Abstract
Objective
To compare the left ventricular Eccentricity Index (EI) and tricuspid valve systolic:diastolic (SD) ratio in infants at risk of bronchopulmonary dysplasia (BPD) and pulmonary hypertension (PH).
Study Design
Review of echocardiograms performed on infants born at ≤28 weeks postmenstrual age, categorized into three cohorts: BPD and PH (n=13); BPD only (n=16); and controls (n=59). EI was measured from a parasternal short axis 2D image. The SD ratio was measured from the continuous wave Doppler tracing. Groups were compared using Kruskal-Wallis and Wilcoxon rank sum tests.
Result
EI and SD ratio were successfully measured in all infants. There were no differences between controls and BPD cohort. In contrast, the BPD and PH cohort had increased systolic EI (1.46 vs 1.00–1.01), diastolic EI (1.47 vs 1.00), and SD ratio (1.12 vs 0.97–1.00) compared to controls and BPD only cohort (P≤0.01 for all).
Conclusion
The EI and SD ratio may be useful as a screening tool for PH in this population.
Introduction
Bronchopulmonary dysplasia (BPD) is a risk factor for developing pulmonary hypertension (PH) in extremely low birth weight infants.1 The pathology underlying BPD, poor development of immature lungs leading to fewer and smaller alveoli, also leads to poorly developed pulmonary vessels, thus increasing the risk for pulmonary hypertension.2 In these infants two year survival after diagnosis of PH is less than 50%, and infants with more severe BPD tend to have more severe PH.1,3
Diagnosis and screening for PH in infants with BPD has proven difficult. The gold standard for establishing the diagnosis of PH is cardiac catheterization, which is impractical for screening as it is invasive, expensive, and is associated with morbidity and mortality.4,5 The more commonly used transthoracic echocardiography has the benefits of being noninvasive and generally available at centers with intensive care nurseries.6,7 However, echocardiographic measurements have not accurately and consistently correlated with PH, especially in infants.4,8,9 Commonly reported echocardiographic indices used to characterize PH in infants include systolic pulmonary artery pressure approximated by tricuspid valve regurgitant and pulmonary valve insufficiency velocities, interventricular septal shape, right atrial and right ventricular size, and direction of color flow Doppler across intra-atrial and ductal shunts. These measurements all have disadvantages. Some measurements are affected by acoustic windows and variable degrees of valve insufficiency, while others are difficult to quantify on a meaningful scale.9
Left ventricle eccentricity index (EI) is a quantifiable measure of the amount of distortion of ventricular septal geometry due to elevated right ventricular systolic or diastolic pressures and/or volumes. Greater degrees of left ventricular EI have been associated with PH in children and adults, but have not been studied in premature infants.10–15 The left ventricular EI has the added benefit of being easily measured from any short axis view of the mid-left ventricle.
Similarly, the tricuspid valve systolic to diastolic (SD) ratio represents a quantifiable echocardiographic measure that can be readily obtained from the tricuspid valve regurgitant jet. This measurement has been evaluated in healthy neonates as a measurement of systolic function and has the advantage of being measured even when there is an incomplete tricuspid regurgitant jet that prevents estimation of the peak systolic regurgitant velocity.10,16 Elevated SD ratios have been associated with worse clinical outcomes in children with PH and has been shown to be abnormal in children with systolic and/or diastolic dysfunction.17 However, this measurement has not been previously described in infants with PH.
Because PH complicating BPD is of growing concern for extremely low birth weight infants, and no consensus, evidence-based guideline exists for what measures to use for screening, we sought to assess systolic and diastolic EI and SD ratio in existing echocardiographic images on 3 groups of extremely low birth weight infants: 1) BPD and previously diagnosed PH; 2) BPD only; and 3) controls with neither BPD nor PH as a first step towards including these measures in a BPD-PH screening guideline.
Methods
Subjects
We performed a retrospective review of infants born ≤28 weeks postmenstrual age admitted to the Duke University Intensive Care Nursery from 2010 to 2012. All infants having a trans-thoracic echocardiogram performed after 36 weeks’ corrected postmenstrual age were screened. Infants with evidence of congenital heart disease (with the exception of patent ductus arteriosus or patent foramen ovale) or structural lung and airway malformations were excluded. Acquired vascular abnormalities, including pulmonary venous stenosis, were excluded as well. Remaining infants were divided into 3 groups: infants with both BPD and PH, infants with BPD only (without PH), and infant controls without BPD or PH. BPD was defined as continued need for respiratory support (supplemental oxygen with FiO2, mechanical ventilation, or provision of continuous positive airway pressure) persisting at 36 weeks corrected postmenstual age.18 PH was defined by cardiac catheterization data, peak tricuspid valve regurgitant jet velocity and/or clinical need/response to pulmonary vasodilators. Ventricular septal geometry was excluded from this definition. Nine of the thirteen infants with BPD and PH were defined as having PH by any echocardiogram demonstrating tricuspid valve regurgitant jet velocity greater than one-half systemic pressure during their stay in the intensive care nursery. The remaining four infants with clinically suspected of having PH were diagnosed by cardiac catheterization after the date of the reviewed echocardiograms of this study. Infants were identified using a clinical database developed and maintained by the Duke Neonatal-Perinatal Medicine department. The indications for echocardiograms varied by groups, noted by associated diagnosis with the ordered study. The BPD and PH group’s and BPD only group’s studies were most commonly obtained for evaluation for pulmonary hypertension. The control group’s echocardiograms were most commonly obtained for evaluation for patent ductus arteriosus. This study was approved after meeting all waivers of informed consent requirements by the Duke University Health System Institutional Review Board.
Echocardiography
Complete echocardiograms with on-axis images were reviewed using the first study performed at >36 weeks corrected postmenstrual age. Images were obtained using Phillips IE33 ultrasound scanner with an 8 or 12 MHz transducer and analyzed using Xcelera (Phillips Medical Systems, Koninklijke, Netherlands) after standard data compression to 30 frames per second. Tricuspid valve Doppler tracings were obtained from the apical 4-chamber view by continuous wave Doppler. A single reviewer performed the initial measurements. A random sample of 20 studies was reviewed by a blinded second reviewer to determine inter-observer reliability, and repeated a second time by the initial observer to determine intra-observer reliability. The echocardiographic variables that were analyzed included the following:
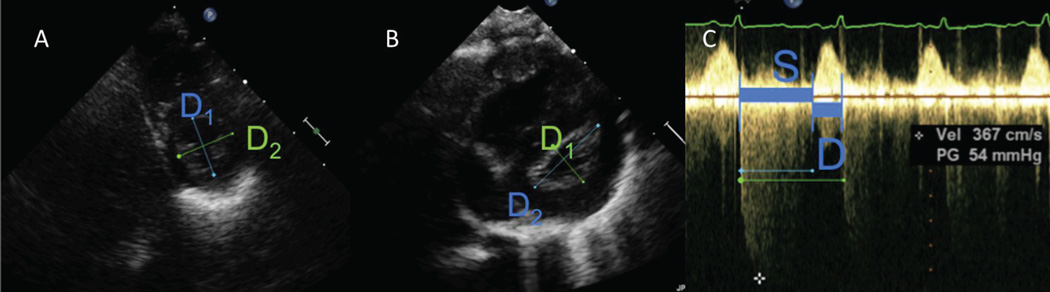
Left Ventricular Eccentricity index (EI), measured at end-systole and end-diastole, from the parasternal short axis 2D image at the mid-papillary muscle level (Figure 1A, 1B). The formula (EI=D2/D1) was used where D1 is the ventricular diameter perpendicular to the interventricular septum bisecting D2, the diameter parallel to the interventricular septum.15 A single cardiac cycle was evaluated for all echocardiograms and reported.
Systolic:Diastolic (SD) Ratio was measured from the continuous wave Doppler tracing of the tricuspid valve regurgitation jet. Appropriate angle of isonation was guided using 2D imaging with simultaneous color flow mapping immediately prior to continuous wave Doppler interrogation, with the infant in a still position. S was defined as the duration of the tricuspid regurgitation flow, and D was defined as the interval between successive tricuspid regurgitation jets10 (Figure 1C), with the ratio calculated using the formula (SD=S/D).
Figure 1. Measurement of Eccentricity Index and Systolic-Diastolic Ratio.
A) Systolic eccentricity index in control, EI=1.00.
B) Systolic eccentricity index in infant with bronchopulmonary dysplasia and pulmonary hypertension, EI=1.89.
C) Systolic-Diastolic ratio in infant with bronchopulmonary dysplasia and pulmonary hypertension, SD=2.36.
Legend: D1 – diameter perpendicular to the interventricular septum; D2- diameter parallel to the interventricular septum; S- systolic duration; D- diastolic duration.
Analysis
Statistical analysis was performed using Stata 13 (College Station, TX). Continuous variables were described using number of observations, median and interquartile range (IQR). Categorical variables were described using counts, proportions, and percentages. Echocardiographic measurements and demographic values between the 3 groups were compared using Kruskal-Wallis tests or Fisher’s Exact Probability Tests, where appropriate. When significant, pairwise comparisons were performed using Wilcoxon rank sum tests. Intra- and inter-observer agreements between two readers were evaluated in a random sample using Bland Altman analysis. A p-value < 0.05 was considered significant. Additionally, a power calculation was performed for our sample size. At an alpha of 0.05, our calculated power was 86%, and our significant values were within our limits of detection.
Results
88 infants were included in the study population. Demographic data and statistical analyses between groups are presented in Table 1. There were 13 infants who had BPD and PH, 16 with BPD only, and 59 controls with neither diagnosis. The median postmenstrual age of the entire cohort at birth was 25 weeks (interquartile range (IQR) 24–26) and the median birthweight was 730 g (628–843). One patient in the study (from the BPD and PH group) had a patent ductus arteriosus at time of first echocardiogram after 36 weeks corrected age. No other patients in any group had a patent ductus arteriosus at the time of the study echocardiogram. This patient subsequently underwent device closure of the patent ductus arteriosus. The analysis was completed separately with the first echocardiogram after 36 weeks corrected postmenstrual age and then repeated with the first echocardiogram after device closure of the patent ductus arteriosus. The statistical significance of the study results was unchanged between the two analyses. For consistency the data from the first echocardiogram after 36 weeks corrected postmenstrual age data were included in the data presented. There was no difference between groups in presence of symptomatic patent ductus arteriosus. All patients had qualitatively normal right ventricular systolic function by visual assessment.
Table 1.
Patient Demographics
| BPD and PH (n=13) | BPD only (n=16) | Controls (n=59) | p (3 groups) |
p (BPD and PH vs BPD only) |
|
|---|---|---|---|---|---|
| Male | 8 (62%) | 11 (69%) | 29 (49%) | 0.33 | 0.71 |
| Birth Weight (g) | 634 (510, 730) | 690 (628, 798) | 760 (665, 860) | 0.014 | 0.25 |
| Postmenstrual Age (weeks) | 24 (24, 25) | 24 (24, 26) | 25 (24.5, 26) | 0.016 | 0.48 |
| Corrected postmenstrual age at echo (weeks) | 45 (41, 48) | 38.5 (37, 40) | 39 (37, 40) | <0.01 | <0.01 |
| Treatment with pulmonary vasodilator1 | 10 (78%) | 0 | 0 | <0.01 | <0.01 |
| Received cardiac catheterization | 6 (46%) | 0 | 0 | <0.01 | <0.01 |
| Treated with hydrocortisone | 8 (62%) | 15 (94%) | 43 (73%) | 0.014 | 0.064 |
| Treated with dexamethasone | 5 (38%) | 6 (38%) | 3 (5%) | 0.041 | 1 |
| Symptomatic PDA, requiring closure2 | 6 (46%) | 5 (31%) | 24 (41%) | 0.68 | 0.47 |
Continuous variables presented as median (25th%, 75th%), categorical variables presented as N (%)
Three infants treated with vasodilator other than oxygen at time of study echocardiogram (nitric oxide or sildenafil)
Indomethacin, coil, or surgical ligation
BPD – bronchopulmonary dysplasia; echo – echocardiogram; PH – pulmonary hypertension; Controls – infants with BPD or PH; PDA – patent ductus arteriosus
The BPD and PH group was significantly older at time of study echocardiogram. Earlier echocardiograms were not available for review due initial treatment of these infants at other facilities. The control group had a larger median birth weight compared with the BPD and PH group and the BPD only group, and may be related to the birth weight as a risk factor for BPD.19 Treatment with a pulmonary vasodilator and cardiac catheterization was limited to the BPD and PH group, while there were no differences between BPD and PH compared to BPD only groups in treatment with hydrocortisone and dexamethasone.
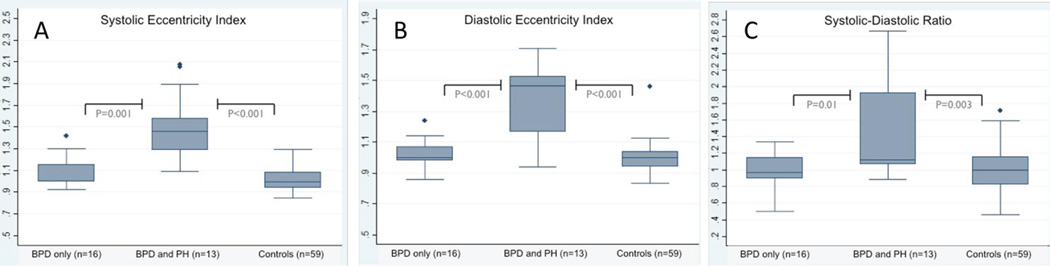
Adequate images for measurement of the eccentricity index were available in all infants. Differences in the Eccentricity Index and SD ratio between groups are shown in Figures 2A, B and C. The end-systolic EI from infants with BPD and PH (1.46, IQR: 1.29–1.59) was significantly higher than the BPD only group (1.01, IQR: 0.99–1.16; p<0.01) and the control group (1.00, IQR: 0.94–1.08; p<0.01) (Figure 2A). The BPD only and control groups were not statistically different (p=0.36). Similarly, the end-diastolic EI from infants with BPD and PH (1.47, IQR: 1.17 – 1.53) was significantly higher than the BPD only group (1.00, IQR: 0.98 – 1.07, p<0.01) and the control group (1.00, IQR: 0.94–1.04; p<0.01) (Figure 2B). The BPD only and control groups were not statistically different (p=0.06). The statistically significant differences in end-systolic and end-diastolic EI between BPD and PH compared to other groups persisted when all three groups were compared using the Kruskal-Wallis test (p<0.01).
The SD ratio was successfully measured in all infants, compared to only 54% of infants with clinical PH having a measurable peak tricuspid regurgitant velocity. The SD ratio from infants with BPD and PH (1.12, IQR: 1.06–1.92) was significantly higher than the BPD only group (0.97, IQR: 0.89–1.15; p=0.01) and the control group (1.00, IQR: 0.82–1.16; p<0.01) (Figure 2C). The BPD only and control groups were not statistically different (p=0.93). The Kruskal-Wallis test comparing the SD ratio among all 3 groups was significant (p=0.01). Since the systolic and diastolic components of the SD ratio will vary with heart rate,20 the mean heart rates from each group were compared with no statistically significant difference detected between groups.
Intra-observer and inter-observer reliability data are presented in Table 2. Intra-observer variability was minimal for all three measurements, while inter-observer variability was minimal for EI, with greater variability present for SD measurements.
Table 2.
Intra- and Inter-observer Variability in Eccentricity Index and Systolic-Diastolic Ratio
| Intra-observer Mean Difference (limits of agreement) |
Inter-observer Mean Difference (limits of agreement) |
|
|---|---|---|
| Systolic EI | −0.032 (−0.275 to 0.211) | 0.025 (−0.263 to 0.313) |
| Diastolic EI | −0.028 (−0.217 to 0.161) | 0.004 (−0.314 to 0.321) |
| SD Ratio | 0.02 (−0.515 to 0.555) | 0.184 (−0.846 to 1.213) |
Data represented: median (25th%, 75th%)
EI- Eccentricity Index; SD- Systolic-Diastolic Ratio; BPD- Bronchopulmonary Dysplasia; PH- Pulmonary Hypertension
Discussion
Despite consistently improving care, ELBW infants, and in particular those at the limits of viability, are at risk for developing BPD. There is a need to effectively screen this population for closer follow-up with attention to the possibility of PH as they graduate from the nursery.21 To date, there have been significant challenges screening and diagnosing PH in infants with BPD. No guidelines exist so individual centers have developed center specific protocols.1,9,22 Echocardiography is typically the primary study, though it may be part of a multipronged approach.21 Timing and frequency of echocardiographic screening is also inconsistent as some centers initiate screening by the first month while others wait until infants reach 36 weeks corrected age.8,21
This study demonstrates that extremely low birth weight infants from our center with BPD and PH have significantly higher left ventricular end-systolic and end-diastolic EI and tricuspid valve SD ratios when compared to similar infants with only BPD or no BPD or PH. We also found that these measurements can be reliably measured in the ELBW population as they approach 40 weeks post-menstrual age.
Abnormal ventricular septal geometry, primarily septal flattening, is an echocardiographic finding associated with elevated right ventricular systolic and diastolic pressures, and in the absence of right ventricular outflow obstruction, reflects pulmonary arterial pressures.14,15,23 Efforts to quantify the degree of change from normal ventricular septal geometry have been described in children and adult patients, but this has not been extensively studied to date in the premature infant population. In contrast to the challenges of obtaining well-aligned Doppler indices in all infants, and especially in infants with coexisting lung disease limiting transthoracic windows, it is possible to obtain a short axis view of the left ventricle at the mid-cavity level in almost all infants. More specificity, the advantages of EI are: 1) an on-axis short axis view of the left ventricle at the appropriate level can be obtained from either the traditional parasternal view (prone to lung artifact) or from the subcostal sagittal view (much less prone to lung artifact); 2) systolic EI and diastolic EI can be measured from the same beat, reducing any confounding data from using non-simultaneous measurements; 3) the measurements are relatively independent of angle of insonation; and 4) there is no dependence on adequate valve regurgitation to capture a complete Doppler envelope. The simplicity of the eccentricity index in providing a quantifiable measurement of ventricular septal flattening, as well as the high correlation between reviewers in this study suggests its utility with a complement of other measurements to diagnose and predict infants at risk of developing PH.
Traditionally, a tricuspid valve regurgitant peak velocity has been the standard for estimating right ventricular systolic pressures by echocardiography. Early studies validated the use of this measure in different populations by simultaneous comparison to cardiac catheterization.24,25 However, in extremely low birth weight infants approaching 40 weeks post menstrual age there is frequently not a well-characterized regurgitant jet. Of note, only six of the thirteen patients with PH in this study had a measurable peak tricuspid valve regurgitant velocity. Mourani et al compared catheterization data with echocardiography in infants with BPD and PH and reported only 61% of echocardiograms with an adequate TR jet.9 This finding was consistent with our study with a similar percentage of complete TR jets permitting a measurable peak velocity. Measurement of the SD ratio from the tricuspid regurgitant jet was much more feasible in our study, with 100% success despite incomplete Doppler envelopes, although with a wider range of values and greater intra- and inter-observer variability.
The greater feasibility in measuring the SD ratio results from it being measurable from a tricuspid regurgitant jet that may display clear acceleration and deceleration edges, but an incomplete peak velocity. The SD ratio has been published as a non-invasive marker of cardiac dysfunction in children with congenital heart disease and impaired systolic function,10,26 and in children with restrictive cardiomyopathy and impaired diastolic function.10 An abnormal SD ratio has been shown to correlate with multiple indicators of poor functional and clinical outcomes in children with pulmonary hypertension.17 In healthy patients, the duration of systole represents approximately 40% of the duration of the cardiac cycle,10 producing an SD ratio <1. With either systolic or diastolic dysfunction the duration of systole increases and diastole decreases, producing an elevated SD ratio >1. Multiple alterations in cardiac structure and function contribute to an elevated SD ratio. These range from prolongation of isovolumic and ejection times due to poor contractility (lengthening systole), to shortening ventricular filling times due to ventricular hypertrophy or fibrosis producing poor ventricular compliance (shortening diastole). The distinction in this study between infants with PH (abnormal SD ratio) and infants with BPD only (normal SD ratio) suggests impaired cardiac function in PH and preserved cardiac function in BPD only patients. In contrast with qualitative assessment of right ventricular function, which did not distinguish between groups, the abnormal SD ratio in patients with PH supports the incremental usefulness of this measurement in a non-invasive PH evaluation.
Limitations of this study include its retrospective nature and small size, as seen in the difference between control and BPD groups for end-diastolic EI that neared statistical significance, despite both being significantly different from infants with both BPD and PH. This highlights both the importance of careful measurement as well as the challenges in assessing ventricular septal geometry in diastole, when the magnitude of difference between ventricular filling pressures will be less. Potential bias may also be introduced by measuring the EI and SD ratio on echocardiograms obtained as part of the clinical assessment of at-risk infants. To reduce this potential bias, all echocardiograms in infants with PH were reviewed by a third, blinded reviewer to confirm that PH was defined by measures other than ventricular septal flattening. Changes in heart rate affect both the systolic and diastolic components of the SD ratio (systole varies linearly with heart rate, diastole varies exponentially with heart rate), which may confound the use of this measurement.20 However, statistical analysis of heart rates between the groups in this study demonstrated no significant difference. This study is also limited by the lack of direct comparison to catheterization data, since cardiac catheterization was not performed in all subjects. However, the risks of cardiac catheterization in the former ELBW population makes this is a common limitation in the majority of hemodynamic studies in this group, and speaks highly to the need to optimize our use of non-invasive tools.
In conclusion, the left ventricular EI and SD ratio are significantly different in extremely low birthweight infants with BPD and PH when compared to infants with BPD or normal controls. The simplicity and reliability of these measurements may facilitate their incorporation into a screening protocol of infants receiving the diagnosis of BPD. Distinguishing patients with PH could significantly change clinical follow up and medication therapies. A larger prospective trial is needed to validate the use of these measurements in identifying extremely low birthweight infants who develop BPD and PH, as well as the changes in measurements after initiation of therapy.
Supplementary Material
BPD- bronchopulmonary dysplasia; PH- pulmonary hypertension; Controls-infants without bronchopulmonary dysplasia or pulmonary dysplasia.
Figure 2. Eccentricity Index and Systolic-Diastolic Ratio.
BPD- bronchopulmonary dysplasia; PH- pulmonary hypertension; Controls-infants without bronchopulmonary dysplasia or pulmonary dysplasia.
Acknowledgments
The authors wish to acknowledge the staff of the Duke Pediatric Echocardiographic Laboratory and the Intensive Care Nursery for their assistance in this project.
Dr. Hill and Dr. Hornik receive grant support from the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
AM designed the study, collected and performed preliminary analysis of the data, prepared the figures, and wrote the main manuscript text.
JM, CC and GT performed additional data collection (neonatology and cardiology) and analysis of the data.
CH performed detailed statistical analysis of the final data.
KH performed additional data collection (cardiac catheterization) and analysis of the data.
PB designed the study, obtained institutional review board approval, performed analysis of the data and assisted in manuscript preparation.
All authors reviewed, edited and approved the manuscript.
References
- 1.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007 Dec;120(6):1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu. Rev. Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J. Perinatol. 2011 Oct;31(10):635–640. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 4.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011 Apr;97(8):612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J. Am. Coll. Cardiol. 2006 Dec 19;48(12):2546–2552. doi: 10.1016/j.jacc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 6.Fraisse A, Geva T, Gaudart J, Wessel DL. Doppler echocardiographic predictors of outcome in newborns with persistent pulmonary hypertension. Cardiol. Young. 2004 Jun;14(3):277–283. doi: 10.1017/S1047951104003051. [DOI] [PubMed] [Google Scholar]
- 7.Ochikubo CG, Waffarn F, Turbow R, Kanakriyeh M. Echocardiographic evidence of improved hemodynamics during inhaled nitric oxide therapy for persistent pulmonary hypertension of the newborn. Pediatr. Cardiol. 1997 Jul-Aug;18(4):282–287. doi: 10.1007/s002469900175. [DOI] [PubMed] [Google Scholar]
- 8.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012 Mar;129(3):e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008 Feb;121(2):317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with heart failure secondary to restrictive cardiomyopathy. J. Am. Soc. Echocardiogr. 2006 Nov;19(11):1326–1331. doi: 10.1016/j.echo.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Lammers AE, Haworth SG, Riley G, Maslin K, Diller GP, Marek J. Value of tissue Doppler echocardiography in children with pulmonary hypertension. J. Am. Soc. Echocardiogr. 2012 May;25(5):504–510. doi: 10.1016/j.echo.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Candales A, Bazaz R, Edelman K, Gulyasy B. Apical systolic eccentricity index: a better marker of right ventricular compromise in pulmonary hypertension. Echocardiography. 2010 May;27(5):534–538. doi: 10.1111/j.1540-8175.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Nakatani S, Kanzaki H, et al. Patterns of the interventricular septal motion can predict conditions of patients with pulmonary hypertension. J. Am. Soc. Echocardiogr. 2008 Apr;21(4):386–393. doi: 10.1016/j.echo.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2002 Apr 3;39(7):1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 15.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J. Am. Coll. Cardiol. 1985 Apr;5(4):918–927. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 16.Jain A, Mohamed A, El-Khuffash A, et al. A comprehensive echocardiographic protocol for assessing neonatal right ventricular dimensions and function in the transitional period: normative data and z scores. J. Am. Soc. Echocardiogr. 2014 Dec;27(12):1293–1304. doi: 10.1016/j.echo.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am. J. Cardiol. 2010 Aug 1;106(3):430–436. doi: 10.1016/j.amjcard.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005 Dec;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 19.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009 May;123(5):1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 20.Sarnari R, Kamal RY, Friedberg MK, Silverman NH. Doppler assessment of the ratio of the systolic to diastolic duration in normal children: relation to heart rate, age and body surface area. J. Am. Soc. Echocardiogr. 2009 Aug;22(8):928–932. doi: 10.1016/j.echo.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Collaco JM, Romer LH, Stuart BD, et al. Frontiers in pulmonary hypertension in infants and children with bronchopulmonary dysplasia. Pediatric pulmonology. 2012 Nov;47(11):1042–1053. doi: 10.1002/ppul.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim GB. Pulmonary hypertension in infants with bronchopulmonary dysplasia. Korean journal of pediatrics. 2010 Jun;53(6):688–693. doi: 10.3345/kjp.2010.53.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman DJ, Harrison DC, Popp RL. Echocardiographic features of primary pulmonary hypertension. Am. J. Cardiol. 1974 Mar;33(3):438–443. doi: 10.1016/0002-9149(74)90329-4. [DOI] [PubMed] [Google Scholar]
- 24.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. Journal of the American College of Cardiology. 1985 Oct;6(4):750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 25.Skinner JR, Stuart AG, O'Sullivan J, Heads A, Boys RJ, Hunter S. Right heart pressure determination by Doppler in infants with tricuspid regurgitation. Archives of disease in childhood. 1993 Aug;69(2):216–220. doi: 10.1136/adc.69.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with hypoplastic left heart syndrome: a novel Doppler index of right ventricular function. J. Am. Soc. Echocardiogr. 2007 Jun;20(6):749–755. doi: 10.1016/j.echo.2006.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BPD- bronchopulmonary dysplasia; PH- pulmonary hypertension; Controls-infants without bronchopulmonary dysplasia or pulmonary dysplasia.