In 2003, Herridge et al. first described poor functional outcomes in working-age, 1-year survivors of Acute Respiratory Distress Syndrome (ARDS) (1). In the ensuring decade, countless studies have confirmed that poor long-term outcomes are the rule, not the exception, after ARDS. Survivorship from critical illness has been heralded as the defining challenge of critical care in the 21st century (2). Recently, helping patients to thrive after critical illness was deemed the “third revolution” of critical care (3).
As the field of critical care medicine increasingly focuses on long-term outcomes, it is important to recognize that long-term outcomes are the result of two distinct components: early and late events (Figure 1). For example, five-year mortality is the result of early mortality (e.g. in-hospital death) and late mortality (e.g. death after hospital discharge, but within 5 years of hospitalization). If early mortality is sufficiently high enough, then long-term mortality will be high even if the critical illness does not increase risk for late death among those who survive to hospital discharge. When considering long-term mortality from critical illness, it is important to differentiate between early versus late deaths because they are driven by different factors (4).
Figure 1. Long-term versus Late Mortality after Critical Illness.
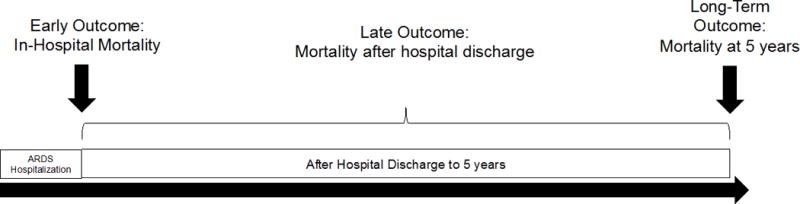
Panel A: Components of Long-Term Mortality
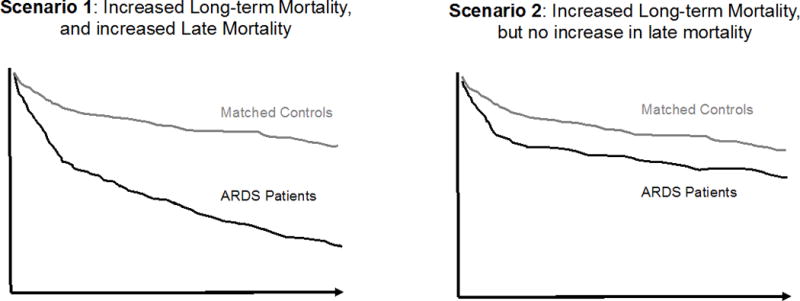
Panels B: Hypothetical Kaplan-Meier Survival Curve for Patients with ARDS and Matched Controls without ARDS
Long-term mortality after critical illness consists of early and late deaths. In this study, early deaths were considered as in-hospital deaths, while late deaths were deaths in the 5 years following survival from ARDS hospitalization. Panel B shows that ARDS patients may have increased long-term mortality with (scenario 1) or without (scenario 2) increased late mortality.
While early mortality from ARDS has been steadily declining with time (5), late mortality—death in the years after ARDS hospitalization—remains high (6). Drivers of this late mortality are not well understood. As would be expected, age, pre-existing chronic medical conditions, and prior nursing home use predict increased risk for late mortality (6). As yet, though, we have not elucidated if or how ARDS itself increases risk for late mortality relative to age and co-morbidity-matched controls.
In studies with careful matching and control for confounding, pneumonia (7), sepsis (8), and all-cause critical illness (9) have each been found to increase survivor’s risk for late mortality, independent of age and baseline health status. So, we can reasonably hypothesize that surviving ARDS also increases one’s risk for late mortality. But, the mechanisms by which any of these acute diseases increases late mortality remain poorly understood. Impaired immunity, weakness, accelerated cardiovascular disease, or heightened risk for malignancy may all play a role.
In this issue of Critical Care Medicine, Dinglas et al (10) examined the relationship of skeletal muscle weakness and late mortality in ARDS survivors—in effect, tying together outcomes which are often considered in isolation: functional disability and late mortality. Skeletal muscle wasting occurs rapidly in critical illness, is a major contributor to functional disability (11), and predicts early mortality (12). But, the impact of muscle weakness on late mortality in ARDS survivors has not been described previously.
One hundred fifty-six ARDS survivors were followed to death or for 5 years following hospital discharge. Muscle strength was measured at hospital discharge and again 3, 6, 12, 24, 36, and 48 months post-discharge using the Medical Research Council sum score (MRC), a 0-to-60 strength scale where a score less than 48 is considered to reflect pathological weakness. The MRC is recommended by several critical care professional societies to measure muscle strength, but is a subjective test (13). To combat the known subjectivity of MRC, this study included rigorous quality control; strength assessors underwent initial training and periodic quality review for the duration of the study to ensure high inter-rater reliability of their assessments.
The authors examined the association of weakness at hospital discharge, and changes in weakness over time (persistent, resolved, new) with 5-year mortality. An initial multivariable model was used to inform a parsimonious set of covariates (age, comorbidity, and mean daily SOFA score) that were included in the final Cox proportional hazard model. Each of these covariates been previously described to affect either skeletal muscle loss/function (14) or mortality (15).
At hospital discharge, 38% of the ARDS survivors in this study had muscle weakness, and one third of the cohort died during the 5-year follow-up period. Muscle weakness at hospital discharge was independently associated with 5-year mortality. The association persisted whether weakness was considered as a binary predictor (being weak at hospital discharge predicted 5-year mortality) or as a continuous predictor (each additional 1-point loss of strength predicted higher 5-year mortality).
Persistent weakness (maintaining MRC<48) over time was associated with worse 5-year survival, compared to maintaining a normal MRC. But curiously, resolving weakness was no better than persistent weakness, suggesting that weakness at hospital discharge is an ominous sign, even if a patient is subsequently able to rehabilitate back to normal strength. In future work, it would be interesting to see the relationship of muscle weakness trajectories to late mortality stratified by physical function at hospital discharge (16) or inflammatory load during acute illness (17). Progressing from normal strength to weakness during the 5-year follow-up period was not associated with worse 5-year survival, but this trajectory had insufficient power to draw strong conclusions.
These data attractively suggest that muscle wasting is a unifying contributor to late mortality and functional disability. An alternative view may be that muscle weakness and weakness trajectories are epiphenomena of the more holistic concept of frailty (18), and that frailty drives late mortality. Yet, it is biologically plausible that muscle weakness itself increases late mortality. Muscle weakness may cause swallowing dysfunction (19), decreased nutritional intake (19), increased aspiration (19), and decreased physical activity (20), which in turn may increase risk for cardiovascular events and malignancy. Individually or in combination these factors may impair muscle protein synthesis, leading to a vicious cycle of maintained cachexia—which is strongly associated with mortality (21).
As in-hospital mortality from ARDS continues to fall, we are increasingly challenged to improve the survivor experience. This study argues that skeletal muscle weakness is a key determinant of not only functional disability, but also of late mortality. Even those patients whose weakness improved over time experienced increased rates of 5-year mortality, underscoring the importance of preventing muscle loss during the acute phase of critical illness.
Footnotes
Copyright form disclosure: Dr. Prescott’s institution received funding from the National Institutes of Health (NIH) and the American Thoracic Society Foundation. She received support for article research from the NIH. Dr. Puthucheary disclosed that he does not have any potential conflicts of interest.
References
- 1.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 2.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153(3):204–5. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Speelmon EC. Advancing a Third Revolution in Critical Care. Am J Respir Crit Care Med. 2016;194(7):782–3. doi: 10.1164/rccm.201603-0619ED. [DOI] [PubMed] [Google Scholar]
- 4.Garland A, Olafson K, Ramsey CD, Yogendran M, Fransoo R. Distinct determinants of long-term and short-term survival in critical illness. Intensive Care Med. 2014;40(8):1097–105. doi: 10.1007/s00134-014-3348-y. [DOI] [PubMed] [Google Scholar]
- 5.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 6.Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40(3):388–96. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317–23. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 8.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lone NI, Gillies MA, Haddow C, et al. Five-Year Mortality and Hospital Costs Associated with Surviving Intensive Care. Am J Respir Crit Care Med. 2016;194(2):198–208. doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingas. Muscle Weakness and 5-Year Survival in Acute Respiratory Distress Syndrome Survivors. Crit Care Med. 2017 doi: 10.1097/CCM.0000000000002208. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parry SM, El-Ansary D, Cartwright MS, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30(5):1151.e9–1151.e14. doi: 10.1016/j.jcrc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Hermans G, Van Mechelen H, Clerckx B, et al. Acute Outcomes and 1-Year Mortality of Intensive Care Unit–acquired Weakness. A Cohort Study and Propensity-matched Analysis. Am J Respir Crit Care Med. 2014;190(4):410–20. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 13.Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15(1):R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthucheary ZA, Rawal J, McPhail M, et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA. 2013;310(15):1591. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 15.Lone NI, Walsh TS. Impact of Intensive Care Unit Organ Failures on Mortality during the Five Years after a Critical Illness. Am J Respir Crit Care Med. 2012;186(7):640–7. doi: 10.1164/rccm.201201-0059OC. [DOI] [PubMed] [Google Scholar]
- 16.Puthucheary ZA, Denehy L. Exercise Interventions in Critical Illness Survivors: Understanding Inclusion and Stratification Criteria. Am J Respir Crit Care Med. 2015;191(12):1464–7. doi: 10.1164/rccm.201410-1907LE. [DOI] [PubMed] [Google Scholar]
- 17.Griffith DM, Lewis S, Rossi AG, et al. Systemic inflammation after critical illness: relationship with physical recovery and exploration of potential mechanisms. Thorax. 2016;71(9):820–9. doi: 10.1136/thoraxjnl-2015-208114. [DOI] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. Can Med Assoc J. 2014;186(2):E95–102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzakhani H, Williams J-N, Mello J, et al. Muscle Weakness Predicts Pharyngeal Dysfunction and Symptomatic Aspiration in Long-term Ventilated Patients. Anesthesiology. 2013;119(2):389–97. doi: 10.1097/ALN.0b013e31829373fe. [DOI] [PubMed] [Google Scholar]
- 20.McNelly AS, Rawal J, Shrikrishna D, et al. An Exploratory Study of Long-Term Outcome Measures in Critical Illness Survivors. Crit Care Med. 2016;44(6):e362–9. doi: 10.1097/CCM.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4(2):89–94. doi: 10.1007/s13539-013-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


