autophagosomes be formed even in the absence of the Atg conjugation system? Tsuboyama et al showed that STX17 positive autophagosomes are not formed in the absence of FIP200, Atg9A or Atg14. However, how these molecules are involved in the initial part of autophagosome formation is unknown. Since membrane tethering or hemifusion can also be achieved through autophagic SNAREs and Atg14 in vitro12, Atg14 may compensate for the role of LC3-PE. In fact, it has been shown that Atg14 is recruited to the ER-mitochondrial contact site to generate autophagosomes in an STX17-dependent manner13. More investigation is required to address this issue.
Third, whatever the initial molecular mechanism through which isolation membranes are formed and expanded, are the autophagosomes observed with STX17 in Atg-deficient cells really the same as autophagosomes in normal cells? Although Tsuboyama et al claim that STX17-positive autophagosomes are qualitatively similar to LC3-positive autophagosomes, it is formally possible that the STX17-positive autophagosomes observed in the authors’ study may not be identical to those observed in cells with an intact Atg conjugation system. Thus, the proposed role of the Atg conjugating system in mediating the closure of autophagosomes and degradation of IAM observed in cells lacking the Atg conjugation system may not be the same as that in ordinary LC3-II-positive autophagosomes. For example, STX17-positive autophagosomes may be generated by “alternative autophagy”, proposed by Nishida et al10, which occurs from the trans-Golgi in an Atg5/7-independent manner and in which autophagosomes do not associate with LC3. Altough Tsuboyama et al proposed that autophagosomes observed in Atg3 KO MEFs do not represent alternative autophagy because Atg5-positive autophagosomes are formed near the ER and not the Golgi area, it alone may not fully exclude the possibility that STX17-positive autophagosomes in Atg-deficient cells could represent alternative autophagy. Furthermore, Parkin-mediated mitophagy, one of the best characterized forms of mitophagy, utilizes adaptor/receptor proteins to connect damaged/ubiquitinated mitochondria with LC3-PE on autophagosomes. It is possible that the behavior of the STX17-positive autophagosomes observed in the Atg-deficient cells may not be representative of the process involved in mitophagy and, thus, the requirement of the Atg conjugation system for closure in mitophagy remains to be clarified.
Potential clinical and translational implications
The study by Tsuboyama et al has shown that downregulation of Atg3, Atg5 or Atg7 affects the closure and the inward fission of autophagosomes. Currently, we do not know whether autophagy is impaired at the step of autophagosome closure in any cardiovascular conditions. It will be interesting to evaluate which pathologically relevant condition in the cardiovascular system, if any, induces downregulation of the Atg conjugation systems and, if so, whether it affects the closure of autophagosomes. Importantly, however, since the complete lack of LC3-II or LC3 puncta does not necessarily indicate that autophagy or autophagic degradation is completely abolished, it is important to investigate whether autophagy is really suppressed when the Atg conjugation system is downregulated and, more practically, whether any back-up mechanism exists and how it contributes to the maintenance of cellular hemostasis. Considering the fundamental importance of autophagy in many cellular functions, it is not surprising to find that cells have such back-up systems, including alternative autophagy10. Investigating how the cell compensates for downregulation of the Atg conjugation system may be another interesting avenue of future research.
Acknowledgments
The authors thank Daniela Zablocki for critical reading of the manuscript. This work was supported in part by U.S. Public Health Service grants HL67724, HL91469, HL102738, HL112330 and AG23039 (J.S.), and by the Leducq Foundation Transatlantic Networks of Excellence (J.S.). T.S. is supported by a Postdoctoral Fellowship from the American Heart Association Founder’s Affiliate.
Glossary
- Atg
autophagy related
- IAM
Inner autophagosomal membrane
- OAM
outer autophagsomal membrane
- PE
phosphatidylethanolamine
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- STX17
syntaxin 17
Footnotes
Disclosures
None.
References
- 1.Ohsumi Y. Historical landmarks of autophagy research. Cell research. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- 3.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 5.Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16:984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 6.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Tsuboyama K, Koyama-Honda I, Sakamaki Y, Koike M, Morishita H, Mizushima N. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science. 2016;354:1036–1041. doi: 10.1126/science.aaf6136. [DOI] [PubMed] [Google Scholar]
- 8.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Matsui M, Yamamoto A, Kuma A, Ohsumi Y, Mizushima N. Organelle degradation during the lens and erythroid differentiation is independent of autophagy. Biochem Biophys Res Commun. 2006;339:485–489. doi: 10.1016/j.bbrc.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 10.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 11.Hirota Y, Yamashita S, Kurihara Y, Jin X, Aihara M, Saigusa T, Kang D, Kanki T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy. 2015;11:332–343. doi: 10.1080/15548627.2015.1023047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, Zhou Q, Wilz LM, Li J, Vivona S, Pfuetzner RA, Brunger AT, Zhong Q. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]