Abstract
Background
Acantholytic squamous cell carcinoma (aSCC) is regarded as a high-risk variant of cutaneous squamous cell carcinoma (SCC). Acantholytic actinic keratosis (aAK) has been regarded as a precursor risk factor for aSCC. However, supporting evidence is limited.
Objective
We sought to document clinical features, histologic features, management, and outcomes in a series of aSCC cases.
Methods
Definitions of aSCC, aAK, and aSCC arising in association with aAK were applied to a consecutive series of aSCC cases. Clinical characteristics and outcomes were obtained from electronic medical records.
Results
Of 115 aSCC cases (103 patients, mean age 71.8 years), actinic keratosis was present in 23% (27/115) but only 7.8% (9/115) exhibited associated aAK. Ten cases (10/115, 9%) fulfilled strict histologic criteria for follicular SCC as previously defined, but 50 of 115 (43%) of our aSCC cases exhibited predominant involvement of follicular epithelium rather than epidermis. Clinical outcome (median follow-up, 36 months) was available in 106 of 115 (92%). One patient experienced regional extension (parotid), and 1 patient experienced a local recurrence (nose). No disease-related metastases or deaths were documented.
Limitations
This was a single-institution retrospective study from the United States.
Conclusions
The presence of acantholysis in cutaneous SCC does not specifically confer aggressive behavior, a finding that may inform clinical practice guidelines.
Keywords: acantholysis, acantholytic actinic keratosis, cutaneous oncology, dermatopathology, follicular squamous cell carcinoma, nonmelanoma skin cancer, outcomes, prognosis, squamous cell carcinoma
Squamous cell carcinoma (SCC) is the second most common form of skin cancer and most common cause of death from nonmelanoma skin cancer.1-3 Acantholytic SCC (aSCC) is a distinctive histologic subtype of SCC first described by Lever4 in 1947 as a form of sweat gland carcinoma. Synonyms in the literature include adenoid SCC (adenoacanthoma of Lever) or pseudoglandular SCC. aSCC also encompasses the rare histologic subsets of pseudovascular SCC, pseudoangiosarcomatous SCC, and small-cell SCC.1,5-10 aSCC has long been regarded as an intermediate- to high-risk form of SCC.1,11 A frequently cited reference in support of the high-risk nature of aSCC is the case series published in 1989 by Nappi and coworkers,11 who reported that 19% of their 49 patients developed fatal metastases. Case reports and small case series have also documented aggressive behavior.12-15 However, comparatively lower mortality was reported by other institutions, with the largest published series of 155 patients by Johnson and Helwig16 reporting only 3% mortality.17 Garcia and Crowson18 recently questioned whether aSCC is truly an aggressive tumor. However, aSCC remains classified as a high-risk form of SCC in the 2016 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for cutaneous SCC (v1.2016)19 and current clinical practice guidelines from the European Organization for Research and Treatment of Cancer (EORTC).20 The emerging role for sentinel lymph node biopsy for high-risk SCC magnifies the importance of accurate risk stratification for cutaneous SCC.3
As a histologic variant of SCC, the diagnosis of aSCC is necessarily a histologic diagnosis. aSCC is regarded as a rare variant of cutaneous SCC. Although diagnosing aSCC has not historically been controversial, there are no validated or standardized minimal criteria for the diagnosis of aSCC. Similarly, a definition of aSCC arising in association with acantholytic actinic keratosis (aAK) (or SCC arising in association with actinic keratosis [AK] generally) has not been proposed. Moreover, acantholysis has been documented in other variants of SCC, including follicular SCC,21 spindle-cell SCC,22,23 and even rarely as an incidental finding in keratoacanthoma (KA),24 despite the fact that some investigators definitionally exclude KA if acantholysis is present.25 Cassarino and colleagues,1 in their comprehensive review of the histopathology of cutaneous SCC, characterized aSCC as exhibiting intratumoral acantholysis “At least focally, but often extensively....” In the largest series to date, Johnson and Helwig16 noted that aSCC tumors typically arose from the upper portion of follicular outer root sheath, but characteristic involvement of follicular epithelium was not mentioned in subsequent descriptions of aSCC11,26 or follicular variants of SCC.21,27,28 Of note, Carr and coworkers29 identified a central acantho lytic mucin pool in over half of their series of 30 cases of follicular SCC and contrasted this finding with the characteristic suprabasilar acantholysis that typifies aAK and aSCC. Multiple authorities state that aSCC is often present in association with aAK.30-32 AK is a widely accepted precursor to, and risk factor for, cutaneous SCC,33 with aAK representing the presumptive precursor of aSCC.16 However, a formal evaluation of the degree of association between aAK and aSCC has not been reported. Similar to aSCC, aAK is a rare variant of AK, representing less than 5% of 402 AKs in the series of Carapeto and Garcia-Perez34 and 10% in the series of 300 AKs by Pensley and Sims.35
In this study, we developed and uniformly applied definitions of aSCC, aAK, and aSCC arising in association with AK/aAK to facilitate review of the associated clinical features, management, and clinical outcomes in aSCC.
METHODS
A natural language search for “acantholytic squamous cell carcinoma” and “squamous cell carcinoma, acantholytic” was performed on the database of an academic medical center's dermatopathology laboratory to identify a consecutive sampling of tissue specimens with a diagnosis of aSCC (2006-2011). Study material was fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin-eosin for histologic diagnosis. All cases were originally interpreted by a board-certified dermatopathologist.
Study definitions were developed to be compatible with existing descriptions in published studies and reviews.1,5,11,16,17 The study definitions were as follows.
Acantholytic SCC
This was defined as SCC containing atypical keratinocytes associated with loss of cohesion between epidermal or adnexal epithelial cells with formation of intraepithelial clefts (most commonly suprabasilar). Acantholytic keratinocytes exhibit a round shape with a central round uniformly staining basophilic nucleus, eosinophilic cytoplasm, and absence of desmosomal attachments on hematoxylin-eosin stain affecting at least half of the cell circumference. A pseudoglandular appearance may result when acantholysis is prominent. Associated spongiosis and dyskeratotic keratinocytes may also be present.
aSCC, follicular type
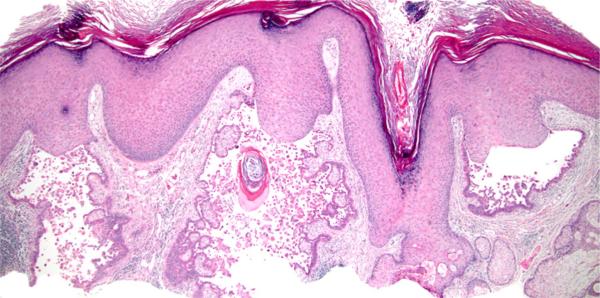
aSCC (Fig 1) appears centered upon 1 or more hair follicles, with or without infundibulocystic differentiation/features, but lacking features of KA (ie, verrucous crateriform profile composed mostly of large keratinocytes with pale eosinophilic glassy cytoplasm) (Fig 2), and lacking involvement of epidermis; ulcerated tumors do not qualify.
Fig 1.
Acantholytic squamous cell carcinoma, follicular type. The tumor involves follicular epithelium but not epidermis. (Hematoxylin-eosin stain; original magnification: ×20; inset, ×100.)
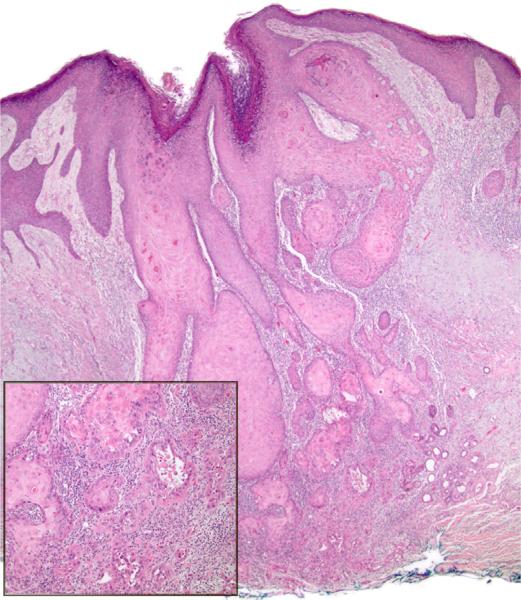
Fig 2.
Acantholytic squamous cell carcinoma with features of keratoacanthoma. The tumor is mostly composed of large keratinocytes with abundant pale eosinophilic (glassy) cytoplasm. Acantholysis is present. (Hematoxylin-eosin stain; original magnification: ×20; inset, ×100.)
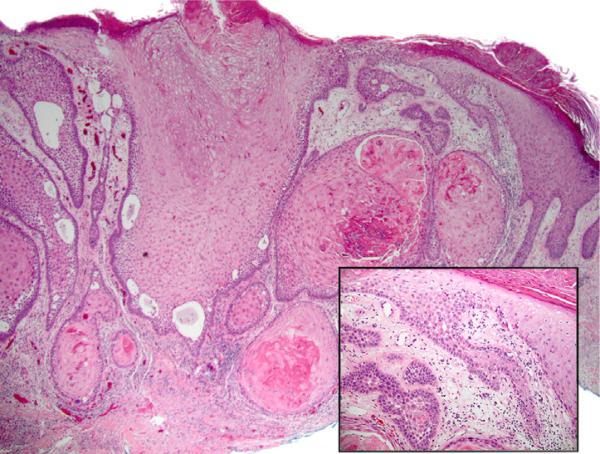
aSCC, follicular pattern
We define this as aSCC predominantly involving and/or predominantly arising from involved follicular epithelium (Fig 3).
Fig 3.
Acantholytic squamous cell carcinoma (aSCC), follicular pattern. The tumor is centered upon follicular epithelium but also involves epidermis. Some features of actinic keratosis (parakeratosis, basilar epidermal atypia) are present; by our definition, this example did not qualify for aSCC arising in association with actinic keratosis because there is invasive carcinoma in the directly underlying dermis. (Hematoxylin-eosin stain; original magnification: ×20; inset, ×100.)
Actinic keratosis
We define this as epidermis (interadnexal) with parakeratosis and atypia/crowding of underlying basilar keratinocytes, involving adjacent epidermis, without directly underlying SCC.
Acantholytic AK
This is defined as AK exhibiting acantholysis (defined above) or intraepidermal clefts (usually suprabasilar).
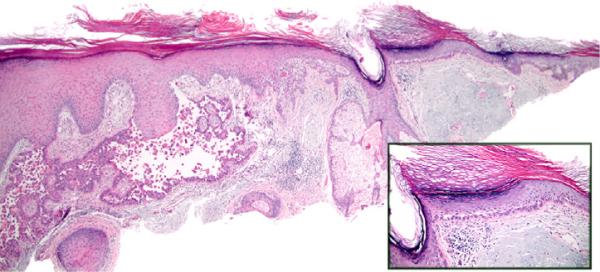
aSCC arising in association with AK
This is defined as aSCC with basilar epidermal atypia and parakeratosis of the epidermis peripheral to the main tumor (same or adjacent section), with any degree of sparing of at least 1 adnexal structure, without directly underlying invasive tumor (Fig 4).
Fig 4.
Acantholytic squamous cell carcinoma (SCC) with associated acantholytic actinic keratosis (aAK). aAK exhibiting parakeratosis, atypical basilar keratinocytes with suprabasilar acantholytic clefting, sparing of adnexal epithelium, severe solar elastosis, and absence of directly underlying SCC. (Hematoxylin-eosin stain; original magnification: ×20; inset, ×100).
Solar elastosis
Mild solar elastosis was defined as discrete individual gray solar elastotic fibers separated by collagen fibers. Severe solar elastosis was defined as solid or nodular accumulations of elastotic material displacing the background collagen.
After establishing the study definitions, all cases were reviewed with concurrence at the time of the study by at least 3 investigators, including the first and corresponding authors plus 1 or 2 additional board-certified dermatopathologists. Inclusion criteria included the fulfillment of minimal histologic criteria as described in the study definition of aSCC. Exclusion criteria included cases that did not fulfill minimal histologic criteria or did not clearly demonstrate invasive carcinoma as a result of superficial sampling.
Histologic attributes included AK (present, absent, indeterminate/insufficient tissue); aAK (present, absent, indeterminate/insufficient tissue); solar elastosis (mild, severe, indeterminate); follicular SCC (yes, no, indeterminate); and follicular pattern in SCC (yes, no, indeterminate).
Clinical attributes were obtained by review of the patient's electronic medical record: age at time of biopsy, gender, ethnicity/race (Caucasian, Hispanic, African American, Asian, other), anatomic site, tumor size, immune status (organ transplantation, radiation exposure, other, not known), treatment (Mohs, standard excision, electrodessication and curettage, radiation, other), follow-up duration, and outcome (alive with no evidence of disease, local recurrence, distant metastasis, death from disease, death from unrelated causes) at most recent follow-up.
Data was recorded in Excel 2010 (Microsoft Corp, Redmond, WA). Institutional review board approval was obtained.
RESULTS
A total of 115 specimens from 103 patients were identified and studied (Table I). The average tumor size among those listed (69% of tumors) was 1.2 cm (range 0.1-5.0 cm). Most patients were treated with Mohs micrographic surgery. No disease-related deaths were recorded. Associated AK was identified in 23% (27/116) of the aSCC tumors, but less than 8% of these (9/116) were aAK (Table II). Most of the specimens were shave biopsies, but acantholysis comprised less than 25% of the aSCC tumor in the tissue sections in nearly all cases. Clinical features of aSCC with a follicular pattern are summarized in Table III. Nearly half of aSCC cases predominantly, if not exclusively, appeared to involve follicular epithelium.
Table I.
Clinical and histologic features of acantholytic squamous cell carcinoma
| Patient gender and mean age | 72 y |
| Male (79%) | |
| Female (21%) | |
| Ethnicity/race | Caucasian (83%) |
| Other/declined to state (17%) | |
| Tumor sites | Face (42%) |
| Ear (14%) | |
| Scalp (13%) | |
| Arm (12%) | |
| Other (19%) | |
| Immune status | Organ transplantation (10%) |
| HIV (3%) | |
| Corticosteroid therapy (6%) | |
| Other (1%) | |
| Treatment | Mohs micrographic surgery (58 cases) |
| Standard excision (17) | |
| Electrodessication and curettage (1) | |
| Mohs + standard excision (1) | |
| Mohs + standard excision + radiation (1) | |
| Other (7) | |
| Unknown (29) | |
| Outcome | NED (90) |
| Dead, unrelated cause (7) | |
| Dead, unknown cause (1) | |
| Recurrence (2 local, 2 distant) | |
| Unknown (7) | |
| Follow-up: mean, median | 34 mo, 29 mo |
| Associated actinic keratosis | 24% |
| Associated acantholytic actinic keratosis | 8% |
| Solar elastosis | Severe (78%) |
| Mild (11%) | |
| Present (mild vs severe) (4%) | |
| Indeterminate (7%) |
NED, Alive, no evidence of disease.
Table II.
Clinical and histologic features of acantholytic squamous cell carcinoma arising in association with acantholytic actinic keratosis
| Age, y/gender | Site | Clinical | Size, cm | Solar elastosis | Immunosuppression | Outcome |
|---|---|---|---|---|---|---|
| 90/F | Forehead | Unknown | Unknown | Severe | No | Dead, unknown cause |
| 83/M | Back | Rough spot | 3.8 | Mild | No | NED |
| 78/M | Scalp | Painful papule | 0.5 | Severe | Chronic lymphocytic leukemia | NED |
| 78/M | Forearm | Papule | 1.7 | Mild | No | NED |
| 77/M | Cheek | Patch | Unknown | Severe | No | NED |
| 73/M | Ear, helix | Unknown | 0.7 | Severe | No | NED |
| 70/M | Ear, rim | Unknown | Unknown | Severe | No | Dead, unrelated cause |
| 68/M | Scalp | Depressed patch | 0.8 | Severe | No | NED |
| 64/M | Forehead | Mobile mass | 3.0 | Severe | No | NED |
F, Female; M, male; NED, alive, no evidence of disease.
Table III.
Selected clinical features of acantholytic squamous cell carcinoma exhibiting a follicular pattern
| Patient gender and mean age | 70 y |
| Male (82%) | |
| Female (18%) | |
| Tumor sites | Face (64%) |
| Other (36%) | |
| Treatment | Mohs micrographic surgery: 30 cases (60%) |
| Outcome | NED: 40 (80%) |
| Dead, unrelated cause: 4 (8%) | |
| Dead, unknown cause: 3 (6%) | |
| Local recurrence: 1 (2%) | |
| Unknown: 2 (4%) | |
| Solar elastosis | Severe (78%) |
| Mild (12%) | |
| Follicular SCC* | Yes (20%) |
| No (80%) |
NED, Alive, no evidence of disease; SCC, squamous cell carcinoma.
See “Methods” section for definition.
DISCUSSION
Evidence-based standardization of clinical practice guidelines is a defining feature of 21st century medical practice. In the realm of high-risk SCC, the recent trend toward consideration of sentinel lymph node biopsy magnifies the importance of accurate risk stratification among SCC subtypes.3 Moreover, although acantholysis is a readily and universally recognized histopathologic feature, minimal criteria for the diagnosis of aSCC, or the relationship of aSCC with other SCC subtypes such as follicular SCC, is uncertain. Yet, a pathologic diagnosis of aSCC is widely accepted among practicing dermatopathologists. Despite multiple reports of aggressive behavior associated with aSCC, the largest aSCC series do not support aSCC as a high-risk subtype of SCC.
Although deductive reasoning supports the assumption that aAK is a common precursor to aSCC, less than 8% were associated with aAK in our series (23% AK overall). Similarly, in the largest aSCC series, only 6.4% (10/155) of aSCC were associated with aAK.16 Given the lack of an explicit or uniformly applied definition of AK-associated SCC, variable prevalence rates may be attributed to variable definitions in addition to presumed true differences in prevalence between populations. For example, the aAK depicted in Fig 8 by Johnson and Helwig16 might not be regarded as classic for AK by some observers (and excluded using the definitions in our study) because there is no epidermal involvement at all. As another example, in a recent case report by Ruini and colleagues36 documenting the progression of AK to SCC, the example of AK provided in their Fig 4, B, shows a lesion entirely lacking parakeratosis but involving follicular epithelium. However, studies have consistently demonstrated that aAK comprises only a minority of AK, whatever the absolute value of the AK “denominator.” The concomitant observation that nearly half of our cases of aSCC predominantly, if not exclusively, appeared to involve follicular epithelium suggests that aAK may not be a required or typical antecedent to aSCC, ie, despite the shared attribute of acantholysis, aAK and aSCC may develop via parallel signaling pathways, rather than in series. The possibility that aAK might be obscured or replaced by aSCC cannot be excluded; however, nearly half of aSCC in our series predominantly involved follicular epithelium, whereas AK (all variants) classically exhibits a reciprocal pattern, primarily involving interadnexal epidermis and sparing adnexal epithelium (hair follicle, eccrine duct), at least initially. The observation by Carr and colleagues29 that a central acantholytic mucin pool characterizes follicular SCC (vs suprabasilar acantholysis, without mucin, in aAK and aSCC) also warrants further study. We did not perform mucin stains in our cases, as ancillary histochemical staining was not required in our routine practice.
The concept of follicular SCC was introduced by Diaz-Cascajo et al21 in 2004 as a variant of cutaneous SCC originating from follicular epithelium; in this series, a few foci of acantholysis were documented in 5 of 16 cases. Kossard28 introduced well-differentiated, moderately differentiated, and infiltrative variants of infundibulocystic SCC in 2012 as histologic subtypes of follicular SCC distinct from KA; in this series, acantholysis was not mentioned at all. In 2011, Misago et al27 reported 8 cases of follicular/infundibular SCC and infundibulocystic SCC, the latter exhibiting an infiltrative pattern of growth and merging histologically with microcystic adnexal carcinoma; in this series, acantholysis was not mentioned at all. However, acantholysis was a relatively common feature in a review that referenced a series of 30 follicular SCC cases.29
Regarding metastatic potential, Cassarino et al1 stratified SCC into categories of low (≤2% metastatic rate), intermediate (3%-10%), and high (>10%), and aSCC was classified as intermediate risk. Thus, “aggressive behavior” in cutaneous SCC can be defined as encompassing intermediate risk (3%-10% metastatic rate) and high risk (>10% metastatic rate) subgroups. NCCN regards aSCC as high risk,19 as does the EORTC and Working Group of the French Dermatology Recommendations Association.20,37 However, in a recent series of 110 cases of SCC, acantholysis was not a significant prognostic factor.38 The 2010 Union for International Cancer Control and 2010 American Joint Committee on Cancer TNM classifications confer elevated risk for cutaneous carcinomas larger than 2 cm and exhibiting deep infiltration (bone and soft tissue), without specifying histologic subtypes, although a recent review of these classifications suggested that histology should be considered, with emphasis on microscopic tumor thickness and perineural invasion (not acantholysis).39
The average clinical tumor size in our series is similar to those previously reported. Specifically, the tumor size in our series (1.2 cm; range 0.5-5.0 cm) is slightly larger than that in the largest series by Johnson and Helwig16 and appears comparable with that of Nappi and coworkers.11 Thus, clinical tumor size alone does not appear to account for the variation in reported mortality. Tumor depth of extension remains a likely relevant prognostic variable in aSCC that bears investigation, because existing case series of aSCC have not recorded tumor thickness (Table IV). The anecdotal recollection of memorably aggressive aSCC cases may correlate with thicker tumors and/or involvement of anatomically high-risk sites such as the ear, where involvement of subcutis or underlying fascia occurs with a relatively smaller depth of extension compared with other sites.
Table IV.
Summary of published acantholytic squamous cell carcinoma case series
| Study | No. of tumors/patients | Clinical tumor size, cm | Tumor thickness | Mortality | Follow-up |
|---|---|---|---|---|---|
| Johnson and Helwig,11 1966 | 213/155 | 0.4-12 | Unknown | 5/155 (3%) | 1 mo-30 y (median, 6.7 y) |
| Nappi et al,6 1989 | 55/49 | 0.4-6 (mean 0.8) | Unknown | 7/36 (19%), all >1.5 cm | 6 mo-10 y |
| Current study | 115/103 | 0.1-5.0 (mean 1.2) | Unknown | 0/88 | (median, 29 mo) |
In conclusion, we applied uniform, detailed definitions of aSCC, aAK, and aSCC arising in association with AK/aAK to report, to our knowledge, the largest case series of aSCC in a half century. We confirmed a common association of aSCC with follicular epithelium, and only infrequent association of aSCC with aAK, and no evidence of increased morbidity or mortality. The presence of acantholysis in SCC per se does not specifically confer aggressive behavior by cutaneous SCC and may inform clinical practice guidelines.
CAPSULE SUMMARY.
Acantholytic actinic keratosis has been regarded as a precursor of acantholytic squamous cell carcinoma (SCC), a reportedly aggressive high-risk form of SCC.
In this series of 115 cases, acantholytic SCC was more commonly associated with follicular epithelium than acantholytic actinic keratosis and did not display aggressive behavior.
The presence of acantholysis, per se, in cutaneous acantholytic SCC does not confer aggressive clinical behavior.
Acknowledgments
Dr Kiuru's involvement in this research was in part supported by the National Cancer Institute, National Institutes of Health, through grant K12CA138464.
Abbreviations used
- aAK
acantholytic actinic keratosis
- AK
actinic keratosis
- aSCC
acantholytic squamous cell carcinoma
- EORTC
European Organization for Research and Treatment of Cancer
- KA
keratoacanthoma
- NCCN
National Comprehensive Cancer Network
- SCC
squamous cell carcinoma
Footnotes
Conflicts of interest: None declared.
A portion of this work was presented at the 52nd Annual Meeting of the American Society of Dermatopathology, San Francisco, CA, October 11, 2015.
REFERENCES
- 1.Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. Part one. J Cutan Pathol. 2006;33(3):191–206. doi: 10.1111/j.0303-6987.2006.00516_1.x. [DOI] [PubMed] [Google Scholar]
- 2.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Navarrete-Dechent C, Veness MJ, Droppelmann N, Uribe P. High-risk cutaneous squamous cell carcinoma and the emerging role of sentinel lymph node biopsy: a literature review. J Am Acad Dermatol. 2015;73(1):127–137. doi: 10.1016/j.jaad.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Lever WF. Adenocanthoma of sweat glands; carcinoma of sweat glands with glandular and epidermal elements: report of four cases. Arch Dermatol Syphilol. 1947;56(2):157–171. doi: 10.1001/archderm.1947.01520080017002. [DOI] [PubMed] [Google Scholar]
- 5.LeBoit PE, Burg G, Weedon D, Sarasin A. Pathology and genetics of skin tumors. 3rd ed. IARC Press; Lyon (France): 2005. World Health Organization classification of tumors. [Google Scholar]
- 6.Toyama K, Hashimoto-Kumasaka K, Tagami H. Acantholytic squamous cell carcinoma involving the dorsum of the foot of elderly Japanese: clinical and light microscopic observations in five patients. Br J Dermatol. 1995;133(1):141–142. doi: 10.1111/j.1365-2133.1995.tb02510.x. [DOI] [PubMed] [Google Scholar]
- 7.Giordano G, D'Adda T, Merisio C, Gnetti L. Vulvar acantholytic squamous carcinoma: a case report with immunohistochemical and molecular study. Int J Gynecol Pathol. 2005;24(3):303–306. doi: 10.1097/01.pgp.0000157919.43978.f3. [DOI] [PubMed] [Google Scholar]
- 8.Driemel O, Muller-Richter UD, Hakim SG, et al. Oral acantholytic squamous cell carcinoma shares clinical and histological features with angiosarcoma. Head Face Med. 2008;4:17. doi: 10.1186/1746-160X-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerawala CJ. Acantholytic squamous cell carcinoma of the oral cavity: a more aggressive entity? Br J Oral Maxillofac Surg. 2009;47(2):123–125. doi: 10.1016/j.bjoms.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Muller SA, Wilhelmj CM, Jr, Harrison EG, Jr, Winkelmann RK. Adenoid squamous cell carcinoma (adenoacanthoma of Lever). Report of seven cases and review. Arch Dermatol. 1964;89:589–597. doi: 10.1001/archderm.1964.01590280089016. [DOI] [PubMed] [Google Scholar]
- 11.Nappi O, Pettinato G, Wick MR. Adenoid (acantholytic) squamous cell carcinoma of the skin. J Cutan Pathol. 1989;16(3):114–121. doi: 10.1111/j.1600-0560.1989.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 12.Weidner N, Foucar E. Adenosquamous carcinoma of the skin. An aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121(6):775–779. [PubMed] [Google Scholar]
- 13.Uchiyama N, Shindo Y. A case of acantholytic squamous cell carcinoma derived from the acantholytic type of solar keratosis. J Dermatol. 1986;13(5):377–380. doi: 10.1111/j.1346-8138.1986.tb02958.x. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Mukawa A, Saito K, Nakanishi I, Tsuda H. Adenoid squamous cell carcinoma of the skin overlying the right breast. An autopsy case clinically manifested with rapid growth and widely spreading metastases. Acta Pathol Jpn. 1986;36(12):1921–1929. doi: 10.1111/j.1440-1827.1986.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 15.Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18(4):227–234. doi: 10.1111/j.1600-0560.1991.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson WC, Helwig EB. Adenoid squamous cell carcinoma (adenoacanthoma). A clinicopathologic study of 155 patients. Cancer. 1966;19(11):1639–1650. doi: 10.1002/1097-0142(196611)19:11<1639::aid-cncr2820191131>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Sajin M, Hodorogea Prisacaru A, Luchian MC, et al. Acantholytic squamous cell carcinoma: pathological study of nine cases with review of literature. Rom J Morphol Embryol. 2014;55(2):279–283. [PubMed] [Google Scholar]
- 18.Garcia C, Crowson AN. Acantholytic squamous cell carcinoma: is it really a more-aggressive tumor? Dermatol Surg. 2011;37(3):353–356. doi: 10.1111/j.1524-4725.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network [September 3, 2016];National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Cutaneous SCC (v1.2016) 2016 Available at: http://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf.
- 20.Stratigos A, Garbe C, Lebbe C, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51(14):1989–2007. doi: 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Cascajo C, Borghi S, Weyers W, Bastida-Inarrea J. Follicular squamous cell carcinoma of the skin: a poorly recognized neoplasm arising from the wall of hair follicles. J Cutan Pathol. 2004;31(1):19–25. doi: 10.1046/j.0303-6987.2004.0134.x. [DOI] [PubMed] [Google Scholar]
- 22.Gray Y, Robidoux HJ, Farrell DS, Robinson-Bostom L. Squamous cell carcinoma detected by high-molecular-weight cytokeratin immunostaining mimicking atypical fibroxanthoma. Arch Pathol Lab Med. 2001;125(6):799–802. doi: 10.5858/2001-125-0799-SCCDBH. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto O, Yasuda H. A case of pseudovascular adenoid squamous cell carcinoma of the skin with spindle cell pattern. J Dermatol. 1997;24(9):587–594. doi: 10.1111/j.1346-8138.1997.tb02298.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez Yus E, Requena L, Simon P, de Hijas CM. Incidental acantholysis. J Cutan Pathol. 1993;20(5):418–423. doi: 10.1111/j.1600-0560.1993.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 25.Shah K, Kazlouskaya V, Lal K, Molina D, Elston DM. Perforating elastic fibers (’elastic fiber trapping’) in the differentiation of keratoacanthoma, conventional squamous cell carcinoma and pseudocarcinomatous epithelial hyperplasia. J Cutan Pathol. 2014;41(2):108–112. doi: 10.1111/cup.12247. [DOI] [PubMed] [Google Scholar]
- 26.Nappi O, Wick MR, Pettinato G, Ghiselli RW, Swanson PE. Pseudovascular adenoid squamous cell carcinoma of the skin. A neoplasm that may be mistaken for angiosarcoma. Am J Surg Pathol. 1992;16(5):429–438. doi: 10.1097/00000478-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Misago N, Inoue T, Toda S, Narisawa Y. Infundibular (follicular) and infundibulocystic squamous cell carcinoma: a clinicopathological and immunohistochemical study. Am J Dermatopathol. 2011;33(7):687–694. doi: 10.1097/DAD.0b013e318205b2c5. [DOI] [PubMed] [Google Scholar]
- 28.Kossard S. Infundibular (follicular) and infundibulocystic squamous cell carcinoma: a clinicopathological and immunohisto-chemical study. Am J Dermatopathol. 2012;34(6):675–676. doi: 10.1097/DAD.0b013e31823fd78b. [DOI] [PubMed] [Google Scholar]
- 29.Carr RA, Taibjee SM, Turnbull N, Attili S. Follicular squamous cell carcinoma is an under-recognized common skin tumor. Diag Histopathol. 2014;20:289–296. [Google Scholar]
- 30.Calonje E, Brenn T, Lazar A, McKee PH. McKee's pathology of the skin. 4th ed. Vol. 2. Elsevier Saunders; Philadelphia: 2012. [Google Scholar]
- 31.Kirkham N, Khadija A. Lever's Histopathology of the Skin. 11th ed. Wolters Kluwer; Philadelphia: 2015. Tumors and cysts of the epidermis. p. 1003. Chapter 29. [Google Scholar]
- 32.Patterson JW. Weedon's Skin Pathology. 4th ed. Churchill Livingstone; London: 2016. [Google Scholar]
- 33.Wyatt AJ, Busam KJ. Tumors of the epidermis. In: Busam KJ, editor. Dermatopathology. 2nd ed. Elsevier Saunders; Philadelphia: 2016. p. 356. [Google Scholar]
- 34.Carapeto FJ, Garcia-Perez A. Acantholytic keratosis. Dermatologica. 1974;148(4):233–239. doi: 10.1159/000251644. [DOI] [PubMed] [Google Scholar]
- 35.Pensley N, Sims CF. Keratosis senilis with epidermal splits. Its resemblance to Darier's disease and its probable significance. Arch Dermatol. 1961;83:951–955. doi: 10.1001/archderm.1961.01580120063015. [DOI] [PubMed] [Google Scholar]
- 36.Ruini C, Witkowski AM, Cesinaro A, Teixeira De Carvalho N, Pellacani G. From actinic keratosis to squamous cell carcinoma: evidence of morphologic and biologic progression. J Am Acad Dermatol. 2015;72(Suppl):S8–S10. doi: 10.1016/j.jaad.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 37.Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol. 2011;25(Suppl):1–51. doi: 10.1111/j.1468-3083.2011.04296.x. [DOI] [PubMed] [Google Scholar]
- 38.Quaedvlieg PJ, Creytens DH, Epping GG, et al. Histopathological characteristics of metastasizing squamous cell carcinoma of the skin and lips. Histopathol. 2006;49(3):256–264. doi: 10.1111/j.1365-2559.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breuninger H, Brantsch K, Eigentler T, Hafner HM. Comparison and evaluation of the current staging of cutaneous carcinomas. J Dtsch Dermatol Ges. 2012;10(8):579–586. doi: 10.1111/j.1610-0387.2012.07896.x. [DOI] [PubMed] [Google Scholar]