Abstract
Objective
Diabetes is one of the most prevalent and costly chronic diseases in the United States. This study analyzed the risk of developing diabetes and the annual cost of diabetes for a U.S. general population.
Methods
Data from the Medical Expenditure Panel Survey (MEPS), 2008–2012, was used to analyze (i) probabilities of developing diabetes and (ii) annual total healthcare expenditures for diabetics. The age-gender-race-body mass index (BMI) category specific risks of developing diabetes were estimated by fitting an exponential survival function to age at first diabetes diagnosis. Annual healthcare expenditures were estimated using a generalized linear model with log-link and gamma variance function. Complex sampling designs in the MEPS were adjusted for. All dollar values are presented in 2012 U.S. dollars.
Results
We observed a >6-fold increase in diabetes risks for class III obese (BMI≥40) individuals, compared to normal weight individuals. Using age 50 as an example, we found a >3-fold increase in annual healthcare expenditures for diabetics ($13,581), compared to non-diabetics ($3,954). Compared to normal weight (18.5≤BMI<25) individuals, class II obese (35≤BMI<40) and class III obese individuals incurred an annual marginal cost of $628 and $756, respectively. The annual healthcare expenditure differentials between diabetics and non-diabetics age 50 were the highest for individuals with class II ($12,907) and class III obesity ($9,703).
Conclusions
This paper highlights the importance of obesity on diabetes burden. Our results suggested that obesity, in particular, BMI ≥35, is associated with a substantial increase in the risk of developing diabetes and imposes a large economic burden.
Keywords: Diabetes, obesity, healthcare expenditures, economic burden
Introduction
Diabetes is one of the most prevalent and costly chronic diseases in the United States. In 2011, it was estimated that 20.8 million U.S. adults (9%) lived with diabetes (1), and about 1.5 million new cases of diabetes were diagnosed (2). Diabetes is not only detrimental to the health and productivity of those who suffer from it, but also incurs substantial economic burden to them and to society. The total estimated cost of diagnosed diabetes in 2012 was $245 billion, including $69 billion in reduced productivity and $176 billion in direct medical costs (3).
Obesity is a significant risk factor of diabetes. It has been shown that 87% of U.S. adults with diabetes are overweight or obese (body mass index, BMI ≥25 kg/m2) (4). Moreover, previous studies have shown a strong association of overweight and obesity with the incidence of type II diabetes (5) and diabetes-related comorbidities (6), which lead to higher medical expenses for the obese population compared to the normal weight population (7). Therefore, it is important to better understand the association of obesity with the risk of developing diabetes and the medical expenditures associated with diabetes and diabetes-related comorbidities.
Past studies have investigated the association of obesity with diabetes and suggested a positive relationship between obesity and the incidence of diabetes. Geiss et al. analyzed the association between the time trend of obesity and incidence of diabetes from 1980 to 2012 (8). Narayan et al. estimated the incidence of diabetes to predict the remaining lifetime risk of developing diabetes by BMI category, rather than the annual risks of developing diabetes (9). Using nationally representative data from 1997 to 2000, Leung et al. computed the life years lost and lifetime healthcare expenditures associated with diabetes by age, gender, race, and BMI category (10). In this study, we report the annual risks of developing diabetes by age-gender-race-BMI category, the results of which can be used to inform future cost-effectiveness analyses of obesity prevention programs or diabetes prevention and control interventions.
There is a substantial amount of literature addressing the costs associated with diabetes. Trogdon et al. analyzed annual medical expenditures as a function of duration of diabetes (11). Zhuo et al. computed the excess medical spending attributable to diabetes, differentiating by types of medical services (12). Other studies have shown that BMI is associated with increased medical expenditures for patients with and without diabetes. A study in Spain showed that an increase in BMI among patients with type 2 diabetes was associated with an increase in direct healthcare costs (13). Cawley et al. used an instrumental variable approach to examine the causal relationship between BMI and savings in medical expenditures by diabetes status. Our study will be the first to compare the differences in total healthcare expenditures by diabetic status across BMI category (14).
The purpose of this study is to examine the age-gender-race specific risks of diabetes and annual total healthcare expenditures by BMI category for both diabetics and non-diabetics in the U.S. population. Because obesity is one of the most important modifiable risk factors of diabetes, our study findings will help inform future decision analysis in diabetes prevention studies when BMI category is considered an important determinant of outcomes.
Methods
Data
We used data from the Medical Expenditure Panel Survey (MEPS) Household Component (HC) Full-Year Consolidated Data files collected by the Agency for Healthcare Research and Quality and the National Center for Health Statistics (15). The MEPS is a complex multistage probability sample design dataset that provides nationally representative data containing detailed information such as healthcare use, expenditures, sources of payment, and health insurance coverage for the U.S. civilian noninstitutionalized population (16). In addition, information regarding respondents’ health status, demographics, and insurance coverage is also provided in the HC data files. The MEPS HC data files were merged with the Medical Conditions Files to supplement the dataset with diabetes-related comorbidities.
Analytic Cohort
We targeted U.S. adults age ≥20 in our study cohort. The analytic cohort was assembled by excluding the following people: (i) individuals with missing values on the target variables; (ii) underweight (BMI<18.5 kg/m2) individuals, because they may include heavy smokers or persons with severe chronic diseases and malignancies (17); (iii) women pregnant at the time of survey, because their BMI levels are unstable during pregnancy (17, 18); (iv) individuals diagnosed with cancer, since their BMI levels are less stable due to cancer treatment and appetite loss (18, 19); and (v) diabetics who reported inconsistent diabetes status across MEPS 2008–2012.
Models and Analyses
The MEPS collect information about diabetes by asking participants whether they had ever been diagnosed with diabetes and when their first diagnosis was. The probabilities of developing diabetes were estimated by fitting an exponential survival function to age at first diabetes diagnosis separately for males and females (10). Covariates included age at survey (20–29, 30–39, 40–49, 50–59, 60–69, ≥70), race (white, black, other), and BMI category (normal weight (18.5≤BMI<25 kg/m2), overweight (25≤BMI<30 kg/m2), class I obese (30≤BMI<35 kg/m2), class II obese (35≤BMI<40 kg/m2), class III obese (BMI ≥40 kg/m2)) (20) based on self-reported BMI at survey. We then predicted the probabilities of developing diabetes for each individual.
To predict BMI at diabetes diagnosis for each diabetes patient, we ran a regression of BMI at survey on duration of diabetes, age category, and race category separately for males and females (see details in part A of the Appendix). We then predicted BMI at diabetes diagnosis for each individual with diabetes. Based on these predicted BMIs at diagnosis for individual diagnosed with diabetes and BMI at survey for individuals without diagnosis, we grouped the population into the five BMI categories using the aforementioned criteria (20). The predicted probabilities of developing diabetes (computed according to the model in the previous paragraph) were then averaged across individuals in each BMI category to obtain the mean probabilities of developing diabetes for each BMI category.
Annual total healthcare expenditures were estimated using a generalized linear model (GLM) with log-link and gamma variance function (21). We controlled for diabetes status, duration of diabetes, duration of diabetes squared, age (at survey), age squared, interactions between diabetes status with age and with age squared, gender, race (white, black, Hispanic, Asian, other), education level (less than high school, high school diploma, college degree, graduate degree, other degree), household income level as a percentage of Federal Poverty Level (<100%, 100–199%, 200–399%, ≥400%), census region (northeast, midwest, south, west), primary source of health insurance (Medicaid, Medicare, private insurance, other public insurance, uninsured), diabetes-related comorbidities (heart disease, stroke, congestive heart failure, hypertension, high cholesterol, renal failure), BMI category (normal weight, overweight, class I, II and III obese) based on self-reported BMI at survey, current smoker or not, survey year dummies (2009, 2010, 2011, 2012), and interactions between BMI categories and diabetes status. We added the last covariate in addition to the covariates included in Trogdon et al. (11) to test for differences in the effect of BMI categories on healthcare expenditures between individuals with and without diabetes.
An alternative model using a two-part model with a logit model in the first part and a separate generalized linear model with log-link and gamma variance function in the second part was also constructed. Sensitivity analyses using the inverse of BMI as a continuous variable in the cost estimation were also performed to examine if our main conclusion is sensitive to the specification of BMI as a categorical versus a continuous variable. We computed healthcare cost differentials associated with diabetes by taking the differences between the predicted healthcare expenditures of the diabetics and the non-diabetics for each age, gender, race, and BMI category.
The MEPS data does not allow us to differentiate between type 1 and 2 diabetes. Since 78% of diabetes incidence before age 20 is type 1 diabetes (22), we conducted sensitivity analyses for the risks of developing diabetes and cost associated with diabetes by excluding diabetes patients who reported age at diabetes diagnosis before 20.
Statistical Analyses
We merged multiple years (2008–2012) of the MEPS data to increase the sample size for analysis, following the analytic guidelines published on the MEPS website. A common variance structure is specified across years that accurately reflects the complex sample design of the MEPS (23). The complex sampling designs in the MEPS were adjusted for in all analyses. All dollar values were deflated using the Personal Health Care Expenditure Price Index and are presented at 2012 price levels (24). STATA version 12 (StataCorp. 2012. College Station, TX) was used in all analyses.
Role of the Funding Source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Analytic Cohort
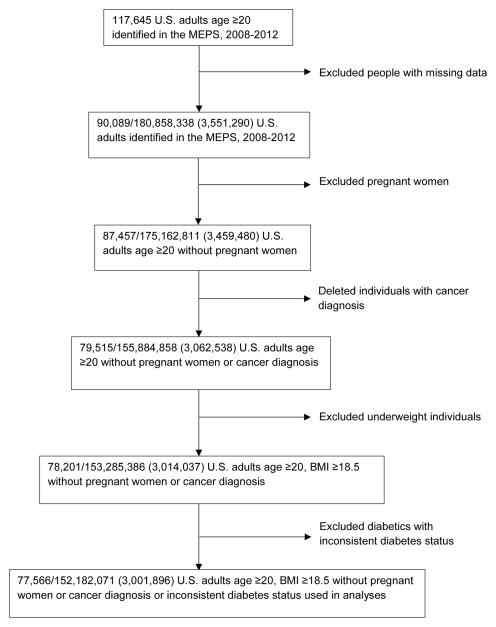
Table 1 reports the summary statistics of the sample and population estimates by diabetes status. Our final analytic sample comprises 77,566 individuals, representing 152,182,071 (s.e. = 3,001,896) U.S. adults in the population. Details of data attrition are shown in Figure 1. Among the population represented by the analytic cohort, 8.1% (N = 12,329,131) were diabetics. Within the population, the average age of diabetics was 59 compared to 45 for non-diabetics. The average age at diabetes diagnosis in our sample was 49, and the average duration of diabetes was 10 years. The average annual total healthcare expenditures in 2012 dollars were $10,015 for diabetics and $3,626 for non-diabetics. Individuals with diabetes were poorer, with lower education levels, and were more likely to be minorities, to be obese, and to have diabetes-related comorbidities, compared to individuals without diabetes.
Table 1.
Descriptive statistics by diabetes status, MEPS 2008–2012
| Diabetics | Non-diabetics | |||
|---|---|---|---|---|
| Variable | n* (%) | N† (%) | n* (%) | N† (%) |
| Total | 7,043 (9.1) | 12,329,131 (8.1) | 70,523 (90.9) | 139,852,940 (91.9) |
| Gender | ||||
| Male | 3,238 (46.0) | 6,234,780 (50.6) | 34,383 (48.8) | 71,248,701 (50.9) |
| Female | 3,805 (54.0) | 6,094,352 (49.4) | 36,140 (51.2) | 68,604,239 (49.1) |
| Race | ||||
| White | 4,488 (63.7) | 9,273,061 (75.2) | 50,031 (70.9) | 113,614,876 (81.2) |
| Black | 1,875 (26.6) | 2,067,832 (16.8) | 13,354 (18.9) | 16,031,182 (11.5) |
| Asian | 419 (5.9) | 517,389 (4.2) | 5,236 (7.4) | 6,955,123 (5.0) |
| Hispanic | 1,899 (27.0) | 2,040,448 (16.5) | 18,368 (26.0) | 20,496,363 (14.7) |
| Other | 209 (3.0) | 411,970 (3.3) | 1,409 (2.0) | 2,654,421 (1.9) |
| BMI category | ||||
| Normal weight | 959 (13.6) | 1,598,203 (13.0) | 23,784 (33.7) | 49,425,424 (35.3) |
| Overweight | 2,145 (30.5) | 3,640,956 (29.5) | 25,783 (36.6) | 50,813,947 (36.3) |
| Class I Obese | 1,922 (27.3) | 3,439,462 (27.9) | 12,949 (18.4) | 24,495,346 (17.5) |
| Class II Obese | 1,094 (15.5) | 2,013,615 (16.3) | 5,098 (7.2) | 9,761,179 (7.0) |
| Class III Obese | 923 (13.1) | 1,636,895 (13.3) | 2,909 (4.1) | 5,357,044 (3.8) |
| Poverty level | ||||
| <100% poverty level | 1,470 (20.9) | 1,868,138 (15.2) | 11,592 (16.4) | 15,993,647 (11.4) |
| 100–199% poverty level | 1,758 (25.0) | 2,682,577 (21.8) | 15,263 (21.6) | 23,611,728 (16.9) |
| 200–399% poverty level | 2,138 (30.4) | 3,713,166 (30.1) | 22,022 (31.2) | 43,179,588 (30.9) |
| ≥400% poverty level | 1,654 (23.5) | 4,027,451 (32.7) | 21,458 (30.4) | 56,696,222 (40.5) |
| Education | ||||
| Less than high school | 2,422 (34.3) | 3,122,852 (25.3) | 16,119 (22.9) | 21,890,288 (15.7) |
| High school diploma | 3,091 (43.9) | 6,004,583 (48.7) | 32,307 (45.8) | 64,433,769 (46.1) |
| College degree | 738 (10.5) | 1,543,332 (12.5) | 11,330 (16.1) | 27,463,379 (19.6) |
| Graduate degree | 322 (4.6) | 691,680 (5.6) | 5,372 (7.6) | 13,621,154 (9.7) |
| Other degree | 470 (6.7) | 966,684 (7.8) | 5,395 (7.6) | 12,444,350 (8.9) |
| Insurance | ||||
| Medicaid | 2,808 (39.9) | 4,990,800 (40.5) | 9,526 (13.5) | 20,067,060 (14.3) |
| Medicare | 1,566(22.2) | 2,080,733 (16.9) | 8,356 (11.8) | 11,405,979 (8.2) |
| Private insurance | 3,589 (51.0) | 7,291,576 (59.1) | 42,845 (60.8) | 97,704,387 (69.9) |
| Other public insurance | 41 (0.6) | 67,794 (0.5) | 242 (0.3) | 399,599 (0.3) |
| Uninsured | 933 (13.2) | 1,211,298 (9,8) | 16,138 (22.9) | 23,813,172 (17.0) |
| Diabetes-related comorbidities | ||||
| Heart disease | 1,985 (28.2) | 3,663,124 (29.7) | 6,746 (9.6) | 14,930,637 (10.7) |
| Stroke | 708 (10.1) | 1,211,131 (9.8) | 1,696 (2.4) | 3,396,530 (2.4) |
| Congestive heart failure | 204 (2,9) | 378,029 (3.1) | 267 (0.4) | 502,338 (0.4) |
| Hypertension | 5,339 (75.8) | 9,239,379 (74.9) | 19,523 (27.7) | 38,957,842 (27.9) |
| High cholesterol | 5,027 (71.4) | 8,899,582 (72.2) | 17,719 (25.1) | 37,390,007 (26.7) |
| Renal Failure | 113 (1.6) | 174,104 (1.4) | 119 (0.2) | 208,514 (0.1) |
| Current Smoker | 1,112 (15.8) | 2,025,479 (16.4) | 13,557 (19.2) | 27,228,617 (19.5) |
| Mean‡ | SE§ | Mean | SE | |
| Annual Healthcare Expenditures (2012$) | 10,015 | 17,635 | 3,626 | 11,731 |
| Age (years) | 58.7 | 14.1 | 44.9 | 16.0 |
| Age of diabetes diagnosis (years) | 48.7 | 15.5 | -- | -- |
| Duration of diabetes (years) | 10.0 | 9.5 | -- | -- |
| BMI (kg/m2) | 32.2 | 7.7 | 27.7 | 5.9 |
Notes: MEPS: Medical Expenditure Panel Survey.
n: sample size,
N: estimated population size,
Mean: estimated mean based on the population,
SE: standard error based on the population.
Figure 1.
Data attrition diagram
Risk of Developing Diabetes
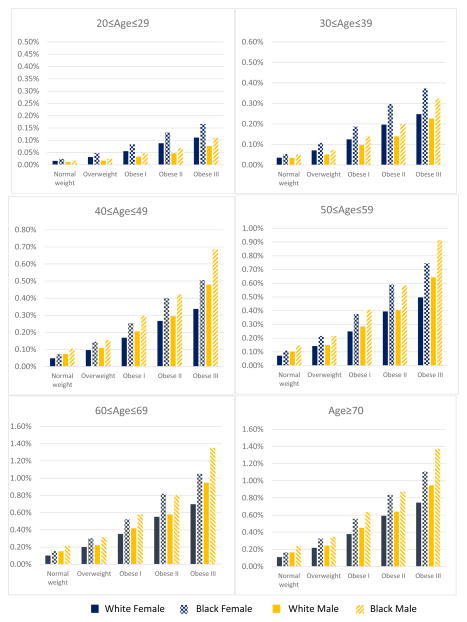
The predicted annual probabilities of developing diabetes by age, gender, race, and BMI category based on the predicted BMI at diagnosis for diabetics and BMI at survey for non-diabetics are presented in Figure 2. Hazard ratios for developing diabetes from the survival analysis are presented in Appendix Table 1. The annual probabilities of developing diabetes ranged from 0.02% to 1.27% for females and 0.01% to 1.77% for males depending on age, race, and BMI category (Figure 2). The probabilities of developing diabetes increased monotonically with BMI category. The average probability of developing diabetes (an arithmetic mean over age, gender, and race) for normal weight individuals was 0.11% compared to 0.69% for obese III individuals (a >6-fold increase in risks for class III obese individuals).
Figure 2.
Mean predicted probabilities of developing diabetes by age, gender, race, and BMI category based on predicted BMI at diagnosis for individuals with diabetes and BMI at survey for individuals without diabetes
| Age | Gender | Race | BMI category | Average | ||||
|---|---|---|---|---|---|---|---|---|
| Normal weight | Overweight | Obese I | Obese II | Obese III | ||||
| 20–29 | Female | White | 0.02 | 0.03 | 0.06 | 0.09 | 0.11 | 0.07 |
| Black | 0.02 | 0.05 | 0.08 | 0.13 | 0.17 | |||
| Other | 0.03 | 0.06 | 0.10 | 0.16 | 0.20 | |||
| Male | White | 0.01 | 0.02 | 0.03 | 0.05 | 0.08 | ||
| Black | 0.02 | 0.02 | 0.05 | 0.07 | 0.11 | |||
| Other | 0.02 | 0.03 | 0.05 | 0.08 | 0.13 | |||
| 30–39 | Female | White | 0.04 | 0.07 | 0.12 | 0.20 | 0.25 | 0.17 |
| Black | 0.05 | 0.11 | 0.19 | 0.30 | 0.37 | |||
| Other | 0.06 | 0.13 | 0.22 | 0.36 | 0.45 | |||
| Male | White | 0.03 | 0.05 | 0.10 | 0.14 | 0.23 | ||
| Black | 0.05 | 0.07 | 0.14 | 0.20 | 0.32 | |||
| Other | 0.06 | 0.08 | 0.16 | 0.23 | 0.37 | |||
| 40–49 | Female | White | 0.05 | 0.10 | 0.17 | 0.27 | 0.34 | 0.29 |
| Black | 0.07 | 0.14 | 0.25 | 0.40 | 0.51 | |||
| Other | 0.09 | 0.17 | 0.31 | 0.48 | 0.60 | |||
| Male | White | 0.07 | 0.11 | 0.21 | 0.29 | 0.48 | ||
| Black | 0.11 | 0.16 | 0.30 | 0.42 | 0.69 | |||
| Other | 0.12 | 0.18 | 0.34 | 0.49 | 0.75 | |||
| 50–59 | Female | White | 0.07 | 0.14 | 0.25 | 0.39 | 0.50 | 0.41 |
| Black | 0.11 | 0.21 | 0.37 | 0.59 | 0.74 | |||
| Other | 0.13 | 0.26 | 0.45 | 0.71 | 0.89 | |||
| Male | White | 0.10 | 0.15 | 0.28 | 0.40 | 0.64 | ||
| Black | 0.15 | 0.22 | 0.41 | 0.58 | 0.91 | |||
| Other | 0.17 | 0.25 | 0.47 | 0.66 | 1.08 | |||
| 60–69 | Female | White | 0.10 | 0.20 | 0.35 | 0.55 | 0.70 | 0.58 |
| Black | 0.15 | 0.30 | 0.52 | 0.82 | 1.05 | |||
| Other | 0.18 | 0.36 | 0.63 | 0.96 | 1.27 | |||
| Male | White | 0.15 | 0.22 | 0.42 | 0.58 | 0.95 | ||
| Black | 0.21 | 0.32 | 0.58 | 0.80 | 1.35 | |||
| Other | 0.25 | 0.36 | 0.66 | 0.92 | 1.42 | |||
| ≥70 | Female | White | 0.11 | 0.22 | 0.38 | 0.59 | 0.74 | 0.62 |
| Black | 0.16 | 0.33 | 0.56 | 0.83 | 1.11 | |||
| Other | 0.20 | 0.39 | 0.68 | 1.10 | 1.24 | |||
| Male | White | 0.16 | 0.24 | 0.45 | 0.64 | 0.95 | ||
| Black | 0.24 | 0.35 | 0.64 | 0.87 | 1.37 | |||
| Other | 0.27 | 0.40 | 0.70 | 1.01 | 1.77 | |||
| Average | 0.11 | 0.18 | 0.32 | 0.48 | 0.69 | |||
Females had slightly higher risks of developing diabetes before age 40 than males. However, after age 40, males had slightly higher probabilities of developing diabetes than females over all BMI categories except for Class III Obese. For people over age 40, Class III Obese males had significantly higher probabilities of developing diabetes compared with females of the same BMI category. We also found that the probabilities of developing diabetes increased monotonically with age. On average, individuals age ≥70 had about 9 times higher risks of developing diabetes compared with individuals age 20–29 (0.62% versus 0.07%). Racial groups other than whites or blacks had the highest risk of developing diabetes (0.44%), followed by blacks (0.37%) and whites (0.26%).
The results from the sensitivity analysis for excluding diabetes patients who reported age at diabetes diagnosis below 20 show that the patterns of developing diabetes are preserved for most of the age at survey groups except the age 20–29 group due to the reduced size of the age 20–29 group resulting from this exclusion (Appendix Figure 1).
Healthcare Expenditures Associated with Diabetes
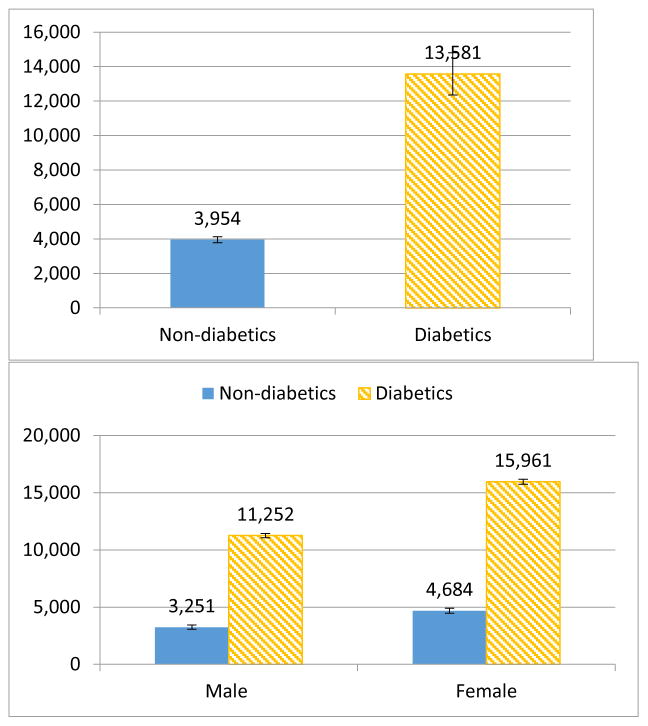
Results from our cost estimation show that diabetics had 127% higher annual healthcare expenditures than non-diabetics regardless of age (Appendix Table 2). The rate of increase in total annual healthcare expenditures slowed with age and duration of diabetes. However, the interactions for diabetes and age were insignificant, which suggested that there was no significant difference in the association of age and total annual healthcare expenditures between diabetics and non-diabetics. Using population age 50 as an example, Figure 3 shows the predicted annual total healthcare expenditures by (i) diabetes status, (ii) gender and diabetes status, (iii) race and diabetes status, and (iv) BMI category and diabetes status (see Appendix Table 3 for populations age 30, 40, 50, 60, and 70). The mean predicted annual total healthcare expenditures were $13,581 for diabetics and $3,954 for non-diabetics age 50, with a differential of $9,627.
Figure 3.
Mean predicted annual total healthcare* expenditures (2012$) for diabetics and non-diabetics age 50**
*All of the graphs were generated from the result of the multivariable analysis, using the GLM model controlling for age, age square, race (white, black, Hispanic, Asian, other), education level (less than high school, high school diploma, college degree, graduate degree, other degree), household income level as a percentage of Federal Poverty Level (<100%, 100–199%, 200–399%, 400%), census region (northeast, Midwest, south, west), primary source of health insurance (Medicaid, Medicare, private insurance, other public insurance, other public insurance), diabetes-related comorbidities (heart disease, stroke, congestive heart failure, hypertension, high cholesterol, renal failure), year dummies (2009, 2010, 2011, 2012), and current smoker or not. **Results for mean predicted annual total healthcare expenditures (2012$) for diabetics and non-diabetics age 40, 50, and 60 are presented in Appendix Table 3.
Our results show that females had significantly higher total annual healthcare expenditures than males in both diabetics and non-diabetics. At age 50, non-diabetic males spent $3,251 on annual healthcare compared with $11,252 for diabetic males, while non-diabetic females spent $4,684 compared with $15,961 for diabetic females.
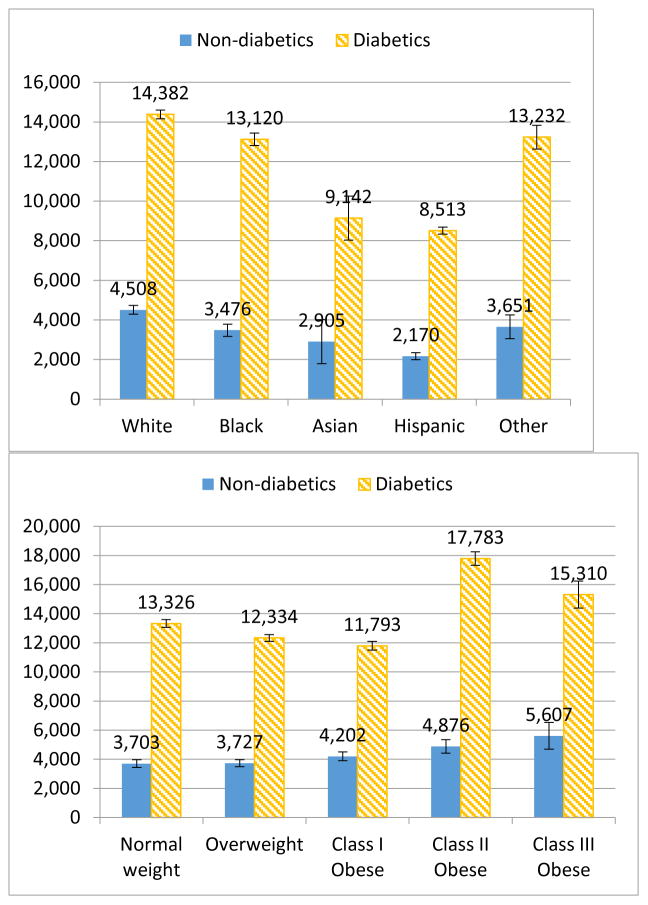
Overweight individuals had a yearly marginal effect of −$65 compared with normal weight individuals, although this difference was not statistically significant (Table 2). Class II and class III obese individuals had significantly higher annual healthcare expenditures than normal weight individuals. Compared to their normal weight counterparts, class II obese individuals spent about 12% more and class III obese individuals spent about 20% more (Appendix Table 2), which corresponds to an average marginal cost of $628 for class II obese individuals and $756 for class III obese individuals (Table 2). Our results also show that interactions between diabetes status and BMI category were insignificant, which suggested that there was no significant difference in the effects of BMI category on annual total healthcare expenditures between the diabetics and the non-diabetics. In terms of the healthcare cost differentials between diabetics and non-diabetics, at age 50, class I obese individuals had the lowest differentials ($7,591), followed by the overweight group ($8,607). Class II obese individuals had the largest healthcare cost differentials associated with diabetes ($12,907) among all BMI categories (see Appendix Table 3).
Table 2.
Marginal effects of annual total healthcare expenditures (2012$) on selected variables: generalized linear model with log-link and gamma variance
| Margin ($) | 95% Confidence Interval | |
|---|---|---|
| Diabetes status | ||
| No diabetes | 0 | [--, --] |
| Diabetes | 6,047 | [5268, 6826] |
| Duration of diabetes (year) | 160 | [75, 246] |
| Age (year) | 27 | [−15, 69] |
| Gender | ||
| Male | 0 | [--, --] |
| Female | 1,317 | [1028, 1607] |
| BMI category | ||
| Normal weight | 0 | [--, --] |
| Overweight | −65 | [-402, 273] |
| Class I Obese | 54 | [-307, 415] |
| Class II Obese | 628 | [190, 1066] |
| Class III Obese | 756 | [43, 1470] |
Other covariates that are controlled for include: race (white, black, Hispanic, Asian, other), education level (less than high school, high school diploma, college degree, graduate degree, other degree), household income level as a percentage of Federal Poverty Level (<100%, 100–199%, 200–399%, ≥400%), year dummies (2009, 2010, 2011, 2012), census region (northeast, Midwest, south, west), primary source of health insurance (Medicaid, Medicare, private insurance, other public insurance, other public insurance), diabetes-related comorbidities (heart disease, stroke, congestive heart failure, hypertension, high cholesterol, renal failure), and current smoker or not.
The results of the sensitivity analyses using the two-part model (Appendix Tables 4 & 5), the inverse of BMI as a continuous variable (Appendix Table 6), and the exclusion of diabetes patients who reported age at diagnosis below 20 (Appendix Table 7) are consistent with the results reported above.
Discussion
We analyzed the burden of diabetes by investigating the associations of BMI category with risk of developing diabetes and annual healthcare expenditures associated with diabetes. We observed a >6-fold increase in probabilities of developing diabetes for class III obese individuals, compared to normal weight individuals. We also found that class II obese individuals and class III obese individuals spent about 12% and 20% higher than normal weight individuals.
Our study differed from others in that we estimated the probabilities of developing diabetes directly, rather than based on the incidence rate of diabetes (8, 9, 25), which has often been used to approximate the probabilities of developing diabetes in decision analyses. Probability describes the likelihood of an event occurring, while the incidence rate describes the proportion of new cases in a given time period. Therefore, a direct comparison cannot be made between these two measures. Moreover, instead of focusing on the time trend of the risk of developing diabetes, as was done by Geiss et al. (8), we estimated and predicted probabilities of developing diabetes by age, gender, race, and BMI category, which were not previously reported. Our probability estimates can be directly used to inform decision analysis models regarding diabetes prevention and interventions when BMI is considered an important determinant of the outcomes of the study.
Our results are consistent with those presented in Geiss et al. in several ways. (i) Geiss et al. showed that males and females had similar diabetes incidence since 1980 (8). We also found that males and females had similar probabilities of developing diabetes over all BMI categories except for Class III obese individuals. (ii) We demonstrated that the probabilities of developing diabetes increased with age. In particular, individuals age ≥70 had a nearly 9 times higher chance of developing diabetes compared with individuals age 20–29. This result is qualitatively comparable with Geiss et al., in which they showed that diabetes incidence increased with age category and individuals age 65–79 had a ~4 times higher incidence rate compared with individuals age 20–44 (8). (iii) Our results suggested that races other than black and white were more likely to develop diabetes than blacks, followed by whites. The average probability over age, gender, and BMI was 0.44% for other races, 0.37% for blacks, and 0.26% for whites. This is consistent with Geiss et al. who showed that Hispanics had higher diabetes incidence than non-Hispanic blacks, and that Non-Hispanic blacks had higher diabetes incidence than non-Hispanic whites from 2008–2012 (8).
Our cost estimation is comparable to Trogdon et al. We found that class II obese individuals and class III obese individuals spent about $628 and $756 more on healthcare annually than normal weight individuals. However, our estimate of the average marginal effect of one additional year of diabetes duration on total healthcare expenditures was $160, 1.8 times higher than that estimated in Trogdon et al. ($75 in 2005$, i.e., $90 in 2012$), in which they used MEPS data from 2000–2004 (11). As suggested by Trogdon et al. and Zhuo et al., the increase in diabetes costs over time was largely due to higher prescription drug expenditures, which might explain the higher cost found in our study.
We found that diabetics age 50 spent an excess of $9,627 on total annual healthcare compared with non-diabetics at the same age. Although this additional cost may be underestimated given the inclusion of renal failure as a covariate in our study, this estimate was still higher than that predicted by Zhuo et al. ($5,378 in 2012$) (12). The difference can be explained by the following reasons. First, in their study, duration of diabetes was not considered in the model, which could underestimate the association of diabetes with healthcare costs, as the costs increase with time since diagnosis (11). Second, Zhuo et al. used MEPS data from 2010–2011 in their analysis, while our study used data from 2008–2012 (12).
Our results were also consistent with Cawley et al. in several ways. Using the restricted-use version of the MEPS data, Cawley et al. established a causal relationship between BMI and medical expenditures by using BMI and BMI squared of the respondent’s oldest biological child as an instrumental variable for the respondent’s BMI and BMI squared. They found that individuals with class I obesity do not have elevated healthcare costs, but healthcare costs rise rapidly with BMI in the range of class II and class III obesity, which is consistent with our findings. Estimating separately for diabetics and non-diabetics, they found that medical care costs for diabetics were greater than non-diabetics at every unit of BMI. We found in our estimation that the highest differentials of the mean predicted annual total healthcare expenditures between diabetics and non-diabetics were in individuals with class II and III obesity.
Our study has several strengths: first, we used the most recent MEPS data at the time of this study to provide the most up-to-date estimates of the probabilities of developing diabetes and total annual healthcare expenditures. Second, we showed that obesity was an important factor in developing diabetes (class III obese individuals have a >6 times higher risk of developing diabetes compared with normal weight individuals). Third, we documented the economic burden of diabetes and obesity by comparing the mean predicted total annual healthcare expenditures jointly by BMI category and diabetes status. Last, the metrics that we reported can be directly applied to decision modeling when BMI is considered an important factor.
Our study also has several limitations. First, our study relies on self-reported information, which may be subject to reporting bias. For example, systematic self-reported error in BMI can lead to an overestimation of the healthcare expenditures associated with obesity (26). Moreover, self-reported information on diabetes diagnosis might not capture the entire diabetes population, in particular, people with undiagnosed diabetes. The direction of this bias is ambiguous, because undiagnosed diabetics are likely in poorer health, which would tend to increase the total healthcare expenditures; yet since people with undiagnosed diabetes likely did not receive diabetes-specific treatment, this lack of treatment could lead to lower total healthcare expenditures. Also, it has been shown that the accuracy of self-reporting data for diabetes data is reasonably high in population surveys (27). Second, missing information of unobserved diabetics who died before the time of survey may create bias similar to survivorship bias due to potential systematic differences between the observed and unobserved diabetes patients in age at diabetes diagnosis. However, to our knowledge there is no clear evidence to suggest such systematic differences. Therefore, the direction of bias of such selection of survivorship in our study is ambiguous. Finally, our work studied the risks and costs associated with diabetes. The readers should be cautious not to interpret our results as causal.
Conclusions
We examined the association of obesity with risk and cost of diabetes by analyzing the probabilities of developing diabetes and total annual healthcare expenditures for diabetics and non-diabetics by BMI categories. We showed that obesity, in particular, class II and III obesity, is associated with a substantial increase in the risk of developing diabetes and imposes a large economic burden on healthcare expenditures. Our study highlights the importance of obesity on the burden of diabetes in the United States. Our results can inform policy makers about the potential benefits of reducing the burden of diabetes by reducing obesity. The metrics that we reported can be directly applied to decision models in diabetes prevention studies when BMI is considered an important factor.
Supplementary Material
Acknowledgments
Funding: The Foundation for Barnes-Jewish Hospital and the National Institutes of Health Grant U54 CA155496 supported this research. S-H. Chang is supported by the Agency for Healthcare Research and Quality Grant K01 HS022330. G.A. Colditz is supported by the American Cancer Society Clinical Research Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez ME, Maltzman T, Marshall JR, Einspahr J, Reid ME, Sampliner R, et al. Risk factors for Ki-ras protooncogene mutation in sporadic colorectal adenomas. Cancer Res. 1999;59(20):5181–5. [PubMed] [Google Scholar]
- 2.Gil MJ, Manu MA, Arteaga C, Migliaccio M, Encio I, Gonzalez A, et al. Synthesis and cytotoxic activity of N-(2-pyridylsulfenyl)urea derivatives. A new class of potential antineoplastic agents. Bioorg Med Chem Lett. 1999;9(16):2321–4. doi: 10.1016/s0960-894x(99)00373-x. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bays HE, Chapman RH, Grandy S, Group SI. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61(5):737–47. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28(7):1599–603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 8.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–26. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 9.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 10.Leung MY, Pollack LM, Colditz GA, Chang SH. Life years lost and lifetime health care expenditures associated with diabetes in the U.S., National Health Interview Survey, 1997–2000. Diabetes Care. 2015;38(3):460–8. doi: 10.2337/dc14-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trogdon JG, Hylands T. Nationally representative medical costs of diabetes by time since diagnosis. Diabetes Care. 2008;31(12):2307–11. doi: 10.2337/dc08-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuo X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW. Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care. 2015;38(4):581–7. doi: 10.2337/dc14-1687. [DOI] [PubMed] [Google Scholar]
- 13.Dilla T, Valladares A, Nicolay C, Salvador J, Reviriego J, Costi M. Healthcare costs associated with change in body mass index in patients with type 2 diabetes mellitus in Spain: the ECOBIM study. Appl Health Econ Health Policy. 2012;10(6):417–30. doi: 10.1007/BF03261876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in Medical Expenditures Associated with Reductions in Body Mass Index Among US Adults with Obesity, by Diabetes Status. Pharmacoeconomics. 2015;33(7):707–22. doi: 10.1007/s40273-014-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medical Expenditure Panel Survey (MEPS) Agency for Healthcare Research and Quality; Rockville, MD: Sep, 2012. [PubMed] [Google Scholar]
- 16.Menarguez J, Goicoechea M, Crist bal E, Arribas B, Martinez ME, Alcazar JA, et al. Lack of relationship between BsmI vitamin D receptor polymorphism and primary hyperparathyroidism in a Spanish female population. Calcif Tissue Int. 1999;65(3):214–6. doi: 10.1007/s002239900685. [DOI] [PubMed] [Google Scholar]
- 17.Chang SH, Pollack LM, Colditz GA. Life Years Lost Associated with Obesity-Related Diseases for U.S. Non-Smoking Adults. PLoS One. 2013;8(6):e66550. doi: 10.1371/journal.pone.0066550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SH, Pollack LM, Colditz GA. Obesity, mortality, and life years lost associated with breast cancer in nonsmoking US Women, National Health Interview Survey, 1997–2000. Prev Chronic Dis. 2013;10:E186. doi: 10.5888/pcd10.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorogood M, Appleby PN, Key TJ, Mann J. Relation between body mass index and mortality in an unusually slim cohort. J Epidemiol Community Health. 2003;57(2):130–3. doi: 10.1136/jech.57.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 21.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? Journal of Health Economics. 2001;20(4):461–94. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. [accessed on June 23, 2016];Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/
- 23.MEPS HC-036: 1996–2013 Pooled Linkage Variance Estimation File. Agency for Healthcare Research and Quality Center for Financing, Access, and Cost Trends; Sep, 2015. [Google Scholar]
- 24.Uriz MS, Gorina N, Martinez-Mejias A, Lopez-Linan MJ, Bella F. Arthritis as complication of Mediterranean boutonneuse fever in infancy. Enferm Infecc Microbiol Clin. 1999;17(6):316–8. [PubMed] [Google Scholar]
- 25.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867–74. doi: 10.1016/S2213-8587(14)70161-5. [DOI] [PubMed] [Google Scholar]
- 26.Cawley J, Maclean JC, Hammer M, Wintfeld N. Reporting error in weight and its implications for bias in economic models. Econ Hum Biol. 2015;19:27–44. doi: 10.1016/j.ehb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor PJ, Rush WA, Pronk NP, Cherney LM. Identifying diabetes mellitus or heart disease among health maintenance organization members: sensitivity, specificity, predictive value, and cost of survey and database methods. Am J Manag Care. 1998;4(3):335–2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.