Abstract
Vascular smooth muscle (VSM) plays an important role in maintaining vascular tone. In addition to Ca2+-dependent myosin light chain (MLC) phosphorylation, protein kinase C (PKC) is a major regulator of VSM function. PKC is a family of conventional Ca2+-dependent α, β, and γ, novel Ca2+-independent δ, ε, θ, and η, and atypical ξ, and ι/λ isoforms. Inactive PKC is mainly cytosolic, and upon activation it undergoes phosphorylation, maturation and translocation to the surface membrane, the nucleus, endoplasmic reticulum, and other cell organelles; a process facilitated by scaffold proteins such as RACKs. Activated PKC phosphorylates different substrates including ion channels, pumps and nuclear proteins. PKC also phosphorylates CPI-17 leading to inhibition of MLC phosphatase, increased MLC phosphorylation and enhanced VSM contraction. PKC could also initiate a cascade of protein kinases leading to phosphorylation of the actin-binding proteins calponin and caldesmon, increased actin-myosin interaction and VSM contraction. Increased PKC activity has been associated with vascular disorders including ischemia-reperfusion injury, coronary artery disease, hypertension, and diabetic vasculopathy. PKC inhibitors could test the role of PKC in different systems, and could reduce PKC hyperactivity in vascular disorders. First generation PKC inhibitors such as staurosporine and chelerythrine are not very specific. Isoform-specific PKC inhibitors such as ruboxistaurin have been tested in clinical trials. Target-delivery of PKC pseudosubstrate inhibitory peptides and PKC siRNA may be useful in localized vascular disease. Further studies of PKC and its role in VSM should help design isoform-specific PKC modulators that are experimentally potent and clinically safe to target PKC in vascular disease.
Keywords: blood vessels, signaling, calcium, protein kinases, contraction, hypertension
1. INTRODUCTION
Protein Kinase C (PKC) is a ubiquitous enzyme found in almost all cell types including the endothelium, VSM and fibroblasts of blood vessels. PKC phosphorylates serine and threonine residues in a large number of protein substrates and regulates many cellular processes. PKC exhibits significant and sometimes opposite effects in different tissues, and is widely implicated in multiple physiological and pathological processes. The versatility of the effects of PKC is best illustrated in the observation that it could induce both vascular contraction and relaxation. For instance, PKC may mediate the release of endothelium-derived contracting factors such as endothelin-1 (ET-1) and promote vasoconstriction, but could also mediate endothelial nitric oxide (NO) synthesis and promote vasodilation (Wang et al., 2015). PKC could also affect vascular fibroblasts causing increases in transforming growth factor-β, and extracellular matrix (ECM) production, thus promoting vascular remodeling (Geraldes and King, 2010; Ding et al., 2011a). In addition to its effects on the endothelium and ECM, PKC plays a major role in the regulation of VSM function.
Several excellent reviews have discussed many of the biochemical aspects of PKC and its substrates (Nishizuka, 1992; Kanashiro and Khalil, 1998; Newton, 2010; Mochly-Rosen et al., 2012; Khalil, 2013; Mukherjee et al., 2016). Also, the multiple effects of PKC in different cellular processes have made it an important target in many diseases. Understanding the basic biochemical properties of PKC and its effects in the vascular system should help to provide the basis for targeting PKC in different vascular disorders. The purpose of this chapter is to highlight the role of PKC as a major regulator of VSM function with emphasis on recent discoveries and their relevance to vascular disease. We used data published in PubMed and other databases, as well as data from our laboratory to first provide a brief background on PKC biochemistry, its different isoforms, tissue distribution, substrates, and different activators and inhibitors. We will discuss some findings that challenged the concept that PKC translocation is necessary for its activation, and other theories on how to modulate PKC activity by targeting different sites in its regulatory and catalytic domains. We will then discuss the potential role of PKC in vascular disorders and the potential benefits of PKC inhibitors in the management of vascular disease. While the focus of the chapter is on PKC in VSM, in the instances that there is little information available in VSM, the effects of PKC on other systems will be described.
2. PKC STRUCTURE AND ISOFORMS
In 1977, Nishizuka and colleagues discovered PKC in rat brain extract (Takai et al., 1977). PKC was initially defined as a kinase that is activated by proteolysis, but was soon found to be activated by diacyglycerol (DAG) (Takai et al., 1979), and later by phorbol ester, a tumor promoter (Castagna et al., 1982). The PKC molecule comprises a N-terminal regulatory domain and a C-terminal catalytic domain between which lies the V3 hinge region (Kishimoto et al., 1989) (Fig. 1). The conventional PKC isoforms α, βI, βII, and γ have four conserved regions (C1, C2, C3 and C4) and five variable regions (V1, V2, V3, V4, and V5). The regulatory domain is comprised of the two conserved C1 and C2 regions. The C1 region contains cysteine-rich zinc finger-like motifs as well as lipid-binding sites surrounded by a band of hydrophobic residues that penetrate the lipid bilayer and provide a strong driving force to anchor PKC to DAG-containing membranes. In addition to the membrane-docking interactions caused by binding of the C1 region to DAG, full-length PKC may stably associate with membranes by a second membrane-targeting interaction involving the C2 region (Steinberg, 2008). Furthermore, the C1 region binds the PKC cofactor phosphatidylserine (PS) (Kim et al., 2013), and PS may also bind to the C2 region (Poli et al., 2014). An autoinhibitory pseudosubstrate sequence, common to all protein kinases, is embedded in the N-terminal regulatory domain immediately preceding the C1 region and has an amino acid sequence between residues 19 and 36 that resembles the PKC substrate phosphorylation site (Newton, 2001). The catalytic or kinase activity domain contains a conserved C3 region, an ATP/Mg-binding site in a narrow hydrophobic pocket, and a binding site for the phospho-acceptor sequence in the substrate (Mochly-Rosen et al., 2012). The C4 region constitutes the substrate-binding part of the PKC molecule (Newton, 1995). The catalytic domain also contains three key phosphorylation and autophosphorylation sites in the C-terminal activation loop, turn-motif and hydrophobic-motif.
Fig. 1.
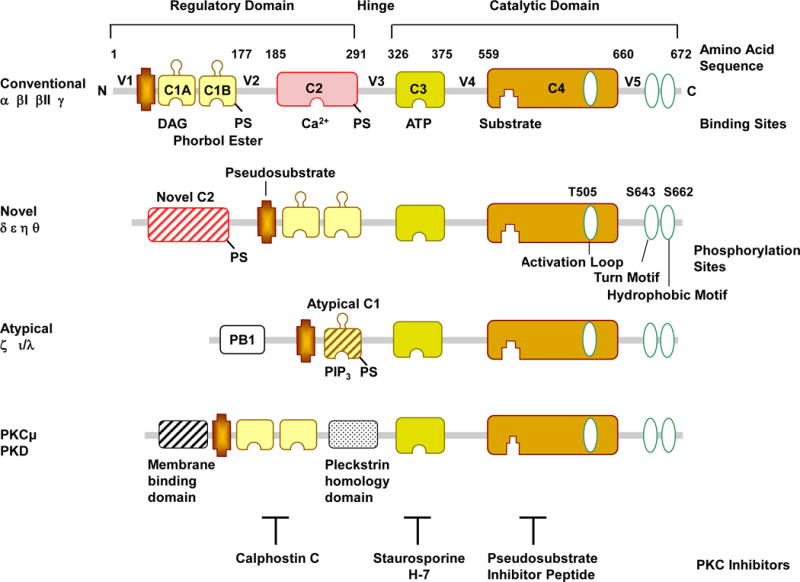
PKC Structure. PKC contains a N-terminal ‘regulatory domain’ and a C-terminal ‘catalytic domain’, between which lies the V3 hinge region. The regulatory domain contains two conserved C1 and C2 regions, and the pseudosubstrate region. The catalytic or kinase activity domain contains a C3 ATP/Mg-binding site and a C4 binding site for the phospho-acceptor sequence in the substrate proteins. The catalytic domain also contains phosphorylation sites in the activation loop, turn-motif and hydrophobic-motif. The figure illustrates the amino acids for PKCδ phosphorylation sites, which may vary among different PKCs. Based on the domain composition of the N-terminals, the PKC family is classified into conventional cPKCs α, βI, βII, and γ; novel nPKCs δ, ɛ, η and θ; and atypical aPKCs ι/λ and ζ isoforms. cPKCs consist of 4 conserved (C1–C4) and 5 variable regions (V1–V5) and are activated by Ca2+, DAG, and PS. The C1 region contains the recognition site for phosphatidylserine (PS), DAG, and phorbol esters, while the C2 region contains the binding site for Ca2+. PS can also be bound by the C2 region. Both cPKCs and nPKCs have twin C1 regions (C1A and C1B) and a C2 region. The order of C1 and C2 regions is switched in nPKCs compared with cPKCs. The nPKCs have a variant form of C2 region that is insensitive to Ca2+ activation, but still binds lipids. The aPKCs do not have a C2 region and hence not activated by Ca2+ or DAG, and have a variant form of C1 that is not duplicated, but retains lipid-binding activity and are activated by DAG and PS. The aPKCs also encode the protein–protein-interacting region Phox and Bem 1 (PB1) that binds with ZIP/p62, Par6, or MEK5 through a PB1-PB1 domain interaction and hence control the localization of aPKCs. Other related kinases include PKCμ (PKD). PKC inhibitors could compete with DAG at the C1 region (calphostin) or ATP at the ATP-binding site (staurosporine) or the true PKC substrate (pseudosubstrate inhibitor peptide).
PKC belongs to the AGC family of serine/threonine protein kinases that are related at their primary sequence and are named after the first identified ‘founding members’ cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA), cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG) and PKC (Linch et al., 2014). PKC is the largest serine/threonine kinase family, comprising ~2% of the human kinome (Mellor and Parker, 1998). The PKC family is encoded by nine different genes and consists of 10 isoforms (Hoque et al., 2014). The N-terminal regulatory domain contains a highly 60–80% homologous C1 region among different PKC isoforms (Mochly-Rosen et al., 2012). Based on the structure of the N-terminal domain, PKC isoforms are classified into conventional cPKCs α, βI, βII, and γ; novel nPKCs δ, ɛ, η and θ; and atypical aPKCs ζ and ι/λ isoforms (Salamanca and Khalil, 2005) (Fig. 1). The cPKCs consist of four conserved regions (C1–C4) and five variable regions (V1–V5), and are activated by calcium (Ca2+), DAG, and PS. The C1 region contains the recognition site for PS, DAG, and phorbol esters while the C2 region is rich in acidic residues and contains the binding site for Ca2+ (Newton, 1995). In cPKCs, the C2 region comprises ~130 residue eight-stranded anti-parallel β-sandwich structures with three inter-strand Ca2+-binding loops responsible for Ca2+-dependent anionic phospholipid binding (Steinberg, 2015). Both cPKCs and nPKCs have twin C1 regions (C1A and C1B) and a C2 region, but the ordering of the C1 and C2 regions is switched in nPKCs compared to cPKCs (Steinberg, 2015). Also, nPKCs have a variant C2 region that lacks the critical Ca2+-coordinating aspartic acid residues that are highly conserved in cPKCs, making it insensitive to Ca2+ (Newton, 2001). As a result, the nPKC C2 region does not bind Ca2+, and nPKCs are activated by DAG and PS, but not Ca2+. The C1 region of nPKCs has a higher affinity for DAG than the C1 region of cPKCs, and functions as a lipid-binding membrane-targeting module in a Ca2+-independent manner (Dries et al., 2007). The C2 region of PKCδ does not bind lipids, but has a protein–protein interaction domain that binds phospho-tyrosine residues flanked by the consensus sequence (Y/F)-(S/A)-(V/I)-pY-(Q/R)-X-(Y/F). PKCδ contains several tyrosine phosphorylation sites throughout its structure, including in the highly conserved regulatory and kinase domains and the intervening more flexible variable hinge region, most of which are unique to PKCδ (Benes et al., 2005). The aPKCs do not have a C2 region but have a variant form of C1 and are therefore activated by PS but not Ca2+ or DAG (Newton, 2001). However, aPKCs do retain lipid-binding activity, and the C1 region confers DAG binding that is not duplicated, unlike the C1A–C1B tandem repeat found in cPKCs and nPKCs (Linch et al., 2014). The aPKCs also uniquely encode the protein–protein-interacting Phox and Bem 1 (PB1) region in the N-terminus domain, which binds ZIP/p62, Par6, or MEK5 through a PB1-PB1 domain interaction that controls the localization of aPKCs (Hirano et al., 2004).
PKCμ and PKCν are often considered a fourth class of PKC isoforms or members of a different family called protein kinase D (PKD) (Rozengurt, 2011; Poli et al., 2014). Other PKC-related-kinases (PRKs) include PRK1–3 and are also considered a fourth group of the PKC family (Mellor and Parker, 1998). PRKs are similar in structure to PKCs except for the C1 region, but do not bind Ca2+, DAG, or phorbol esters, and have homology region 1 (HR1) motifs responsible for RhoA binding (Hage-Sleiman et al., 2015).
The greatest homology between PKC isoforms is in the highly conserved catalytic domain (~70%). Also, similar to other Ser/Thr protein kinases, PKC isoforms have a highly conserved ATP-binding site. The exception to the catalytic domain homology is the variable V5 region, consisting of 60–70 different amino acids. PKC isoforms also differ in the intervening V3 hinge region and the C2 region of the regulatory domain (Mochly-Rosen et al., 2012). PKCβI and βII are generated by alternative splicing from a single gene, but differ at their C-terminal 50 (βI) or 52 (βII) residues (Newton, 1995). The amino acid for each phosphorylation site also varies in different PKCs. For example, the activation loop contains a phosphorylatable Thr497 in PKCα, T500 in PKCβII, T505 in PKCδ and T538 in PKCθ, and the turn motif contains a T638 in PKCα, T641 in PKCβI and PKCβII, S643 in PKCδ and S676 and S685 in PKCθ, while the hydrophobic motif contains a Ser657 in PKCα, S660 in PKCβII, S662 in PKCδ and S695 flanked by bulky hydrophobic residues in PKCθ (Xu et al., 2004; Steinberg, 2008).
3. PKC DISTRIBUTION AND TRANSLOCATION
PKCs are found in varying amounts in different tissues and cells, including various vascular beds. PKCα, δ and ζ are universally expressed in almost all blood vessels examined, while other PKCs show specific distribution in specific vascular beds (Kanashiro and Khalil, 1998; Khalil, 2013) (Table 1). In human VSMCs, the expression of PKCα, β, δ and ɛ, but not PKCζ, is relatively high (Grange et al., 1998). In endothelial cells, however, the levels of PKCδ are lower than PKCζ, demonstrating how PKC distribution varies depending on the vascular cell type (Mattila et al., 1994; Magid and Davies, 2005). In resting cells, PKCα, β and γ, are localized mainly in the cytosolic fraction, and activated PKC undergoes translocation from the cytosolic to the particulate and membrane fraction (Kraft and Anderson, 1983) (Fig. 2); although other PKCs may show redistribution in specific cell membranes (Khalil, 2013).
Table 1.
Distribution of PKC isoforms and potential scaffold proteins in major tissues and representative blood vessels
| PKC | MW (kDa) | Main Tissue Distribution | Blood vessel Examined | Location in Resting State | Location in Activated State | Potential Scaffold | Reference |
|---|---|---|---|---|---|---|---|
| cPKCs | |||||||
| α | 74–82 | Universally expressed | Rat aorta | Cytosolic | Nuclear | RACK1 | 1(Watanabe et al., 1989; Wetsel et al., 1992; Haller et al., 1994; Khalil et al., 1994; Ohanian et al., 1996; Kanashiro et al., 2000a; Hoque et al., 2014) |
| Rat mesenteric artery | Cytosolic/Membrane | Cytosolic/Membrane | p32 | ||||
| Rat carotid, porcine coronary artery, bovine aorta, ferret portal vein | Cytosolic | Plasma membrane | RACK1, AKAPs, HSP, p32 | ||||
| β | 80–82 | Adipose tissue, liver, kidney, spleen, skeletal muscle, brain, | Rat aorta | Cytosolic | Nuclear | RACK1, p32 | (Haller et al., 1994; Hoque et al., 2014; Mehta, 2014) |
| Rat carotid | Cytosolic | Membrane | RACK1, AKAPs, HSP, p32, 14-3-3 | ||||
| γ | 70–82 | Adrenal gland, brain | Rat mesenteric artery | Cytosolic | Cytosolic | RACK1, AKAPs, HSP Importins, 14-3-3 | (Wetsel et al., 1992; Ohanian et al., 1996; Hoque et al., 2014) |
| nPKCs | |||||||
| δ | 76–82 | Universally expressed | Rat aorta | Cytoskeleton/Organelle | Cytoskeleton/Organelle | RACK1, p32 | (Liou and Morgan, 1994; Ohanian et al., 1996; Zhao et al., 2012; Hoque et al., 2014) |
| Rat mesenteric artery | Membrane | Membrane | AKAPs, HSP, p32, 14-3-3 | ||||
| ε | 90–97 | Pancreas, kidney, brain | Rat mesenteric artery, porcine coronary artery | Cytosolic/Membrane | Cytosolic/Membrane | AKAPs, p32 | (Khalil et al., 1992; Wetsel et al., 1992; Ohanian et al., 1996; Kanashiro et al., 2000a; Hoque et al., 2014) |
| Ferret aorta | Cytosolic | Surface membrane | RACK1, RACK2 AKAPs, HSP, p32, 14-3-3 |
||||
| η | 80 | Lung, skin, brain | NIH 3T3 fibroblasts | Cytosolic/Membrane | Membrane | (Goodnight et al., 1995; Suzuki et al., 2009; Hoque et al., 2014) | |
| θ | 79 | Lymphoid and hematopoetic cells, skeletal muscle | AKAPs, HSP, Importins, p32, 14-3-3 | (Hoque et al., 2014; Hage-Sleiman et al., 2015) | |||
| aPKCs | |||||||
| ζ | 64–82 | Universally expressed | Rat aorta, ferret aorta and portal vein | Perinuclear | Intranuclear | AKAPs, HSP, Importins, p32, 14-3-3 | (Khalil et al., 1992; Wetsel et al., 1992; Liou and Morgan, 1994; Ohanian et al., 1996; Hoque et al., 2014) |
| Rat mesenteric artery | Cytosolic | Cytosolic | |||||
| ι/λ | 70 | Testis, ovary, kidney, brain | Rabbit femoral artery and portal vein | Cytosolic | Cytosolic | AKAPs, HSP, Importins, 14-3-3 | (Akimoto et al., 1994; Gailly et al., 1997; Hoque et al., 2014) |
MW, molecular weight
Fig. 2.
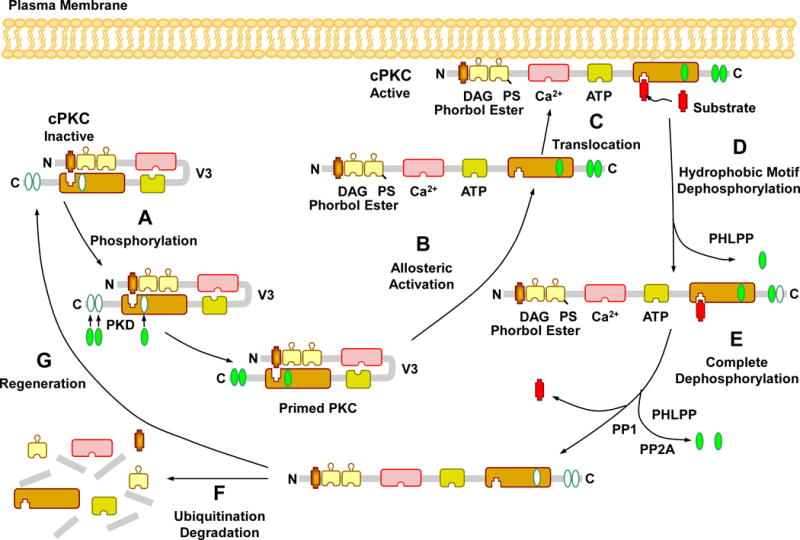
Activation, translocation, substrate interaction and deactivation of cPKCs. In the PKC cytosolic and inactive state, the pseudosubstrate binds the catalytic site in the C4 region, leading to folding of the regulatory and catalytic domain. Before it becomes catalytically competent, nascent PKC undergoes phosphorylation at three phosphorylation sites. The first and rate-limiting phosphorylation of the activation loop is catalyzed by phosphoinositide-dependent kinase (PDK). Consequently, a negative charge is introduced that properly aligns residues to form a competent catalytic domain and facilitate subsequent autophosphorylation at the turn motif and hydrophobic motif, a process that keeps PKC in a catalytically competent and protease resistant conformation. PKC activators such as PS, DAG, phorbol esters, and Ca2+ promote full allosteric activation and translocation of PKC to the plasma membrane. Allosteric activation also induces an open conformation state, making PKC susceptible to phosphatases and proteases and allows PKC to either enter an autophosphorylation/dephosphorylation cycle, or undergoes proteolytic degradation, PKC dephosphorylation terminates its kinase activity and is carried out by the PP2C member pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP) at the hydrophobic motif, starting the process that consequently drives complete dephosphorylation of PKC by PP1/PP2A protein phosphatases at the turn motif. Dephosphorylation also predisposes “naked” PKC to ubiquitination and degradation, requiring new synthesis of the enzyme.
The mechanisms of PKC movement from the cytosol to the membrane are not fully understood. Simple diffusion and other physico-chemical forces may drive the movement of PKC inside the cell, while specific targeting mechanisms would allow its translocation to different cell membranes and tight binding to its target location. Targeting mechanisms include conformation changes, altered hydrophobicity, lipid modification, protein-protein interaction, targeting sequences, and phosphorylation (Saito et al., 2003; Khalil, 2013).
Binding of Ca2+ or DAG may cause conformational changes that unfold the PKC molecule and result in exposure of the substrate region, increased PKC hydrophobicity and binding to membrane lipids (Newton, 1995). Changes in the plasma membrane lipid domains could also influence the subcellular distribution of PKC. The VSM plasma membrane is composed of several domains of focal adhesions alternating with zones rich in caveolae, and both harbor a subset of membrane-associated proteins. Caveolae appear to be a major cell surface location for PKC. For instance, PKCα is constitutively present and exhibits binding activity in caveolae, and does not bind to non-caveolae membranes, which constitute over 90% of the plasma membrane (Mineo et al., 1998). Local fluctuations in [Ca2+] may affect the amount of a specific PKC isoforms retained in caveolae, reflecting the ion requirement for PKC binding to caveolae. For instance, caveolae contain PKCα only in the presence of Ca2+, and PKCλ only in the absence of Ca2+ from the isolation buffer, while retention of PKCɛ in caveolae is not dependent on Ca2+ (Mineo et al., 1998). Caveolins are scaffold proteins that could help PKCα and ζ localize to the caveolar microdomains where they are subsequently activated (Oka et al., 1997). In rabbit femoral and renal arteries at rest, PKCζ is localized in punctate plasma membrane aggregates alternating with vinculin (supporting its location in caveolae), and in a perinuclear location, and these locations may be conducive to regulating VSM [Ca2+]i (Ratz and Miner, 2009).
The plasma membrane lipids are also segregated into cholesterol-rich lipid rafts and glycerophospholipid-rich non-raft regions, an arrangement that is critical for preserving the membrane protein architecture and for the translocation of proteins. In VSMC membrane, lipid segregation is supported by annexins that target membrane sites of distinct lipid composition, and each annexin requires different [Ca2+] for its translocation to the plasma membrane, thus allowing a spatially confined graded response to external stimuli and plasmalemmal localization of PKC (Draeger et al., 2005). Several members of the annexin family function as PKC substrates and can promote membrane association of PKC (Dubois et al., 1996; Xu and Rumsby, 2004). PKC isoforms interact with unique members of the annexin family, and PKCβ, ɛ and α interact with annexin I, II and VI, respectively (Mochly-Rosen et al., 1991). Also, a transient interaction between annexin V and PKCδ occurs in cells after PKCδ stimulation, but before its translocation to the particulate fraction, suggesting that PKCδ requires binding to annexin V for its translocation, and whether other PKCs require annexin binding before translocation is unclear (Kheifets et al., 2006).
Myristoylated alanine-rich C kinase substrate (MARCKS) may play a role in PKC membrane binding. MARCKS is a major PKC substrate that is bound to F-actin and may function as a cross-bridge between cytoskeletal actin and the plasma membrane (Hartwig et al., 1992). Phosphorylation of MARKS by PKC may have an electrostatic effect that affects its protein affinity to the plasma membrane and consequently interferes with its actin cross-linking and causes its displacement from the plasma membrane. On the other hand, dephosphorylation of MARCKS causes its re-association with the plasma membrane via its stably attached myristic acid membrane-targeting moiety (Thelen et al., 1991).
Protein-protein interactions are crucial in signal transduction, and binding sites for arginine-rich polypeptides have been identified in the PKC molecule distal to its catalytic site, allowing targeting of PKC to precise substrates at specific cellular locations. Scaffold proteins could participate in the compartmentalization of PKC to the membrane, and include receptor for activated C kinase (RACK), substrates that interacts with C kinase (STICK), receptor for inactive C kinase (RICK), and A-kinase activating proteins (AKAPs) (Ron and Kazanietz, 1999). RACKs and STICKs bind to active PKCs, whereas RICKs and AKAPs interact with inactive PKCs. Binding of a specific activated PKC to its RACK provides access to and phosphorylation of its substrates (Qvit and Mochly-Rosen, 2010). Also, the binding of RACK increases the phosphorylation capacity of PKC several-fold independently from the substrate identity (Ron and Mochly-Rosen, 1994). RACKs may also target PKC to cytoskeletal elements (Ron and Mochly-Rosen, 1994). The interaction of PKC and RACK is isoform specific and is largely mediated by the C2 region of cPKCs (Ron D, 1995), and peptide fragments of this region have been developed as modulators of PKC activity (Mochly-Rosen and Kauvar, 2000). These short peptides induce activation and translocation of the corresponding PKC isoform by mimicking the action of the RACK on the isoform and, therefore, are termed ‘pseudo RACKs’ (ΨRACK) (Dorn et al., 1999; Churchill et al., 2009). Disruption of the interaction between the ψεRACK and the RACK-binding site is a critical rate-limiting step in translocation of PKCε (Schechtman et al., 2004). Other scaffold proteins including 14-3-3, heat shock protein (HSP), importins, and even actin, have been suggested to tether PKC isozymes to organelles and membranes (Toker et al., 1990; Prekeris et al., 1996; Welch et al., 2010; Adwan et al., 2011; Lum et al., 2013). Additional unique protein-protein interactions between specific PKC isoforms and their substrates might provide further anchoring and specificity at the subcellular sites. For instance, for PKCɛ, a myofilament-binding site in the C2 region (Huang and Walker, 2004), an intra-sarcoplasmic reticulum calsequestrin-binding site (Rodriguez et al., 1999), a neurocytoskeletal elements-binding site (Zeidman et al., 1999), a unique actin-binding site within the C1 region of ɛPKC and a Golgi-binding site (Prekeris et al., 1996; Csukai et al., 1997) have been identified (Churchill et al., 2009). PKCε exhibits a unique association with Golgi membranes via its zinc finger domain and specifically modulates Golgi function, and the zinc finger domain might act as a specific localization signal (Lehel et al., 1995a). PKCε may also translocate from Golgi to the plasma membrane by two distinct mechanisms; as a rapid, vesicle-independent process was observed with holo PKCε (which requires the presence of the pseudosubstrate and/or hinge regions), and a slow, vesicle-dependent pathway was observed with the zinc finger fragment (Lehel et al., 1996).
Different parts of the PKC molecule play varying roles in mediating PKC activation and translocation. The pseudosubstrate and hinge regions facilitate plasma membrane and cytoskeletal association (Lehel et al., 1995b). Also, the V5 variable region contributes to the regulation of PKCα activity through multiple mechanisms involving stabilizing the kinase through direct interactions with its N-terminal, interacting with the pseudosubstrate in the N-terminal regulatory domain, and mediating subcellular localization through interaction with RACK (Yang and Igumenova, 2013).
Although the interaction of cPKCs at the plasma membrane has been well-studied, less is known about the activity of nPKCs and aPKCs at the plasma membrane and other cell membranes including those of the nucleus and other cell organelles such as the mitochondria, endoplasmic reticulum (ER) and Golgi (Fig. 3). For instance, c-src-dependent phosphorylation of tyrosine Y256 in PKCι, through enhanced interaction with the nuclear transporter protein importin-β, results in its translocation to the nucleus (White et al., 2002). Also, the ER membrane may represent the main target for PKCδ recruitment rather than Golgi or mitochondrial membranes. Other nPKCs such as PKCɛ also display the same translocation pattern following ATP binding. Considering the importance of the ER in protein synthesis and modification, the functions of nPKCs at the ER membrane need further investigations (Hui X, 2014).
Fig. 3.
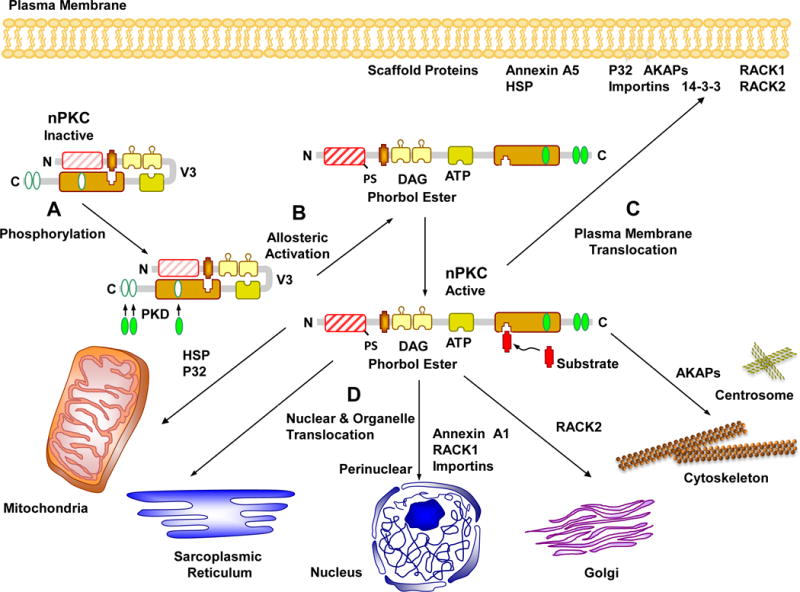
Activation and translocation of novel PKCs (nPKC). After undergoing phosphorylation (A) and allosteric activation (B), nPKCs may remain cytosolic or use scaffold proteins to translocate to the plasma membrane (C), or to the perinuclear region, the nucleus and cell organelles (D) such as the mitochondria, sarcoplasmic reticulum, Golgi, the cytoskeleton and centrosome.
While translocation to cell membranes was traditionally considered the hallmark of PKC activation, this allosteric model for PKC activation by lipid cofactors and the concept that membrane translocation is essential to PKC activation have been challenged. For instance, the model assumes that the cellular actions of PKC are membrane-limited, as it focuses on the role of lipid cofactor in mediating translocation and delivery of the enzyme to the membranes. However, PKC may be localized in the cell periphery in both resting and stimulated tissues. Immunohistochemical studies have shown that the distribution of PKCα in the longitudinal and circular layers of the swine stomach tonic fundus and phasic antrum under resting conditions does not differ, being predominantly localized near the smooth muscle plasma membrane, and stimulation of either tissue with PDBu or carbachol does not alter this peripheral PKCα distribution (Zhang et al., 2013). Also, PKC is found in other cell compartments like the mitochondria and soluble fraction of cells subjected to oxidative stress, a known activator of PKC (Konishi et al., 2001; Steinberg, 2015). PKCs in the soluble fraction of VSM could also phosphorylate sarcomeric proteins in the contractile apparatus, located distant from the membrane lipid bilayer (Steinberg, 2015). PKC translocation may also be dependent on cytoskeletal elements and active transport along the cytoskeleton, suggesting that other forms of protein-protein interactions may be involved in the translocation process (Schmalz et al., 1996; Dykes et al., 2003; Kheifets and Mochly-Rosen, 2007). Another misconception of the canonical model of PKC activation is related to the concept that PKC’s catalytic activity is an inherent property of the enzyme that is not altered by the activation process; a model that does not adequately explain the diverse and, in some cases, opposing actions of certain PKCs (Steinberg, 2015).
4. PKC PHOSPHORYLATION
In the inactive state of PKC, the pseudosubstrate binds the catalytic site in the C4 region, and both the regulatory and catalytic domains are folded together (House and Kemp, 1987). In the activated state, the PKC molecule is unfolded, the pseudosubstrate is dissociated from the C4 region, and PKC is ready to target its specific substrate. Before it becomes catalytically competent and able to respond to its allosteric activators, nascent PKCs undergo conformational changes at three conserved serine/threonine residue phosphorylation sites in the C-terminal domain (Newton, 1995) (see Fig. 2). Phosphorylation could change protein conformation or electric charge and consequently affect its lipid affinity and binding to the plasma membrane. Phosphorylation of PKC itself via autophosphorylation or by a putative PKC kinase may determine its localization. The cPKCs, nPKCs and probably aPKCs are dependent to varying degrees on phosphorylation of the sites at the activation loop, turn motifs, and hydrophobic motif (Parekh et al., 2000; Newton, 2003). These phosphorylations are thought to keep PKC in a catalytically competent and protease resistant conformation. Full activation of PKC by allosteric activators induces an open conformation that makes the enzyme susceptible to both phosphatases and proteases, leading to either repeated autophosphorylation/dephosphorylation cycles, or proteolytic degradation of the PKC molecule and the synthesis of de novo enzyme (Parekh et al., 2000; Newton, 2010).
The first and rate-limiting phosphorylation of the activation loop at the conserved threonine, is catalyzed by phosphoinositide-dependent kinase (PDK), and is critical for activation of PKC (Le Good et al., 1998; Newton, 2001). In the absence of PDK-1, PKC is prone to rapid degradation before turning into catalytically competent enzyme (Balendran et al., 2000). Mutation of phosphorylatable Thr-residues in the activation loop abolishes PKC activity, supporting its essential role in PKC activation (Cazaubon et al., 1994; Liu et al., 2002). As a result of phosphorylation of the activation loop, a negative charge is introduced that properly aligns residues to form a competent catalytic domain and facilitate the subsequent autophosphorylation of 2 sites in the C-terminus, one at the ‘turn motif’, so named because it corresponds to a phosphorylation site in PKA localized at the apex of a turn, and the other at the more C-terminal hydrophobic motif (Behn-Krappa and Newton, 1999). The hydrophobic motif is an important and direct mediator of PKC stability, functioning as a docking-site for PDK-1 through its repeated negatively charged aspartate sequence called PDK-1 interacting fragment (Balendran et al., 2000; Newton, 2003); an interaction that allows PDK-1 to access the activation loop (Hage-Sleiman et al., 2015).
There are differences in the phosphorylation process in different PKCs. In cPKCs, both the turn motif and the hydrophobic motif are autophosphorylated, whereas in nPKCs autophosphorylation occurs only in the turn motif, and phosphorylation in the hydrophobic motif is carried out by other kinases (Hage-Sleiman et al., 2015). For PKCδ, autophosphorylation of its turn motif contributes to its relative stability and solubility. In VSM, autophosphorylation of PKCα and ɛ may be regulated by α-adrenergic receptor agonists, and the actin-binding protein calponin (CaP) may be involved as α-adrenergic agonists induce translocation of CaP from the contractile filaments to the cortex of VSMCs (Kim et al., 2013). Also, aPKCs are phosphorylated at the activation loop and turn motif, but naturally contain glutamate ‘phosphomimetic’ residues in their hydrophobic motif (Parekh et al., 2000; Newton, 2003; Cameron et al., 2007), while the hydrophobic motif of nPKCs contains an aspartate residue (Cameron et al., 2007).
PKC phosphorylation may occur only during maturation of the newly synthesized enzyme, as has been shown with PKCα, or is dynamically regulated, as has been shown with nPKCs (Cenni et al., 2002; Rybin et al., 2003; Rybin et al., 2004). For example, phosphorylation of multiple sites may be required for activation of mature PKCs as has been shown during H2O2-induced tyrosine phosphorylation of PKCδ (Konishi et al., 1997). Also, in cardiomyocytes, PKCδ and ɛ appear to undergo phosphorylation of the activation loop and the hydrophobic motif even in the absence of allosteric regulators (Rybin et al., 2003), supporting that the regulatory pathways of PKC are isoform- and cell-specific.
There has been some discussion whether phosphorylation of the hydrophobic motif of cPKCs and nPKCs occur via autophosphorylation or through trans-phosphorylation by upstream kinases (Ziegler et al., 1999; Cameron et al., 2007). PDK1 and mTOR are potential upstream kinases that may be key to these phosphorylations (Dutil et al., 1998; Le Good et al., 1998; Jacinto and Lorberg, 2008). For instance, phosphorylation of the turn motif by the mTORC2 complex may trigger autophosphorylation of the hydrophobic motif (Sarbassov et al., 2004; Ikenoue et al., 2008).
The scaffold protein 14-3-3 has been identified as a partner of phosphorylated PKCɛ in mammalian cells. Phosphorylation of PKCɛ on Ser346 and Ser368 is required for binding to 14-3-3, and in turn locks the enzyme in an open, active and lipid-independent conformation (Saurin et al., 2008; Linch et al., 2014). On the other hand, direct interaction between PKCθ and 14-3-3 tau has been observed in T cells, and 14-3-3 overexpression inhibits PKCθ translocation and function (Meller et al., 1996).
Other phosphorylation patterns may be specific to certain PKC isoforms. For instance, tyrosine phosphorylation has been implicated as a mechanism to regulate PKCδ catalytic activity, and the tyrosine-phosphorylated enzyme is constitutively active and no longer requires DAG as a cofactor (Konishi et al., 2001). PKCδ has tyrosine phosphorylation sites, and a pathway involving tyrosine phosphorylation may underlie redox control of PKCδ activity. A Src family kinase (Lck)-driven phosphorylation at Tyr311 in rodent PKCδ (Tyr313 in human PKCδ) is believed to mediate H2O2-dependent increase in PKCδ activity (Konishi et al., 2001; Steinberg, 2015), and has been implicated in different PKCδ-dependent cellular responses (Lu et al., 2007; Nakashima et al., 2008; Steinberg, 2015).
PKC phosphorylation is often used as a marker of its activation and in testing whether certain effects are mediated by PKC. For instance, a PKCα autophosphorylation site has been used as a marker of its activity (Ng et al., 1999; Durgan et al., 2007). However, the use of PKC phosphorylation as a marker of PKC activation may not be definitive. For example, while phospho-S299 could be a useful marker of activated PKCδ, PKCδ is phosphorylated at other sites and undergoes in vitro autophosphorylation at three sites within its V3 region (S299, S302, S304), each of which is evolutionarily conserved and unique to PKCδ. S299-phosphorylated PKCδ is localized at both the plasma and nuclear membranes, making it the best marker of the activated enzyme (Durgan et al., 2007). While S643 is also an important PKCδ autophosphorylation site (Li et al., 1997), it is not ideally used as a marker of activation because it is relatively resistant to dephosphorylation and remains phosphorylated even when PKCδ releases its activator DAG and adopts a ‘closed’ conformation (Parekh et al., 2000; Durgan et al., 2007).
The kinase activity of PKCs is terminated by dephosphorylation, and this usually occurs when PKC is in an “open” conformation, unbound by the pseudosubstrate or constitutively active (Dutil et al., 1994; Lee et al., 1996; Gao et al., 2008). For cPKCs and nPKCs, dephosphorylation is carried out by the PP2C member pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP) at the hydrophobic motif, which starts the process that drives PKC to be totally dephosphorylated and degraded by PP1/PP2A protein phosphatases at the turn motif (Sontag et al., 1997; Ahn et al., 2007; Gao et al., 2008; Newton, 2010). In some instances, phosphatases may have an indirect effect on PKC, for example dephosphorylation of the PKCθ downstream molecules CARMA1 by PP2A leads to PKCθ deactivation (Eitelhuber et al., 2011). Dephosphorylation predisposes “naked” protein kinases to ubiquitination and degradation (Katzmann et al., 2002). Partial inhibition of phosphorylation is caused by binding with HSP70, thus promoting rephosphorylation of PKCs and their subsequent reactivation (Gao and Newton, 2006; Poli et al., 2014).
PKC-priming phosphorylation may also be influenced by the inferred allosteric behavior caused by MgATP binding. Nucleotide pocket occupation confers on PKC a conformation that is both conducive to the action of upstream kinases and protective from the action of antagonistic phosphatases. Studies of kinase-inactive mutant forms of PKC isotypes that typically comprise mutation of the highly conserved lysine residue responsible for coordination of the -β phosphates of ATP, have shown that when the kinase domain is compromised through mutation of the highly conserved lysine it fails to be primed, but can be fully primed upon binding an ATP-competitive high-affinity inhibitor. In one study, investigators expressed an inactive green fluorescent protein (GFP) tagged PKCε K437M mutant (where K437 refers to the conserved lysine that contacts the ATP α-β phosphates) in HEK cells using a tetracycline-inducible system. This led to the accumulation in the cells of inactive PKCε lacking appreciable phosphorylation of the priming sites, while wild-type PKCε expressed under the same conditions was constitutively phosphorylated at all priming sites. Importantly, treatment with the PKC inhibitor bisindolylmaleimide I induced rapid phosphorylation of the priming sites of PKCε K437M–expressing cells, but did not increase phosphorylation of wild-type PKCε, supporting that the conformation induced by occupation of the nucleotide pocket of PKCε K437M with an inhibitor might be sufficient to promote priming. This may be relevant to the natural occupation of the nucleotide pocket with MgATP, as the PKCɛ M468A gatekeeper mutant confers sensitivity to the PKD inhibitor 1-naphthyl-PP1 (NaPP1) and becomes fully primed at steady-state. However, activation with phorbol esters, which relieves the regulatory domain inhibition, permits catalytic action and turnover of MgATP/ADP, leading to rapid dephosphorylation. This is likely due to reduced steady-state occupancy of the nucleotide-binding pocket, as the mutant PKCɛ dephosphorylation can be blocked by NaPP1. Also, following complete phorbol ester-induced dephosphorylation of the PKCɛ M468A mutant, phosphorylation can be re-established by treatment with NaPP1 (Cameron et al., 2009; Linch et al., 2014).
5. PKC ACTIVATORS
PKCs are activated by a variety of hormones such as adrenaline and angiotensin II (AngII), growth factors including epidermal growth factor and insulin, and neurotransmitters like dopamine and endorphin (Mochly-Rosen et al., 2012). These stimulants generally interact with their plasma membrane receptors leading to activation of phospholipase C and hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and DAG. IP3 stimulates Ca2+ release from the intracellular stores in the endoplasmic reticulum, while DAG activates PKC. Activation of PKC can also occur in the absence of receptor activation, as high levels of cytosolic Ca2+ can directly activate phospholipase C and lead to activation of PKC (Mochly-Rosen et al., 2012). PKC isoforms respond differently to Ca2+, PS, DAG, and other phospholipid degradation products. cPKCs bind Ca2+ in a phospholipid-dependent manner, and Ca2+ may form a “bridge” holding the protein and phospholipid complex together at the membrane (Bazzi and Nelsestuen, 1990). PS is indispensable for activation of PKC. Phosphatidylinositol and phosphatidic acid activate PKC at high Ca2+ concentrations. DAG activates PKC by reducing its Ca2+ requirement and enhancing its membrane association (Nishizuka, 1992). PKC activators also include lipids derived from sources other than glycerolipid hydrolysis such as cis-unsaturated free fatty acids and lysophosphatidylcholine, ceramide (a sphingomyelinase product), phosphatidylinositol 3,4,5-trisphosphate, and cholesterol sulfate (Nishizuka, 1995).
Phorbol esters such as phorbol 12,13-dibutyrate (PDBu), phorbol 12-myristate 13-acetate (PMA) and 12-O-tetradecanoylphorbol-13-acetate (TPA) can substitute for DAG in PKC activation. Phorbol esters stabilize PKC–membrane association by reducing its apparent Km for Ca2+ (Kanashiro and Khalil, 1998). PMA binds to PKC 1000-fold more strongly than DAG (Dries and Newton, 2008; Sanchez-Bautista et al., 2009). Interestingly, the binding of PMA to the C1B domain of PKCs alone may not induce a significant conformational change in the protein or release the pseudosubstrate domain from the catalytic core, but may generate a hydrophobic cap covering polar groups and thus helping PKCs to insert into membrane lipid bilayer (Zhang et al., 1995).
DAG analogs and phorbol esters are not specific for a particular PKC isoform, and may have other effects unrelated to PKC. For example, PMA, a widely used PKC activator, recruits members of both cPKCs and nPKCs to the plasma membrane (Hui et al., 2014). Also, 1,2-dioctanoyl-sn-glycerol (DiC8), a DAG analog used commonly to activate PKC, blocks Kv, BKCa and KATP channels of mesenteric artery VSM in a PKC-independent manner. 1-oleoyl-2-acetyl-sn-glycerol (OAG) is a related compound that activates PKC but without blocking K+ channels, and is therefore a preferred pharmacological tool over DiC8 (Rainbow et al., 2011).
Post-translational modifications could activate certain PKC isoforms. For example, proteolysis between the regulatory and the catalytic domains activates PKCδ (Persaud et al., 2005). Other post-translational modifications including oxidation, acetylation and nitration and phosphorylation could also activate PKC (Steinberg, 2008). Oxidants such as H2O2 can directly activate PKC, and both the regulatory and catalytic domains of PKC are susceptible to oxidative modification (Gopalakrishna and Anderson, 1989). PKC contains unique structural features that are especially susceptible to oxidative modification, like the zinc-binding cysteine-rich motifs of the N-terminal regulatory domain (Gopalakrishna and Jaken, 2000). Also, hydroquinone, catechol, and whole cigarette smoke condensate have been shown to activate PKC in Lewis lung carcinoma cells (Gopalakrishna et al., 1994).
6. PKC SUBSTRATES
When PKC is not catalytically active, the basic autoinhibitory pseudosubstrate is protected from proteolysis by an acidic patch in the substrate-binding site. When PKC is activated, it phosphorylates arginine-rich protein substrates, which neutralize the acidic patch and displace the pseudosubstrate from its binding site in the kinase core (House and Kemp, 1987; Newton, 1995). The amino acid sequence near the substrate phosphorylation site may assist in PKC substrate recognition. Several PKC substrates have been identified (Table 2), and PKC isotypes may show some substrate specificity. For instance, PKCα, β, and γ are potent histone IIIS kinases, while PKCδ, ε, and η have a poor capacity to phosphorylate histone (Kanashiro and Khalil, 1998). However, PKC isoforms may show overlapping specificities for substrates derived from modification of their pseudosubstrate regions. For example, the PKC targeting protein AKAP79 binds the catalytic core of all PKCs through a pseudosubstrate-like mechanism (Faux et al., 1999; Bogard and Tavalin, 2015)
Table 2.
Representative PKC substrates and the effect of their phosphorylation
| Substrate | Effect of Substrate Phosphorylation | Reference |
|---|---|---|
| Histones | ||
| H3T45 | DNA fragmentation, apoptosis | (Hurd et al., 2009) |
| H3T6 | Prevents LSD1 from demethylating H3K4 during androgen receptor-dependent gene activation. Promotes cell proliferation | (Metzger et al., 2010) |
| Membrane-bound proteins | ||
| MARCKS (myristoylated, alanine-rich C kinase substrate) | MARCKS is bound to F-actin. Functions as cross-bridge between cytoskeletal actin and plasma membrane | (Hartwig et al., 1992) |
| The inhibitory GTP-binding protein Gi | Facilitates the dissociation of the αi subunit from adenylyl cyclase and thereby relieves it from inhibition. | (Kanashiro and Khalil, 1998) |
| Ion Channels | ||
| BKCa channels | Inhibition, leading to membrane depolarization, activation of L-type voltage-gated Ca2+ channels, and increased [Ca2+]i and vascular tone, e.g. in pulmonary artery and porcine coronary artery. | (Minami et al., 1993; Lange et al., 1997; Taguchi et al., 2000; Barman et al., 2004; Crozatier, 2006; Ledoux et al., 2006; Zhu et al., 2013) |
| Voltage-gated K+ channel | Inhibition. Increases vascular tone | (Cogolludo et al., 2003; Novokhatska et al., 2013; Zhu et al., 2013; Brueggemann et al., 2014) |
| KATP channels | Inhibition. Alters the channel properties by modifying kinetics and/or the number of channels at the cell membrane, e.g. in mesenteric artery | (Levitan, 1994; Bonev and Nelson, 1996; Light, 1996; Zhu et al., 2013) |
| Store-operated Ca2+ channel | HEK293 cells. Inhibition. | (Shi et al., 2004) |
| Ion Pumps & Exchangers | ||
| Ca2+-ATPase activation | Activation. Promotes Ca2+ extrusion. Explains transient nature of agonist-induced increase in VSM [Ca2+]i. | (Salamanca and Khalil, 2005) |
| α1 subunit of Na+/K+-ATPase | Inhibition. Alters membrane potential and intracellular concentrations of Na+ and K+ | (Bertorello et al., 1991) |
| Na+/H+ antiport exchanger | Activation. Increases cytoplasmic pH, which increases contraction | (Aviv, 1994; Austin and Wray, 2000; Wray and Smith, 2004) |
| Cytoskeletal & Regulatory Proteins | ||
| Vinculin | Controls cell shape and adhesion | (Perez-Moreno et al., 1998) |
| Vimentin | Recycles β1-integrins to plasma membrane | (Ivaska et al., 2005) |
| CPI-17 | Enhances myofilament force sensitivity to Ca2+. Inhibits MLC phosphatase, increases MLC phosphorylation and enhances VSM contraction, e.g. in rabbit femoral artery | (Woodsome et al., 2001) |
| Calponin | Allows actin-myosin interaction and enhances VSM contraction | (Parker et al., 1994) |
| Raf | Initiates a cascade involving MAPK kinase (MEK) and MAPK, and phosphorylation of the actin-binding protein caldesmon (CaD) which reverses its inhibition of MgATPase activity and thus increases actin–myosin interaction and VSM contraction | (Khalil et al., 1995; Kim et al., 2008) |
| 20-kDa MLC and MLCK | Counteracts Ca2+-induced actin–myosin interaction and force development, e.g. in In rabbit mesenteric artery | (Inagaki et al., 1987) |
| Ribosomal Protein Kinases | ||
| S6KβII | Nucleocytoplasmic shuttling of S6KβII. Regulates protein synthesis and the G1/S transition in the cell cycle | (Valovka et al., 2003) |
| Other | ||
| Arginine-rich protein substrates | Neutralizes the acidic patch in the substrate binding site. Displaces PKC pseudosubstrate from the kinase core | (House and Kemp, 1987; Newton, 1995) |
PKC substrates include the anchoring proteins STICKs such as MARCKs, MacMARCKs, α-, β-, and γ-adducin, clone 72 (SseCKs), GTP-binding proteins and cytoskeletal proteins (Ron and Mochly-Rosen, 1994; Ron and Kazanietz, 1999; Hage-Sleiman et al., 2015). PKC causes phosphorylation of the inhibitory GTP-binding protein Gi, facilitating the dissociation of its αi subunit from adenylyl cyclase and thereby relieves it from inhibition (Kanashiro and Khalil, 1998). PKC could also phosphorylate and activate cell migration-related molecules such as focal adhesion kinase, paxillin, and vinculin (Lewis et al., 1996; Li et al., 2003; Kappert et al., 2010; Ding et al., 2011b). PKC phosphorylation of vinculin, a cytoskeletal protein localized at adhesion plaques, could control cell shape and adhesion (Perez-Moreno et al., 1998). PKC could also phosphorylate substrates involved in protein trafficking. Efficient recycling of β1-integrins to the plasma membrane requires PKCɛ-regulated phosphorylation of vimentin, an intermediate filament protein upregulated upon epithelial cell transformation. Inhibition of PKC and vimentin phosphorylation causes integrins to become trapped in vesicles and attenuates directional cell motility. In vitro reconstitution assays showed that PKCε dissociates from integrin containing endocytic vesicles in a selectively phosphorylated vimentin-containing complex. Mutations of PKC-regulated sites on vimentin lead to the accumulation of intracellular PKCε/integrin positive vesicles, while introduction of wild-type vimentin promotes cell motility in a PKCε-dependent manner, supporting that PKC-mediated phosphorylation of vimentin is a key process in integrin trafficking and cell motility (Ivaska et al., 2005). PKC also plays a role in phosphorylation and nucleocytoplasmic shuttling of S6KβII, one of the forms of the ribosomal protein S6 kinase (S6K) involved in the regulation of protein synthesis and the G1/S transition in the cell cycle, and this PKC-mediated phosphorylation is induced by mitogens such as PMA, EGF, IGF-1, and PDGF (Valovka et al., 2003).
The list of PKC substrates is growing and many of these substrates could be playing a role in VSM contraction and growth (Table 2).
7. PKC INHIBITORS
PKC inhibitors include compounds that could interact with the PKC molecule, interfere with PKC binding to its substrates, decrease PKC synthesis, or counteract the effects of PKC. Several PKC inhibitors interact directly with PKC at different sites of the PKC molecule (Table 3). The first generation PKC inhibitors such as H7 and staurosporine are nonspecific pan-PKC inhibitors that block all PKC isoforms and are toxic for clinical use (Clarke and Dodson, 2007). H7 and staurosporine are ATP-competitive small molecule inhibitors that bind to and compete with ATP at the ATP site of the catalytic domain, and therefore display severe side effects in vivo (Mochly-Rosen et al., 2012). The poor selectivity of ATP-binding drugs is also due to their interaction with ATP-binding kinases other than PKC, since the hydrophobic pocket is conserved throughout the kinome (Roffey et al., 2009). Some PKC inhibitors targeting the ATP-binding site such as indolcarbazole and bisindoylmaleimide have shown selectivity to specific PKC isoforms. Ruboxistaurin is a class of bisindoylmaleimide and a relatively selective PKCβ inhibitor (Koya et al., 1997; Geraldes and King, 2010). PKC inhibitors competing at the DAG/phorbol ester or the PS binding site may be more specific. Calphostin C bind to the C1 domain, mimicking DAG-binding (Mochly-Rosen et al., 2012). Interestingly, extended exposure to phorbol esters can specifically downregulate PKCα, β and γ (Kanashiro et al., 2000a), but the tumor-promoting properties of phorbol esters limit their clinical use.
Table 3.
PKC Inhibitors
| Class/ Inhibitor |
Chemistry | Site of Action | Isoform Selectivity |
Kd or IC50 | Reference |
|---|---|---|---|---|---|
|
Isoquinolines H-7 |
1-(5-isoquinolinesulfonyl)-2-methylpiperazines | ATP-binding site | PKCβI 3.5
μM PKCζ 6 μM |
(Howcroft and Lindquist, 1991) | |
|
Benzophenones Chelerythrine |
1,2-dimethoxy-12-methyl[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium | ATP-binding site | Pan-PKCs | 0.66 μm | (Ding et al., 2011b) |
| Balanol | 2-{[2,6-dihydroxy-4-({[(3S,4R)-3-[(4-hydroxybenzene)amido]azepan-4-yl]oxy}carbonyl)phenyl]carbonyl}-3-hydroxybenzoic acid | ATP-binding site | Pan-PKCs PKCβII> βI> η> δ> α> ɛ |
4–9 μm | (Pande et al., 2008; Mochly-Rosen et al., 2012) |
|
Indolocarbazoles Gö6976 |
5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile | Catalytic domain | PKCα, β1 | PKCα 2.3, βI 6.2 nM | (Martiny-Baron et al., 1993; Grandage et al., 2006) |
| Gö6983 | 1H-Pyrrole-2,5-dione, 3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)- | ATP-binding site Suppresses PKCμ auto-phosphorylation |
Pan-PKC inhibitor Potent: PKCα,
β, γ, δ Less potent: PKCζ |
PKCα 7, β 7, γ 6, δ 10, ζ 60 nM | (Gschwendt et al., 1996; Peterman et al., 2004) |
| Enzastaurin (LY317615) | 3-(1-methyl-1H-indol-3-yl)-4-(1-(1-(pyridin-2-ylmethyl)piperidin-4-yl)-1H-indol-3-yl)-1H-pyrrole-2,5-dione | ATP-binding site | Potent: PKCβ Less potent: PKCα, γ, ɛ |
PKCα 39, β 6, γ 83, ɛ 110 nM | (Graff et al., 2005; Rovedo et al., 2011) |
| LY379196 | ATP-binding site | PKCβ | 3–6 μM | (Slosberg et al., 2000) | |
| Staurosporine (CGP41251) | 9,13-Epoxy-1H,9H-diindolo[1,2,3-gh:3′,2′,1′-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, [9S-(9α,10β,11β,13α)]- | ATP-binding site | Pan-PKCs Potent: PKCα, γ, η Less potent: PKCδ, ɛ |
PKCα 2, γ 5, δ 20, η 4 nM | (Tamaoki et al., 1986; Meggio et al., 1995) |
| CGP53353 | 5,6-bis[(4-Fluorophenyl)amino]-1H-isoindole-1,3(2H)-dione | ATP-binding site | PKCβ | PKCβI 3.8, βII 0.41 μM | (Deng et al., 2012) |
| UCN-01 | 7-hydroxystaurosporine | ATP-binding site | cPKCs | 25–50 nM | (Tamaoki, 1991) |
| Sotrastaurin (AEB071) | 3-(1H-indol-3-yl)-4-(2-(4-methylpiperazin-1-yl)quinazolin-4-yl)-1H-pyrrole-2,5-dione | ATP-binding site | Pan-PKC, especially PKCθ | PKCα 0.95, βI 0.64, δ 2.1, ɛ 3.2, η 1.8, θ 0.22 nM (Ki) | (Evenou et al., 2009; Naylor et al., 2011) |
|
Staurosporine
Analogs Ruboxistaurin (LY333531) |
(9S)-9-[[(Dimethyl-d6)amino]methyl]-6,7,10,11-tetrahydro-9H,18H-5,21:12,17-Dimethenodibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecine-18,20(19H)-dione Hydrochloride | ATP-binding site | PKCβI, βII. | PKCβI 4.7, βII: 5.9 nM | (Aiello et al., 2011) |
| Midostaurin (PKC412, CGP41251) | (9S,10R,11R,13R)-2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-11-(methylamino)-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3′,2′,1′-lm]pyrrolo[3,4-j][1,7]benzodiamzonine-1-one | ATP-binding site | Pan-PKCs | 12 nM | (Millward et al., 2006) |
| Bisindolylmaleimide (GF 109203X, Gö 6850) | 3-(1-(3-(Dimethylamino)propyl)-1H-indol-3-yl)-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione | ATP-binding site | Pan-PKC, especially PKCα, βI | PKCα 8.4, βI 18, βII 16, γ 20, δ 210, ɛ 132, ζ 5800 nM | (Toullec et al., 1991; Gekeler et al., 1996) |
| Ro 31-8220 | Carbamimidothioic acid, 3-[3-[2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl]-1H-indol-1-yl]propyl ester, methanesulfonate | Catalytic domain | Pan-PKC: PKCα, βI, βII, γ, ɛ | PKCα 5, βI 24, βII: 14, γ 27, ɛ 24 nM | (Wilkinson et al., 1993; Davies et al., 2000) |
| SCH47112 | ATP-binding site | (Reynolds et al., 1997) | |||
|
Dicationic, lipophilic
compounds Dequalinium Cl |
Quinolinium, 1,1′-(1,10-decanediyl)bis[4-amino-2-methyl-, chloride (1:2) | Covalently modifies the C2-domain | All PKC | 7 μM-18 μM | (Castle et al., 1993; Manetta et al., 1993; Roffey et al., 2009) |
|
Flavonoid Myricitrin |
4H-1-Benzopyran-4-one, 3-[(6-deoxy-α-L-mannopyranosyl)oxy]-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)- | Prevents PKCα and PKCɛ activation by phorbol esters | PKCα, ɛ | (Meotti et al., 2006) | |
| Quercetin | 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy- | Slight PKC inhibitor | (Navarro-Nunez et al., 2010) | ||
|
Benzothiazole Riluzole |
6-(trifluoromethoxy)benzothiazol-2-amine | ATP-binding site | PKCα | (Noh et al., 2000) | |
|
Perylenequinone Calphostin C (UCN-1028C) |
1-[3,10-dihydroxy-12-[2-(4-hydroxyphenoxy)carbonyloxypropyl]-2,6,7,11-tetramethoxy-4,9-dioxoperylen-1-yl]propan-2-yl benzoate | Regulatory domain: Competes at the binding site for DAG and phorbol esters. |
cPKCs, nPKCs | 50 nM | (Ogiwara et al., 1998) |
|
Phenolic ketone Rottlerin (Mallotoxin) |
5,7-dihydroxy-2,2-dimethyl-6-(2,4,6-trihydroxy-3-methyl-5-acetylbenzyl)-8-cinnamoyl-1,2-chromene) | ATP-binding site | PKCδ Other nPKCs |
PKCδ 5 μM Other PKCs 30 μM |
(Gschwendt et al., 1994) |
|
Macrolactone Bryostatin 1 (NSC 339555) |
(1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(Acetyloxy)-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.13,7.111,15]nonacos-8-en-12-yl (2E,4E)-2,4-octadienoate | C1 domain of PKC: competes with phorbol ester and diacylglycerol binding | Twofold selectivity for PKCɛ over PKCα and PKCδ (short term administration activates PKC, long term inhibits) | (Kraft et al., 1986; Roffey et al., 2009; Mochly-Rosen et al., 2012) | |
|
Membrane
lipids Sphingosine (D-erythro-Sphingosine) |
2-Amino-4-octadecene-1,3-diol; trans-4-Sphingenine | Regulatory domain: Competitive inhibitor with phosphatidylserine | 2.8 μM | (Khan et al., 1990) | |
| N,N-Dimethyl-D-erythro-sphingosine | (E,2S,3R)-2-(Dimethylamino)octadec-4-ene-1,3-dio | 12 μM | (Kim and Im, 2008) | ||
|
Taxol Tamoxifen |
2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine | Regulatory domain | cPKCs | (Zarate et al., 2007) | |
|
Purine
nucleoside Sangivamycin |
4-amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine | ATP-binding site | 10 μM | (Osada et al., 1989) | |
|
Carbonitrile 5-vinyl-3-pyridinecarbonitriles |
Catalytic domain | PKCθ | PKCθ 4.7 nM | (Tumey et al., 2009) | |
|
Pyrimidine 2,4-Diamino-5-nitropyrimidine |
Catalytic domain | PKCθ | (Cywin et al., 2007) | ||
|
Sterols Spheciosterol sulfate A |
Catalytic domain | PKCζ | PKCζ 1.59 μM | (Whitson et al., 2009) | |
| Spheciosterol sulfate B | Catalytic domain | PKCζ | PKCζ 0.53 μM | ||
| Spheciosterol sulfate C | Catalytic domain | PKCζ | PKCζ 0.11 μM | ||
|
Antisense
oligonucleotides Isis3521 (CGP64128A, Aprinocarsen) |
20-mer phosphorothioate oligodeoxynucleotide | Inhibits PKCα mRNAexpression | PKCα | – | (Lahn et al., 2003) |
| Isis9606 | 19-mer phophorothioate oligodeoxynucleotide | Inhibits PKCα mRNA | PKCα | – | (Levesque et al., 1997) |
|
Short
peptides Myristoylated-pseudosubstrate peptide inhibitor |
Peptide sequence: myr-FARKGALRQ | Substrate-binding site | cPKCs | – | (Eichholtz et al., 1993) |
| αV5-3 | Peptide sequence: QLVIAN | Site: aa 642–647 | PKCα | – | (Kim et al., 2011b) |
| βIV5-3 | Peptide sequence: KLFIMN | Inhibits PKC translocation Site: aa 646–651 |
PKCβI | – | (Ferreira et al., 2011) |
| βIIV5-3 | Peptide sequence: QEVIRN | Inhibits PKC translocation Site: aa 645–650 |
PKCβII | – | (Stebbins and Mochly-Rosen, 2001) |
| βC2-4 | Peptide sequence: SLNPEWNET | Site: aa 218–226 | All cPKCs | – | (Ron et al., 1995) |
| δV1-1 (KAI-9803, Delcasertib) | Peptide sequence: SFNSYELGSL | RACK-binding site Inhibits translocation Site: aa 8–17 |
PKCδ | – | (Chen et al., 2001) |
| ɛV1-2 (KAI-1678) | Peptide sequence: EAVSLKPT | RACK-binding site Inhibits translocation Site: aa 14–21 |
PKCɛ | – | (Gray et al., 1997) |
| KCe-12 and KCe-16 | Substrate-binding site | PKCɛ | – | (Yonezawa et al., 2009) | |
| ZIP | Peptide sequence: SIYRRGARRWRKL | ζ–pseudo substrate | PKCζ and aPKCs | – | (Braun and Mochly-Rosen, 2003) |
| γV5-3 | Peptide sequence: RLVLAS | Site: aa 659–664 | PKCγ | – | (Sweitzer et al., 2004) |
|
Other α-tocopherol, adriamycin, aminoacridine, apigenin, cercosporin, chlorpromazine, dexniguldipine, polymixin B, trifluoperazine, UCN-02 | |||||
aa, amino acid
Peptides that interfere with the intramolecular interactions within PKC have been developed (Churchill et al., 2009). For instance, myr-ΨPKC is a myristoylated peptide based on the substrate motif of PKCα and β that inhibits TPA-Induced PKC activation and phosphorylation of MARCKS (Eichholtz et al., 1993). Other peptides disrupt protein/protein interactions between the PKC regulatory domain and RACK (Mochly-Rosen et al., 2012). The interaction of PKC and RACK is isoform selective and largely involves the C2 region of cPKC, and peptide fragments of this region may function as selective cPKCs inhibitors (Ron et al., 1995). Also, a peptide derived from the PKC binding proteins annexin I and RACKI inhibits translocation of PKCβ (Ron and Mochly-Rosen, 1994).
Peptides derived from the pseudosubstrate region show autoinhibitory effect on PKC activity and are attractive PKC inhibitors (House and Kemp, 1987; Eichholtz et al., 1993; Bogard and Tavalin, 2015). The autoinhibitory role of the PKC pseudosubstrate has been suggested as deletion of the pseudosubstrate site abrogates the inhibitory effect of the regulatory domain of PKCα on the full-length enzyme (Parissenti AM, 1998). Synthetic oligopeptides based on pseudosubstrate sequence are specific PKC inhibitors because they exploit its substrate specificity and do not interfere with ATP binding. The synthetic peptide (19–36) inhibits PKC autophosphorylation and protein substrate phosphorylation. Replacement of Arg-27 with alanine in the peptide [Ala-27] PKC (19–31) increases the IC50 for inhibition of substrate phosphorylation. A structure-function study of the PKC pseudosubstrate sequence R19FARK-GALRQKNV31 examined the role of specific residues using an alanine substitution scan. Arg-22 was the most important determinant in the inhibitor sequence, since substitution of this residue by alanine gave a 600-fold increase in the IC50. Substitutions of other basic residues with Ala-19, Ala-23 and Ala-27 also increased the IC50 5-, 11- and 24-fold, respectively. The importance of basic residues in determining the potency of the pseudosubstrate peptide reflects the requirement of these residues in peptide substrate phosphorylation. Gly-24, Leu-26 and Gln-28 residues were also important for pseudosubstrate inhibitor potency. The large increase in the IC50 for the [A22]PKC(19–31) peptide makes it a valuable control in studies utilizing the pseudosubstrate peptide to examine functional roles of PKC (House and Kemp, 1990). Another reason pseudosubtrate inhibitors were thought to be more specific inhibitors for PKC isoforms is that the pseudosubstrate region provides a large interface for multiple points of contact (Churchill et al., 2009; Bogard and Tavalin, 2015). However, this is not always the case as a cell-penetrating myristoylated peptide derived from the pseudosubstrate domain of PKCζ, and termed PKCζ pseudosubstrate inhibitor peptide (ZIP) shows affinity for all PKC isoforms causing disruption of PKC targeting and translocation, suggesting that pseudosubstrates of PKC isoforms may possess several invariant well-conserved residues (Bogard and Tavalin, 2015). Also, mutation of the alanine in the pseudosubstrate with serine or glutamate, mimics the charge of a phosphorylated residue and in effect activates PKC (Pears et al., 1990; Parissenti et al., 1998; Kheifets and Mochly-Rosen, 2007).
Compounds that counteract the effects of PKC include activators of β-adrenoceptors and antioxidants. For example, in portal vein, stimulation of β-adrenoceptors opposes the effects of PKC and causes vasodilatation and reduces the activity of store-operated channels via a cAMP-dependent protein kinase (PKA) pathway (Liu et al., 2005; Albert and Large, 2006). Also, antioxidants may inactivate PKC. The PKC catalytic domain contains several reactive cysteines that can be targeted by antioxidants such as selenocompounds, vitamin E, and polyphenolic agents such as curcumin (Boscoboinik et al., 1991; Liu et al., 1993; Gopalakrishna and Jaken, 2000). In VSM, α-tocopherol inhibits the expression, activity, and phosphorylation of PKCα and decrease VSM proliferation, and PKC activity in VSM gradually declines as the α-tocopherol level rises. These effects are not mimicked by β-tocopherol or probucol (Engin, 2009), and, in effect, β-tocopherol may oppose the inhibitory effects of α-tocopherol (Clement et al., 1997). Interestingly, hyperglycemia-induced retinal vascular dysfunction in different animal models can be prevented by α-tocopherol via inhibition of the DAG-PKC pathway (Engin, 2009). Also, high doses of vitamin E may decrease hyperglycemia-induced DAG and PKC activity and reverse some of the changes in the retinal and renal vessels in diabetes (Bursell and King, 1999). On the other hand, glutathione may inhibit PKC by a nonredox mechanism (Ward et al., 1998).
Post-translational modifications of PKC may alter its function. S-nitrosylation, a ubiquitous protein modification in redox-based signaling that forms S-nitrosothiol from nitric oxide (NO) on cysteine residues, decreases PKC activity and signaling and impairs contraction in mouse aorta, and may represent a key mechanism in conditions associated with decreased vascular reactivity (Choi et al., 2011).
Transgenic animals, knockout mice and antisense techniques have been useful in studying the effects of PKC down-regulation in vivo (Table 4). Isoform-specific PKC knockout mice have demonstrated a critical role of PKC in several tissues including endocrine and vascular cells, and further characterization of the PKC knockout vascular phenotype should shed more light on the role of PKC in the vascular system. Also, antisense and siRNA for specific PKC isoforms are now available and can be used to study the role of PKC in various cell functions. ISSI-3521 is a phosphorothioate antisense oligonucleotide that has been targeted to the 3′-untranslated region of PKCα mRNA, and has shown a highly specific reduction of PKCα protein expression in cancer cell lines and human tumor xenograft models (Song et al., 2003; Roffey et al., 2009).
Table 4.
PKC knockout mouse models, their prominent phenotype, and major implications
| PKC Knockout | Prominent Phenotype | Implications | Reference |
|---|---|---|---|
| PKCα−/− | Increased BP in knockout mice fed a high-salt diet. Principal cells of renal cortical collecting ducts show increased number of epithelial Na channel (ENaC) per cell-attached patch clamp, increased membrane localization of α-, β-, and γ-subunits of ENaC, and increased open probability of ENaC channel. | PKCα reduces ENaC membrane accumulation and open probability | (Bao et al., 2014) |
| In skeletal muscles and adipocytes, enhanced insulin signaling to insulin receptor substrate (IRS) 1-dependent PI3K, PKB, and PKCλ, and downstream processes, glucose transport and activation of ERK | PKCα serves as a tonic endogenous inhibitor of IRS-1-dependent PI3K, PKB, and PKCλ during insulin stimulation of glucose transport and ERK | (Leitges et al., 2002) | |
| Peripheral CD3(+)T cells show impaired CD3/CD28 Ab- and MHC alloantigen-induced T cell proliferation and IFN-γ production. PKCα−/− mice give diminished OVA-specific IgG2a and IgG2b responses following OVA immunization experiments | PKCα is necessary for T cell-dependent IFN-γ production and IgG2a/2b Ab responses | (Pfeifhofer et al., 2006) | |
| PKCβ−/−− | ApoE−/− and PKCβ−/−/ApoE−/− mice rendered diabetic with streptozotocin, Diabetes accelerated atherosclerosis in the aorta, increased the level of phosphorylated ERK1/2 and Jun-N-terminus kinase MAPK and augmented vascular expression of inflammatory mediators, and monocyte/macrophage infiltration and CD11c(+) cells accumulation, and processes were diminished by pharmacological inhibition of PKCβ and in diabetic PKCβ(−/−)/ApoE(−/−) mice. | PKCβ is linked to diabetic atherosclerosis through modulation of gene transcription, cell signaling and inflammation in the vascular wall. PKCβ could be a potential therapeutic target for prevention and treatment of diabetic atherosclerosis. | (Kong et al., 2013) |
| PKCγ−/− | Exposure to hyperbaric oxygen was associated
with increased thicknesses of the inner nuclear and ganglion cell layers
of the retina. Destruction of the outer plexiform layer. Significant
degradation of the retina Damage to the outer segments of the photoreceptor layer and ganglion cell layer |
PKCγ may protect retina from damage by hyperbaric oxygen. Hyperbaric oxygen, should be used with care particularly in patients with a genetic disease such as spinocerebellar ataxia type 14 with nonfunctional PKCγ. | (Yevseyenkov et al., 2009) |
| PKCδ−/− | Thickening of the articular cartilage and
calcified bone-cartilage interface. Increased number of hypertrophic
chondrocytes in the articular cartilage. -Loss of demarcation between
articular cartilage and bone was concomitant with irregular chondrocyte
morphology and arrangement. -Increased intensity of calcein labeling in
the interface of the growth plate and metaphysis. -Reduced level of glycosaminoglycan production. |
PKCδ plays a role in the osteochondral plasticity of the interface between articular cartilage and the osteochondral junction. | (Yang et al., 2015) |
| Increased white blood cells and platelet
counts, and bone marrow and splenic megakaryocytes. Increased megakaryocyte number and DNA content. Altered thrombopoietin-induced signaling and increased ERK and Akt308 phosphorylation in megakaryocytes. -Faster recovery and heightened rebound thrombocytosis after thrombocytopenic challenge. |
PKCδ is important for megakaryopoiesis by regulating thrombopoietin-induced signaling. | (Kostyak et al., 2014) | |
| Fertility analysis has shown that mating pairs produce fewer pups per litter than wild-type pairs. Reduced number of total implantations in females. Sperms showed decreased capacity to penetrate the zona pellucida. Pregnant females exhibit a high incidence of embryonic loss post-implantation. | PKCδ is important for key reproductive functions and fertility in both males and females | (Ma et al., 2015) | |
| PKCɛ−/− | Embryonic fibroblasts exhibit reduced insulin
uptake which was associated with decreased insulin receptor
phosphorylation. Changed localization of insulin receptor with
colocalization with membrane microdomains marker flotillin-1. Reduced
redistribution of insulin receptor by insulin
stimulation -Reduced expression of CEACAM1, a receptor substrate which modulates insulin clearance. |
PKCɛ affects insulin uptake through promotion of receptor-mediated endocytosis, and that this may be mediated by regulation of CEACAM1 expression. | (Pedersen et al., 2013) |
| PKCη−/− | -Poor proliferation of T cells in response to
stimulation by antigen -Defective homeostatic proliferation, a function requiring recognition of self antigens. -Higher ratio of CD4+ to CD8+ T cells compared to that of wild-type mice. |
PKCη performs functions that are important for homeostasis and activation of T cells. | (Fu et al., 2011) |
| PKCθ−/− | -The thymus contains less mature single
positive T cells than wildtype. Thymocytes show defective activation of
transcription factors AP-1, NFAT and NFκB and impaired phosphorylation of ERK after T cell receptor stimulation in vitro. |
PKCθ plays a role in positive selection of thymocytes in a pathway leading to the activation of ERK, AP-1, NFAT, and NFκB. | (Gruber et al., 2010) |
| PKCζ−/− | -Impaired secretion of T helper 2 (Th2)
cytokines, as well as the nuclear translocation and tyrosine
phosphorylation of Stat6 and Jak1 activation, essential downstream
targets of IL-4 signaling. -Dramatic inhibition of ovalbumin-induced allergic airway disease. |
PKCζ is critical for IL-4 signaling and Th2 differentiation. Asthma is a disease of chronic airway inflammation in which T helper (Th) 2 cells play a critical role, and PKCζ can be a therapeutic target in asthma. | (Martin et al., 2005) |
| PKCλ−/− | Tissue-specific knockout in muscle shows
impaired insulin-stimulated glucose transport (M) and insulin
resistance. Knockout in liver shows impaired insulin-stimulated lipid synthesis and insulin-hypersensitivity. Knockout in adipocytes shows diminished Insulin-stimulated activity and glucose transport, ERK levels and activity. -Diminished adiposity and serum leptin levels. |
PKCλ plays a role in insulin-stimulated glucose transport and ERK signaling in muscle, liver and adipocytes. | (Sajan et al., 2014) |
Thus some challenges remain with the development of drugs that target specific PKC isoforms. These challenges are largely posed by the ~70% homologous structure of the catalytic domain within the PKC family. Pharmacological tools that target the C2 region could be more selective, as the C2 region is the less conserved among different PKCs (Mochly-Rosen et al., 2012). The V5 region may also be a good target for isoform-specific modulators of PKC activity. PKC isoforms interact with their substrates at sequences unique for the individual isoforms and this interaction can be selectively disrupted by peptide inhibitors that share the same substrate sequence. Also, protein-protein interactions can regulate the subcellular localization of specific PKC isoforms. Further research of PKC substrate interaction sites and PKC protein-protein interactions would shed more light on the various PKC-mediated effects in different systems and provide more specific targets for future therapy of PKC-related disorders (Mochly-Rosen et al., 2012).
8. Vascular Effects of PKC
PKC isoforms have diverse effects in different vascular cell types, with prominent effects on VSM. The role of each PKC isoform in certain vascular responses has been supported by measuring PKC gene expression, protein levels and PKC activity, and by determining the effects of pharmacological isoform-specific PKC inhibitors as well as knockout mice and transgenic rats (Mehta, 2014).
PKC and VSM Contraction
It is widely accepted that Ca2+-dependent myosin light chain (MLC) phosphorylation is a major determinant of VSM contraction (Rembold and Murphy, 1988; Kamm and Stull, 1989) (Fig. 4). Agonist-induced activation of membrane receptors causes an increase in intracellular free Ca2+ concentration ([Ca2+]i) due to initial Ca2+ release from the sarcoplasmic reticulum and maintained Ca2+ entry from the extracellular space. Ca2+ binds calmodulin (CAM) to form a Ca2+-CAM complex, which activates MLC kinase (MLCK), causes phosphorylation of the 20-kDa MLC, and increases the activity of actin-activated Mg2+-ATPase, leading to actin-myosin interaction and VSM contraction (Rembold and Murphy, 1988; Kamm and Stull, 1989). VSM relaxation is initiated by a decrease in [Ca2+]i due to Ca2+ uptake by the sarcoplasmic reticulum and Ca2+ extrusion by the plasmalemmal Ca2+ pump and Na+-Ca2+ exchanger. The decrease in [Ca2+]i causes dissociation of the Ca2+-CAM complex and the phosphorylated MLC is dephosphorylated by MLC phosphatase.
Fig. 4.
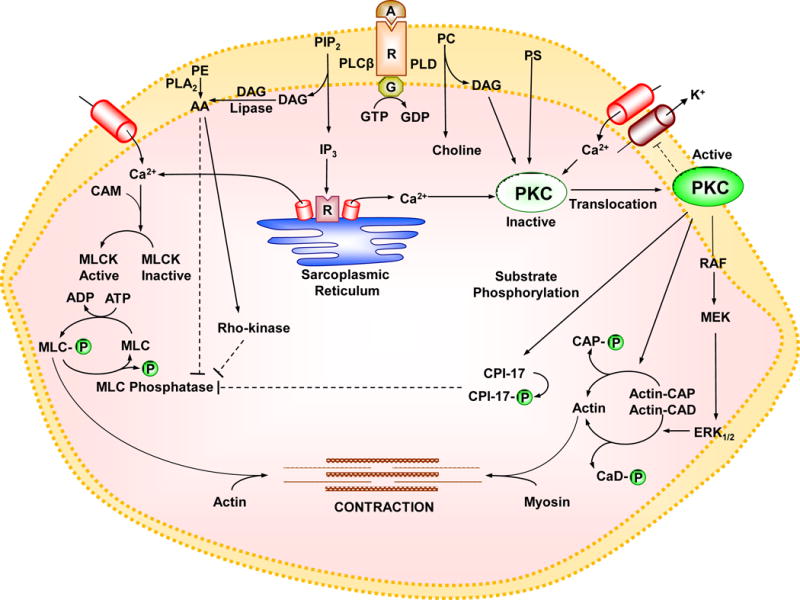
Pathways of VSM contraction. The interaction of an agonist (A) with its specific α-adrenergic receptor (R) and its coupled heterotrimeric GTP-binding protein (G) activates phospholipase C (PLCβ) which stimulates the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) as well as phospholipase D (PLD) which stimulates the hydrolysis of phosphatidylcholine (PC) into choline and DAG. IP3 stimulates Ca2+ release from the sarcoplasmic reticulum. Agonists also stimulate Ca2+ influx through Ca2+ channels. Ca2+ binds calmodulin (CAM), activates myosin light chain (MLC) kinase (MLCK), causes MLC phosphorylation, and initiates VSM contraction. DAG in the presence of PS, and in case of cPKCs Ca2+, cause activation and translocation of PKC. PKC could inhibit K+ channels leading to membrane depolarization and activation of voltage-gated Ca2+ channels, but could also inhibit Ca2+ entry through store-operated Ca2+ channels (SOCs) and transient receptor potential channels (TRPCs). PKC could cause phosphorylation of CPI-17, which in turn inhibits MLC phosphatase and increases MLC phosphorylation and VSM contraction. PKC-induced phosphorylation of the actin-binding protein calponin (CaP) allows more actin to bind myosin and enhances contraction. PKC may also activate a protein kinase cascade involving Raf, MAPK kinase (MEK) and MAPK (ERK1/2) leading to phosphorylation of the actin-binding protein caldesmon (CaD) and enhanced contraction. DAG is transformed by DAG lipase into arachidonic acid (AA). Also, activation of phospholipase A2 (PLA2) increases the hydrolysis of phosphatidylethanolamine (PE) into AA. AA could activate RhoA/Rho-kinase, which in turn inhibits MLC phosphatase and further enhances the Ca2+ sensitivity of contractile proteins. Dashed line indicates inhibition.
PKC can affect VSM contraction by several mechanisms including regulation of ion channels and pumps and in turn [Ca2+]i, Ca2+ sensitization of the contractile proteins, or activation of Ca2+ independent contraction pathways. PKC translocation to the cell surface could also trigger a cascade of protein kinases that ultimately interact with the contractile myofilaments and cause VSM contraction. In some instances, PKC may inhibit VSM contraction.
PKC, ion Channels, and [Ca2+]i
PKC can change [Ca2+]i by modulating the activity of plasmalemmal K+ and Ca2+ channels. K+ channels play a role in the regulation of the resting membrane potential, and inactivation of K+ channels in VSMCs causes membrane depolarization, elevation of [Ca2+]i and VSM contraction (Nelson and Quayle, 1995). Membrane depolarization activates Ca2+ entry via L-type voltage-gated Ca2+ channels (L-VGCC) and may also cause Ca2+ release from IP3- and ryanodine-sensitive intracellular Ca2+ stores leading to increase in [Ca2+]i (Nauli et al., 2001; Kizub et al., 2014). Large conductance Ca2+-activated K+ channels (BKCa) are the predominant K+ channels in VSMCs (Nelson and Quayle, 1995; Ghatta et al., 2006). PKC activators such as PDBu inhibit BKCa leading to increases in vascular tone in both physiological and pathophysiological conditions (Taguchi et al., 2000; Barman et al., 2004; Kizub et al., 2010; Novokhatska et al., 2013), and in various vascular beds including pulmonary (Barman et al., 2004), coronary (Minami et al., 1993), cerebral (Lange et al., 1997), and uterine vessels (Hu et al., 2011). PKC activators inhibit BKCa by phosphorylation of the channel protein and decreasing its sensitivity to activation by cGMP-dependent protein kinase (Crozatier, 2006; Ledoux et al., 2006).
Voltage-gated K+ channels (Kv) also play a role in the regulation of VSM function, and can be modulated by vasoconstrictors such as arginine vasopressin, ET-1 and AngII via a mechanism involving PKC. In rat mesenteric artery VSMCs, vasopressin regulates Kv7.4 and Kv7.5 subunits of Kv7 channels via activation of PKC. PKCα-dependent phosphorylation of the K+ channel proteins on serine residues is sufficient to reduce Kv7 channel activity, and the extent of PKC-mediated Kv7.4 and Kv7.5 phosphorylation and K+ current suppression depends on the subunit composition of the channel proteins (Brueggemann et al., 2014). Also, thromboxane A2 may induce pulmonary vasoconstriction by a mechanism involving PKCζ and inhibition of Kv (Cogolludo et al., 2003). PKC isoforms may contribute differently to the vasoconstrictor-induced effects on different K+ channels. In rabbit coronary arterial VSMCs, ET-1 and AngII inhibit Kv currents by activating PKCɛ, and inhibit KIR channel activity by activating PKCα (Park et al., 2005; Park et al., 2006).
PKC may also regulate KATP channels, and vasoconstrictor agonists may inhibit KATP through PKC signaling (Nelson and Quayle, 1995; Quayle et al., 1997). Phorbol esters inhibit KATP currents in mesenteric arteries (Bonev and Nelson, 1996). Although the mechanism via which PKC regulates KATP is not well defined, in human embryonic kidney cells (HEK293) PKC-mediated AngII- and PDBu- induced inhibition of KATP channel may involve channel complexes composed of four Kir6.1 and their associated SUR2B subunits (Thorneloe et al., 2002). Also, trafficking studies have shown that PKC may initiate internalization of the channel complex leading to decreased KATP channel activity (Manna et al., 2010). PKC-mediated phosphorylation of KATP may also alter the channel properties, kinetics and/or number at the cell membrane (Levitan, 1994; Light, 1996).
PKC, Ion Pumps and Co-transporters, and [Ca2+]i
Plasmalemmal Ca2+-ATPase (PMCA) and sarcoplasmic reticulum Ca2+-ATPase (SERCA) are important Ca2+ homeostasis mechanisms in VSM. PKC may activate PMCA or SERCA, an action that promotes Ca2+ extrusion and re-uptake and lead to a decrease in VSM [Ca2+]i. In isolated cardiac sarcoplasmic reticulum preparations, PKC activates the Ca2+-transport ATPase (Limas, 1980). Also, the α1 subunit of Na+/K+-ATPase may serve as a PKC substrate, and PKC-mediated inhibition of Na+/K+ pump causes changes in the membrane potential and the intracellular concentrations of Na+ and K+ (Bertorello et al., 1991). PKC activation by phorbol esters and permeable DAG analogs may also phosphorylate and activate the Na+/H+ antiport exchanger and thereby increase the cytoplasmic pH leading to alkalinization, which generally increases vascular contraction (Rosoff et al., 1984; Aviv, 1994; Austin and Wray, 2000; Wray and Smith, 2004).
KC and Ca2+-Sensitization of Contractile Proteins
Activation of PKC could increase the myofilament force sensitivity to [Ca2+]i, thereby maintaining VSM contraction with smaller increases in [Ca2+]i. The nPKC isoforms play an important role in mediating VSM contraction through a Ca2+ sensitizing pathway, and inhibition of nPKCs attenuates norepinephrine-induced VSM contraction (Wang et al., 2015). PKC-induced Ca2+ sensitization could involve phosphorylation of regulatory proteins in the VSM contractile myofilaments and the cytoskeleton. PKC phosphorylates CPI-17, which in turn inhibits MLC phosphatase, increases MLC phosphorylation, and thereby enhances VSM contraction (Woodsome et al., 2001). PKC could also inhibit MLC-phosphatase via the phosphorylation of the myosin targeting subunit of myosin phosphatase (MYPT1) (El-Yazbi et al., 2015). Activation of PKCα could cause phosphorylation of CaP, a VSM differentiation marker and an actin-associated regulatory protein, and thereby reverses its inhibition of actin-activated myosin ATPase, allowing more actin to bind myosin, and enhancing VSM contraction. Interestingly, CaP may activate PKC in vitro in the absence of lipid cofactors, and knockdown of CaP inhibits PKC-dependent contraction in ferret arterial VSM (Je et al., 2001; Kim et al., 2008).
PKC may contribute to VSM force production in a MLC phosphorylation-independent manner. In rat middle cerebral artery, PKC activation by PDBu is associated with sustained force generation and vasoconstriction that is much larger than that expected with the same level of MLC phosphorylation achieved by 5-HT (El-Yazbi et al., 2015).
PKC may also be involved in mechanical stretch-induced vascular myogenic response. In rat cerebral artery VSMCs, PKC activators increase stretch-activated channel activity and induce depolarization, and these effects are blocked by PKC inhibitors (Slish et al., 2002). It is possible that increases in tension on vascular myocytes lead to stimulation of phospholipase C (PLC), hydrolysis of phosphoinositides and production of DAG, which activates PKC and stimulate the myogenic response (Albert and Large, 2006). PKC may primarily affect the maintained phase of stretch-induced contraction by changing the Ca2+ sensitivity of the contractile elements (Nakayama and Tanaka, 1993). Some studies suggest that PKCθ and μ may participate in stretch-induced VSM mechanotransduction, as cyclic-stretch of VSM specifically activates these PKC (Yang et al., 2014). However, other studies have shown activation of PKCδ by cyclic stretch in VSM (Li et al., 2003) and PKCɛ activation by mechanical stretch in cardiomyocytes (Klein et al., 2005; Bullard et al., 2007).
The nature and extent of the PKC-activated pathway could vary depending on the vasoconstrictor tested, the vascular bed examined and in arteries versus veins. Vasoconstrictors such as AngII, ET-1, serotonin, norepinephrine, and neuropeptide Y activate PKC-dependent pathways, causing VSMC membrane depolarization and contraction (Quayle et al., 1997; Cole et al., 2000; Zhu et al., 2013). Of note, AngII activates multiple PKC isoforms in VSM (Griendling et al., 1997) and PKC may increase VSM contraction via other pathways involving downregulation of atrial natriuretic peptide (ANP) receptor and the binding of ANP to VSM, and thereby preventing ANP-induced inhibition of contraction (Jiao and Yang, 2015). Although the role of venous function in blood pressure (BP) control has been underappreciated, its contribution is significant in the deoxycorticosterone salt rat model of HTN where ET-1 was found to elevate venomotor tone and contribute to HTN (Tykocki et al., 2014). The PKC inhibitor chelerythrine attenuates ET-1-induced contraction in both the aorta and vena cava, suggesting that ET-1 acts via PKC to mediate VSM contraction of both arteries and veins. However, in the aorta, ET-1-induced contraction is largely dependent on PLC activation and IP3-mediated Ca2+ release, while in the vena cava ET-1 induced contraction is unaffected by the IP3 receptor antagonist 2-APB. Also, only the vena cava contracts in response to the DAG analog OAG, highlighting the differences in the venous and arterial pathways of contraction (Tykocki et al., 2014). It should be noted that endothelium-derived NO regulates VSM tone by activating guanylate cyclase, increasing cGMP and producing vasodilation, and PKC could inhibit NO-mediated vasodilation by inhibiting guanylate cyclase, leading to decreases in intracellular cGMP and increased vasoconstriction (Johnson and Barman, 2004).
PKC and Cytoskeletal Proteins
Studies in cerebral resistance arteries have shown that PKC could mediate myogenic constriction through dynamic reorganization of the cytoskeleton and increased actin polymerization (Moreno-Dominguez et al., 2014). Also, both in the presence and absence of Ca2+, PKC may promote cerebral vasoconstriction by increasing the phosphorylation of paxillin and HSP27, reducing G-actin content, and promoting actin cytoskeleton reorganization (El-Yazbi et al., 2015). The relative contribution of PKC to cytoskeletal modification versus other mechanisms of VSM contraction appears to be more significant in the cerebral circulation. In rat middle cerebral arteries, PDBu-induced PKC constriction is more sensitive to disruption of actin cytoskeleton compared to inhibition of cross-bridge cycling, providing evidence for the pivotal contribution of PKC-mediated cytoskeletal actin polymerization to force generation in cerebral resistance arteries (El-Yazbi et al., 2015).
PKC may also modulate certain genes that code for structural proteins such as fibronectin and type IV collagen, by changing the binding of nuclear transcription factors to the promoter regions on responsive genes (Clarke and Dodson, 2007). PKC also affects the gene expression of the regulator of G-protein signaling 2 (RGS2), which may affect vascular tone. In cultured VSMCs, adrenotensin increases RGS2 expression, while the PKC inhibitor chelerythrine reduces RGS2 expression, suggesting that adrenotensin increases gene expression via a PKC-dependent pathway (Mao et al., 2013).
PKC-Dependent Signaling Cascades
The interaction of PKC with its substrate may trigger a cascade of protein kinases that ultimately stimulate VSM contraction (Fig. 4). PKC may affect Akt signaling PKC (Radhakrishnan et al., 2008; Ding et al., 2011b). Also, mitogen-activated protein kinases (MAPK) such as extracellular signal-regulated kinase (ERK), p38 and JNK, are common downstream effectors of PKC (Yamaguchi et al., 2004; Ginnan and Singer, 2005). PKC, MAPK, and c-Raf-1 have been implicated in VSM growth. MAPK is a Ser/Thr kinase that is activated by its dual phosphorylation at Thr and Tyr residues. In quiescent undifferentiated cultured VSMCs, MAPK is mainly cytosolic, but translocates to the nucleus during activation by mitogens (Mii et al., 1996). Tyrosine kinase and MAPK activities have also been identified in differentiated contractile VSM. MAPK transiently translocates to the surface membrane during early activation of VSM, but undergoes redistribution to the cytoskeleton during maintained VSM activation (Khalil et al., 1995). It has been suggested that during VSM activation, DAG promotes translocation of cytosolic PKCε to the surface membrane, where it is fully activated. Activated PKCε stimulates the translocation of cytosolic MAPK kinase (MEK) and MAPK to the plasmalemma, where they form a surface membrane kinase complex. PKC causes phosphorylation and activation of MEK, which in turn phosphorylates MAPK at both Thr and Tyr residues. Tyrosine phosphorylation targets MAPK to the cytoskeleton, where it phosphorylates the actin-binding protein caldesmon (CaD) and reverses its inhibition of MgATPase activity and thus increases actin-myosin interaction and VSM contraction (Khalil et al., 1995; Kim et al., 2008). This is supported by the observations that in aortic VSM, phenylephrine activates a pathway involving CaP-dependent PKC autophosphorylation and activation followed by a much delayed ERK activation, CaD phosphorylation and VSM contraction (Kim et al., 2013). These PKC-dependent pathways occurs in parallel with the previously described transient spike in [Ca2+]i and MLC phosphorylation in VSM (Kim et al., 2013). Interestingly, biochemical studies have shown that in either the presence or absence of extracellular Ca2+, PKC activation by PDBu does not directly change the phospho-content of the thin filament proteins CaP or CaD (El-Yazbi et al., 2015), supporting that other kinases downstream of PKC may be needed to cause phosphorylation of CaP or CaD in vivo, and further demonstrating the complexity of signaling at the whole cell level (Kim et al., 2013).
PKC and Vasodilation
PKC may directly affect the permeability of VSM Ca2+ channels. In VSMC, agonists of G-protein-coupled receptors could activate receptor-operated Ca2+ channels (ROCs) including store-operated Ca2+ channels (SOCs) and transient receptor potential channels (TRPCs), and PKC may modulate these channels. Studies have suggested that low levels of DAG could activate TRPC6 via a PKC-independent mechanism, while high levels of DAG inhibit TRPC6 SOCs activity. In mesenteric and ear artery VSMC, DAG exerts an inhibitory action on TRPCs through a PKC-dependent pathway, and such mechanism may limit ROC activity at high agonist concentrations (Large et al., 2009). PKC inhibits the TRPC6 SOCs activity in a Ca2+-dependent manner (Shi et al., 2004; Albert and Large, 2006).
The 20-kDa MLC and MLCK also serve as substrates for PKC, and their phosphorylation could counteract the Ca2+-induced actin-myosin interaction and force development (Inagaki et al., 1987). In human VSMCs, PKC activation stimulates secretion of C-type natriuretic peptide (CNP) (Mendonca et al., 2006), which could function as an endogenous vasodilator (Ahluwalia et al., 2004). PKCα and δ mediate most of the increase in CNP mRNA induced by the PKC activator PMA, and PDGF increases CNP in SMCs via a PKCδ-dependent pathway (Mendonca et al., 2012).
9. PHYSIOLOGICAL CHANGES IN PKC
PKC levels may vary with certain physiological changes such as age, exercise, gender, sex hormone status and pregnancy.
Age-related Changes in PKC
Studies have shown age-dependent decrease in PKC activity and its translocation has in postmortem human brains (Wang et al., 1994). Also, in platelets, PKC activity in both the cytosolic and membrane fractions and its redistribution in response to stimulation of cell surface receptors are reduced in elderly men. Interestingly, age-related decrease in PKC activity is mitigated in older men who maintain moderately high levels of aerobic fitness as they age (Wang et al., 1995). Also, in rats, PKCɛ expression decreases gradually with age particularly among male rats (Li et al., 2014). Thus, PKC activity and its translocation may serve as biological markers of aging, and physical exercise may slow the changes in PKC during the aging process (Wang et al., 2000).
Sex Differences in PKC
Sex hormone status has emerged as an important modulator of vascular physiology and cardiovascular risk, and PKC expression/activity may be different in males versus females. Low testosterone levels in men may be associated with a higher risk of cardiovascular disease (Weidemann and Hanke, 2002), and testosterone reduces neointimal plaque development in male rabbit aortas (Hanke et al., 2001). PKCδ plays a role in mediating testosterone-induced apoptosis and inhibition of VSMC proliferation (Bowles et al., 2007). Overexpression of PKCδ in rat aortic VSMCs inhibits growth and proliferation, decreases thymidine incorporation, induces G0/G1 arrest, reduces cyclin D1 and E, and increases p27kip1, and PKCδ knockdown with siRNA diminishes the downregulation of cyclin D1 and E, and the upregulation of p21cip1 (Fukumoto et al., 1997; Bowles et al., 2007). Cleavage of PKCδ by caspase 3 and nuclear accumulation of catalytic PKCδ could be an important component of the apoptotic response induced by testosterone. It is believed that testosterone-induced increase in full-length PKCδ could cause an increase in caspase 3-mediated production of the 40-kDa catalytic fragment of PKCδ and lead to VSMC apoptosis (Bowles et al., 2007). PKCδ null mice exhibit decreased VSMC apoptosis and exacerbated vein graft arteriosclerosis (Leitges et al., 2001). The testosterone-induced PKCδ-dependent G1/S cell cycle arrest and stimulation of apoptosis may explain some of its beneficial effects on coronary vasculoproliferative disease, restenosis and atherosclerosis (Bowles et al., 2007). While local conversion of testosterone to estrogen via aromatase could mediate some of the beneficial effects of testosterone (Yamada et al., 1990), it may not be involved in testosterone-induced PKCδ-mediated inhibition of coronary VSMC proliferation (Bowles et al., 2007).
PKC may also mediate some of the vascular effects of female sex hormones. For instance, females tolerate shock and sepsis better than males likely through a protective GPR30-estrogen receptor mediated vascular response involving PKC (Angele et al., 2006; Li et al., 2014). Also, in mesenteric arteries from normal and shocked rats, estrogen increases the expression/activity of PKCɛ, and PKCɛ psuedosubstrate inhibitory peptide antagonizes the effect of estrogen on vascular reactivity in shocked rats (Li et al., 2014).
Sex differences in the expression/activity of PKC isoforms have also been observed in aortic VSM of male and female Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHR). VSM contraction and the expression/activity of PKCα, δ and ζ are less in intact female compared with male WKY, and the sex-related differences are greater in VSM from SHR compared with WKY rats (Kanashiro and Khalil, 2001). PDBu-induced contraction and PKC activity are greater in ovariectomized (OVX) females than in intact female rats, and treatment of OVX females with 17β-estradiol subcutaneous implants reduces PDBu contraction and PKC activity to a greater extent in SHR than WKY rats. These observations have suggested sex-related reduction in VSM contraction and the expression/activity of PKCα, δ and ζ in females compared with males, and that these differences are possibly mediated by estrogen and are enhanced in hypertension (Kanashiro and Khalil, 2001).
Pregnancy-related Changes in PKC
Normal pregnancy is associated with physiological changes in uterine blood flow caused by changes in uterine arterial Ca2+-dependent phasic contraction and maintained DAG/PKC -mediated tonic contraction (Ford, 1995). PKC inhibitors decrease thromboxane A2-induced contraction in uterine and mesenteric arteries of non-pregnant rats and in mesenteric of pregnant rats, supporting a role of PKC in mediating VSM contraction during pregnancy (Goulopoulou et al., 2012). PKC activity changes during the course of pregnancy, and PKC activity and vascular contraction are reduced in uterine artery of late pregnant ewes and gilts and aorta of late pregnant rats (Magness et al., 1991; Farley and Ford, 1992; Kanashiro et al., 2000b). Also, the expression and subcellular redistribution of PKCα, δ and ζ are reduced in aortic VSM of late pregnant rats (Kanashiro et al., 1999; Kanashiro et al., 2000b). A decrease in PKC signaling is chiefly responsible for the decreased contractions in pregnant uterine arteries in order to maintain low basal uterine vascular tone and to accommodate the increased uterine blood flow during pregnancy (Xiao et al., 2006). The pregnancy-associated decrease in uterine vascular tone and increase in uterine blood flow may be caused by increased steroid hormones and their receptors. The sex steroids estrogen and progesterone have been shown to attenuate PKC-mediated signaling in uterine arterial VSMCs and uterine artery contraction and myogenic tone, partly through upregulation of K+ channel expression/activity (Xiao et al., 2006; Zhu et al., 2013).
10. PKC IN VASCULAR INJURY AND DISEASE
In addition to its effects on vascular contraction/relaxation mechanisms, PKC has been implicated in multiple pathological processes involving VSM growth/proliferation, angiogenesis/vasculogenesis, apoptosis, vascular inflammation, restenosis, oxidative stress and ischemia-reperfusion injury. Pathological changes in PKC expression/activity could cause vascular hyper-reactivity and vascular remodeling leading to vascular disorders such as systemic and pulmonary HTN, preeclampsia, diabetic vasculopathy, atherosclerosis, and coronary artery disease (Fig. 5).
Fig. 5.
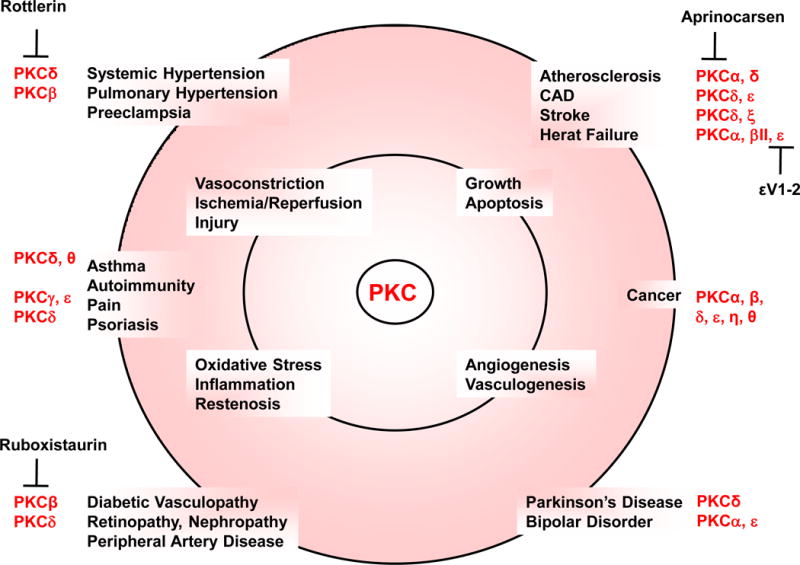
Implications for PKC in pathological processes and diseases. PKC mediates many pathological processes in the vascular system and other tissue and organs, which could contribute to vascular diseases such as hypertension and atherosclerosis, and other diseases such as cancer, asthma and autoimmune disease. The pathological changes in vascular PKC could also participate in diseases of other tissues and organs. For example, PKC through promoting angiogenesis could play a role in tumor growth. Also, PKC through promoting inflammation could play a role in asthma. PKC isoforms show varying contributions to different diseases, and isoform-specific PKC inhibitors such as the PKCα inhibitor aprinocarsen may be the key to combating their pathological effects while minimizing side effects. CAD, coronary artery disease.
PKC, VSM Growth, and Angiogenesis/Vasculogenesis
Studies have shown PKC translocation and localization to the nucleus in different cell types including VSM, suggesting interaction with nuclear factors and genes and a role in the regulation of VSM growth and proliferation (Salamanca and Khalil, 2005). PKC isoforms exert different effects leading to either stimulation or suppression of cell growth (Clarke and Dodson, 2007). PKC regulates vascular endothelial growth factor (VEGF) at the gene transcription level (Monti et al., 2013; Carracedo et al., 2014). Also, PKCɛ is a powerful oncogene promoting cell growth and proliferation (Nishizuka, 1995) and has been used as a tumor biomarker (Gorin and Pan, 2009; Duquesnes et al., 2011). PKCβII is also an upstream regulator of Early growth response-1 (Egr-1), a master switch that orchestrates the expression of diverse gene families that elicit a pathological response to hypoxia, ischemia/reperfusion, and vascular stress (Yan et al., 2006). In contrast, PKCδ is pro-apoptotic, anti-oncogene and tumor suppressor (Reddig et al., 1999; Duquesnes et al., 2011), that suppresses the expression of positive regulatory factors required for cell cycle progression (Fukumoto et al., 1997; Bowles et al., 2007).
PKC may be involved in the angiogenesis and vasculogenesis associated with cancer and metastasis (Kim et al., 2011a; Mochly-Rosen et al., 2012). VEGF activates PKCɛ in endothelial cells, and the selective PKCɛ agonist ψɛRACK promotes fibroblast growth factor-2 (FGF-2) release and export to cell membrane, and induces pro-angiogenic responses in endothelial cells and the formation of capillary-like structures and endothelial cell growth, proliferation and sprouting (Monti et al., 2013), PKCɛ-dependent formation of blood vessels may involve downstream signaling cascades including Akt and eNOS (Rask-Madsen and King, 2008). Double null mutation of PKCδ and ɛ causes embryonic lethality with defective blood vessel formation, impaired endothelial cell organization, dilated vessels, reduced endothelial-specific adherent junctions, decreased contact of endothelial cells with mural cells, deficient angiogenesis related transcripts, and almost undetectable α-smooth muscle actin, a classical marker for VSMC (Carracedo et al., 2014). On the other hand, PKCδ-deficient mice show increased number of SMCs and macrophages, accelerated neointimal lesions and intimal hyperplasia and delayed reendothelialization in mouse wire-injured femoral artery. PKCδ knockdown using small hairpin RNA (shRNA) in cultured endothelial cells is also associated with reduced cell migration and accumulation of the antiangiogenesis protein vasohibin-1, and downregulation of vasohibin-1 restores the migration rate in PKCδ-deficient cells (Bai et al., 2010).
PKC and VSM Apoptosis
Apoptosis has been observed in cardiovascular diseases such as myocardial infarction, aneurysm and ischemia/reperfusion injury. Whether apoptosis is beneficial or detrimental in vascular disease has been debated, but the finding of marked endothelial cell apoptosis in patients with peripheral vascular disease suggest that it may induce cell and tissue damage in certain conditions (Gardner et al., 2014).
PKCδ plays a role in apoptosis, and overexpression of the catalytic fragment of PKCδ alone is sufficient to induce apoptosis (Zhao et al., 2012). PKCδ is activated by a variety of pro-apoptotic stimuli including DNA damaging agents, ultraviolet (UV) radiation, the phorbol ester PMA and reactive oxygen species (ROS). VSMCs from PKCδ null mice are resistant to apoptosis induced by UV, TNFα, or H2O2, and show defective caspase-3 activation in response to oxidative stress (Zhao et al., 2012). PKCδ is an early regulator of apoptosis, and may function upstream of the mitochondria as an integrator for various death signals in multiple cell types and under various stimuli. Cytosolic PKCδ may function at the initial stages of apoptosis. Tyrosine phosphorylation of PKCδ also occurs at the beginning of apoptosis and may be responsible for its translocation to the cell membrane, mitochondria, ER, and lysosomes (Zhao et al., 2012). A positive loop between mitochondrial-mediated caspase activation and PKCδ cleavage and activation supports the pro-apoptotic role of PKCδ in the cytoplasm. The nucleus may also act as a major target for PKCδ to amplify the apoptosis signal. Translocation of PKCδ to the nucleus may be essential for inducing apoptosis, and the proteolytically cleaved constitutively active catalytic fragment of PKCδ accumulates in the nucleus (DeVries et al., 2002; Zhao et al., 2012). PKCδ knockout mice are resistant to apoptosis in models of abdominal aortic aneurysm, and adenovirus-mediated delivery of PKCδ locally to the arterial wall is sufficient to restore aneurysm development in PKCδ knockout mice (Morgan et al., 2012).
In contrast with the pro-apoptotic properties of PKCδ, PKCδ may have anti-apoptotic effects, as demonstrated in the response to the cytokine TNFα (Lu et al., 2009; Ren et al., 2014). Silencing PKCδ expression by siRNA inhibits TNFα-mediated ERK1/2 activation (Kilpatrick et al., 2006; Ren et al., 2014). PKCδ also interacts with the mitochondrial protein Smac, and exposure to apoptotic stimuli such as paclitaxel, disrupts the PKCδ-Smac interaction resulting in the release of Smac into the cytosol, activation of caspases in the cytochrome c/Apaf-1/caspase-9 pathway and promotion of apoptosis. On the other hand, activation of PKCδ rescues the PKCδ-Smac interaction and suppresses paclitaxel-induced cell death (Masoumi et al., 2012; Ren et al., 2014). The factors that determine whether PKCδ exerts a pro- or anti-apoptotic role in a given cell remain to be examined.
PKC and Vascular Inflammation
Vascular inflammation is observed in cardiovascular diseases such as atherosclerosis and myocardial infarction (Ross, 1999). Pro-inflammatory chemokines released by VSMCs play a role in vascular inflammation and recruit inflammatory cells to the vascular wall (Brasier, 2010; Ren et al., 2014). PKCδ is upregulated in VSMCs of injured arteries such as in aneurysmal aortic tissues and in restenotic lesions, and could be involved in vascular inflammation (Morgan et al., 2012; Si et al., 2012; Ren et al., 2014). PKCδ knockout mice show diminished expression of VSM pro-inflammatory factors and inflammatory cell infiltration (Morgan et al., 2012). PKCδ may promote chemokine expression at the transcription level by activating NF-κB through an IκB-independent cytosolic interaction, which subsequently leads to enhanced p65 phosphorylation and DNA binding affinity (Ren J, 2014). Delivery of PKCδ to the aortic wall of PKCδ−/− mice restores aneurysm, whereas overexpression of a dominant negative PKCδ mutant in the aorta of wild-type mice attenuates aneurysm. Monocyte chemoattractant protein-1 (MCP-1) is one of several inflammatory chemokines in VSMCs induced by PKCδ-regulated genes, and could be involved in the PKCδ role in aneurysm formation (Ren et al., 2014). This is supported by reports that PKCδ gene deficiency reduces the production of MCP-1 and other cytokines by aortic VSMCs, and the ectopic administration of MCP-1 to the aortic wall of PKCδ knockout mice restores aneurysm development (Morgan et al., 2012).
PKCɛ is also likely involved in inflammation, as PKCɛ inhibition both by knockout in mice and peptide modulators suppress the acute and chronic inflammatory pain response (Hucho et al., 2005; Koyanagi et al., 2007). Also, selective inhibition of PKCɛ with ɛV1-2 prolongs graft survival and improves functional recovery of the heart in cardiac transplantation models. PKCɛ inhibition attenuates the inflammatory response, decreases infiltration of macrophages and T cells and the attachment of mononuclear inflammatory cells to the arterial wall, and reduces luminal narrowing and parenchymal fibrosis, thereby preserving cardiac tissue architecture after transplantation (Koyanagi et al., 2007).
PKC may play a role in the inflammation caused by prolonged Mg2+ deficiency (Altura et al., 2012). PKC is activated in rat VSMC exposed to short-term Mg2+ deficiency. In Mg2+ deficient animals there may be cross-talk between PKC and the ceramide, sphingosine, NF-κB and cytokine pathways in vascular cells. PKCζ, in particular, plays a role in de novo formation of ceramide, through a sphingolipid salvage pathway. Mg2+ supplements in drinking water prevents the upregulation of PKC isoforms in VSMCs when exposed to low [Mg2+]o providing an effective solution to prevent inflammation induced by Mg2+ deficiency (Altura et al., 2014).
PKC and Vascular Restenosis
Long-term success of vascular bypass and angioplasty procedures is limited by restenosis particularly in obese and diabetic patients and PKC may contribute to vascular restenosis through the initial thrombosis and inflammation and the subsequent VSMC migration and proliferation (Ding et al., 2011a).
Thrombosis is involved in the early stages of vascular restenosis. PKCα, β, δ and θ are expressed in platelets, and cPKCs may promote while nPKCs inhibit platelet aggregation and thrombus formation (Gilio et al., 2010). This is illustrated by reports that knocking out PKCδ or PKCθ potentiates murine platelet aggregation, and that the PKCδ inhibitor rottlerin potentiates human platelet aggregation (Pula et al., 2006; Ding et al., 2011a).
PKCα, β and ζ also potentiate the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), a key step in leucocyte recruitment, that leads to VSMC migration and proliferation and vascular stenosis (Javaid et al., 2003; Kouroedov et al., 2004; Abdala-Valencia and Cook-Mills, 2006). PKCα, β and δ further affect VSMC migration by promoting actin polymerization and enhancing cell adhesion (Okazaki et al., 2000; Campbell and Trimble, 2005; Liu et al., 2007). PKCɛ promotes VSMC migration by upregulating matrix metalloproteinases (MMPs), particularly MMP-2 and -9 (Rodriguez-Pla et al., 2005; Thomas and Newby, 2010; Ding et al., 2011a).
PKC also contributes to VSMC proliferation, the final step of vascular restenosis. PKCβ mediate synergistic proliferative effect of PDGF and high glucose on human coronary VSMCs (Ling et al., 2002), and the selective PKCβ inhibitor LY-379196 attenuates DNA synthesis and cell growth (Ding et al., 2011b). PKCɛ seems to be involved in the development of neointimal hyperplasia. In rat models of aortic balloon injury the PKCɛ activator ψɛRACK promotes neointimal development, while the PKCɛ inhibitor ɛV1-2 reduces luminal narrowing, neointimal proliferation and VSMC ERK phosphorylation in vivo, and PDGF-induced VSMC proliferation/migration in vitro (Deuse et al., 2010).
PKC and Oxidative Stress
Oxidative agents such as H2O2 and superoxide activate PKC independent of classical PKC cofactors such as DAG. H2O2-induced activation of PKC may cause stimulation of arterial VSM L-type Ca2+ channels, and these effects are abolished by PKC inhibition. Also, hypoxia and vasoconstrictors such as AngII increase mitochondrial ROS production via PKC-mediated activation of NADPH oxidase (Nox) in pulmonary artery VSMCs (Doughan et al., 2008; Rathore et al., 2008; Perez-Vizcaino et al., 2010).
PKC and ROS appear to be tightly coupled in causing vascular dysfunction, as ROS can activate PKC and vice versa (Novokhatska et al., 2013). Also, a positive feedback mechanism may amplify the production of ROS and PKC. For instance, excess ROS production may occur through PKC activation, and subsequent phosphorylation of p47 phox subunit and activation of NADPH oxidase. ROS then creates a positive feedback loop through activation of c-Src, which then amplifies NADPH oxidase activity to produce more ROS (Lyle and Griendling, 2006; Novokhatska et al., 2013).
ROS activates different PKC isoforms. In isolated pulmonary artery, H2O2-induced Ca2+ sensitization and constriction is associated with PKCα activation and abolished by PKC inhibitors (Pourmahram et al., 2008; Perez-Vizcaino et al., 2010). Also, exogenous H2O2, mimics hypoxia and increases PKCɛ activity (Rathore et al., 2006; Perez-Vizcaino et al., 2010). In pulmonary artery VSMCs, mitochondrial-derived ROS may activate PKCɛ, which subsequently activates Nox-dependent ROS generation, further illustrating the positive feedback mechanism involved in hypoxia-induced increase in ROS (Rathore et al., 2008; Perez-Vizcaino et al., 2010).
PKC isoforms affect ROS production via different pathways. For instance, PKCɛ siRNA knockdown blocks ROS production by sphingosylphosphorylcholine (Shaifta et al., 2015). On the other hand, PKCζ appears to increase ROS through an Insulin Growth factor IGF-I–stimulated pathway, as high glucose induces NADPH oxidase 4 (Nox4) upregulation in a PKCζ/NF-κB–dependent manner in VSMCs and diabetic mice (Xi et al., 2012).
Oxidative stress may have other PKC-mediated vascular effects. In pulmonary artery, H2O2 may inhibit Kv channel by activating PKCα and ɛ. Also, in mesenteric artery, PKCα and ɛ may mediate the Kv channel inhibitory effect of ET-1 and AngII, respectively (Rainbow et al., 2009; Perez-Vizcaino et al., 2010). PKCζ also plays a role in the inhibition of Kv channels by U46619 and hypoxia in rat pulmonary artery VSMCs (Cogolludo et al., 2003; Cogolludo et al., 2009). Although PKC is involved in hypoxia-induced ROS generation, PKCζ does not mediate Kv channel inhibition through ROS (Perez-Vizcaino et al., 2010).
PKC and Ischemia/Reperfusion Injury
PKC activity has been observed in ischemic injury in multiple tissues including the heart (Speechly-Dick et al., 1994), liver (Piccoletti et al., 1992) and kidney (Padanilam, 2001). The regulation of cellular viability during an ischemic event may be influenced by the ratio of PKCδ and ɛ, as they display detrimental and protective effects, respectively (Churchill and Mochly-Rosen, 2007; Duquesnes et al., 2011).
Prolonged ischemia and reperfusion activates PKCδ more than PKCɛ, leading to translocation of PKCδ into the mitochondria and phosphorylation of pyruvate dehydrogenase kinase, which in turn phosphorylates pyruvate dehydrogenase, leading to a reduction in the tricarboxylic acid (TCA) cycle and ATP regeneration (Inagaki et al., 2003; Churchill et al., 2005; Mochly-Rosen et al., 2012). Mitochondrial dysfunction causes increases in ROS production and lipid peroxidation leading to accumulation of ROS and toxic aldehydes, such as 4-hydroxynonenal (4HNE), that interact and inactivate macromolecules including proteins, DNA and lipids. Mitochondrial dysfunction and increase in ROS leads to apoptosis, necrosis and severe cardiac dysfunction (Armstrong and Whiteman, 2007; Mochly-Rosen et al., 2012).
Short bouts of ischemia and reperfusion prior to the prolonged ischemic event (ischemic preconditioning) provides cardioprotection by preferentially activating PKCɛ (Inagaki et al., 2006), which translocates into the mitochondria and prevents mitochondrial dysfunction induced by prolonged ischemia and reperfusion (Budas et al., 2010; Mochly-Rosen et al., 2012). PKCɛ-mediated protection occurs, in part, by PKCɛ-induced phosphorylation and activation of aldehyde dehydrogenase 2 (ALDH2) (Chen et al., 2008), which metabolizes aldehydes such as 4HNE, thus reducing the aldehyde load and the mitochondrial and cellular damage (Mochly-Rosen et al., 2012). In addition, mitochondrial function is preserved in preconditioned hearts through the inhibition of the mitochondrial permeation pore and KATP channel opening (Costa et al., 2006; Duquesnes et al., 2011). The reduced 4HNE levels also prevent direct inactivation of peroxisome and thus enable fast removal of aggregated proteins. Furthermore, ischemic preconditioning prevents I/R injury at reperfusion by protecting ATP-dependent 26S proteasomal function. The active proteasome also selectively degrades activated PKCδ, thus decreasing the accumulation of the pro-apoptotic PKCδ at cardiac mitochondria and increasing the balance in favor of the cardiac protective and pro-survival PKCɛ. (Budas et al., 2007; Churchill et al., 2010; Mochly-Rosen et al., 2012). PKC-induced closure of connexons may also participate in ischemic preconditioning by an unclear mechanism (Naitoh et al., 2009; Duquesnes et al., 2011).
Ischemic stroke represents a major cause of death and disability among elderly, and the presence or absence of reperfusion is an important variable affecting outcome (Aronowski and Labiche, 2003). PKCβI and βII are increased in infarcted tissue of an ischemic stroke, whereas PKCγ increases 2 to 24 fold in the ischemic penumbra, but not in the infarcted tissues (Krupinski et al., 1998; Young et al., 2005). PKCγ may play a contrasting role in regulating the vulnerability of tissue to I/R-induced damage, as it functions first as a deleterious factor during evolution of intra-ischemic neuronal damage, then as a neuroprotective factor during post-ischemic reperfusion (Aronowski and Labiche, 2003). PKCγ may carry out its neuroprotective role in reversible focal ischemia by protein phosphorylation, as impaired protein phosphorylation in PKCγ knockout mice influences the overall infarct volume. Studies have shown larger infarct volumes in PKCγ knockout compared with wild-type, and inhibitors of the protein phosphatase calcineurin reduced infarct volume in the PKCγ knockout mice (Aronowski et al., 2000; Young et al., 2005).
In the cerebral circulation, PKCδ is believed to have a deleterious role in cerebral reperfusion. A model of transient middle cerebral artery occlusion demonstrated that PKCδ-null mice showed a 70% reduction in stroke size compared with wild-type mice (Chou et al., 2004; Young et al., 2005). PKCδ may mediate its detrimental effects in cerebral reperfusion by affecting neutrophil migration into ischemic tissue. PKCδ null mice show impaired neutrophil function and decreased neutrophil migration into ischemic tissues, and transplantation of bone marrow from the PKCδ-null mice into the wildtype mice reduces infarct size while bone marrow transplantation from wildtype donors increased infarction size and worsened neurological scores in PKCδ-null mice (Chou et al., 2004; Young et al., 2005). Inhibition of PKCδ improves microvascular pathology and function in transient focal ischemia in normotensive animals and chronic hypertension, and reduces ischemic damage following an ischemic event. Thus PKCδ could be an important therapeutic target for the preservation of microcerebrovascular function following stroke, and its inhibition may reduce stroke risk and damage in hypertensive patients (Bright et al., 2007). PKCζ also appears to be a downstream component of NMDA-induced excito-toxic neuronal cell death, as inhibiting PKCζ and its translocation, prevents NMDA-induced cell death. PKCζ mRNA is also induced in the cerebral cortex after focal brain ischemia (Koponen et al., 2003; Young et al., 2005). In contrast, PKCɛ is activated during cerebral ischemia in vivo and may play a role in mediating the early cellular response to ischemic stress, possibly mediating ischemic tolerance. Systemic delivery of PKCɛ-selective peptide activator ψɛRACK confers neuroprotection against a subsequent cerebral ischemic event when delivered immediately prior to stroke. In addition, activation of PKCɛ by ψɛRACK decreases vascular tone and microvascular cerebral blood flow, which may contribute to the conferred protection (Bright et al., 2008).
PKC and Coronary Artery Disease
PKCδ may contribute to coronary artery disease, through increased ROS formation, decreased ATP generation and increased apoptosis and necrosis (Inagaki et al., 2003; Churchill and Mochly-Rosen, 2007; Mochly-Rosen et al., 2012). PKCɛ, on the other hand, is protective, as it protects mitochondrial functions and proteasomal activity, activates ALDH2 and reduces aldehyde load (Mochly-Rosen and Kauvar, 2000; Budas et al., 2007; Chen et al., 2008; Mochly-Rosen et al., 2012). A combination of a PKCδ inhibitor and a PKCɛ activator could be useful for organ preservation and in prevention of ischemia-reperfusion injury and graft coronary artery disease in cardiac transplantation (Tanaka et al., 2004). In a case-control study, ischemia-reperfusion injury was the strongest alloantigen-independent factor for the subsequent development of graft coronary artery disease (Gaudin et al., 1994), and PKC modulators could modulate this pathological process.
PKC and Hypertension
Increased PKC activity could play a role in HTN, and mutations of PKC may influence the individual susceptibility to vascular hyper-reactivity. For instance, a consistent association is found between the single nuclear polymorphism (SNP) rs9922316 in PKCβ gene (PRKCB) and inter-individual variation in the constriction responses of dorsal hand vein to the selective α2 adrenergic receptor agonist dexmedetomidine (Posti et al., 2013). Also, PKCδ mRNA expression and protein levels are increased in VSM from SHR rats. PKC could increase VSM contraction in HTN by altering BKCa channel conductance, as the PKC inhibitor chelerythrine restores K+ channel activity in SHR (Novokhatska et al., 2013).
Sleep apnea could cause systemic and pulmonary HTN (Campen et al., 2005; Snow et al., 2011). Rat models of sleep apnea produced by exposure to eucapnic intermittent hypoxia display increased circulating ET-1 levels and ET-1-dependent systemic HTN, that is likely mediated by PKCδ-dependent VSM Ca2+ sensitization in systemic arteries (Allahdadi et al., 2008; Snow et al., 2011). On the other hand, in the pulmonary circulation, intermittent hypoxia appears to mediate a PKCβ-dependent increase in reactivity to different receptor-mediated vasoconstrictor agonists including ET-1 (Snow et al., 2008).
PKC isoforms have been implicated in hypoxia-associated pulmonary HTN by affecting both Ca2+ influx and Ca2+ sensitization in pulmonary artery VSM. In normal small pulmonary arteries, PKC inhibitors attenuate ET-1 induced constriction and [Ca2+]i as well as vasoconstrictor responses associated with store-operated Ca2+ entry, suggesting that PKC contributes to both Ca2+ sensitization and Ca2+ influx (Jernigan and Resta, 2014). Also, in fawnhooded rat model of pulmonary HTN, PKC inhibits BKCa, resulting in indirect activation of VGCC and pulmonary vasoconstriction (Zhu et al., 2008). PKC-mediated Ca2+ sensitization is also demonstrated by an increase in total and phosphorylated active CPI-17 levels in pulmonary arteries from newborn swine exposed to hypoxia (Dakshinamurti et al., 2005).
PKCɛ may have divergent effects in pulmonary HTN. PKCɛ null mice show decreased acute hypoxic pulmonary vasoconstriction, increased Kv3.1b channel expression and membrane hyperpolarization (Littler et al., 2003). On the other hand, PKCɛ null mice show a greater increase in pulmonary arterial pressure compared to wild-type mice following chronic hypoxia exposure. The increase in pressure is reversed by inhaled NO suggesting that PKCɛ may be an important signaling intermediate in the hypoxic regulation of NO synthase (Littler et al., 2005).
Hypertension in pregnancy and preeclampsia are major complications of pregnancy and placental ischemia/hypoxia could be an initiating event. Chronic hypoxia enhances uterine vascular tone in pregnant sheep and is associated with an increase in PKC activity (Chang et al., 2009). Hypoxia during pregnancy may attenuate the effects of sex steroid hormones/receptors, leading to enhanced PKC activation in pregnant uterine arteries. Increased BKCa channel activity inhibits PKC-mediated contraction in ovine uterine arteries during pregnancy, and gestational hypoxia may upregulate PKC and inhibit BKCa (Xiao et al., 2014). Hypoxia may also inhibit KIR channels via PKC-dependent mechanism, and may contribute to the maladaptation of uterine vascular hemodynamics in preeclampsia and the fetal intrauterine growth restriction in response to hypoxia (Zhu et al., 2013). Also, in cultured rat cardiomyocytes, treatment with IgG obtained from preeclamptic women enhances AT1R-mediated response, which is ameliorated with the PKC inhibitor calphostin C, further supporting a role of PKC in preeclampsia (Wallukat et al., 1999).
PKC and Diabetic Vasculopathy
Diabetes mellitus is a complex syndrome of multiple disorders including vascular dysfunction. PKC could play a role in diabetes-related vascular pathology through multiple mechanisms including cell growth and proliferation, cell permeability, oxidative stress, increased vascular reactivity, inhibition of K+ channels and Na+-K+-ATPase, activation of cytosolic phospholipase A2, vascular remodeling and increased ECM, and vascular inflammation and increased pro-inflammatory cytokines (Nishizuka, 1992; Koya and King, 1998; Meier and King, 2000). In diabetes, PKC is activated by advanced glycation end (AGE) products and polyol pathway flux (Thallas-Bonke et al., 2008; Geraldes and King, 2010; Kizub et al., 2014). Also, chronic hyperglycemia stimulates synthesis of DAG and activates DAG-dependent cPKCs and nPKCs in cultured bovine aortic endothelial cells and VSM (Inoguchi et al., 1992). Fatty acids, especially the unesterified forms and their coenzyme A (CoA) esters, work synergistically with DAG to activate PKC (Clarke and Dodson, 2007). PKC is also activated by ROS generated by different oxidases and the mitochondrial electron transport chain, and following AGE:RAGE (AGE receptor) interactions (Liu and Heckman, 1998).
High glucose-induced activation of PKC could cause vascular dysfunction by altering the expressions of growth factors such as VEGF, PDGF, and transforming growth factor-β (Yokota et al., 2003; Lizotte et al., 2013), which in turn affect the expression of ECM proteins (Jakus and Rietbrock, 2004). Also, in diabetes, activated PKC increases endothelial cell permeability and decreases blood flow and the production of and responsiveness to angiogenic factors, and this may contribute to the loss of capillary pericytes, retinal permeability, ischemia, and neovascularization (Aiello et al., 1994; Huang and Yuan, 1997; Williams et al., 1997; Pomero et al., 2003; Lizotte et al., 2013). PKC also activates NADPH oxidases and increases ROS (Inoguchi et al., 2000; Gao and Mann, 2009; Kizub et al., 2014). In hyperglycemia, VSMCs show increased DNA synthesis and contraction to PMA and reduced apoptosis, and these effects are blocked by the PKC inhibitor calphostin C (Hall et al., 2000; Geraldes and King, 2010). In diabetes, PKC may enhance vascular reactivity by inhibition of K+ channels and promoting Ca2+ sensitization in VSM myofilaments (Nelson and Quayle, 1995; Kizub et al., 2014). High glucose via PKC activation and oxidative stress also reduces arterial SMC Kv current resulting in VSM depolarization and vasoconstriction (Liu et al., 2001; Rainbow et al., 2006; Straub et al., 2009). Diabetic patients also often have reduced nocturnal BP dip and increased vascular complications regardless of the average BP, partly due to lack of diurnal PKC inhibition (Nakano et al., 1991; Palmas et al., 2008).
PKC may contribute to diabetic nephropathy by increasing endothelial permeability to albumin and other macromolecules. PKC also induces ECM protein synthesis by mesangial cells and promotes sclerosis (Rovin et al., 1992; Henry et al., 1999; Heilig et al., 2013). In cultured mesangial cells, cGMP suppresses PKC-mediated actions including matrix protein production, and impaired NO-mediated cGMP generation in mesangial cells could amplify the PKC signal and increase matrix protein synthesis in diabetes (Williamson et al., 1993; Craven et al., 1994; Derubertis and Craven, 1994). Podocyte injury or loss is a hallmark of diabetic nephropathy and PKC contributes to the progression of glomerular injury (Teng et al., 2014). PKC also mediates diabetic glomerulosclerosis partially through its interaction with GLUT1, which facilitates the movement of glucose into the cell (Koya et al., 1997; Heilig et al., 2013). PKC stimulates TGF-β-mediated effects including activation of the highly pro-fibrotic cytokine CTGF, and engagement of the TGF-β receptor, triggering more GLUT1 synthesis via MAPK, and ECM protein synthesis via Smads (Twigg et al., 2001; Qi et al., 2005; Heilig et al., 2013). Also, AngII interaction with AT1 receptor stimulates DAG/PKC causing additional GLUT1 synthesis, and AngII and GLUT1 activation of PKC could promote glomerulosclerosis through both TGF-β-dependent and -independent pathways (Koya et al., 1997; Henry et al., 1999; Heilig et al., 2013).
PKC isoforms play different roles in diabetic vasculopathy. In VSMCs, high glucose activates PKCα, β, δ, and ɛ but not PKCζ (Haller et al., 1995; Igarashi et al., 1999; Lizotte et al., 2013). PKCβ and δ appear to be the dominant PKCs involved in diabetes. In rat VSMCs, high glucose increases the membrane fraction expression of PKCβ and PKCδ, p38 MAPK phosphorylation and arachidonic acid release (Igarashi et al., 1999; Geraldes and King, 2010). PKCβ is implicated in insulin resistance. Transgenic mice overexpressing PKCβII exhibit decreased Akt activation in vascular cells after insulin stimulation (Naruse et al., 2006; Geraldes and King, 2010). PKC could prevent insulin actions on the PI3K pathway at the insulin receptor substrate (IRS) level (Sampson and Cooper, 2006), but could accentuate insulin actions on the ERK1/2 pathway (Bakker et al., 2008). Thus, PKC could mediate selective insulin resistance by enhancing insulin’s pro-atherosclerotic mechanisms via ERK1/2 signaling or inhibiting its anti-atherosclerotic mechanisms by inhibiting the PI3K/Akt pathway (Geraldes and King, 2010). PKCβ activation by hyperglycemia may play a role in mediating the microvascular disease complications of retinopathy, nephropathy, and neuropathy. Hyperglycemia leads to chronic activation of PKCβ, causing aberrant signaling and other pathologies including cytokine activation and inhibition, vascular alterations, cell cycle and transcriptional factor dysregulation, and abnormal angiogenesis (Geraldes and King, 2010; Mochly-Rosen et al., 2012). PKCβ is chiefly responsible in causing diabetic retinopathy by affecting VEGF expression through the mRNA-stabilizing human embryonic lethal abnormal vision protein, HuR, in the retina (Amadio et al., 2010; Gogula et al., 2013). PKCβ may mediate diabetes-induced increase in vascular contraction by inhibiting BKCa channel, and PKCβ inhibition restores BKCa-mediated vasodilation in diabetic mice. Also, reduced expression of the BKβI channel subunit in arteries of STZ-induced diabetic mice and in human coronary artery VSMCs cultured with high glucose has been related to increased PKCβ expression (Lu et al., 2012; Kizub et al., 2014).
The nPKCs could also contribute to insulin resistance by serine phosphorylation and inhibition of IRS1 (Yu et al., 2002; Ritter et al., 2015). PKCδ plays a role in islet cell function and insulin response, and changes in PKCδ expression/activity among mice strains correlate with insulin resistance and glucose intolerance. Also, mice with global or liver-specific downregulation of the PKCδ gene (PRKCD) display increased hepatic insulin signaling and improved glucose tolerance with aging. Conversely, mice with liver-specific overexpression of PKCδ develop hepatic insulin resistance and decreased insulin signaling (Bezy et al., 2011). Diabetes-induced PKCδ activation also decreases responsiveness to PDGF leading to pericyte apoptosis, acellular capillaries, and diabetic retinopathy (Geraldes et al., 2009). PKCδ is also likely involved in poor collateral vessel formation in diabetes, as the ischemic adductor muscles of diabetic PRKCD knockout mice show increased blood flow and capillary density compared with diabetic PRKCD+/+ mice. The poor angiogenesis response in ischemic diabetic muscles could be caused by PKCδ-induced expression of Src homology-2 domain-containing phosphatase-1 (SHP-1), contributing to VEGF and PDGF unresponsiveness (Lizotte et al., 2013). PKCδ may also mediate inhibition of K+ current in aortic SMCs, and PKCδ gene silencing by siRNAs restores VSMCs K+ current and endothelium-dependent vasodilatation in aorta of streptozotocin-induced diabetic rats (Kizub et al., 2014; Klymenko et al., 2014). Endothelium-independent vasoconstriction mediated by EP1-/EP3-receptors activation is also enhanced in mesenteric arteries of diabetic rats and highly sensitive to PKCδ inhibition (Ishida et al., 2012; Kizub et al., 2014).
Ruboxistaurin (LY333531) is an oral PKCβII inhibitor commonly used in cellular, animal and human studies (Geraldes and King, 2010). Ruboxistaurin has been tested in diabetic retinopathy, nephropathy and neuropathy, and is well-tolerated (Mehta et al., 2009; Kizub et al., 2010; Aiello et al., 2011). Ruboxistaurin decreases vessel permeability and the onset of diabetic macular edema, improves retinal condition in diabetic patients, and prevents reduction of visual acuity (Geraldes and King, 2010; Gogula et al., 2013). While ruboxistaurin preserves visual acuity by decreasing capillary permeability or targeting the neural retina it may not delay the progression of diabetic retinopathy, and inhibiting PKCβ alone may not be sufficient to stop the early metabolic changes that drive the progression of pre-proliferative diabetic retinopathy (Geraldes and King, 2010). Indolylmaleimide and its derivatives are nonselective PKC inhibitors that reduced diabetic complications such as nephropathy, cardiomyopathy and neuropathy in clinical trials (Sobhia et al., 2013; Kizub et al., 2014), but the lack of selectivity on PKC isoforms raises concerns regarding safety. Interestingly, some of the drugs already in clinical use for vascular disease may mediate some of their effects through inhibition of PKC. For instance, metformin and liraglutide (a glucagon like peptide-1 (GLP-1)) could prevent diabetic cardiovascular complications and atherosclerosis (Batchuluun et al., 2013). In cultured human endothelial cells, both metformin and liraglutide prevent high glucose-induced oxidative stress through inhibition of PKC-NADPH oxidase pathway, and these effects are enhanced when the drugs are combined. In cells treated with metformin and liraglutide, hyperglycemia fails to induce PKCβII translocation and phosphorylation of endogenous PKC. Also, both drugs inhibit p47phox translocation and NADPH oxidase activation, and prevent high glucose-induced changes in intracellular DAG level and phosphorylation of AMP-activated protein kinase (AMPK) (Batchuluun et al., 2013).
PKC and Atherosclerosis
Atherosclerosis results from deposition of lipid and chronic inflammation in the arterial wall, and PKC has been linked to many of the pathways involved in atherosclerosis. PKC expression is higher in plaques from atherosclerotic patients compared with control subjects. Also, PKC expression is higher in atherosclerotic rabbit aorta VSMC than in the control group and in unstable versus stable plaques (Sirikci et al., 1996). PKC is also positively correlated with the increase in adipose differentiation-related protein, a protein present in higher amounts in unstable atherosclerotic versus stable plaques. PKC may further potentiate plaque-formation through endothelial dysfunction, foam cell formation, and VSMC proliferation. PKC may also contribute to the increased thickness of the intima and media of the vessel wall in atherosclerosis due to an imbalance between proliferation and apoptosis (Xu et al., 2015).
PKC isoforms play a varying role in the atherosclerotic process. Both PKCβ and δ are potential therapeutic targets as PKCβ potentiates atherosclerotic formation, and PKCδ appears to have an opposite effect (Fan et al., 2014). Depletion of PKCβ gene or treatment with LY333531 in apolipoprotein E-deficient mice decreases atherosclerosis by inhibiting the early growth response (Egr)-1 protein, which regulates VCAM expression and matrix metalloproteinase-2 activity (Harja et al., 2009; Geraldes and King, 2010). On the other hand, PKCδ deletion promotes arteriosclerosis, partly due to the lack of PKCδ-mediated VSMC apoptosis (Leitges et al., 2001; Geraldes and King, 2010). Also, PKCδ mediates collagen I secretion in VSMCs, and tight regulation of collagen is critical to the stability of atherosclerotic plaque. PKCδ knockout mice show marked reduction of collagen I in the arterial wall. PKCδ may also regulate the trafficking of collagen by controlling its exit from the trans-Golgi network through a mechanism involving cell division cycle 42 (Cdc42) protein (Lengfeld et al., 2012).
Vascular calcification contributes to atherosclerosis, as it reduces elasticity of blood vessels, and PKC may coordinate between cytoskeletal changes and hyperphosphatemia-induced vascular calcification. Expression and phosphorylation of both PKCα and δ decreases during inorganic phosphate (Pi)-induced VSMC calcification. Knockdown of PKCα and δ accelerates Pi-induced calcification in VSMCs and aorta in culture through upregulation of osteogenic signaling. Inhibition of PKCα and δ may also induce disassembly of microtubule and actin, respectively (Lee et al., 2014).
11. CONCLUSION AND PERSPECTIVE
PKC is a major regulator of vascular function and a potential target in several pathological processes. Although significant information is currently available on PKC, it is important to further our knowledge of the role of PKC in vascular disease and the mechanisms behind its contribution. Research efforts have been limited by the existence of several PKC isoforms, the non-uniform expression and distribution of PKC throughout the vascular tree, and the poor specificity of chemical inhibitors (Schubert et al., 2008). Continued research should further define the specific characteristics of the different PKC isoforms that determine their subcellular localization, phosphorylation pattern and potential substrates. The precise knowledge of the structural aspects of PKC isoforms should allow the development of new tools to evaluate PKC function and potential new therapies. The development of FRET-based reporters of PKC activity (Violin et al., 2003; Braun et al., 2005) and new peptides directed towards other domains than those presently utilized in the V1 region is a step in the right direction (Churchill et al., 2009; Duquesnes et al., 2011). Also, while PKC could play a role in vascular disease, this should not minimize its role in other pathological processes and diseases (Fig. 5). It is important to continue research of the role of PKC isoforms in various diseases, as there are still many uncertainties about their exact mechanism of action. For instance, while pro-apoptotic PKCδ likely acts as a tumor suppressor (Hampson et al., 2005; Zhao et al., 2012), in some cases it enhances tumorigenesis, and PKCδ-deficient mice are protected from urethane-induced lung tumor (Symonds et al., 2011; Zhao et al., 2012).
In order to enhance selectivity, it is important to determine the precise cellular location of various PKC isoforms, both in the resting and active state. The subcellular location of PKC may determine the state of VSM activity, and may be useful in the diagnosis/prognosis of HTN (Salamanca and Khalil, 2005). Since some PKC-mediated pathways involve PKC translocation to the nucleus, such as in apoptosis-induction by PKCδ, new nuclear targets of PKCδ such as the recently identified C/EBPa and hnRNPK could limit apoptosis (Gao et al., 2009; Zhao et al., 2012). Similarly, the translocation activator peptide ψδRACK attenuates Ccl2 production, providing a way to specifically block PKCδ-regulated proinflammatory chemokines (Ren et al., 2014). Genetic differences in PKC may also alter its effects, and studies have suggested a new role for PKC in inhibiting store-operated Ca2+ entry in the hypertensive pulmonary circulation of Sprague-Dawley, but not Wistar rats. The precise genetic differences responsible for this discrepancy in VSM Ca2+ regulation, as well as in other PKC-mediated effects, should be further explored (Snow et al., 2009).
Modulation of PKC activity presents an attractive target for drug development in vascular disease and other related conditions. Despite the promise of PKC modulators, results in clinical trials have been mixed and often negative (Mochly-Rosen et al., 2012). Isoform-specific PKC inhibitors have shown some promise in clinical trials (Table 5). Partial prevention of the progress of malignancies were found in early phases of clinical trials of the PKC inhibitors UCN-01 and CGP41251 (Shen, 2003). PKC inhibitors could also be useful in treatment of Ca2+ antagonist-resistant forms of HTN where the Ca2+ independent PKC isoforms could be targeted (Salamanca and Khalil, 2005). Inhibitors of PKCβ and δ may reduce fat accumulation, improve glucose tolerance, decrease hepatosteatosis, and suppress foam cell formation in obesity and hyperlipidemia-induced atherosclerosis (Bezy et al., 2011; Huang et al., 2012; Fan et al., 2014). Also, a PKCβ inhibitor or PKCɛ activator may reduce damage secondary to endothelial dysfunction and VSMC proliferation in patients with atherosclerosis caused by long-term smoking, HTN or diabetes (Harja et al., 2009; Huang et al., 2010; Monti et al., 2010; Fan et al., 2014). Activators of PKCɛ may also be useful in coronary artery disease, and the PKCɛ activator acadesine reduced the 2 year mortality in patients with postoperative acute myocardial infarction after coronary bypass grafting (Mochly-Rosen et al., 2012).
Table 5.
PKC Isoforms Involved in Specific Vascular Diseases and PKC Modulators Used as Potential Therapeutic Tools in Clinical Trials
| PKC | Vascular Disease | Role of PKC | Drug | Effect on PKC | Outcome | Comments | Reference |
|---|---|---|---|---|---|---|---|
| PKCβ | Diabetic Retinopathy | Detrimental. Cytokine activation and inhibition, vascular alterations, cell cycle and transcriptional factor dysregulation, abnormal angiogenesis, increased matrix protein synthesis | Ruboxistaurin | Inhibit | Reduced sustained moderate vision loss in large studies | Under review by FDA for diabetic retinopathy | (Tuttle et al., 2005; Vinik et al., 2005; Geraldes and King, 2010; Aiello et al., 2011; Mochly-Rosen et al., 2012) |
| Ruboxistaurin | Inhibit | Failed to improve kidney outcomes | Studied as secondary outcome in large retinopathy trials | ||||
| Ruboxistaurin | Inhibit | Mild decrease in symptoms | Requires validation in larger study | ||||
| PKCδ | Ischemic Heart Disease | Detrimental. Increases ROS production, decreases ATP generation, increases apoptosis and necrosis | Delcasertib for acute myocardial infarction (MI) | Inhibit | No benefit when given intravenously | Positive biomarker trend when given to patients with TIMI 0/1 flow | (Inagaki et al., 2003; Churchill and Mochly-Rosen, 2007; Bates et al., 2008; Mochly-Rosen et al., 2012) |
| KAI-9803: Phase I clinical trial, intracoronary injection during primary percutaneous coronary intervention | Selective PKCδ RACK antagonist | Signs of potential drug activity (not dose-dependent) | Acceptable safety and tolerability | ||||
| PKCɛ | Ischemic Heart Disease | Protective. Protection of mitochondrial functions & proteasomal activity, activation of ALDH2 and reduction of aldehydic load | Adenosine for acute MI | ↑PKCɛ | Reduced infarct size from 27% to 11% when given at 70 mcg/kg/min | No reduction in composite endpoint of death and CHF | (Mochly-Rosen and Kauvar, 2000; Ross et al., 2005; Budas et al., 2007; Chen et al., 2008; Mochly-Rosen et al., 2012) |
| Adenosine for coronary bypass grafting | ↑PKCɛ | Reduction in composite AMI, mortality, need for pressors postoperatively. | Requires validation in larger study | ||||
| Acadesine for coronary bypass grafting | ↑PKCɛ | Reduced two year mortality in the small group of patients who had a post-operative acute MI. | No reduction in death, acute MI, or stroke |
Since PKC modulates many physiological functions, unwanted effects may occur when non-selective PKC inhibitors are administered systemically. Sustained delivery of peptide inhibitors of PKC for two months is safe in animals (Tanaka et al., 2004; Inagaki et al., 2008; Ding et al., 2011b). Nevertheless, local delivery of PKC inhibitors may be a better approach. For example, to prevent restenosis, PKC inhibitors could be coated onto stents or balloons to be directly released into the injured area at effective concentrations. PKCβII and δ inhibitors coated stents or balloons showed efficacy and safety in animal trials (Ding et al., 2011b).
PKC siRNA may hold the promise to target specific PKC isoforms in vascular disease. PKCδ gene silencing with the short hairpin RNAs (shRNAs)-plasmid delivery system administered intravenously restores the vasodilator potential and normalize vascular function and high BP in SHR (Novokhatska et al., 2013). Also, PKCδ siRNA attenuates the proinflammatory effect of human CRP in diabetic rats (Jialal et al., 2013). Further research should help design more specific and effective remedies of PKC-mediated vascular disease and other PKC-related conditions.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-98724, HL-111775).
ABREVIATIONS
- AGE
Advanced Glycation End products
- ALDH2
aldehyde dehydrogenase 2
- AngII
angiotensin II
- BKCa
large conductance Ca2+-activated K+ channel
- BP
blood pressure
- Ca2+
calcium
- [Ca2+]i
intracellular free Ca2+ concentration
- CaD
caldesmon
- CAM
calmodulin
- CaP
calponin
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CNP
C-type natriuretic peptide
- CPI-17
PKC-potentiated phosphatase inhibitor protein-17
- DAG
diacyglycerol
- ECM
extracellular matrix
- ER
endoplasmique reticulum
- ERK
extracellular signal-regulated kinase
- ET-1
endothelin-1
- HSP
heat shock protein
- HR1
homology region 1
- ICAM-1
intercellular adhesion molecule-1
- IP3
inositol 1,4,5-trisphosphate
- IRS1
insulin receptor substrate 1
- Kv
voltage-gated K+ channel
- MARCKS
myristoylated alanine-rich C kinase substrate
- MCP-1
monocyte chemoattractant protein-1
- MLC
myosin light chain
- PDBu
phorbol 12,13-dibutyrate
- PDGF
platelet-derived growth factor
- PDK
phosphoinositide-dependent kinase
- PKC
protein kinase C
- PKG
cGMP-dependent protein kinase
- PMA
phorbol 12-myristate 13-acetate
- PLC
phospholipase C
- PS
phosphatidylserine
- RAGE
AGE receptor
- ROS
reactive oxygen species
- VCAM-1
vascular cell adhesion molecule-1
- VEGF
vascular endothelial growth factor
- VGCC
voltage-gated Ca2+ channel
- VSM
vascular smooth muscle
Footnotes
CONFLICT OF INTEREST
None
References
- Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation. J Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adwan TS, Ohm AM, Jones DN, Humphries MJ, Reyland ME. Regulated binding of importin-alpha to protein kinase Cdelta in response to apoptotic signals facilitates nuclear import. J Biol Chem. 2011;286:35716–35724. doi: 10.1074/jbc.M111.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004;99:83–89. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD, Wolka AM, Shahri N, Milton RC. Oral protein kinase c beta inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31:2084–2094. doi: 10.1097/IAE.0b013e3182111669. [DOI] [PubMed] [Google Scholar]
- Akimoto K, Mizuno K, Osada S, Hirai S, Tanuma S, Suzuki K, Ohno S. A new member of the third class in the protein kinase C family, PKC lambda, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem. 1994;269:12677–12683. [PubMed] [Google Scholar]
- Albert AP, Large WA. Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J Physiol. 2006;570:45–51. doi: 10.1113/jphysiol.2005.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCdelta. Am J Physiol Heart Circ Physiol. 2008;294:H920–927. doi: 10.1152/ajpheart.01264.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Shah NC, Shah G, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells: cross-talk among cytokines, Mg2+, NF-kappaB, and de novo ceramide. Am J Physiol Heart Circ Physiol. 2012;302:H319–332. doi: 10.1152/ajpheart.00453.2011. [DOI] [PubMed] [Google Scholar]
- Altura BM, Shah NC, Shah GJ, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term Mg deficiency upregulates protein kinase C isoforms in cardiovascular tissues and cells; relation to NF-kB, cytokines, ceramide salvage sphingolipid pathway and PKC-zeta: hypothesis and review. Int J Clin Exp Med. 2014;7:1–21. [PMC free article] [PubMed] [Google Scholar]
- Amadio M, Bucolo C, Leggio GM, Drago F, Govoni S, Pascale A. The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochem Pharmacol. 2010;80:1230–1237. doi: 10.1016/j.bcp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Angele MK, Frantz MC, Chaudry IH. Gender and sex hormones influence the response to trauma and sepsis: potential therapeutic approaches. Clinics (Sao Paulo) 2006;61:479–488. doi: 10.1590/s1807-59322006000500017. [DOI] [PubMed] [Google Scholar]
- Armstrong JS, Whiteman M. Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol. 2007;80:355–377. doi: 10.1016/S0091-679X(06)80018-X. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Grotta JC, Strong R, Waxham MN. Interplay between the gamma isoform of PKC and calcineurin in regulation of vulnerability to focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:343–349. doi: 10.1097/00004647-200002000-00016. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Labiche LA. Perspectives on reperfusion-induced damage in rodent models of experimental focal ischemia and role of gamma-protein kinase C. ILAR J. 2003;44:105–109. doi: 10.1093/ilar.44.2.105. [DOI] [PubMed] [Google Scholar]
- Austin C, Wray S. Interactions between Ca(2+) and H(+) and functional consequences in vascular smooth muscle. Circ Res. 2000;86:355–363. doi: 10.1161/01.res.86.3.355. [DOI] [PubMed] [Google Scholar]
- Aviv A. Cytosolic Ca2+, Na+/H+ antiport, protein kinase C trio in essential hypertension. Am J Hypertens. 1994;7:205–212. doi: 10.1093/ajh/7.2.205. [DOI] [PubMed] [Google Scholar]
- Bai X, Margariti A, Hu Y, Sato Y, Zeng L, Ivetic A, Habi O, Mason JC, Wang X, Xu Q. Protein kinase C{delta} deficiency accelerates neointimal lesions of mouse injured artery involving delayed reendothelialization and vasohibin-1 accumulation. Arterioscler Thromb Vasc Biol. 2010;30:2467–2474. doi: 10.1161/ATVBAHA.110.215723. [DOI] [PubMed] [Google Scholar]
- Bakker W, Sipkema P, Stehouwer CD, Serne EH, Smulders YM, van Hinsbergh VW, Eringa EC. Protein kinase C theta activation induces insulin-mediated constriction of muscle resistance arteries. Diabetes. 2008;57:706–713. doi: 10.2337/db07-0792. [DOI] [PubMed] [Google Scholar]
- Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- Bao HF, Thai TL, Yue Q, Ma HP, Eaton AF, Cai H, Klein JD, Sands JM, Eaton DC. ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Calpha knockout mice. Am J Physiol Renal Physiol. 2014;306:F309–320. doi: 10.1152/ajprenal.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L149–155. doi: 10.1152/ajplung.00207.2003. [DOI] [PubMed] [Google Scholar]
- Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, Miura D, Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2013;232:156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, Grimes K, Harrington R, Huber K, Kleiman N, Mochly-Rosen D, Roe M, Sadowski Z, Solomon S, Widimsky P. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation. 2008;117:886–896. doi: 10.1161/CIRCULATIONAHA.107.759167. [DOI] [PubMed] [Google Scholar]
- Bazzi MD, Nelsestuen GL. Protein kinase C interaction with calcium: a phospholipid-dependent process. Biochemistry. 1990;29:7624–7630. doi: 10.1021/bi00485a012. [DOI] [PubMed] [Google Scholar]
- Behn-Krappa A, Newton AC. The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Curr Biol. 1999;9:728–737. doi: 10.1016/s0960-9822(99)80332-7. [DOI] [PubMed] [Google Scholar]
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P. Phosphorylation of the catalytic subunit of Na+,K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991;88:11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, Goldfine AB, Patti ME, King GL, Kahn CR. PKCdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest. 2011;121:2504–2517. doi: 10.1172/JCI46045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard AS, Tavalin SJ. Protein Kinase C (PKC)zeta Pseudosubstrate Inhibitor Peptide Promiscuously Binds PKC Family Isoforms and Disrupts Conventional PKC Targeting and Translocation. Mol Pharmacol. 2015;88:728–735. doi: 10.1124/mol.115.099457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- Bowles DK, Maddali KK, Dhulipala VC, Korzick DH. PKCdelta mediates anti-proliferative, pro-apoptic effects of testosterone on coronary smooth muscle. Am J Physiol Cell Physiol. 2007;293:C805–813. doi: 10.1152/ajpcell.00127.2007. [DOI] [PubMed] [Google Scholar]
- Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DC, Garfield SH, Blumberg PM. Analysis by fluorescence resonance energy transfer of the interaction between ligands and protein kinase Cdelta in the intact cell. J Biol Chem. 2005;280:8164–8171. doi: 10.1074/jbc.M413896200. [DOI] [PubMed] [Google Scholar]
- Braun MU, Mochly-Rosen D. Opposing effects of delta- and zeta-protein kinase C isozymes on cardiac fibroblast proliferation: use of isozyme-selective inhibitors. J Mol Cell Cardiol. 2003;35:895–903. doi: 10.1016/s0022-2828(03)00142-1. [DOI] [PubMed] [Google Scholar]
- Bright R, Steinberg GK, Mochly-Rosen D. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 2007;1144:146–155. doi: 10.1016/j.brainres.2007.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Sun GH, Yenari MA, Steinberg GK, Mochly-Rosen D. epsilonPKC confers acute tolerance to cerebral ischemic reperfusion injury. Neurosci Lett. 2008;441:120–124. doi: 10.1016/j.neulet.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, Byron KL. Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem. 2014;289:2099–2111. doi: 10.1074/jbc.M113.527820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55:523–536. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bullard TA, Hastings JL, Davis JM, Borg TK, Price RL. Altered PKC expression and phosphorylation in response to the nature, direction, and magnitude of mechanical stretch. Can J Physiol Pharmacol. 2007;85:243–250. doi: 10.1139/y07-023. [DOI] [PubMed] [Google Scholar]
- Bursell SE, King GL. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res Clin Pract. 1999;45:169–182. doi: 10.1016/s0168-8227(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Cameron AJ, De Rycker M, Calleja V, Alcor D, Kjaer S, Kostelecky B, Saurin A, Faisal A, Laguerre M, Hemmings BA, McDonald N, Larijani B, Parker PJ. Protein kinases, from B to C. Biochem Soc Trans. 2007;35:1013–1017. doi: 10.1042/BST0351013. [DOI] [PubMed] [Google Scholar]
- Cameron AJ, Escribano C, Saurin AT, Kostelecky B, Parker PJ. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat Struct Mol Biol. 2009;16:624–630. doi: 10.1038/nsmb.1606. [DOI] [PubMed] [Google Scholar]
- Campbell M, Trimble ER. Modification of PI3K- and MAPK-dependent chemotaxis in aortic vascular smooth muscle cells by protein kinase CbetaII. Circ Res. 2005;96:197–206. doi: 10.1161/01.RES.0000152966.88353.9d. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Shimoda LA, O’Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol (1985) 2005;99:2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- Carracedo S, Sacher F, Brandes G, Braun U, Leitges M. Redundant role of protein kinase C delta and epsilon during mouse embryonic development. PLoS One. 2014;9:e103686. doi: 10.1371/journal.pone.0103686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- Castle NA, Haylett DG, Morgan JM, Jenkinson DH. Dequalinium: a potent inhibitor of apamin-sensitive K+ channels in hepatocytes and of nicotinic responses in skeletal muscle. Eur J Pharmacol. 1993;236:201–207. doi: 10.1016/0014-2999(93)90590-e. [DOI] [PubMed] [Google Scholar]
- Cazaubon S, Bornancin F, Parker PJ. Threonine-497 is a critical site for permissive activation of protein kinase C alpha. Biochem J. 1994;301(Pt 2):443–448. doi: 10.1042/bj3010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni V, Doppler H, Sonnenburg ED, Maraldi N, Newton AC, Toker A. Regulation of novel protein kinase C epsilon by phosphorylation. Biochem J. 2002;363:537–545. doi: 10.1042/0264-6021:3630537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1840–1849. doi: 10.1152/ajpheart.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Tostes RC, Webb RC. S-nitrosylation Inhibits protein kinase C-mediated contraction in mouse aorta. J Cardiovasc Pharmacol. 2011;57:65–71. doi: 10.1097/FJC.0b013e3181fef9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill EN, Mochly-Rosen D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35:1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Qvit N, Mochly-Rosen D. Rationally designed peptide regulators of protein kinase C. Trends Endocrinol Metab. 2009;20:25–33. doi: 10.1016/j.tem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Dodson PM. PKC inhibition and diabetic microvascular complications. Best Pract Res Clin Endocrinol Metab. 2007;21:573–586. doi: 10.1016/j.beem.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Clement S, Tasinato A, Boscoboinik D, Azzi A. The effect of alpha-tocopherol on the synthesis, phosphorylation and activity of protein kinase C in smooth muscle cells after phorbol 12-myristate 13-acetate down-regulation. Eur J Biochem. 1997;246:745–749. doi: 10.1111/j.1432-1033.1997.t01-2-00745.x. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003;93:656–663. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Frazziano G, Moral-Sanz J, Menendez C, Castaneda J, Gonzalez C, Villamor E, Perez-Vizcaino F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2009;82:296–302. doi: 10.1093/cvr/cvn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats. Evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994;93:311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier B. Central role of PKCs in vascular smooth muscle cell ion channel regulation. J Mol Cell Cardiol. 2006;41:952–955. doi: 10.1016/j.yjmcc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Csukai M, Chen CH, De Matteis MA, Mochly-Rosen D. The coatomer protein beta’-COP, a selective binding protein (RACK) for protein kinase Cepsilon. J Biol Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- Cywin CL, Dahmann G, Prokopowicz AS, 3rd, Young ER, Magolda RL, Cardozo MG, Cogan DA, Disalvo D, Ginn JD, Kashem MA, Wolak JP, Homon CA, Farrell TM, Grbic H, Hu H, Kaplita PV, Liu LH, Spero DM, Jeanfavre DD, O’Shea KM, White DM, Woska JR, Jr, Brown ML. Discovery of potent and selective PKC-theta inhibitors. Bioorg Med Chem Lett. 2007;17:225–230. doi: 10.1016/j.bmcl.2006.09.056. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulmonol. 2005;40:398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Xie S, Wang J, Xia Z, Nie R. Inhibition of protein kinase C beta(2) prevents tumor necrosis factor-alpha-induced apoptosis and oxidative stress in endothelial cells: the role of NADPH oxidase subunits. J Vasc Res. 2012;49:144–159. doi: 10.1159/000332337. [DOI] [PubMed] [Google Scholar]
- Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- Deuse T, Koyanagi T, Erben RG, Hua X, Velden J, Ikeno F, Reichenspurner H, Robbins RC, Mochly-Rosen D, Schrepfer S. Sustained inhibition of epsilon protein kinase C inhibits vascular restenosis after balloon injury and stenting. Circulation. 2010;122:S170–178. doi: 10.1161/CIRCULATIONAHA.109.927640. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D, Zhou W. Matrix metalloproteinases modulated by protein kinase Cepsilon mediate resistin-induced migration of human coronary artery smooth muscle cells. J Vasc Surg. 2011a;53:1044–1051. doi: 10.1016/j.jvs.2010.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding RQ, Tsao J, Chai H, Mochly-Rosen D, Zhou W. Therapeutic potential for protein kinase C inhibitor in vascular restenosis. J Cardiovasc Pharmacol Ther. 2011b;16:160–167. doi: 10.1177/1074248410382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Draeger A, Wray S, Babiychuk EB. Domain architecture of the smooth-muscle plasma membrane: regulation by annexins. Biochem J. 2005;387:309–314. doi: 10.1042/BJ20041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- Dries DR, Newton AC. Kinetic analysis of the interaction of the C1 domain of protein kinase C with lipid membranes by stopped-flow spectroscopy. J Biol Chem. 2008;283:7885–7893. doi: 10.1074/jbc.M709943200. [DOI] [PubMed] [Google Scholar]
- Dubois T, Oudinet JP, Mira JP, Russo-Marie F. Annexins and protein kinases C. Biochim Biophys Acta. 1996;1313:290–294. doi: 10.1016/0167-4889(96)00102-4. [DOI] [PubMed] [Google Scholar]
- Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol. 2011;51:665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Durgan J, Michael N, Totty N, Parker PJ. Novel phosphorylation site markers of protein kinase C delta activation. FEBS Lett. 2007;581:3377–3381. doi: 10.1016/j.febslet.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Dutil EM, Keranen LM, DePaoli-Roach AA, Newton AC. In vivo regulation of protein kinase C by trans-phosphorylation followed by autophosphorylation. J Biol Chem. 1994;269:29359–29362. [PubMed] [Google Scholar]
- Dutil EM, Toker A, Newton AC. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1) Curr Biol. 1998;8:1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Dykes AC, Fultz ME, Norton ML, Wright GL. Microtubule-dependent PKC-alpha localization in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2003;285:C76–87. doi: 10.1152/ajpcell.00515.2002. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, de Bont DB, de Widt J, Liskamp RM, Ploegh HL. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem. 1993;268:1982–1986. [PubMed] [Google Scholar]
- Eitelhuber AC, Warth S, Schimmack G, Duwel M, Hadian K, Demski K, Beisker W, Shinohara H, Kurosaki T, Heissmeyer V, Krappmann D. Dephosphorylation of Carma1 by PP2A negatively regulates T-cell activation. EMBO J. 2011;30:594–605. doi: 10.1038/emboj.2010.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Yazbi AF, Abd-Elrahman KS, Moreno-Dominguez A. PKC-mediated cerebral vasoconstriction: Role of myosin light chain phosphorylation versus actin cytoskeleton reorganization. Biochem Pharmacol. 2015;95:263–278. doi: 10.1016/j.bcp.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Engin KN. Alpha-tocopherol: looking beyond an antioxidant. Mol Vis. 2009;15:855–860. [PMC free article] [PubMed] [Google Scholar]
- Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, Welzenbach KA, Weitz-Schmidt G, Guntermann C, Towbin H, Cottens S, Kaminski S, Letschka T, Lutz-Nicoladoni C, Gruber T, Hermann-Kleiter N, Thuille N, Baier G. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J Pharmacol Exp Ther. 2009;330:792–801. doi: 10.1124/jpet.109.153205. [DOI] [PubMed] [Google Scholar]
- Fan HC, Fernandez-Hernando C, Lai JH. Protein kinase C isoforms in atherosclerosis: pro- or anti-inflammatory? Biochem Pharmacol. 2014;88:139–149. doi: 10.1016/j.bcp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Farley DB, Ford SP. Evidence for declining extracellular calcium uptake and protein kinase C activity in uterine arterial smooth muscle during gestation in gilts. Biol Reprod. 1992;46:315–321. doi: 10.1095/biolreprod46.3.315. [DOI] [PubMed] [Google Scholar]
- Faux MC, Rollins EN, Edwards AS, Langeberg LK, Newton AC, Scott JD. Mechanism of A-kinase-anchoring protein 79 (AKAP79) and protein kinase C interaction. Biochem J. 1999;343(Pt 2):443–452. [PMC free article] [PubMed] [Google Scholar]
- Ferreira JC, Koyanagi T, Palaniyandi SS, Fajardo G, Churchill EN, Budas G, Disatnik MH, Bernstein D, Brum PC, Mochly-Rosen D. Pharmacological inhibition of betaIIPKC is cardioprotective in late-stage hypertrophy. J Mol Cell Cardiol. 2011;51:980–987. doi: 10.1016/j.yjmcc.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SP. Control of blood flow to the gravid uterus of domestic livestock species. J Anim Sci. 1995;73:1852–1860. doi: 10.2527/1995.7361852x. [DOI] [PubMed] [Google Scholar]
- Fu G, Hu J, Niederberger-Magnenat N, Rybakin V, Casas J, Yachi PP, Feldstein S, Ma B, Hoerter JA, Ampudia J, Rigaud S, Lambolez F, Gavin AL, Sauer K, Cheroutre H, Gascoigne NR. Protein kinase C eta is required for T cell activation and homeostatic proliferation. Sci Signal. 2011;4:ra84. doi: 10.1126/scisignal.2002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Nishizawa Y, Hosoi M, Koyama H, Yamakawa K, Ohno S, Morii H. Protein kinase C delta inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J Biol Chem. 1997;272:13816–13822. doi: 10.1074/jbc.272.21.13816. [DOI] [PubMed] [Google Scholar]
- Gailly P, Gong MC, Somlyo AV, Somlyo AP. Possible role of atypical protein kinase C activated by arachidonic acid in Ca2+ sensitization of rabbit smooth muscle. J Physiol. 1997;500(Pt 1):95–109. doi: 10.1113/jphysiol.1997.sp022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FH, Wu YL, Zhao M, Liu CX, Wang LS, Chen GQ. Protein kinase C-delta mediates down-regulation of heterogeneous nuclear ribonucleoprotein K protein: involvement in apoptosis induction. Exp Cell Res. 2009;315:3250–3258. doi: 10.1016/j.yexcr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- Gao T, Newton AC. Invariant Leu preceding turn motif phosphorylation site controls the interaction of protein kinase C with Hsp70. J Biol Chem. 2006;281:32461–32468. doi: 10.1074/jbc.M604076200. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, Csiszar A, Sonntag WE. Greater endothelial apoptosis and oxidative stress in patients with peripheral artery disease. Int J Vasc Med. 2014;2014:160534. doi: 10.1155/2014/160534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin PB, Rayburn BK, Hutchins GM, Kasper EK, Baughman KL, Goodman SN, Lecks LE, Baumgartner WA, Hruban RH. Peritransplant injury to the myocardium associated with the development of accelerated arteriosclerosis in heart transplant recipients. Am J Surg Pathol. 1994;18:338–346. doi: 10.1097/00000478-199404000-00002. [DOI] [PubMed] [Google Scholar]
- Gekeler V, Boer R, Uberall F, Ise W, Schubert C, Utz I, Hofmann J, Sanders KH, Schachtele C, Klemm K, Grunicke H. Effects of the selective bisindolylmaleimide protein kinase C inhibitor GF 109203X on P-glycoprotein-mediated multidrug resistance. Br J Cancer. 1996;74:897–905. doi: 10.1038/bjc.1996.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatta S, Nimmagadda D, Xu X, O’Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther. 2006;110:103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Gilio K, Harper MT, Cosemans JM, Konopatskaya O, Munnix IC, Prinzen L, Leitges M, Liu Q, Molkentin JD, Heemskerk JW, Poole AW. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J Biol Chem. 2010;285:23410–23419. doi: 10.1074/jbc.M110.136176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginnan R, Singer HA. PKC-delta-dependent pathways contribute to PDGF-stimulated ERK1/2 activation in vascular smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1193–1201. doi: 10.1152/ajpcell.00499.2004. [DOI] [PubMed] [Google Scholar]
- Gogula SV, Divakar C, Satyanarayana C, Kumar YP, Lavanaya VS. Computational investigation of pkcbeta inhibitors for the treatment of diabetic retinopathy. Bioinformation. 2013;9:1040–1043. doi: 10.6026/97320630091040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnight JA, Mischak H, Kolch W, Mushinski JF. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R, Chen ZH, Gundimeda U. Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells. Proc Natl Acad Sci U S A. 1994;91:12233–12237. doi: 10.1073/pnas.91.25.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- Gorin MA, Pan Q. Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulopoulou S, Hannan JL, Matsumoto T, Webb RC. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am J Physiol Heart Circ Physiol. 2012;302:H2477–2488. doi: 10.1152/ajpheart.00900.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, Banks C, Capen A, Goode R, Lewis JE, Sams L, Huss KL, Campbell RM, Iversen PW, Neubauer BL, Brown TJ, Musib L, Geeganage S, Thornton D. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- Grandage VL, Everington T, Linch DC, Khwaja A. Go6976 is a potent inhibitor of the JAK 2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol. 2006;135:303–316. doi: 10.1111/j.1365-2141.2006.06291.x. [DOI] [PubMed] [Google Scholar]
- Grange JJ, Baca-Regen LM, Nollendorfs AJ, Persidsky Y, Sudan DL, Baxter BT. Protein kinase C isoforms in human aortic smooth muscle cells. J Vasc Surg. 1998;27:919–926. doi: 10.1016/s0741-5214(98)70273-3. discussion 926–917. [DOI] [PubMed] [Google Scholar]
- Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Ushio-Fukai M, Lassegue B, Alexander RW. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29:366–373. doi: 10.1161/01.hyp.29.1.366. [DOI] [PubMed] [Google Scholar]
- Gruber T, Pfeifhofer-Obermair C, Baier G. PKCtheta is necessary for efficient activation of NFkappaB, NFAT, and AP-1 during positive selection of thymocytes. Immunol Lett. 2010;132:6–11. doi: 10.1016/j.imlet.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Hage-Sleiman R, Hamze AB, Reslan L, Kobeissy H, Dbaibo G. The Novel PKCtheta from Benchtop to Clinic. J Immunol Res. 2015;2015:348798. doi: 10.1155/2015/348798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL, Matter CM, Wang X, Gibbons GH. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase C-dependent pathway. Circ Res. 2000;87:574–580. doi: 10.1161/01.res.87.7.574. [DOI] [PubMed] [Google Scholar]
- Haller H, Baur E, Quass P, Behrend M, Lindschau C, Distler A, Luft FC. High glucose concentrations and protein kinase C isoforms in vascular smooth muscle cells. Kidney Int. 1995;47:1057–1067. doi: 10.1038/ki.1995.152. [DOI] [PubMed] [Google Scholar]
- Haller H, Quass P, Lindschau C, Luft FC, Distler A. Platelet-derived growth factor and angiotensin II induce different spatial distribution of protein kinase C-alpha and -beta in vascular smooth muscle cells. Hypertension. 1994;23:848–852. doi: 10.1161/01.hyp.23.6.848. [DOI] [PubMed] [Google Scholar]
- Hampson P, Chahal H, Khanim F, Hayden R, Mulder A, Assi LK, Bunce CM, Lord JM. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood. 2005;106:1362–1368. doi: 10.1182/blood-2004-10-4117. [DOI] [PubMed] [Google Scholar]
- Hanke H, Lenz C, Hess B, Spindler KD, Weidemann W. Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall. Circulation. 2001;103:1382–1385. doi: 10.1161/01.cir.103.10.1382. [DOI] [PubMed] [Google Scholar]
- Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Heilig CW, Deb DK, Abdul A, Riaz H, James LR, Salameh J, Nahman NS., Jr GLUT1 regulation of the pro-sclerotic mediators of diabetic nephropathy. Am J Nephrol. 2013;38:39–49. doi: 10.1159/000351989. [DOI] [PubMed] [Google Scholar]
- Henry DN, Busik JV, Brosius FC, 3rd, Heilig CW. Glucose transporters control gene expression of aldose reductase, PKCalpha, and GLUT1 in mesangial cells in vitro. Am J Physiol. 1999;277:F97–104. doi: 10.1152/ajprenal.1999.277.1.F97. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Yoshinaga S, Ogura K, Yokochi M, Noda Y, Sumimoto H, Inagaki F. Solution structure of atypical protein kinase C PB1 domain and its mode of interaction with ZIP/p62 and MEK5. J Biol Chem. 2004;279:31883–31890. doi: 10.1074/jbc.M403092200. [DOI] [PubMed] [Google Scholar]
- Hoque M, Rentero C, Cairns R, Tebar F, Enrich C, Grewal T. Annexins – scaffolds modulating PKC localization and signaling. Cell Signal. 2014;26:1213–1225. doi: 10.1016/j.cellsig.2014.02.012. [DOI] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein kinase C pseudosubstrate prototope: structure-function relationships. Cell Signal. 1990;2:187–190. doi: 10.1016/0898-6568(90)90022-3. [DOI] [PubMed] [Google Scholar]
- Howcroft TK, Lindquist RR. The protein kinase C inhibitor 1-(5-isoquinolinesulfonyl)-2-methylpiperazine dihydrochloride (H-7) inhibits PMA-induced promiscuous cytolytic activity but not specific cytolytic activity by a cloned cytolytic T lymphocyte. Biochem Biophys Res Commun. 1991;179:720–725. doi: 10.1016/0006-291x(91)91876-e. [DOI] [PubMed] [Google Scholar]
- Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Chang JS, Xu Y, Li Q, Zou YS, Yan SF. Reduction of PKCbetaII activity in smooth muscle cells attenuates acute arterial injury. Atherosclerosis. 2010;212:123–130. doi: 10.1016/j.atherosclerosis.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- Huang W, Bansode RR, Bal NC, Mehta M, Mehta KD. Protein kinase Cbeta deficiency attenuates obesity syndrome of ob/ob mice by promoting white adipose tissue remodeling. J Lipid Res. 2012;53:368–378. doi: 10.1194/jlr.M019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Walker JW. Myofilament anchoring of protein kinase C-epsilon in cardiac myocytes. J Cell Sci. 2004;117:1971–1978. doi: 10.1242/jcs.01044. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui X, Reither G, Kaestner L, Lipp P. Targeted activation of conventional and novel protein kinases C through differential translocation patterns. Mol Cell Biol. 2014;34:2370–2381. doi: 10.1128/MCB.00040-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd PJ, Bannister AJ, Halls K, Dawson MA, Vermeulen M, Olsen JV, Ismail H, Somers J, Mann M, Owen-Hughes T, Gout I, Kouzarides T. Phosphorylation of histone H3 Thr-45 is linked to apoptosis. J Biol Chem. 2009;284:16575–16583. doi: 10.1074/jbc.M109.005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–1569. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Yokokura H, Itoh T, Kanmura Y, Kuriyama H, Hidaka H. Purified rabbit brain protein kinase C relaxes skinned vascular smooth muscle and phosphorylates myosin light chain. Arch Biochem Biophys. 1987;254:136–141. doi: 10.1016/0003-9861(87)90089-0. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Protein kinase C delta contributes to increase in EP3 agonist-induced contraction in mesenteric arteries from type 2 diabetic Goto-Kakizaki rats. Pflugers Arch. 2012;463:593–602. doi: 10.1007/s00424-012-1088-9. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Lorberg A. TOR regulation of AGC kinases in yeast and mammals. Biochem J. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–142. [PubMed] [Google Scholar]
- Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circ Res. 2003;92:1089–1097. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- Je HD, Gangopadhyay SS, Ashworth TD, Morgan KG. Calponin is required for agonist-induced signal transduction–evidence from an antisense approach in ferret smooth muscle. J Physiol. 2001;537:567–577. doi: 10.1111/j.1469-7793.2001.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan NL, Resta TC. Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation. 2014;21:259–271. doi: 10.1111/micc.12096. [DOI] [PubMed] [Google Scholar]
- Jialal I, Machha A, Devaraj S. Small interfering-RNA to protein kinase C-delta reduces the proinflammatory effects of human C-reactive protein in biobreeding diabetic rats. Horm Metab Res. 2013;45:326–328. doi: 10.1055/s-0032-1327643. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Yang Q. Downregulation of natriuretic peptide clearance receptor mRNA in vascular smooth muscle cells by angiotensin II. Fundam Clin Pharmacol. 2015;29:260–268. doi: 10.1111/fcp.12111. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Barman SA. Protein kinase C modulation of cyclic GMP in rat neonatal pulmonary vascular smooth muscle. Lung. 2004;182:79–89. doi: 10.1007/s00408-003-1046-6. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Alexander BT, Granger JP, Khalil RA. Ca(2+)-insensitive vascular protein kinase C during pregnancy and NOS inhibition. Hypertension. 1999;34:924–930. doi: 10.1161/01.hyp.34.4.924. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Altirkawi KA, Khalil RA. Preconditioning of coronary artery against vasoconstriction by endothelin-1 and prostaglandin F2alpha during repeated downregulation of epsilon-protein kinase C. J Cardiovasc Pharmacol. 2000a;35:491–501. doi: 10.1097/00005344-200003000-00021. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol. 2000b;278:R295–303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol. 1998;25:974–985. doi: 10.1111/j.1440-1681.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280:C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- Kappert K, Furundzija V, Fritzsche J, Margeta C, Kruger J, Meyborg H, Fleck E, Stawowy P. Integrin cleavage regulates bidirectional signalling in vascular smooth muscle cells. Thromb Haemost. 2010;103:556–563. doi: 10.1160/TH09-07-0478. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Khalil RA. Protein Kinase C Inhibitors as Modulators of Vascular Function and their Application in Vascular Disease. Pharmaceuticals (Basel) 2013;6:407–439. doi: 10.3390/ph6030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil RA, Lajoie C, Morgan KG. In situ determination of [Ca2+]i threshold for translocation of the alpha-protein kinase C isoform. Am J Physiol. 1994;266:C1544–1551. doi: 10.1152/ajpcell.1994.266.6.C1544. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Lajoie C, Resnick MS, Morgan KG. Ca(2+)-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol. 1992;263:C714–719. doi: 10.1152/ajpcell.1992.263.3.C714. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Menice CB, Wang CL, Morgan KG. Phosphotyrosine-dependent targeting of mitogen-activated protein kinase in differentiated contractile vascular cells. Circ Res. 1995;76:1101–1108. doi: 10.1161/01.res.76.6.1101. [DOI] [PubMed] [Google Scholar]
- Khan WA, Dobrowsky R, el Touny S, Hannun YA. Protein kinase C and platelet inhibition by D-erythro-sphingosine: comparison with N,N-dimethylsphingosine and commercial preparation. Biochem Biophys Res Commun. 1990;172:683–691. doi: 10.1016/0006-291x(90)90728-6. [DOI] [PubMed] [Google Scholar]
- Kheifets V, Bright R, Inagaki K, Schechtman D, Mochly-Rosen D. Protein kinase C delta (deltaPKC)-annexin V interaction: a required step in deltaPKC translocation and function. J Biol Chem. 2006;281:23218–23226. doi: 10.1074/jbc.M602075200. [DOI] [PubMed] [Google Scholar]
- Kheifets V, Mochly-Rosen D. Insight into intra- and inter-molecular interactions of PKC: design of specific modulators of kinase function. Pharmacol Res. 2007;55:467–476. doi: 10.1016/j.phrs.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LE, Sun S, Mackie D, Baik F, Li H, Korchak HM. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of delta-PKC and ERK1/2. J Leukoc Biol. 2006;80:1512–1521. doi: 10.1189/jlb.0406284. [DOI] [PubMed] [Google Scholar]
- Kim HL, Im DS. N, N-dimethyl-D-erythro-sphingosine increases intracellular Ca2+ concentration via Na+-Ca2+-exchanger in HCT116 human colon cancer cells. Arch Pharm Res. 2008;31:54–59. doi: 10.1007/s12272-008-1120-y. [DOI] [PubMed] [Google Scholar]
- Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Gallant C, Morgan KG. Regulation of PKC autophosphorylation by calponin in contractile vascular smooth muscle tissue. Biomed Res Int. 2013;2013:358643. doi: 10.1155/2013/358643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Koyanagi T, Mochly-Rosen D. PKCdelta activation mediates angiogenesis via NADPH oxidase activity in PC-3 prostate cancer cells. Prostate. 2011a;71:946–954. doi: 10.1002/pros.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Thorne SH, Sun L, Huang B, Mochly-Rosen D. Sustained inhibition of PKCalpha reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene. 2011b;30:323–333. doi: 10.1038/onc.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- Kizub IV, Klymenko KI, Soloviev AI. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int J Cardiol. 2014;174:230–242. doi: 10.1016/j.ijcard.2014.04.117. [DOI] [PubMed] [Google Scholar]
- Kizub IV, Pavlova OO, Ivanova IV, Soloviev AI. Protein kinase C-dependent inhibition of BK(Ca) current in rat aorta smooth muscle cells following gamma-irradiation. Int J Radiat Biol. 2010;86:291–299. doi: 10.3109/09553000903564042. [DOI] [PubMed] [Google Scholar]
- Klein G, Schaefer A, Hilfiker-Kleiner D, Oppermann D, Shukla P, Quint A, Podewski E, Hilfiker A, Schroder F, Leitges M, Drexler H. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCepsilon. Circ Res. 2005;96:748–755. doi: 10.1161/01.RES.0000161999.86198.1e. [DOI] [PubMed] [Google Scholar]
- Klymenko K, Novokhatska T, Kizub I, Parshikov A, Dosenko V, Soloviev A. PKC-delta isozyme gene silencing restores vascular function in diabetic rat. J Basic Clin Physiol Pharmacol. 2014:1–9. doi: 10.1515/jbcpp-2013-0147. [DOI] [PubMed] [Google Scholar]
- Kong L, Shen X, Lin L, Leitges M, Rosario R, Zou YS, Yan SF. PKCbeta promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol. 2013;33:1779–1787. doi: 10.1161/ATVBAHA.112.301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, Ohmae K, Kikkawa U, Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci U S A. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen S, Kurkinen K, Akerman KE, Mochly-Rosen D, Chan PH, Koistinaho J. Prevention of NMDA-induced death of cortical neurons by inhibition of protein kinase Czeta. J Neurochem. 2003;86:442–450. doi: 10.1046/j.1471-4159.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- Kostyak JC, Bhavanasi D, Liverani E, McKenzie SE, Kunapuli SP. Protein kinase C delta deficiency enhances megakaryopoiesis and recovery from thrombocytopenia. Arterioscler Thromb Vasc Biol. 2014;34:2579–2585. doi: 10.1161/ATVBAHA.114.304492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroedov A, Eto M, Joch H, Volpe M, Luscher TF, Cosentino F. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–96. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115–126. doi: 10.1172/JCI119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- Koyanagi T, Noguchi K, Ootani A, Inagaki K, Robbins RC, Mochly-Rosen D. Pharmacological inhibition of epsilon PKC suppresses chronic inflammation in murine cardiac transplantation model. J Mol Cell Cardiol. 2007;43:517–522. doi: 10.1016/j.yjmcc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kraft AS, Anderson WB. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983;301:621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kraft AS, Smith JB, Berkow RL. Bryostatin, an activator of the calcium phospholipid-dependent protein kinase, blocks phorbol ester-induced differentiation of human promyelocytic leukemia cells HL-60. Proc Natl Acad Sci U S A. 1986;83:1334–1338. doi: 10.1073/pnas.83.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Slevin MA, Kumar P, Gaffney J, Kaluza J. Protein kinase C expression and activity in the human brain after ischaemic stroke. Acta Neurobiol Exp (Wars) 1998;58:13–21. doi: 10.55782/ane-1998-1254. [DOI] [PubMed] [Google Scholar]
- Lahn M, Sundell K, Moore S. Targeting protein kinase C-alpha (PKC-alpha) in cancer with the phosphorothioate antisense oligonucleotide aprinocarsen. Ann N Y Acad Sci. 2003;1002:263–270. doi: 10.1196/annals.1281.029. [DOI] [PubMed] [Google Scholar]
- Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium. 2009;45:574–582. doi: 10.1016/j.ceca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Lee HW, Smith L, Pettit GR, Bingham Smith J. Dephosphorylation of activated protein kinase C contributes to downregulation by bryostatin. Am J Physiol. 1996;271:C304–311. doi: 10.1152/ajpcell.1996.271.1.C304. [DOI] [PubMed] [Google Scholar]
- Lee K, Kim H, Jeong D. Protein kinase C regulates vascular calcification via cytoskeleton reorganization and osteogenic signaling. Biochem Biophys Res Commun. 2014;453:793–797. doi: 10.1016/j.bbrc.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Lehel C, Olah Z, Jakab G, Anderson WB. Protein kinase C epsilon is localized to the Golgi via its zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci U S A. 1995a;92:1406–1410. doi: 10.1073/pnas.92.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehel C, Olah Z, Jakab G, Szallasi Z, Petrovics G, Harta G, Blumberg PM, Anderson WB. Protein kinase C epsilon subcellular localization domains and proteolytic degradation sites. A model for protein kinase C conformational changes. J Biol Chem. 1995b;270:19651–19658. doi: 10.1074/jbc.270.33.19651. [DOI] [PubMed] [Google Scholar]
- Lehel C, Olah Z, Petrovics G, Jakab G, Anderson WB. Influence of various domains of protein kinase C epsilon on its PMA-induced translocation from the Golgi to the plasma membrane. Biochem Biophys Res Commun. 1996;223:98–103. doi: 10.1006/bbrc.1996.0852. [DOI] [PubMed] [Google Scholar]
- Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–858. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- Lengfeld J, Wang Q, Zohlman A, Salvarezza S, Morgan S, Ren J, Kato K, Rodriguez-Boulan E, Liu B. Protein kinase C delta regulates the release of collagen type I from vascular smooth muscle cells via regulation of Cdc42. Mol Biol Cell. 2012;23:1955–1963. doi: 10.1091/mbc.E11-06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque L, Dean NM, Sasmor H, Crooke ST. Antisense oligonucleotides targeting human protein kinase C-alpha inhibit phorbol ester-induced reduction of bradykinin-evoked calcium mobilization in A549 cells. Mol Pharmacol. 1997;51:209–216. doi: 10.1124/mol.51.2.209. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Cheresh DA, Schwartz MA. Protein kinase C regulates alpha v beta 5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wernig F, Leitges M, Hu Y, Xu Q. Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J. 2003;17:2106–2108. doi: 10.1096/fj.03-0150fje. [DOI] [PubMed] [Google Scholar]
- Li T, Xiao X, Zhang J, Zhu Y, Hu Y, Zang J, Lu K, Yang T, Ge H, Peng X, Lan D, Liu L. Age and sex differences in vascular responsiveness in healthy and trauma patients: contribution of estrogen receptor-mediated Rho kinase and PKC pathways. Am J Physiol Heart Circ Physiol. 2014;306:H1105–1115. doi: 10.1152/ajpheart.00645.2013. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang J, Bottaro DP, Pierce JH. Identification of serine 643 of protein kinase C-delta as an important autophosphorylation site for its enzymatic activity. J Biol Chem. 1997;272:24550–24555. doi: 10.1074/jbc.272.39.24550. [DOI] [PubMed] [Google Scholar]
- Light P. Regulation of ATP-sensitive potassium channels by phosphorylation. Biochim Biophys Acta. 1996;1286:65–73. doi: 10.1016/0304-4157(96)00004-4. [DOI] [PubMed] [Google Scholar]
- Limas CJ. Phosphorylation of cardiac sarcoplasmic reticulum by a calcium-activated, phospholipid-dependent protein kinase. Biochem Biophys Res Commun. 1980;96:1378–1383. doi: 10.1016/0006-291x(80)90103-5. [DOI] [PubMed] [Google Scholar]
- Linch M, Riou P, Claus J, Cameron AJ, de Naurois J, Larijani B, Ng T, McDonald NQ, Parker PJ. Functional implications of assigned, assumed and assembled PKC structures. Biochem Soc Trans. 2014;42:35–41. doi: 10.1042/BST20130192. [DOI] [PubMed] [Google Scholar]
- Ling S, Little PJ, Williams MR, Dai A, Hashimura K, Liu JP, Komesaroff PA, Sudhir K. High glucose abolishes the antiproliferative effect of 17beta-estradiol in human vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2002;282:E746–751. doi: 10.1152/ajpendo.00111.2001. [DOI] [PubMed] [Google Scholar]
- Liou YM, Morgan KG. Redistribution of protein kinase C isoforms in association with vascular hypertrophy of rat aorta. Am J Physiol. 1994;267:C980–989. doi: 10.1152/ajpcell.1994.267.4.C980. [DOI] [PubMed] [Google Scholar]
- Littler CM, Morris KG, Jr, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2003;284:H1321–1331. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- Littler CM, Wehling CA, Wick MJ, Fagan KA, Cool CD, Messing RO, Dempsey EC. Divergent contractile and structural responses of the murine PKC-epsilon null pulmonary circulation to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1083–1093. doi: 10.1152/ajplung.00472.2004. [DOI] [PubMed] [Google Scholar]
- Liu B, Ryer EJ, Kundi R, Kamiya K, Itoh H, Faries PL, Sakakibara K, Kent KC. Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. J Vasc Surg. 2007;45:160–168. doi: 10.1016/j.jvs.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Lin SJ, Lin JK. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14:857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- Liu M, Large WA, Albert AP. Stimulation of beta-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mechanism in rabbit portal vein myocytes. J Physiol. 2005;562:395–406. doi: 10.1113/jphysiol.2004.077602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Graham C, Li A, Fisher RJ, Shaw S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem J. 2002;361:255–265. doi: 10.1042/bj3610255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- Lizotte F, Pare M, Denhez B, Leitges M, Guay A, Geraldes P. PKCdelta impaired vessel formation and angiogenic factor expression in diabetic ischemic limbs. Diabetes. 2013;62:2948–2957. doi: 10.2337/db12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Chai Q, Yu L, d’Uscio LV, Katusic ZS, He T, Lee HC. Reactive oxygen species signaling facilitates FOXO-3a/FBXO-dependent vascular BK channel beta1 subunit degradation in diabetic mice. Diabetes. 2012;61:1860–1868. doi: 10.2337/db11-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Finnis S, Xiang C, Lee HK, Markowitz Y, Okhrimenko H, Brodie C. Tyrosine 311 is phosphorylated by c-Abl and promotes the apoptotic effect of PKCdelta in glioma cells. Biochem Biophys Res Commun. 2007;352:431–436. doi: 10.1016/j.bbrc.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZG, Liu H, Yamaguchi T, Miki Y, Yoshida K. Protein kinase Cdelta activates RelA/p65 and nuclear factor-kappaB signaling in response to tumor necrosis factor-alpha. Cancer Res. 2009;69:5927–5935. doi: 10.1158/0008-5472.CAN-08-4786. [DOI] [PubMed] [Google Scholar]
- Lum MA, Balaburski GM, Murphy ME, Black AR, Black JD. Heat shock proteins regulate activation-induced proteasomal degradation of the mature phosphorylated form of protein kinase C. J Biol Chem. 2013;288:27112–27127. doi: 10.1074/jbc.M112.437095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Ma W, Baumann C, Viveiros MM. Lack of protein kinase C-delta (PKCdelta) disrupts fertilization and embryonic development. Mol Reprod Dev. 2015;82:797–808. doi: 10.1002/mrd.22528. [DOI] [PubMed] [Google Scholar]
- Magid R, Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ Res. 2005;97:443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260:E464–470. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- Manetta A, Emma D, Gamboa G, Liao S, Berman M, DiSaia P. Failure to enhance the in vivo killing of human ovarian carcinoma by sequential treatment with dequalinium chloride and tumor necrosis factor. Gynecol Oncol. 1993;50:38–44. doi: 10.1006/gyno.1993.1161. [DOI] [PubMed] [Google Scholar]
- Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J Biol Chem. 2010;285:5963–5973. doi: 10.1074/jbc.M109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Su J, Lei L, Meng L, Qi Y, Huo Y, Tang C. Adrenomedullin and adrenotensin increase the transcription of regulator of Gprotein signaling 2 in vascular smooth muscle cells via the cAMPdependent and PKC pathways. Mol Med Rep. 2013;9:323–327. doi: 10.3892/mmr.2013.1751. [DOI] [PubMed] [Google Scholar]
- Martin P, Villares R, Rodriguez-Mascarenhas S, Zaballos A, Leitges M, Kovac J, Sizing I, Rennert P, Marquez G, Martinez AC, Diaz-Meco MT, Moscat J. Control of T helper 2 cell function and allergic airway inflammation by PKCzeta. Proc Natl Acad Sci U S A. 2005;102:9866–9871. doi: 10.1073/pnas.0501202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Masoumi KC, Cornmark L, Lonne GK, Hellman U, Larsson C. Identification of a novel protein kinase Cdelta-Smac complex that dissociates during paclitaxel-induced cell death. FEBS Lett. 2012;586:1166–1172. doi: 10.1016/j.febslet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Mattila P, Majuri ML, Tiisala S, Renkonen R. Expression of six protein kinase C isotypes in endothelial cells. Life Sci. 1994;55:1253–1260. doi: 10.1016/0024-3205(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, Meyer T, Mett H, Fabbro D, Furet P, et al. Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem. 1995;234:317–322. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- Mehta KD. Emerging role of protein kinase C in energy homeostasis: A brief overview. World J Diabetes. 2014;5:385–392. doi: 10.4239/wjd.v5.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NN, Sheetz M, Price K, Comiskey L, Amrutia S, Iqbal N, Mohler ER, Reilly MP. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc Drugs Ther. 2009;23:17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M, King GL. Protein kinase C activation and its pharmacological inhibition in vascular disease. Vasc Med. 2000;5:173–185. doi: 10.1177/1358836X0000500307. [DOI] [PubMed] [Google Scholar]
- Meller N, Liu YC, Collins TL, Bonnefoy-Berard N, Baier G, Isakov N, Altman A. Direct interaction between protein kinase C theta (PKC theta) and 14-3-3 tau in T cells: 14-3-3 overexpression results in inhibition of PKC theta translocation and function. Mol Cell Biol. 1996;16:5782–5791. doi: 10.1128/mcb.16.10.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca MC, Doi SQ, Glerum S, Sellitti DF. Increase of C-type natriuretic peptide expression by serum and platelet-derived growth factor-BB in human aortic smooth muscle cells is dependent on protein kinase C activation. Endocrinology. 2006;147:4169–4178. doi: 10.1210/en.2006-0239. [DOI] [PubMed] [Google Scholar]
- Mendonca MC, Koles N, Sellitti DF. Protein kinase C-delta (PKC-delta) and PKC-alpha mediate Ca(2+)-dependent increases in CNP mRNA in human vascular cells. Vascul Pharmacol. 2012;57:98–104. doi: 10.1016/j.vph.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Meotti FC, Luiz AP, Pizzolatti MG, Kassuya CA, Calixto JB, Santos AR. Analysis of the antinociceptive effect of the flavonoid myricitrin: evidence for a role of the L-arginine-nitric oxide and protein kinase C pathways. J Pharmacol Exp Ther. 2006;316:789–796. doi: 10.1124/jpet.105.092825. [DOI] [PubMed] [Google Scholar]
- Metzger E, Imhof A, Patel D, Kahl P, Hoffmeyer K, Friedrichs N, Muller JM, Greschik H, Kirfel J, Ji S, Kunowska N, Beisenherz-Huss C, Gunther T, Buettner R, Schule R. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2010;464:792–796. doi: 10.1038/nature08839. [DOI] [PubMed] [Google Scholar]
- Mii S, Khalil RA, Morgan KG, Ware JA, Kent KC. Mitogen-activated protein kinase and proliferation of human vascular smooth muscle cells. Am J Physiol. 1996;270:H142–150. doi: 10.1152/ajpheart.1996.270.1.H142. [DOI] [PubMed] [Google Scholar]
- Millward MJ, House C, Bowtell D, Webster L, Olver IN, Gore M, Copeman M, Lynch K, Yap A, Wang Y, Cohen PS, Zalcberg J. The multikinase inhibitor midostaurin (PKC412A) lacks activity in metastatic melanoma: a phase IIA clinical and biologic study. Br J Cancer. 2006;95:829–834. doi: 10.1038/sj.bjc.6603331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1993;190:263–269. doi: 10.1006/bbrc.1993.1040. [DOI] [PubMed] [Google Scholar]
- Mineo C, Ying YS, Chapline C, Jaken S, Anderson RG. Targeting of protein kinase Calpha to caveolae. J Cell Biol. 1998;141:601–610. doi: 10.1083/jcb.141.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Kauvar LM. Pharmacological regulation of network kinetics by protein kinase C localization. Semin Immunol. 2000;12:55–61. doi: 10.1006/smim.2000.0207. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M, Donnini S, Giachetti A, Mochly-Rosen D, Ziche M. deltaPKC inhibition or varepsilonPKC activation repairs endothelial vascular dysfunction by regulating eNOS post-translational modification. J Mol Cell Cardiol. 2010;48:746–756. doi: 10.1016/j.yjmcc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M, Donnini S, Morbidelli L, Giachetti A, Mochly-Rosen D, Mignatti P, Ziche M. PKCepsilon activation promotes FGF-2 exocytosis and induces endothelial cell proliferation and sprouting. J Mol Cell Cardiol. 2013;63:107–117. doi: 10.1016/j.yjmcc.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Dominguez A, El-Yazbi AF, Zhu HL, Colinas O, Zhong XZ, Walsh EJ, Cole DM, Kargacin GJ, Walsh MP, Cole WC. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J Biol Chem. 2014;289:20939–20952. doi: 10.1074/jbc.M114.553743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S, Yamanouchi D, Harberg C, Wang Q, Keller M, Si Y, Burlingham W, Seedial S, Lengfeld J, Liu B. Elevated protein kinase C-delta contributes to aneurysm pathogenesis through stimulation of apoptosis and inflammatory signaling. Arterioscler Thromb Vasc Biol. 2012;32:2493–2502. doi: 10.1161/ATVBAHA.112.255661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Roy S, Saha B, Mukherjee D. Spatio-Temporal Regulation of PKC Isoforms Imparts Signaling Specificity. Front Immunol. 2016;7:45. doi: 10.3389/fimmu.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naitoh K, Yano T, Miura T, Itoh T, Miki T, Tanno M, Sato T, Hotta H, Terashima Y, Shimamoto K. Roles of Cx43-associated protein kinases in suppression of gap junction-mediated chemical coupling by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2009;296:H396–403. doi: 10.1152/ajpheart.00448.2008. [DOI] [PubMed] [Google Scholar]
- Nakano S, Uchida K, Kigoshi T, Azukizawa S, Iwasaki R, Kaneko M, Morimoto S. Circadian rhythm of blood pressure in normotensive NIDDM subjects. Its relationship to microvascular complications. Diabetes Care. 1991;14:707–711. doi: 10.2337/diacare.14.8.707. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Frank GD, Shirai H, Hinoki A, Higuchi S, Ohtsu H, Eguchi K, Sanjay A, Reyland ME, Dempsey PJ, Inagami T, Eguchi S. Novel role of protein kinase C-delta Tyr 311 phosphorylation in vascular smooth muscle cell hypertrophy by angiotensin II. Hypertension. 2008;51:232–238. doi: 10.1161/HYPERTENSIONAHA.107.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Tanaka Y. Stretch-induced contraction and Ca2+ mobilization in vascular smooth muscle. Biol Signals. 1993;2:241–252. doi: 10.1159/000109505. [DOI] [PubMed] [Google Scholar]
- Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Williams JM, Akopov SE, Zhang L, Pearce WJ. Developmental changes in ryanodine- and IP(3)-sensitive Ca(2+) pools in ovine basilar artery. Am J Physiol Cell Physiol. 2001;281:C1785–1796. doi: 10.1152/ajpcell.2001.281.6.C1785. [DOI] [PubMed] [Google Scholar]
- Navarro-Nunez L, Lozano ML, Martinez C, Vicente V, Rivera J. Effect of quercetin on platelet spreading on collagen and fibrinogen and on multiple platelet kinases. Fitoterapia. 2010;81:75–80. doi: 10.1016/j.fitote.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Naylor TL, Tang H, Ratsch BA, Enns A, Loo A, Chen L, Lenz P, Waters NJ, Schuler W, Dorken B, Yao YM, Warmuth M, Lenz G, Stegmeier F. Protein kinase C inhibitor sotrastaurin selectively inhibits the growth of CD79 mutant diffuse large B-cell lymphomas. Cancer Res. 2011;71:2643–2653. doi: 10.1158/0008-5472.CAN-10-2525. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T, Squire A, Hansra G, Bornancin F, Prevostel C, Hanby A, Harris W, Barnes D, Schmidt S, Mellor H, Bastiaens PI, Parker PJ. Imaging protein kinase Calpha activation in cells. Science. 1999;283:2085–2089. doi: 10.1126/science.283.5410.2085. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- Novokhatska T, Tishkin S, Dosenko V, Boldyriev A, Ivanova I, Strielkov I, Soloviev A. Correction of vascular hypercontractility in spontaneously hypertensive rats using shRNAs-induced delta protein kinase C gene silencing. Eur J Pharmacol. 2013;718:401–407. doi: 10.1016/j.ejphar.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Ogiwara T, Negishi T, Chik CL, Ho AK. Differential effects of two protein kinase C inhibitors, calphostin C and Go6976, on pineal cyclic nucleotide accumulation. J Neurochem. 1998;71:1405–1412. doi: 10.1046/j.1471-4159.1998.71041405.x. [DOI] [PubMed] [Google Scholar]
- Ohanian V, Ohanian J, Shaw L, Scarth S, Parker PJ, Heagerty AM. Identification of protein kinase C isoforms in rat mesenteric small arteries and their possible role in agonist-induced contraction. Circ Res. 1996;78:806–812. doi: 10.1161/01.res.78.5.806. [DOI] [PubMed] [Google Scholar]
- Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem. 1997;272:33416–33421. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- Okazaki J, Mawatari K, Liu B, Kent KC. The effect of protein kinase C and its alpha subtype on human vascular smooth muscle cell proliferation, migration and fibronectin production. Surgery. 2000;128:192–197. doi: 10.1067/msy.2000.108062. [DOI] [PubMed] [Google Scholar]
- Osada H, Sonoda T, Tsunoda K, Isono K. A new biological role of sangivamycin; inhibition of protein kinases. J Antibiot (Tokyo) 1989;42:102–106. doi: 10.7164/antibiotics.42.102. [DOI] [PubMed] [Google Scholar]
- Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int. 2001;59:1789–1797. doi: 10.1046/j.1523-1755.2001.0590051789.x. [DOI] [PubMed] [Google Scholar]
- Palmas W, Pickering T, Teresi J, Schwartz JE, Eguchi K, Field L, Weinstock RS, Shea S. Nocturnal blood pressure elevation predicts progression of albuminuria in elderly people with type 2 diabetes. J Clin Hypertens (Greenwich) 2008;10:12–20. doi: 10.1111/j.1524-6175.2007.07170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande V, Ramos MJ, Gago F. The Protein kinase inhibitor balanol: structure-activity relationships and structure-based computational studies. Anticancer Agents Med Chem. 2008;8:638–645. doi: 10.2174/187152008785133056. [DOI] [PubMed] [Google Scholar]
- Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parissenti AM, Kirwan AF, Kim SA, Colantonio CM, Schimmer BP. Inhibitory properties of the regulatory domains of human protein kinase Calpha and mouse protein kinase Cepsilon. J Biol Chem. 1998;273:8940–8945. doi: 10.1074/jbc.273.15.8940. [DOI] [PubMed] [Google Scholar]
- Park WS, Han J, Kim N, Youm JB, Joo H, Kim HK, Ko JH, Earm YE. Endothelin-1 inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase C. J Cardiovasc Pharmacol. 2005;46:681–689. doi: 10.1097/01.fjc.0000182846.08357.ed. [DOI] [PubMed] [Google Scholar]
- Park WS, Kim N, Youm JB, Warda M, Ko JH, Kim SJ, Earm YE, Han J. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun. 2006;341:728–735. doi: 10.1016/j.bbrc.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Parker CA, Takahashi K, Tao T, Morgan KG. Agonist-induced redistribution of calponin in contractile vascular smooth muscle cells. Am J Physiol. 1994;267:C1262–1270. doi: 10.1152/ajpcell.1994.267.5.C1262. [DOI] [PubMed] [Google Scholar]
- Pears CJ, Kour G, House C, Kemp BE, Parker PJ. Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem. 1990;194:89–94. doi: 10.1111/j.1432-1033.1990.tb19431.x. [DOI] [PubMed] [Google Scholar]
- Pedersen DJ, Diakanastasis B, Stockli J, Schmitz-Peiffer C. Protein kinase Cepsilon modulates insulin receptor localization and trafficking in mouse embryonic fibroblasts. PLoS One. 2013;8:e58046. doi: 10.1371/journal.pone.0058046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Avila A, Islas S, Sanchez S, Gonzalez-Mariscal L. Vinculin but not alpha-actinin is a target of PKC phosphorylation during junctional assembly induced by calcium. J Cell Sci. 1998;111(Pt 23):3563–3571. doi: 10.1242/jcs.111.23.3563. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F, Cogolludo A, Moreno L. Reactive oxygen species signaling in pulmonary vascular smooth muscle. Respir Physiol Neurobiol. 2010;174:212–220. doi: 10.1016/j.resp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Persaud SD, Hoang V, Huang J, Basu A. Involvement of proteolytic activation of PKCdelta in cisplatin-induced apoptosis in human small cell lung cancer H69 cells. Int J Oncol. 2005;27:149–154. [PubMed] [Google Scholar]
- Peterman EE, Taormina P, 2nd, Harvey M, Young LH. Go 6983 exerts cardioprotective effects in myocardial ischemia/reperfusion. J Cardiovasc Pharmacol. 2004;43:645–656. doi: 10.1097/00005344-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Pfeifhofer C, Gruber T, Letschka T, Thuille N, Lutz-Nicoladoni C, Hermann-Kleiter N, Braun U, Leitges M, Baier G. Defective IgG2a/2b class switching in PKC alpha−/− mice. J Immunol. 2006;176:6004–6011. doi: 10.4049/jimmunol.176.10.6004. [DOI] [PubMed] [Google Scholar]
- Piccoletti R, Bendinelli P, Arienti D, Bernelli-Zazzera A. State and activity of protein kinase C in postischemic reperfused liver. Exp Mol Pathol. 1992;56:219–228. doi: 10.1016/0014-4800(92)90038-d. [DOI] [PubMed] [Google Scholar]
- Poli A, Mongiorgi S, Cocco L, Follo MY. Protein kinase C involvement in cell cycle modulation. Biochem Soc Trans. 2014;42:1471–1476. doi: 10.1042/BST20140128. [DOI] [PubMed] [Google Scholar]
- Pomero F, Allione A, Beltramo E, Buttiglieri S, D’Alu F, Ponte E, Lacaria A, Porta M. Effects of protein kinase C inhibition and activation on proliferation and apoptosis of bovine retinal pericytes. Diabetologia. 2003;46:416–419. doi: 10.1007/s00125-003-1044-5. [DOI] [PubMed] [Google Scholar]
- Posti JP, Salo P, Ruohonen S, Valve L, Muszkat M, Sofowora GG, Kurnik D, Stein CM, Perola M, Scheinin M, Snapir A. A polymorphism in the protein kinase C gene PRKCB is associated with alpha2-adrenoceptor-mediated vasoconstriction. Pharmacogenet Genomics. 2013;23:127–134. doi: 10.1097/FPC.0b013e32835d247f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pula G, Schuh K, Nakayama K, Nakayama KI, Walter U, Poole AW. PKCdelta regulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035–4044. doi: 10.1182/blood-2006-05-023739. [DOI] [PubMed] [Google Scholar]
- Qi W, Twigg S, Chen X, Polhill TS, Poronnik P, Gilbert RE, Pollock CA. Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am J Physiol Renal Physiol. 2005;288:F800–809. doi: 10.1152/ajprenal.00179.2004. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Qvit N, Mochly-Rosen D. Highly Specific Modulators of Protein Kinase C Localization: Applications to Heart Failure. Drug Discov Today Dis Mech. 2010;7:e87–e93. doi: 10.1016/j.ddmec.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan Y, Maile LA, Ling Y, Graves LM, Clemmons DR. Insulin-like growth factor-I stimulates Shc-dependent phosphatidylinositol 3-kinase activation via Grb2-associated p85 in vascular smooth muscle cells. J Biol Chem. 2008;283:16320–16331. doi: 10.1074/jbc.M801687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow R, Parker A, Davies N. Protein kinase C-independent inhibition of arterial smooth muscle K(+) channels by a diacylglycerol analogue. Br J Pharmacol. 2011;163:845–856. doi: 10.1111/j.1476-5381.2011.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow RD, Hardy ME, Standen NB, Davies NW. Glucose reduces endothelin inhibition of voltage-gated potassium channels in rat arterial smooth muscle cells. J Physiol. 2006;575:833–844. doi: 10.1113/jphysiol.2006.114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow RD, Norman RI, Everitt DE, Brignell JL, Davies NW, Standen NB. Endothelin-I and angiotensin II inhibit arterial voltage-gated K+ channels through different protein kinase C isoenzymes. Cardiovasc Res. 2009;83:493–500. doi: 10.1093/cvr/cvp143. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. Differential regulation of VEGF signaling by PKC-alpha and PKC-epsilon in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:919–924. doi: 10.1161/ATVBAHA.108.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCepsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006;351:784–790. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratz PH, Miner AS. Role of protein kinase Czeta and calcium entry in KCl-induced vascular smooth muscle calcium sensitization and feedback control of cellular calcium levels. J Pharmacol Exp Ther. 2009;328:399–408. doi: 10.1124/jpet.108.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- Rembold CM, Murphy RA. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988;63:593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Ren J, Wang Q, Morgan S, Si Y, Ravichander A, Dou C, Kent KC, Liu B. Protein kinase C-delta (PKCdelta) regulates proinflammatory chemokine expression through cytosolic interaction with the NF-kappaB subunit p65 in vascular smooth muscle cells. J Biol Chem. 2014;289:9013–9026. doi: 10.1074/jbc.M113.515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NJ, McCombie SW, Shankar BB, Bishop WR, Fisher GJ. SCH 47112, a novel staurosporine derivative, inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and epidermal hyperplasia in hairless mouse skin. Arch Dermatol Res. 1997;289:540–546. doi: 10.1007/s004030050236. [DOI] [PubMed] [Google Scholar]
- Ritter O, Jelenik T, Roden M. Lipid-mediated muscle insulin resistance: different fat, different pathways? J Mol Med (Berl) 2015;93:831–843. doi: 10.1007/s00109-015-1310-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pla A, Bosch-Gil JA, Rossello-Urgell J, Huguet-Redecilla P, Stone JH, Vilardell-Tarres M. Metalloproteinase-2 and -9 in giant cell arteritis: involvement in vascular remodeling. Circulation. 2005;112:264–269. doi: 10.1161/CIRCULATIONAHA.104.520114. [DOI] [PubMed] [Google Scholar]
- Rodriguez MM, Chen CH, Smith BL, Mochly-Rosen D. Characterization of the binding and phosphorylation of cardiac calsequestrin by epsilon protein kinase C. FEBS Lett. 1999;454:240–246. doi: 10.1016/s0014-5793(99)00697-3. [DOI] [PubMed] [Google Scholar]
- Roffey J, Rosse C, Linch M, Hibbert A, McDonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–279. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. [PubMed] [Google Scholar]
- Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of beta protein kinase C in vivo. J Biol Chem. 1995;270:24180–24187. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- Ron D, Mochly-Rosen D. Agonists and antagonists of protein kinase C function, derived from its binding proteins. J Biol Chem. 1994;269:21395–21398. [PubMed] [Google Scholar]
- Rosoff PM, Stein LF, Cantley LC. Phorbol esters induce differentiation in a pre-B-lymphocyte cell line by enhancing Na+/H+ exchange. J Biol Chem. 1984;259:7056–7060. [PubMed] [Google Scholar]
- Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rovedo MA, Krett NL, Rosen ST. Inhibition of glycogen synthase kinase-3 increases the cytotoxicity of enzastaurin. J Invest Dermatol. 2011;131:1442–1449. doi: 10.1038/jid.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992;148:2148–2153. [PubMed] [Google Scholar]
- Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin VO, Guo J, Sabri A, Elouardighi H, Schaefer E, Steinberg SF. Stimulus-specific differences in protein kinase C delta localization and activation mechanisms in cardiomyocytes. J Biol Chem. 2004;279:19350–19361. doi: 10.1074/jbc.M311096200. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Sabri A, Short J, Braz JC, Molkentin JD, Steinberg SF. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J Biol Chem. 2003;278:14555–14564. doi: 10.1074/jbc.M212644200. [DOI] [PubMed] [Google Scholar]
- Saito K, Ito E, Takakuwa Y, Tamura M, Kinjo M. In situ observation of mobility and anchoring of PKCbetaI in plasma membrane. FEBS Lett. 2003;541:126–131. doi: 10.1016/s0014-5793(03)00324-7. [DOI] [PubMed] [Google Scholar]
- Sajan MP, Jurzak MJ, Samuels VT, Shulman GI, Braun U, Leitges M, Farese RV. Impairment of insulin-stimulated glucose transport and ERK activation by adipocyte-specific knockout of PKC-lambda produces a phenotype characterized by diminished adiposity and enhanced insulin suppression of hepatic gluconeogenesis. Adipocyte. 2014;3:19–29. doi: 10.4161/adip.26305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson SR, Cooper DR. Specific protein kinase C isoforms as transducers and modulators of insulin signaling. Mol Genet Metab. 2006;89:32–47. doi: 10.1016/j.ymgme.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bautista S, Corbalan-Garcia S, Perez-Lara A, Gomez-Fernandez JC. A comparison of the membrane binding properties of C1B domains of PKCgamma, PKCdelta, and PKCepsilon. Biophys J. 2009;96:3638–3647. doi: 10.1016/j.bpj.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Saurin AT, Durgan J, Cameron AJ, Faisal A, Marber MS, Parker PJ. The regulated assembly of a PKCepsilon complex controls the completion of cytokinesis. Nat Cell Biol. 2008;10:891–901. doi: 10.1038/ncb1749. [DOI] [PubMed] [Google Scholar]
- Schechtman D, Craske ML, Kheifets V, Meyer T, Schechtman J, Mochly-Rosen D. A critical intramolecular interaction for protein kinase Cepsilon translocation. J Biol Chem. 2004;279:15831–15840. doi: 10.1074/jbc.M310696200. [DOI] [PubMed] [Google Scholar]
- Schmalz D, Kalkbrenner F, Hucho F, Buchner K. Transport of protein kinase C alpha into the nucleus requires intact cytoskeleton while the transport of a protein containing a canonical nuclear localization signal does not. J Cell Sci. 1996;109(Pt 9):2401–2406. doi: 10.1242/jcs.109.9.2401. [DOI] [PubMed] [Google Scholar]
- Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Shaifta Y, Snetkov VA, Prieto-Lloret J, Knock GA, Smirnov SV, Aaronson PI, Ward JP. Sphingosylphosphorylcholine potentiates vasoreactivity and voltage-gated Ca2+ entry via NOX1 and reactive oxygen species. Cardiovasc Res. 2015;106:121–130. doi: 10.1093/cvr/cvv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen GX. Selective protein kinase C inhibitors and their applications. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:301–307. doi: 10.2174/1568006033481375. [DOI] [PubMed] [Google Scholar]
- Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Ren J, Wang P, Rateri DL, Daugherty A, Shi XD, Kent KC, Liu B. Protein kinase C-delta mediates adventitial cell migration through regulation of monocyte chemoattractant protein-1 expression in a rat angioplasty model. Arterioscler Thromb Vasc Biol. 2012;32:943–954. doi: 10.1161/ATVBAHA.111.244921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikci O, Ozer NK, Azzi A. Dietary cholesterol-induced changes of protein kinase C and the effect of vitamin E in rabbit aortic smooth muscle cells. Atherosclerosis. 1996;126:253–263. doi: 10.1016/0021-9150(96)05909-6. [DOI] [PubMed] [Google Scholar]
- Slish DF, Welsh DG, Brayden JE. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2196–2201. doi: 10.1152/ajpheart.00605.2002. [DOI] [PubMed] [Google Scholar]
- Slosberg ED, Yao Y, Xing F, Ikui A, Jirousek MR, Weinstein IB. The protein kinase C beta-specific inhibitor LY379196 blocks TPA-induced monocytic differentiation of HL60 cells the protein kinase C beta-specific inhibitor LY379196 blocks TPA-induced monocytic differentiation of HL60 cells. Mol Carcinog. 2000;27:166–176. [PubMed] [Google Scholar]
- Snow JB, Gonzalez Bosc LV, Kanagy NL, Walker BR, Resta TC. Role for PKCbeta in enhanced endothelin-1-induced pulmonary vasoconstrictor reactivity following intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2011;301:L745–754. doi: 10.1152/ajplung.00020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JB, Kanagy NL, Walker BR, Resta TC. Rat strain differences in pulmonary artery smooth muscle Ca(2+) entry following chronic hypoxia. Microcirculation. 2009;16:603–614. doi: 10.1080/10739680903114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol (1985) 2008;104:110–118. doi: 10.1152/japplphysiol.00698.2005. [DOI] [PubMed] [Google Scholar]
- Sobhia ME, Grewal BK, Ml SP, Patel J, Kaur A, Haokip T, Kokkula A. Protein kinase C inhibitors: a patent review (2008 – 2009) Expert Opin Ther Pat. 2013;23:1297–1315. doi: 10.1517/13543776.2013.805205. [DOI] [PubMed] [Google Scholar]
- Song HF, Tang ZM, Yuan SJ, Zhu BZ, Liu XW. Antisense candidates against protein kinase C-alpha designed based on phylogenesis and simulant structure of mRNA. Acta Pharmacol Sin. 2003;24:269–276. [PubMed] [Google Scholar]
- Sontag E, Sontag JM, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ Res. 1994;75:586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem. 2001;276:29644–29650. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF. Mechanisms for redox-regulation of protein kinase C. Front Pharmacol. 2015;6:128. doi: 10.3389/fphar.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SV, Girouard H, Doetsch PE, Hannah RM, Wilkerson MK, Nelson MT. Regulation of intracerebral arteriolar tone by K(v) channels: effects of glucose and PKC. Am J Physiol Cell Physiol. 2009;297:C788–796. doi: 10.1152/ajpcell.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci U S A. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Wong SM, Peters MC, Mochly-Rosen D, Yeomans DC, Kendig JJ. Protein kinase C epsilon and gamma: involvement in formalin-induced nociception in neonatal rats. J Pharmacol Exp Ther. 2004;309:616–625. doi: 10.1124/jpet.103.060350. [DOI] [PubMed] [Google Scholar]
- Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA, Reyland ME. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res. 2011;71:2087–2097. doi: 10.1158/0008-5472.CAN-10-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Kaneko K, Kubo T. Protein kinase C modulates Ca2+-activated K+ channels in cultured rat mesenteric artery smooth muscle cells. Biol Pharm Bull. 2000;23:1450–1454. doi: 10.1248/bpb.23.1450. [DOI] [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979;91:1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tamaoki T. Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991;201:340–347. doi: 10.1016/0076-6879(91)01030-6. [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Terry RD, Mokhtari GK, Inagaki K, Koyanagi T, Kofidis T, Mochly-Rosen D, Robbins RC. Suppression of graft coronary artery disease by a brief treatment with a selective epsilonPKC activator and a deltaPKC inhibitor in murine cardiac allografts. Circulation. 2004;110:II194–199. doi: 10.1161/01.CIR.0000138389.22905.62. [DOI] [PubMed] [Google Scholar]
- Teng B, Duong M, Tossidou I, Yu X, Schiffer M. Role of protein kinase C in podocytes and development of glomerular damage in diabetic nephropathy. Front Endocrinol (Lausanne) 2014;5:179. doi: 10.3389/fendo.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Thomas AC, Newby AC. Effect of matrix metalloproteinase-9 knockout on vein graft remodelling in mice. J Vasc Res. 2010;47:299–308. doi: 10.1159/000265564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Maruyama Y, Malcolm AT, Light PE, Walsh MP, Cole WC. Protein kinase C modulation of recombinant ATP-sensitive K(+) channels composed of Kir6.1 and/or Kir6.2 expressed with SUR2B. J Physiol. 2002;541:65–80. doi: 10.1113/jphysiol.2002.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A, Ellis CA, Sellers LA, Aitken A. Protein kinase C inhibitor proteins. Purification from sheep brain and sequence similarity to lipocortins and 14-3-3 protein. Eur J Biochem. 1990;191:421–429. doi: 10.1111/j.1432-1033.1990.tb19138.x. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tumey LN, Bhagirath N, Brennan A, Brooijmans N, Lee J, Yang X, Boschelli DH. 5-Vinyl-3-pyridinecarbonitrile inhibitors of PKCtheta: optimization of enzymatic and functional activity. Bioorg Med Chem. 2009;17:7933–7948. doi: 10.1016/j.bmc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28:2686–2690. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Chen MM, Joly AH, Chakrapani SD, Tsubaki J, Kim HS, Oh Y, Rosenfeld RG. Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology. 2001;142:1760–1769. doi: 10.1210/endo.142.5.8141. [DOI] [PubMed] [Google Scholar]
- Tykocki NR, Wu B, Jackson WF, Watts SW. Divergent signaling mechanisms for venous versus arterial contraction as revealed by endothelin-1. J Vasc Surg. 2014;62:721–733. doi: 10.1016/j.jvs.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valovka T, Verdier F, Cramer R, Zhyvoloup A, Fenton T, Rebholz H, Wang ML, Gzhegotsky M, Lutsyk A, Matsuka G, Filonenko V, Wang L, Proud CG, Parker PJ, Gout IT. Protein kinase C phosphorylates ribosomal protein S6 kinase betaII and regulates its subcellular localization. Mol Cell Biol. 2003;23:852–863. doi: 10.1128/MCB.23.3.852-863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ., 3rd Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005;27:1164–1180. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Bashore TR, Friedman E. Exercise reduces age-dependent decrease in platelet protein kinase C activity and translocation. J Gerontol A Biol Sci Med Sci. 1995;50A:M12–16. doi: 10.1093/gerona/50a.1.m12. [DOI] [PubMed] [Google Scholar]
- Wang HY, Bashore TR, Tran ZV, Friedman E. Age-related decreases in lymphocyte protein kinase C activity and translocation are reduced by aerobic fitness. J Gerontol A Biol Sci Med Sci. 2000;55:B545–551. doi: 10.1093/gerona/55.11.b545. [DOI] [PubMed] [Google Scholar]
- Wang HY, Pisano MR, Friedman E. Attenuated protein kinase C activity and translocation in Alzheimer’s disease brain. Neurobiol Aging. 1994;15:293–298. doi: 10.1016/0197-4580(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou H, Wu B, Zhou Q, Cui D, Wang L. Protein Kinase C Isoforms Distinctly Regulate Propofol-induced Endothelium-dependent and Endothelium-independent Vasodilation. J Cardiovasc Pharmacol. 2015;66:276–284. doi: 10.1097/FJC.0000000000000275. [DOI] [PubMed] [Google Scholar]
- Ward NE, Pierce DS, Chung SE, Gravitt KR, O’Brian CA. Irreversible inactivation of protein kinase C by glutathione. J Biol Chem. 1998;273:12558–12566. doi: 10.1074/jbc.273.20.12558. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hachiya T, Hagiwara M, Hidaka H. Identification of type III protein kinase C in bovine aortic tissue. Arch Biochem Biophys. 1989;273:165–169. doi: 10.1016/0003-9861(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Weidemann W, Hanke H. Cardiovascular effects of androgens. Cardiovasc Drug Rev. 2002;20:175–198. doi: 10.1111/j.1527-3466.2002.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WO, Seibenhener ML, Wooten MW. Phosphorylation of tyrosine 256 facilitates nuclear import of atypical protein kinase C. J Cell Biochem. 2002;85:42–53. [PubMed] [Google Scholar]
- Whitson EL, Bugni TS, Chockalingam PS, Concepcion GP, Feng X, Jin G, Harper MK, Mangalindan GC, McDonald LA, Ireland CM. Fibrosterol sulfates from the Philippine sponge Lissodendoryx (Acanthodoryx) fibrosa: sterol dimers that inhibit PKCzeta. J Org Chem. 2009;74:5902–5908. doi: 10.1021/jo900844r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Gallacher B, Patel H, Orme C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes. 1997;46:1497–1503. doi: 10.2337/diab.46.9.1497. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Smith RD. Mechanisms of action of pH-induced effects on vascular smooth muscle. Mol Cell Biochem. 2004;263:163–172. doi: 10.1023/B:MCBI.0000041858.78005.d2. [DOI] [PubMed] [Google Scholar]
- Xi G, Shen X, Maile LA, Wai C, Gollahon K, Clemmons DR. Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCzeta-dependent manner in vascular smooth muscle cells. Diabetes. 2012;61:104–113. doi: 10.2337/db11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol. 2006;290:H2337–2343. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- Xiao D, Zhu R, Zhang L. Gestational hypoxia up-regulates protein kinase C and inhibits calcium-activated potassium channels in ovine uterine arteries. Int J Med Sci. 2014;11:886–892. doi: 10.7150/ijms.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zhao H, Wang S, Sun X, Qin X. Increased ADRP expression in human atherosclerotic lesions correlates with plaque instability. Int J Clin Exp Med. 2015;8:5414–5421. [PMC free article] [PubMed] [Google Scholar]
- Xu TR, Rumsby MG. Phorbol ester-induced translocation of PKC epsilon to the nucleus in fibroblasts: identification of nuclear PKC epsilon-associating proteins. FEBS Lett. 2004;570:20–24. doi: 10.1016/j.febslet.2004.05.080. [DOI] [PubMed] [Google Scholar]
- Xu ZB, Chaudhary D, Olland S, Wolfrom S, Czerwinski R, Malakian K, Lin L, Stahl ML, Joseph-McCarthy D, Benander C, Fitz L, Greco R, Somers WS, Mosyak L. Catalytic domain crystal structure of protein kinase C-theta (PKCtheta) J Biol Chem. 2004;279:50401–50409. doi: 10.1074/jbc.M409216200. [DOI] [PubMed] [Google Scholar]
- Yamada S, Kimura R, Harada Y, Nakayama K. Calcium channel receptor sites for (+)-[3H]PN 200–110 in coronary artery. J Pharmacol Exp Ther. 1990;252:327–332. [PubMed] [Google Scholar]
- Yamaguchi H, Igarashi M, Hirata A, Sugae N, Tsuchiya H, Jimbu Y, Tominaga M, Kato T. Altered PDGF-BB-induced p38 MAP kinase activation in diabetic vascular smooth muscle cells: roles of protein kinase C-delta. Arterioscler Thromb Vasc Biol. 2004;24:2095–2101. doi: 10.1161/01.ATV.0000144009.35400.65. [DOI] [PubMed] [Google Scholar]
- Yan SF, Harja E, Andrassy M, Fujita T, Schmidt AM. Protein kinase C beta/early growth response-1 pathway: a key player in ischemia, atherosclerosis, and restenosis. J Am Coll Cardiol. 2006;48:A47–55. doi: 10.1016/j.jacc.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Yang X, Teguh D, Wu JP, He B, Kirk TB, Qin S, Li S, Chen H, Xue W, Ng B, Chim SM, Tickner J, Xu J. Protein kinase C delta null mice exhibit structural alterations in articular surface, intra-articular and subchondral compartments. Arthritis Res Ther. 2015;17:210. doi: 10.1186/s13075-015-0720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Igumenova TI. The C-terminal V5 domain of Protein Kinase Calpha is intrinsically disordered, with propensity to associate with a membrane mimetic. PLoS One. 2013;8:e65699. doi: 10.1371/journal.pone.0065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Wang XD, Huang K, Wang L, Jiang ZL, Qi YX. Temporal phosphoproteomics to investigate the mechanotransduction of vascular smooth muscle cells in response to cyclic stretch. J Biomech. 2014;47:3622–3629. doi: 10.1016/j.jbiomech.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Yevseyenkov VV, Das S, Lin D, Willard L, Davidson H, Sitaramayya A, Giblin FJ, Dang L, Takemoto DJ. Loss of protein kinase Cgamma in knockout mice and increased retinal sensitivity to hyperbaric oxygen. Arch Ophthalmol. 2009;127:500–506. doi: 10.1001/archophthalmol.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes. 2003;52:838–845. doi: 10.2337/diabetes.52.3.838. [DOI] [PubMed] [Google Scholar]
- Yonezawa T, Kurata R, Kimura M, Inoko H. PKC delta and epsilon in drug targeting and therapeutics. Recent Pat DNA Gene Seq. 2009;3:96–101. doi: 10.2174/187221509788654205. [DOI] [PubMed] [Google Scholar]
- Young LH, Balin BJ, Weis MT. Go 6983: a fast acting protein kinase C inhibitor that attenuates myocardial ischemia/reperfusion injury. Cardiovasc Drug Rev. 2005;23:255–272. doi: 10.1111/j.1527-3466.2005.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA, Manji HK. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9:561–570. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Lofgren B, Pahlman S, Larsson C. PKCepsilon, via its regulatory domain and independently of its catalytic domain, induces neurite-like processes in neuroblastoma cells. J Cell Biol. 1999;145:713–726. doi: 10.1083/jcb.145.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Kazanietz MG, Blumberg PM, Hurley JH. Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hermanson ME, Eddinger TJ. Tonic and phasic smooth muscle contraction is not regulated by the PKCalpha – CPI-17 pathway in swine stomach antrum and fundus. PLoS One. 2013;8:e74608. doi: 10.1371/journal.pone.0074608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Xia L, Chen GQ. Protein kinase cdelta in apoptosis: a brief overview. Arch Immunol Ther Exp (Warsz) 2012;60:361–372. doi: 10.1007/s00005-012-0188-8. [DOI] [PubMed] [Google Scholar]
- Zhu R, Xiao D, Zhang L. Potassium channels and uterine vascular adaptation to pregnancy and chronic hypoxia. Curr Vasc Pharmacol. 2013;11:737–747. doi: 10.2174/1570161111311050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, White RE, Barman SA. Role of phosphodiesterases in modulation of BKCa channels in hypertensive pulmonary arterial smooth muscle. Ther Adv Respir Dis. 2008;2:119–127. doi: 10.1177/1753465808091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler WH, Parekh DB, Le Good JA, Whelan RD, Kelly JJ, Frech M, Hemmings BA, Parker PJ. Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr Biol. 1999;9:522–529. doi: 10.1016/s0960-9822(99)80236-x. [DOI] [PubMed] [Google Scholar]


