Abstract
Background
Helminth infections can negatively affect the immunologic host control, which may increase the risk of progression from latent Mycobacterium tuberculosis infection to tuberculosis (TB) disease and alter the clinical presentation of TB. We assessed the prevalence and determined the clinical relevance of helminth co-infection among TB patients and household contact controls in urban Tanzania.
Methodology
Between November 2013 and October 2015, we enrolled adult (≥18 years) sputum smear-positive TB patients and household contact controls without TB during an ongoing TB cohort study in Dar es Salaam, Tanzania. We used Baermann, FLOTAC, Kato-Katz, point-of-care circulating cathodic antigen, and urine filtration to diagnose helminth infections. Multivariable logistic regression models with and without random effects for households were used to assess for associations between helminth infection and TB.
Principal findings
A total of 597 TB patients and 375 household contact controls were included. The median age was 33 years and 60.2% (585/972) were men. The prevalence of any helminth infection among TB patients was 31.8% (190/597) and 25.9% (97/375) among controls. Strongyloides stercoralis was the predominant helminth species (16.6%, 161), followed by hookworm (9.0%, 87) and Schistosoma mansoni (5.7%, 55). An infection with any helminth was not associated with TB (adjusted odds ratio (aOR) 1.26, 95% confidence interval (CI): 0.88–1.80, p = 0.22), but S. mansoni infection was (aOR 2.15, 95% CI: 1.03–4.45, p = 0.040). Moreover, S. mansoni infection was associated with lower sputum bacterial load (aOR 2.63, 95% CI: 1.38–5.26, p = 0.004) and tended to have fewer lung cavitations (aOR 0.41, 95% CI: 0.12–1.16, p = 0.088).
Conclusions/Significance
S. mansoni infection was an independent risk factor for active TB and altered the clinical presentation in TB patients. These findings suggest a role for schistosomiasis in modulating the pathogenesis of human TB. Treatment of helminths should be considered in clinical management of TB and TB control programs.
Author summary
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, and parasitic worm infections are typical diseases of poverty. They often overlap geographically, and can occur in the same individual. Parasitic worm infections contribute to the down-regulation of the essential immune response against TB, and therefore can increase progression from latent M. tuberculosis infection to active TB. We conducted a case-control study in Dar es Salaam, the economic capital of Tanzania, where TB and helminths constitute a considerable burden. We found that infection with the blood fluke Schistosoma mansoni was associated with active TB, while none of the other parasitic worms showed such an association. Interestingly, TB patients infected with S. mansoni had significantly lower sputum bacterial load at diagnosis and tended to have fewer lung cavitations compared with TB patients without any parasitic worm infection. Diagnosis and treatment of parasitic worm infections, particularly schistosomiasis, should be considered during the management of TB patients and in the context of TB control programs. This could help to reduce the TB burden in settings where TB and parasitic worms co-exist.
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis remains a challenging disease to control. Indeed, over two billion people are estimated to be infected with M. tuberculosis worldwide [1]. Moreover one billion people are infected with soil-transmitted helminths, schistosomes, filarial worms, and food-borne trematodes [2–4]. In 2014, an estimated 9.6 million new TB patients were notified and 1.5 million TB patients died from the disease [1]. TB is a leading cause of deaths from an infectious disease [5].
TB and helminthiases overlap geographically, particularly in areas where poverty persists, for example in countries of sub-Saharan Africa [1,6]. Where TB and helminth infections co-occur, they can affect the same individual and thus exacerbate the course of disease [6]. Several conditions such as diabetes mellitus, malnutrition, and malignancies are known to increase the risk of progressing from latent M. tuberculosis infection to active TB [7]. Human immunodeficiency Virus (HIV)-induced immunodeficiency is by far the most important risk factor for developing TB [1,8], but parasitic co-infections such as with helminths can also contribute to the development of TB [9–11]. Immune dysregulations caused by helminth infections are known to negatively affect the prognosis of HIV and malaria [6,12]. The immune response to helminth infections is characterized by the induction of CD4+ T-helper 2 (Th2) and down-regulation of CD4+ T-helper 1 (Th1) cells [12–15]. This immunological imbalance has been suggested to increase the risk of progression from latent M. tuberculosis infection to active TB and to worsen the clinical outcomes.
We aimed to study the interaction between TB and helminth co-infections by comparing the prevalence of helminth infections, using a suite of diagnostic techniques, between TB patients and household contact controls without TB in an ongoing cohort study in Dar es Salaam, Tanzania, and to assess the effects of helminth infection on the clinical presentation and outcomes of TB disease.
Methods
Ethics statement
The study protocol was approved by the institutional review board of the Ifakara Health Institute (IHI; reference no. IHI/IRB/No 04–2015) and the Medical Research Coordinating Committee of the National Institute of Medical Research (NIMR; reference no. NIMR/HQ/R.8c/Vol.I/357) in Tanzania, and the ethics committee of north-west and central Switzerland (EKNZ; reference no.: UBE-15/42). Written informed consent was obtained from all study participants. TB patients were treated according to the National TB and Leprosy Programme (NTLP) treatment guideline [8]. Individuals with a Schistosoma spp. infection were treated with praziquantel (40 mg/kg). Other helminth infections were treated with albendazole (400 mg) immediately after diagnosis, as recommended by the national treatment guidelines [16]. HIV-positive patients were clinically managed according to the Tanzania National HIV and acquired immune deficiency syndrome (AIDS) treatment guideline [17].
Study setting
The study was conducted in the densely populated urban setting of Temeke district in Dar es Salaam, which is the economic capital of Tanzania. The population of Temeke is estimated at 1.4 million. In 2014, about one third of all TB patients from Dar es Salaam were notified in Temeke district (4,373; 32%) [18]. The overall HIV prevalence in the general adult population in Dar es Salaam is 5.2% [19]. The study area includes two TB sub-districts, Wailes I and Wailes II, whose patients are clinically managed at the Temeke district hospital and the two associated TB diagnostic and treatment centers of Tambukareli and Pasada [20].
Study design
The study was conducted within the frame of an ongoing prospective cohort study of TB patients and household contact controls in Dar es Salaam (TB-DAR). We assessed the association of TB and helminth infection in a case-control study design of TB patients (sputum smear-positives for acid-fast bacilli [AFB]) and household contact controls (Xpert MTB/RIF negative), who were matched by age (±5 years) and whenever possible by sex. We prospectively followed-up TB patients and assessed the clinical outcomes comparing TB patients with and without helminth infection at 6 and 12 months after recruitment.
Study population and sample size
We consecutively enrolled study participants starting in November 2013 until October 2015 to reach the required sample size. Over this period, we included adult TB patients (≥18 years of age and sputum-smear positive) and household contact controls. Any individual living in the same household as the index TB patients enrolled in the study is referred to as a household contact control. Controls at recruitment were free of symptoms and signs suggestive of TB, healthy on physical examination, and had a negative Xpert MTB/RIF result (Cepheid; California, United States of America).
Assuming a helminth prevalence of 45% in TB patients and 26% in controls based on results from previous publications [21] and a power of 80%, the target sample size was 109 study participants (for each group) to detect a prevalence difference of 19% between the two groups with a significance level of test 0.05, two-tailed and calculated with Stata version 14.0 (Stata Corp; Texas, United States of America).
Study procedures
TB patients and household contact controls were interviewed and underwent physical examination during recruitment at the study site (see under “Data Collection and Definitions”). We collected skinfold measurements from four body sites (biceps, triceps, subscapular, and suprailiac) using the Harpenden skinfold caliper [22]. The percentage body fat was calculated as previously described [23]. Household contacts with no symptoms or signs of TB submitted a sputum sample for Gene Xpert MTB/RIF to rule-out TB. We collected blood, stool, and urine samples from TB patients and controls for subsequent laboratory investigations. Chest X-rays for TB patients were done at the Temeke district hospital and were interpreted by an experienced board certified radiologist who was blinded to patients’ clinical data. Trained field workers collected geographic coordinates (global positioning system [GPS]) from the patients’ homes using Samsung Tab 4 android tablets (Samsung; Suwon, South Korea).
Laboratory procedures
Microbiological investigations
A patient was considered as having TB when any of the two submitted sputum samples were positive for AFB by staining sputum smears using the Ziehl-Nielsen (ZN) method, and a positive mycobacterial culture. Sputum smear microscopy was done at the Temeke district hospital under continuous quality control by the central tuberculosis reference laboratory (Dar es Salaam, Tanzania). AFB smear-positive results were graded according to World Health Organization/International Union Against Tuberculosis and Lung Disease (WHO/IUATLD) guidelines: “scanty” with 1–9 AFB per 100 oil immersion fields; “1+” with 10–99 AFB per 100 immersion fields; “2+” with 1–10 AFB per 1 immersion field, and “3+” with >10 AFB per immersion field [8,24]. To rule out TB among household controls, an additional sputum sample from TB patients and controls was sent to the TB laboratory at the Bagamoyo Research and Training Center (BRTC), IHI, for GeneXpert MTB/RIF (controls) and for culture on Löwenstein-Jensen media (TB patients and controls).
Helminthological investigations
For the diagnosis of helminth infections, single stool and urine samples were collected from each participant before the start of TB treatment (TB patients) and at the time of enrolment (controls). All stool and urine samples were transferred to the Helminth Unit at BRTC and examined for helminth infections using standardized, quality-controlled procedures as described elsewhere [25–27]. The Kato-Katz (triplicate thick smears per stool sample) and the FLOTAC methods were used to diagnose Ascaris lumbricoides, hookworm, S. mansoni, and Trichuris trichiura infections. The Baermann method was used to identify Strongyloides stercoralis infections [28]. The adhesive tape test was used to diagnose Enterobius vermicularis infections [26]. In addition, a rapid point-of-care circulating cathodic antigen (POC-CCA) urine cassette test was employed for the diagnosis of S. mansoni [29]. The urine filtration method was applied to detect S. haematobium infections [26]. For quality control, 10% of Kato-Katz slides were randomly selected and re-examined by a second reader.
Blood testing
In line with national HIV testing algorithms, screening was done using the Alere Determine HIV rapid test (Alere, USA). The Uni-gold HIV (Trinity Biotech; Wicklow, Ireland) rapid test served as a confirmatory test in case of a positive screening test. The CD4+ T-cells counts were determined using a FACSCount machine (Becton Dickinson Biosciences; California, United States of America). A full blood cell count was done with a MS4 Vet hematology analyzer (Diamond Diagnostics; Massachusetts, United States of America). All blood tests were performed at the Temeke district hospital laboratory, which is under supervision and quality control by the regional laboratory technician.
Data collection and definitions
We collected socio-demographic indicators including age, sex, ethnicity, education, and household income. Anthropometric data included weight, height, and skinfold measurements. Clinical data collected pertained to presenting symptoms of TB patients, TB treatment category, and treatment outcomes. Laboratory data included ZN sputum smear results and Gene Xpert MTB/RIF results, helminth species infections, HIV status, full blood cell count, and CD4+ cell count. All study participants were asked about their use of anthelmintic treatment in the last 12 months prior to the enrollment into the study. Study data were captured by electronic case report forms using the open-source data collection software ODK on Android PC tablets [30]. Data management was done using the eManagement tool “odk_planner”, as previously described [30]. Data were uploaded to a password protected secure server with regular back-ups.
In order to grade the clinical severity of TB, we adopted a previously published clinical TB score [31], with the following modification: 12 points TB score parameters instead of 13 points as tachycardia was not systematically measured. The following TB score parameters were used: (i) coughing; (ii) hemoptysis; (iii) chest pain; (iv) dyspnea; (v) night sweating; (vi) anemic conjunctivae; (vii) positive finding at auscultation; (viii) axillary temperature >37.0°C; (ix) mid upper arm circumference (MUAC) <220 mm; (x) MUAC <200 mm; (xi) body mass index (BMI) <18 kg/m2; and (xii) BMI <16 kg/m2. TB score was then categorized into mild (score of 1–5) and severe (score of ≥6). Low BMI was defined as BMI <18 kg/m2; high sputum bacterial load as AFB sputum smear result ≥2+ (quantitative scoring), which correlates with GeneXpert Ct values [32]. To assess the clinical outcomes among TB patients, we defined poor gain as a change in absolute body weight (<7 and ≥7 kg), BMI (<2.6 and ≥2.6 kg/m2) and body fat (<0 and ≥0%) from recruitment to month 6 of follow-up.
“Any helminth infection” was defined as infection with any of the following helminth species: A. lumbricoides, E. vermicularis, hookworm, Hymenolepis diminuta, S. haematobium, S. mansoni, S. stercoralis and T. trichiura. High occupational risk for schistosomiasis was defined as working in rice fields, sand harvesting, washing cars, and fishing in freshwater. The intensity of helminth infection was defined according to WHO classification [33]. The average egg counts from the triplicate Kato-Katz thick smears per stool sample and per individual were multiplied by a factor of 24 to obtain eggs per gram (EPG) of stool [25].
Statistical analysis
We compared the characteristics of TB patients and household contact controls at the time of TB diagnosis or enrolment. The prevalence of helminth infection was calculated from the generalized estimations equation adjusting for clustering at the household level. We used multilevel mixed-effects logistic regression with random intercepts at the level of households to assess risk factors for helminth infection. To assess risk factors for TB, we compared cases and controls using unconditional logistic regression because not all TB cases could be assigned a control. In addition, we also performed conditional logistic regression among matched pairs to confirm the results. Additional analyses assessed the association of TB and with specific helminth species separately. We also examined whether the association between the presence of a helminth infection and a recent history of deworming drugs depended on HIV infection status by including an interaction term in the logistic regression model. Among TB patients, logistic regression models were used to study associations between helminth infection and clinical presentation at the time of TB diagnosis (such as TB score, high sputum bacterial load, lung infiltration, and cavitation), and to study the association between helminth infection and clinical outcomes after 6 months of TB treatment (change in absolute weight, BMI, and percentage body fat). Associations were expressed as crude odds ratios (ORs) and adjusted ORs (aORs). All analyses were performed in Stata version 14.0 (Stata Corp; Texas, United States of America).
We used the geographic coordinates of the TB patients’ homes to analyze the spatial distribution of TB and helminth co-infections. The prevalence of helminths and helminth species was analyzed at the ward level for optimal readability. The average area per ward in the Dar es Salaam region is 15.5 km2 [19]. The maps were produced using the software package ArcGIS Desktop version 10.2 (ESRI; California, United States of America) and the shape files from the National Bureau of Statistics of Tanzania [34].
Results
Characteristics of study participants
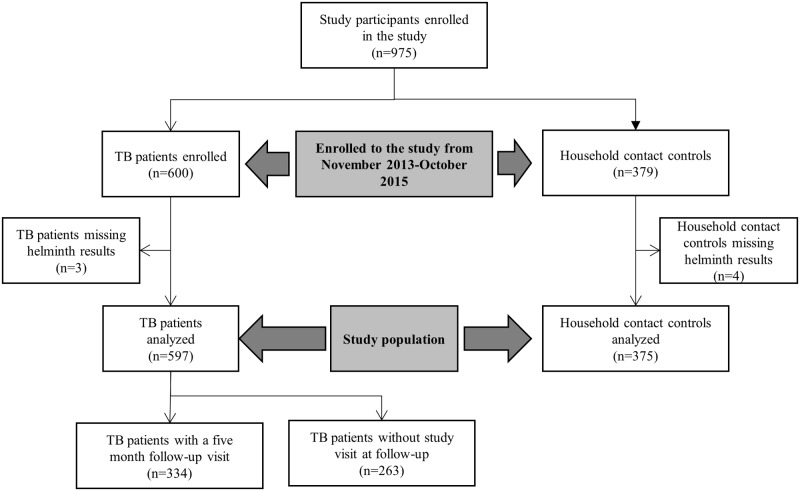
A total of 597 TB patients and 375 household contact controls were included. Table 1 summarizes the socio-demographic and clinical characteristics of TB patients and controls. The study participants’ flow diagram is shown in Fig 1. Among all study participants, the median age was 33 years (interquartile range [IQR]: 26–41 years) and 60.2% (585/972) were men. HIV prevalence was 20.4% (95% confidence interval (CI): 17.9–23.0%). TB patients were more frequently male compared with controls (68.8% [411/597] vs. 46.4% [174/375]), HIV-positive (27.3% [163] vs. 9.3% [35]), and smokers (18.1% [108] vs. 8.8% [33]). TB patients also had a lower median BMI (18.3 kg/m2, IQR: 16.5–20.4 kg/m2 vs. 23.9 kg/m2, IQR: 21.6–28.1 kg/m2) and a lower median hemoglobin level (11.3 g/dl, IQR: 9.9–12.7 g/dl vs. 12.8 g/dl, IQR: 11.5–14.1 g/dl). The patient characteristics, stratified by HIV status, are shown in S1 Table.
Table 1. Socio-demographic and clinical characteristics of tuberculosis (TB) patients and household contact controls without TB.
| Characteristics | Total (n = 972) |
TB patient (n = 597) |
Controls (n = 375) |
|---|---|---|---|
| Age in years, median (IQR) | 33 (26–41) | 33 (26–40) | 33 (26–42) |
| Age groups (years) | |||
| 18–24 | 194 (20.0) | 107 (17.9) | 87 (23.2) |
| 25–34 | 347 (35.7) | 226 (37.9) | 121 (32.3) |
| 35–44 | 266 (27.4) | 169 (28.3) | 97 (25.9) |
| ≥45 | 165 (17.0) | 95 (15.9) | 70 (18.7) |
| Sex | |||
| Female | 387 (39.8) | 186 (31.2) | 201 (53.6) |
| Male | 585 (60.2) | 411 (68.8) | 174 (46.4) |
| HIV status | |||
| Negative | 774 (79.6) | 434 (72.7) | 340 (90.7) |
| Positive | 198 (20.4) | 163 (27.3) | 35 (9.3) |
| Education level | |||
| No/primary | 806 (82.9) | 500 (83.8) | 306 (81.6) |
| Secondary/University | 166 (17.1) | 97 (16.2) | 69 (18.4) |
| Occupation | |||
| Unemployed | 349 (35.9) | 204 (34.2) | 145 (38.7) |
| Employed | 623 (64.1) | 393 (65.8) | 230 (61.3) |
| Smoking status | |||
| No | 831 (85.5) | 489 (81.9) | 342 (91.2) |
| Yes | 141 (14.5) | 108 (18.1) | 33 (8.8) |
| People in the household | |||
| ≤3 | 731 (75.2) | 442 (74.0) | 289 (77.1) |
| >3 | 241 (24.8) | 155 (26.0) | 86 (22.9) |
| Household income per month (US$) | |||
| ≤100 | 763 (78.5) | 473 (79.2) | 290 (77.3) |
| >100 | 209 (21.5) | 124 (20.8) | 85 (22.7) |
| Body weight at diagnosis, [in kg], (IQR) | 54 (48–61) | 51 (46–57) | 59 (53–67) |
| BMI (kg/m2), median (IQR) | 20.0 (17.6–23.4) | 18.3 (16.6–20.4) | 23.9 (21.6–28.1) |
| BMI categories (kg/m2) | |||
| Underweight <18.5 | 337 (34.7) | 318 (53.3) | 19 (5.1) |
| Normal, 18.5–24.9 | 454 (46.7) | 256 (42.9) | 198 (52.8) |
| Overweight 25.0–29.9 | 119 (12.2) | 21 (3.5) | 98 (26.1) |
| Obese ≥30 | 62 (6.4) | 2 (0.3) | 60 (16.0) |
| Body fat (%) | 10.1 (7.7–14.7) | 9.5 (6.8–13.7) | 11.5 (8.5–17.0) |
| MUAC (cm), median (IQR) | 24.3 (22.7–26.2) | 23.3 (22.0–25.3) | 25.3 (23.7–28.0) |
| Waist hip ratio, median (IQR) | 0.89 (0.86–0.94) | 0.89 (0.86–0.94) | 0.89 (0.86–0.94) |
| Occupational riska | |||
| No | 521 (54.2) | 322 (54.2) | 199 (54.1) |
| Yes | 441 (45.8) | 272 (45.8) | 169 (45.9) |
| Individual deworming (past 12 months) | |||
| Yes | 797 (82.0) | 484 (81.1) | 313 (83.5) |
| No | 175 (18.0) | 113 (18.9) | 62 (16.5) |
| Hb level (g/dl), median (IQR) | 12 (10.4–13.3) | 11.3 (9.9–12.7) | 12.8 (11.5–14.1) |
a Occupational risk for acquiring schistosomiasis (working in rice fields, sand harvesting, washing cars, and fishing)
BMI, body mass index; HIV, human immunodefiency virus; Hb, hemoglobin level; IQR, inter-quartile range; MUAC, mid-upper arm circumference; US$, United States dollars (1 US$ = 2,190 Tanzanian Shillings in March 2016)
Fig 1. Study participants’ flow diagram.
Prevalence and risk factors for helminth infection
Among all participants, the prevalence of any helminth infection was 29.5% (95% CI: 26.7–32.6%). S. stercoralis (16.5%, 161) was the predominant helminth species, followed by hookworm (9.0%, 87), S. mansoni (5.7%, 55) and S. haematobium (2.0%, 19). Overall, TB patients were more frequently co-infected with any helminth species compared with controls (OR 1.34, 95% CI: 1.00–1.78, p = 0.048; Table 2). The prevalence of helminth infection was lower in HIV-positive (22.7%, 45) compared with HIV-negative study participants (31.3%, 242; S1 Table). Similarly, helminth infection was lower among TB patients co-infected with HIV (22.7%, 37) compared with HIV-negative TB patients (35.3%, 153; S2 Table). We found that most study participants had light-intensity helminth infection. For example, 96.4% (54) of study participants had light-intensity hookworm infection as determined by the Kato-Katz method (S3 Table). The prevalence and geographic distribution of species-specific helminth infections in the study area is shown in S1 Fig.
Table 2. Frequency distribution of helminth infections, stratified by TB patients and household contact controls.
| Helminth infection | All | TB patients | Controls | Comparing TB patients and controlsa | |
|---|---|---|---|---|---|
| (n = 972) | (n = 597) | (n = 375) | |||
| n (%) | n (%) | n (%) | OR (95% CI) | p-value | |
| Any helminth | 287 (29.5) | 190 (31.8) | 97 (25.9) | 1.34 (1.00–1.78) | 0.048 |
| Helminth species | |||||
| Strongyloides stercoralis | 161 (16.6) | 111 (18.6) | 50 (13.3) | 1.48 (1.03–2.13) | 0.032 |
| Hookworm | 87 (9.0) | 55 (9.2) | 32 (8.5) | 1.09 (0.69–1.72) | 0.72 |
| Ascaris lumbricoides | 6 (0.6) | 3 (0.5) | 3 (0.8) | 0.63 (0.13–3.12) | 0.57 |
| Enterobius vermicularis | 5 (0.5) | 1 (0.2) | 4 (1.1) | NA | NA |
| Trichuris trichiura | 9 (0.9) | 6 (1.0) | 3 (0.8) | 1.25 (0.31–5.06) | 0.75 |
| Hymenolepis diminuta | 2 (0.2) | 1 (0.2) | 1 (0.3) | NA | NA |
| Schistosoma spp. | 70 (7.2) | 49 (8.2) | 21 (5.6) | 1.51 (0.89–2.56) | 0.13 |
| Schistosoma mansoni | 55 (5.7) | 40 (6.7) | 15 (4.0) | 1.72 (0.94–3.17) | 0.079 |
| Schistosoma haematobium | 19 (2.0) | 11 (1.8) | 8 (2.1) | 0.86 (0.34–2.16) | 0.75 |
| Helminth infection | 0.13 | ||||
| None | 685 (70.5) | 407 (68.2) | 278 (74.1) | 1 | |
| Mono-infection | 237 (24.4) | 158 (26.5) | 79 (21.1) | 1.37 (1.00–1.86) | |
| Infection with ≥2 species | 50 (5.1) | 32 (5.3) | 18 (4.8) | 1.21 (0.67–2.21) | |
a Estimates from an unadjusted mixed-effect models with household as a random intercept
NA, not applicable; OR, odds ratio
Study participants with occupational risk for acquiring schistosomiasis, such as working in rice fields, sand harvesting, washing cars, and fishing had higher odds of being infected with any helminth species (aOR 1.42, 95% CI: 1.04–1.95, p = 0.029). HIV-positive patients were less likely to be infected with any helminth species (aOR 0.57, 95% CI: 0.37–0.87, p = 0.010; Table 3). Study participants who did not take anthelmintic treatment in the past 12 months did not have significant higher odds of being co-infected with any helminth species (aOR 1.35, 95% CI: 0.92–1.99, p = 0.12). There was no statistically significant interaction between the effects of HIV infection and deworming status on TB incidence (P-value from test for interaction: 0.5). When analyzing the risk factors for helminth infection separately for TB patients and household controls without TB, we found similar results (see S5 and S6 Tables).
Table 3. Risk factors for any helminth infection among TB patients and household controls without TB.
| Characteristic | Helminth infection, n (%) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Yes | No | OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Participant | 0.054 | 0.18 | ||||
| Controls | 97 (33.8) | 278 (40.6) | 1.00 | 1.00 | ||
| TB patients | 190 (66.2) | 407 (59.4) | 1.35 (1.00–1.82) | 1.29 (0.88–1.87) | ||
| Age group (years) | 0.30 | 0.46 | ||||
| 18–24 | 50 (17.4) | 144 (21.0) | 1.00 | 1.00 | ||
| 25–34 | 115 (40.1) | 232 (33.9) | 1.46 (0.96–2.23) | 1.38 (0.89–2.17) | ||
| 35–44 | 75 (26.1) | 191 (27.9) | 1.13 (0.72–1.78) | 1.11 (0.68–1.82) | ||
| ≥45 | 47 (16.4) | 118 (17.2) | 1.16 (0.70–1.92) | 1.18 (0.69–2.03) | ||
| Sex | 0.003 | 0.24 | ||||
| Female | 93 (32.4) | 294 (42.9) | 1.00 | 1.00 | ||
| Male | 194 (67.6) | 391 (57.1) | 1.60 (1.17–2.18) | 1.23 (0.87–1.75) | ||
| HIV status | 0.022 | 0.010 | ||||
| Negative | 242 (84.3) | 532 (77.7) | 1.00 | 1.00 | ||
| Positive | 45 (15.7) | 153 (22.3) | 0.63 (0.43–0.94) | 0.57 (0.37–0.87) | ||
| BMI category (kg/m2) | 0.077 | 0.47 | ||||
| BMI ≥18 | 175 (61.0) | 460 (67.2) | 1.00 | 1.00 | ||
| BMI <18 | 112 (39.0) | 225 (32.8) | 1.32 (0.97–1.79) | 1.14 (0.79–1.64) | ||
| Education level | 0.28 | 0.50 | ||||
| No/primary | 243 (84.7) | 563 (82.2) | 1.00 | 1.00 | ||
| Secondary/University | 44 (15.3) | 122 (17.8) | 0.80 (0.53–1.20) | 0.86 (0.55–1.34) | ||
| Employment status | 0.13 | 0.42 | ||||
| Unemployed | 93 (32.4) | 256 (37.4) | 1.00 | 1.00 | ||
| Employed | 194 (67.6) | 429 (62.6) | 1.28 (0.93–1.76) | 1.16 (0.81–1.65) | ||
| Number of people in the household | 0.66 | 0.97 | ||||
| ≤3 | 218 (76.0) | 69 (24.0) | 1.00 | 1.00 | ||
| >3 | 513 (74.9) | 172 (25.1) | 0.93 (0.65–1.32) | 0.99 (0.69–1.42) | ||
| Household income per month (US$) | 0.47 | 0.75 | ||||
| ≤100 | 229 (79.8) | 534 (78.0) | 1.00 | 1.00 | ||
| >100 | 58 (20.2) | 151 (22.0) | 0.87 (0.60–1.26) | 0.94 (0.63–1.40) | ||
| Individual deworming (past 12 months) | 0.043 | 0.12 | ||||
| Yes | 224 (78.0) | 573 (83.6) | 1.00 | 1.00 | ||
| No | 63 (22.0) | 112 (16.4) | 1.48 (1.01–2.15) | 1.35 (0.92–1.99) | ||
| Occupational riska | 0.009 | 0.029 | ||||
| No | 136 (47.7) | 385 (56.9) | 1.00 | 1.00 | ||
| Yes | 149 (52.3) | 292 (43.1) | 1.50 (1.11–2.03) | 1.42 (1.04–1.95) | ||
a Occupational risk for acquiring schistosomiasis (working in rice fields, sand harvesting, washing cars, and fishing)
BMI, body mass index; HIV, human immunodeficieny virus; US$, United States dollars (1 US$ = 2,190 Tanzanian Shillings in March 2016)
Multilevel mixed-effects logistic regression model with household as a random intercept, adjusted for TB status, age-groups, sex, HIV status, BMI, education level, employment status, number of people living in the same household, individual deworming status, occupational risk, and income level.
Note: interaction between the effect of HIV and deworming status on the risk for any helminth infection: p = 0.50
Helminth infection as a risk factor for TB
Multiple logistic regression models adjusted for patient characteristics and known risk factors for TB showed that any helminth infection was not statistically significantly associated with TB (aOR 1.26, 95% CI: 0.88–1.80, p = 0.22, Table 4 and S7 Table). However, when analyzing each helminth species separately, we found that S. mansoni infection was significantly associated with TB (aOR 2.15, 95% CI: 1.03–4.45, p = 0.040), but there was no significant association between TB and S. stercoralis or hookworm infection (S8 Table). Other co-factors that were significantly associated with TB included: male sex, HIV co-infection, smoking, living in a household with ≥3 people, and a low BMI (Table 4). The unadjusted and adjusted ORs for any helminth infection and S. mansoni are shown in S7 Table. Results were more pronounced when using a conditional logistic regression model (S9 Table).
Table 4. Associations of TB disease with helminth infection and other patient characteristics.
| Characteristics | Any helminth infection (n = 972) | S. mansoni infection (n = 972) | ||||
|---|---|---|---|---|---|---|
| TB patients | Controls | Adjusted | Adjusted | |||
| n (%) | n (%) | aOR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Helminth infection | 0.22 | 0.040 | ||||
| No | 407 (68.2) | 278 (74.1) | 1.00 | 1.00 | ||
| Yes | 190 (31.8) | 97 (25.9) | 1.26 (0.88–1.80) | 2.15 (1.03–4.45) | ||
| Age group (years) | 0.49 | 0.25 | ||||
| 18–24 | 107 (17.9) | 87 (23.2) | 1.00 | 1.00 | ||
| 25–34 | 226 (37.9) | 121 (32.3) | 1.22 (0.77–1.94) | 1.24 (0.78–1.97) | ||
| 35–44 | 169 (28.3) | 97 (25.9) | 1.00 (0.60–1.67) | 1.02 (0.61–1.7) | ||
| ≥45 | 95 (15.9) | 70 (18.7) | 0.85 (0.48–1.48) | 0.88 (0.51–1.54) | ||
| Sex | <0.001 | <0.001 | ||||
| Female | 186 (31.2) | 201 (53.6) | 1.00 | 1.00 | ||
| Male | 411 (68.8) | 174 (46.4) | 3.12 (2.13–4.56) | 3.16 (2.16–4.63) | ||
| HIV status | <0.001 | <0.001 | ||||
| Negative | 434 (72.7) | 340 (90.7) | 1.00 | 1.00 | ||
| Positive | 163 (27.3) | 35 (9.3) | 6.18 (3.83–9.95) | 6.23 (3.86–10.05) | ||
| Education level | 0.55 | 0.57 | ||||
| No/primary | 500 (83.8) | 306 (81.6) | 1.00 | 1.00 | ||
| Secondary/University | 97 (16.2) | 69 (18.4) | 1.15 (0.73–1.80) | 1.14 (0.72–1.79) | ||
| Employment status | 0.63 | 0.66 | ||||
| Unemployed | 204 (34.2) | 145 (38.7) | 1.00 | 1.00 | ||
| Employed | 393 (65.8) | 230 (61.3) | 0.91 (0.62–1.33) | 0.92 (0.63–1.34) | ||
| Smoking status | 0.012 | 0.011 | ||||
| No | 489 (81.9) | 342 (91.2) | 1.00 | 1.00 | ||
| Yes | 108 (18.1) | 33 (8.8) | 1.92 (1.15–3.21) | 1.95 (1.16–3.25) | ||
| Number of people in the household | 0.018 | 0.015 | ||||
| ≤3 people | 442 (74.0) | 289 (77.1) | 1.00 | 1.00 | ||
| >3 people | 155 (26.0) | 86 (22.9) | 1.58 (1.08–2.30) | 1.60 (1.09–2.34) | ||
| Household income per month (US$) | 0.85 | 0.95 | ||||
| ≤100 | 473 (79.2) | 290 (77.3) | 1.00 | 1.00 | ||
| >100 | 124 (20.8) | 85 (22.7) | 1.04 (0.69–1.56) | 1.01 (0.68–1.52) | ||
| BMI category (kg/m2) | <0.001 | <0.001 | ||||
| BMI ≥18 | 279 (46.7) | 318 (53.3) | 1.00 | 1.00 | ||
| BMI <18 | 356 (94.9) | 19 (5.1) | 23.20 (13.91–38.69) | 23.52 (14.1–39.24) | ||
| Occupational riska | 0.24 | 0.26 | ||||
| No | 322 (54.2) | 199 (54.1) | 1.00 | 1.00 | ||
| Yes | 272 (45.8) | 169 (45.9) | 0.82 (0.59–1.15) | 0.83 (0.59–1.15) | ||
| Individual deworming (past 12 months) | 0.20 | 0.21 | ||||
| Yes | 484 (81.1) | 313 (83.5) | 1.00 | 1.00 | ||
| No | 113 (18.9) | 62 (16.5) | 0.75 (0.48–1.16) | 0.76 (0.49–1.17) | ||
BMI, body mass index; CI, confidence interval; HIV, human immunodeficieny virus; OR, odds ratio; US$, United States dollars (1 US$ = 2,190 Tanzanian Shillings in March 2016)
a Occupational risk for acquiring schistosomiasis (working in rice fields, sand harvesting, washing cars, and fishing)
Logistic regression model for TB disease status as the outcome. Model adjusted for any helminth infection/S. mansoni, age, sex, HIV status, BMI, education level, employment status, smoking status, number of people living in the same household, individual deworming status, helminth risk occupation and income level.
The full table with unadjusted and adjusted odds ratios is shown in the Supplementary Information (S7 Table).
Effect of helminth infection on clinical presentation and disease severity in TB patients
TB patients co-infected with any helminth infection were more likely than helminth un-infected TB patients to present with hemoptysis (74 [38.9%] vs. 123 [30.2%]), had higher median hemoglobin levels (11.7 g/dl, IQR: 10.1–13.0 g/dl vs. 11.3 g/dl, IQR: 9.8–12.5 g/dl) and higher median eosinophil counts (0.2, IQR: 0.1–0.4 cells/μl vs. 0.1, IQR: 0.05–0.2 cells/μl; Table 5). TB patients co-infected with S. mansoni were more likely to have lower sputum bacterial load than helminth-uninfected TB patients (aOR 2.63; 95% CI: 1.38–5.26, p = 0.004). Furthermore, we found that TB patients co-infected with S. mansoni tended to have fewer lung cavities, although this association lacked statistical significance (aOR 0.41, 95% CI: 0.12–1.16, p = 0.088; Table 6). There were no statistically significant differences in radiological features between TB patients with and without any helminth infection as shown in S10 Table.
Table 5. Patient characteristics of TB patients infected and not infected with helminths at the time of TB diagnosis.
| Characteristics | Total | TB and helminth | TB only | p-value |
|---|---|---|---|---|
| (n = 597) | (n = 190) | (n = 407) | ||
| Age, median (IQR) (years) | 33 (26–40) | 31 (26–39) | 34 (27–40) | 0.22 |
| Age groups (years) | 0.13 | |||
| 18–24 | 107 (17.9) | 35 (18.4) | 72 (17.7) | |
| 25–34 | 226 (37.9) | 81 (42.6) | 145 (35.6) | |
| 35–44 | 169 (28.3) | 42 (22.1) | 127 (31.2) | |
| ≥45 | 95 (15.9) | 32 (16.8) | 63 (15.5) | |
| Sex | 0.007 | |||
| Female | 186 (31.2) | 45 (23.7) | 141 (34.6) | |
| Male | 411 (68.8) | 145 (76.3) | 266 (65.4) | |
| HIV status | 0.003 | |||
| Negative | 434 (72.7) | 153 (80.5) | 281 (69.0) | |
| Positive | 163 (27.3) | 37 (19.5) | 126 (31.0) | |
| CD4+ count, cells/mla | 202 (94–273) | 185 (90–259) | 203 (100–273) | 0.74 |
| Education level | 0.49 | |||
| No/primary | 500 (83.8) | 162 (85.3) | 338 (83.0) | |
| Secondary/University | 97 (16.2) | 28 (14.7) | 69 (17.0) | |
| Occupation | 0.99 | |||
| Unemployed | 204 (34.2) | 65 (34.2) | 139 (34.2) | |
| Employed | 393 (65.8) | 125 (65.8) | 268 (65.8) | |
| Number of people in the household | 0.89 | |||
| ≤3 people | 442 (74.0) | 140 (73.7) | 302 (74.2) | |
| > 3 people | 155 (26.0) | 50 (26.3) | 105 (25.8) | |
| Smoking status | <0.004 | |||
| No | 489 (81.9) | 143 (75.3) | 346 (85.0) | |
| Yes | 108 (18.1) | 47 (24.7) | 61 (15.0) | |
| Household income per month (US$) | 0.45 | |||
| ≤100 | 473 (79.2) | 154 (81.1) | 319 (78.4) | |
| >100 | 124 (20.8) | 36 (18.9) | 88 (21.6) | |
| Body weight (kg), median (IQR) | 51 (46–57) | 50.9 (46–56) | 51.7 (46–57.5) | 0.40 |
| BMI (kg/m2), median(IQR) | 18.3 (16.6–20.4) | 18.2 (16.5–20.2) | 18.5 (16.6–20.4) | 0.22 |
| BMI (kg/m2) groups, n (%) | 0.40b | |||
| Underweight <18.5 | 318 (53.3) | 108 (56.8) | 210 (51.6) | |
| Normal, 18.5–24.9 | 256 (42.9) | 78 (41.1) | 178 (43.7) | |
| Overweight 25.0–29.9 | 21 (3.5) | 4 (2.1) | 17 (4.2) | |
| Obese ≥30 | 2 (0.3) | 0 | 2 (0.5) | |
| Body fat (%) | 9.5 (6.8–13.7) | 9.1 (6.0–12.7) | 9.8 (7.4–14.0) | 0.008 |
| MUAC (cm), median (IQR) | 23.3 (22.0–25.3) | 23.7 (22.0–25.0) | 23.3 (22.0–25.7) | 0.99 |
| Waist hip ratio, median (IQR) | 0.89 (0.85–0.94) | 0.89 (0.85–0.94) | 0.89 (0.86–0.94) | 0.75 |
| Occupational risk | 0.095 | |||
| No | 322 (54.2) | 93 (49.2) | 229 (56.5) | |
| Yes | 272 (45.8) | 96 (50.8) | 176 (43.5) | |
| Individual deworming (past 12 months) | 0.013 | |||
| Yes | 484 (81.1) | 143 (75.3) | 341 (83.8) | |
| No | 113 (18.9) | 47 (24.7) | 66 (16.2) | |
| Symptomsc | ||||
| Cough | 594 (99.5) | 189 (99.5) | 405 (99.5) | 0.96 |
| Fever | 551 (92.3) | 174 (91.6) | 377 (92.6) | 0.65 |
| Weight loss | 573 (96.0) | 181 (95.3) | 392 (96.3) | 0.54 |
| Night sweats | 566 (94.8) | 184 (96.8) | 382 (93.9) | 0.13 |
| Hemoptysis | 197 (33.0) | 74 (38.9) | 123 (30.2) | 0.035 |
| TB score, median (IQR) | 5 (4–6) | 5 (4–6) | 5 (4–6) | 0.22 |
| TB score (0–5) | 372 (62.3) | 115 (60.5) | 257 (63.1) | |
| TB score (6–12) | 225 (37.7) | 75 (39.5) | 150 (36.9) | |
| TB treatment categories | 0.40 | |||
| Retreatment | 14 (2.3) | 3 (1.6) | 11 (2.7) | |
| New patients | 583 (97.7) | 187 (98.4) | 396 (97.3) | |
| Blood parameters c | ||||
| Hemoglobin level | 11.3 (9.9–12.7) | 11.7 (10.1–13) | 11.3 (9.8–12.5) | 0.044 |
| Eosinophil, cells per μld | 0.15 (0.06–0.32) | 0.2 (0.1–0.4) | 0.1 (0.05–0.2) | 0.003 |
AFB, acid-fast bacilli; BMI, body mass index; HIV, human immunodefiency virus; IQR, interquartile range; MUAC, mid-upper arm circumference; US$, United States dollars (1 US$ = 2,190 Tanzanian Shillings in March 2016)
Helminth infection occupation risk (working in rice fields, sand harvesting, washing cars, and fishing)
a TB patient co-infected with HIV and have CD4+ count values (n = 80)
b Fisher’s exact test
c“Symptoms”, and “blood parameters”: categories not mutually exclusive
d TB patients with an available full blood count result (n = 322)
Table 6. Effect of helminth infection on the clinical severity and clinical presentation in TB patients at the time of TB diagnosis.
| Helminth infection | Severe TB scorea | High sputum bacterial loadb | Lung infiltration | Lung cavitation | ||||
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Any helminth infection | 0.55 | 0.12 | 0.42 | 0.82 | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1.12 (0.78–1.61) | 0.75 (0.51–1.08) | 0.82 (0.50–1.33) | 0.95 (0.60–1.50) | ||||
| Strongyloides stercoralisc | 0.44 | 0.39 | 0.17 | 0.76 | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1.19 (0.76–1.86) | 0.82 (0.52–1.29) | 1.56 (0.83–2.92) | 1.09 (0.62–1.91) | ||||
| Schistosoma mansoni d | 0.75 | 0.004 | 0.15 | 0.088 | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 0.89 (0.45–1.78) | 0.37 (0.19–0.72) | 0.51 (0.21–1.27) | 0.41 (0.12–1.16) | ||||
| Hookworme | 0.55 | 0.40 | 0.086 | 0.54 | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1.20 (0.67–2.15) | 0.77 (0.42–1.42) | 0.51 (0.23–1.10) | 0.79 (0.37–1.69) | ||||
| Multiple infections | 0.82 | 0.020 | 0.19 | 0.40 | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Mono | 1.13 (0.77–1.67) | 0.88 (0.59–1.31) | 0.85 (0.51–1.43) | 1.06 (0.66–1.72) | ||||
| Double or more | 1.04 (0.48–2.22) | 0.34 (0.16–0.73) | 0.67 (0.24–1.84) | 0.50 (0.17–1.44) | ||||
Logistic regression model adjusted for age, sex, HIV infection, and smoking status.
a TB score (mild [score of 1–5] and severe [score of 6–12])
b Sputum bacterial load (according to qualitative AFB smear microscopy grading): mild (scanty and 1+) and severe (≥+2)
c 79 TB patients with any helminth infection other than S. stercoralis were excluded
d 150 TB patients with helminth co-infection other than S. mansoni were excluded
e 72 TB patients with helminth co-infection other than hookworm were excluded
Effect of helminth infection on clinical outcomes in TB patients
Overall, 81.7% (273 TB patients) were cured at the end of TB treatment (at 6 months), 17.4% (58) completed treatment (AFB smear results not available at 6 months, but documented completion of treatment), and 0.9% (3) were treatment failures (positive AFB smear result at 6 months). We found no significant associations between helminth infection (at time of recruitment) and poor gain in absolute weight (aOR 0.89, 95% CI: 0.55–1.45, p = 0.63), BMI (aOR 0.74, 95% CI: 0.46–1.21, p = 0.23), and body fat percentage (aOR 0.92, 95% CI: 0.55–1.56, p = 0.78) after 6 months on TB treatment, as shown in S11 Table.
Discussion
We present findings on the prevalence and association of TB and helminth co-infection among adult TB patients and household contact controls in a highly-urbanized setting of Dar es Salaam, Tanzania. We found that S. mansoni infection was a risk factor for TB disease. This association remained significant after adjustment for other known risk factors for TB, such as HIV infection, smoking, and underweight [35]. None of the other investigated helminth species or the surrogate measure of “any helminth infection” were significantly associated with TB. Importantly, associations between any helminth co-infection and TB were reported in previous epidemiologic studies [21,36,37], as well as in experimental work using animal or macrophage infection models [9,13,15]. In line with our findings, a recent study with human peripheral mononuclear cells exposed to M. tuberculosis and S. mansoni antigens showed that S. mansoni-induced CD4+ T cells disrupt the control of M. tuberculosis in infected macrophages [9].
Several studies in humans suggested that helminth infections may increase the risk for progression of latent M. tuberculosis infection to active TB [15,21,37] as well as for exacerbating the disease [15]. However, the results of these studies are conflicting, and no differentiation at the helminth species level was made in these analyses. Indeed, the hypothesis of a helminth species-specific impact on the host response is supported by a recent systematic review, which revealed a trend toward an association between a decrease in HIV viral loads and treatment for S. mansoni, but not for other helminth species [38]. A case-control study from Ethiopia also found an association between TB and helminth infections, and the association was stronger in patients that were infected with multiple helminth species [21]. The small number of study participants with S. mansoni infection (31 among TB cases, nine among controls) may have masked an association between TB and schistosomiasis in that study [21]. In contrast, a cohort study from India showed no difference in TB incidence rates in helminth-infected and helminth-free individuals after 2.5 years of follow-up [39].
We also found that S. mansoni, but not other helminth species, was associated with the clinical presentation among TB patients. Patients co-infected with S. mansoni had lower sputum bacterial loads at the time of TB diagnosis than S. mansoni-negative TB patients. Similarly, a study in Ethiopia observed lower sputum bacterial loads at TB diagnosis in TB patients co-infected with any helminth species [40]. Interestingly, our observation in TB patients co-infected with S. mansoni resembles the paucibacillary disease in HIV-positive individuals with severe immunosuppression, who frequently have negative or low bacterial M. tuberculosis loads in the sputum compared with HIV-negative patients [40,41]. Hence, the helminth-induced Th1 immunological impairment might have an effect on the sputum bacterial load. Moreover, TB patients with an impaired host immune system rarely present with lung cavitation resulting in fewer M. tuberculosis bacilli being expectorated in the sputum [40,41]. This is in line with our findings that TB patients co-infected with S. mansoni tended to present less frequently with lung cavitations compared with S. mansoni-negative TB patients. Any helminth co-infection did not appear to have an effect on clinical outcomes during follow-up. We found no evidence for an effect of helminth co-infection on the gain in the percentage of body fat and BMI after 6 months (e.g., at the time of completed TB treatment). This might be explained by the fact that the administration of anthelmintic treatment offered to the study participants after diagnosis might have reversed the Th1 immune response [15], and thus attenuated the effect of helminth infections on clinical outcomes. However, the effect of a reversal of the Th1 immune response could be minimal as the anthelmintic drugs target the worms [42], which are less immunogenic compared with deposited S. mansoni eggs [9].
We found that TB patients had a higher crude prevalence of helminth infections, as compared with household contact controls. The higher prevalence of helminth infections among TB patients could be the result of the pathogenic role of helminth infection in the progression from M. tuberculosis infection to active TB. The higher prevalence of helminth co-infection in TB patients has also been noted in other studies from different settings [9,43]. For example, a study conducted in Ethiopia reported a higher prevalence of helminth infection among TB patients as compared with household contact controls [21]. Overall, the prevalence of helminth infection in our study was 32% and lower compared with the 71% observed in the latter study [21]. It is conceivable that the high proportion of self-reported previous use of anthelmintic drugs in our study (approximately 80%) could have reduced the overall prevalence of helminth infection. Hence, we may have underestimated the effects of helminth infection seen in our study.
We also found that occupation exposing people to regular water contacts (for instance rice field workers, sand harvesters, car washers, and fishermen) were associated with helminth infections. Being exposed to freshwater bodies and being involved in water-related activities have previously been reported to increase the risk of helminth infections [44]. In the current study, HIV-positive individuals were less likely to be co-infected with helminths. A lower prevalence of helminth infections in HIV-positive patients has also been reported in a study conducted in Mwanza in northern Tanzania, which is a highly endemic area for helminthiases [45]. Of note, current clinical practice in Tanzania is to treat any helminth infection in HIV-positive patients at enrolment into HIV care and in case of clinical suspicion of helminth infection during follow-up, as specified in the HIV/AIDS management guideline [17]. The use of anthelmintic drugs is safe and might be beneficial in HIV-positive patients by possibly reducing the HIV-RNA viral load and subsequently improving clinical outcomes [46]. Furthermore, cotrimoxazole preventive therapy (CPT), which is recommended for HIV-positive patients, has also been reported to have limited anthelmintic properties [43,47]. This might explain the lower prevalence of helminth infection among HIV-positive individuals in our study [17].
Our research has several strengths and limitations that warrant consideration. An important strength of our study is the large sample size and the recruitment of both TB patients and household contact controls with similar socioeconomic profiles and exposure patterns to both TB and helminth infection. Our findings may well apply to other settings with a similar prevalence of TB, HIV, and helminth infections in sub-Saharan Africa. Furthermore, we used recommended TB diagnostics and a suite of standardized, quality-controlled helminth diagnostics, which have comparable diagnostic performance to resource-intensive molecular test assays [25].
Study limitations include the following. First, this is an observational study which cannot establish a causal relationship between helminth infections and TB disease. Second, we could not fully verify whether or not the household contact controls were latently infected with M. tuberculosis, which is a prerequisite to develop TB. However, because Dar es Salaam is a high-burden setting for TB with considerable risk of transmission, and because living with a TB patient is a strong risk factor for TB [35], it is reasonable to assume that the controls have previously been exposed and infected with M. tuberculosis. Third, we did not check the helminth infection status for TB patients during and after completion of TB treatment, which could influence the clinical outcomes. However, we do not expect a high helminth re-infection rate after 6 months in our study area [48]. Fourth, we did not use molecular diagnostics such as polymerase chain reaction (PCR) which might have identified some more cases, but one of our previous studies revealed that also PCR approaches miss in particular very light intensity infections. Moreover, its performance and sensitivity vary with the helminth species under examination [25]. Hence, also a PCR cannot be considered as the diagnostic gold-standard.
In conclusion, co-infection with S. mansoni, but not other helminth species, was found to be an independent risk factor for active TB in our study and was associated with the clinical presentation in TB patients. These findings suggest a role for S. mansoni, or helminth infection in general, in immunomodulation of human TB. Treatment of helminth infections should be considered in the clinical management of TB patients, and helminthiasis control/elimination through preventive chemotherapy might prove to be useful as an additional component of TB control programs. Further research is needed to establish the underlying mechanisms, and compare helminth-induced immune regulation by different helminth species. Prospective cohort studies that evaluate the effect of preventive anthelmintic chemotherapy on the incidence of M. tuberculosis infection and active TB could further help to understand the interaction between these diseases at the population level. Helminthiasis control measures, in combination with traditional TB control strategies, could potentially contribute to the global efforts to reduce TB incidence by 80% until 2030, as stipulated in WHO’s ambitious End TB Strategy [5].
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Associations of TB disease with helminth infection and other patient characteristics comparing TB patients and household contact controls without TB.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(A) The prevalence of helminth infection summarized at the ward level. (B) The helminth species distribution at the study area. Other helminth infections include: Ascaris lumbricoides, Enterobius vermicularis, Trichuris trichiura, and Hymenolepis diminuta.
(DOCX)
(DOCX)
Acknowledgments
We thank all the study participants whose data were used in this study. We are grateful for the assistance and support from the office of the Temeke District Medical Officer, the Temeke district hospital staff, and the National TB Program and the District TB coordinators of the Temeke district, Dar es Salaam, Tanzania. We also thank the study team at the Temeke district hospital for recruiting patients.
Data Availability
Due to ethical restrictions imposed by the Ifakara Health Institute Institutional Review Board and National Health Research Ethics sub-Committee of the National Institute for Medical Research of Tanzania on protecting patient confidentiality, data cannot be made publicly available. Some of identified reasons include many unique identifiers such as TB district number which is the unique number assigned to TB patients who are diagnosed and treated at the health facility where also this study is being conducted. In addition, the dataset has the following variables that may breach patient privacy such as; place of treatment, gender, geographical position system (GPS) of residence of TB patients enrolled into this study, household size, and year of birth. Interested researchers should contact Dr. Frederick Haraka (fharaka@ihi.or.tz) for further information related to data access.
Funding Statement
LF received funding from Rugolf Geigy Foundation (http://www.geigystiftung.ch/en/). FM received funding for his PhD studies from Ifakara Health Institute (www.ihi.or.tz) and Amt für Ausbildungsbeiträge (http://www.hochschulen.bs.ch/ueber-uns/organisation/amt-ausbildungsbeitraege.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Knopp S, Steinmann P, Keiser J, Utzinger J. Nematode infections: soil-transmitted helminths and Trichinella. Infect Dis Clin North Am. 2012;26: 341–358. 10.1016/j.idc.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37 10.1186/1756-3305-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utzinger J, Becker S, Knopp S, Blum J, Neumayr A, Keiser J, et al. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly. 2012; 142:w13727 10.4414/smw.2012.13727 [DOI] [PubMed] [Google Scholar]
- 5.WHO. The end TB strategy. Geneva: World Health Organization; 2015. [Google Scholar]
- 6.Simon GG. Impacts of neglected tropical disease on incidence and progression of HIV/AIDS, tuberculosis, and malaria: scientific links. Int J Infect Dis. 2016;42: 54–57. 10.1016/j.ijid.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 7.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68: 2240–2466. 10.1016/j.socscimed.2009.03.041 [DOI] [PubMed] [Google Scholar]
- 8.NTLP, MoHSW. Manual for the management of tuberculosis and leprosy. 6th ed Dar es Salaam: Ministry of Health and Social Welfare, Dar es Salaam; 2013. [Google Scholar]
- 9.DiNardo AR, Mace EM, Lesteberg K, Cirillo JD, Mandalakas AM, Graviss EA, et al. Schistosome soluble egg antigen decreases Mycobacterium tuberculosis-specific CD4+ T-cell effector function with concomitant arrest of macrophage phago-lysosome maturation. J Infect Dis. 2016;214: 479–488. 10.1093/infdis/jiw156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208: 1863–1874. 10.1084/jem.20091473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafi W, Ribeiro-Rodrigues R, Ellner JJ, Salgame P. Coinfection-helminthes and tuberculosis. Curr Opin HIV AIDS. 2012;7: 239–244. 10.1097/COH.0b013e3283524dc5 [DOI] [PubMed] [Google Scholar]
- 12.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14: 1118–1126. 10.1038/ni.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu S, Nutman TB. Helminth-tuberculosis co-infection: an immunologic perspective. Trends Immunol. 2016;37: 597–607. 10.1016/j.it.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014;7: 753–762. 10.1038/mi.2014.23 [DOI] [PubMed] [Google Scholar]
- 15.Monin L, Griffiths KL, Lam WY, Gopal R, Kang DD, Ahmed M, et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J Clin Invest. 2015;125: 4699–4713. 10.1172/JCI77378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MoHSW. Standard treatment guidelines and essential medicines list. 4th ed Dar es Salaam: Ministry of Health and Social Welfare; 2013. [Google Scholar]
- 17.NACP. National guidelines for the management of HIV and AIDS. 5th ed Dar es Salaam: Ministry of Health and Social Welfare; 2015. [Google Scholar]
- 18.NTLP, MoHSW. National Tuberculosis and Leprosy 2014 Annual Report. Dar es Salaam: Ministry of Health and Social Welfare; 2015. [Google Scholar]
- 19.PMORALG. Dar es Salaam region socio-economic profile. Dar es Salaam: Prime Minister’s Office, Regional Administration and Local Government; 2014. [Google Scholar]
- 20.Mhimbira F, Hella J, Maroa T, Kisandu S, Chiryamkubi M, Said K, et al. Home-based and facility-based directly observed therapy of tuberculosis treatment under programmatic conditions in urban Tanzania. PLoS One. 2016;11: e0161171 10.1371/journal.pone.0161171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health. 2006;11: 551–558. 10.1111/j.1365-3156.2006.01578.x [DOI] [PubMed] [Google Scholar]
- 22.Baty International (BI). The Harpenden skinfold caliper HSB-BI. Baty International, West Sussex; 2007. [Google Scholar]
- 23.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32: 77–97. [DOI] [PubMed] [Google Scholar]
- 24.WHO. The handbook: laboratory diagnosis of tuberculosis by sputum microscopy. Global ed Adelaide: Stop TB Partnership-Global Laboratory Initiative Working Group; 2013. [Google Scholar]
- 25.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, et al. Diagnostic accuracy of Kato–Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg. 2014;90: 535–545. 10.4269/ajtmh.13-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salim N, Knopp S, Lweno O, Abdul U, Mohamed A, Schindler T, et al. Distribution and risk factors for Plasmodium and helminth co-infections: a cross-sectional survey among children in Bagamoyo district, Coastal Region of Tanzania. PLoS Negl Trop Dis. 2015;9: e0003660 10.1371/journal.pntd.0003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salim N, Schindler T, Abdul U, Rothen J, Genton B, Lweno O, et al. Enterobiasis and strongyloidiasis and associated co-infections and morbidity markers in infants, preschool- and school-aged children from rural coastal Tanzania: a cross-sectional study. BMC Infect Dis. 2014;14: 644 10.1186/s12879-014-0644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia LS, Bruckner DA (2001) Diagnostic medical Parasitology; Garcia LS, Bruckner DA, eds. Washington D.C.: American Society for Microbiology; pp 1–791. [Google Scholar]
- 29.Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuenté L-A, N’Goran EK, et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg. 2013;88: 426–432. 10.4269/ajtmh.12-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner A, Hella J, Grüninger S, Mhalu G, Mhimbira F, Cercamondi CI, et al. Managing research and surveillance projects in real-time with a novel open-source eManagement tool designed for under-resourced countries. J Am Med Inform Assoc. 2016; ocv185. [DOI] [PubMed] [Google Scholar]
- 31.Wejse C, Gustafson P, Nielsen J, Gomes VF, Aaby P, Andersen PL, et al. TB score: signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand J Infect Dis. 2008;40: 111–120. 10.1080/00365540701558698 [DOI] [PubMed] [Google Scholar]
- 32.Blakemore R, Nabeta P, Davidow AL, Vadwai V, Tahirli R, Munsamy V, et al. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med. 2011;184: 1076–1084. 10.1164/rccm.201103-0536OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Helminth control in school-age children: a guide for managers of control programmes. Geneva: World Health Organization; 2011. [Google Scholar]
- 34.Tanzania: shapefiles for EAs, villages, districts and regions. In: openmicrodata.org [Internet]. [cited 3 Apr 2016]. https://openmicrodata.wordpress.com/2010/12/16/tanzania-shapefiles-for-eas-villages-districts-and-regions/
- 35.Rieder HL. Epidemiologic basis of tuberculosis control. 1st ed Paris: International Union Against Tuberculosis and Lung Disease; 1999. [Google Scholar]
- 36.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2006;147: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tristão-Sá R, Ribeiro-Rodrigues R, Johnson LT, Pereira FEL, Dietze R. Intestinal nematodes and pulmonary tuberculosis. Rev Soc Bras Med Trop. 2002;35: 533–535. [DOI] [PubMed] [Google Scholar]
- 38.Sangaré LR, Herrin BR, John-Stewart G, Walson JL. Species-specific treatment effects of helminth/HIV-1 co-infection: a systematic review and meta-analysis. Parasitology. 2011;138: 1546–1558. 10.1017/S0031182011000357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatterjee S, Kolappan C, Subramani R, Gopi PG, Chandrasekaran V, Fay MP, et al. Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One. 2014;9: e94603 10.1371/journal.pone.0094603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abate E, Belayneh M, Idh J, Diro E, Elias D, Britton S, et al. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and Th-2 responses and a lower sputum smear positivity. PLoS Negl Trop Dis. 2015;9: e0003994 10.1371/journal.pntd.0003994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis and management. Indian J Med Res. 2005;121: 550–567. [PubMed] [Google Scholar]
- 42.Martin RJ, Puttachary S, Buxton SK, Verma S, Robertson AP. The Conqueror Worm: recent advances with cholinergic anthelmintics and techniques excite research for better therapeutic drugs. J Helminthol. 2015;89: 387–397. 10.1017/S0022149X1400039X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abate E, Belayneh M, Gelaw A, Idh J, Getachew A, Alemu S, et al. The impact of asymptomatic helminth co-infection in patients with newly diagnosed tuberculosis in North-West Ethiopia. PLoS One. 2012;7: e42901 10.1371/journal.pone.0042901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodburn PW, Muhangi L, Hillier S, Ndibazza J, Namujju PB, Kizza M, et al. Risk factors for helminth, malaria, and HIV infection in pregnancy in Entebbe, Uganda. PLoS Negl Trop Dis. 2009;3: e473 10.1371/journal.pntd.0000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Range N, Magnussen P, Mugomela A, Malenganisho W, Changalucha J, Temu MM, et al. HIV and parasitic co-infections in tuberculosis patients: a cross-sectional study in Mwanza, Tanzania. Ann Trop Med Parasitol. 2007;101: 343–351. 10.1179/136485907X176373 [DOI] [PubMed] [Google Scholar]
- 46.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10: 455–463. 10.1016/S1473-3099(10)70093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssen S, Hermans S, Knap M, Moekotte A, Rossatanga EG, Adegnika AA, et al. Impact of anti-retroviral treatment and cotrimoxazole prophylaxis on helminth infections in HIV-Infected patients in Lambaréné, Gabon. PLoS Negl Trop Dis. 2015;9: e0003769 10.1371/journal.pntd.0003769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tukahebwa EM, Vennervald BJ, Nuwaha F, Kabatereine NB, Magnussen P. Comparative efficacy of one versus two doses of praziquantel on cure rate of Schistosoma mansoni infection and re-infection in Mayuge District, Uganda. Trans R Soc Trop Med Hyg. 2013;107: 397–404. 10.1093/trstmh/trt024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Associations of TB disease with helminth infection and other patient characteristics comparing TB patients and household contact controls without TB.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(A) The prevalence of helminth infection summarized at the ward level. (B) The helminth species distribution at the study area. Other helminth infections include: Ascaris lumbricoides, Enterobius vermicularis, Trichuris trichiura, and Hymenolepis diminuta.
(DOCX)
(DOCX)
Data Availability Statement
Due to ethical restrictions imposed by the Ifakara Health Institute Institutional Review Board and National Health Research Ethics sub-Committee of the National Institute for Medical Research of Tanzania on protecting patient confidentiality, data cannot be made publicly available. Some of identified reasons include many unique identifiers such as TB district number which is the unique number assigned to TB patients who are diagnosed and treated at the health facility where also this study is being conducted. In addition, the dataset has the following variables that may breach patient privacy such as; place of treatment, gender, geographical position system (GPS) of residence of TB patients enrolled into this study, household size, and year of birth. Interested researchers should contact Dr. Frederick Haraka (fharaka@ihi.or.tz) for further information related to data access.