Abstract
Cyclase enzymes weave simple polyprenyl chains into the elaborate polycyclic ring systems of terpenes, a sequence that is often difficult to emulate under abiotic conditions. Herein we report a disparate synthetic approach to complex terpenes whereby simple prenyl-derived chains are cyclized using radical, rather than cationic, reaction pathways. This strategy efficiently forges challenging 5/8/5-fused ring systems found in numerous complex natural product classes and enabled a nine-step total synthesis of (−)-6-epi-ophiobolin N, a member of the large family of cytotoxic ophiobolin sesterterpenes. A small-molecule thiol catalyst was found to override the inherent diastereoselectivity observed during a reductive radical cascade cyclization process. This work lays the foundation for efficiently accessing historically challenging terpenoid ring systems of interest in medicinal research.
Main Text
Terpenes represent a highly diverse class of natural products whose derivatives have been developed into numerous FDA-approved drugs for the treatment of cancer, bacterial infection, malaria, and various other human diseases (1,2). Despite these successes, terpenes can pose unique challenges to medicinal research owing to often-limited commercial availability, difficulties in making deep-seated structural modifications, and incompletely elucidated biosynthetic pathways. Owing to their non-modular chemical structures, a unifying strategy for the chemical synthesis of terpenes does not exist and such compounds have historically posed significant challenges to the field (3). Terpenes arise via the enzymatic conversion of simple polyprenyl chains into highly intricate polycyclic carbon networks of extraordinary diversity (4). Biomimetic synthesis, the act of emulating nature’s bond construction process, would be an ideal synthetic tool in this context (5,6), and indeed cationic polyene cyclizations are perhaps the best studied of all biomimetic cyclization reactions (7). To date however, a very limited subset of terpenoid carbocyclic diversity can be accessed in this manner in a laboratory setting, and the synthesis of many medium and larger ring terpene frameworks has proven particularly problematic. In contrast, shape-restricted enzyme cavities in terpene cyclases have evolved with appropriately placed amino acid residues to stabilize selective transition states, and in concert, dictate cyclization pathways. As a result of modulating this environment, the formation of myriad terpene skeletons, elaborate rearrangement processes, and various termination modes all become chemically possible (4). Herein we describe a disparate approach, whereby simple polyprenyl-derived chains are cyclized via radical, not cationic–based, methods. Utilizing different reagent combinations, we show that the termination modes of these cyclizations can be controlled, and importantly, with a chiral small-molecule thiol catalyst, their inherent stereochemical preferences altered.
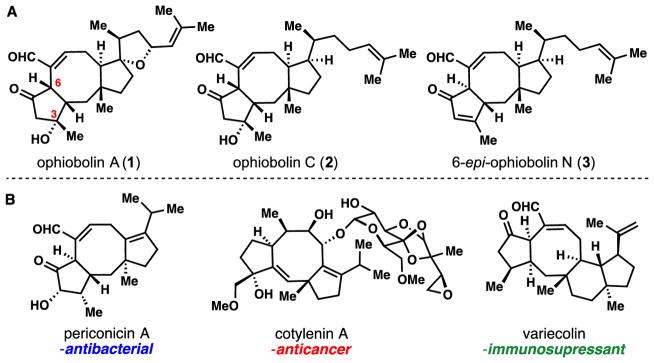
Complex 5/8/n-fused ring systems (n = 5, 6) are found in numerous di- and sesterterpenes possessing notable antibiotic (8), cytotoxic (9), and immunosuppressant properties among others (10) (Fig. 1). Central to this structural type is the continually expanding family of ophiobolin sesterterpenes featuring a stereochemically rich and synthetically formidable 5/8/5-fused ring system (Fig. 1A). While initially heavily investigated for their phytotoxic effects which negatively impact agricultural production, ophiobolin A (1) was later discovered to be a powerful inhibitor of calmodulin and remains an important tool for studying this calcium signaling protein today (11). Most recently, these fungal metabolites have attracted much attention for their potent cytotoxic effects against multiple cancer cell lines, including the highly drug resistant human brain tumor glioblastoma multiforme (12–14). While over thirty distinct members have been identified to date, often in minute and varying quantities, ophiobolin A (1), C (2), and 6-epi N (3) highlight the major structural variations found in this terpenoid family, namely: i) hydroxylation at carbon-3 or dehydration to an enone system, ii) epimeric stereochemistry at carbon-6 for nearly all members, and iii) myriad side-chain oxidation motifs, sometimes resulting in tetrahydrofuran ring formation (see 1 for example) (11,15). Despite decades of research by numerous laboratories (16–22), only the Kishi (23), and more recently Nakada (24) groups have charted fully synthetic routes to various ophiobolin members. Despite these achievements, the long step-counts required (38 steps to 2, 47 steps to 1, respectively) are limitations for accessing numerous family members, conducting in-depth structure activity studies, and ultimately producing superior derivatives. These limitations are no doubt a direct consequence of the unique challenges posed by the carbogenic complexity of the ophiobolins.
Fig. 1. Complex terpenoids containing 5/8/n-fused carbocyclic skeletons.
(A) Members of the ophiobolin sesterterpenes. (B) Di- and sesterterpene classes of relevance to multiple therapeutic areas.
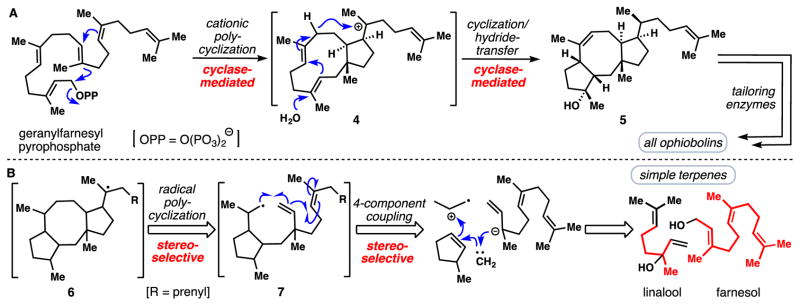
In analyzing previous syntheses of ophiobolins, the lengthy, stepwise construction of the 5/8/5-fused ring system contrasts strikingly with the concise cyclase-mediated biosynthetic pathway (Fig. 2A). It has been proposed that the biosynthesis of the ophiobolins involves a cationic cyclization of geranylfarnesyl pyrophosphate to carbocation 4, which through a hydride shift, transannular cyclization, and hydration process, is then converted into the 5/8/5-fused skeleton of 5 (the process is shown in one step for simplicity) (25). In developing a retrosynthesis of ophiobolins, we were inspired by hypothetical carbon-centered radical 6 and considered its formation by the 8-endo/5-exo-cascade cyclization process shown (see 7→6, Fig. 2B). Further disconnection, via the hypothetical 4-component coupling sequence shown, led to the identification of linalool (C-10) and farnesol (C-15) as suitable materials wherein the carbons labeled in red are incorporated into the final target. Thus the central tenet of our synthetic strategy was a desire to utilize the biochemical building blocks, but to forge the bonds in an abiotic fashion – a strategy we have previously found to facilitate the synthesis of simpler terpenoids (26).
Fig. 2. Synthetic approaches to access complex ophiobolin ring systems.
(A) Nature’s strategy employing cyclase-mediated carbocationic cascades (25). (B) Retrosynthetic analysis employing a strategic radical cascade involving simple polyprenyl building blocks.
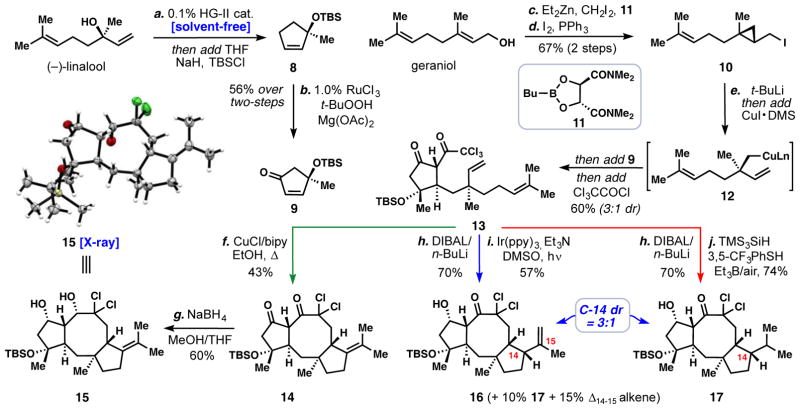
Seeking to realize aspects of this synthetic blueprint, we prepared cyclopentenone 9 via a short sequence from the abundant monoterpene (−)-linalool (Fig. 3). A solvent-free ring closing metathesis reaction catalyzed by 0.1 mol% of the Hoveyda-Grubbs second-generation catalyst (HG-II) was first used to construct the cyclopentene ring (27), and upon complete consumption of linalool, the reaction mixture was diluted with THF and silylated in-situ (NaH, TBSCl) affording chiral cyclopentene 8 in near quantitative yield (>95%). After examining a variety of allylic oxidation conditions, we found that modifications to a ruthenium-catalyzed procedure developed by researchers at Merck (28) smoothly furnished 9 in reasonable yield and on multi-gram scales (57% isolated). In a separate vessel, we prepared highly sensitive alkyl iodide 10 from geraniol utilizing Charette’s asymmetric cyclopropanation methodology (29) in conjunction with a modified Appel procedure. With two-step access to chiral fragments 9 and 10, we developed and optimized a challenging 3-component coupling reaction, bearing strong analogy to our retrosynthetic blueprint (Fig. 2B), but having already incorporated the methylene (:CH2) unit. Treating 10 with tert-butyllithium induced lithium-halogen exchange and subsequent anionic cyclopropane fragmentation (30), and following transmetallation with copper iodide dimethylsulfide complex, an intermediate organocopper species (12) was formed which cleanly added to cyclopentenone 9. This 1,4-addition occurred in a diastereoselective fashion (diastereomeric ratio (dr) = 3:1) with the nucleophile approaching opposite to the bulky OTBS group, and following quenching with trichloroacetyl chloride, cyclopentenone 13 could be isolated after column chromatography. While this reaction proved sensitive to temperature and mixing efficiency, we found it highly reproducible on half-gram scales.
Fig. 3. Four-step entry into complex 5/8/5-fused ring systems.
Reagents and conditions: step (a) HG-II (0.1 mol %), 25 °C, 1 h, then add NaH (3.0 equiv), THF, 0 °C, 5 min, then add TBSCl (1.5 equiv), 65 °C, 2 h, 98%; (b) RuCl3 (1 mol %), Mg(OAc)2·4H2O (2.0 equiv), t-BuOOH (10.9 equiv), CH2Cl2/H2O, 25 °C, 57%; (c) geraniol (1 equiv), boronic ester 11 (1.5 equiv), Et2Zn (2 equiv), CH2I2 (4 equiv), CH2Cl2, 0 °C → 25 °C, 5 h, 89%; (d) I2 (1.1 equiv), PPh3 (1.1 equiv), imidazole (1.8 equiv), CH2Cl2, 0 °C, 1 h, 75%; (e) 10 (1.3 equiv), t-BuLi (2.7 equiv), pentane/Et2O, −78 °C, 2.5 h, then add CuI (0.53 equiv), Me2S (2.1 equiv), −78 °C, 15 min, then add 9 (1.0 equiv), −78 °C → −40 °C, 4 h, then add Cl3CCOCl (3.3 equiv), −78 °C → −40 °C, 2 h, 60%, dr = 3:1; (f) CuCl (0.7 equiv), bipy (0.9 equiv), EtOH, 80 °C, 1.5 h, 43%; (g) NaBH4 (2 equiv), MeOH/THF, 0 °C, 1 h, 60%; (h) DIBAL (1.0 equiv), n-BuLi (1.0 equiv), toluene/Et2O, 2 h, then AcOH (6.0 equiv), 70%; (i) Ir(ppy)3 (1 mol %), Et3N (0.5 equiv), DMSO, Blue LEDs, 25 °C, 2 h, 57%, dr = 3:1; (j) Et3B (1.0 equiv), air, (TMS)3SiH (1.2 equiv), 3,5-bis(CF3)-PhSH (2.0 equiv), PhH, 25 °C, 74%, dr = 3:1. HG-II = Hoveyda Grubb’s second generation catalyst = (1,3-Bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(o-isopropoxyphenylmethylene) ruthenium; DIBAL = diisobutylaluminum hydride; ppy = 2-phenylpyridinato; bipy = 2,2′-bipyridine; TMS = trimethylsilyl; TBS = tert-butyldimethylsilyl
Our efforts to effect the key radical cyclization of trichloroketone 13 initially focused on copper-mediated atom-transfer radical cyclization processes to construct the 5/8/5-fused ring system. While molecular models gave credence to the feasibility of this cascade process, both 8-endo and 7-exo cyclization pathways have been observed previously (31, 32); the outcome of this transformation, especially with regard to stereochemistry, was therefore by no means certain (33). Gratifyingly however, when 13 was heated with copper(I) chloride and bipyridine, polycycle 14, which contains the desired trans 5/8 ring junction, was formed in 43% yield. The stereochemistry of this cascade, as well as of prior steps, was confirmed by X-ray crystallographic analysis of the sodium borohydride reduction product 15. If a tertiary chloride intermediate is formed under these cyclization conditions, it eliminates selectively to the tetrasubstituted olefin isomer – a process we envision to be crucial in efforts to synthesize periconicin and cotylenin A which possess internal alkenes in this sector (see Fig 1B). With respect to the ophiobolin synthetic problem, this termination mode is not ideal, as a key stereocenter on the newly forged cyclopentane ring is lost. After surveying a variety of options, we perceived two interesting solutions. First, we found that through selective reduction of 13 (DIBAL/n-Buli), the keto alcohol subsequently formed could be converted into 16 via an Ir-catalyzed photoredox cyclization (34). Under these cyclization conditions, the exocyclic alkene product predominates (57%) and the tetrasubstituted alkene isomer is minimized (15%). This experiment also confirmed that the key cyclopentane stereocenter was correctly set during the cyclization cascade (dr = 3:1, see supplementary material). A small amount of reductive cyclization product 17 (10%) was also formed under these conditions. Given that a reductive process could potentially construct the basic ophiobolin side chain in its correct oxidation state directly, we optimized this transformation. By employing polarity reversal conditions (35), utilizing tris(trimethylsilyl)silane and a thiol additive (3,5-CF3PhSH was found to be optimal), reductive cyclization product 17 could be formed in good yield (74%). Triethylborane/air-mediated initiation was found to be crucial as the temperatures needed to cajole AIBN-mediated processes resulted in lower diastereoselectivity at the isopropyl-containing stereocenter (dr ~ 1:1, T = 90 °C). Overall, these three cyclization modes offer rapid (4 to 5 steps) synthetic entry into complex 5/8/5-fused tricycles with requisite handles to be converted into diverse terpenoid structures.
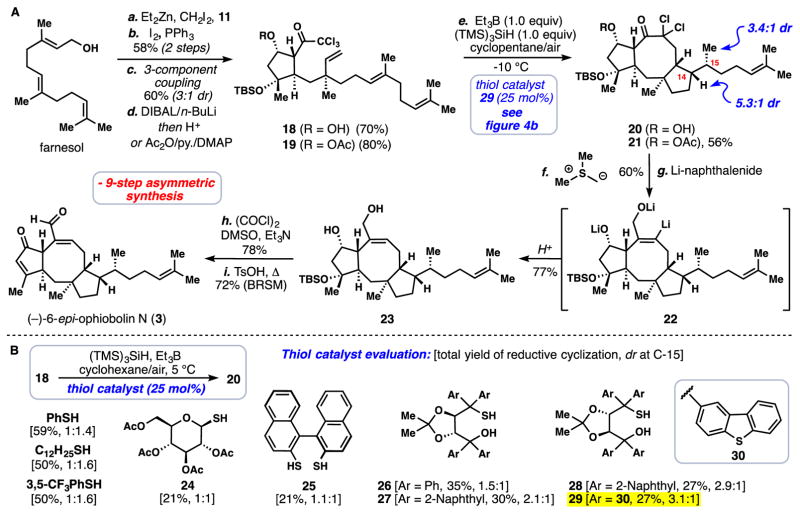
In an attempt to translate these results into the synthesis of full ophiobolin natural products, we subjected farnesol to an analogous 4-step sequence previously described for geraniol (Fig. 4). After the final hydride reduction step either free alcohol 18, or its acetylated variant 19, could be isolated in one pot. Much to our delight, subjecting 18 to the previously discovered conditions for reductive radical cyclization afforded tricycle 20 featuring the complete eastern sector of all minimally oxidized ophiobolins. While we were pleased to find that the diastereoselectivity at the cyclopentane stereocenter (C-14) was slightly improved relative to the geraniol-based system (dr ~ 4:1, T = 5 °C), our excitement was quickly diminished when it was determined that the major product of the cascade possessed the incorrect stereochemistry at the neighboring C-15 methyl stereocenter. In particular, when using previously employed 3,5-CF3PhSH as a catalyst (25 mol%), we obtained a 1.6:1 mixture favoring 15-epi 20 (Fig. 4B). Given that the thiol is believed to donate the final hydrogen atom under these conditions (35), we examined the effect of thiol structure on the diastereoselectivity of the process 18 → 20 (Fig 4B). All achiral aromatic, and aliphatic thiols tested favored the incorrect isomer with slightly varying preferences (1.4:1–1.6:1 dr in favor of 15-epi 20). Taking inspiration from the work of Roberts on enantioselective radical hydrosilylation (36), we began to examine the potential of chiral thiol catalysts to override the inherent substrate bias of this terminating hydrogen atom abstraction event (37). Although known glucose-based thiol 24 and BINOL dithiol 25 led to slight improvements, we found that when employing TADDOL-based monothiols 26 and 27 (derived from l-tartrate), the desired isomer was significantly favored (dr =1.5:1 and 2.1:1 respectively). Somewhat to our surprise, the opposite enantiomer of thiol 27 (i.e 28) was even better in this regard, resulting in a 2.9:1 diastereomeric ratio at C-15 favoring 20. The benzothiophene-based TADDOL monothiol 29 proved optimal, resulting in 3.1:1 dr in favor of 20. Optimization of the reaction conditions and use of acetate substrate 19 afforded a 56% combined yield of reductively cyclized material favoring 21 (Fig 4A). The diastereoselectivity at the cyclopentane stereocenter (C-14) was further increased to greater than 5:1 by conducting the transformation at −10 °C in cyclopentane solvent. It was not possible to chromatographically separate the individual isomers of 21 at this stage and the reported diastereomeric ratios were determined after synthetic step h in Figure 4. (see also supplementary material).
Fig. 4. Total synthesis of an ophiobolin sesterterpene.
(A) Nine-step asymmetric synthesis of (−)-6-epi-ophiobolin N (3) (yields reported for synthetic steps e-h are for the diastereomeric mixture) (B) Evaluation of thiol catalysts for the transformation of 18→20 (yields and selectivity determined by 1H NMR analysis, dr at C-14 was approximately 4:1). Reagents and conditions: (a-d) see Figure 3 for analogous conditions, (e) 19 (1.0 equiv), TMS3SiH (1.0 equiv), 29 (25 mol%), Et3B (1.0 M solution in THF, 1.25 equiv) added over 12 hours, air, cyclopentane (0.009 M), −10 °C, 12 hours, 56% combined yield of reductively cyclized material (the reported dr values at C-14 (5.3:1) and C-15 (3.4:1) were determined after synthetic step h (see SM); (f) Me3SI (24.0 equiv), n-BuLi (6.0 equiv), THF, 0 °C, 15 min, then add 21 (1.0 equiv), 10 min, 60%; (g) Lithium naphthalenide (1.0 M solution in THF, 40 equiv.), THF, −78 °C, 20 min, 77%; (h) (COCl)2 (10.0 equiv), DMSO (15.0 equiv), Et3N (20.0 equiv), CH2Cl2, −78 °C→0 °C, 3 h, 78%; (i) p-TsOH (3.0 equiv), t-BuOH/CH2Cl2, 40 °C, 24 h, 59% + 19% recovered starting material. p-TSOH = para-toluenesulfonic acid, py = pyridine, DMAP = 4-dimethylaminopyridine.
Tricycle 21, accessible in only five steps from readily abundant farnesol, requires only one carbon to complete the full C-25 skeleton of the target. This task was readily accomplished via the Corey-Chaykovsky epoxidation reaction (38), which fortuitously also removed the acetate group in the process (Fig 4A). A second reductive cascade process was then designed to convert this spiro-epoxide intermediate (not shown) into the requisite ophiobolin cyclooctene ring system. Thus, treatment with excess lithium naphthalenide induced reductive ring opening of the epoxide (39), with concomitant dehalogenation of the remaining chloride, presumably forming trianionic intermediate 22. Following aqueous work-up, diol 23 could be isolated in excellent yield (77%). The extraneous chlorine atoms, which are required for efficient atom transfer cyclization, are seamlessly converted into the requisite olefinic functionality found in the final target with minimal chemical expenditure – a task that may find use in other settings. Finally, double Swern oxidation of diol 23, followed by treatment with para-toluenesulfonic acid under slightly elevated temperatures, forged (−)-6-epi-ophiobolin N (3), thus completing a nine-step enantioselective total synthesis in 2% overall yield. We have prepared 15 milligrams of (−)-3 using this sequence to date.
The described synthesis of 6-epi-ophiobolin N (3) lays the foundation for the efficient synthesis of other complex 5/8/n-fused terpenes for which no simple chemical or synthetic biological solutions exist. Moreover, this work also represents one of the shortest total syntheses of any known sesterterpene natural product (40). In addition, this strategy represents a departure from biomimetic, cationic cascade processes in that the chiral reagent exerts its influence during the termination of the cascade, rather than its initiation (7); combinations of both may prove even more powerful. Nevertheless, the synthesis described is not without flaw; in particular, a lack of complete diastereocontrol is noted in several steps. Myriad reductive radical cyclizations terminate with a hydrogen atom abstraction step which creates a stereogenic carbon center (41); using elements of the work demonstrated herein, it may be possible to create previously inaccessible stereochemical permutations – a process ideal from the vantage point of diverse analog preparation.
Supplementary Material
Acknowledgments
Financial support for this work was provided by UC-Berkeley, The UC-Berkeley Hellman Fellows fund, The Alfred P. Sloan Foundation, and the NIH General Medical Sciences (R01GM116952). ZGB acknowledges UC-Berkeley and the National Science Foundation for a Berkeley Fellowship and NSF Graduate Research Fellowship respectively (DGE-1106400). Mr. Nick Shin is acknowledged for helpful technical assistance. Dr. Antonio DiPasquale is acknowledged for X-ray crystallographic analysis and support from NIH Shared Instrument Grant (S10-RR027172). We thank N. Burns and F. Seidl (Stanford University) for a generous supply of various TADDOL diols used in early stages of this work. Metrical parameters for the structures of compounds 15 and SI-6 are available free of charge from the Cambridge Crystallographic Data Centre under accession numbers CCDC-1474968 and CCDC-1474969 respectively.
Footnotes
References and Notes
- 1.Stockdale TP, Williams CM. Chem Soc Rev. 2015;44:7737–7763. doi: 10.1039/c4cs00477a. [DOI] [PubMed] [Google Scholar]
- 2.Breitmaier E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. Wiley-VCH; Weinhem: 2006. [Google Scholar]
- 3.Maimone TJ, Baran PS. Nat Chem Biol. 2007;3:396–407. doi: 10.1038/nchembio.2007.1. [DOI] [PubMed] [Google Scholar]
- 4.Christianson DW. Chem Rev. 2006;106:3412–3442. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]
- 5.Poupon E, Nay B. Biomimetic Organic Synthesis. 1–2 Wiley-VCH; Weinheim: 2011. [Google Scholar]
- 6.Razzak M, De Brabander JK. Nat Chem Biol. 2011;7:865–875. doi: 10.1038/nchembio.709. [DOI] [PubMed] [Google Scholar]
- 7.Yoder RA, Johnston JS. Chem Rev. 2005;105:4730–4756. doi: 10.1021/cr040623l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Shin DS, Lee T, Oh KB. J Nat Prod. 2004;67:448–450. doi: 10.1021/np030384h. [DOI] [PubMed] [Google Scholar]
- 9.Kasukabe T, Okabe-Kado J, Honma Y. Cancer Sci. 2008;99:1693–1698. doi: 10.1111/j.1349-7006.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto H, Nakamura E, Okuyama E, Ishibashi M. Chem Pharm Bull. 2000;48:1436–1441. doi: 10.1248/cpb.48.1436. [DOI] [PubMed] [Google Scholar]
- 11.Au TK, Wallace SH, Leung PC. Life Sciences. 2000;67:733–742. doi: 10.1016/s0024-3205(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang T, Lu Z, Meng L, Wei S, Hong K, Zhu W, Huang C. Bioorg Med Chem Lett. 2012;22:579–585. doi: 10.1016/j.bmcl.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 13.Sun W, Lv C, Zhu T, Yang X, Wei S, Sun J, Hong K, Zhu W, Huang C. Mar Drugs. 2013;11:4570–4584. doi: 10.3390/md11114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bury M, Girault A, Mégalizzi V, Spiegl-Kreinecker S, Mathieu V, Berger W, Evidente A, Kornienko A, Gailly P, Vandier C, Kiss R. Cell Death and Disease. 2013;4:e561. doi: 10.1038/cddis.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei H, Itoh T, Kinoshita M, Nakai Y, Kurotaki M, Kobayashi M. Tetrahedron. 2004;60:6015–6019. [Google Scholar]
- 16.Dauben WG, Hart DJ. J Org Chem. 1977;42:922–923. [Google Scholar]
- 17.Paquette LA, Colapret JA, Andrews DR. J Org Chem. 1985;50:201–205. [Google Scholar]
- 18.Rigby JH, Senanayake C. J Org Chem. 1987;52:4634–4635. [Google Scholar]
- 19.Michalak M, Michalak K, Urbanczyk-Lipkowska Z, Wicha J. J Org Chem. 2011;76:7497–7509. doi: 10.1021/jo201357p. [DOI] [PubMed] [Google Scholar]
- 20.Wender PA, Nuss JM, Smith DB, Suárez-Sobrino A, Vågberg J, Decosta D, Bordner J. J Org Chem. 1997;62:4908–4909. [Google Scholar]
- 21.Li K, Wang C, Yin G, Gao S. Org Biomol Chem. 2013;11:7550–7558. doi: 10.1039/c3ob41693c. [DOI] [PubMed] [Google Scholar]
- 22.Mehta G, Singh V. Chem Rev. 1999;99:881–930. doi: 10.1021/cr9800356. [DOI] [PubMed] [Google Scholar]
- 23.Rowley M, Tsukamoto M, Kishi Y. J Am Chem Soc. 1989;111:2735–2737. [Google Scholar]
- 24.Tsuna K, Noguchi N, Nakada M. Angew Chem Int Ed. 2011;50:9452–9455. doi: 10.1002/anie.201104447. [DOI] [PubMed] [Google Scholar]
- 25.Chiba R, Minami A, Gomi K, Oikawa H. Org Lett. 2013;15:594–597. doi: 10.1021/ol303408a. [DOI] [PubMed] [Google Scholar]
- 26.Zhao YM, Maimone TJ. Angew Chem Int Ed. 2015;54:1223–1226. doi: 10.1002/anie.201410443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meylemans HA, Quintana RL, Goldsmith BR, Harvey BG. ChemSusChem. 2011;4:465–469. doi: 10.1002/cssc.201100017. [DOI] [PubMed] [Google Scholar]
- 28.Miller RA, Li W, Humphrey GR. Tetrahedron Lett. 1996;37:3429–3432. [Google Scholar]
- 29.Charette AB, Juteau H, Lebel H, Molinaro C. J Am Chem Soc. 1998;120:11943–11952. [Google Scholar]
- 30.Charette AB, Naud J. Tetrahedron Lett. 1999;39:7259–7262. [Google Scholar]
- 31.Sato T, Ishida S, Ishibashi H, Ikeda M. J Chem Soc Perkin Trans 1. 1991:353–359. [Google Scholar]
- 32.Liu L, Chen Q, Wu YD, Li C. J Org Chem. 2005;70:1539–1544. doi: 10.1021/jo0481349. [DOI] [PubMed] [Google Scholar]
- 33.RajanBabu TV. Acc Chem Res. 1991;24:139–145. [Google Scholar]
- 34.Tucker JW, Nguyen JD, Narayanam JMR, Krabbe SW, Stephenson CRJ. Chem Commun. 2010;46:4985–4987. doi: 10.1039/c0cc00981d. [DOI] [PubMed] [Google Scholar]
- 35.Roberts BP. Chem Soc Rev. 1999;28:25–35. [Google Scholar]
- 36.Cai Y, Roberts BP, Tocher DA. J Chem Soc Perkin Trans 1. 2002:1376–1386. [Google Scholar]
- 37.Lewis CA, Miller SJ. Angew Chem Int Ed. 2006;45:5616–5619. doi: 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]
- 38.Corey EJ, Chaykovsky M. J Am Chem Soc. 1965;87:1353–1364. [Google Scholar]
- 39.Concellón JM, Llavona L, Bernad PL., Jr Tetrahedron. 1995;51:5573–5584. [Google Scholar]
- 40.Hog DT, Webster R, Trauner D. Nat Prod Rep. 2012;29:752–779. doi: 10.1039/c2np20005h. [DOI] [PubMed] [Google Scholar]
- 41.McCarrol AJ, Walton JC. Angew Chem Int Ed. 2001;40:2224–2248. doi: 10.1002/1521-3773(20010618)40:12<2224::aid-anie2224>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Charette AB, Lebel H. Organic Syntheses. 1999;76:86–100. [Google Scholar]
- 43.Zou L, Wang B, Hongfang M, Song H, Qu Y. Org Lett. 2013;15:3106–3109. doi: 10.1021/ol401306h. [DOI] [PubMed] [Google Scholar]
- 44.Beck AK, Gysi P, La Vecchia L, Seebach D. Organic Syntheses. 1999;76:12–22. [Google Scholar]
- 45.Je JT, Uk S, Park SB. Spiro compounds as electroluminescent material for organic electroluminescent device. Repub Korean Kongkae Taeho Kongbo. 2012 KR2012072785. [Google Scholar]
- 46.Bruneau A, Roche M, Hamze A, Brion JD, Alami M, Messaoudi S. Chem Eur J. 2015;21:8375–8379. doi: 10.1002/chem.201501050. [DOI] [PubMed] [Google Scholar]
- 47.Hatano M, Maki T, Moriyama K, Arinobe M, Kazuaki I. J Am Chem Soc. 2008;130:16858–16860. doi: 10.1021/ja806875c. [DOI] [PubMed] [Google Scholar]
- 48.De Lucchi O, Maglioli P, Delogu G, Valle G. Synlett. 1991;11:841–844. [Google Scholar]
- 49.Morra NA, Pagenkopf BL. Organic Syntheses. 2008;85:53–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.