Abstract
Background
Many of the mosquito species responsible for malaria transmission belong to a sibling complex; a taxonomic group of morphologically identical, closely related species. Sibling species often differ in several important factors that have the potential to impact malaria control, including their geographical distribution, resistance to insecticides, biting and resting locations, and host preference. The aim of this study was to define the geographical distributions of dominant malaria vector sibling species in Africa so these distributions can be coupled with data on key factors such as insecticide resistance to aid more focussed, species-selective vector control.
Results
Within the Anopheles gambiae species complex and the Anopheles funestus subgroup, predicted geographical distributions for Anopheles coluzzii, An. gambiae (as now defined) and An. funestus (distinct from the subgroup) have been produced for the first time. Improved predicted geographical distributions for Anopheles arabiensis, Anopheles melas and Anopheles merus have been generated based on records that were confirmed using molecular identification methods and a model that addresses issues of sampling bias and past changes to the environment. The data available for insecticide resistance has been evaluated and differences between sibling species are apparent although further analysis is required to elucidate trends in resistance.
Conclusions
Sibling species display important variability in their geographical distributions and the most important malaria vector sibling species in Africa have been mapped here for the first time. This will allow geographical occurrence data to be coupled with species-specific data on important factors for vector control including insecticide resistance. Species-specific data on insecticide resistance is available for the most important malaria vectors in Africa, namely An. arabiensis, An. coluzzii, An. gambiae and An. funestus. Future work to combine these data with the geographical distributions mapped here will allow more focussed and resource-efficient vector control and provide information to greatly improve and inform existing malaria transmission models.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1734-y) contains supplementary material, which is available to authorized users.
Keywords: Species distribution model, Maps, Susceptibility bioassays
Background
Over 100 anopheline mosquito species can transmit human malaria parasites but there are important differences among these species that influence their role in malaria transmission. Many of these species belong to a sibling complex; a complex is a taxonomic group of morphologically identical, closely related species. In the past, sibling species have been hard to distinguish and complexes have often been treated as a single entity despite important differences among sibling species. In Africa, Anopheles arabiensis, Anopheles coluzzii and Anopheles gambiae from the Gambiae complex and Anopheles funestus from the Funestus subgroup are undoubtedly the most important vectors transmitting both Plasmodium falciparum and Plasmodium vivax parasites to humans [1–3]. Within the Gambiae complex, Anopheles melas and Anopheles merus are also considered dominant vectors (“dominant” is defined as a vector species that has been identified as the main, dominant or important vector in at least one region) whereas there is no strong evidence that other species from this complex play any role in malaria transmission [4, 5].
In addition to differences in the vector status of each species, sibling species also have important differences in their geographical distributions. Previous studies that estimated the geographical distributions of the dominant malaria vectors were hampered by low volumes of data for individual sibling species and had to choose between mapping complexes or incorporating species records that had been determined on the basis of morphology alone and were therefore potentially misidentified [6]. Furthermore, insecticide resistance in vector species currently threatens the efficacy of vector control [7], making this a critical factor that needs to be understood within each vector species. In the past, many studies that used susceptibility assays to measure prevalence of resistance in vector populations did not fully identify sibling species. Thus, the mortality values obtained related to the species complex as a whole and potentially important differences among sibling species were not identified.
In recent years, the importance of species identification alongside the availability of accurate molecular identification methods has increased the number of studies reporting reliably identified sibling species. The aim of this study was to use the increasing availability of sibling species records, and an improved species distribution model, to define the geographical distributions of individual vector species within the Gambiae complex and Funestus subgroup in Africa. The available evidence for insecticide resistance was then examined in these species to assess the feasibility of combining insecticide resistance data with the geographical distributions generated. The distributions of An. gambiae and An. coluzzii are modelled separately for the first time and An. funestus is modelled for the first time as the type species distinct from other members of the subgroup.
Methods
Summary of species distribution map generation
Records of sibling species occurrence, where species were identified using molecular methods, were retrieved from the published literature (from both resistance and behavioural studies) and from unpublished sources to compile a set of presence records for each species. A larger dataset, including all Anopheles surveys in the region, was used as a background dataset that captured sampling bias in the presence records. Both datasets informed a species distribution model that identified the combinations of environmental variables that best distinguished areas supporting species presence from the range of environments sampled. This model was then used to estimate the relative probability of species presence at all locations within the species range.
Species background and presence data
Data from two previously collated and publicly available databases of dominant malaria vector species occurrence and bionomics [8, 9] were combined with a new database of insecticide resistance records (described below) and duplicate records were removed. Searches of the more recent literature were conducted from the dates that the earlier searches finished (2009, 2013 and 2015 respectively) to 29 September 2016, to fill any gaps in the dataset. The new searches used the Web of Sciences bibliography and the search terms “[species name]” and “[country name]”. New records of occurrence that matched the inclusion criteria were extracted and added to the composite database. Only studies that provided the location and time of collection, and gave details of the identification method(s) used, were included.
Geographical coordinates for the collection locations were converted to decimal degrees. For sites where no coordinates were given, coordinates were assigned using the site name and contextual information, such as the district or distance to a major city, using online gazetteers including GeoNames, Google Maps, and OpenStreetMap. All coordinates provided by the source or generated as part of this project were checked to ensure that they matched the sampling design described, fell on land and fell in the correct country, using the geographical information software ArcMAP. If collection dates were missing for a data point, the year of collection was assumed to be two years before the article publication year based on the trend seen for data with a known collection date, for the purposes of this study. For each species, the full species occurrence dataset was classified into (1) studies that used molecular identification methods capable of detecting that species, and (2) studies that would not have detected that species using molecular methods.
To generate a presence dataset for each species, all records that used appropriate molecular identification methods and recorded presence of the species were extracted from the dataset described above. For all studies that identified the species formerly known as An. gambiae (An. gambiae/An. coluzzii or M/S forms combined) using molecular methods but did not identify the M and S forms, occurrences were labelled “An. gambiae (old)” to distinguish them from the newly defined An. gambiae (formerly An. gambiae S form). Records of “An. gambiae (old)” outside the An. coluzzii range plus a 300 km buffer were designated An. gambiae and records inside the overlapping An. gambiae and An. coluzzii ranges, plus buffer, were discarded.
It is not possible to test empirically whether spatial clustering in the presence data is due to habitat suitability or spatial bias in sampling effort, so this was accounted for a priori through the selection of background data with the same spatial bias in sampling effort [10]. The full mosquito occurrence dataset was used as a source of background data that captures the sampling bias in the environments surveyed for Anopheles species. Most of the species modelled have ranges that border on desert areas where Anopheles are known to be absent but no surveys are performed, so 210 pseudo-absences were generated by randomly selecting locations within the desert biome defined by the United Nations Environment Programme [11].
Summary of available insecticide resistance data for sibling species
A literature search was performed in the Web of Science bibliographic database using the search terms “insecticide resistance” and “anopheles”. Articles of potential interest were identified and their abstracts were scanned to identify studies that had performed a bioassay on field-collected mosquitoes (up to the F1 generation). Full texts were obtained for these articles and data extracted for all bioassays that had used an insecticide from one of the four major neurotoxic classes: carbamates, organochlorines, organophosphates and pyrethroids. Unpublished data were also requested from authors of the published articles and groups working on insecticide resistance. Data fields extracted from both published and unpublished sources covered: species; collection dates; collection location; method(s) of capture; method(s) of identification; insecticide; bioassay protocol; percent mortality; and source citation(s). Data from all bioassays identified were extracted and any deviations from a standard published protocol (for example non-standard exposure times) were noted. Records that did not identify sibling species using molecular methods were discarded for the purposes of the current study. Summary statistics were calculated, based on the mortalities obtained for samples where >95% of the mosquitoes were identified as a single species, to give an indication of bioassay data availability and variation among sibling species.
Species ranges
In order to model the geographical distribution within each species range, previously defined ranges for An. arabiensis, An. funestus, An. melas and An. merus [6, 12] were used to limit the extent of the model outputs. These ranges were compared to the presence dataset for each species described above and if confirmed records of the species were found outside the previously defined range, the range was extended to encompass the new location(s). For An. coluzzii the presence dataset and a previous map showing records of the M form of An. gambiae [13] were used to define its range. One record of a single An. coluzzii mosquito in Zimbabwe shown on the previous map was discarded after a thorough search of the literature found no other record of this species within over 500 km of this location since the original record was published. Individual species ranges and presence points were combined to generate ranges for the Gambiae complex, Funestus subgroup and Funestus group. A 300 km buffer was added to each species range to reflect uncertainty in the exact ranges of these mosquitoes.
Environmental data
The modelling approach used here relies on the relationship between species occurrence and combinations of environmental covariates. Covariate values were extracted from an existing set of spatial data layers for environmental covariates believed to be of importance to mosquito occurrence and malaria transmission [14]. Full details are provided in Additional file 1. Briefly, data layers at a 5 × 5 km resolution were included for land surface temperature, seasonality in temperature, measures of wetness/greenness, seasonality in wetness/greenness, elevation, proportional cover of 14 land classes, and human population density.
Species distribution model
Each species was modelled separately using the same species distribution model. The approach used was a boosted regression trees method that combines both regression trees (which build a set of decision rules on the predictor variables by portioning the data into successively smaller groups with binary splits) and boosting (which selects sets of trees that minimise the loss function) to best capture the variables that define the distribution of the input data [15–17]. The boosted regression trees methods has been used in previous malaria vector studies [6] and has recently been updated to use background data that characterises sampling bias in the presence records, and to include changes in land cover over time [18, 19]. For each model, the presence records for that species and the background data points located within the range of that species, excluding survey records found in the presence dataset, were used. The background data were classified into (1) records from studies that used methods that would have identified the sibling species being modelled (had that species been present in the sample collected), and (2) all other records. Data points linked to a random 10% of locations from both the presence and background datasets were withheld for use in the model validation. Together the remaining presence and background data formed the model training dataset.
The boosted regression trees method requires both presence and absence data, or background data can be used when true absence data is not available. Mosquito occurrence datasets are subject to spatial bias and if unaccounted for this survey bias can translate into environmental bias in the fitted model. The background data used in the study reflected the same survey bias found in the presence data so the model could identify suitable environments for the species within the sampled space, rather than just areas that are more heavily sampled. This approach does not eliminate sampling bias issues entirely but improved model performance has been demonstrated [10]. The model was updated further in order to weight the background data so that records from surveys using molecular methods that would have identified the species being modelled (had that species been present in the sample collected) received twice the weight of other background data points. Presence and background data from 2001 to 2012 were linked to covariate values for the relevant year in order to improve the predictions where possible. For all records prior to 2001, covariate values for 2001 were used, and for any data collected after 2012, covariate values for 2012 were used. Model predictions were made to the most contemporary covariate data available.
For each species, 200 submodels were then fitted trained to a bootstrap of the presence/background dataset. Each submodel generated a predicted value for the relative probability of species occurrence at every 5 km × 5 km pixel and together the ensemble of submodels generated a distribution of predicted values for every pixel. Mean values together with 0.025 and 0.975 quantile values were then derived from the distribution of predictions at every 5 × 5 km pixel.
Model validation
Withheld data (the test data) from each presence and background dataset were used to validate each mean map generated. The area under the receiver operator curve (AUC) was calculated to assess the mean map’s ability to distinguish species presence points from background points that are representative of the locations surveyed for Anopheles vectors, whilst marginalising the arbitrary choice of a classification threshold [20]. An AUC of 0 means the model ranked all sites the wrong way round, 0.5 means the model was no better than random, and an AUC of 1 means it made a perfect prediction. The same test presence and background datasets were used to calculate the AUC value for previously published maps of these species to allow us to compare model performance.
Results
Compiled species occurrence database
The data volumes available for each species are given in Table 1, the presence and background data that went into the training and test datasets are provided in Additional file 2, and maps showing the distributions of these datasets are provided in Additional file 3.
Table 1.
The volumes of data collated
| Species | Number of presence points | Number of background points | |
|---|---|---|---|
| Class 1 | Class 2 | ||
| An. arabiensis | 2106 (505) | 1066 | 784 |
| An. coluzzii | 1086 (172) | 385 | 762 |
| An. funestus | 720 (172) | 50 | 2991 |
| An. gambiae | 1703 (420) | 1070 | 1058 |
| An. merus | 111 (71) | 447 | 0 |
| An. melas | 178 (58) | 1021 | 3 |
The total number of presence points for each species is provided and the subtotal that fell outside the time range for which the annual covariate data is given in parentheses. Background data points are split into those that used molecular methods that would have identified the species modelled (class 1) and those that did not (class 2)
Geographical distributions of the sibling species
The mean estimated relative probability of occurrence for each modelled sibling species is shown in a set of predictive maps (Fig. 1a–f). In addition, predictive maps for the Gambiae complex as a whole, Funestus subgroup and Funestus group are provided in Additional file 4.
Fig. 1.
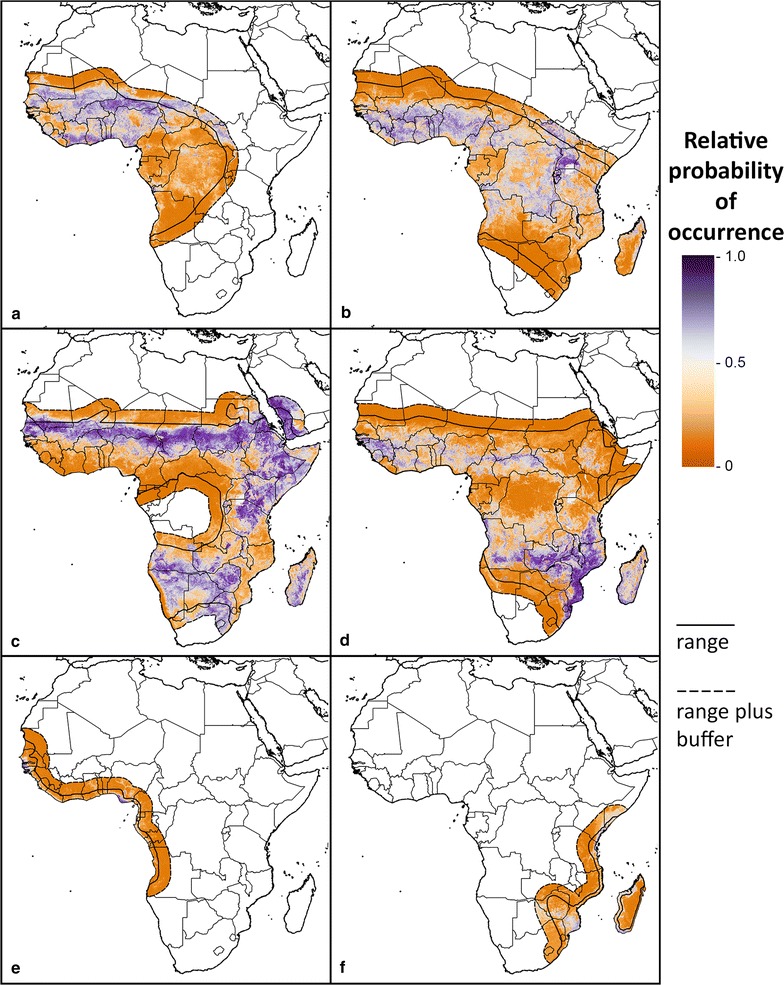
Predictive maps for occurrence of sibling species. The relative probability of occurrence for each species is shown within its range plus a 300 km buffer. a An. coluzzii. b An. gambiae c An. arabiensis. d An. funestus. e An. melas. f An. merus
The AUC values for the Gambiae complex model outputs were 0.870 for An. arabiensis, 0.783 for An. coluzzii, 0.778 for An. gambiae, 0.866 for An. melas, and 0.804 for An. merus. These values are consistently higher than those obtained for the previously published maps (available for An. arabiensis, An. melas and An. merus only) as shown in Additional file 5. The AUC values for the modelled An. funestus map was 0.824, for the Funestus subgroup it was 0.806 and for the Funestus group it was 0.796. The AUC value for the Funestus subgroup was higher than the value obtained for the previously published map (Additional file 5). Maps showing uncertainty in these predictions, in the form of the range from the 2.5th to the 97.5th centile, are provided in Additional file 5. The GeoTIFF files containing the mean, median and quantile predictions for every 5 × 5 km pixel are provided in Additional files 6, 7, 8, 9, 10, 11, 12, 13, 14.
The environmental variables that proved to be the top predictors in each species model are also provided in Additional file 5. For members of the Gambiae complex the top two predictors were always related to temperature and/or wetness, or elevation which is closely correlated with both. For An. funestus, land cover types were also strong predictors.
For the most part, there are few contiguous areas of high relative probability of occurrence that cross from the known range of a species into the 300 km outside this range, indicating biogeographical barriers are limiting the species ranges (Fig. 1). For An. coluzzii, however, an area of high relative probability of occurrence, or environmental suitability, can be seen running from within its range in Chad out to the northwest. A similar area in South Sudan can be seen for An. gambiae. This could indicate areas suitable for future expansion of the ranges of these species, or it could reflect uncertainty about the current species ranges in this part of Africa. The same pattern can be seen at the southern end of the An. arabiensis range where this species may be restricted by factors that have not been captured in this model.
Insecticide resistance in sibling species
Bioassay results from samples where more than 95% of the mosquitoes were identified as belonging to a single species were included in the final insecticide resistance dataset, providing 2437 records. The results for each insecticide class and species are given in Additional file 15 and the results for pyrethroid resistance in members of the Gambiae complex are shown in Table 2. A further 1156 records provided mortality records for mixed samples of identified sibling species within the Gambiae complex and 424 records came from studies that used molecular methods to identify species but did not provide the species composition of the samples.
Table 2.
Available data on pyrethroid resistance for sibling species of the Gambiae complex
| Year range | No. records | Mortality (%) | Countries |
|---|---|---|---|
| An. arabiensis | |||
| Up to 2000 (first year = 1996) | 3 | Min 100 | South Africa |
| Max 100 | |||
| Mean 100 | |||
| 2001–2005 | 67 | Min 75 | Cameroon, Kenya, Madagascar, Mozambique, Nigeria, South Africa, Sudan, Tanzania |
| Max 100 | |||
| Mean 95.6 | |||
| 2006–2010 | 218 | Min 0 | Burkina Faso, Cameroon, Chad, Ethiopia, Kenya, Mozambique, Senegal, Sudan, Tanzania, The Gambia, Uganda, Zambia, Zimbabwe |
| Max 100 | |||
| Mean 79.3 | |||
| 2011–2015 | 161 | Min 9 | Ethiopia, Kenya, Malawi, Mali, South Africa, Senegal, Sudan, Tanzania, Uganda |
| Max 100 | |||
| Mean 74.7 | |||
| An. coluzzii | |||
| 2001–2005 | 38 | Min 19 | Benin, Cameroon, Nigeria |
| Max 100 | |||
| Mean 89.7 | |||
| 2006–2010 | 144 | Min 0.9 | Benin, Burkina Faso, Cameroon, Côte d’Ivoire, Ghana, Mali, Nigeria |
| Max 100 | |||
| Mean 73.2 | |||
| 2011–2015 | 44 | Min 1 | Benin, Burkina Faso, Cameroon, Côte d’Ivoire, Mali, Mozambique, Liberia |
| Max 100 | |||
| Mean 60.6 | |||
| An. gambiae | |||
| Up to 2000 (first year = 1999) | 10 | Min 100 | Zambia |
| Max 100 | |||
| Mean 100 | |||
| 2001–2005 | 55 | Min 27 | Angola, Burundi, Cameroon, Equatorial Guinea, Mozambique, Nigeria, Uganda |
| Max 100 | |||
| Mean 88.82 | |||
| 2006–2010 | 139 | Min 0 | Benin, Burkina Faso, Burundi, Cameroon, Congo, Ghana, Guinea, Kenya, Malawi, Mozambique, Uganda, Zambia |
| Max 100 | |||
| Mean 75.2 | |||
| 2011–2015 | 48 | Min 0 | Cameroon, DRC, Ghana, Kenya, Mali, Tanzania, Uganda |
| Max 100 | |||
| Mean 48.7 | |||
| An. melas | |||
| All years (2005) | 4 | Min 100 | Cameroon |
| Max 100 | |||
| Mean 100 | |||
| An. quadriannulatus | |||
| All years (2002) | 1 | Min 100 | South Africa |
| Max 100 | |||
| Mean 100 | |||
A record is defined as a mortality value for a single mosquito population sampled at a specified time and place by a unique study. For species with <10 bioassay records, records for all years were aggregated and the year range is noted in parentheses. For species with >10 bioassay records, the data was divided into three year ranges and the year of the first record is given in parentheses. The mean (given in italics), minimum (min) and maximum (max) mortality values across all records for that time period are given together with a list of the countries where the field collections were taken
When the criterion of >95% of the mosquitoes confirmed as a single sibling species is applied, it is apparent that since the year 2000 over 100 bioassay results from multiple countries have been made available for each of the following: pyrethroid resistance in An. arabiensis, An. coluzzii, An. gambiae and An. funestus; carbamate resistance in An. arabiensis and An. coluzzii; organochlorine resistance in An. arabiensis and An. gambiae; and organophosphate resistance in An. arabiensis. Far fewer data points are available for the years up to 2000 and the first results for sibling species start in the late 1990s. For other African vector species, available data are limited or were not found at all. The search included malaria vector species outside the Gambiae complex and Funestus group, but no available data were found for An. coustani (An. coustani data are available but not confirmed using molecular identification methods), An. moucheti, species of the Nili complex, or An. pharoensis.
Consistent susceptibility to organophosphates was found for An. funestus across all 11 countries sampled from 1999 to the current time, and was also seen in the small number of results for other members of this group. Within the Gambiae complex, differences are apparent among sibling species in terms of their resistance to each of the four major classes of insecticide (Table 2; Additional file 14). Caution is needed, however, when identifying apparent variation or trends in the insecticide resistance data. For example, the summary data in Table 2 appears to show that resistance to pyrethroids has consistently increased over time within members of the Gambiae complex but Table 2 also shows that there is substantial bias in the locations sampled and in the times sampled. These biases are likely also to be present at finer spatial and temporal scales, and need to be incorporated in any analysis of the patterns of resistance in sibling species. Further, the values shown are derived from bioassays that used a range of protocols and these differences need to be captured and included in the data in order to perform a robust analysis of the dataset.
Discussion
This study provides full modelled geographical distributions for An. coluzzii and An. gambiae (as now defined) for the first time and clear differences can be seen between these two sibling species, formerly considered a single species. Estimates for the distributions of An. arabiensis, An. melas and An. merus (also within the Gambiae complex) are provided based on improved methods and updated data, resulting in notably better model performance than seen with a previous mapping study [6]. The geographical distribution of An. arabiensis has also been modelled in recent years by an independent group [21]. Their aim was to extrapolate into the future when environmental conditions not currently in existence may occur so they selected a low bias bootstrap aggregation for one class data (LOBAG-OC) model. The data output by their model were not released so a quantitative comparison is not possible but a visual comparison shows broader habitat suitability in the earlier modelled map compared to the current study. The AUC value generated by that study was marginally lower than the value generated here (0.77 compared to 0.78, although caution is needed because the data used to generate these values differed), the data volumes were much lower, and the data for each environmental variable used in the earlier model was a single average over a long time period (1950–2000), meaning the current map is based on a more robust approach.
Although the full geographical distributions of An. coluzzii and An. gambiae have not been modelled previously, a recent study modelled the probability of An. coluzzii presence relative to the probability of An. gambiae presence in their sympatric range [22]. It is difficult to compare (1) an analysis of the relative occurrence of two species with (2) two independent species maps, but the results presented here are consistent with the predictions made by the earlier study. Both show An. coluzzii extending further north and closer to the coast than An. gambiae within west Africa. An interesting extension of the current work would be to characterize the locations where both species are sympatric while maintaining reproductive isolation.
A modelled distribution for An. funestus (distinct from the subgroup) has also been generated here for the first time. Only range maps were previously available for this species but the geographical distribution of the Funestus subgroup has been modelled previously [6]. The geographical distribution for the Funestus subgroup generated by the current study, using the same methods as the current An. funestus map, showed better model performance than the earlier work.
It is well established that Anopheles species are strongly influenced by temperature and humidity or wetness [23–25], and previous studies have found differences in the relationships between these variables and individual sibling species [26–32]. The study presented here used a model that provides strong predictive power to generate robust species distributions but it cannot elucidate relationships with individual environmental variables. It was clear, however, that the variables with the strongest influence on each model were wetness and temperature or factors strongly correlated with these two variables, namely elevation and a vegetation index, and the exact set of top predictors varied among each sibling species. Relationships between species and vegetation type have also been found by previous studies [33, 34] and pollution is known to play a role [22]. Fourteen land classes were included in this work and some of these were important predictors as was human population density, which is linked to pollution, but caution is needed when interpreting the ranking of covariate influence by the model. The modelling framework used here is not an appropriate tool to confirm the specific relationships with environmental variables found by detailed field studies and the maps also need to be viewed in the context of the scale used. The aim here was to produce continent-wide maps at a 5 × 5 km resolution and the land cover data used by the model were expressed as the proportion of square kilometres within each 5 × 5 km assigned to a particular land cover type. All other environmental and socioeconomic variables were provided to the model at 5 × 5 km resolution. This approach does not capture microscale variation that may be important to these species locally. At the other end of the spectrum, however, for vector and malaria control plans devised at a national or subnational level, the data from the maps presented here can be aggregated to provide information for the areas used for planning.
The temporal resolution of the data used to generate these maps was annual and thus seasonal variation is not captured here and the maps presented provide the relative probability of a species occurring at each location during at least one time of the year. Strong seasonal fluctuations in mosquito abundance occur, particularly in West Africa, and these differ among species [35–38]. If the dataset used here incorporated a systematic bias towards collection times that would miss particular species then this could impact the maps generated, however, the collated data provide good coverage for each species within areas with strong seasonal patterns as well as regions with smaller fluctuations (Additional file 3).
The geographical distribution of a species is not sufficient information alone to inform vector control programmes and these distributions need to be used in combination with data on the key attributes of each species. Use of indoor insecticide-based control measures has resulted in important reductions in vector populations and malaria prevalence [39, 40] but at the same time the relative abundance of individual malaria vectors has changed [41–44] leading to greater importance placed on mosquitoes that bite or rest outdoors or have less restricted feeding preferences [8, 45, 46]. Also critically important is resistance to the major insecticide classes and data is essential to provide evidence for insecticide resistance management planning [47]. Changes in the prevalence of insecticide resistance over time and differences among sibling species are apparent in the dataset presented here but a full analysis of this data must take account of the strong spatial bias in sampled locations and potential confounding factors such as variation in the protocols used. The one exception is susceptibility to organophosphates in An. funestus, which has remained constant over time at all locations, in agreement with earlier reviews [48]. It is clear that there are far fewer data available for individual species than for complexes [49]. There is, however, sufficient data available for the most important vectors species to allow variation in resistance at the species level to be considered and combined with species distributions.
Conclusions
Sibling species within the Gambiae complex display important differences in their geographical distributions and the same appears to be true for prevalence of insecticide resistance. The most important malaria vector sibling species in Africa have been mapped here for the first time and the evidence for insecticide resistance in these species has been summarised. The species-specific distributions can now be coupled with data on insecticide resistance, behaviour and vector status to make better informed decisions on vector control policy.
Authors’ contributions
CLM designed the study. AW performed the experiments with support from JL, FS and CLM. KG compiled the insecticide resistance data overview. AW, KG and NCM processed and geopositioned the data with input from CLM, MES and MC. All authors read and approved the final manuscript.
Acknowledgements
The authors are extremely grateful to the following people who contributed unpublished datasets: Kounbobr Roch Dabiré, Geraldine Foster, Christophe Antonio Nkondjio, Hilary Ranson, Jacob Riveron and Charles Wondji. We are also very grateful to all of the many authors who provided additional information linked to their published work and who are cited in Additional file 2.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data used to generate the maps presented here is provided in Additional file 2. The model output data is provided per species in Additional files 6, 7, 8, 9, 10, 11, 12, 13, 14. The model code is available from the GitHub repository [50].
Funding
This work was funded by a Wellcome Trust grant (108440/Z/15/Z) awarded to CLM, PWG, MC and JH. The funder had no role in the design of the study or collection, analysis or interpretation of the data, or in the writing of the manuscript.
Additional files
Additional file 1. Environmental covariates used in the model.
Additional file 2. Presence and background datasets for each species. Each dataset is provided in a separate worksheet within the workbook.
Additional file 3. Maps showing the spatial distribution of the data that went into the model.
Additional file 4. Additional maps for the Gambiae complex, Funestus subgroup, and Funestus group.
Additional file 5. Tables of AUC values and top predictors, and maps showing the prediction ranges.
Additional file 6. Geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for An. arabiensis. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 7. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for An. coluzzii. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 8. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for An. funestus. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 9. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for An. gambiae. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 10. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for An. melas. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 11. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for An. merus. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 12. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for the Gambiae complex. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 13. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for the Funestus subgroup. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 14. geotiff that can be opened in GIS software such as QGIS (http://www.qgis.org/) or ArcMap (http://www.esri.com/software/arcgis). Model output data for the Funestus group. Band 1 contains the mean values per pixel, band 2 contains the median values, band 3 contains the 2.5% quantile values, and band 4 contains the 97.5% quantile values.
Additional file 15. Tables of summary insecticide resistance data. A separate worksheet is provided for each insecticide class for each of the Gambiae complex and the Funestus group.
Contributor Information
Antoinette Wiebe, Email: antoinette.wiebe@gmail.com.
Joshua Longbottom, Email: joshua.longbottom@gmail.com.
Katherine Gleave, Email: katherine.gleave@lstmed.ac.uk.
Freya M. Shearer, Email: freya.m.shearer@gmail.com
Marianne E. Sinka, Email: marianne.sinka@gmail.com
N. Claire Massey, Email: claire.massey@zoo.ox.ac.uk.
Ewan Cameron, Email: dr.ewan.cameron@gmail.com.
Samir Bhatt, Email: bhattsamir@gmail.com.
Peter W. Gething, Email: peter.gething@well.ox.ac.uk
Janet Hemingway, Email: janet.hemingway@lstmed.ac.uk.
David L. Smith, Email: smitdave@uw.edu
Michael Coleman, Email: michael.coleman@lstmed.ac.uk.
Catherine L. Moyes, Email: catherinemoyes@gmail.com
References
- 1.Battle KE, Gething PW, Elyazar IRF, Moyes CL, Sinka ME, Howes RE, et al. The global public health significance of Plasmodium vivax. Adv Parasitol. 2012;80:1–111. doi: 10.1016/B978-0-12-397900-1.00001-3. [DOI] [PubMed] [Google Scholar]
- 2.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 4.Kipyab PC, Khaemba BM, Mwangangi JM, Mbogo CM. The bionomics of Anopheles merus (Diptera: Culicidae) along the Kenyan coast. Parasit Vectors. 2013;6:37. doi: 10.1186/1756-3305-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebenezer A, Noutcha AEM, Okiwelu SN. Relationship of annual entomological inoculation rates to malaria transmission indices, Bayelsa State, Nigeria. J Vector Borne Dis. 2016;53:46–53. [PubMed] [Google Scholar]
- 6.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–1788. doi: 10.1016/S0140-6736(15)00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massey NC, Garrod G, Wiebe A, Henry AJ, Huang Z, Moyes CL, et al. A global bionomic database for the dominant vectors of human malaria. Sci Data. 2016;3:160014. doi: 10.1038/sdata.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyes CL, Temperley WH, Henry AJ, Burgert CR, Hay SI. Providing open access data online to advance malaria research and control. Malar J. 2013;12:161. doi: 10.1186/1475-2875-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips SJ, Dudik M, Elith J, Graham CH, Lehmann A, Leathwick J, et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 11.United Nations Environmental Programme. Biomes of Africa. In Africa Atlas of Our Changing Environment. Johannesburg; 2008.
- 12.Dia I, Guelbeogo MW, Ayala D. Advances and perspectives in the study of the malaria mosquito Anopheles funestus. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. Rijeka: In Tech Publisher; 2013. p. 197–220.
- 13.della Torre A, Tu ZJ, Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem Mol Biol. 2005;35:755–769. doi: 10.1016/j.ibmb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Weiss DJ, Mappin B, Dalrymple U, Bhatt S, Cameron E, Hay SI, et al. Re-examining environmental correlates of Plasmodium falciparum malaria endemicity: a data-intensive variable selection approach. Malar J. 2015;14:68. doi: 10.1186/s12936-015-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De’ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88:243–251. doi: 10.1890/0012-9658(2007)88[243:BTFEMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- 17.Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- 18.Moyes CL, Shearer FM, Huang Z, Wiebe A, Gibson HS, Nijman V, et al. Predicting the geographical distributions of the macaque hosts and mosquito vectors of Plasmodium knowlesi malaria in forested and non-forested areas. Parasit Vectors. 2016;9:242. doi: 10.1186/s13071-016-1527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearer FM, Huang Z, Weiss DJ, Wiebe A, Gibson HS, Battle KE, et al. Estimating geographical variation in the risk of zoonotic Plasmodium knowlesi infection in countries eliminating malaria. PLoS Negl Trop Dis. 2016;10:e0004915. doi: 10.1371/journal.pntd.0004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleiss J, Levin B, Paik M. Statistical methods for rates and proportions. Hoboken: Wiley; 2003. [Google Scholar]
- 21.Drake JM, Beier JC. Ecological niche and potential distribution of Anopheles arabiensis in Africa in 2050. Malar J. 2014;13:213. doi: 10.1186/1475-2875-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fossog BT, Ayala D, Acevedo P, Kengne P, Mebuy INA, Makanga B, et al. Habitat segregation and ecological character displacement in cryptic African malaria mosquitoes. Evol Appl. 2015;8:326–345. doi: 10.1111/eva.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamou A, Dao A, Timbine S, Kassogue Y, Yaro AS, Diallo M, et al. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar J. 2011;10:151. doi: 10.1186/1475-2875-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Meneses CR, Fofana A, Lanzaro GC. Desiccation resistance among subpopulations of Anopheles gambiae s.s. from Selinkenyi, Mali. J Med Entomol. 2009;46:316–320. doi: 10.1603/033.046.0216. [DOI] [PubMed] [Google Scholar]
- 25.Lyons CL, Coetzee M, Chown SL. Stable and fluctuating temperature effects on the development rate and survival of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Parasit Vectors. 2013;6:104. doi: 10.1186/1756-3305-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Souza D, Kelly-Hope L, Lawson B, Wilson M, Boakye D. Environmental factors associated with the distribution of Anopheles gambiae s.s in Ghana; an important vector of lymphatic filariasis and malaria. PLoS ONE. 2010;5:e9927. doi: 10.1371/journal.pone.0009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huestis DL, Yaro AS, Traore AI, Adamou A, Kassogue Y, Diallo M, et al. Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J Exp Biol. 2011;214:2345–2353. doi: 10.1242/jeb.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly-Hope LA, Hemingway J, McKenzie FE. Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malar J. 2009;8:268. doi: 10.1186/1475-2875-8-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipyab PC, Khaemba BM, Mwangangi JM, Mbogo CM. The physicochemical and environmental factors affecting the distribution of Anopheles merus along the Kenyan coast. Parasit Vectors. 2015;8:221. doi: 10.1186/s13071-015-0819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby MJ, Lindsay SW. Effect of temperature and inter-specific competition on the development and survival of Anopheles gambiae sensu stricto and An. arabiensis larvae. Acta Trop. 2009;109:118–123. doi: 10.1016/j.actatropica.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Lyons CL, Coetzee M, Terblanche JS, Chown SL. Thermal limits of wild and laboratory strains of two African malaria vector species, Anopheles arabiensis and Anopheles funestus. Malar J. 2012;11:226. doi: 10.1186/1475-2875-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paaijmans KP, Huijben S, Githeko AK, Takken W. Competitive interactions between larvae of the malaria mosquitoes Anopheles arabiensis and Anopheles gambiae under semi-field conditions in western Kenya. Acta Trop. 2009;109:124–130. doi: 10.1016/j.actatropica.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in western Kenya highlands: effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77:660–666. [PubMed] [Google Scholar]
- 34.Minakawa N, Dida GO, Sonye GO, Futami K, Njenga SM. Malaria vectors in Lake Victoria and adjacent habitats in Western Kenya. PLoS ONE. 2012;7:e32725. doi: 10.1371/journal.pone.0032725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dery DB, Brown C, Asante KP, Adams M, Dosoo D, Amenga-Etego S, et al. Patterns and seasonality of malaria transmission in the forest-savannah transitional zones of Ghana. Malar J. 2010;9:314. doi: 10.1186/1475-2875-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guelbeogo WM, Sagnon N, Grushko O, Yameogo MA, Boccolini D, Besansky NJ, et al. Seasonal distribution of Anopheles funestus chromosomal forms from Burkina Faso. Malar J. 2009;8:239. doi: 10.1186/1475-2875-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simard F, Lehmann T, Lemasson JJ, Diatta M, Fontenille D. Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Molec Biol. 2000;9:467–479. doi: 10.1046/j.1365-2583.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Athrey G, Hodges TK, Reddy MR, Overgaard HJ, Matias A, Ridl FC, et al. The effective population size of malaria mosquitoes: large impact of vector control. PLoS Genet. 2012;8:e1003097. doi: 10.1371/journal.pgen.1003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crook SE, Baptista A. The effect of permethrin-impregnated wall-curtains on malaria transmission and morbidity in the suburbs of Maputo, Mozambique. Trop Geogr Med. 1995;47:64–67. [PubMed] [Google Scholar]
- 42.Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, Phillips-Howard PA, et al. Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68:16–22. [PubMed] [Google Scholar]
- 43.Govella NJ, Chaki PP, Killeen GF. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar J. 2013;12:124. doi: 10.1186/1475-2875-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinka ME, Golding N, Massey NC, Wiebe A, Huang Z, Hay SI, et al. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar J. 2016;15:142. doi: 10.1186/s12936-016-1187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Killeen GF, Kiware SS, Okumu FO, Sinka ME, Moyes CL, Massey NC, et al. Going beyond personal protection against mosquito bites to eliminate malaria transmission: population suppression of malaria vectors that exploit both human and animal blood. BMJ Glob Health. http://archive.lstmed.ac.uk/6378/ (in press). [DOI] [PMC free article] [PubMed]
- 46.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chanda E, Thomsen EK, Musapa M, Kamuliwo M, Brogdon WG, Norris DE, et al. An operational framework for insecticide resistance management planning. Emerg Infect Dis. 2016;22:773–779. doi: 10.3201/eid2205.150984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coetzee M, Koekemoer LL. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. An Rev Entomol. 2013;58:393–412. doi: 10.1146/annurev-ento-120811-153628. [DOI] [PubMed] [Google Scholar]
- 49.Coleman M, Hemingway J, Gleave K, Wiebe A, Gething PW, Moyes CL. Developing global maps of insecticide resistance risk to improve vector control. Malar J. doi:10.1186/s12936-017-1733-z. [DOI] [PMC free article] [PubMed]
- 50.SEEG-Oxford SDM code at the GitHub repository. https://github.com/SEEG-Oxford/seegSDM.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to generate the maps presented here is provided in Additional file 2. The model output data is provided per species in Additional files 6, 7, 8, 9, 10, 11, 12, 13, 14. The model code is available from the GitHub repository [50].


