Abstract
The inability of the adult heart to repair or regenerate is manifested in prevalent morbidity and mortality related to myocardial infarction and heart failure. Cardiomyocyte proliferation, especially after myocardial infarction or in the context of heart failure, has been an area of intense research by many investigators for decades. As a researcher working in this area for the past 25 years, this has been an exciting time, requiring an open mind in leaving old accepted ideas behind and moving forward towards exploiting this new knowledge of cardiomyocyte plasticity.
Keywords: Cardiac myocyte, proliferation, regeneration, developmental biology
Too often, the heart does not have the capacity to overcome loss of cardiac muscle cells after injury, thus contributing to heart failure and death in the long term. For many years, the prevailing dogma was that adult mammalian cardiomyocytes are post-mitotic due to irreversible cell cycle withdrawal and a switch to hypertrophic growth soon after birth. More recently, low levels of cardiomyocyte proliferation have been found in adult mammalian hearts, including humans. In addition, genetic manipulations in mice provide evidence that barriers to adult cardiomyocyte proliferation can be overcome based on knowledge of normal developmental transitions of cardiomyocyte maturation. Studies of regenerating zebrafish and neonatal mouse hearts also have identified mechanisms of cardiomyocyte dedifferentiation and proliferation with implications for promoting cardiomyocyte proliferation in adults. The accumulating evidence that resident cardiomyocytes can be stimulated to proliferate holds promise for the development of new and more effective treatments for the most devastating types of cardiovascular disease.
Evolving views of cardiomyocyte proliferation and regeneration
In the 1900s, adult mammalian cardiomyocytes were viewed as post-mitotic and refractory to regeneration or repair, originally based on cytology and later on electron microscopy.1 The prevailing dogma, based largely on studies in rodents, was that differentiated cardiomyocytes could proliferate in utero, but after birth, cardiomyocytes undergo 1–2 rounds of proliferation, become binucleated, and withdraw from the cell cycle.2 Subsequent heart growth was viewed as due to hypertrophy of existing cardiomyocytes in juveniles and adults with essentially no turnover or addition of new muscle cells. The adult heart was thought to respond to physiologic or pathologic stresses by undergoing hypertrophy, with reactivation of aspects of the fetal program, but was considered unable to generate new muscle through proliferation.
In the 1990s and early 2000s, multiple studies provided evidence that adult mammalian cardiomyocytes can proliferate. However, the rate of proliferation was an area of debate in the literature and at scientific meetings. Rigorous analysis of cardiomyocyte proliferation based on 14C exposure in humans and 15N labeling in mice demonstrated low levels of cardiomyocyte proliferation in children and adults.3, 4 In mice, the new myocytes arise from existing differentiated cardiomyocytes, supporting their ability to proliferate.4 Over time, the field came to accept that adult cardiomyocytes could proliferate, albeit at low frequencies (<1%).3 While this low level of cardiomyocyte turnover is not sufficient to overcome damage after myocardial infarction or prevent heart failure, these observations suggested that rare proliferative events could be harnessed at a larger scale for cardiac repair if the underlying mechanisms were fully understood.
Unlike adult mammalian hearts, hearts of anurans have long been known to regenerate after injury. Interest in cardiac regeneration intensified in the early 2000s with the report by Poss et al.5 demonstrating adult zebrafish heart regeneration after injury. Notably, the regenerating cardiomyocytes in zebrafish arise from dedifferentiation and proliferation of existing CMs.6 Mammalian cardiac regenerative ability was found in neonatal mice within the first week after birth, but cardiac injury in mice more than 7 days-old resulted in scarring, not new muscle formation.7 Again, the new muscle generated after neonatal injury arises from differentiated cardiomyocytes, further supporting the ability of differentiated cardiomyocytes to produce new muscle cells. While less is known of human cardiomyocyte maturation and proliferation, there is evidence that cardiomyocytes from infants have an increased capacity to proliferate and respond to regenerative signals relative to older children or adults.8 Studies in large animal models will be necessary to determine the time window of potential cardiomyocyte proliferation after birth with important clinical implications for surgical repair of congenital heart malformations in infants.
Developmental regulation of cardiomyocyte proliferation
Prior to birth, the heart is one of the few organs in which differentiated cells proliferate in the context of a functioning organ.8 During embryogenesis, mitotic figures are obvious in differentiated cardiomyocytes with sarcomeric structures, and the heart grows primarily by hyperplasia. Thus, differentiated cardiomyocytes have the ability to proliferate before birth. The proliferative rates of embryonic and fetal cardiomyocytes are distinct for diversified subpopulations of cardiomyocytes in compact and trabecular ventricular layers, atria, atrioventricular canal and conduction system. These differential rates of cardiomyocyte proliferation are critical for morphogenesis and organogenesis of the heart before birth. Thus, unlike many other developing tissues, differentiated cardiomyocytes continue to proliferate in the context of a functioning organ throughout prenatal development.
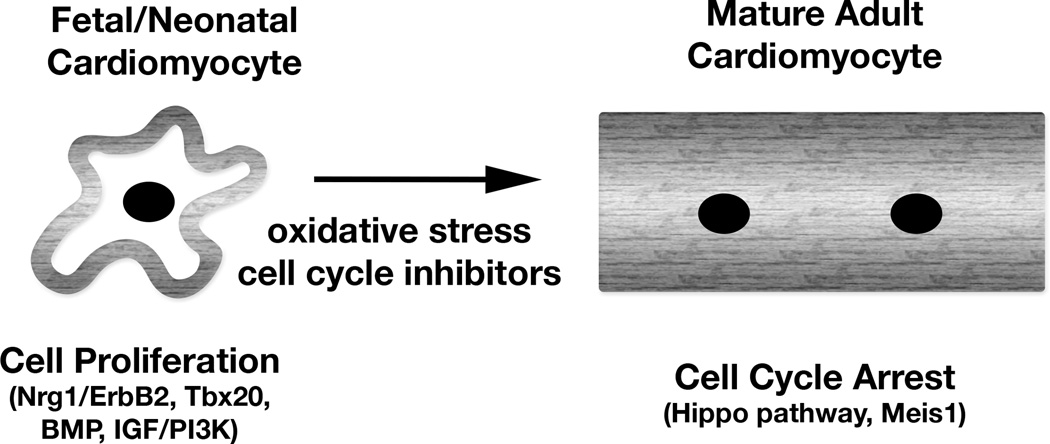
In the developing heart, cardiomyocyte proliferation is regulated by several different pathways (Figure).8 Neuregulin/ErbB/ERK signaling is the primary proliferative pathway in the embryonic heart. After midgestation, BMP signaling and IGF/PI3K pathways are required for hyperplastic growth of cardiomyocytes. Throughout development and after birth, the Hippo/Yap pathway is a critical regulator of cardiomyocyte proliferation and organ size. Early cardiomyocyte differentiation and specialization are regulated by several transcription factors, including members of Nkx, GATA, Mef2, Tbx, and HAND gene families. Notably, some of these same factors have been used in various combinations for reprogramming of iPS and cardiac fibroblasts into immature cardiomyocyte lineages.9 Thus, these factors not only regulate initial differentiation, but they also control specialization of cardiomyocytes, including late fetal proliferating cardiomyocytes.
Figure.
Regulatory mechanisms of cardiomyocyte proliferation and post-natal cell cycle arrest. Genetic activation of proliferative pathways or inhibition of arrest mechanisms can promote cardiomyocyte cell cycle activity and improve cardiac function after injury in adult mice.
In the days after birth, mouse cardiomyocytes undergo 1–2 rounds of cell division, become binucleated, and switch to predominantly hypertrophic growth.2 At the same time contractile protein isoforms switch from fetal to adult, metabolism switches from glycolytic to oxidative, cell cycle activators are repressed, cell cycle inhibitors are upregulated, and cardiomyocytes lose their ability to regenerate after injury.10 Cardiomyocyte proliferation is essentially undetectable 7–14 days after birth in rodents, as they undergo a round of nuclear division without cytokinesis, leading to binucleation and cell cycle arrest.2 The mechanism of postnatal cardiomyocyte cell cycle withdrawal is not fully understood. Proproliferative signaling pathways and cell cycle proteins are decreased in the week after birth, while expression of cell cycle inhibitors is increased. Oxidative stress after birth has been implicated in the loss of proliferative or regenerative ability of cardiomyocytes after birth in mice.10 Interestingly, both zebrafish and mouse fetal/neonatal cardiomyocytes are in hypoxic environments, which has been linked to the ability to regenerate.
Inducing adult cardiomyocyte proliferation by manipulation of developmental factors
Manipulation of signaling molecules important for developmental regulation of cardiomyocyte proliferation, such as Yap and neuregulin pathway proteins, can overcome post-natal cell cycle arrest and promote adult cardiomyocyte proliferation.11 Inhibition of the Hippo pathway promotes adult cardiomyocyte cell cycle activity, cytoskeletal remodeling, dedifferentiation, and cardioprotection after injury.12, 13 Activation of neuregulin (Nrg) signaling through activated ErbB2 also promotes robust cardiomyocyte proliferation and dedifferentiation in juvenile and adult mice.14 Conversely, the homeobox protein Meis1 promotes neonatal cardiomyocyte withdrawal through activation of multiple cell cycle inhibitors, and loss of Meis1 prolongs proliferation of cardiomyocytes after birth.15 Our lab generated mice with Tbx20 overexpression in adulthood that exhibit increased cardiomyocyte proliferation and repair after injury, characterized by induction of multiple proliferative pathways, repression of inhibitory pathways, and increased fetal characteristics.16 In the Tbx20 overexpressing mice, we saw alterations in Yap1, Akt and BMP signaling, but we still do not understand how the multiple pathways that control cardiomyocyte proliferation intersect. It is becoming increasingly clear that postnatal induction of cardiomyocyte proliferation requires both induction of proliferative pathways and repression of cell cycle inhibitory pathways.
A limitation of induction of cardiomyocyte proliferation after birth is that cardiac hypertrophy or cardiomegaly, ultimately leading to heart failure, can occur. While manipulation of Hippo pathway proteins, cell cycle regulators, or microRNAs promotes adult cardiomyocyte cell cycling, these mice also exhibit cardiac dysfunction and failure in the long term.11 Ultimately, mice with enlarged hearts due to decreased Hippo signaling or associated microRNAs exhibit pathologic hypertrophy and heart failure. In contrast, loss of Meis1 or overexpression of the developmental factor Tbx20 in adult differentiated cardiomyocytes leads to increased numbers of small proliferative cardiomyocytes, without causing hypertrophy or apparent cardiac dysfunction.15, 16 While overexpression of the activated ErbB2 oncogene leads to cardiomegaly and, ultimately, heart failure and death if unrestrained, transient induction ErbB2 promotes limited cardiomyocyte proliferation and dedifferentiation, followed by redifferentiation, after injury.14 The success of this approach provides evidence that adult cardiomyocytes can be induced to dedifferentiate and proliferate for repair after injury and then mature into the more normal adult quiescent state for restoration of normal function in the long term. Thus, there is accumulating evidence that manipulation of cell cycle genes, inhibitors of proliferation, and secreted factors in adult mammalian hearts can promote cardiomyocyte proliferation and repair after injury. However, these pathways must be controlled for successful cardiac repair.
New prospects for cardiac repair
Genetic manipulation of cardiomyocytes resulting in increased proliferation and repair after injury has been achieved using a variety of approaches in mice. While transgenesis and gene targeting are not currently feasible in humans, cardiac delivery via emerging recombinant AAV or modified RNA (modRNA) technologies could be applied in large animals or clinical settings.17 Likewise, administration of paracrine factors via an epicardial patch might be an effective way to stimulate resident cardiomyocyte proliferation and repair after myocardial infarction. For current trials of cell-based therapies, it is tempting to speculate that secreted factors stimulate resident cardiomyocytes to proliferate. However, much is still not known about the fundamental biology of adult cardiomyocyte proliferation, especially in the context of the human heart. It is still not entirely clear if hypertrophied binucleated cardiomyocytes can divide or if there are primitive subpopulations that account for adult cardiomyocyte proliferative activity. Application of findings from cardiac development to the post-natal heart likely will continue to provide new insights into adult homeostasis and potential repair mechanisms in the years ahead.
In the current era of cellular reprogramming, the concept of terminal differentiation has been called into question. Thus, the traditional view of irreversible cell lineage commitment during development has been modified to include new levels of cellular plasticity. The induction of fetal characteristics in adult cardiomyocytes by transcription factors or signaling pathways active in the developing heart is not as much of a leap as creating new cardiomyocytes by reprogramming of multipotential stem cells or fibroblasts. However, potential limitations include cardiomegaly or hypertrophy, as was observed in some mouse models, as well as possible arrhythmias due to the immaturity of myocyte conduction or poor coupling with existing myocardium. In order to be effective clinically, efforts to promote proliferation will need to include strategies to prevent unrestrained cardiac growth in the long term. While we are far from clinical translation of our new knowledge of adult cardiomyocyte proliferation, the possibility that resident cardiomyocytes in an injured heart could be stimulated for repair is an exciting new direction to explore for both basic scientists and cardiologists.
Acknowledgments
Sources of funding
This work is supported by NIH/NHLBI R01 HL082716, P01 HL069779 and a Cincinnati Children’s Research Foundation endowed chair to KEY.
Footnotes
Disclosures
None.
References
- 1.Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiac myogenesis and regeneration. Int Rev Cytol. 1977;51:187–273. [PubMed] [Google Scholar]
- 2.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271:H2183–H2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardway RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;234:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2012;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729–740. doi: 10.1242/dev.132910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis of adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14:529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uygur A, Lee RT. Mechanisms of cardiac regeneration. Dev Cell. 2016;36:362–374. doi: 10.1016/j.devcel.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERRB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang F-L, Guo M, Yutzey KE. Overexpression of Tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation. 2016;133:1081–1092. doi: 10.1161/CIRCULATIONAHA.115.019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6:239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]