Abstract
Metformin is widely used for the treatment of type II diabetes mellitus. It was reported to be substrate of OCT3/Oct3, which is expressed in the brush boarder membrane of the enterocytes. However, the role of OCT3/Oct3 in the intestinal absorption process of metformin remains obscure. In the present study, we aimed to clarify the impact of Oct3 on the oral bioavailability and pharmacokinetics of metformin in mice, by means of in vivo pharmacokinetic study using wild-type (Oct3+/+) and Oct3-knockout (Oct3−/−) mice. When metformin (8.0 mg/kg) was intravenously administered to male Oct3+/+ and Oct3−/− mice, AUC0–∞ of metformin was evaluated to be 659 ± 133 µg h/mL and 734 ± 213 µg h/mL, respectively. In the case of orally administered metformin (15 mg/kg), AUC0–∞ was 578 ± 158 µg h/mL and 449 ± 101 µg h/mL in Oct3+/+ and Oct3−/− mice, respectively. Based on these pharmacokinetic parameters, absolute bioavailability (F) of metformin in Oct3+/+ mice was evaluated as 46.8%, and it was significantly decreased to 32.6% in Oct3−/− mice. Taking into account the fact that metformin undergoes negligible metabolism, these results imply that intestinal absorption of metformin is mediated at least in part by Oct3 in mice.
Keywords: Metformin, OCT3, Transporter, Intestinal absorption, Bioavailability, Mouse
1. Introduction
Metformin is widely used for the treatment of hyperglycemia in patients with type II diabetes mellitus. It undergoes neither hepatic metabolism nor biliary excretion and over 90% of the absorbed metformin is eliminated unchanged in the urine. Therefore, the fraction absorbed of metformin is considered to be very close to its bioavailability of 50–60% in human. This relatively high absorption rate of metformin, in spite of its high hydrophilicity (log P value of −1.43), is likely to be due to the involvement of transporter-mediated processes in intestinal absorption [1].
Metformin has been well characterized in vitro as a substrate of organic cation transporters (OCTs) in the SLC22A family. OCTs play an important role in cellular uptake and accumulation of metformin in various organs [2]. In the intestine, OCT3 and OCT1 have been reported to be localized in the apical and basolateral membrane of enterocytes, respectively [3–6]. However, Han et al. also reported that OCT1/Oct1 is apically expressed in Caco-2 cells and human and mouse intestines [7]. Therefore, the localization of OCT1 in the intestinal epithelium still remains inconclusive. On the other hand, OCT3 is expressed unequivocally in the apical membrane of enterocytes and mediates the uptake of organic cations from the lumen into the enterocytes [5,6]. These findings indicate the possibility that OCT/Oct transporters contribute to intestinal absorption of metformin. However, the roles of OCTs in intestinal transport and oral absorption of metformin in vivo remains to be elucidated.
In the present study, we focused on mouse Oct3 and aimed to evaluate the impact of OCT3/Oct3 transporter on intestinal absorption of metformin in vivo. Here, we show, by means of in vivo pharmacokinetic study using Oct3-knockout (Oct3−/−) mice, that Oct3 contributes to the oral bioavailability and pharmacokinetics of metformin in vivo.
2. Materials and methods
2.1. Materials
[14C]Metformin hydrochloride was purchased from Moravek Biochemicals, Inc. (Brea, CA). 1,1-Dimethylbiguanide hydrochloride (metformin hydrochloride) was purchased from Sigma–Aldrich (St. Louis, MO). All other chemicals and general reagents were of analytical grade or better and were obtained from various commercial sources such as Invitrogen (Carlsbad, CA) or Applied Biosystems (Foster City, CA).
2.2. Animals
The Oct3 (Slc22a3) null mice of the FVB inbred strain were originally developed by Dr. Denise Barlow [8] and maintained by Dr. Alfred Schinkel (Netherlands Cancer Institute). After rederivation at Charles River Laboratories (16), breeding pairs of wild-type (Oct3+/+) and Oct3-knockout (Oct3−/−) mice were kindly provided to us by Dr. John Markowitz (University of Florida) with approval from Dr. Schinkel. These mice were housed in the specific pathogen-free facility at the University of Washington (Seattle, WA). Male Oct3+/+ and Oct3−/− mice (10–12 weeks old) were housed five per cage with free access to commercial chow and tap water, and were maintained on a 12-h light/dark cycles in an air-controlled room. All animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Research Council. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee at the University of Washington.
2.3. In vivo pharmacokinetic study
Mice were randomly divided into two groups: an intravenous metformin group (8 mg/kg; 4 mL/kg metformin dissolved in saline) and an oral metformin group (15 mg/kg; 10 mL/kg metformin dissolved in saline). For pharmacokinetic study of intravenous metformin, male Oct3+/+ and Oct3−/− mice (10–12 weeks old) were fasted for ~10 h. Under anesthesia (2–5% isoflurane), mice were administered 8 mg/kg metformin containing 0.1 mCi/kg [14C] metformin by retro-orbital injection. At various time points (0–480 min), mice (n = 6–9 mice at each time point) were anesthetized, followed by the submandibular puncture (as submandibular bleeding method). Blood was collected using a heparinized micro blood collecting tube (Thermo Fisher Scientific Inc, Waltham, MA) and centrifuged at 5000 × g for 10 min at 4 °C. Plasma was collected and stored at −80 °C until further analysis.
The pharmacokinetic study of orally administered metformin in Oct3+/+ and Oct3−/− mice has been previously described [2]. The plasma concentration data obtained from this study were used to obtain oral pharmacokinetic parameters in the present study. Mice were administered 15 mg/kg metformin containing 0.2 mCi/kg [14C]metformin by oral gavage. Plasma metformin concentrations were determined by liquid scintillation counting. Plasma concentration of metformin was expressed as ng/mL.
2.4. Data analysis
Plasma concentration–time curves of metformin were plotted and analyzed. Because metformin concentrations in plasma were sampled in different animals at each time point (1–3 points sampling), the population pharmacokinetics and Bayesian estimation was used to calculate the mean and standard deviation of the area under the concentration–time curves (AUCs) and other pharmacokinetic parameters, using the numerical analysis program for pharmacokinetics NAPP (Version 2.3) [9]. The extent of absolute oral bioavailability (F) expressed as a percentage was directly calculated by dividing the oral AUC by the intravenous AUC normalized to dose:
| (1) |
where AUCIV and AUCoral are the area under the plasma concentration-versus-time curve after intravenous and oral administrations (ng · h/mL), respectively. Doseoral and DoseIV represent oral and intravenous dosage amounts, respectively.
3. Results and discussion
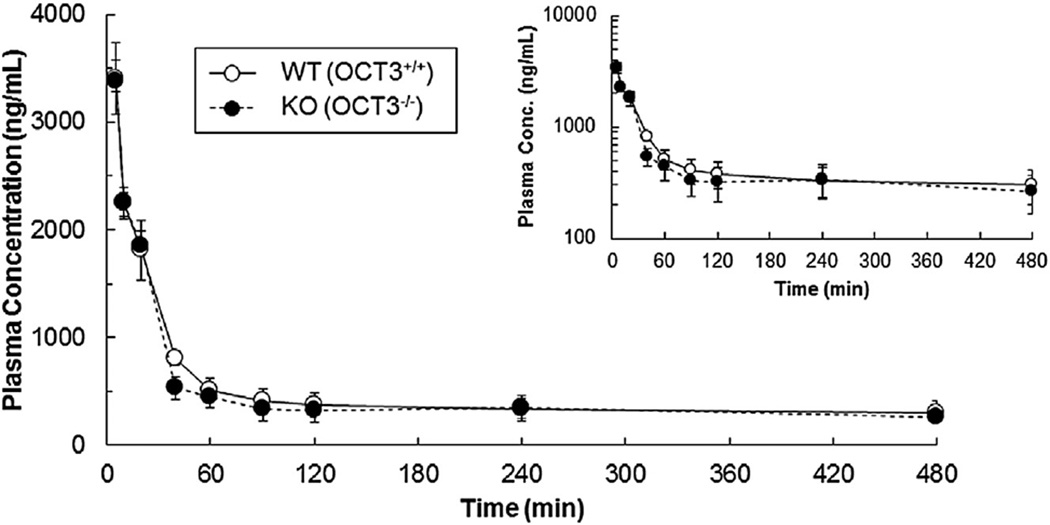
The impact of Oct3 on the oral bioavailability and pharmacokinetics of metformin in mice was assessed by means of in vivo pharmacokinetic study using Oct3−/− mice. When metformin (8.0 mg/kg) was intravenously administered to male Oct3+/+ and Oct3−/− mice, AUC0–∞ of metformin was evaluated to be 659 ± 133 µg h/mL and 734 ± 213 µg h/mL, respectively (Fig. 1 and Table 1). After oral administration (15 mg/kg), the AUC0–∞ of metformin was 578 ± 158 µg h/mL and 449 ± 101 µg h/mL in Oct3+/+ and Oct3−/− mice, respectively. Based on these pharmacokinetic parameters, absolute bioavailability (F) of metformin in Oct3+/+ mice was estimated as 46.8%, and it was markedly decreased to 32.6% in Oct3−/− mice (Table 1). Taking into account the fact that metformin undergoes negligible metabolism (hepatic or nonhepatic), the result implies that intestinal absorption of metformin is mediated at least in part by Oct3 in mice
Fig. 1.
Mean plasma concentration–time profiles of metformin in male wild-type (Oct3+/+) and Oct3-knockout (Oct3−/−) mice after intravenous administration. Metformin (8 mg/kg containing 0.1 mCi/kg [14C] was intravenously administered by retro-orbital injection in male Oct3+/+ (open circles) and Oct3−/− mice (filled circles). Data are shown as the mean ± SEM (n = six to nine mice at each time point). A total of 26 Oct3+/+ and 26 Oct3−/− male mice were used for the pharmacokinetic studies.
Table 1.
Pharmacokinetic parameters of metformin in male wild-type (Oct3+/+) and Oct3-knockout (Oct3−/−) mice after intravenous and oral administration.
| Pharmacokinetic parametersa | Oct3+/+ mice | Oct3−/− mice | |||
|---|---|---|---|---|---|
| IVb | Oralc | IVb | Oralc | ||
| Dose | (mg/kg) | 8.0 | 15 | 8.0 | 15 |
| Cmax | (µg/mL) | – | 2.71 ± 0.72 | – | 2.97 ± 0.66 |
| tmax | (min) | – | 19.2 ± 0.1 | – | 25.7 ± 8.4* |
| AUC0–∞ | (µg min/mL) | 659 ± 133 | 578 ± 158 | 734 ± 213* | 449 ± 101* |
| t1/2 | (min) | 1085 ± 916 | 135 ± 2 | 1860 ± 1197* | 85.6 ± 3.9* |
| MRTtot | (min) | 1372 ± 1125 | 199 ± 3 | 2406 ± 1504* | 133 ± 8.4* |
| CLtot | (mL/min/kg) | 12.7 ± 2.7 | – | 11.8 ± 3.4 | – |
| CLtot/F | (mL/min/kg) | – | 27.8 ± 7.3 | – | 35.0 ± 7.7* |
| Vd | (L/kg) | 1.74 ± 0.28 | – | 1.86 ± 0.12* | – |
| Vd/F | (L/kg) | – | 5.40 ± 1.38 | – | 4.30 ± 0.46* |
| F | (%) | 46.8 | 32.6 | ||
Metformin was intravenously (8 mg/kg) and orally (15 mg/kg) administered to male Oct3+/+ and Oct3−/− mice.
P < 0.05, significantly different from values in Oct3+/+ mice. Data are shown as means ± SD (n = three to six mice at each time point).
Definitions of disposition parameters: AUC0–∞, area under plasma concentration–time curve from 0 to infinity; Cmax, peak plasma drug concentration; tmax, time to reach maximum plasma concentration; CLtot, total clearance; F, oral bioavailability; Vd, volume of distribution; t1/2, elimination half-life.
Pharmacokinetic parameters of metformin after a single intravenous dose were obtained from Fig. 1.
Pharmacokinetic parameters of metformin after a single oral dose were derived using data from Lee et al. [2].
The lower bioavailability of metformin in Oct3−/− mice is considered to be mainly due to a reduction of oral AUC0–∞ caused by a lack of Oct3-mediated absorption process of metformin (Table 1). In addition, it may also result from an increase in AUC0–∞ of metformin in Oct3−/− mice after intravenous administration (Table 1). The higher AUC0–∞ of metformin in Oct3−/− mice may be presumably due to a reduced distribution of metformin to peripheral tissues in Oct3−/− mice. This consideration is supported by previous report demonstrating that overall metformin exposures in salivary gland, skeletal muscle and heart were significantly reduced in Oct3−/− mice compared to Oct3+/+ mice [2]. However, Vd of metformin showed unexpected significant increase in Oct3−/− mice compared to Oct3+/+ mice (1.74 ± 0.28 L/kg vs. 1.86 ± 0.12 L/kg), while Vd/F was significantly lower in Oct3−/− mice than in Oct3+/+ mice (5.40 ± 1.38 L/kg vs. 4.30 ± 0.46 L/kg) (Table 1). The apparently inconsistent effects of Oct3 on the distribution volume of metformin between after intravenous (Vd) and oral (Vd/F) administrations may be explained by a complicated intestinal accumulation of metformin due to apical expression of Oct3 in enterocytes. Assuming that after intravenous administration intestinal Oct3 is mainly involved in efflux transport of metformin from enterocyte to intestinal lumen, absence of Oct3 may lead to higher cellular accumulation of metformin, resulting in an apparent enhancement of metformin distribution to intestine in Oct3−/− mice. In contrast, taking into consideration the finding that metformin absorption may be mediated by Oct3, absence of Oct3 may cause lower intracellular concentration of metformin in the intestine after oral administration, resulting in apparent reduction of metformin distribution to intestine in Oct3−/− mice. In order to prove the validity of these hypotheses, further studies with Oct3−/− mice are needed.
As shown in Table 1, CLtot of metformin after intravenous administration is almost comparable between Oct3+/+ and Oct3−/− mice. Therefore, it is reasonable to consider that Oct3 deletion does not affect metformin clearance. Although Oct3 is reportedly expressed in the liver, it is considered that Oct3 does not contribute to hepatic clearance because metformin undergoes neither hepatic metabolism nor biliary excretion [2]. Meanwhile, recent report also showed slight expression of Oct3 in the kidney, which is mainly responsible for metformin elimination [2]. Therefore, the possibility that renal Oct3 contributes to metformin clearance cannot be ruled out. The membrane localization of Oct3 in kidney cells has not been determined. If Oct3 is involved in apical secretion of metformin in kidney, Oct3 deficiency may not apparently change CLtot of metformin, because the distribution volume as well as t1/2 would both be increased. Although we have little information about the Oct3-mediated excretion and the distribution volume of metformin in the kidney, this consideration appears to be consistent with our findings that MRTtot value after intravenous administration of metformin was significantly higher for the Oct3−/− mice when compared with the Oct3+/+ mice, which may imply a prolonged elimination of metformin due to absence of Oct3 (Table 1).
On the other hand, MRTtot value of metformin in the Oct3−/− mice after oral administration was significantly lower than that in the Oct3+/+ mice Table 1). However, since a large difference in the t1/2 of metformin was observed between intravenous and oral administrations, it is hard to obtain MAT or ka values and thereby impossible to discuss directly the differences in absorption phase between Oct3+/+ and Oct3−/− mice Table 1). At present, the effect of the administration route on elimination half-life has not been clarified yet. However, this apparently paradoxical observation may be explained by the complex and long terminal phase due to a multi-compartment that metformin enters and leaves slowly [1]. Initially, the plasma concentrations of metformin decrease rapidly after intravenous administration, and therefore it may be possible to view terminal phase within 480 min, resulting in a meaningful elimination half-life (Fig.1). In contrast, when metformin was orally administered to mice, existence of an absorption phase might further delay the terminal phase, implying that 480 min was not long enough to quote a true terminal t1/2 of metformin [2]. In addition, the oral pharmacokinetics of metformin has been suggested to be flip-flop kinetics due to very slow intestinal absorption. These considerations simply mean that pharmacokinetic study with orally administered metformin may require a longer time course of metformin plasma concentrations to quote a terminal t1/2. Recent report showed that the terminal t1/2 determined from the rate of metformin excretion in urine in humans is much longer than from plasma and ranges from about 9 to 19 h [1,10]. Furthermore, a terminal t1/2 of about 20 h is supported from the determination of the plasma t1/2 following cessation of multiple dosage regimens of metformin [1,11]. It is also reported that metformin is taken up by erythrocytes from which the t1/2 of loss is about 20 h [1,10,12]. To clarify reliable terminal half-life of metformin, further investigation on multi-compartments that metformin enters and leaves slowly should be investigated.
Although the present study strongly suggested that OCT3/Oct3 contribute to the intestinal absorption of metformin, we cannot rule out the possibility that some other influx and/or efflux transporter(s) also plays a role in the intestinal absorption of metformin. Previously metformin was clarified to be a substrate of OCT1, carnitine/organic cation transporter 1 (OCTN1), multidrug and toxin extrusion protein 1 (MATE1) and the plasma membrane monoamine transporter (PMAT), which are all expressed in the small intestine [2,3,7,13,14]. Except one study [7], OCT1 was reported by several other studies to be localized at the basolateral membrane of enterocytes [3–6]. Pharmacokinetic study in Oct1−/− mice revealed that distribution of metformin after intravenous administration to the small intestine in Oct1−/− mice was significantly lower than that in Oct1+/+ mice, suggesting that Oct1 has a possible role in the basolateral transport of metformin [3]. It is possible that apical Oct3 may work together with basolateral Oct1 to mediate transepithelial transport of metformin in the intestine. Further investigation would be needed to examine the cooperative role of OCT1/Oct1 and OCT3/Oct3 in intestinal absorption process of metformin. In addition, OCTN1 and PMAT were reported to be expressed at apical membranes and may be presumably involved in the apical transport of metformin in intestine [13,14]. Although the localization of MATE1 in the small intestine has not been fully clarified yet, intestinal expression of MATE1 may contribute to the intestinal absorption of a variety of drugs including metformin. The existence of these transporters susceptible to metformin may result in a complex multiple transport mechanisms in the intestinal absorption and disposition of metformin. Therefore, to examine the contribution of these transporters to intestinal absorption of metformin, further studies would be needed.
In conclusion, the present study has clearly demonstrated a remarkable influence of Oct3 on the oral bioavailability and pharmacokinetics of metformin in mice. Although contributions of other influx/efflux transporters to metformin absorption cannot be ruled out, our findings support that Oct3 is one of the major determinants of the intestinal absorption processes of metformin.
Acknowledgments
This work was supported in part by Grant from NIH [R01 GM066233], UW DMTPR funding, and Grants for the Uehara Memorial Foundation Research Fellowship [201340221] and for Basic Science Research Projects from the Sumitomo Foundation 150556].
Abbreviations
- AUC
area under the plasma concentration–time curve
- CL
clearance
- Cmax
maximum concentration
- OCT/Oct
organic cation transporter
- SLC
solute carrier
- tmax
time to maximum concentration
Footnotes
Conflict of interest
The authors have declared no conflict of interest.
Authorship contributions
Participated in research design: Shirasaka, Wang.
Conducted experiments: Shirasaka, Lee, Zha.
Performed data analysis: Shirasaka, Wagner, Wang.
Wrote or contributed to the writing of the manuscript: Shirasaka, Wang.
References
- 1.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Lee N, Duan H, Hebert MF, Liang CJ, Rice KM, Wang J. Taste of a pill: organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. J Biol Chem. 2014;289:27055–27064. doi: 10.1074/jbc.M114.570564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Sawano T, Endo T, Sakata M, Sato J. Studies on intestinal absorption of sulpiride (2): transepithelial transport of sulpiride across the human intestinal cell line Caco-2. Biol Pharm Bull. 2002;25:1345–1350. doi: 10.1248/bpb.25.1345. [DOI] [PubMed] [Google Scholar]
- 5.Müller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 7.Han TK, Everett RS, Proctor WR, Ng CM, Costales CL, Brouwer KL, et al. Organic cation transporter 1 (OCT1/mOct1) is localized in the apical membrane of Caco-2 cell monolayers and enterocytes. Mol Pharmacol. 2013;84:182–189. doi: 10.1124/mol.112.084517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisaka A, Sugiyama Y. Analysis of nonlinear and nonsteady state hepatic extraction with the dispersion model using the finite difference method. J Pharmacokinet Biopharm. 1998;26:495–519. doi: 10.1023/a:1023294632129. [DOI] [PubMed] [Google Scholar]
- 10.Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambol NC, Chiang J, O'Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–1021. doi: 10.1177/009127009603601105. [DOI] [PubMed] [Google Scholar]
- 12.Robert F, Fendri S, Hary L, Lacroix C, Andréjak M, Lalau JD. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29:279–283. doi: 10.1016/s1262-3636(07)70037-x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamichi N, Shima H, Asano S, Ishimoto T, Sugiura T, Matsubara K, et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J Pharm Sci. 2013;102:3407–3417. doi: 10.1002/jps.23595. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35:1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]