Abstract
The three endogenous gaseous transmitters — nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) — regulate a number of key biological functions. Emerging data identify several new mechanisms of each of these three gasotransmitters in tumour biology. It is now appreciated that they show bimodal pharmacological character in cancer, in that not only the inhibition of their biosynthesis, but also elevation of the concentration of each gasotransmitter beyond a certain threshold can exert anticancer effects. This Review discusses the role of each gasotransmitter in cancer and the effects of compounds — some of which are in early-stage clinical studies — that modulate the levels of each gasotransmitter. A clearer understanding of the pharmacological character of these three gases and their underlying biological mechanisms is expected to guide further clinical translation.
Introduction
The three small, diffusible gaseous mediators nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) play multiple roles in normal physiology and in the pathogenesis of many diseases. Although a significant amount of work has been conducted on the role of NO, CO and H2S in cancer, the field is full of paradoxes and controversies, which presents a significant obstacle for clinical translation. One of the biggest obstacles to understanding the roles of these gasotransmitters in cancer was the seeming discrepancy between some studies showing that these mediators have pro-tumour effects, and others that demonstrated antitumour effects. Owing to more recent research, it is now recognized that, in cancer, these three gases exhibit a bell-shaped (often also termed ‘biphasic’, ‘bimodal’ or ‘Janus-faced’) pharmacological character.
A greater appreciation of the complex pharmacological character of these mediators has important implications for a deeper understanding of the pathophysiology of cancer. It also resolves some of these controversies in the field, thereby facilitating the formulation of novel therapeutic concepts, either based on pharmacological inhibition of the formation of these transmitters, or on their therapeutic donation.
This article reviews the major roles of NO, CO and H2S in tumour pathophysiology, illustrating how either lower or higher concentrations can affect tumour growth, angiogenesis and survival. It also highlights the potential therapeutic value in cancer of compounds that modulate gasotransmitter levels by either inhibiting their production or acting as donors.
Nitric oxide
NO, a free radical mediator, has been implicated in a plethora of biological processes. It is produced from L-arginine in various tissues by a family of enzymes called nitric oxide synthases (NOSs) (Table 1).1–4 Endothelial NOS (eNOS; also known as NOS3) and the neuronal NOS (nNOS; also known as NOS1) are constitutive, low-output enzymes, whereas the macrophage-type, or inducible, NOS isoform (iNOS; also known as NOS2) is an inducible, high-output enzyme. NOS enzymes use molecular O2 and require a number of cofactors for their activity. For instance, calmodulin binds tightly with iNOS such that the enzyme is in a continuous activated state.2 NO biosynthesis by the three NOS isoforms can be suppressed using various small-molecule inhibitors, some of which have selectivity for individual NOS isoforms. NG-methyl- L-arginine (L-NMA) inhibits all NOS isoforms and L-NG-nitroarginine methyl ester (L-NAME) has some selectivity for the constitutive NOS isoforms, whereas other inhibitors (aminoguanidine, 1400W and many others) exhibit selectivity for iNOS.5,6
Table 1.
NO, CO and H2S: biological properties and effects on tumour cells
| NO | CO | H2S | |
|---|---|---|---|
| Biological sources |
|
Haem oxygenases |
|
| Chemical properties | Diffusible, labile free radical gas | Diffusible, labile gas | Diffusible, labile gas |
| Biological half-life | Short (a few seconds) | Long (minutes) | Medium (seconds to minutes) |
| Elimination |
|
Mainly unaltered, via the exhaled air |
|
| Key biological reactions |
|
|
|
| Selected signalling pathways |
|
|
|
| Vascular effects in tumours |
|
|
|
| Cellular bioenergetic effects in tumour cells |
|
Inhibits cytochrome c oxidase, impairing cellular bioenergetics |
|
| Direct effects on tumour cell viability |
|
|
|
In its ‘classical’ physiological pathway, NO binds to the haem group of guanylyl cyclase to induce an elevation of intracellular cyclic GMP (cGMP), which leads to downstream effects via cGMP-dependent protein kinases (PKGs). At physiological concentrations, NO also opens ATP-dependent potassium (KATP) channels, and mediates various post-translational protein modifications via S-nitrosylation (Table 1). At higher concentrations, NO can exert deleterious effects, including: inhibition of mitochondrial enzymes; initiation of DNA damage; and the activation of p53 and poly(ADP-ribose polymerase) (Table 1). In biological systems, many of these adverse effects are the consequence of the simultaneous production of NO and oxygen-derived reactive oxidative species (ROS); one pathway that has been implicated in this process involves the generation of peroxynitrite (ONOO–) from one molecule of NO and one molecule of superoxide, followed by the generation of a hydroxyl-radical-type reactive species.1–4,7,8
The cellular (micro)environment strongly influences the biological profile of NO. For example, acidosis increases the half-life and diffusibility of NO. Under acidic conditions, NO can also be generated from its semi-stable metabolite, nitrite (NO2–). Moreover, when levels of the intracellular antioxidants and/or certain NOS co-factors (for instance, tetrahydrobiopterin [BH4]) are depleted, NOS produces superoxide (O2–) ions instead of NO. Such effects of environment and concentration must be borne in mind when interpreting the biological roles of NOS and NO. 1–4,9,10
At low concentrations, NO tends to exert antioxidant type responses, thereby protecting against oxidative cell injury and cell death. 1–4,9,10 It is thought to do this through: neutralizing deleterious redox responses (for example, by inactivating superoxide); cGMP-mediated signalling; phosphorylation of extracellular signal-regulated kinase (ERK); and inhibition of caspase activation. By contrast, higher NO levels (directly or indirectly) lead to DNA damage, impaired cellular metabolism and can initiate various (apoptotic, necrotic or mixed-type) forms of cell death. 1–4,11,12
Antitumour effects of nitric oxide
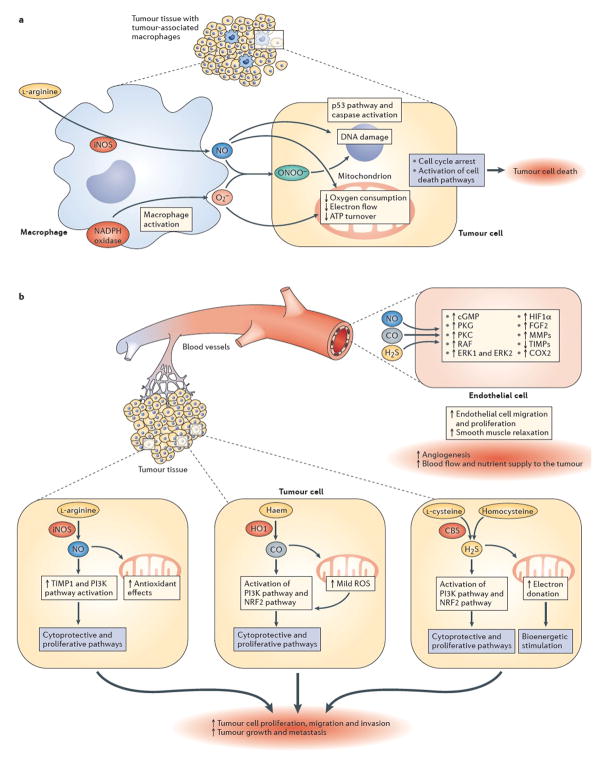
Several decades ago it was observed that the killing of tumour cells by activated macrophages is associated with a massive production of nitrite and nitrate. However, the underlying pathomechanism for this was not understood until it was noticed that the macrophage-mediated killing depends on the presence of L-arginine in the culture medium.13 Subsequent studies have shown that the NOS inhibitor L-NMA inhibits macrophage-mediated killing, and that NO (or a closely related species) is responsible for the tumour cell lysis.14,15 Importantly, the importance of NO in macrophage-mediated cell death is highly dependent on the type of tumour cell.16 The molecular mechanisms involved in NO-mediated cell death are multiple and, as mentioned above, involve high local concentrations or fluxes of NO, which together with ROS, induce metabolic inhibition and DNA damage13–16 (Fig. 1a).
Figure 1. Effects of NO, CO and H2S on tumour survival and growth.
[a | NO-mediated mechanisms of tumour cell killing by tumour-associated macrophages. Upregulation of inducible nitric oxide synthase (iNOS) in activated tumour-associated macrophages leads to the production of high local levels of NO. At the same time, macrophages also produce superoxide (O2–) from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and other cellular sources. Together, NO and superoxide form peroxynitrite (ONOO–), a reactive oxidant species. The resulting combination of nitrosative and oxidative stress can be cytostatic or cytotoxic to certain tumour cell types (the NO-associated component of cell killing is tumour-cell-type dependent). In susceptible tumour cells, the NO-mediated cell killing involves the inhibition of mitochondrial activity, DNA damage and activation of downstream pathways including such as the p53 and caspase activation pathways, culminating in tumour cell lysis. These mechanisms can be enhanced by various immunostimulatory therapies and/or by supplementation of L-arginine, the substrate of NOS. b | Pro-tumour effects of low levels of endogenously produced NO, carbon monoxide (CO) and hydrogen sulfide (H2S). Survival and proliferation of the tumour cell is stimulated by gasotransmitter production within the tumour. Within the tumour, induction of iNOS and consequently elevated levels of NO (top right), induction of haem oxygenase 1 (HO1) and elevated levels of CO (middle cancer cell on top of the right side of the graph) and/or induction of cystathionine-β-synthase (CBS) and elevated levels of H2S (bottom right) can exert pro-survival and pro-proliferative effects. Depending on the gasotransmitter, these signalling mechanisms can culminate in upregulation of fibroblast growth factor 2 (FGF2), activation of matrix metalloproteinases (MMPs), upregulation of tissue inhibitors of matrix metalloproteinases (TIMPs), activation of PI3K, and/or the stimulation of the inducible isoform of cyclooxygenase (COX2). In addition to tumour-autonomous effects, each gasotransmitter can diffuse out from the tumour cells and can stimulate intra- and peritumour angiogenesis through paracrine actions on endothelial cells, for instance by stimulating various pro-angiogenic pathways (including the cyclic GMP–protein kinase G (PKG) signalling pathway, activation of protein kinase C (PKC), RAF, extracellular signal-regulated kinase 1 (ERK1) and ERK2, and stabilization of hypoxia inducible factor 1α (HIF1α)). Although the signalling mechanisms are gasotransmitter- and condition-dependent, the ultimate result is the stimulation of peritumour angiogenesis and an increase in tumour blood flow.
The in vivo correlate of this paradigm is the immune-mediated tumour cell killing in tumour-bearing, immunocompetent (or even immunologically hyperactivated) mice. In a mouse model of Bacillus Calmette–Guérin (BCG)-induced tumour resistance, the BCG-induced clearance of a syngeneic ovarian tumour was attenuated by treatment with L-NMA, suggesting that NO contributes to the antitumour immune effector response.17 Likewise, interferon-β (IFNβ)-overexpressing metastatic murine pancreatic adenocarcinoma cells and 3-methylcholanthrene-induced fibrosarcoma lines grew much faster in iNOS−/− mice than in wild-type control hosts.18,19 Similarly, treatment with the selective iNOS inhibitor 1400W produced a 50% reduction in the antitumour effect of tumour necrosis factor-α (TNFα) therapy against MethA mouse fibrosarcoma.20 The antitumour effect of interleukin-13 (IL-13) against various head and neck tumours was also attenuated by L-NMA.21 Finally, treatment of mice bearing pancreatic adenocarcinoma tumours (which only express low levels of iNOS) with N6-(1-iminoethyl)- L-lysine (L-NIL; another NOS inhibitor with limited selectivity for iNOS) increased the formation of liver metastases.22
In line with in vitro work demonstrating the marked variation in the susceptibility of tumour cells to NO-mediated killing,23–25 several other in vivo studies have shown that the growth of implanted tumours depends on the type of tumour and the immune status of the host. For instance, the growth of B16-BL6 melanoma and M5076 ovarian sarcoma was only enhanced by 20% in iNOS−/− mice21 whereas the growth of B16-F1 melanoma cells was in fact slightly reduced in iNOS−/− mice,22 perhaps indicating that the growth of these different tumour types may depends on the presence or relative scarcity of NO.
Interestingly, factors (that have not been characterized yet) in the environment of some tumours can attenuate the host’s NO-mediated antitumour action by suppressing the ability of M2 macrophages to convert into pro-inflammatory M1 macrophages, which produce higher levels of NO.25,26 This response can protect some tumours from macrophage-mediated cell death.25,26
There are several ways in which the antitumour effects of NO can be exploited therapeutically. The first approach involves the on-demand upregulation of intratumour levels of NO (and/or associated reactive nitrogen species) to extremely high — cytotoxic — levels, a strategy that can be used alongside tumour immunotherapy to boost the natural antitumour immune response. The most successful strategy to do this relates to the immunotherapy — for instance, with IFNα or with BCG — of bladder cancer, whereby the upregulation of the local antitumour immune response results in the marked upregulation of iNOS, NO and peroxynitrite, which contribute to the antitumour efficacy of the therapy.27–30
Since iNOS is a high-output source of NO that significantly relies on substrate availability (as opposed to eNOS and nNOS, which produce lower amounts of NO under more-regulated conditions), the macrophage production of NO by iNOS may also be enhanced by supplementation with its substrate, L-arginine. In preclinical studies, L-arginine treatment was found to stimulate anticancer immune responses and reduce tumour growth.30–32 In clinical trials, oral L-arginine supplementation was found to counteract tumour-induced immunosuppression and/or to enhance the antitumour immune response.33–37 L-arginine restored antitumour immunity and improving long-term survival in malnourished patients with gastric cancer or head and neck cancer receiving neoadjuvant chemotherapy.35 Moreover, treatment of individuals with colorectal cancer with 30 g per day L-arginine for 3 days reduced the expression of survivin (a nuclear antigen found on proliferating cells); increased iNOS expression in tumour cell biopsies; and increased the serum levels of NO metabolites.37 Although these findings suggest that L-arginine supplementation attenuates the development of colorectal tumours by increasing NO levels within the tumour tissue, one must keep in mind that L-arginine is also a secretagogue for growth hormone, insulin-like growth factor 1, insulin and prolactin. Thus, the relative contributions of NO-dependent and the NO-independent actions of L-arginine in cancer remain to be separated in future studies.
The very fact that cancer develops in the first place proves that the innate immune system is often inadequate to defeat the growth of the tumour. One mechanism by which the tumour may evade the NO-mediated immune response of the host involves the increase in the expression of microRNA-146a in the tumour cell.38 MicroRNA-146a leads to an inhibition of iNOS translation in the tumour cell, which, in turn, (via mechanisms that remain to be further characterized) suppresses the ability of tumour-infiltrating macrophages to kill the tumour cell.38 Further understanding and pharmacological correction of these ‘evasive actions’ of the tumour may open new therapeutic avenues.
Another distinct but related therapeutic approach involves increasing NO levels in the tumour microenvironment in ways that are independent of the host immune system. Traditionally, NO could be delivered using NO donors, but additional approaches to doing this may involve therapeutic overexpression of iNOS in the tumour (for instance, via gene therapy). In some cases, these approaches can be combined with antitumour chemotherapy, whereas in other cases, they rely on ‘hybrid’ or ‘multifunctional’ NO donor molecules in which a NO donor group is linked to an existing drug (such as a non-steroidal anti-inflammatory drug). In other cases, NO donors can be used in combination or sequentially with radiotherapy. The use of therapeutic donation of NO for cancer is discussed in specialized reviews.39–45
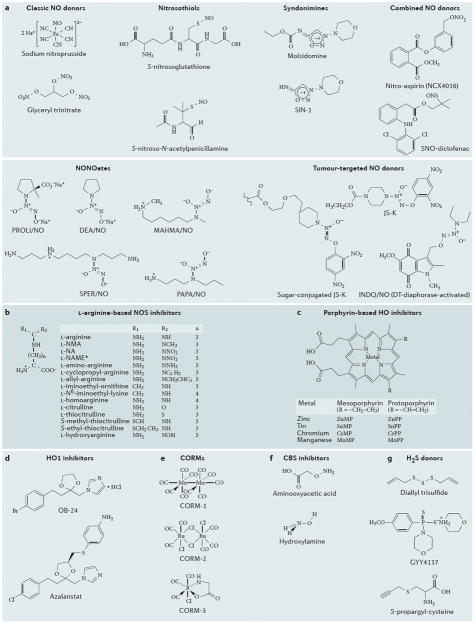
Killing tumour cells with high concentrations of NO donors in vitro is relatively straightforward. Moreover, in vivo, depending on the dose and type of the NO donor and type of tumour tissue involved, some degree of NO-mediated vasodilation may help with the delivery of cytotoxic drugs to the tumour. However, since NO potently induces vasodilation at concentrations that are well below those required to elicit cytotoxity, one of the central challenges associated with NO donor therapies is to deliver high concentrations of NO into the tumour, without ‘spilling’ too much NO into the circulation, where it induces a dose-limiting systemic hypotensive side effect. There are several approaches to selectively directing NO to tumour cells. One involves exploiting the specific metabolic activity of cancer cells by using glutathione-S-transferase (GST)-activated NO donors, such as para-aminobenzoic acid (PABA)–NO and JS-K46,47 — in some cases, in combination with multi-arm polymeric nanocarriers.48,49 Another strategy is to use photoactivated NO donors, in some cases applied as supramolecular complexes.49–51 One study tested a combination of the bacterial enzyme Escherichia coli nitroreductase and NO-producing prodrugs directed to the tumour,52 as well as NO donors activated by tumour-cell esterase or DT-diaphorase.52 Another strategy exploits the hypoxic nature of the tumour cell by delivering NO in the form of S-NO-haemoglobin or nitrite, both of which preferentially release NO in hypoxic or acidic environments.42 The structures of some non-tumour-targeted and tumour-targeted NO donors are shown in Fig. 2a.
Figure 2. Chemical structures of selected compounds that affect levels of gasotransmitters.
a | Chemical structures of selected NO donor molecules. SNP and glyceryl trinitrate are considered “classic molecules”, which have been used by cardiologists for several decades. Nitrosothiols, syndonimines and NONOates have different half-lives/NO release profiles, but do not offer tumor-cell selectivity. The examples indicated in the figure are research compounds, rather than clinical development candidates. The “combined NO donors” (selected examples of which are shown here) offer the combined pharmacological action of the parent compound (e.g. a nonsteroidal anti-inflammatory) and the NO donating group; several members of this class are in various stages or preclinical or clinical development. The tumour-targeted NO donors utilise specific features of the tumour microenvironment to direct the release of NO within the tumour cells, in order to increase tumour cell specificity and to reduce potential systemic side effects of NO; these compounds are currently in preclinical testing. b | Chemical structures of selected L-arginine-based NOS inhibitors. In L-NAME, the acid functional group (-COO–) becomes CO-O-CH3; **GW273629 is (3-[[2-[(1-iminoethyl)amino]ethyl]sulfonyl]-L-alanine); the central portion of the molecule contains a sulfonyl group within a 3-membered carbon chain; cindunistat is S-[2-(ethanimidoylamino)ethyl]-2-methyl- L-cysteine, where the central portion of the molecule contains a sulfur atom within a 3-membered carbon chain. c | Chemical structures of porphyrin-based haem oxygenase 1 (HO1) inhibitor compounds. Zinc, tin, manganese and chromium protoporphyrins have been described as competitive inhibitors for HO1 in the liver, spleen, kidney and other tissues. Most studies in cancer utilize ZnPP and SnPP. Sn-mesoporphyrin (Stanate; InfaCare Pharmaceutical Corporation) is noteworthy, as it has already been used in human studies. d | Chemical structures of selected non-porphyrin-based HO1 inhibitor compounds. OB24, an imidazole-dioxolane compound, is a member of a large group of compounds (that also contains imidazole ketones and imidazole alcohols) that are competitive inhibitors of HO1. OB24 has demonstrated efficacy in tumour-bearing mice models in vivo. Azalanstat is another potent HO1 inhibitor (IC50 values: 6 and 28 μM for rat HO1 and HO2, respectively). e | Chemical structures of selected CO-releasing molecules (CORMs). Each CORM molecule releases 1 molecule of CO; CORM1 and CORM2 releases CO rapidly (half-life: approx. 1 minute); CORM3 is a slower releaser of CO (half-life: approx. 1 hour). f | Chemical structures of two inhibitors of the hydrogen sulfide-producing enzyme cystathionine-β-synthase (CBS). G. Chemical structures of commonly used H2S donors.
Although the upregulation of NO levels can be exploited as an antitumour approach, in many forms of cancer immunotherapy (for example, TNFα, IFNγ or IL-2 therapies), it represents a side effect, because these immunotherapies induce an increase in NO production in the systemic vasculature Box 1. In these instances, therefore, the inhibition of systemic NOS production could be an approach to mitigate the dose-limiting adverse haemodynamic effects (that is, systemic vasodilation and hypotension) of these therapies.
Box 1. iNOS induction in the vasculature and systemic hypotension.
Immunotherapy of cancer — for instance, with interleukin-2 (IL-2) — is associated with a severe, often dose-limiting hypotension that some studies have suggested is partly attributable to systemic overproduction of nitric oxide (NO). Although initial observations suggested a role for inducible nitric oxide synthase (iNOS), which is expressed in vascular smooth muscle, in this hypotension,200,201 more-recent work has implicated endothelial NOS (eNOS)-derived NO.202,203 The formation of peroxynitrite has also been implicated in mediating part of the IL-2-induced organ-damage side effects.204 These findings indicate that systemic NO synthesis inhibition and/or the neutralization of peroxynitrite may be used to reduce the systemic toxicity of cancer immunotherapy. In a Phase I clinical trial, the non-isoform-selective NOS inhibitor NG-methyl- L-arginine (L-NMA) was tested in 23 patients with cancer,205 the majority of whom developed hypotension in response to IL-2. L-NMA exhibited marked antihypotensive activity at all dose levels (3–36 mg per kg), and the duration of the effect was proportional to the dose of the NOS inhibitor used. At the highest dose tested (36 mg per kg), adverse effects of NOS inhibition were also observed, such as an increase in pulmonary vascular resistance and a decrease in cardiac output. These observations suggest that NOS inhibition may be useful to alleviate the hypotensive effects of high-dose IL-2 therapy (or of other immunotherapies) in individuals with cancer. According to preclinical data, non-isoform selective or eNOS-selective NOS inhibition does not interfere with the anticancer effect of IL-2.202
Pro-tumour effects of tumour-derived NO
Several tumours (including gastrointestinal cancers, breast cancer, ovarian cancer, bladder cancer and glioma) express high levels of iNOS and produce increased amounts of NO53–57 (Table 2), and this may affect the profiles of these tumours. iNOS-overexpressing colonic adenocarcinoma tumours implanted into nude mice grew markedly faster, exhibited a more invasive phenotype and showed a higher degree of intra- and peritumoural vascularization than did wild-type control tumours.58 In vitro, the growth of many iNOS-overexpressing tumours can be reduced by NOS inhibitors (e.g. L-NMA)57 or by the iNOS inhibitor aminoguanidine,56 suggesting that endogenous, tumour-derived NO can support tumour growth56 - although there are also notable counterexamples where in vitro the growth of certain tumours cannot be attenuated by NOS inhibitors.58 Moreover, the growth of glioma xenografts was markedly reduced after silencing of iNOS within the tumour cells prior to implantation, and this was associated with a substantial decrease in tumour perfusion.59 Similar effects were noted after silencing of iNOS in melanoma cells prior to implantation into nude mice.60,61 Together, these findings strongly suggest that the enhanced growth of the iNOS-overexpressing tumours may be, at least in part, attributable to effects of NO that reach outside the tumour cell — such as induction of angiogenesis, which would enhance tumour perfusion and supply of nutrients to the tumour. These effects are consistent with the well-established role of NO as an endogenous pro-angiogenic mediator.62–64
Table 2.
Changes in the expression of various NO-, CO- and H2S-producing enzymes in various forms of cancer.
| NO | CO | H2S | |
|---|---|---|---|
| Biliary tract carcinoma | iNOS ↑ | – | CBS ↑ |
| Breast cancer | iNOS ↑↑ | HO1 ↑ | CBS ↑ |
| Colon cancer | iNOS ↑↑ | HO1 ↑ | CBS ↑↑, CSE ↑ |
| Gastric cancer | iNOS ↑ | – | – |
| Glioma | nNOS ↑, iNOS ↑ | HO1 ↑ | 3-MST ↑ |
| Hepatic cholangiocarcinoma | iNOS ↑ | – | – |
| Hepatocellular carcinoma | iNOS ↑, iNOS ↓ | HO1 ↑ | – |
| Leukemia, lymphoma | iNOS ↑ | HO1 ↑ | – |
| Melanoma | nNOS ↑, iNOS ↑ | HO1 ↑ | CSE ↑, 3-MST ↑ |
| Myeloma | iNOS ↑↑, nNOS ↑ | – | CBS ↑ |
| Ovarian cancer | iNOS ↑ | – | CBS ↑↑ |
| Pancreatic cancer | eNOS ↑ | HO1 ↑ | – |
| Prostate cancer | iNOS ↑ | HO1 ↑ | CBS ↑↑, CSE ↑ |
| Renal cell carcinoma | eNOS ↑, iNOS ↑ | HO1 ↑ | CBS ↑ |
| Sarcoma | eNOS ↑, iNOS ↑ | HO1 ↑ | – |
| Squamous carcinoma | iNOS ↑ | HO1 ↑ | – |
The notion that tumour-derived, iNOS-mediated overproduction of NO supports tumour angiogenesis and tumour growth in vivo (Fig. 1b) has subsequently been confirmed using selective iNOS inhibitors. 1400W, a NOS inhibitor of high iNOS-selectivity (approximately 5000-fold over other isoforms), reduced the growth rate of iNOS-overexpressing mammary adenocarcinoma cells in nude mice, as well as of other tumours that spontaneously express high levels of iNOS.65 Moreover, L-NMA inhibited the proliferation of L3.6pl human pancreatic cancer cells implanted into nude mice66 and the proliferation of melanoma cells in a chorioallantoic membrane model;67 aminoguanidine inhibited the growth of subcutaneously implanted MCF forestomach carcinoma cells in athymic mice;68 L-NIL and 1,3-PBIT slowed the growth of human melanoma in immunodeficient mice,60 and aminoguanidine (as well as L-NMA) slowed growth of oestrogen-receptor-negative breast cancers in mice.56,57 In most cases, the antitumour responses of the NOS inhibitors were associated with a suppression of tumour angiogenesis, further supporting the notion that the inhibition of the paracrine effects of tumour-derived NO underlies the NOS inhibitors’ action. However, in some cases (for instance, in the oestrogen-receptor-negative breast cancer cells and melanoma cells), iNOS-derived NO was also shown to play tumour-autonomous roles — for example, supporting tumour cell proliferation and migration — by stimulating proliferative pathways such as the phosphatidylinositol 3-kinase (PI3K)–AKT–mTOR pathway or the tissue inhibitor of metalloproteinases 1 (TIMP1) activation pathway, by upregulating pro-tumour components such as S100A8 in the tumour microenvironment56 and by promoting epithelial-mesenchymal transition (EMT, a process by which epithelial cells lose their cell polarity and cell-cell adhesion, and assume migratory and invasive phenotype) through activating EMT-inducing transcription factors such as Snail, Slug, and Twist1.57
Selective inhibition of tumour iNOS may therefore help to combat the actions of iNOS-derived tumour NO by reducing the tumour-cell-autonomous actions of NO (including cytoprotection, stimulation of proliferation and migration) as well as the paracrine actions of iNOS-derived NO (such as stimulation of tumour angiogenesis) (Fig. 1b). Although such an approach can only be expected to exert anticancer effects in tumour cells with a high level of iNOS expression, iNOS-dependent tumours are fairly common (Table 2). Inhibition of iNOS and/or NO scavenging have stand-alone effects in these susceptible tumour types, but it is more likely that it will be used to enhance the antitumour effect of other chemotherapies56–70 — although probably not with radiotherapy, as NOS inhibitors can produce tumour ischaemia or hypoxia, which can create tumour radioresistance71,72). The measurement of NO metabolite levels in the blood of people with cancer, and/or immunohistochemical detection of iNOS of the resected primary tumour may be used in the future to identify patients who will be likely to respond to iNOS inhibitor therapy.
iNOS inhibition has long been clinically investigated for its potential to treat various forms of local inflammation (such as arthritis, colitis, asthma) and systemic inflammation (such as circulatory shock).1–4 The potential for iNOS inhibition in treating cancer only emerged later on. Preclinical efficacy studies for iNOS inhibitors in cancer have been carried out for 1400W,61 a research compound synthesized by Glaxo Wellcome; aminoguanidine,56 a several-decade-old compound with mixed pharmacological actions (which, nevertheless, includes some selectivity for iNOS, especially in vivo);5 and L-NIL,60 another L-arginine-based NOS inhibitor that is not a clinical development candidate. Of these, the only compound that may be immediately available for clinical trials is aminoguanidine, which has already been tested in humans for non-oncological indications (including diabetic nephropathy and chronic obstructive pulmonary disease) and shows an acceptable safety profile73,74 (Box 2). However, the intellectual property status of aminoguanidine (which is not a ‘novel structure of matter’) may diminish industry’s interest in developing it.
Box 2. Drug repurposing in the area of gasotransmitters and cancer.
A significant remaining challenge for translational and clinical work is the identification of suitable clinical development candidates. For each gasotransmitter, future clinical trials may be made possible through the revitalization or repurposing of various clinical-stage drugs. Compared with most of the indications previously considered for these compounds, the regulatory guidelines for cancer require a relatively small regulatory ‘package’; thus, it can be hoped that clinical work with such compounds will be feasible in the future. Repurposing is an approach that is therefore often advocated, both for the pharmaceutical industry, as well as for academic clinical translational efforts, and has been successfully used for the experimental therapy of cancer, as demonstrated by the cases of topoisomerase inhibitors, metformin, and others.206–209 In the area of gasotransmitter research, the production of each of the three gasotransmitters may be modulated by compounds that have already been in clinical trials for different indications.
For inhibition of inducible nitric oxide synthase (iNOS), the use of aminoguanidine is a possibility. Although this compound does not have a high degree of selectivity for iNOS, it has a reasonably good inhibitory potency for iNOS.5 Its use in cancer is supported by in vivo data that show a marked reduction of tumour growth in mammary adenocarcinoma models.56 Aminoguanidine has previously been used in clinical trials (experimental therapy of diabetic complications), both for its iNOS inhibitory actions, and for its NOS-independent actions as an inhibitor of the formation of advanced glycation end products.73,74
For inhibition of carbon monoxide (CO) production from haem oxygenase 1 (HO1), Sn-mesoporphyrin (a porphyrin-based HO1 inhibitor) has already been in clinical trials for the experimental therapy of infant hyperbilirubinaemia,113–115 and may be available for future trials in cancer.
For inhibition of hydrogen sulfide (H2S) production, the cystathionine-β-synthase (CBS) inhibitor aminooxyacetic acid (AOAA) has already been tested in humans in the contexts of Huntington disease and tinnitus.157,158 Although the intended target in these trials was not CBS, but γ-amino butyric acid (GABA) aminotransferase GABAT (a pyridoxal phosphate (PLP)-dependent enzyme involved in the biosynthesis of GABA in the nervous system), these trials have yielded useful human safety and tolerability information on AOAA.157,158
Generally, the interest of the pharmaceutical industry in NOS inhibitors has diminished over the past decade. Historically, this may be related to the failure of L-NMA in Phase III clinical trials in patients with circulatory shock;75 however. L-NMA is not a selective inhibitor of iNOS, and newer-generation NOS inhibitors that are more selective for iNOS may have markedly different safety and efficacy profiles. GlaxoSmithKline has completed several small clinical trials with GW273629 (another selective iNOS inhibitor);76 the compound failed to show clinical efficacy in migraine77 and asthma,78 but exhibited some efficacy in rheumatoid arthritis79 Pfizer’s iNOS inhibitor cindunistat (which is structurally closely related to GW273629) failed to show efficacy in a 2-year trial in osteoarthritis.80
There are no published data on GW273629 or cindunistat in preclinical or clinical cancer models. According to a recent patent filing81 Astellas’ iNOS inhibitor FK330 (also known as FR260330), shows antitumour efficacy in combination with taxol; however, the development stage of this compound has not been publicly disclosed. Structures of some of the NOS-inhibiting compounds are shown in Fig. 2b.
Pro-angiogenic effects of host-derived NO
The inhibitory effect of selective iNOS inhibitors on tumour growth is not universal. For example, whereas aminoguanidine, aminoethyl isothiourea and 1400W were found to inhibit the growth of rat carcinosarcoma, the eNOS-selective NOS inhibitors L-NAME and L-NNA, as well as various ruthenium-based NO scavengers, did.69.82 These observations may indicate that although some tumour tissues do not overproduce NO, and although NO that emanates from the tumour tissue may not always be essential for tumour angiogenesis, eNOS-derived NO that is produced by the blood vessels of the host can, nevertheless, increase tumour blood flow and/or peritumour angiogenesis. In such instances, therapeutic inhibition of host eNOS may be of potential therapeutic benefit. The fact that L-NAME exerts marked reductions in tumour blood flow, and consequently induces tumour hypoxia, has long been established;71,72,83,84 subsequent studies also demonstrated that L-NAME can reduce tumour angiogenesis.85,86 Melanomas grown in eNOS-deficient mice had decreased vessel numbers (but increased vessel perimeters and numbers of endothelial nuclei per vessel cross-section) compared with those grown in wild-type animals.87 By contrast, in the same experimental model, the deletion of iNOS (instead of eNOS) in the host stromal cells did not affect angiogenesis and vessel morphology.87 Similarly, the deficiency of host eNOS reduced the growth of pancreatic cancer.88
Thus, regardless of the level of NOS expression or NO production by the tumour tissue, inhibition of host eNOS may be a stand-alone target for antitumour therapy. Nevertheless, it is likely that in most cases both the tumour tissue and the host tissue contribute to NO levels in the tumour microenvironment. Moreover, tumour-cell-localized nNOS and eNOS (Table 2)89,90 may also contribute to the elevation of NO levels in the tumour microenvironment. Thus, NOS inhibition needs to be tailored and adjusted to the relevant source(s) of NO within the specific tumour type.
Several early-stage clinical trials have now been conducted to target host eNOS or to non-selectively inhibit NOS generally to suppress tumour angiogenesis. Advantages of this approach include its broad applicability for vascularized tumours, and the fact that some of the NOS inhibitors (for instance, L-NMA) have already been in human clinical trials. In a Phase I clinical study in 19 patients with non-small-cell lung cancer, prostate cancer or cervical cancer, L-NMA (0.1–0.9 mg per kg) was administered intravenously, and caused a significant decrease in tumour blood flow that was maintained for 24 hours.91 The side effects were relatively minor: mild bradycardia and slight hypertension.91 Although these findings are encouraging, haemodynamic side effects may become problematic if eNOS inhibitors were to be used chronically. This concern, coupled with the fact that the typical non-isoform-selective or eNOS-selective inhibitors (that is, L-NAME and L-NMA, among others) no longer have proprietary patent status, makes it less likely that this approach will ultimately succeed in its clinical translation.
Carbon monoxide
CO is a stable free radical mediator that, like NO, has multiple physiological roles. It is produced in various mammalian cells and tissues by a family of enzymes called haem oxygenases (HOs) (Table 1) that catalyse the oxidative degradation of haem (reviewed in92–96). The inducible HO isoform (HO1) can be upregulated in response to various stimuli, including haem, oxidative stress, ultraviolet irradiation, heat shock, hypoxia and NO. The constitutive isoform, HO2, is expressed in several tissues including the brain, kidney, liver and spleen. The enzymatic activity of HOs depends on NADPH and requires oxygen. Importantly, HO-dependent CO production can be inhibited with various small-molecule HO inhibitor protoporphyrins (PPs) such as SnPP and ZnPP, and mesoporphyrins (MPs) such as Zn deuteroporphyrin (ZnDP).92–96
The ‘classical’ pathways of the physiological actions of CO involve the stimulation of the guanylyl cyclase–cGMP pathway, although the affinity of CO for the haem group of guanylyl cyclase is much lower than that of NO. Low CO concentrations also activate (open) KATP channels and influence various intracellular kinase pathways, including phosphatidylinositol 3-kinase–AKT and p38 MAPK signalling (Table 1). At higher concentrations, CO exerts adverse biological effects, which, in vivo, are mainly attributed to the binding of CO to haemoglobin; the resulting carboxyhaemoglobin (COHb) reduces the oxygen-carrying capacity of the blood and leads to tissue hypoxia. In vitro, CO inhibits mitochondrial electron transport by irreversibly inhibiting cytochrome c oxidase (complex IV). The combination of these deleterious actions are considered the central modes of CO inhalation poisoning, a relatively common clinical problem.96
Some of the best-characterized physiological effects of CO include anti-inflammatory, antiproliferative, antiapoptotic, and anticoagulative responses; by contrast, at higher concentrations, CO becomes cytotoxic (Table 1). In contrast to NO, the cytoprotective and cytotoxic effects of CO are intimately intertwined. For example, a slight degree of CO-mediated inhibition of mitochondrial activity, followed by a slight increase in intracellular ROS production has been shown to be key in CO-mediated cytoprotective signalling events (such as activation of kinase pathways and stabilization of hypoxia-inducible factor 1α [HIF1α]).97,98 In a way, the cytoprotective effects of CO resemble the protective effects of pharmacological preconditioning, whereby a short, relatively mild (and survivable) insult triggers a secondary cytoprotective phenotype, for instance via activation of the prototypical antioxidant response element nuclear factor erythroid 2-related factor 2 (NRF2). Thus, a protective cellular phenotype is maintained in the cell for a long time after CO has already been cleared from the biological system.
Pro-tumour effects of CO
Several tumours (including prostate cancer, renal cell carcinoma, hepatoma, glioblastoma, melanoma, Kaposi sarcoma, pancreatic cancer and CML) contain high levels of HO1, either within the tumour cells themselves, and/or in the tumour-infiltrating macrophages99,100 (Table 2).
The functional importance of intratumour CO is well illustrated by studies showing that siRNA-mediated suppression of HO1 expression reduces the viability and the rate of proliferation of pancreatic cancer cells in vitro and in vivo,101 as well as reducing cellular survival and increasing apoptosis in mouse hepatoma cell lines.102 Tumours in which HO1 was silenced grew slower than did tumours expressing normal levels of the enzyme, and this reduced growth was associated with a reduced microvessel density, consistent with the notion that HO1 (and CO), facilitates intra- and peritumour angiogenesis.102 Likewise, when implanted into severe combined immunodeficient (SCID) mice, HO1 short-hairpin RNA (shRNA)-silenced prostate tumours grew significantly slower than did wild-type control tumours, exhibited a less invasive phenotype and showed a lower degree of metastatic activity.103,104
Studies investigating the effects of HO1 inhibitors on tumour angiogenesis and growth have further confirmed the role of CO overproduction in cancer (Fig. 1b). Treatment of tumour-bearing mice with ZnPP IX reduced tumour growth in several different studies with ovarian, pancreatic and colon carcinoma cell lines,105–110 and OB-24, an imidazole-based inhibitor of HO1, inhibited growth of prostate tumours implanted in mice.103 OB-24 also exerted additive or synergistic effects when administered in combination with taxol therapy,103 possibly indicating that the inhibition of CO biosynthesis may be therapeutically applicable in combination with antitumour chemotherapy.
Despite this evidence that HO1-derived CO has cytoprotective and pro-angiogenic effects, it should be noted that in a few reports, HO1 silencing increased, rather than decreased tumour growth,111,112 implying the role of HO1 and CO in cancer may be very much dependent on the tumour type. As discussed elsewhere,93–96 the biological effects of HO1 inhibition or silencing cannot be equated to the pharmacological inhibition of biological CO production, as the roles of HO1 go beyond CO and involve the modulation of cellular levels of bilirubin and haem, with consequent changes in cellular redox status. Moreover, the selectivity of the most commonly used HO1 inhibitor, ZnPP IX is limited (as with most currently known HO inhibitors); the pharmacological actions of such drugs extend well beyond HO1 inhibition.
Nevertheless, the validity of the approach of therapeutically inhibiting HO1 to reduce the protective actions of CO (cytoprotection, stimulation of proliferation and migration) and the paracrine actions (stimulation of tumour angiogenesis) is supported by several lines of preclinical data. Confirmation of HO1 overexpression in the tumour tissue of a patient prior to therapy would be expected to increase the likelihood of therapeutic success. The clinical or translational progression of HO1 inhibition for cancer would require a HO1 inhibitor of suitable potency, selectivity and safety for clinical development. SnMP, an HO inhibitor with a reasonably good potency Box 2, was tested clinically in the experimental therapy of hyperbilirubinaemia and acute porphyric crisis113–115 and may be a potential candidate for clinical repurposing for cancer. Infacare Pharmaceuticals currently holds the intellectual property rights for the compound (under the brand names Stannsoporphrin and Stanate).
Another compound, a PEGylated form of ZnPP, showed improved pharmacological properties in cancer models, compared to the non-pegylated ZnPP molecule;105 further improvements to its structure were later published.116,117 OB-24 has also been shown to exert antitumour effects in vivo against prostate cancer.111 Additional potential avenues may include the discovery and development of novel HO1 inhibitors and approaches focusing on silencing or suppressing the induction of HO1. The structures of several novel HO1 inhibitors have recently been disclosed, including that of azalanstat.118 However, these agents have not yet been evaluated in cancer models. Structures of selected HO inhibitors are shown in Fig. 2c.
Antitumour effects of CO through metabolic exhaustion of tumour cells
Beyond a certain threshold, high levels of CO (owing to, for instance, CO gas, high concentrations of CO releasing molecules (CORMs) or overexpression of HO1) can be detrimental to cell viability. At such high concentrations (typically produced by millimolar concentrations of CORMs in vitro), CO reduces mitochondrial activity, triggers generation of mitochondrial ROS, inhibits cellular protein synthesis and decreases cell viability, proliferation and survival.119–125 Accordingly, in vivo exposure of tumour-bearing mice to inhaled CO (250 parts per million (ppm) for 1 hour every day) suppressed the growth of prostate cancer xenografts, and this effect was associated with increased tumour cell apoptosis and reduced tumour vascularization.121 Similar effects of CO were observed in two models of spontaneously developed tumours (the transgenic adenocarcinoma mouse prostate (TRAMP) cancer model and the lung tumour KRAS mouse models).121 Moreover, inhaled CO (500 ppm, 1 hour per day every day) attenuated the growth rate and the peritumour angiogenic response of CAPAN-2 pancreatic cancer cells in CD-1 athymic mice;123 the effects of inhaled CO were recapitulated by the CO releasing molecule CORM2 (35 mg per kg per day, via intraperitoneal injection).123
The above data raise the notion of using therapeutic CO donation for experimental therapy in cancer. Although CO has a ‘bad reputation’ with physicians, owing to its well-known toxicity profile in the context of CO poisoning, over the past decade, experimental therapeutic CO administration for many conditions — from transplant rejection to pulmonary diseases — has been explored in some detail.126,127 However, recently, the development of inhaled CO (Covox) by Ikaria Inc. was stopped in Phase II clinical stage, and the CORMs developed by Hemocorm/Alfama have not yet entered clinical testing. The reasons for clinical development hurdles have previously been discussed elsewhere126,127 and include regulatory issues, potential concerns related to therapeutic indices, as well as (real or perceived) issues around clinicians’ willingness to use such an ‘obviously highly toxic’ molecule therapeutically. Examples of various CORMs (that are currently only used as preclinical experimental tools) are shown in Fig. 2e.
Inhaled CO gas is widely available in the hospital environment — it is used in pulmonary function tests that are based on the measurement of the diffusing capacity of CO128 — and thus, it is available for small, investigator-initiated trials, similar to ones previously conducted in Europe and Japan. For instance, CO inhalation at doses that exhibit preclinical efficacy in murine models of cancer (250–500 ppm for 1 h) has already been tested in humans129–132 and appears to be well tolerated, at least in short-term regimens.
Hydrogen sulfide
H2S is produced in various mammalian cells and tissues by three principal enzymes: cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) (Table 1, reviewed in133–137). CBS and CSE are pyridoxal phosphate (PLP)-dependent enzymes that use L-cysteine and homocysteine as their substrates, whereas the substrate of 3-MST is 3-mercaptopyruvate, which is produced from L-cysteine via cysteine aminotransferase. An additional enzymatic pathway for H2S production that involves 3-MST and D-amino acid oxidase has recently been identified in the kidney.138 CBS, CSE and 3-MST are constitutive enzymes, with differential expression levels in different tissues and organs, but their levels can also be up- or downregulated in various conditions. Importantly, CBS and CSE can be inhibited with small molecules of varying degree of selectivity; propargylglycine (PAG) is the most commonly used research tool to inhibit CSE, and aminooxyacetic acid (AOAA) and hydroxylamine are research compounds most often used to inhibit CBS139 (Fig. 2f).
H2S activates many intracellular signalling pathways; it opens KATP channels, and indirectly stimulates the guanylyl cyclase–cGMP pathway by inhibiting cGMP phosphodiesterases; these actions promote vasodilation and angiogenesis.137–141 In addition, at low concentrations, H2S stimulates the cytoprotective PI3K–Akt, p38–MAPK and NRF2 pathways.133–137 Many of the biological actions of H2S, including KATP channel opening, occur, at least in part, via sulfhydration, a post-translational modification of specific protein cysteines. At physiological concentrations, H2S can also stimulate cellular bioenergetic function by donating electrons to the mitochondrial electron transport chain at complex II, and by increasing mitochondrial levels of cAMP.142 At higher concentrations, H2S inhibits cytochrome c oxidase (Complex IV), thus disrupting the mitochondrial electron transport; it can also exert pro-oxidant and DNA-damaging effects.133,142
Like NO and CO, H2S at low concentrations (owing to low production rates, low fluxes, or shorter duration of exposure tends to exert cytoprotective, antioxidant-type responses, whereas higher concentrations can lead to mitochondrial inhibition or poisoning and cell death. Importantly, whereas low concentrations of H2S are generally anti-inflammatory, higher concentrations of H2S can stimulate various pro-inflammatory pathways (Table 1).133–137,142
Pro-tumour effects of CBS-derived H2S
Colon cancer cells,143 ovarian cancer cells,144 prostate cancer cells145 and breast cancer cells146 exhibit high expression levels of CBS and produce increased amounts of H2S compared with adjacent non-cancerous tissue or non-transformed cells (Table 2). The functional role of CBS-derived H2S in the regulation of proliferation, migration, and invasion in colon cancer and ovarian cancer has been studied in vitro using a combination of genetic approaches (for example, siRNA-mediated stable silencing of CBS) and pharmacologic agents (for example, AOAA).143–146 Downregulation or inhibition of CBS inhibited cell proliferation and, at higher concentrations, AOAA reduced tumour cell metabolism and viability. Mechanistically, downregulation or inhibition of CBS suppresses cellular bioenergetics (both via mitochondrial electron transport and via oxidative phosphorylation and glycolysis), and — as shown in ovarian cancer models — reduces intracellular levels of the antioxidant glutathione, and triggers apoptotic cascades through modulation of the NF-κB and p53 pathways.143–146 Another important consequence of silencing or inhibiting CBS is an increase in cellular ROS levels, which may be secondary to intracellular antioxidant depletion.144 This mechanism may contribute to the sensitization of the tumour cells to macrophage-mediated killing after silencing of tumour CBS, a phenomenon, which has been demonstrated in breast cancer cells in vitro. 146
Subsequent studies in nude mice transplanted with colon cancer or ovarian cancer xenografts extended the findings into in vivo models. ShRNA-mediated stable silencing of CBS expression in the tumour cells prior to implantation into the mice reduced in vivo tumour growth by approximately 40–50% and led to a marked decrease in the size and number of tumour nodules143–146 and inhibited peritumour angiogenesis.143,144 These effects were recapitulated by AOAA; indeed, the efficacy of AOAA was superior to that of CBS silencing, probably reflecting additional, CBS-independent actions of this compound.143,144 Importantly, inhibition or silencing of tumour CBS also sensitized the cancer cells to concomitant chemotherapy.144,147
The findings above suggest that CBS-derived H2S creates a supportive environment for the tumour cell (Fig. 1B). It must be pointed out, nevertheless, that in a glioma model, CBS silencing increased, rather than decreased tumour growth,148 illustrating the different tumour-cell-type dependent roles of H2S. Notably, because CBS activity affects the cellular levels of cysteine and homocysteine and modulates oxidative status, the biological effects of CBS inhibition or silencing cannot be simply equated to the pharmacological inhibition of H2S production.149 Moreover, the pharmacological effects of the most commonly used CBS inhibitor, AOAA, go well beyond CBS inhibition.149
The literature on the functional role of CSE and 3-MST in cancer is less developed than the role of CBS149–155 (Table 2). Upregulation of CSE has been demonstrated in several tumours, including melanoma, prostate cancer and lung cancer, whereas 3-MST upregulation has been reported in astrocytoma and melanoma. CSE silencing suppressed tumour cell proliferation in vitro and in vivo in a colon cancer model156 but CSE inhibition or CSE silencing failed to affect tumour cell proliferation in melanoma.150 The functional role of changes of the levels of the various H2S-producing enzymes in most other types of cancer has not yet been explored.
Inhibition of CBS, CSE (or both) is expected to exert antitumour effects, although therapeutic inhibition of CBS in cancer is expected to induce less ‘collateral damage’ than inhibition of CSE, as CSE is broadly expressed in the cardiovascular system, whereas CBS is restricted to a smaller number of organs (including the liver and the brain). Ideally, patients with tumours that produce high levels of H2S should be identified as probable responders to CBS-inhibiting treatments. Exhaled H2S is increased in many cancer patients,149 and measurement of exhaled H2S (or quantification of the intratumour expression of CBS) could be combined with CBS inhibition in a ‘theranostic’ approach. The most potent CBS inhibitor identified to date is AOAA, with an IC50 of approximately 3–10 μM on human recombinant CBS; however, AOAA also inhibits other transaminases.149 Preclinical data from tumour-bearing nude mice demonstrate that AOAA prodrugs have better cellular uptake and higher anticancer potency than does the parent compound AOAA.157
Future medicinal chemistry efforts could be targeted to identify new CBS-inhibitor scaffolds with higher potency and/or selectivity for CBS to test and develop as drugs. AOAA was in clinical trials several decades ago for non-oncological indications (including Huntington disease and tinnitus)158,159 Box 2, and as such, could potentially be repurposed. Since CBS is the main enzyme involved in the biological degradation of homocysteine, chronic CBS inhibition is expected to induce hyperhomocysteinaemia. This can be either viewed as a systemic side effect of CBS inhibition (which, in the short-to-mid-term, is purportedly reasonably well tolerated), or it could even be used as a biomarker of CBS inhibition to confirm therapeutic target engagement.
Antitumour effects of H2S donors
Similar to NO and CO, elevation of cellular H2S levels beyond a certain threshold (typically achieved by millimolar concentrations of H2S donors) becomes detrimental to cell viability.149 Accordingly, H2S donors — either as stand-alone agents, or as H2S-donating functional moieties of pharmacologically active base scaffolds — have been investigated as potential cancer therapies. It is relatively easy to kill cancer cells with high concentrations of H2S donors in vitro; however, such experiments are of limited value in predicting the in vivo utility of the compound. Therefore, the discussion below focuses on in vivo studies.
At low or medium concentrations, GYY4137 — a ‘slow-release’ H2S donor160 — induces vasodilation, hypotension, cytoprotection and anti-inflammatory effects; however, at higher concentrations, it is antiproliferative and becomes detrimental to the viability of cells via various mechanisms, including mitochondrial inhibition, activation of cell death signalling and intracellular acidification, culminating in activation of caspase 9 and apoptosis.161–163 In SCID mice, daily administration of up to 300 mg per kg GYY4137 attenuated the growth of subcutaneous tumours.163 GYY4137 also exerted antitumour efficacy in a subcutaneous hepatic carcinoma xenograft model in mice.164 GYY4137 is currently the most specific H2S donor with confirmed in vivo anticancer effects.163,164
Many studies have also demonstrated the anticancer effect of the naturally occurring H2S donor compounds diallyl sulfide, diallyl disulfide and diallyl trisulfide in vivo. These compounds generate H2S in the cellular environment via glutathione-dependent release processes and elevate intracellular and circulating H2S levels.165–167 The pharmacological actions of these compounds extend beyond H2S donation,168 making the interpretation of the findings in terms of gasotransmitter biology difficult. Nevertheless, anticancer effects of these compounds have been shown in vivo, in mice bearing glioblastoma, melanoma, gastric cancer, osteosarcoma, pulmonary adenocarcinoma or colon cancer.169–172 Additional H2S donor compounds with in vivo anticancer actions include S-propargyl-cysteine173 and various H2S-donating acetylsalicylic acid derivatives.174 Multifunctional H2S donors — which contain an H2S-donating moiety conjugated with a previously known drug (such as a nonsteroidal anti-inflammatory drug) — have been reviewed elsewhere.175,176
Despite the large body of preclinical work investigating various naturally occurring polysulfides in cancer, and the fact that these compounds can be considered ‘natural supplements’ (as they are abundant in, for instance, garlic oil), the pharmacology of these compounds is complex, and H2S donation represents only one of their many modes of action. Future drug discovery efforts could exploit the specific environment of the tumours to produce specific H2S donors that are selectively bioactivated in tumour tissues.
Summary and future directions
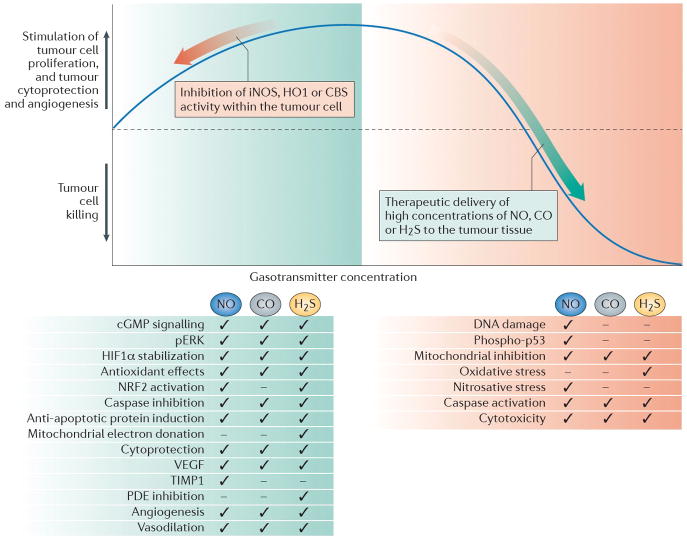
Three decades of preclinical studies in the field of the three gasotransmitters NO, CO and H2S have identified several pathophysiological paradigms and associated experimental therapeutic approaches that may be ultimately suitable for use in clinical translation. Specifically in cancer, the initially confusing concept whereby, superficially looking at the literature, both gasotransmitter donors and gasotransmitter synthesis inhibitors seem to have anticancer effects can be explained by the complex biology and bell-shaped pharmacology of NO, CO and H2S (Fig. 3), and should not be viewed as a barrier for translation.
Figure 3. The implications of the bell-shaped pharmacological profile of NO, CO and H2S for the therapy of cancer.
Low concentrations of nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) that are produced endogenously by inducible NO synthase (iNOS), haem oxygenase 1 (HO1) and cystathionine-β-synthase (CBS), respectively, can support tumour growth and tumour angiogenesis through the mediators and effects listed in the green box. Inhibition of these responses (depicted by the red arrow on the left side of the graph) can be of therapeutic benefit, either on its own, or to sensitize the tumour cell to standard anticancer therapies. High concentrations of the gasotransmitters can be cytostatic or cytotoxic; thus, therapeutic administration of each gasotransmitter (depicted by the green arrow on the right side of the graph), to sufficiently high concentrations in the tumour cell can be used to induce anticancer effects (listed in red box) and/or to potentiate anticancer chemo-or radiotherapy. Ticks indicate key pathways or mechanisms involved in the biological actions of low or high levels of each gasotransmitter. Please note that the figure incorporates some generalization; the pathways and mechanisms involved in the action of each gasotransmitter can be dependent on the cell-type and the experimental condition used in the various studies.
With several caveats in mind, therapeutic inhibition of gasotransmitter biosynthesis can generally be warranted if the three following conditions are met. First, the tumour should express high levels of gasotransmitter-producing enzymes. Second the tumour should produce significant amounts of the gasotransmitter, with which it defends itself from the host and fosters its own growth, proliferation and angiogenesis. Third, the gasotransmitter that is targeted should not constitute a major component of the host’s own antitumour immune defence mechanism. This can be conceptualized by shifting the dose–response curve in Fig. 3 to the left. On the other hand — and fairly independently from the expression level of gasotransmitter-producing enzymes in the tumour — donation of cytotoxic levels of gasotransmitter may be warranted in some forms of tumours, akin to shifting the dose–response curve to the right on the right side of Fig. 3. The relatively younger fields of CO donors and H2S donors may thus receive inspiration from the field of NO and may consider creating tumour-selective donors that rely on tumour-associated enzymes for tumour-specific bioactivation.
It must be stressed that, with each of the three gaseous transmitters discussed in this article, inhibition or silencing of each of the enzymes that produces the transmitters can have biological effects because the intervention will influence L-arginine metabolism (in the case of NOS), haem metabolism (in the case of HO isoforms) or L-cysteine metabolism (in the case of the H2S-producing enzymes). These effects (or pseudophenomena) must be dissected from the biological effects mediated by NO, CO or H2S through careful control experiments — for example, by pharmacological replacement of the gaseous transmitter after inhibition of the enzyme that produces it, or by comparing the results of enzyme inhibitor experiments with studies that test the effects of scavengers of the gaseous transmitters.
Another point to consider is that the selection of the preclinical cancer model used (for instance, using an immunocompetent versus immunosuppressed host into which the tumour is implanted) markedly influences the conclusions of the preclinical studies. For instance, whereas in immunocompetent (or immunologically hyperstimulated) hosts, the host macrophage iNOS-derived antitumour component can be considerable, the results of experiments that use immunosuppressed hosts (for example, nude mice) will highlight the biological character of the implanted tumour, often at the expense of recognizing relevant properties of the host organism.
We must remain realistic about how much stand-alone antitumour action can be expected from gasotransmitter-related therapeutic approaches. Inhibiting gasotransmitter production by the tumour cell will create a ‘less nourishing’ environment for the tumour, but may not induce ‘all-out’ cell death (as illustrated by the examples of partial, but not complete, suppression of tumour growth after silencing of tumour iNOS, HO1 or CBS silencing). Likewise, although high local concentrations of NO, CO and H2S can be cytostatic or cytotoxic, the intrinsic toxicity of these molecules is much lower than that of most ‘professional’ chemotherapeutic agents. Therefore, most success in gasotransmitter modulation in cancer is expected to be from uses in combination with chemo- or radiotherapy or with ‘targeted’ therapies.
Indeed, one area of particularly significant translational promise is radiosensitization through the use of NO donors Box 3. For each gasotransmitter, the most recent developments (for instance, the tumour-targeted NO donors, intermittent low-dose CO inhalation and slow-acting H2S donors) hold the most substantial scientific novelty and translational promise. Nevertheless, much preclinical work remains to be conducted, to further establish dose–response relationships, to identify drugs that might be repurposed (Box 2) and/or most likely responder populations, and to develop suitable combinations with chemotherapy, radiotherapy or targeted therapies.
Box 3. NO as a radiosensitizer.
Studies in the early 1990s demonstrated that administration of nitric oxide (NO) donors sensitizes tumours to radiotherapy-mediated killing, whereas inhibition of NO synthase (NOS) can cause tumour hypoxia, which confers tumour radioresistance.71,72 The mechanisms of NO-mediated radiosensitization are multiple and include effects on haemodynamics — that is, an increase in tumour blood flow via vasodilation and rheological effects on red blood cells210,211 — but may also involve signalling processes within the tumour cells, including modulation of the p53 and hypoxia-inducible factor 1α (HIF1α) pathways.40,211 These data indicate that administration of NO donors may be a potential way of achieving radiosensitization and more-effective elimination of the tumours through radiotherapy. The clinical translation of this concept has already begun. In a Phase II trial, 25 patients with locally advanced non-small-cell lung cancer were treated with the cytotoxic cisplatin and the antimitotic vinorelbine plus concurrent nitroglycerin (which is converted to NO) with radiotherapy. A 25-mg nitroglycerin patch was administered to the patients for 5 days during radiotherapy. Nitroglycerin exhibited an acceptable toxicity profile;212 larger trials will be required to study its efficacy. RRx-001, a novel, hypomethylating and free-radical-inducing anticancer agent that activates nitrite reduction to NO under hypoxia, is currently in Phase I trials.213 In addition to pharmacological NO donation, upregulation of inducible NOS (iNOS) in tumour-associated macrophages may also confer radiosensitization.214 The clinical translation of this approach may be possible, for instance by using ONO-4007 (a non-specific immunostimulant synthetic lipid A analogue) that was designed as a pharmacological upregulator of iNOS expression, and a compound with established antitumor effects in preclinical models, which has already completed Phase I clinical trials.215
One must also bear in mind that the three gaseous transmitters do not act in isolation, but rather, in concert — sometimes by utilizing overlapping signalling pathways (for example, both NO and CO stimulates the guanylyl cyclase pathway),198 and other times by enhancing each other’s action (for example, NO directly stimulates the guanylyl cyclase pathway, while H2S concurrently blocks the degradation of cGMP via inhibition of cGMP phosphodiesterases).141 These interactions remain to be studied in the context of cancer, and may be exploited in the future for therapeutic benefit. As one such effort, a recent study demonstrated the in vitro and in vivo anticancer effect of a combined NO- and H2S-donating compound, NOSH-aspirin.199
An area in which significant progress can be expected is the field of multifunctional compounds: clinically used drugs with added NO- or H2S-donating moieties. The pharmacology of some of these compounds is challenging. One reason for this is that the amount of the gaseous transmitter released from them is very small. Another is that that the contributions of different parts of the molecule may need defining; for example, in the case of the NO-aspirin, the NO only serves as a leaving group, and the spacer group has been shown to be responsible for some of the added pharmacological action.45 Despite these challenges, further work in this field is expected to produce additional, pharmacologically active compounds and potential drug development candidates. Some of the multifunctional NO donors with significant anticancer effects in vitro and in vivo include GIT-27NO, which is an NO-donating version of the isoxazole acetic acid derivative compound VGX-1027,177,178 and the NO-donating derivative of the protease inhibitor saquinavir.179,180 CO-donating multifunctional donors have not yet been characterized. There are, however, multiple examples for H2S-donating multifunctional donors with direct anticancer effects (see above),174–176 as well as an H2S-releasing version of naproxen.181
As NO, CO and H2S can leave the tumour (sometimes to form new compounds) and can even be measured in the exhaled breath, future gasotransmitter inhibition therapy may be combined with measurement of the levels of these mediators. Indeed, there are several reports of: increased circulating and exhaled NO levels in patients with cancer;182,183 increased carboxyhaemoglobin levels in colon cancers;184 and increased exhaled H2S levels in patients with cancer (reviewed in148).
There are several further reasons to continue research into the cancer biology of each of the three gasotransmitters covered here. Some of the finer-detailed changes in intracellular localization of the various gasotransmitter-producing enzymes in tumours — such as the nuclear translocation of HO1 in certain forms of cancer121 or the mitochondrial translocation of CBS in others143,144 — may further modify the complex role of each gasotransmitter in tumour biology. Moreover, emerging in vitro evidence suggests that the upregulation of gasotransmitter production in tumours can also be a reactive phenomenon that occurs in response to chemo- or radiotherapy, conferring tumour cell resistance and/or dedifferentiation.185–189 Further, there is increasing evidence for the role of the three gasotransmitters in the biology of tumour stem cells, the expansion and proliferation of which is promoted by these mediators,190–192 thereby providing potential additional future therapeutic targets. There is also much to be considered about the potential role of the three gasotransmitters in carcinogenesis or tumorigenesis (which may occur in part owing to the pro-inflammatory effects of high concentrations of each of the three gasotransmitters), and in chemoprevention; these stand-alone fields are the subjects of dedicated review articles.193–197
Taken together, gasotransmitter biology offers an opportunity for a diverse set of therapeutic approaches (Table 3). Each of these approaches has advantages and potentially disadvantages, and should be tailored to the biological character of the tumour to be targeted. I hope that this article will help to define distinct pathophysiological and therapeutic paradigms that characterize the roles of the three gaseous transmitters in cancer, and may stimulate the formulation of novel therapeutic concepts and the revitalization of drug discovery efforts and drug development programmes in this area.
Table 3.
Advantages and disadvantages of approaches to modulate NO, CO and H2S in cancer.
| Biological observation or mechanism | Therapeutic approaches | Advantages | Disadvantages |
|---|---|---|---|
| NO | |||
| The innate immune system uses NO produced by tumour-infiltrating macrophages to exert antitumour effects | Upregulation of the antitumour immune response, by inducing NOS activity (for example, using cytokines or non-specific immunostimulation) to promote NO production, or by supplementing NOS substrate (for example, with L-arginine) |
|
|
| NO at high local concentrations can kill tumour cells | NO donation (systemic or tumour-cell-targeted) |
|
|
| NO overproduction in certain tumour cells (usually owing to iNOS overexpression) exerts cytoprotective, pro-proliferative and pro-angiogenic effects | Selective inhibition of iNOS, via small-molecule iNOS inhibitors |
|
|
| NO produced by eNOS in the host vasculature contributes to the maintenance of tumour blood supply and stimulates tumour angiogenesis | Inhibition of eNOS, or scavenging of NO in the host organism |
|
Systemic side effects of non-isoform-selective or eNOS-selective NOS inhibition include vasoconstiction and hypertension; loss of the vascular protective effects of NO (including increased platelet and mononuclear cell adhesion and activation); and perhaps enhancement of cardiovascular adverse events |
| CO | |||
| CO overproduction within the tumour (usually owing to HO1 overexpression in the tumour cell and/or the tumour-infiltrating immune cells) exerts cytoprotective, pro-proliferative and pro-angiogenic effects | Selective inhibition of HO1, using small-molecule HO inhibitors |
|
|
| High tumour levels of CO impair tumour cell metabolism and exert antitumour effects | Donation of CO (via CO gas inhalation and/or parenteral CORM administration) |
|
|
| H2S | |||
| H2S overproduction in the tumour cells (usually owing to CBS overexpression) stimulates tumour cell bioenergetics, activates growth and proliferation, and exerts cytoprotective and pro-angiogenic effects | Selective inhibition of CBS, using small-molecule inhibitors |
|
Systemic side effects of CBS inhibition may include elevation of circulating homocysteine levels, a known cardiovascular risk factor |
| H2S donation impairs tumour cell metabolism and exerts antitumour effects. | Treatment with H2S donor compounds |
|
|
Online summary.
Nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) are labile gaseous mediators that mediate multiple biological functions in the tumour cell and in the host tissue. Each of these gases is produced by specific enzyme systems, and regulates (among other aspects) cell viability, cell division, mitochondrial activity, angiogenesis and vascular tone.
Upregulation of the various gasotransmitter-producing enzymes occurs in many tumours; most commonly, NO is overproduced by upregulation of inducible NO synthase (iNOS), CO is overproduced by haem oxygenase 1 (HO1) and H2S is overproduced by cystathionine-β-synthase (CBS).
Selective genetic silencing or pharmacological inhibition of iNOS, HO1 or CBS has been shown to exert anticancer effects in various in vitro and in vivo models. Many of these approaches also sensitize the tumour to chemo- and/or radiotherapy.
Because of the bell-shaped pharmacological character of the gasotransmitters, not only inhibition of gasotransmitter biosynthesis, but also elevation of gasotransmitter levels beyond a certain threshold can exert antitumour effects; preclinical data show that tumour-targeted NO donors, CO donors or CO inhalation therapy, and H2S donors of various classes exert antitumour effects.
Although the clinical translation of the findings of gasotransmitters in the field of tumour biology has been slow, several compounds can be identified that may be suitable for clinical repurposing and translational research activity.
Acknowledgments
The research of the author in the field of H2S and cancer is supported by a grant from the US National Institutes of Health (NIH; R01CA175803).
Glossary
- peroxynitrite
is the product of the diffusion-controlled reaction of nitric oxide (·NO) with superoxide radical (O2·−), a short-lived cytotoxic oxidant species.
- Bacillus Calmette–Guérin
is a live attenuated strain of Mycobacterium bovis and an FDA-approved therapy of in situ bladder carcinoma.
- methylcolanthrene
is a highly carcinogenic polycyclic aromatic hydrocarbon; its topical administration in mice is often used as an experimental cancer model
- secretagogue
is a term that indicates a biological substance that induces the secretion of another substance. For example, angiotensin II is a secretagogue for aldosterone.
- glutathione-S-transferases
are soluble proteins with molecular masses of approximately 50 kDa. They represent a major group of detoxification enzymes. They catalyse the conjugation of the reduced form of glutathione (GSH) to various cellular substrates.
- multi-arm polymeric nanocarriers
or star polymers are branched, globular nanoscale materials exhibiting a large surface area. They are commonly used for targeted drug delivery.
- chorioallantoic membrane model of melanoma
is a common experimental model where melanoma cells are grown on chick chorioallantoic membranes (CAMs), a model with a significant angiogenesis component.
- protoporphyrins
are tetrapyrroles containing 4 methyl side chains, 2 propionic acid side chains and 2 vinyl side chains. The iron complex of protoporphyrins occurs in a number of proteins including haemoglobin, myoglobin, and several electron transport proteins of the mitochondrial respiratory chain.
- transgenic adenocarcinoma mouse prostate (TRAMP) cancer model
One of the most well-known prostate cancer mouse models which, according to its many proponents, closely mirrors the pathogenesis of human prostate cancer. In this model, expression of both the large and small SV40 tumor antigens is regulated by the prostate-specific rat probasin promoter.
- theranostic
approaches (or “theranostics”) incorporates the development of molecular diagnostic tests in combination with with targeted therapeutics. The approach is an integral component of the personalized medicine concept.
- prodrugs
are inactive precursors of active therapeutic agents. The conversion into the active form occurs through normal metabolic processes, often involving the hydrolysis of an ester group. For example, Enalapril is the prodrug of the angiotensin-converting enzyme inhibitor antihypertensive agent enalaprilat.
Biography
Csaba Szabo
Csaba Szabo obtained his medical degree at the Semmelweis University in Budapest, Hungary, and conducted his postdoctoral training in pharmacology with John Vane at the William Harvey Research Institute in London, UK. He then became Research Director of the Division of Critical Care at Children’s Hospital Medical Center, Cincinnati, Ohio, USA. In 1999, he became full professor at the University of Medicine and Dentistry of New Jersey, USA, and in 2008 he moved his laboratory to University of Texas Medical Branch in Galveston, Texas, USA. His original scientific research in the area of nitric oxide, peroxynitrite and poly(ADP-ribose) polymerase in cardiovascular and inflammatory diseases is widely recognized, and has opened up several new areas of research and drug development. For the past decade, many of his scientific efforts focus on investigating the biological effects of hydrogen sulfide. In addition to his academic efforts, he has a translational and biotech interest as well. As Chief Scientific Officer of various biotechnological companies, he has directed the progression of several drug targets into development candidates and into clinical trials. His work has received several academic and commercialization awards, including the Texas Star Award and the Novartis Prize. He is an Elected Fellow of the British Pharmacological Society and an Elected Member of the American Society for Clinical Investigation. With over 500 full publications, with over 45,000 independent citations received, he is one of the top 10 most cited pharmacologists in the world today.
Footnotes
Competing interests
The author declares competing interests: see Web version for details.
CFI statement
The author is a principal and a shareholder of CBS Therapeutics Inc., a start-up company involved in the research and development of CBS inhibitors for cancer therapy.
References
- 1.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 2.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 3.Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit Care Med. 2000;28:N37–52. doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro LJ, editor. Nitric Oxide, Biology and Pathobiology. 2. Academic Press; 2009. [Google Scholar]
- 5.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 6.Joubert J, Malan SF. Novel nitric oxide synthase inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:537–560. doi: 10.1517/13543776.2011.556619. [DOI] [PubMed] [Google Scholar]
- 7.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kröncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection--how, why, when, and where? Nitric Oxide. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 10.Wink DA, et al. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DD, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese V, et al. Nitric oxide in cell survival: a janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 13.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 14.Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. A pioneering study implicating a role of NO in immune activation-mediated tumour cell killing in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui S, Reichner JS, Mateo RB, Albina JE. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994;54:2462–2467. [PubMed] [Google Scholar]
- 16.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 17.Farias-Eisner R, Sherman MP, Aeberhard E, Chaudhuri G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci USA. 1994;91:9407–9411. doi: 10.1073/pnas.91.20.9407. Another pioneering study implicating a role of NO in immune activation-mediated tumor cell killing in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, et al. Intact nitric oxide synthase II gene is required for interferon-beta-mediated suppression of growth and metastasis of pancreatic adenocarcinoma. Cancer Res. 2001;61:71–75. [PubMed] [Google Scholar]
- 19.Wei D, et al. Direct demonstration of negative regulation of tumor growth and metastasis by host-inducible nitric oxide synthase. Cancer Res. 2003;63:3855–3859. [PubMed] [Google Scholar]
- 20.Menon C, et al. Tumoricidal activity of high-dose tumor necrosis factor-alpha is mediated by macrophage-derived nitric oxide burst and permanent blood flow shutdown. Int J Cancer. 2008;123:464–475. doi: 10.1002/ijc.23499. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami K, Kawakami M, Puri RK. Nitric oxide accelerates interleukin-13 cytotoxin-mediated regression in head and neck cancer animal model. Clin Cancer Res. 2004;10:5264–5270. doi: 10.1158/1078-0432.CCR-04-0314. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, et al. A novel model system for studying the double-edged roles of nitric oxide production in pancreatic cancer growth and metastasis. Oncogene. 2003;22:1771–1782. doi: 10.1038/sj.onc.1206386. [DOI] [PubMed] [Google Scholar]
- 23.Shi Q, et al. Influence of nitric oxide synthase II gene disruption on tumor growth and metastasis. Cancer Res. 2000;60:2579–2583. [PubMed] [Google Scholar]
- 24.Konopka TE, et al. Nitric oxide synthase II gene disruption: implications for tumor growth and vascular endothelial growth factor production. Cancer Res. 2001;61:3182–3187. [PubMed] [Google Scholar]
- 25.Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183:1323–1329. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigert A, Brüne B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide. 2008;19:95–102. doi: 10.1016/j.niox.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Böhle A, Brandau S. Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. J Urol. 2003;170:964–969. doi: 10.1097/01.ju.0000073852.24341.4a. [DOI] [PubMed] [Google Scholar]
- 28.Hosseini A, et al. Enhanced formation of nitric oxide in bladder carcinoma in situ and in BCG treated bladder cancer. Nitric Oxide. 2006;15:337–343. doi: 10.1016/j.niox.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Shah G, et al. iNOS expression and NO production contribute to the direct effects of BCG on urothelial carcinoma cell biology. Urol Oncol. 2014;32:45.e1–9. doi: 10.1016/j.urolonc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992;149:2709–2714. [PubMed] [Google Scholar]
- 31.Ma Q, Hoper M, Anderson N, Rowlands BJ. Effect of supplemental L-arginine in a chemical-induced model of colorectal cancer. World J Surg. 1996;20:1087–1091. doi: 10.1007/s002689900165. [DOI] [PubMed] [Google Scholar]
- 32.Yeh CL, Pai MH, Li CC, Tsai YL, Yeh SL. Effect of arginine on angiogenesis induced by human colon cancer: in vitro and in vivo studies. J Nutr Biochem. 2010;21:538–543. doi: 10.1016/j.jnutbio.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Brittenden J, Heys SD, Ross J, Park KG, Eremin O. Natural cytotoxicity in breast cancer patients receiving neoadjuvant chemotherapy: effects of L-arginine supplementation. Eur J Surg Oncol. 1994;20:467–472. [PubMed] [Google Scholar]
- 34.Heys SD, et al. Dietary supplementation with L-arginine: modulation of tumour-infiltrating lymphocytes in patients with colorectal cancer. Br J Surg. 1997;84:238–241. [PubMed] [Google Scholar]
- 35.Buijs N, et al. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am J Clin Nutr. 2010;92:1151–1156. doi: 10.3945/ajcn.2010.29532. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, et al. Randomized clinical trial of arginine-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing gastric cancer surgery. J Cancer Res Clin Oncol. 2013;139:1465–1470. doi: 10.1007/s00432-013-1466-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Ma Q, Wang Y, Gao X, Ma Z, Song Z. L-arginine reduces cell proliferation and ornithine decarboxylase activity in patients with colorectal adenoma and adenocarcinoma. Clin Cancer Res. 2007;13:7407–7412. doi: 10.1158/1078-0432.CCR-07-0751. This study shows that L-arginine upregulates NO production in colorectal tumours to confer inhibitory effects on tumour progression in vivo. [DOI] [PubMed] [Google Scholar]
- 38.Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front Physiol. 2013;4:144. doi: 10.3389/fphys.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huerta S, Chilka S, Bonavida B. Nitric oxide donors: novel cancer therapeutics. Int J Oncol. 2008;33:909–927. [PubMed] [Google Scholar]
- 40.Yasuda H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: nitric oxide donor as a therapeutic enhancer. Nitric Oxide. 2008;19:205–216. doi: 10.1016/j.niox.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Jeannin JF, et al. Nitric oxide-induced resistance or sensitization to death in tumor cells. Nitric Oxide. 2008;19:158–163. doi: 10.1016/j.niox.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 42.De Ridder M, Verellen D, Verovski V, Storme G. Hypoxic tumor cell radiosensitization through nitric oxide. Nitric Oxide. 2008;19:164–169. doi: 10.1016/j.niox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Sonveaux P, Jordan BF, Gallez B, Feron O. Nitric oxide delivery to cancer: why and how? Eur J Cancer. 2009;45:1352–1369. doi: 10.1016/j.ejca.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds MM, et al. Applications for nitric oxide in halting proliferation of tumor cells. Biochem Biophys Res Commun. 2013;431:647–651. doi: 10.1016/j.bbrc.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 45.Rigas B, Williams JL. NO-donating NSAIDs and cancer: an overview with a note on whether NO is required for their action. Nitric Oxide. 2008;19:199–204. doi: 10.1016/j.niox.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Findlay VJ, et al. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol Pharmacol. 2004;65:1070–1079. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMurtry V, et al. JS-K, a nitric oxide-releasing prodrug, induces breast cancer cell death while sparing normal mammary epithelial cells. Int J Oncol. 2011;38:963–971. doi: 10.3892/ijo.2011.925. This study demonstrates the anticancer efficacy of a tumour-targeted NO donor in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duan S, Cai S, Yang Q, Forrest ML. Multi-arm polymeric nanocarrier as a nitric oxide delivery platform for chemotherapy of head and neck squamous cell carcinoma. Biomaterials. 2012;33:3243–3253. doi: 10.1016/j.biomaterials.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpenter AW, Schoenfisch MH. Nitric oxide release: part II. Therapeutic applications. Chem Soc Rev. 2012;41:3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapozzi V, et al. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide. 2013;30:26–35. doi: 10.1016/j.niox.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Heilman B, Mascharak PK. Light-triggered nitric oxide delivery to malignant sites and infection. Philos Trans A Math Phys Eng Sci. 2013;371:20120368. doi: 10.1098/rsta.2012.0368. [DOI] [PubMed] [Google Scholar]
- 52.Sharma K, Chakrapani H. Site-directed delivery of nitric oxide to cancers. Nitric Oxide. 2014;43:8–16. doi: 10.1016/j.niox.2014.07.005. This study demonstrates the anticancer efficacy of tumour-targeted NO donors. [DOI] [PubMed] [Google Scholar]
- 53.Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073–6078. [PubMed] [Google Scholar]
- 54.Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev. 1998;17:107–118. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 55.Jahani-Asl A, Bonni A. iNOS: a potential therapeutic target for malignant glioma. Curr Mol Med. 2013;13:1241–1249. doi: 10.2174/1566524011313080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinecke JL, et al. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc Natl Acad Sci USA. 2014;111:6323–6328. doi: 10.1073/pnas.1401799111. This study demonstrates the suppression of tumour growth by selective iNOS inhibition with aminoguanidine in vivo, and the pro-proliferative, tumour-autonomous roles of iNOS-derived NO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granados-Principal S, et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015;17:25. doi: 10.1186/s13058-015-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenkins DC, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. This study pioneered the concept that iNOS in tumour cells can promote tumour growth in vivo, at least in part via stimulation of tumour angiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kostourou V, et al. The role of tumour-derived iNOS in tumour progression and angiogenesis. Br J Cancer. 2011;104:83–90. doi: 10.1038/sj.bjc.6606034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sikora AG, et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–1844. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juang SH, et al. Use of retroviral vectors encoding murine inducible nitric oxide synthase gene to suppress tumorigenicity and cancer metastasis of murine melanoma. Cancer Biother Radiopharm. 1997;12:167–175. doi: 10.1089/cbr.1997.12.167. [DOI] [PubMed] [Google Scholar]
- 62.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. This study defined the role of NO as a pro-angiogenic mediator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost. 2009;7:s35–37. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 64.Ziche M, Morbidelli L. Molecular regulation of tumour angiogenesis by nitric oxide. Eur Cytokine Netw. 2009;20:164–170. doi: 10.1684/ecn.2009.0169. [DOI] [PubMed] [Google Scholar]
- 65.Thomsen LL, et al. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. This study shows that selective iNOS inhibition with 1400W leads to suppression of in vivo tumour growth. [PubMed] [Google Scholar]
- 66.Camp ER, et al. Roles of nitric oxide synthase inhibition and vascular endothelial growth factor receptor-2 inhibition on vascular morphology and function in an in vivo model of pancreatic cancer. Clin Cancer Res. 2006;12:2628–2633. doi: 10.1158/1078-0432.CCR-05-2257. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Rivera E, et al. Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. Cancer Res. 2014;74:1067–1078. doi: 10.1158/0008-5472.CAN-13-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang GY, Ji B, Wang X, Gu JH. Anti-cancer effect of iNOS inhibitor and its correlation with angiogenesis in gastric cancer. World J Gastroenterol. 2005;11:3830–3833. doi: 10.3748/wjg.v11.i25.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tozer GM, et al. Nitric oxide synthase inhibition enhances the tumor vascular-damaging effects of combretastatin a-4 3-o-phosphate at clinically relevant doses. Clin Cancer Res. 2009;15:3781–3790. doi: 10.1158/1078-0432.CCR-08-2906. [DOI] [PubMed] [Google Scholar]
- 70.Cardnell RJ, Mikkelsen RB. Nitric oxide synthase inhibition enhances the antitumor effect of radiation in the treatment of squamous carcinoma xenografts. PLoS One. 2011;6:e20147. doi: 10.1371/journal.pone.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood PJ, et al. Modification of energy metabolism and radiation response of a murine tumour by changes in nitric oxide availability. Biochem Biophys Res Commun. 1993;192:505–510. doi: 10.1006/bbrc.1993.1444. This study provides evidence that modulation of NO homeostasis can affect tumour radiation responses in vivo. [DOI] [PubMed] [Google Scholar]
- 72.Wood PJ, et al. Induction of hypoxia in experimental murine tumors by the nitric oxide synthase inhibitor, NG-nitro-L-arginine. Cancer Res. 1994;54:6458–6463. [PubMed] [Google Scholar]
- 73.Bolton WK, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 74.Brindicci C, Ito K, Torre O, Barnes PJ, Kharitonov SA. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy control subjects, healthy smokers, and COPD patients. Chest. 2009;135:353–367. doi: 10.1378/chest.08-0964. [DOI] [PubMed] [Google Scholar]
- 75.López A, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 76.Alderton WK, et al. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 2005;145:301–312. doi: 10.1038/sj.bjp.0706168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh D, et al. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- 78.Høivik HO, et al. Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache. Cephalalgia. 2010;30:1458–1467. doi: 10.1177/0333102410370875. [DOI] [PubMed] [Google Scholar]
- 79.Seymour M, et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double-blind, randomised, placebo and comparator controlled phase IIa trial of GW274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin Exp Rheumatol. 2012;30:254–261. [PubMed] [Google Scholar]
- 80.Hellio le Graverand MP, et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2013;72:187–195. doi: 10.1136/annrheumdis-2012-202239. [DOI] [PubMed] [Google Scholar]
- 81.Arai Y. (WO2013018829) Method for treating cancer by combined use of drugs. 2014/0066385. US Patent.
- 82.Flitney FW, et al. Antitumor actions of ruthenium(III)-based nitric oxide scavengers and nitric oxide synthase inhibitors. Mol Cancer Ther. 2011;10:1571–1580. doi: 10.1158/1535-7163.MCT-10-0840. [DOI] [PubMed] [Google Scholar]
- 83.Andrade SP, Hart IR, Piper PJ. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992;107:1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer RE, et al. Nitric oxide synthase inhibition irreversibly decreases perfusion in the R3230Ac rat mammary adenocarcinoma. Br J Cancer. 1995;71:1169–1174. doi: 10.1038/bjc.1995.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gallo O, et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- 86.Jadeski LC, Lala PK. Nitric oxide synthase inhibition by NG-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol. 1999;155:1381–1390. doi: 10.1016/S0002-9440(10)65240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kashiwagi S, et al. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lampson BL, et al. Targeting eNOS in pancreatic cancer. Cancer Res. 2012;72:4472–4482. doi: 10.1158/0008-5472.CAN-12-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kashiwagi S, et al. Perivascular nitric oxide gradients normalize tumor vasculature. Nat Med. 2008;14:255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- 90.Yang Z, et al. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid Redox Signal. 2013;19:433–447. doi: 10.1089/ars.2012.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ng QS, et al. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol. 2007;8:111–118. doi: 10.1016/S1470-2045(07)70001-3. This study shows that NOS inhibition suppresses tumour blood flow in patients with cancer. [DOI] [PubMed] [Google Scholar]
- 92.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 93.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 94.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 95.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 96.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 97.Zuckerbraun BS, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. This study connects the mitochondrial inhibitory effect of CO to CO-mediated cytoprotective signaling in vitro. [DOI] [PubMed] [Google Scholar]
- 98.Chin BY, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schacter BA, Kurz P. Alterations in hepatic and splenic microsomal electron transport system components, drug metabolism, heme oxygenase activity, and cytochrome P-450 turnover in Murphy-Sturm lymphosarcoma-bearing rats. Cancer Res. 1982;42:3557–3564. [PubMed] [Google Scholar]
- 100.Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets. 2010;11:1551–1570. doi: 10.2174/1389450111009011551. [DOI] [PubMed] [Google Scholar]
- 101.Berberat PO, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res. 2005;11:3790–3798. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 102.Sass G, et al. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int J Cancer. 2008;123:1269–1277. doi: 10.1002/ijc.23695. This study shows that silencing of HO1 suppresses tumour growth in vivo. [DOI] [PubMed] [Google Scholar]
- 103.Alaoui-Jamali MA, et al. A novel experimental heme oxygenase-1-targeted therapy for hormone-refractory prostate cancer. Cancer Res. 2009;69:8017–8024. doi: 10.1158/0008-5472.CAN-09-0419. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, et al. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J Pathol. 2011;224:90–100. doi: 10.1002/path.2855. [DOI] [PubMed] [Google Scholar]
- 105.Sahoo SK, et al. Pegylated zinc protoporphyrin: a water-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjug Chem. 2002;13:1031–1038. doi: 10.1021/bc020010k. [DOI] [PubMed] [Google Scholar]
- 106.Fang J, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63:3567–3574. This study shows that inhibition of HO1 suppresses tumour growth in vivo. [PubMed] [Google Scholar]
- 107.Hirai K, Sasahira T, Ohmori H, Fujii K, Kuniyasu H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int J Cancer. 2007;120:500–505. doi: 10.1002/ijc.22287. [DOI] [PubMed] [Google Scholar]
- 108.Nowis D, et al. Zinc protoporphyrin IX, a heme oxygenase-1 inhibitor, demonstrates potent antitumor effects but is unable to potentiate antitumor effects of chemotherapeutics in mice. BMC Cancer. 2008;8:197. doi: 10.1186/1471-2407-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyake M, et al. Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of the urinary bladder. Oncol Rep. 2011;25:653–660. doi: 10.3892/or.2010.1125. [DOI] [PubMed] [Google Scholar]
- 110.Deng R, et al. Inhibition of tumor growth and alteration of associated macrophage cell type by an HO-1 inhibitor in breast carcinoma-bearing mice. Oncol Res. 2013;20:473–482. doi: 10.3727/096504013x13715991125684. [DOI] [PubMed] [Google Scholar]
- 111.Gueron G, et al. Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol Cancer Res. 2009;7:1745–1755. doi: 10.1158/1541-7786.MCR-08-0325. [DOI] [PubMed] [Google Scholar]
- 112.Zou C, et al. Heme oxygenase-1: a molecular brake on hepatocellular carcinoma cell migration. Carcinogenesis. 2011;32:1840–1848. doi: 10.1093/carcin/bgr225. [DOI] [PubMed] [Google Scholar]
- 113.Kappas A, Drummond GS, Manola T, Petmezaki S, Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics. 1988;81:485–497. [PubMed] [Google Scholar]
- 114.Dover SB, Moore MR, Fitzsimmons EJ, Graham A, McColl KE. Tin protoporphyrin prolongs the biochemical remission produced by heme arginate in acute hepatic porphyria. Gastroenterology. 1993;105:500–506. doi: 10.1016/0016-5085(93)90726-s. [DOI] [PubMed] [Google Scholar]
- 115.Kappas A, Drummond GS, Henschke C, Valaes T. Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics. 1995;95:468–474. [PubMed] [Google Scholar]
- 116.Nakamura H, Fang J, Gahininath B, Tsukigawa K, Maeda H. Intracellular uptake and behavior of two types zinc protoporphyrin (ZnPP) micelles, SMA-ZnPP and PEG-ZnPP as anticancer agents; unique intracellular disintegration of SMA micelles. J Control Release. 2011;155:367–375. doi: 10.1016/j.jconrel.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 117.Tsukigawa K, Nakamura H, Fang J, Otagiri M, Maeda H. Effect of different chemical bonds in pegylation of zinc protoporphyrin that affects drug release, intracellular uptake, and therapeutic effect in the tumor. Eur J Pharm Biopharm. 2015;89:259–270. doi: 10.1016/j.ejpb.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 118.Pittalà V, Salerno L, Romeo G, Modica MN, Siracusa MA. A focus on heme oxygenase-1 (HO-1) inhibitors. Curr Med Chem. 2013;20:3711–3732. doi: 10.2174/0929867311320300003. [DOI] [PubMed] [Google Scholar]
- 119.Bergstraesser C, et al. Inhibition of VCAM-1 expression in endothelial cells by CORM-3: the role of the ubiquitin-proteasome system, p38, and mitochondrial respiration. Free Radic Biol Med. 2012;52:794–802. doi: 10.1016/j.freeradbiomed.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 120.Schwer CI, et al. Carbon monoxide releasing molecule-2 CORM-2 represses global protein synthesis by inhibition of eukaryotic elongation factor eEF2. Int J Biochem Cell Biol. 2013;45:201–212. doi: 10.1016/j.biocel.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 121.Wegiel B, et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. This study demonstrates the inhibitory effects of CO inhalation therapy on tumour growth in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Long R, Salouage I, Berdeaux A, Motterlini R, Morin D. CORM-3, a water soluble CO-releasing molecule, uncouples mitochondrial respiration via interaction with the phosphate carrier. Biochim Biophys Acta. 2014;1837:201–209. doi: 10.1016/j.bbabio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Vítek L, et al. Antiproliferative effects of carbon monoxide on pancreatic cancer. Dig Liver Dis. 2014;46:369–375. doi: 10.1016/j.dld.2013.12.007. This study demonstrates the inhibitory effects of CO inhalation therapy or parenteral CORM therapy on tumour growth in vivo. [DOI] [PubMed] [Google Scholar]
- 124.Stamellou E, et al. Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation. Redox Biol. 2014;2:739–748. doi: 10.1016/j.redox.2014.06.002. This paper introduces the concept of enzyme-triggered CORM release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee WY, et al. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol Appl Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 126.Foresti R, Bani-Hani MG, Motterlini R. Use of carbon monoxide as a therapeutic agent: promises and challenges. Int Care Med. 2008;34:649–658. doi: 10.1007/s00134-008-1011-1. [DOI] [PubMed] [Google Scholar]
- 127.Otterbein LE. Quoth the Raven: carbon monoxide and nothing more. Med Gas Res. 2013;3:7. doi: 10.1186/2045-9912-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Plummer AL. The carbon monoxide diffusing capacity: clinical implications, coding, and documentation. Chest. 2008;134:663–667. doi: 10.1378/chest.07-1771. [DOI] [PubMed] [Google Scholar]
- 129.Vesely AE, et al. The effects of carbon monoxide on respiratory chemoreflexes in humans. Environ Res. 2004;94:227–233. doi: 10.1016/S0013-9351(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 130.Mayr FB, et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 131.Resch H, Zawinka C, Weigert G, Schmetterer L, Garhöfer G. Inhaled carbon monoxide increases retinal and choroidal blood flow in healthy humans. Invest Ophthalmol Vis Sci. 2005;46:4275–4280. doi: 10.1167/iovs.05-0417. [DOI] [PubMed] [Google Scholar]
- 132.Bathoorn E, et al. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 133.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 134.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 135.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 136.Wallace JL, Ferraz JG, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal. 2012;17:58–67. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 138.Shibuya N, et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 139.Asimakopoulou A, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br J Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Papapetropoulos A, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coletta C, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. This study shows evidence for cooperative signaling between NO and H2S in the control of angiogenesis and vascular relaxation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szabo C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I Biochemical and physiological mechanisms. Br J Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szabo C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. This study shows that silencing or inhibition of CBS suppresses colon cancer bioenergetics and growth in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bhattacharyya S, et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. This study shows that silencing or inhibition of CBS suppresses ovarian cancer bioenergetics and growth in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo H, et al. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology. 2012;79:483.e1–5. doi: 10.1016/j.urology.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 146.Sen S, et al. Role of cystathionine β-synthase in human breast cancer. Free Radic Biol Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 147.Chao C, et al. Cystathionine-β-synthetase inhibition in combination with standard-chemotherapy decreases colorectal cancer metastasis to the liver. Nitric Oxide. 2014;39:S21–22. [Google Scholar]
- 148.Takano N, et al. Decreased expression of cystathionine β-synthase promotes glioma tumorigenesis. Mol Cancer Res. 2014;12:1398–1406. doi: 10.1158/1541-7786.MCR-14-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hellmich MR, Coletta C, Chao C, Szabo C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal. 2015;22:424–48. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Panza E, et al. Role of the cystathionine γ lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28:61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 151.De Vos J, et al. Comparison of gene expression profiling between malignant and normal plasma cells with oligonucleotide arrays. Oncogene. 2002;21:6848–6857. doi: 10.1038/sj.onc.1205868. [DOI] [PubMed] [Google Scholar]
- 152.Hansel DE, et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003;163:217–229. doi: 10.1016/S0002-9440(10)63645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang W, et al. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 2005;65:1554–1560. doi: 10.1158/0008-5472.CAN-04-1554. [DOI] [PubMed] [Google Scholar]
- 154.Jurkowska H, Placha W, Nagahara N, Wróbel M. The expression and activity of cystathionine-γ-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids. 2011;41:151–158. doi: 10.1007/s00726-010-0606-3. [DOI] [PubMed] [Google Scholar]
- 155.Szczesny B, Brunyanszki A, Hellmich MR, Szabo C. Hydrogen sulfide in lung adenocarcinoma: A tumor cell survival factor, an enhancer of mitochondrial DNA repair and a cellular bioenergetic stimulator. Nitric Oxide. 2015;47:S46. [Google Scholar]
- 156.Fan K, et al. Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell Signal. 2014;26:2801–2808. doi: 10.1016/j.cellsig.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 157.Zatarain JR, et al. H2S inhibition of cystathionine-β-synthase (CBS) using a novel prodrug decreases colorectal cancer xenograft growth with less toxicity than aminooxyacetic acid (AOAA) Gastroenterology. 2015;148:S950. [Google Scholar]
- 158.Perry TL, et al. Failure of aminooxyacetic acid therapy in Huntington disease. Neurology. 1980;30:772–775. doi: 10.1212/wnl.30.7.772. [DOI] [PubMed] [Google Scholar]
- 159.Guth PS, et al. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann Otol Rhinol Laryngol. 1990;99:74–79. doi: 10.1177/000348949009900113. [DOI] [PubMed] [Google Scholar]
- 160.Li L, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 161.Han Y, et al. Hydrogen sulfide inhibits abnormal proliferation of lymphocytes via AKT/GSK3β signal pathway in systemic lupus erythematosus patients. Cell Physiol Biochem. 2013;31:795–804. doi: 10.1159/000350097. [DOI] [PubMed] [Google Scholar]
- 162.Lee ZW, et al. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br J Pharmacol. 2014;171:4322–4336. doi: 10.1111/bph.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee ZW, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. This study demonstrates the inhibitory effects of a slow-releasing H2S donor on tumour growth in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu S, Gao Y, Huang X, Wang X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int J Oncol. 2014;44:1259–1267. doi: 10.3892/ijo.2014.2305. [DOI] [PubMed] [Google Scholar]
- 165.Benavides GA, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Insko MA, Deckwerth TL, Hill P, Toombs CF, Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br J Pharmacol. 2009;157:944–951. doi: 10.1111/j.1476-5381.2009.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Predmore BL, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem Toxicol. 2013;57:362–370. doi: 10.1016/j.fct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 169.Singh SV, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–9511. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu PP, et al. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomedicine. 2011;18:672–676. doi: 10.1016/j.phymed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 171.Li W, et al. Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 2012;44:577–583. doi: 10.1093/abbs/gms033. [DOI] [PubMed] [Google Scholar]
- 172.Wallace GC, et al. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J Neurooncol. 2013;114:43–50. doi: 10.1007/s11060-013-1165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma K, et al. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chattopadhyay M, et al. Hydrogen sulfide-releasing aspirin suppresses NF-κB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2012;83:723–732. doi: 10.1016/j.bcp.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 175.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gemici B, et al. H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide. 2015;46:25–31. doi: 10.1016/j.niox.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 177.Maksimovic-Ivanic D, et al. Anticancer properties of the novel nitric oxide-donating compound (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid-nitric oxide in vitro and in vivo. Mol Cancer Ther. 2008;7:510–520. doi: 10.1158/1535-7163.MCT-07-2037. [DOI] [PubMed] [Google Scholar]
- 178.Mijatovic S, et al. Induction of caspase-independent apoptotic-like cell death of mouse mammary tumor TA3Ha cells in vitro and reduction of their lethality in vivo by the novel chemotherapeutic agent GIT-27NO. Free Radic Biol Med. 2010;48:1090–1099. doi: 10.1016/j.freeradbiomed.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 179.Donia M, et al. In vitro and in vivo anticancer action of Saquinavir-NO, a novel nitric oxide-derivative of the protease inhibitor saquinavir, on hormone resistant prostate cancer cells. Cell Cycle. 2011;10:492–499. doi: 10.4161/cc.10.3.14727. [DOI] [PubMed] [Google Scholar]
- 180.Donia M, et al. Unique antineoplastic profile of Saquinavir-NO, a novel NO-derivative of the protease inhibitor Saquinavir, on the in vitro and in vivo tumor formation of A375 human melanoma cells. Oncol Rep. 2012;28:682–688. doi: 10.3892/or.2012.1840. [DOI] [PubMed] [Google Scholar]
- 181.Elsheikh W, Blackler RW, Flannigan KL, Wallace JL. Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer. Nitric Oxide. 2014;41:131–137. doi: 10.1016/j.niox.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 182.Moriyama A, et al. Plasma nitrite/nitrate concentrations as a tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2000;296:181–191. doi: 10.1016/s0009-8981(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 183.Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: novel approach for early detection of lung cancer. Lung Cancer. 2009;63:164–168. doi: 10.1016/j.lungcan.2008.05.020. This paper presents an approach of measuring patient-exhaled levels of NO for the detection of lung cancer by. [DOI] [PubMed] [Google Scholar]
- 184.Yin H, Fang J, Liao L, Maeda H, Su Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer. 2014;14:436. doi: 10.1186/1471-2407-14-436. This study indicates there are increased levels of the CO metabolite carboxyhemoglobin in the circulation of cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bonavida B, Baritaki S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-kB/Snail/YY1/RKIP circuitry. Nitric Oxide. 2011;24:1–7. doi: 10.1016/j.niox.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 186.Bhowmick R, Girotti AW. Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic Biol Med. 2013;57:39–48. doi: 10.1016/j.freeradbiomed.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Furfaro AL, et al. HO-1 up-regulation: a key point in high-risk neuroblastoma resistance to bortezomib. Biochim Biophys Acta. 2014;1842:613–622. doi: 10.1016/j.bbadis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 188.Sanokawa-Akakura R, Ostrakhovitch EA, Akakura S, Goodwin S, Tabibzadeh S. A H2S-Nampt dependent energetic circuit is critical to survival and cytoprotection from damage in cancer cells. PLoS One. 2014;9:e108537. doi: 10.1371/journal.pone.0108537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ostrakhovitch EA, Akakura S, Sanokawa-Akakura R, Goodwin S, Tabibzadeh S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp Cell Res. 2015;330:135–150. doi: 10.1016/j.yexcr.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 190.Eyler CE, et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Herrmann H, et al. The Hsp32 inhibitors SMA-ZnPP and PEG-ZnPP exert major growth-inhibitory effects on D34+/CD38+ and CD34+/CD38− AML progenitor cells. Curr Cancer Drug Targets. 2012;12:51–63. doi: 10.2174/156800912798888992. [DOI] [PubMed] [Google Scholar]
- 192.Kim RK, et al. Fractionated radiation-induced nitric oxide promotes expansion of glioma stem-like cells. Cancer Sci. 2013;104:1172–1177. doi: 10.1111/cas.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107–119. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 194.Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resist Updat. 2006;9:157–173. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 195.Burke AJ, Sullivan FJ, Giles FJ, Glynn SA. The yin and yang of nitric oxide in cancer progression. Carcinogenesis. 2013;34:503–512. doi: 10.1093/carcin/bgt034. [DOI] [PubMed] [Google Scholar]
- 196.Grochot-Przeczek A, Dulak J, Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond) 2012;122:93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 197.Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:504–518. doi: 10.1038/nrgastro.2012.85. [DOI] [PubMed] [Google Scholar]
- 198.Szabo C. Gaseotransmitters: new frontiers for translational science. Sci Transl Med. 2010;2:59ps54. doi: 10.1126/scitranslmed.3000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 200.Kilbourn RG, et al. Inhibition of interleukin-1-alpha-induced nitric oxide synthase in vascular smooth muscle and full reversal of interleukin-1-alpha-induced hypotension by N omega-amino-L-arginine. J Natl Cancer Inst. 1992;84:1008–1016. doi: 10.1093/jnci/84.13.1008. [DOI] [PubMed] [Google Scholar]
- 201.Kilbourn RG, Szabo C, Traber DL. Beneficial versus detrimental effects of nitric oxide synthase inhibitors in circulatory shock: lessons learned from experimental and clinical studies. Shock. 1997;7:235–246. doi: 10.1097/00024382-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 202.Kondapaneni M, McGregor JR, Salvemini D, Laubach VE, Samlowski WE. Inducible nitric oxide synthase (iNOS) is not required for IL-2-induced hypotension and vascular leak syndrome in mice. J Immunother. 2008;31:325–333. doi: 10.1097/CJI.0b013e31816112e8. [DOI] [PubMed] [Google Scholar]
- 203.Samlowski WE, et al. Endothelial nitric oxide synthase is a key mediator of interleukin-2-induced hypotension and vascular leak syndrome. J Immunother. 2011;34:419–427. doi: 10.1097/CJI.0b013e31821dcb50. [DOI] [PubMed] [Google Scholar]
- 204.Maybauer DM, et al. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem Biophys Res Commun. 2008;377:786–791. doi: 10.1016/j.bbrc.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kilbourn RG, Fonseca GA, Trissel LA, Griffith OW. Strategies to reduce side effects of interleukin-2: evaluation of the antihypotensive agent NG-monomethyl-L-arginine. Cancer J Sci Am. 2000;6:S21–30. This study shows that inhibition of NOS production by the non-isoform-selective NOS inhibitor L-NMA reduces the hypotensive side effects of IL-2 during the immunotherapy of cancer patients. [PubMed] [Google Scholar]
- 206.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–83. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 207.Blatt J, Corey SJ. Drug repurposing in pediatrics and pediatric hematology oncology. Drug Discov Today. 2013;18:4–10. doi: 10.1016/j.drudis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 208.Stenvang J, et al. Biomarker-guided repurposing of chemotherapeutic drugs for cancer therapy: a novel strategy in drug development. Front Oncol. 2013;3:313. doi: 10.3389/fonc.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quinn BJ, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–80. doi: 10.1016/j.tem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 210.Jordan BF, et al. Nitric oxide as a radiosensitizer: evidence for an intrinsic role in addition to its effect on oxygen delivery and consumption. Int J Cancer. 2004;109:768–773. doi: 10.1002/ijc.20046. [DOI] [PubMed] [Google Scholar]
- 211.Oronsky BT, Knox SJ, Scicinski JJ. Is nitric oxide (NO) the last word in radiosensitization? A review. Transl Oncol. 2012;5:66–71. doi: 10.1593/tlo.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Arrieta O, et al. Phase II study. Concurrent chemotherapy and radiotherapy with nitroglycerin in locally advanced non-small cell lung cancer. Radiother Oncol. 2014;111:311–315. doi: 10.1016/j.radonc.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 213.Reid T, et al. Two case reports of resensitization to previous chemotherapy with the novel hypoxia-activated hypomethylating anticancer agent RRx-001 in metastatic colorectal cancer patients. Case Rep Oncol. 2014;7:79–85. doi: 10.1159/000358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jiang H, et al. Activated macrophages as a novel determinant of tumor cell radioresponse: the role of nitric oxide-mediated inhibition of cellular respiration and oxygen sparing. Int J Radiat Oncol Biol Phys. 2010;76:1520–1527. doi: 10.1016/j.ijrobp.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 215.de Bono JS, et al. Phase I study of ONO-4007, a synthetic analogue of the lipid A moiety of bacterial lipopolysaccharide. Clin Cancer Res. 2000;6:397–405. [PubMed] [Google Scholar]