Abstract
Background
The lifetime risk of heart failure is higher in the African-American population than in other racial groups in the United States.
Methods and Results
We measured the Life’s Simple 7 ideal cardiovascular health metrics in 4195 African-Americans in the Jackson Heart Study (2000–2004). We evaluated the association of Simple 7 metrics with incident HF and left ventricular (LV) structure and function by cardiac magnetic resonance (CMR; n=1188). Mean age at baseline was 54.4 years (65% women). Relative to 0–2 Simple 7 factors, African-Americans with 3 factors had 47% lower incident HF risk (HR 0.53, 95% CI 0.39–0.73, P<0.0001); those with ≥ 4 factors had 61% lower HF risk (HR 0.39, 95% CI 0.24–0.64, P=0.0002). Higher blood pressure (HR 2.32, 95% CI 1.28–4.20, P=0.005), physical inactivity (HR 1.65, 95% CI 1.07–2.55, P=0.02), smoking (HR 2.04, 95% CI 1.43–2.91, P<0.0001) and impaired glucose control (HR 1.76, 95% CI 1.34–2.29, P<0.0001) were associated with incident HF. The age-/sex-adjusted population attributable risk for these Simple 7 metrics combined was 37.1%. Achievement of ideal blood pressure, ideal body mass index, ideal glucose control, and non-smoking was associated with less likelihood of adverse cardiac remodeling by CMR.
Conclusions
Cardiovascular risk factors in mid-life (specifically elevated blood pressure, physical inactivity, smoking and poor glucose control) are associated with incident HF in African Americans, and represent targets for intensified HF prevention.
Keywords: Heart failure, African-Americans, Life’s Simple 7, magnetic resonance imaging
Journal Subject Terms: Epidemiology, Obesity, Computerized Tomography (CT), Magnetic Resonance Imaging (MRI)
African-Americans have a disproportionately higher risk1 and earlier onset2 of heart failure (HF) and cardiovascular mortality, relative to the overall American population. A prime contributor to this racial disparity is a higher prevalence and earlier onset of cardiometabolic disease, including obesity, hypertension, diabetes, and physical inactivity3. Though professional guidelines reinforcing ideal cardiovascular health target improvements in cardiometabolic health to prevent disease (“Life’s Simple 7”), recent data in over 5,000 African-Americans from the Jackson Heart Study (JHS) indicate a low prevalence of ideal cardiovascular health characteristics (specifically diet, body mass index, and physical activity) with only modest improvements over time4. In largely non-African American populations, achievement of fewer Life’s Simple 7 components is associated with more adverse left ventricular (LV) remodeling5 and greater cardiovascular and non-cardiovascular disease (CVD) mortality6, 7. Nevertheless, most prior work examining Simple 7 metrics has not focused on African-Americans to define axes of cardiometabolic health that most impact cardiovascular disease progression.
To understand the impact of cardiometabolic health on HF in African-Americans, we studied the relationship of Life’s Simple 7 metrics with incident HF and myocardial structure and function (by cardiac magnetic resonance, CMR) in the Jackson Heart Study. Given prior results in JHS documenting a low prevalence of physical activity, greater body mass index (BMI), and poor diet4, we hypothesized that achievement of fewer ideal components of Life’s Simple 7 would be associated with greater risk of incident HF and more adverse cardiovascular remodeling by CMR.
Methods
Study population
The Jackson Heart Study is a population-based prospective study of African-Americans 21 years or older from the Jackson, Mississippi, tricounty area (Hinds, Madison, and Rankin). The study was designed to identify causes of CVD among African-Americans8, 9. Study subjects were examined at a baseline clinic visit (2000–2004) and during two additional visits: Visit 2 (2005–2008), Visit 3 (2009–2013). Follow-up telephone interviews were performed annually. The study was Institutional Review Board approved. All participants provided written informed consent.
There were 5306 study participants who attended the initial study visit. Our first aim was to examine the association between achievement of Life’s Simple 7 metrics and incident HF. For this aim, we sequentially excluded participants with (1) incomplete data on Life’s Simple 7 components (n=824 participants) and (2) prevalent coronary heart disease [CHD: self-reported history of myocardial infarction (MI) or MI by 12-lead electrocardiogram (ECG) using the Minnesota Code Classification system8] at the baseline clinic visit (n=287 of remaining participants), leaving 4195 participants in our final analytic sample for this first aim.
Our second aim was to assess the relationship of Simple 7 attainment with cardiovascular remodeling by CMR. Of the 4195 individuals included in our first aim, 3112 attended Visit 3, of whom 1188 participants had a CMR with complete circumferential systolic strain data. Of note, participants were excluded from CMR imaging for pregnancy, metallic hazards (e.g., implanted electrical devices, pacemaker, orbital metal), inability to fit in the scanner, claustrophobia or refusal to undergo CMR. In addition, we did not include individuals who had CMR at Visit 2, as we wanted to ensure that the CMR outcome was measured at the same (and most contemporary) time-point across all individuals.
Covariate and exposure definition
We constructed the Simple 7 score for each JHS participant by assigning a score of “1” (ideal status) or “0” (non-ideal) for each of the Simple 7 metrics (Supplemental Table 1) as previously published4, 10. For consistency with prior reports4, we used metrics already adjudicated within the Jackson Heart Study (as opposed to ideal, intermediate, and poor categories as defined in other work4). Smoking was assessed at baseline by questionnaire (current, never or former smoker). Former smokers were further subdivided into those who quit smoking <12 months or >12 months prior to the interview. Family income was adjusted for family size and calendar-year-specific poverty level and stratified into two groups: (1) a composite poor and lower-middle income group and (2) an upper-middle and affluent group. Upper-middle income was defined as at least 1.6 times poverty level.11 Weight was measured (to the nearest kilogram) using a balance scale. Height was measured in a standing position (to the nearest centimeter) with a vertical ruler. Dietary intake was assessed with a regionally specific food frequency questionnaire designed for the study population.12 Physical activity was obtained from a modified Kaiser physical activity survey, and the time per week engaged in moderate or vigorous sports and exercise activity was used to derive the physical activity ideal health score.4 Sitting blood pressure was calculated as the average of two resting blood pressure recordings. Prevalent diabetes was defined according to the American Diabetes Association (ADA) criteria as fasting glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5% or use of medications for diabetes13. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications.
Venous blood samples were drawn from each subject at JHS baseline examination as previously described14. Lipids, fasting plasma glucose, hemoglobin A1c, insulin, high-sensitivity C-reactive protein (hs-CRP) were measured using standard laboratory techniques. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated in molar units as [fasting blood glucose (milligram per deciliter)/18.1 × insulin (microunits per milliliter)/22.5]15. Estimated glomerular filtration rate (in ml/min/1.73 m2) was calculated based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula16. Assays for aldosterone17, renin18, leptin17, and adiponectin19 have been previously described.
Primary outcome ascertainment
The construction of the cohort for outcomes ascertainment is shown in Figure 1. HF adjudication has been previously described20. In brief, incident HF hospitalizations were identified through annual follow-up telephone interviews and compared with annual hospital discharge lists and death certificates by trained and certified HF abstractors. The annual hospital discharge lists were reviewed for (1) International Classification of Diseases, 9th and 10th Revision HF diagnosis codes (ICD-9 code 428 or ICD-10 code I50); and (2) radiographic findings consistent with HF, increased jugular venous pressure, dilated ventricle, LV ejection fraction < 0.40 by echocardiogram or nuclear scan; or (3) autopsy finding of pulmonary edema or HF. Death certificates were reviewed for causes of death that were suggestive of HF. Trained medical personnel performed final event adjudication.
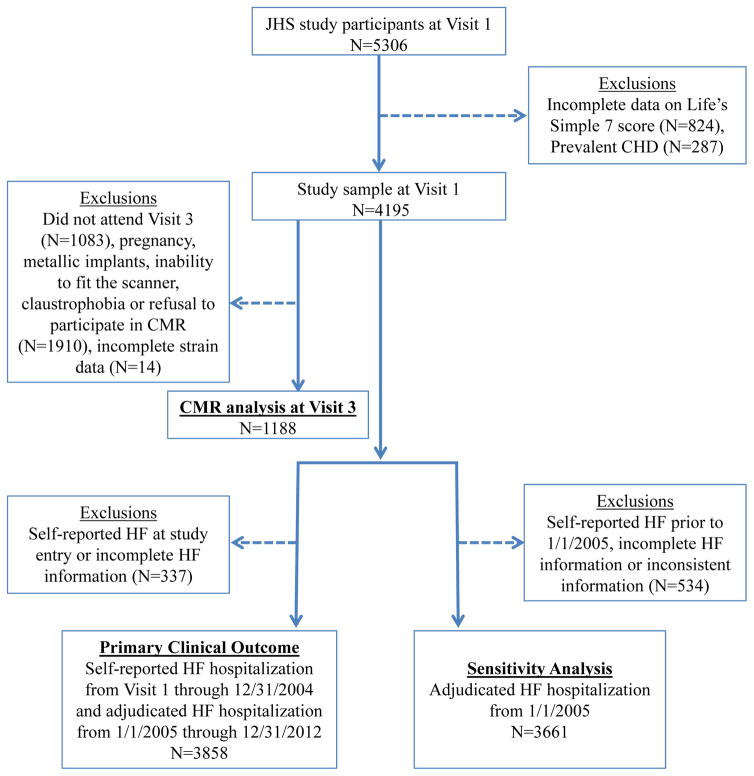
Figure 1. Consort diagram.
Out of 4195 participants without prevalent CHD with Life’s Simple 7 metrics measured at baseline, 1188 were included in our CMR analyses, and 3858 were followed for incident HF. “Incomplete HF information” refers to missing self-reported HF hospitalization or self-reported physician diagnosis of heart failure. CHD, Coronary Heart Disease; CMR, Cardiac Magnetic Resonance; HF, Heart Failure; JHS, Jackson Heart Study.
There was a gap in the period between the baseline visit (9/26/2000) and the start of formal HF adjudication (in 1/1/2005). Events were formally adjudicated by the Jackson Heart Study from 1/1/2005 to 12/31/2012. We have therefore provided analysis for (1) all incident HF from the time of enrollment, either self-reported or by formal adjudication (after 1/1/2005) and (2) formally adjudicated HF only (from 1/1/2005 to endpoint or censor).
Cardiac magnetic resonance imaging
CMR scans included in this study were performed at Visit 3 using a 1.5-T system (Siemens Espree, Siemens, Erlangen Germany, 70 cm bore, advanced cardiac package, TIM Matrix surface coil). The CMR protocol was based and developed in collaboration with the Multi-Ethnic Study of Atherosclerosis CMR protocol to enhance comparability21. Assessment of ventricular function and mass was performed using electrocardiographically-gated fast-gradient echo cine images with steady state free precession (TrueFISP or TRUFI, Siemens sequence variant Tfi2d1_18) with parameters: repetition time TR 45.5 ms, echo time 1.1 ms, flip angle 78–82°, 8-mm slice thickness, matrix 109 × 192 and field of view (FOV) 400 mm. LV volume and mass were determined by short-axis volumetric coverage and LV mass was indexed to height in meters raised to the 2.7 power22. Papillary muscles were included in the LV volumes and excluded from LV mass23. CIM software (University of Auckland, New Zealand) was used to analyze CMR function and morphology data24. CMR tagged images were acquired at the base, mid, and apex of the left ventricle using a cine radiofrequency grid tagging sequence [FOV 400 mm, slice thickness 8 mm, 192 × 256 matrix, TR 60 msec, TE 4 sec, FA of 12° (Siemens Sequence: Tl2d1r5)]. Tagging analysis was performed using HARP (Diagnosoft, Morrisville, NC). Global strain was calculated as the average peak circumferential systolic Eularian strain (Ecc) of the basal, mid-cavity and apical segments of the left ventricle. Aortic pulse wave velocity (PWV) was calculated from phase contrast images acquired in the ascending and descending thoracic aorta, as previously described25. ICC for interobserver reliability based on 96 scans that were analyzed as new scans after relabeling (to blind analysts) was 0.95, 0.88, 0.85, and 0.96 for LV end-diastolic volume, LV end-systolic volume, LV stroke volume, and LV mass, respectively. The ICC for HARP Ecc measures was 0.78 (and for PWV analyses was 0.82) in repeated, blinded analyses of 96 scans.
Statistical analysis
Baseline clinical, demographic, biochemical and imaging characteristics were stratified by categories of Life’s Simple 7 score (0–2, 3, 4–6). We chose these categories to facilitate an approximately equal number of participants in each bin (0–2: N=1936; 3: N=1354; 4–6: N=905). The Kruskal-Wallis test (non-normal continuous data) or chi-square test (categorical) was used for comparisons.
We assessed the relationship between Life’s Simple 7 and incident HF using Cox proportional hazards models and Kaplan Meier survival analysis. For Kaplan-Meier analysis, we stratified Simple 7 score into categories (0–2, 3 and 4–6 ideal factors). To identify which components of Simple 7 were most closely associated with HF, we constructed univariate and multivariate Cox proportional hazards regression models including each individual Simple 7 component, age and sex. To limit confounding by coronary heart disease, we performed additional analyses excluding fatal CHD and incident MI throughout the study period. Fatal CHD and MI were adjudicated by trained personnel on review of medical records20. The proportional hazards assumption was verified by the Kolmogorov-type supremum test.
Finally, we used a pooled logistic regression (splitting follow-up time into 5 year bins for each participant) to estimate an adjusted population attributable risk (PAR) for each of the 4 Simple 7 factors that were significantly associated with incident HF in multivariable Cox regression as specified26. The Simple 7 factors were left constant through the follow-up period (each 5-year bin). To calculate the adjusted (partial) PAR for each of the 4 Simple 7 metrics, we treated age, sex, and the three other Simple 7 variables as non-modifiable. The SAS macro %PAR26 was used for this analysis.
To measure the association of Life’s Simple 7 with cardiac remodeling by CMR, we constructed separate univariate and age- and sex-adjusted linear models for cardiac structure/function by CMR (measured a median 8.1 years after baseline study visit) as a function of each Simple 7 component. CMR indices included LV mass index, LV mass-to-volume ratio (“LV concentric remodeling index”), aortic PWV, and global circumferential LV strain. We performed a Bonferroni correction for multiplicity (to account for multiple models).
We evaluated effect modification by age (median-stratified), sex, and obesity status (defined as obese BMI ≥30 kg/m2 vs. non-obese BMI < 30 kg/m2) on the association between the Simple 7 score (modeled using categories of 0–2, 3 and 4–6 factors) and CMR parameters using a multiplicative interaction term. The least-squares means in each category of age, sex, and obesity status for each cardiac parameter was graphed to facilitate visual interpretation. Of note, we performed log transformations of LV mass index, LV mass-to-volume ratio and aortic PWV to approximate normality prior to regression; least-squares means were exponentiated back after regression to provide clinically meaningful LV mass, concentricity and aortic PWV values.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A two-tailed P value of 0.05 was considered significant.
Results
Baseline characteristics
Demographic, clinical, biochemical and imaging characteristics of our study population stratified by Simple 7 score are presented in Table 1. Mean age in the overall study population was 54.4±12.8 years, with 65% female. Median BMI was at least overweight across all Simple 7 score categories, with low prevalence of ideal nutrition and ideal physical activity, as previously reported.4 Overall, JHS participants with lower Simple 7 scores were more often female and had lower education and income. JHS participants with lower Simple 7 scores had a more adverse cardiometabolic profile, with greater pro-atherogenic dyslipidemia, insulin resistance (HOMA-IR), systemic inflammation (hs-CRP), a more dysfunctional adiposity phenotype (higher leptin, lower adiponectin), and greater renin-angiotensin-aldosterone system activation (all P<0.0001). Of note, individuals who had CMR imaging performed in JHS were in general younger, with a higher Simple 7 score (Supplemental Table 2).
Table 1.
Baseline characteristics for all JHS participants meeting inclusion criteria, stratified by Simple 7 score category.
| Simple 7 score 0–2 | Simple 7 score 3 | Simple 7 score 4–6 | p value | |
|---|---|---|---|---|
| n ≤ 1936* | n ≤ 1354* | n ≤ 905* | ||
| Demographics | ||||
| Age, y | 59.2 (50.1 – 66.3) | 53.0 (43.9 – 63.7) | 45.5 (38.8 – 54.8) | <0.0001 |
| Female | 1288 (66.5) | 874 (64.6) | 554 (61.2) | 0.02 |
| High-school graduate or GED and beyond | 1087 (81.2) | 891 (86.8) | 654 (93.2) | <0.0001 |
| Upper middle income or greater | 956 (57.7) | 719 (63.1) | 541 (69.1) | <0.0001 |
| Ever smoker | 725 (37.5) | 359 (26.5) | 164 (18.1) | <0.0001 |
| Weight, kg | 90.7 (80.0 – 104.0) | 87.4 (75.0 – 102.0) | 79.8 (67.5 – 92.0) | <0.0001 |
| BMI, kg/m2 | 31.9 (28.5 – 36.5) | 30.1 (26.6 – 35.3) | 27.3 (23.9 – 31.9) | <0.0001 |
| Systolic BP, mmHg | 129.3 (122.0 – 140.3) | 124.8 (116.5 – 134.8) | 114.7 (107.3 – 122.0) | <0.0001 |
| Estimated GFR, mL/min | 92.9 (79.1 – 106.6) | 96.7 (83.2 – 110.4) | 103.2 (90.5 – 116.2) | <0.0001 |
| Prevalent disease | ||||
| Hypertension | 1369 (70.7) | 691 (51.0) | 181 (20.0) | <0.0001 |
| Diabetes | 605 (31.3) | 108 (8.0) | 25 (2.8) | <0.0001 |
| Simple 7 component | ||||
| Non - Smoking | 1545 (79.8) | 1222 (90.3) | 861 (95.1) | <0.0001 |
| Ideal BMI | 64 (3.3) | 207 (15.3) | 340 (37.6) | <0.0001 |
| Ideal Nutrition | 3 (0.2) | 11 (0.8) | 16 (1.8) | <0.0001 |
| Ideal Physical Activity | 100 (5.2) | 288 (21.3) | 444 (49.1) | <0.0001 |
| Ideal BP | 53 (2.7) | 253 (18.7) | 585 (64.6) | <0.0001 |
| Ideal Fasting Plasma Glucose | 1112 (57.4) | 1275 (94.2) | 896 (99.0) | <0.0001 |
| Ideal Cholesterol | 358 (18.5) | 806 (59.5) | 760 (84.0) | <0.0001 |
| CMR parameters (n ≤ 1188)† | ||||
| LV mass index, g/m2.7 | 32.0 (28.2 – 36.9) | 31.1 (27.0 – 35.1) | 29.0 (25.3 – 32.5) | <0.0001 |
| LV concentric remodeling index, g/mL | 1.1 (1.0 – 1.3) | 1.1 (0.9 – 1.3) | 0.9 (0.8 – 1.1) | <0.0001 |
| Height-indexed LVEDV, mL/m | 68.9 (59.1 – 82.6) | 71.7 (59.4 – 85.0) | 75.1 (62.7 – 86.2) | 0.02 |
| Stroke volume, mL | 69.1 (56.9 – 86.2) | 72.9 (61.8 – 88.1) | 74.2 (63.1 – 88.6) | 0.03 |
| LV ejection fraction, % | 61.8 (54.7 – 66.8) | 61.4 (55.6 – 66.6) | 59.8 (54.4 – 65.1) | 0.16 |
| Aortic PWV, m/s | 6.2 (5.0 – 8.8) | 5.9 (4.3 – 8.2) | 5.3 (4.0 – 7.4) | <0.0001 |
| Global circumferential strain, % | −15.4 (−16.9 to −13.6) | −15.8 (−17.6 to −14.3) | −16.4 (−17.9 to −14.7) | <0.0001 |
| Biomarkers‡ | ||||
| LDL, mg/dL | 140.5 (115.0 – 162.0) | 120.0 (99.0 – 141.0) | 110.0 (92.0 – 128.0) | <0.0001 |
| HDL, mg/dL | 49.0 (41.0 – 60.0) | 51.0 (41.0 – 60.0) | 50.0 (42.0 – 59.0) | 0.66 |
| Triglycerides, mg/dL | 103.0 (76.0 – 145.5) | 84.0 (62.0 – 117.0) | 69.0 (50.0 – 97.0) | <0.0001 |
| hs–CRP, mg/dL | 0.7 (0.7 – 0.8) | 0.7 (0.6 – 0.8) | 0.7 (0.6 – 0.7) | <0.0001 |
| Hemoglobin A1c, % | 5.9 (5.5 – 6.5) | 5.5 (5.2 – 5.8) | 5.4 (5.1 – 5.6) | <0.0001 |
| HOMA-IR | 3.5 (2.5 –5.0) | 3.0 (2.1 – 4.2) | 2.5 (1.8 – 3.4) | <0.0001 |
| Plasma renin Activity, ng/mL/hr | 0.5 (0.2 – 1.4) | 0.4 (0.2 – 0.9) | 0.3 (0.2 – 0.8) | <0.0001 |
| Aldosterone, ng/dL | 4.7 (2.9 – 8.1) | 4.2 (2.5 – 6.8) | 3.7 (2.2 – 5.8) | <0.0001 |
| Adiponectin, ng/mL | 4041.5 (2557.7 – 6277.4) | 4443.6 (2842.8 – 6895.2) | 4434.0 (2771.4 – 7032.1) | <0.0001 |
| Leptin, ng/mL | 27.0 (13.5 – 43.0) | 23.5 (9.7 – 39.4) | 15.6 (6.2 – 30.1) | <0.0001 |
Values are median (25th–75th percentile) or No. (%).
Numbers are for Simple 7 score category out of a total 4195 participants in our sample population.
The greatest number of missing participants with CMR was in aortic PWV with 93/1188 missing.
The greatest number of missing participants was in plasma renin activity with 2321/4195 missing.
Abbreviations: BMI = Body mass index; BP = blood pressure; CMR = Cardiac magnetic resonance; GED = graduate equivalency diploma; GFR = Glomerular filtration rate; HDL = high-density lipoprotein; HOMA-IR = Homeostatic Model Assessment of insulin resistance; hs-CRP= high-sensitivity C-reactive protein; JHS = Jackson Heart Study; LDL = low-density lipoprotein; LV = Left ventricle; LVEDV= Left ventricular end-diastolic volume; PWV= pulse wave velocity.
Life’s Simple 7 is associated with incident HF
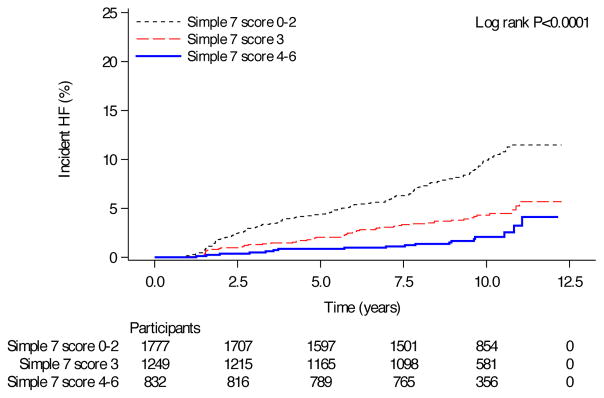
Over a median 9.9-year follow-up from study enrollment (25th–75th percentile 9.0–10.7 years), 239 incident HF events occurred in 3858 participants. After adjustment for age and sex, achievement of more components of ideal health was associated with a reduced hazard of incident HF (HR 0.53, 95% CI 0.39–0.73, P<0.0001 for Simple 7 score of 3 and HR 0.39, 95% CI 0.24–0.64, P=0.0002 for Simple 7 score of ≥4 relative to a Simple 7 score 0–2; Table 2, Figure 2). These associations remained after incident MI or fatal CHD were excluded (Table 2). Finally, when only adjudicated HF hospitalizations beginning 1/1/2005 were included (N=3661 total subjects; see Figure 1 for details), we found a similar association between Life’s Simple 7 and incident HF (Supplemental Table 3).
Table 2.
Association of Life’s Simple 7 with incident HF.
| Model 1 HR | 95% CI | p value | Model 2 HR | 95% CI | p value | |||
|---|---|---|---|---|---|---|---|---|
| Simple 7 score 0–2 | referent | referent | referent | referent | referent | referent | ||
| Simple 7 score 3 | 0.53 | (0.39, | 0.73) | <0.0001 | 0.52 | (0.36, | 0.74) | 0.0003 |
| Simple 7 score 4–6 | 0.39 | (0.24, | 0.64) | 0.0002 | 0.42 | (0.24, | 0.72) | 0.002 |
| Number of participants | 3858 | 3749 | ||||||
| Number of events | 239 | 185 | ||||||
Model 1 is adjusted for age and sex. Model 2 excludes adjudicated incident MI and fatal CHD at any time in JHS and is adjusted for age and sex. Incident HF was defined as self-reported HF hospitalizations (before 1/1/2005) and formally adjudicated HF (after 1/1/2005). Abbreviations: CI = confidence interval; HR = hazard ratio; HF = heart failure.
Figure 2. Survival curves for incident HF by Simple 7 score category.
Kaplan-Meier survival curves for HF-free survival stratified by Simple 7 score 0–2, 3 and ≥ 4. In this analysis, incident HF was defined as either self-reported or formally adjudicated incident HF. 239 incident HF events occurred in 3858 participants.
When evaluating all Simple 7 components simultaneously in an age- and sex-adjusted Cox model, elevated blood pressure (HR 2.32, 95% CI 1.28–4.20, P=0.005), physical inactivity (HR 1.65, 95% CI 1.07–2.55, P=0.02), smoking (HR 2.04, 95% CI 1.43–2.91, P<0.0001) and impaired glucose control (HR 1.76, 95% CI 1.34–2.29, P<0.0001) were associated with incident HF (Table 3). After adjustment for age and sex, the population attributable fraction for elevated blood pressure, physical inactivity, smoking and impaired glucose control was 37.1% (95% CI 20.0 to 51.9%; Table 3, with the greatest PAR% for elevated blood pressure (16.0%, 95% CI 5.4 to 26.3).
Table 3.
Simple 7 metrics associated with incident HF.
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | Adjusted PAR % | |||
| Age | 1.08 | (1.06, | 1.09) | <0.0001 | 1.07 | (1.06, | 1.09) | <0.0001 | |
| Female Sex | 1.14 | (0.86, | 1.49) | 0.36 | 1.08 | (0.81, | 1.43) | 0.61 | |
| Smoking | 1.43 | (1.03, | 2.01) | 0.04 | 2.04 | (1.43, | 2.91) | <0.0001 | 12.5 (6.7, 18.2) |
| Non-ideal BMI | 1.07 | (0.74, | 1.55) | 0.72 | 1.11 | (0.75, | 1.64) | 0.62 | |
| Non-ideal Nutrition | 1.67 | (0.24, | 11.93) | 0.61 | 1.82 | (0.25, | 13.00) | 0.55 | |
| Non-ideal Physical Activity | 2.37 | (1.55, | 3.65) | <0.0001 | 1.65 | (1.07, | 2.55) | 0.02 | 6.2 (1.0, 11.4) |
| Non-ideal BP | 5.25 | (2.94, | 9.38) | <0.0001 | 2.32 | (1.28, | 4.20) | 0.005 | 16.0 (5.4, 26.3) |
| Non-ideal Fasting Plasma Glucose | 2.44 | (1.88, | 3.16) | <0.0001 | 1.76 | (1.34, | 2.29) | <0.0001 | 8.7 (5.0, 12.4) |
| Non-ideal Total Cholesterol | 1.41 | (1.09, | 1.84) | 0.01 | 1.01 | (0.77, | 1.31) | 0.96 | |
| All 4 significant Simple 7 factors | 37.1 (20.0, 51.9) | ||||||||
All ideal cardiovascular health factors were included simultaneously in the multivariate model. PAR = population-attributable risk percent. Each PAR was adjusted for the remaining three Simple 7 factors (that were significantly associated with HF; e.g., BMI, physical activity, blood pressure, and glucose), age, and sex.
Simple 7 components are associated with LV function and structure in African-Americans
In 1188 JHS participants with CMR imaging over a median 8.1 years (25th–75th percentile 7.5–8.9 years) after study entry, ideal blood pressure, BMI, glucose control and non-smoking were consistently associated with cardiac phenotypes (Table 4). Ideal blood pressure was associated with lower LV mass and lower concentricity (both P<0.0001). Ideal BMI was associated with lower LV mass, lower concentricity and strain (all p<0.0001). Non-smoking was associated with lower LV concentric remodeling (P<0.0001), lower LV strain (P=0.0002) and lower aortic PWV (P=0.0001). Ideal glucose was associated with lower LV mass (P=0.002).
Table 4.
Associations between Life’s Simple 7 components and CMR imaging indices.
| LV Mass Index (g/m2.7)* | LV concentric remodeling index (g/mL)* | Peak Global strain (%) | Aortic PWV (m/s)* | |||||
|---|---|---|---|---|---|---|---|---|
| Ideal Cardiovascular Health | LS mean | p value | LS mean | p value | LS mean | p value | LS mean | p value |
| Non-Smoking | 28.95 | 0.01 | 0.98 | <0.0001 | −16.11 | 0.0002 | 5.20 | 0.0001 |
| Smoking | 30.32 | 1.07 | −15.31 | 6.09 | ||||
| Ideal BMI | 26.65 | <0.0001 | 0.97 | <0.0001 | −16.12 | <0.0001 | 5.90 | 0.01 |
| Non-ideal BMI | 32.93 | 1.09 | −15.29 | 5.36 | ||||
| Ideal Nutrition | 29.65 | 0.98 | 0.98 | 0.34 | −15.89 | 0.66 | 4.61 | 0.02 |
| Non-ideal Nutrition | 29.60 | 1.07 | −15.53 | 6.87 | ||||
| Ideal Physical Activity | 29.61 | 0.94 | 1.03 | 0.95 | −15.78 | 0.37 | 5.71 | 0.33 |
| Non-ideal Physical Activity | 29.64 | 1.03 | −15.64 | 5.54 | ||||
| Ideal BP | 28.38 | <0.0001 | 0.98 | <0.0001 | −15.80 | 0.28 | 5.51 | 0.17 |
| Non-ideal BP | 30.92 | 1.07 | −15.62 | 5.75 | ||||
| Ideal Fasting Plasma Glucose | 28.86 | 0.002 | 1.01 | 0.15 | −15.95 | 0.01 | 5.70 | 0.51 |
| Non-ideal Fasting Plasma Glucose | 30.41 | 1.04 | −15.47 | 5.56 | ||||
| Ideal Total Cholesterol | 29.97 | 0.06 | 1.02 | 0.35 | −15.72 | 0.90 | 5.62 | 0.94 |
| Non-ideal Total Cholesterol | 29.29 | 1.03 | −15.70 | 5.63 | ||||
Linear models were constructed for each imaging parameter (dependent variable) as a function of age, sex, and each Simple 7 component to obtain least-squares means. All Simple 7 components were included simultaneously in the same model.
Least-squares means of log-transformed variables (LV mass index, LV concentric remodeling index and Aortic PWV) were exponentiated. P values of <0.007 were considered significant using Bonferroni correction. BMI = body mass index; BP = blood pressure; LV = left ventricle; LS=least-squares; SE = standard error; PWV = pulse wave velocity.
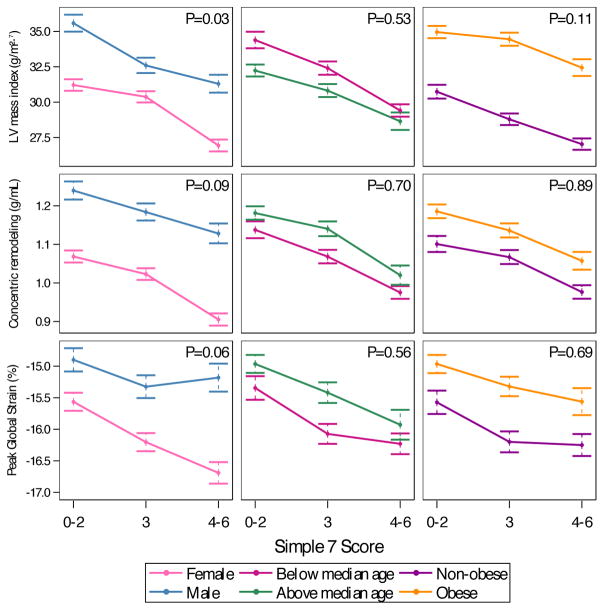
We further examined whether sex, age or BMI at the time of CMR modified the relationship between categories of Simple 7 score and these parameters. While males and obese individuals had greater hypertrophy, concentric remodeling and decreased strain (relative to female and non-obese counterparts), we did not observe any evidence of effect modification (Figure 3).
Figure 3. CMR measures of cardiac remodeling, stratified by median age, sex and obesity.
Data points represent least-squares means (obtained in adjusted linear models), with error bars representing standard error of the mean. P values for interaction between Simple 7 score category and each stratum are presented.
Discussion
In a large, community-based population of African-Americans with comprehensive cardiometabolic phenotyping, we found that achievement of fewer components of Life’s Simple 7 at baseline was strongly associated with incident HF over approximately 10 years of follow-up. Specifically, elevated blood pressure, physical inactivity, smoking, and impaired glucose control contributed to HF risk development, highlighting these factors as essential for HF prevention. In addition, non-ideal cardiovascular health status was strongly associated with adverse cardiac remodeling in African-Americans up to 8 years later, including LV hypertrophy, concentric remodeling and LV dysfunction—all subclinical phenotypes contributing to HF development. Collectively, in a large, contemporary, community-based African-American cohort, cardiovascular health practices in mid-life in African-Americans influence HF progression across the spectrum of subclinical cardiovascular disease to manifest HF. Improved cardiovascular health behaviors early in life may impact long-term HF development in African-Americans.
Since the definition of “ideal cardiovascular health” as 7 separate targets of prevention by the American Heart Association10, a wealth of data attesting to achievement of ideal Life’s Simple 7 metrics and its consequences on cardiometabolic disease and CVD has emerged27, 28. African-Americans may be at especially high risk of achieving fewer components of ideal health relative to the general population: recent results from Djousse and colleagues in over 5000 African-Americans in JHS4 demonstrate that over 60% of JHS participants had fewer than 3 components of Life’s Simple 7, with a dramatically low prevalence of ideal diet (particularly sodium intake). The higher prevalence of HF and LV remodeling in African-Americans warrants a direct investigation of the impact of health characteristics and behaviors on comprehensive cardiometabolic phenotypes.
In the Atherosclerosis Risk in Communities (ARIC) study, Shah and colleagues used echocardiography to demonstrate greater LV mass, impaired LV strain, increased HF and CVD risk with a decline in the number of ideal health components achieved over time5. While this study included 24% African-Americans (1416 from Jackson, given overlap between ARIC and JHS), it did not address specific CMR-based parameters of structure/function and it did not investigate associations with individual components of ideal cardiovascular health. Our study expands these prior efforts by focusing squarely on the largest cohort of African-Americans with detailed CMR assessments of phenotypes central to HF development. We found that achievement of fewer components of ideal health was associated with greater incident HF risk, independent of age, sex, or interval CHD development. In multivariable models for HF, we found that elevated blood pressure, physical inactivity, smoking, and impaired glucose control, were associated with incident HF. While hypertension, smoking and diabetes were significant contributors to incident HF risk in both African American and White Americans in ARIC29, the impact of physical activity is not well defined. In our study, physical inactivity was not associated with LV structure and function but was associated with incident HF, suggesting a possible myocardial-independent contribution of physical inactivity on HF risk30, a finding that motivates further prospective investigation.
Of the different pillars of Life’s Simple 7, similar factors to those associated with incident HF including hypertension, impaired glucose control, smoking but also BMI in the overweight or obese range, were associated with a constellation of myocardial phenotypes classically observed before the onset of frank HF (specifically with preserved ejection fraction; HF-pEF), including greater hypertrophy31, concentric LV remodeling, and higher LV strain by CMR. In the African-American community, hypertension is highly prevalent and an established risk factor for LV hypertrophy and ensuing HF development 32, 33. We add to the current literature by identifying obesity and insulin resistance as correlates of unfavorable LV phenotypes and possible targets for personalized HF prevention in African-Americans in keeping with a prior report by Shah5 and from others34, 35.
The strengths of our study include the use of a large cohort of African-Americans with long-term follow-up, extensive cardiometabolic phenotyping, and careful adjudication of Life Simple’s 7 ideal health metrics. Nevertheless, the results of our study should be viewed in the context of its design. Although low rates of incident HF in JHS may have limited our ability to assess relationships in select subgroups (e.g., by presence/absence of atherosclerotic cardiovascular disease), we nonetheless observed significant associations between achievement of fewer ideal health metrics and HF. Future studies might focus on the prognostic relevance of Life’s Simple 7 metrics in African Americans with established atherosclerotic cardiovascular disease. Though we did not observe significant associations between dietary habits and incident HF, we may have been limited by power, given the low proportion of participants achieving ideal nutrition. An inherent limitation of the JHS study is that CMR imaging was performed in a subset of the JHS participants who achieved more ideal health metrics in general compared to participants that did not undergo CMR imaging. Despite these limitations, the study population represents the largest population of African Americans with detailed imaging-based phenotyping of cardiometabolic risk. In addition, we chose to investigate cardiovascular phenotypes as a function of baseline Life’s Simple 7 metrics and did not consider changes in these metrics over time, which require further longitudinal follow-up and are a fruitful area of future investigation. The population attributable risks denoted here are adjusted for age and sex, and there may be other unmeasured confounders that influence the magnitude of attributable risk; nevertheless, our results are consistent with the common theme of hypertension and diabetes as critical to HF development. Finally, although HF determination was complex (formal adjudication after 1/1/2005 and self-report before 1/1/2005), observed associations remained robust to exclusion of self-reported HF.
In conclusion, in this contemporary, large cohort of African Americans, achievement of fewer components of ideal health was associated with adverse cardiovascular remodeling and HF development. These findings highlight the importance of ideal cardiovascular health in African-Americans and provide potential, specific targets for personalized HF prevention in this population.
Supplementary Material
Acknowledgments
Sources of Funding: The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Shah is funded by grants from the National Institutes of Health (K23HL127099) and the American Heart Association (16SFRN31740000).
The authors thank the participants and data collection staff of the Jackson Heart Study for their tireless efforts in prevention of cardiovascular disease in African Americans. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. Author Contributions: Aferdita Spahillari had full access to the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Aferdita Spahillari, Ravi Shah, Venkatesh Murthy, Adolfo Correa, Katherine Tucker, Sameera Talegawkar. Acquisition, analysis, or interpretation of data: Adolfo Correa, Ravi Shah, Venkatesh Murthy, Aferdita Spahillari, Katherine Tucker, J. Jeffrey Carr, James G. Terry, Stanford Mwasongwe. Drafting of the manuscript: Ravi Shah, Aferdita Spahillari, Venkatesh Murthy, J. Jeffrey Carr, James G. Terry, Adolfo Correa, Jane E. Freedman, Sarah de Ferranti.
Critical revision of the manuscript for important intellectual content: All authors. Study supervision: Ravi Shah, Venkatesh Murthy, Adolfo Correa, Katherine Tucker.
Footnotes
Conflict of Interest Disclosures: Dr. Murthy has minor stockholdings in General Electric.
References
- 1.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djousse L, Petrone AB, Blackshear C, Griswold M, Harman JL, Clark CR, Talegawkar S, Hickson DA, Gaziano JM, Dubbert PM, Correa A, Tucker KL, Taylor HA. Prevalence and changes over time of ideal cardiovascular health metrics among African-Americans: the Jackson Heart Study. Prev Med. 2015;74:111–116. doi: 10.1016/j.ypmed.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal Cardiovascular Health During Adult Life and Cardiovascular Structure and Function Among the Elderly. Circulation. 2015;132:1979–1989. doi: 10.1161/CIRCULATIONAHA.115.017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, Investigators AS. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6-4-17. [PubMed] [Google Scholar]
- 9.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6-18-29. [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task F, Statistics C. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 11.Bruce MA, Beech BM, Crook ED, Sims M, Wyatt SB, Flessner MF, Taylor HA, Williams DR, Akylbekova EL, Ikizler TA. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2010;55:1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, Zaghloul S, Carithers T, Bogle ML. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutrition. 2005;8:87–96. [PubMed] [Google Scholar]
- 13.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, Rifai N, Liu S. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care. 2007;30:1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musani SK, Vasan RS, Bidulescu A, Liu J, Xanthakis V, Sims M, Gawalapu RK, Samdarshi TE, Steffes M, Taylor HA, Fox ER. Aldosterone, C-reactive protein, and plasma B-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013;36:3084–3092. doi: 10.2337/dc12-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, Casanova R, Sims M, Correa A, Wu WC, Wand GS, Golden SH. Aldosterone, Renin, and Diabetes Mellitus in African Americans: The Jackson Heart Study. J Clin Endocrinol Metab. 2016;101:1770–1778. doi: 10.1210/jc.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bidulescu A, Liu J, Musani SK, Fox ER, Samdarshi TE, Sarpong DF, Vaccarino V, Wilson PW, Arnett DK, Din-Dzietham R, Taylor HA, Gibbons GH. Association of adiponectin with left ventricular mass in blacks: the Jackson Heart Study. Circ Heart Fail. 2011;4:747–753. doi: 10.1161/CIRCHEARTFAILURE.110.959742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keku E, Rosamond W, Taylor HA, Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6-62-70. [PubMed] [Google Scholar]
- 21.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 22.de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB Strong Heart Study I. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung J, Francois C, Nelson NA, Young A, Cowan BR, Jerecic R, Carr J. Cardiac image modeling tool for quantitative analysis of global and regional cardiac wall motion. Invest Radiol. 2009;44:271–278. doi: 10.1097/RLI.0b013e31819c96e3. [DOI] [PubMed] [Google Scholar]
- 25.Noda C, Ambale Venkatesh B, Ohyama Y, Liu CY, Chamera E, Redheuil A, Teixido-Tura G, Chugh AR, Wu CO, Hundley GW, Bluemke DA, Lima JA. Reproducibility of functional aortic analysis using magnetic resonance imaging: the MESA. Eur Heart J Cardiovasc Imaging. 2016;17:909–917. doi: 10.1093/ehjci/jev215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 27.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang J, Yang Q, Hong Y, Loustalot F. Status of cardiovascular health among adult Americans in the 50 States and the District of Columbia, 2009. J Am Heart Assoc. 2012;1:e005371. doi: 10.1161/JAHA.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folsom AR, Yamagishi K, Hozawa A, Chambless LE Atherosclerosis Risk in Communities Study I. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA, Berry JD. Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis. Circulation. 2015;132:178617–94. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 31.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Writing Group M; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 34.Abbasi SA, Hundley WG, Bluemke DA, Jerosch-Herold M, Blankstein R, Petersen SE, Rider OJ, Lima JA, Allison MA, Murthy VL, Shah RV. Visceral adiposity and left ventricular remodeling: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25:667–676. doi: 10.1016/j.numecd.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, Kwong RY, Bluemke DA, Lima JA, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1698–1706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.