Abstract
Background
Human Immunodeficiency Virus (HIV)-associated sensory neuropathy is a common neurological complication of HIV infection affecting up to 30% of HIV-positive individuals. However, the exact neuropathological mechanisms remain unknown, which hinders our ability to develop effective treatments for HIV-neuropathic pain (NP). In this study, we tested the hypothesis that inhibition of proinflammatory factors with overexpression of interleukin (IL)-10 reduces HIV-related NP in a rat model.
Methods
NP was induced by the application of recombinant HIV-1 envelope protein gp120 into the sciatic nerve. The hindpaws of rats were inoculated with nonreplicating herpes simplex virus (HSV) vectors expressing antiinflammatory cytokine IL-10 or control vector. Mechanical threshold was tested using von Frey filaments before and after treatments with the vectors. The mechanical threshold response was assessed over time using the area under curves (AUC). The expression of phosphorylated p38 mitogen-activated kinase, as tumor necrosis factor alpha, stromal cell-derived factor-1α (SDF-1α), and C-X-C chemokine receptor type 4 in both the lumbar spinal cord and the L4/5 dorsal root ganglia (DRG) was examined at 14 and 28 days after vector inoculation using Western blots.
Results
We found that in the gp120-induced NP model, IL-10 overexpression mediated by the HSV vector resulted in a significant elevation of the mechanical threshold that was apparent on day 3 after vector inoculation compared with the control vector (P<0.0001). The antiallodynic effect of the single HSV vector inoculation expressing IL-10 lasted more than 28 days. The AUC in the HSV vector expressing IL-10 was increased compared with that in the control vector (P<0.0001). HSV vectors expressing IL-10 reversed the upregulation of phosphorylated p38 mitogen-activated kinase, as tumor necrosis factor alpha, stromal cell-derived factor-1α (SDF-1α), and C-X-C chemokine receptor type 4 expression at 14 and/or 28 days in the DRG and/or the spinal dorsal horn.
Conclusions
Our studies demonstrate that blocking the signaling of these proinflammatory molecules in the DRG and/or the spinal cord using the HSV vector expressing IL-10 is able to reduce HIV-related NP. These results provide new insights on the potential mechanisms of HIV-associated NP and a proof-of concept for treating painful HIV sensory neuropathy with this type of gene therapy.
Introduction
Human immunodeficiency virus-1 (HIV)-related neuropathic pain (NP) is severe and unrelenting and is an important unmet need in medicine. Painful HIV sensory neuropathy can greatly impact quality of life in patients with HIV/ acquired immunodeficiency syndrome (AIDS).1 Despite extensive research, the exact neuropathological mechanisms of painful sensory neuropathy responsible for the development of this devastating condition remain unknown. Neurologic dysfunction is believed to depend on cellular entry of HIV, which requires sequential interactions between the envelope protein gp120 with CD4 glycoprotein2–5 and chemokine receptors on the cell surface.6–9 HIV gp120 is believed to exert both direct and indirect neurotoxic effects in the nervous system through the release of proinflammatory molecules.10 A growing body of evidence in a variety of animal models suggests that proinflammatory cytokines, such as tumor necrosis factor alpha (TNFα) released by the activated spinal glial cells and in the dorsal root ganglia (DRG), are critical to enhancing pain.11–16 Phosphorylated p38 mitogen-activated kinase (p-p38), a member of the family of mitogen-activated protein kinases (MAPKs), is strongly associated with TNFα expression in the chronic pain state.17 The expression of p-p38 in the DRG is well characterized after peripheral nerve injury associated with pathological pain.17,18 The C-X-C chemokine receptor type 4 (CXCR4) acts as an important inflammatory factor in the neuropathogenesis of HIV/AIDS.19,20 CXCR4 is required for gp120-induced cell death.21 Importantly, our studies show that stromal cell-derived factor-1α (SDF-1α) and its receptor CXCR4 are involved in the gp120-induced NP.22
Interleukin 10 (IL-10) is a critical pleiotropic antiinflammatory cytokine that blocks phosphorylation of MAPK pathways, resulting in the inhibition of MAPK actions.23 IL-10 diminishes the levels of TNF mRNAs after the onset of stimulation of polymorphonuclear leukocytes with lipopolysaccharide (LPS, a very common proinflammatory inducer), identifying the biological actions of IL-10 as a suppressor of the inflammatory response.24,25 Lumbosacral administration of the IL-10 transgene or protein leads to robust suppression of tactile allodynia induced by sciatic nerve injury, as well as spinal inflammation after a single intrathecal injection of gp120 protein.26–28 Hyperalgesic responses to TNFα or carrageenan are inhibited by intraplantar administration of IL-10.12
The highly replication-defective herpes simplex virus (HSV) genomic vectors can establish a persistent state that is used to deliver and express transgenes in the DRG neurons. The DRG neurons transduced with the HSV vector transport transgene-coded enkephalin centrally in the bipolar axon of the primary sensory afferents to the spinal dorsal horn (SDH).29 Transduction of the DRG sensory neurons by footpad inoculation with the HSV-based vectors encoding human preproenkephalin gene, produces an antihyperalgesic effect in models of acute thermal pain30 and inflammatory pain.31,32 HSV vectors encoding preproenkephalin gene also induce physiological improvement in visceral pain induced by bladder irritation.33 Inoculation of the HSV vectors expressing the IL-10 gene significantly reduces mechanical allodynia below the level of injury after blunt trauma to the spinal cord.34 HIV NP with a sizeable morbidity is difficult to manage.1 In this study, we tested the hypothesis that HSV vector-mediated IL-10 expression could treat NP induced by HIV gp120. We found that this IL-10 gene therapy produced antinociceptive effects on NP induced by the gp120 application to the sciatic nerve and reduced proinflammatory molecules p-p38, TNFα, and CXCR4/SDF1 in the DRG and/or the SDH.
Methods and Materials
A nonreplicating HSV-based vector expressing IL10
HSV vector expressing IL-10 contains the full-length rat IL-10 gene tagged with hemagglutinin (HA) under the control of the human cytomegalovirus immediate-early promoter (HCMC IEp); the control vector contains the lacZ gene (Q0ZHG) in place of IL10-HA.34 The construction of IL-10 vectors has been described (designated in that report as QHIL10).35 In our previous studies, we demonstrated that the vector produces IL-10 from the primary DRG neurons infected in vitro and in the spinal cord in vivo.16
Animals
Male Sprague-Dawley rats weighing 225 to 250 g were housed 1 to 3 per cage approximately 7 days before the beginning of the study. Rats were maintained with free access to food and water and were on a 12:12, light:dark schedule at 21 °C and 60% humidity. All housing conditions and experimental procedures were approved by the University Animal Care and Use Committee and were conducted in accordance with the ethical guidelines of the International Association for the Study of Pain.
Perineural gp120 neuropathic pain model
Under anesthesia, the rat’s left sciatic nerve was exposed in the popliteal fossa without damaging the nerve construction. A 2 × 6 mm strip of oxidized regenerated cellulose was previously soaked in 250 μl of a 0.1% rat serum albumin in saline, containing 400 ng of gp120 (Immunodiagnostics, Bedford, MA) or 0.1% rat serum albumin in saline for the sham surgery. A length of 3–4 mm of the sciatic nerve was wrapped loosely with the previously soaked cellulose, proximal to the trifurcation not to cause any nerve constriction and left in situ.36,37 The incision was closed with 4/0 sutures.
Mechanical threshold
Rats were placed in nontransparent plastic cubicles on a mesh floor for an acclimatization period of at least 30 min on the morning of the test day. Mechanical threshold was determined by assessing paw withdrawal to von Frey filaments (Stoelting, Wood Dale, IL) of graded tensile strength. A series of calibrated von Frey filaments were presented serially to the hindpaw in ascending order of strength, with each filament applied for 6 s with sufficient force to cause slight bending against the paw. A positive response was defined as a rapid withdrawal and/or licking of the paw immediately on application of the stimulus. Whenever a positive response to a stimulus occurred, the next smaller von Frey hair was applied, and whenever a negative response occurred, the next higher force was applied. In the absence of a response at a pressure of 15.1 g, animals were assigned to this cutoff value. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the up-and-down method.11
Western Blots
The lumbar L4-5 DRG and the SDH ipsilateral to gp120 application were removed rapidly under deep anesthesia, frozen on dry ice, and stored at −80°C. These tissues were homogenized in protein lysis buffer (150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 8.0) containing protease inhibitors and phosphatase inhibitors (Phosphatase Inhibitor Cocktails 1/2). The homogenate was centrifuged at 18,000 g for 20 min at 4°C. The supernatant was collected and assayed for protein concentration using the DC protein assay (Bio-Rad, Hercules, CA). Aliquots containing 30 μg of protein were dissolved in Laemmli buffer and denatured at 95 °C for 5 min; the proteins were separated by 10% Tris-glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% nonfat dry milk in phosphate buffered saline, and then incubated with primary antibodies overnight at 4 °C, including rabbit polyclonal anti- p-p38 IgG (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-TNFα (1 : 500, Chemicon, Temecula, CA), goat anti-CXCR4 (1:1000, Santa Cruz Biotechnology), rabbit anti-SDF1α (1:500, ABCAM, Cambridge, MA), and mouse anti-β-actin (1 : 8000, Sigma, St Louis, MO). The blots were incubated with secondary antibodies (Santa Cruz Biotechnology), developed in chemiluminescence solution (Pierce Biotechnology, Rockford, IL). Quantification of Western blots was done from the obtained chemiluminescence values (ChemiDoc, Bio-Rad). Target protein bands were normalized using β-actin.
Drug and Data Analysis
Statistical analysis was performed with SPSS version 21 (IMB Corporation, Armonk, NY). The results describing behavioral responsiveness to von Frey stimulation that indicates mechanical sensitivity were given as a mechanical threshold. The values from each test group were graphed as mean ± SEM. The data, which incorporated the main effects for group and time, were analyzed using a two-way analysis of variance (ANOVA) repeated measure. The group means and 95% confidence interval (95% CI) were calculated using SPSS. Differences in mean changes at the same time points were evaluated individually for the assessment of treatment effects on hypersensitivity with two-tailed t tests. The area under curves (AUC) data, depicting the mechanical threshold (g) over time, were calculated by the trapezoidal rule in order to express the overall magnitude and duration of effect, and analyzed between 2 different treatments using a t test. The actual P-values were shown in the figures.
The data for the effects of the HSV vector on the neurochemical changes among three groups were compared with one-way ANOVA with post hoc Fisher PLSD (Protected Least Significant Difference) test (StatView5). Given the small sample size and consequently the inability to assess the statistical assumptions for normality and equal variance, a stringent P value ≤ 0.01 was considered to be statistically significant in all ANOVA tests.38
Results
The effect of IL-10 mediated by HSV vector on mechanical allodynia induced by gp120
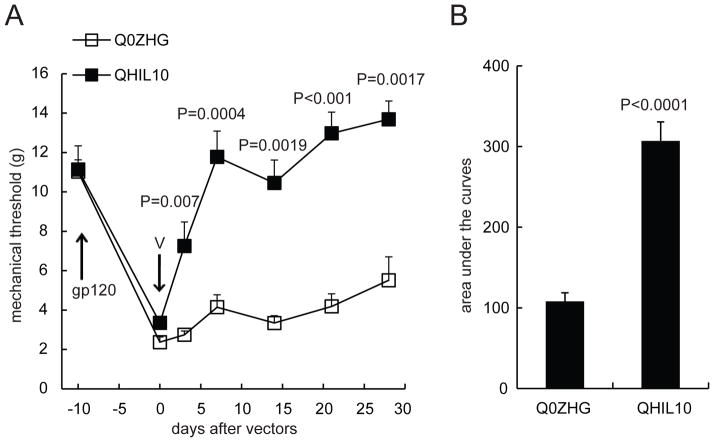
Evidence has demonstrated that peripheral gp120 application into the sciatic nerve results in neuropathic pain characterized by mechanical allodynia12,36,37 and that gp120 induces upregulation of proinflammatory cytokines/chemokines (e.g., TNFα, SDF1, etc) in the spinal cord and the sciatic nerve.12,22,36 The knockdown of TNFα using spinal TNFα siRNA reduces mechanical allodynia.12 The principal antiinflammatory activity of IL-10 is to inhibit the production of proinflammatory cytokines.24 We have demonstrated that IL-10 mediated by the HSV vector reversed formalin-induced inflammatory pain and spinal p-p38 and TNFα.16 Recent studies show that inoculation with the HSV vector expressing IL-10 reduces mechanical allodynia induced by spinal cord injury, which correlates with a significant decrease in spinal TNFα.34 In this study, we further examined whether overexpression of IL-10 mediated by the HSV vector reduced NP induced by HIV gp120. Subcutaneous inoculation with QHIL10 (30 μl containing 1 × 109 plaque-forming units/ml) was performed in the plantar surface of the hindpaw. QHIL10 resulted in a statistically significant elevation of the mechanical threshold that was apparent on day 3 after vector inoculation compared with the control vector (F(1,13) = 43.82, P < 0.0001, repeated measures ANOVA, n=7–8) (Figure 1A). The antiallodynic effect of the HSV vector lasted for more than 28 days. The group means of mechanical threshold with 95% CI were 11.23 (9.61–12.84) in QHIL10 and 3.99 (2.26–5.71) in Q0ZHG. For comparison of the differences at individual time points between groups, we used a two-tailed t test (P values are shown in Figure 1A). The area under curves (AUC) in the QHIL10 group was significantly higher than in the Q0ZHG group (P < 0.0001, t test, n=7–8, Figure 1B).
Figure 1.
The antiallodynic effect of interleukin (IL)-10 mediated by herpes simplex virus (HSV) vectors on neuropathic pain induced by human immunodeficiency virus (HIV) gp120. Mechanical allodynia in rats was shown day 7 after the gp120 administration (A). The gp120 injection (gp120) and the HSV vector (V) inoculation times are indicated by arrows. (A) QHIL10 resulted in a statistically significant elevation of the mechanical threshold (g) compared with the control vector (F(1,12) = 43.818, P < 0.001, repeated measures ANOVA, SPSS, n=7–8 rats, Figure 1A). For the comparison of differences at individual time points between groups, we used a two-tailed t test. Every P value is shown in the Figure. (B) The area under curves (AUC) in QHIL10 was significantly increased compared with Q0ZHG (P < 0.0001, t test, n=7–8 rats).
The effect of HSV vector overexpressing IL-10 on p-p38 in the DRG and SDH in the gp120 model
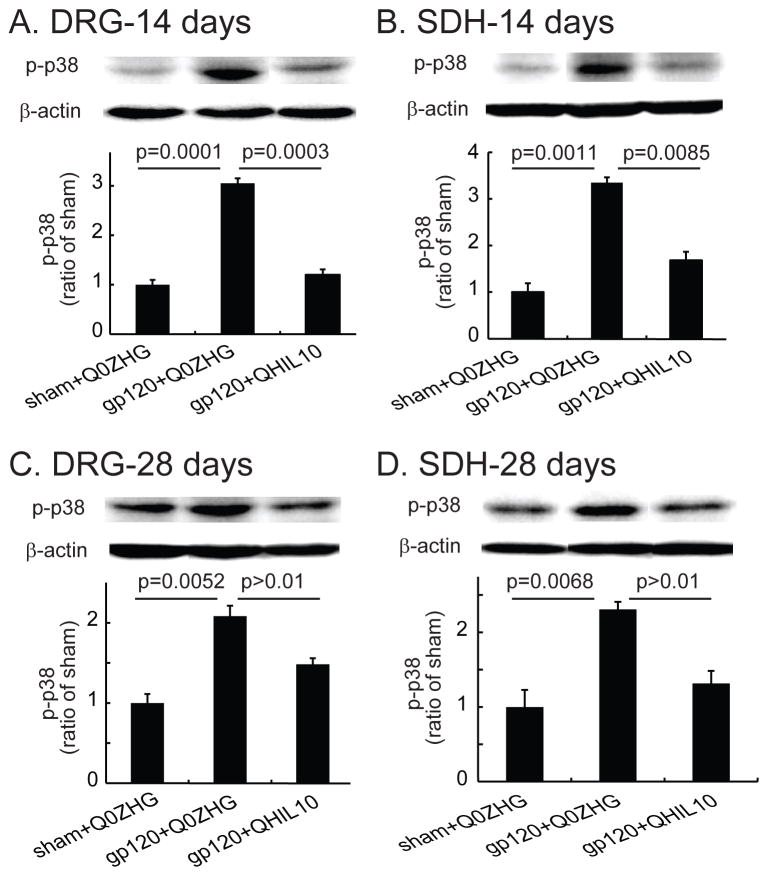
In this study, we investigated whether the overexpression of IL10 mediated by the HSV vector reduced p-p38 in the HIV-related NP state. The L4/5 DRG and spinal cord were harvested on day 14 after vector injection. Pooled L4/5 DRG and SDH were used for Western blots. In the DRG samples from day 14 after vector injection, expression of p-p38 in the gp120+Q0ZHG group was significantly increased compared with that in the sham+Q0ZHG group (P=0.0001, Figure 2A); expression of p-p38 in the gp120+QHIL10 group was markedly lower than that in the gp120+Q0ZHG group (P = 0.0003, Figure 2A). In the SDH samples from day 14 after vector injection, expression of p-p38 in the gp120+Q0ZHG group was markedly increased compared with that in the sham+Q0ZHG group (P = 0.0011, Figure 2B); the expression level of p-p38 in the gp120+QHIL10 group was less than that in the gp120+Q0ZHG group (P=0.0085, Figure 2B).
Figure 2.
The effect of interleukin (IL)-10 mediated by herpes simplex virus (HSV) vectors on the expression of phosphorylated p38 mitogen-activated kinase (p-p38) in the dorsal root ganglia (DRG) and spinal cord at 14 or 28 days. Rats with neuropathic pain were inoculated with QHIL10 or Q0ZHG on day 7 after gp120 administration. In the sham group, rats received Q0ZHG. (A and B) On day 14 after vector injection, the L4/5 DRG (A) and spinal dorsal horn (SDH) (B) were harvested, and expression of p-p38 was tested using Western blots. The data among three groups were compared using one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats. The actual P values between sham+Q0ZHG and gp120+Q0ZHG group, or between gp120+Q0ZHG and gp120+QHIL10 are shown in Figure A and B. (C and D) On day 28 after vector injection, L4/5 DRG (C) and SDH (D) were harvested, and the expression of p-p38 was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in Figure C and D, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats.
Similarly, in a separate set of experiments, on day 28 after vector injection, the L4/5 DRG and spinal cord were harvested for Western blots. In the DRG, there was a significant increase in p-p38 in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P = 0.0052, Figure 2C). However, the expression of p-p38 in the gp120+QHIL10 group was not significantly different from that in the gp120+Q0ZHG group (P = 0.073, Figure 2C). In SDH samples from day 28 after vector injection, p-p38 in the gp120+Q0ZHG group was markedly increased compared with that in the sham+Q0ZHG group (P =0.0068, Figure 2D). In the gp120+QHIL10 group, p-p38 was less than that in the gp120+Q0ZHG group, but the difference was not statistically significant (P>0.01, Figure 2D).
The effect of HSV vector overexpressing IL-10 on TNFα in the DRG and SDH in the gp120 model
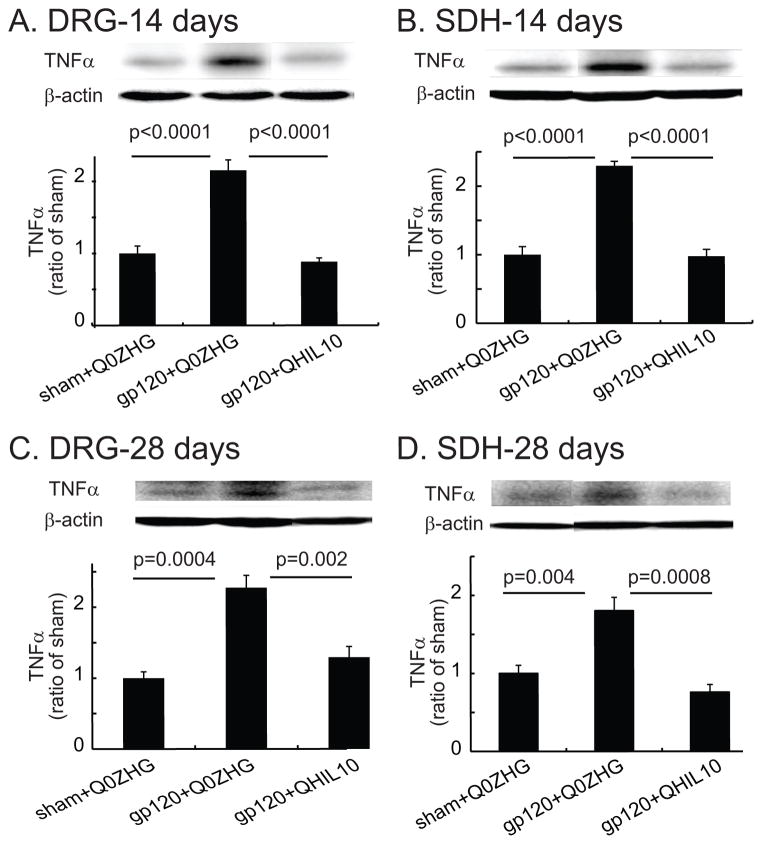
Accumulating evidence indicates that HIV gp120 mediated-NP increases TNFα in the spinal cord and DRG.12,22 In the current study, we examined whether overexpression of IL10 mediated by the HSV vector reduced TNFα in NP induced by gp120. L4/5 DRG and spinal cord were harvested for Western blots on day 14 after vector injection. In the DRG, there was a marked increase in TNFα in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P < 0.0001, Figure 3A). TNFα in the gp120+QHIL10 group was significantly decreased compared with that in the gp120+Q0ZHG group (P < 0.0001, Figure 3A). In the SDH, there was a marked increase in TNFα in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P < 0.0001, Figure 3B). TNFα expression in the gp120+QHIL10 group was significantly lower than that in the gp120+Q0ZHG group (P < 0.0001, Figure 3B). Recent studies show that the HSV vector expressing IL-10 not only reduced mechanical allodynia induced by spinal cord injury, but also decreased TNFα to the level of the sham group,34 which was similar to the current study.
Figure 3.
The effect of interleukin (IL)-10 mediated by herpes simplex virus (HSV) vectors on the expression of tumor necrosis factor alpha (TNFα) in the dorsal root ganglia (DRG) and spinal cord at 14 or 28 days. (A and B) On day 14 after vector injection, L4/5 DRG (A) and the spinal dorsal horn (SDH) (B) were harvested, and the expression of TNFα was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in Figure A and B, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats. (C and D) On day 28 after vector injection, L4/5 DRG (C) and SDH (D) were harvested, and the expression of TNFα was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in Figure C and D, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats.
In similar studies, on day 28 after injection of the vector, L4/5 DRG and spinal cord were harvested for Western blots. In the DRG, there was a significant increase in TNFα in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P = 0.0004, Figure 3C). The expression of TNFα in the DRG in the gp120+QHIL10 group was decreased compared with that in the gp120+Q0ZHG group (P = 0.0024, Figure 3C). In the SDH samples from day 28 after injection of the vector, there was a marked increase in TNFα in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG (P = 0.004, Figure 3D); TNFα in the gp120+QHIL10 group was significantly lower than that in the gp120+Q0ZHG group (P = 0.0008, Figure 3D).
The effect of HSV vector overexpressing IL-10 on SDF1α in the DRG and SDH in the gp120 model
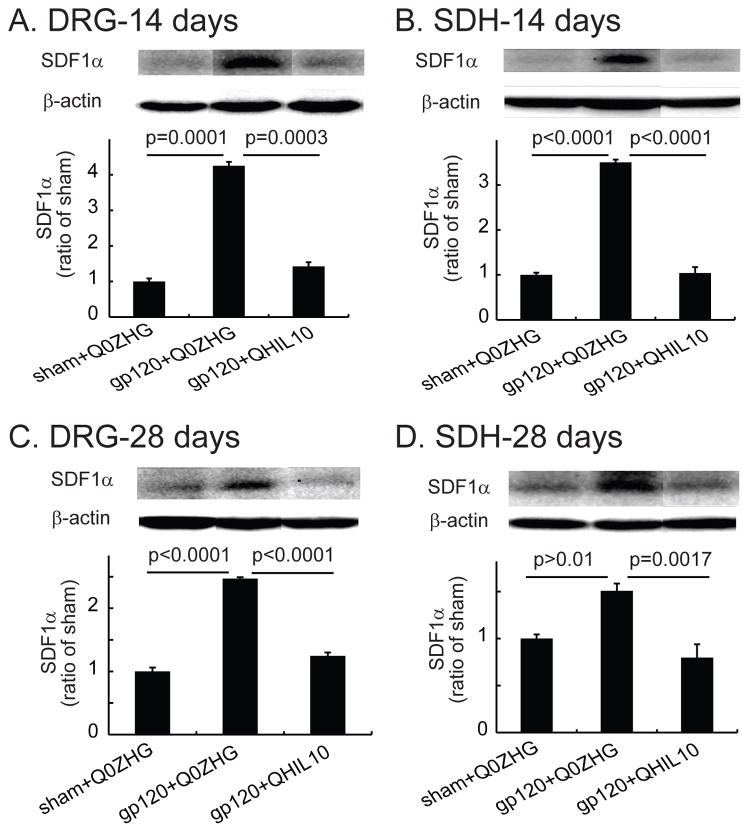
TNFα enhances expression of CXCR4, which facilitates the chemotactic invasiveness of cultured human mesenchymal stem cells toward SDF1α.39 We have reported that IL-10 is able to suppress overexpression of mRNA and protein of TNFα induced by formalin into the hindpaw.16 However, it is not known if IL-10 reduces production of SDF1α in vivo in the gp120-induced NP state. In this study, we investigated whether the overexpression of IL-10 mediated by the HSV vector reduced SDF1α in the NP state. In the DRG samples from day 14 after vector injection, there was a significant increase in SDF1α in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P = 0.0001, Figure 4A). The expression of SDF1α in the DRG in the gp120+QHIL10 group was markedly decreased compared with that in the gp120+Q0ZHG group (P = 0.0003, Figure 4A). In SDH samples from day 14 after vector injection, there was a significant increase in SDF1α in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P < 0.0001, Figure 4B). SDF1α in the gp120+QHIL10 group was markedly decreased compared with that in the gp120+Q0ZHG group (P< 0.0001, Figure 4B).
Figure 4.
The effect of interleukin (IL)-10 mediated by herpes simplex virus (HSV) vectors on the expression of stromal cell-derived factor-1α (SDF-1α) in the dorsal root ganglia (DRG) and spinal cord at 14 or 28 days. (A and B) On day 14 after vector injection, L4/5 DRG (A) and spinal dorsal horn (SDH) (B) were harvested, and the expression of SDF1α was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in Figure A and B, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats. (C and D) On day 28 after vector injection, L4/5 DRG (C) and SDH (D) were harvested, and the expression of SDF1α was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in Figure C and D, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats.
In the DRG on day 28 after injection of vectors, there was a significant increase in SDF1α in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P < 0.0001, Figure 4C). The expression of SDF1α in the gp120+QHIL10 group was decreased compared with that in the gp120+Q0ZHG group (P < 0.0001, Figure 4C). In SDH samples from day 28 after injection of vectors, there was a statistically nonsignificant increase in SDF1α in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P > 0.01, Figure 4D). However, SDF1α in the gp120+QHIL10 group was markedly lower than that in the gp120+Q0ZHG group (P=0.0017, Figure 4D).
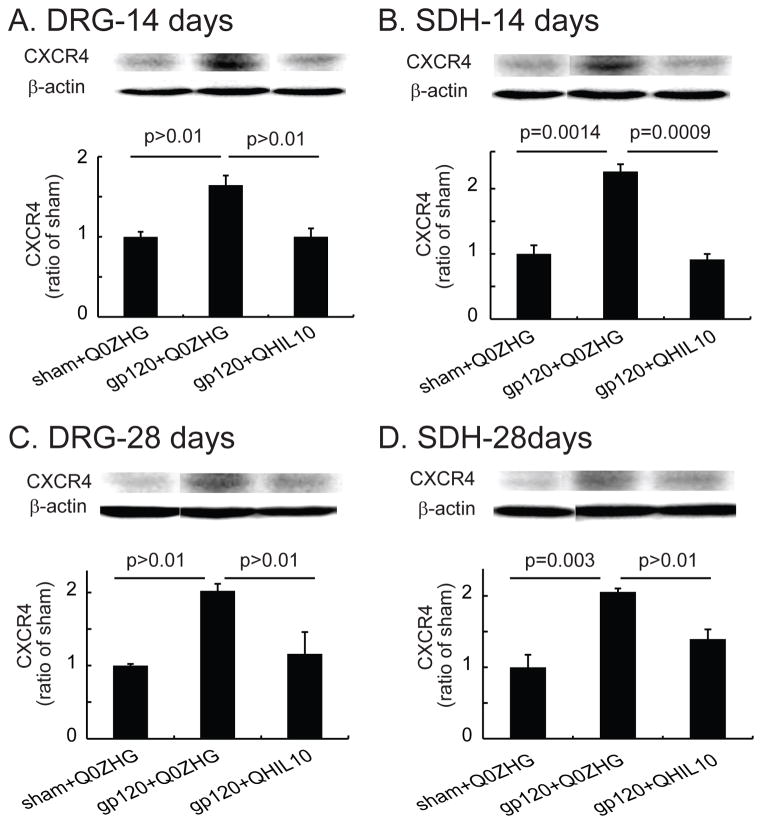
The effect of the HSV vector overexpressing IL-10 on CXCR4 in the DRG and SDH in the gp120 model
In the DRG on day 14 after vector injection, there was a statistically nonsignificant increase in CXCR4 in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (Figure 5A). The expression of CXCR4 in the gp120+QHIL10 group was less than that in the gp120+Q0ZHG group but the difference was not statistically significant (Figure 5A). In the SDH, there was a significant increase in CXCR4 in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P = 0.0014, Figure 5B). CXCR4 in the gp120+QHIL10 group was markedly less than that in the gp120+Q0ZHG group (P= 0.0009, Figure 5B).
Figure 5.
The effect of interleukin (IL)-10 mediated by herpes simplex virus (HSV) vectors on the expression of C-X-C chemokine receptor type 4 (CXCR4) in the dorsal root ganglia (DRG) and the spinal cord at 14 or 28 days. (A and B) On day 14 after vector injection, L4/5 DRG (A) and spinal dorsal horn (SDH) (B) were harvested, and the expression of CXCR4 was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in Figure A and B, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats. (C and D) On day 28 after vector injection, L4/5 DRG (C) and SDH (D) were harvested, and expression of CXCR4 was tested using Western blots. The actual P values between sham+Q0ZHG and gp120+Q0ZHG groups, or between gp120+Q0ZHG and gp120+QHIL10 groups are shown in the Figure C and D, one way ANOVA with post hoc Fisher PLSD test, mean ± SEM, n=4 rats.
In the DRG on day 28 after vector injection, CXCR4 tended to increase in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group, but the difference was not statistically significant (Figure 5C). In the expression of CXCR4, there was statistically nonsignificant difference between the gp120+QHIL10 and gp120+Q0ZHG groups (Figure 5C). In the SDH samples from day 28 after vector injection, there was a significant increase in CXCR4 in the gp120+Q0ZHG group compared with that in the sham+Q0ZHG group (P = 0.003, Figure 5D). CXCR4 in the gp120+QHIL10 group was less than that in the gp120+Q0ZHG group, but the difference was not statistically significant (Figure 5D).
Discussion
The current study demonstrated that in a gp120-induced NP model, IL-10 mediated by the HSV vector resulted in a significant elevation of the mechanical threshold that was apparent on day 3 after vector inoculation. The antiallodynic effect of the HSV vector lasted more than 28 days. The area under curves (AUC) in the HSV vectors expressing IL-10 was increased compared with the control vector. The HSV vector expressing IL-10 reversed upregulation of p-p38, TNFα, SDF1α, and CXCR4 induced by gp120 in the lumbar SDH and/or the DRG at 14 and/or 28 days.
HIV-1 envelope protein gp120 has been implicated directly and indirectly in the pathogenesis of HIV-associated neurocognitive disorders. MAPKs such as p38 are important for intracellular signal transduction and play critical roles in regulating neural plasticity and inflammatory responses.40 In in vitro studies, HIV gp120 activity unravels the involvement of p38 and resultant neurotoxic activity.41,42 The signaling of p38 is critical upon exposure to HIV gp120 for the neurotoxic phenotype of monocytic cells.43,44 HIV uses the p38 pathway to produce new viruses and to deplete CD4+ T cells from the host’s immune system.45 In in vivo studies, Milligan et al. have reported that the systemic p38 inhibitor CNI-1493 blocks intrathecal gp120-induced mechanical allodynia.46
HIV infection is able to increase production of several cytokines.47 It is reported that there is an increased level of TNFα in the human cerebrospinal fluid,48 blood plasma,49 spinal cord,50 and brain51 in patients with HIV. The early presence of cytokines may be involved in induction and/or progression of HIV-sensory neuropathy. For example, the HIV protein influences neuronal survival by increasing TNFα production.52 We and others have reported that application of recombinant gp120 to the sciatic nerve increases TNFα in the DRG and spinal cord.12,36 Furthermore, intrathecal TNFα siRNA or TNF soluble receptor reduces gp120 application-induced mechanical allodynia, indicating that TNFα in the spinal cord and/or the DRG are involved in NP induced by HIV gp120.12 TNF soluble receptor mediated by the HSV vector suppresses gp120-induced NP and reduces TNFα.22 Taken together, these data highlight the importance of TNFα in the development of the exaggerated pain state related to HIV gp120.
A growing body of evidence shows that chemokines and their receptors play an important role in inducing and maintaining NP.22,53,54 The interplay of TNFα and HIV-1 leads to enhanced expression of toxic chemokines.55 SDF-1α has multiple effects on neuronal activity, survival, and death under conditions that generate a proinflammatory microenvironment within the nervous system through its receptor CXCR4.56 Considering the widespread expression of CXCR4 in the nervous system, CXCR4 and its ligand SDF1α are important factors in the neuropathogenesis of HIV/AIDS.19 HIV gp120 binds to and activates CXCR4 expressed by DRG neurons in a CD-4-independent manner,57,58 suggesting the direct neurotoxic effects of gp120 on neurons.59 Recent studies have shown HIV gp120 induces upregulation of SDF1 and CXCR4 in the spinal cord and DRG at 2 weeks.22 In the current studies, although neuropathic rats with Q0ZHG did not show a significant increase in spinal SDF1α at 4 weeks (Figure 4D), there was a significant increase in the DRG (Figure 4C); neuropathic rats with Q0ZHG had a trend to increase CXCR4, but did not significantly increase CXCR4 in the DRG at either 2 weeks or 4 weeks (Figure 5).
The principal antiinflammatory activities of IL-10 are to inhibit the production of proinflammatory cytokines.24,25 IL-10 diminishes TNF mRNA after the onset of stimulation of polymorphonuclear leukocytes with LPS, identifying the biological action of IL-10 as a suppressor of the inflammatory response.24 In in vivo studies, IL-10 inhibited the writhing response induced by acetic acid or zymosan in mice, and knee joint incapacitation induced by zymosan in rats. IL-10 also inhibited the release of TNFα from mice peritoneal macrophages obtained after local injection of zymosan.60 Acute intrathecal administration of rat IL-10 protein briefly reverses chronic constriction injury (CCI)-induced mechanical allodynia.61 Hyperalgesic responses to TNFα or carrageenan are inhibited by intraplantar administration of IL-10.62 We have shown that IL-10 reduces p-p38 and expression of full-length membrane spanning TNFα after LPS stimulation of microglia in vitro; IL-10 also reduces intracellular cleavage of membrane TNFα and release of soluble TNFα.16 In in vitro studies, hypoxia-mediated increases in CXCR4 expression and cell survival are lower in IL-10-deficient othelial progenitor cells.63 IL-10 also downregulates CXCR4 mRNA expression in CD4+ T lymphocytes.64 In the present study, we report for the first time that IL-10 suppresses SDF1/CXCR4 in the NP state induced by gp120.
Viral vectors or plasmids overexpressing IL-10 may be a new approach to producing analgesia and antiallodynia associated with a variety of pain states. Intrathecal delivery of plasmid DNA encoding IL-10 gene prevents and progressively reverses the allodynic state induced by paclitaxel (a chemotherapy drug), and markedly decreases paclitaxel-induced expression of TNF mRNA in the lumbar DRG.65 Repeated intrathecal delivery of the plasmid DNA vectors encoding IL-10 gene abolishes NP induced by sciatic CCI.26 Adenoviral vectors encoding the human IL-10 gene prevent and reverse mechanical allodynia in the CCI model;61 the adenoviral vectors expressing cytokine IL-10 also enhances acute morphine analgesia and attenuate the development of morphine tolerance, hyperalgesia, and allodynia.66 Highly effective gene transfer to the primary sensory neurons of the DRG with self-complementary recombinant adeno-associated virus serotype 8 expressing IL-10, leads to significant reversal of mechanical allodynia in chronic NP induced by L5 spinal nerve ligation.67
Our group has previously reported the expression of IL-10 by HSV vectors-transduced cells.16 The cultured primary DRG neurons with these IL-10 vectors result in the release of IL-10.16 We have found that transduction of DRG neurons in vivo achieved by subcutaneous inoculation of the vector in the foot results in production of transgene-coded IL-10 in DRG neurons and transport of the gene product to terminals in the spinal cord suppresses the formalin-induced nociceptive effect and reduces spinal TNFα and p-p38 expression.16 In the present studies, IL-10 mediated by HSV significantly reversed the upregulation of TNFα at 2 and 4 weeks; however, IL-10 reversed the upregulation of p-p38 at 2 weeks, but not at 4 weeks. The exact mechanisms by which IL-10 suppresses TNFα or p-p38 are still not clear. IL-10 mediated by HSV almost totally reversed the upregulation of mRNA of TNFα in the spinal cord in the formalin pain model.16 Recent studies have shown that the HSV vectors expressing IL-10 not only reduced mechanical allodynia induced by spinal cord injury, but also decreased TNFα to the level of their sham group,34 which is similar to the current study (Figure 3). In our current study, we extend our previous results showing that IL-10 expressed by HSV vectors reduced NP induced by HIV gp120 administration into the sciatic nerve, and reversed the upregulation of p-p38, TNFα, SDF1α, and CXCR4 induced by gp120 in the lumbar SDH and/or DRG at 14 and/or 28 days. Future work will study the detailed mechanisms/pathways by which IL-10 suppresses those inflammatory factors.
In summary, blocking proinflammatory molecules reduced HIV gp120-induced NP. These results support that the current novel proof-of-concept approach to gene therapy with HSV-mediated overexpression of IL-10 is an important new strategy for treating HIV-associated NP.
Acknowledgments
Funding: The study was supported by the NIH DA026734 (S.H.), DA025527 (S.H.), NS066792 (S.H.) and DA34749 (S.H.). R.C.L was supported by NIH DCR022903.
We greatly acknowledge Dr. David Fink and Dr. Marina Mata providing the high-quality HSV vectors and the excellent technical assistance of Vikram Thakur (Department of Neurology, University of Michigan, Ann Arbor, MI). We acknowledge Kaming Lo, M.P.H. (Biostatistician, Division of Biostatistics, Department of Public Health Sciences, University of Miami Miller School of Medicine) for his very helpful suggestions in the statistics analysis.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES:
Name: Wenwen Zheng, PhD
Contribution: Conducted the study, and analyzed the data.
Attestation: Reviewed the original data and the analysis of the data, and approved the final manuscript.
Name: Wan Huang, MD, PhD
Contribution: Conducted the study, analyzed the data, and participated in writing the manuscript.
Attestation: Reviewed the original data and the analysis of the data, and approved the final manuscript
Name: Shue Liu, BS
Contribution: Conducted the study, and analyzed the data.
Attestation: Approved the final manuscript.
Name: Roy C. Levitt, MD
Contribution: Participated in writing the manuscript.
Attestation: Approved the final manuscript.
Name: Keith A. Candiotti, MD
Contribution: Helped write the manuscript.
Attestation: Approved the final manuscript.
Name: David A. Lubarsky, MD, MBA
Contribution: Helped write the manuscript.
Attestation: Approved the final manuscript.
Name: Shuanglin Hao, MD, PhD
Contribution: Designed the whole study, analyzed the data, and wrote the manuscript.
Attestation: Be the archival author/corresponding author. Approved the final manuscript.
Contributor Information
Wenwen Zheng, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida.
Wan Huang, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida; Department of Anesthesiology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China.
Shue Liu, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida.
Roy C. Levitt, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida; Hussman Institute of Human Genomics, University of Miami Miller School of Medicine, Miami, Florida; Veterans Affairs Medical Center, Miami, Florida.
Keith A. Candiotti, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida.
David A. Lubarsky, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida.
Shuanglin Hao, Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida.
References
- 1.Gabbai AA, Castelo A, Oliveira AS. HIV peripheral neuropathy. Handb Clin Neurol. 2013;115:515–29. doi: 10.1016/B978-0-444-52902-2.00029-1. [DOI] [PubMed] [Google Scholar]
- 2.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78:1198–203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 3.Berman JW, Carson MJ, Chang L, Cox BM, Fox HS, Gonzalez RG, Hanson GR, Hauser KF, Ho WZ, Hong JS, Major EO, Maragos WF, Masliah E, McArthur JC, Miller DB, Nath A, O’Callaghan JP, Persidsky Y, Power C, Rogers TJ, Royal W., 3rd NeuroAIDS, drug abuse, and inflammation: building collaborative research activities. J Neuroimmune Pharmacol. 2006;1:351–99. doi: 10.1007/s11481-006-9048-9. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan SD, Schwartz SA, Nair MP. Immunological assays for chemokine detection in in-vitro culture of CNS cells. Biol Proced Online. 2003;5:90–102. doi: 10.1251/bpo50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams KC, Burdo TH. HIV and SIV infection: the role of cellular restriction and immune responses in viral replication and pathogenesis. APMIS. 2009;117:400–12. doi: 10.1111/j.1600-0463.2009.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelman BB, Spencer JA, Holzer CE, 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J Neuroimmune Pharmacol. 2006;1:410–20. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- 7.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–72. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 8.Meeker RB. Feline immunodeficiency virus neuropathogenesis: from cats to calcium. J Neuroimmune Pharmacol. 2007;2:154–70. doi: 10.1007/s11481-006-9045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 11.Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–6. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Molecular pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao S. The Molecular and Pharmacological Mechanisms of HIV-Related Neuropathic Pain. Curr Neuropharmacol. 2013;11:499–512. doi: 10.2174/1570159X11311050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor alpha to the normal and mechanically compressed lumbar ganglia in the rat. Pain. 2002;95:239–46. doi: 10.1016/S0304-3959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 15.Narita M, Shimamura M, Imai S, Kubota C, Yajima Y, Takagi T, Shiokawa M, Inoue T, Suzuki M, Suzuki T. Role of interleukin-1beta and tumor necrosis factor-alpha-dependent expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced by chronic inflammation in mice. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–90. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–21. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 19.Mocchetti I, Campbell LA, Harry GJ, Avdoshina V. When Human Immunodeficiency Virus Meets Chemokines and Microglia: Neuroprotection or Neurodegeneration? J Neuroimmune Pharmacol. 2012;8:14. doi: 10.1007/s11481-012-9353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–96. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 21.Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–9. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang W, Zheng W, Liu S, Zeng W, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. HSV-mediated p55TNFSR reduces neuropathic pain induced by HIV gp120 in rats through CXCR4 activity. Gene Ther. 2014 doi: 10.1038/gt.2013.90. [DOI] [PubMed] [Google Scholar]
- 23.Haddad JJ, Saade NE, Safieh-Garabedian B. Interleukin-10 and the regulation of mitogen-activated protein kinases: are these signalling modules targets for the anti-inflammatory action of this cytokine? Cell Signal. 2003;15:255–67. doi: 10.1016/s0898-6568(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 24.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–11. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard M, O’Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 26.Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–82. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 28.Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, Wieseler-Frank J, Hammack SE, Maier SF, Flotte TR, Forsayeth JR, Leinwand LA, Chavez R, Watkins LR. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Molecular pain. 2005;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antunes bras J, Becker C, Bourgoin S, Lombard M, Cesselin F, Hamon M, Pohl M. Met-enkephalin is preferentially transported into the peripheral processes of primary afferent fibres in both control and HSV1-driven proenkephalin A overexpressing rats. Neuroscience. 2001;103:1073–83. doi: 10.1016/s0306-4522(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 30.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96:3211–6. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8:551–6. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 32.Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, Pohl M. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881–8. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama H, Sasaki K, Franks ME, Goins WF, Goss JR, de Groat WC, Glorioso JC, Chancellor MB, Yoshimura N. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2009;20:63–71. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau D, Harte SE, Morrow TJ, Wang S, Mata M, Fink DJ. Herpes simplex virus vector-mediated expression of interleukin-10 reduces below-level central neuropathic pain after spinal cord injury. Neurorehabil Neural Repair. 2012;26:889–97. doi: 10.1177/1545968312445637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol. 2009;220:183–90. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 37.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130:2688–702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafer SL, Dexter F. Publication bias, retrospective bias, and reproducibility of significant results in observational studies. Anesth Analg. 2012;114:931–2. doi: 10.1213/ANE.0b013e31824a0b5b. [DOI] [PubMed] [Google Scholar]
- 39.Egea V, von Baumgarten L, Schichor C, Berninger B, Popp T, Neth P, Goldbrunner R, Kienast Y, Winkler F, Jochum M, Ries C. TNF-alpha respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ. 2011;18:853–63. doi: 10.1038/cdd.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Xu C, Chen L, Xu P, Xiong H. Involvement of Kv1. 3 and p38 MAPK signaling in HIV-1 glycoprotein 120-induced microglia neurotoxicity. Cell Death Dis. 2012;3:e254. doi: 10.1038/cddis.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Yao H, Lu Y, Wang C, Buch S. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PLoS One. 2010;5:e13427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol. 2010;185:4883–95. doi: 10.4049/jimmunol.0902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao H, Allen JE, Zhu X, Callen S, Buch S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J Neurovirol. 2009;15:164–75. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furler RL, Uittenbogaart CH. Signaling through the P38 and ERK pathways: a common link between HIV replication and the immune response. Immunol Res. 2010;48:99–109. doi: 10.1007/s12026-010-8170-1. [DOI] [PubMed] [Google Scholar]
- 46.Milligan ED, O’Connor KA, Armstrong CB, Hansen MK, Martin D, Tracey KJ, Maier SF, Watkins LR. Systemic administration of CNI-1493, a p38 mitogen-activated protein kinase inhibitor, blocks intrathecal human immunodeficiency virus-1 gp120-induced enhanced pain states in rats. J Pain. 2001;2:326–33. doi: 10.1054/jpai.2001.26174. [DOI] [PubMed] [Google Scholar]
- 47.Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2391–7. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- 48.Grimaldi LM, Martino GV, Franciotta DM, Brustia R, Castagna A, Pristera R, Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol. 1991;29:21–5. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- 49.de Larranaga GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis. 2003;14:15–8. doi: 10.1097/00001721-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012;32:10833–40. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–60. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 52.Sui Z, Sniderhan LF, Schifitto G, Phipps RP, Gelbard HA, Dewhurst S, Maggirwar SB. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J Immunol. 2007;178:3226–36. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- 53.Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007;21:581–91. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Molecular pain. 2009;5:48. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–43. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shepherd AJ, Loo L, Gupte RP, Mickle AD, Mohapatra DP. Distinct modifications in Kv2. 1 channel via chemokine receptor CXCR4 regulate neuronal survival-death dynamics. J Neurosci. 2012;32:17725–39. doi: 10.1523/JNEUROSCI.3029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein 120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–35. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–49. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–21. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 60.Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S, Cunha FQ, Ribeiro RA. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304:102–8. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- 61.Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–48. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 62.Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br J Pharmacol. 1995;115:684–8. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–9. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jinquan T, Quan S, Jacobi HH, Madsen HO, Glue C, Skov PS, Malling HJ, Poulsen LK. CXC chemokine receptor 4 expression and stromal cell-derived factor-1alpha-induced chemotaxis in CD4+ T lymphocytes are regulated by interleukin-4 and interleukin-10. Immunology. 2000;99:402–10. doi: 10.1046/j.1365-2567.2000.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–98. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS, Morrison JH, Beutler AS. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A. 2008;105:1055–60. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]