Abstract
Hepatotoxicity is a serious problem during drug development and for the use of many established drugs. For example, acetaminophen overdose is currently the most frequent cause of acute liver failure in the United States and Great Britain. Evaluation of the mechanisms of drug-induced liver injury indicates that mitochondria are critical targets for drug toxicity, either directly or indirectly through formation of reactive metabolites. The consequence of these modifications is generally a mitochondrial oxidant stress and peroxynitrite formation, which leads to structural alterations of proteins and mitochondrial DNA and eventually to the opening of mitochondrial membrane permeability transition (MPT) pores. MPT pore formation results in collapse of the mitochondrial membrane potential and cessation of ATP synthesis. In addition, the release of intermembrane proteins such as apoptosis-inducing factor and endonuclease G and their translocation to the nucleus leads to nuclear DNA fragmentation. Together these events trigger necrotic cell death. Alternatively, release of cytochrome c and other pro-apoptotic factors from mitochondria can promote caspase activation and apoptotic cell death. Drug toxicity can also induce an inflammatory response with formation of reactive oxygen species by Kupffer cells and neutrophils. If not properly detoxified, these extracellularly generated oxidants can diffuse into hepatocytes and trigger mitochondrial dysfunction and oxidant stress, which then induces the MPT and necrotic cell death. This review addresses the formation of oxidants and the defense mechanisms available for the cells and applies this knowledge to better understand mechanisms of drug hepatotoxicity, especially acetaminophen-induced liver injury.
Keywords: Acetaminophen, peroxynitrite, lipid peroxidation, hepatotoxicity, c-jun-N-terminal kinase, membrane permeability transition pore, cyclophilin D, neutrophils, Kupffer cells
INTRODUCTION
Formation of reactive oxygen species (ROS) is one of the most commonly invoked cell death mechanisms in all organ injury, including drug-induced liver damage. Despite the ubiquitous involvement of ROS in most pathophysiologies, no drugs are available that specifically target ROS. The reason for this discrepancy is likely the limited understanding of the specific role of ROS under most circumstances. In order to improve this, it is important to: (a) identify the specific ROS involved, (b) assess when and where they are generated, (c) what specific signaling effect or damage is caused by them and (d) how this impacts overall cell and organ survival. Importantly, this needs to be investigated in models relevant for animal and human pathophysiology. Drug hepatotoxicity is an ideal example of how progress can be made in the understanding of mechanisms of ROS toxicity when these objectives are kept in mind. The current review will attempt to provide a brief overview of ROS formation and defense mechanisms as a background to the in-depth discussion of the role of oxidant stress in acetaminophen (APAP) hepatotoxicity, currently the most frequent cause of drug-induced liver failure in humans (Larson et al., 2005) and the most studied hepatotoxic drug. The importance of intracellular (mitochondria) and extracellular (inflammatory cells) sources of ROS will be discussed. In addition, mitochondrial dysfunction and oxidant stress as mechanisms of toxicity will be evaluated for a number of lesser known examples of hepatotoxic drugs.
OXIDANT STRESS AND CELLULAR DEFENSE MECHANISMS
Sources of ROS formation
Single electron reductions of molecular oxygen results in formation of ROS including superoxide (+1e), hydrogen peroxide (+2e) and hydroxyl radicals (+3e) (Figure 1). Additional ROS can be generated by myeloperoxidase from hydrogen peroxide (hypochlorous acid) and by lipid peroxidation (LPO), which produces a number of fatty acid hydroperoxides and alkoxy radicals. A combination of the two radicals superoxide and nitric oxide results in the formation of peroxynitrite, a highly reactive nitrogen species (RNS). The most relevant sources of ROS in the cell are electron leaks from the mitochondrial electron transport chain (mainly superoxide) (Murphy, 2009), from the P450 system and its reductases (superoxide and hydrogen peroxide), and from oxidases within the cell, e.g. fatty acid oxidases in peroxisomes. Under pathophysiological conditions, additional oxidases, such as xanthine oxidase, can be generated and become a source of ROS. Phagocytes contain NADPH oxidase (NOX2), which releases superoxide into the phagosome or to the outside of the cell when the phagocyte is activated (Bedard and Krause, 2007). The concomitantly released myeloperoxidase then forms hypochlorous acid (Figure 1). Certain drugs or chemicals (diquat, paraquat) can be reduced by P450 reductase to form unstable radicals, which then transfer their electrons to molecular oxygen and form superoxide. This redox-cycling of the chemical results in the formation of large amounts of superoxide in the cell. Thus, there are a large number of ways to form various ROS and RNS in different locations within the cell or outside.
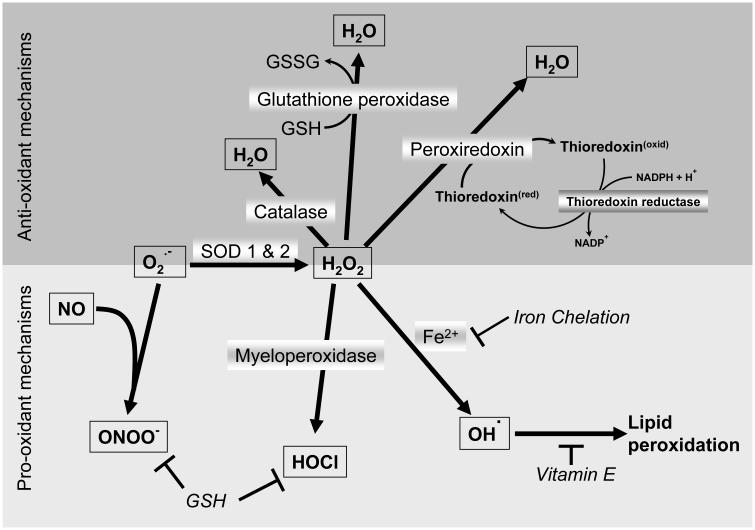
Figure 1. Cellular mechanisms of oxidant generation and scavenging.
Superoxide produced mainly from the mitochondrial electron transport chain can either react with nitric oxide to produce peroxynitrite or be converted to hydrogen peroxide by superoxide dismutases. The hydrogen peroxide thus formed can initiate lipid peroxidation by conversion to the hydroxyl radical or generate hypochlorous acid through the action of myeloperoxidase. Scavenging of hydrogen peroxide can occur through action of catalase, glutathione peroxidase or peroxyredoxin, which uses thioredoxin for its action. Glutathione helps as an anti-oxidant by functioning with glutathione peroxidase or directly scavenging species such as peroxynitrite and hypochlorous acid, while vitamin E interrupt the free radical chain reaction leading to lipid peroxidation.
Antioxidant defenses
Any ROS formation bears the risk of substantial damage to macromolecules within the cell including protein oxidation, lipid peroxidation and DNA/RNA damage (Kehrer, 1993). These changes can vary in degree from complete destruction and functional loss of the molecule to minor modifications (e.g. sulfhydryl oxidation). However, even minor modifications can have adverse functional consequences. Therefore, cells have developed a sophisticated, multi-layered defense system that is vital for cell survival in an oxygen environment (Figure 1) (Jaeschke, 2010). Superoxide can be removed by catalyzing the transfer of an electron from one superoxide molecule to another by Cu/Zn - superoxide dismutase (Cu/Zn-SOD, SOD1), which is located in the cytosol, or by Mn-SOD (SOD2) present in mitochondria. Although the SOD-catalyzed reaction also yields hydrogen peroxide and oxygen similar to the spontaneous dismutation reaction, the enzyme reaction is more efficient and is able to limit the formation of peroxynitrite and prevent the reductive mobilization of iron from ferritin (Figure 1) (Jaeschke, 2010). Hydrogen peroxide can be reduced by glutathione peroxidases in the cytosol and mitochondria using electrons from glutathione (GSH) or peroxiredoxin, which can be regenerated by thioredoxin or sulfiredoxin depending on the degree of oxidation (Watson et al., 2004). Hydrogen peroxide generated predominantly in peroxisomes can be reduced by catalase, which draws electrons from hydrogen peroxide (catalase reaction) or low molecular weight alcohols (peroxidase reaction) (Calabrese and Canada, 1989) (Figure 1). Additional defense systems that limit potential detrimental effects of oxidant stress include metal chelators, which keep heavy metal ions tightly bound. In the case of iron, ferritin and transferrin are the main storage and transport proteins, respectively, which chelate iron in the ferric (Fe3+) state. In addition, lipid soluble antioxidants such as vitamin E are able to interrupt free radical chain reactions in membranes (lipid peroxidation) and water-soluble antioxidants such as GSH can spontaneously react with peroxynitrite, hypochlorous acid and hydrogen peroxide and detoxify them. If some free radical damage does occur, downstream repair mechanisms can be activated. For example, thioredoxins (Trx1 in the cytosol; Trx2 in mitochondria) reduce protein disulfides (Watson et al., 2004), glutathione peroxidase-4 can reduce fatty acid hydroperoxides and phospholipases can remove oxidized fatty acids from membranes (Toppo et al., 2009). If the damage to the protein or organelle is too extensive, the modified protein can be degraded by the proteasome system or the organelle can be removed by autophagy. Together these systems ensure that oxidant stress-mediated cell injury is limited and any minor damage is quickly repaired (Jaeschke, 2010).
Lipid peroxidation
When considering ROS-induced liver injury in any pathogenesis including drug-induced toxicity, lipid peroxidation (LPO) is the most invoked mechanism of cell death (Kehrer, 1993; Negre-Salvayre et al., 2010; Sevanian and Hochstein, 1985). Typical experimental evidence for this mechanism is a significant increase in a parameter that is thought to reflect LPO in the injured tissue (e.g. thiobarbituric acid-reactive substances, TBARS) and a reduction of this parameter with an intervention that is thought to act as antioxidant. Based on these data, it is frequently assumed that LPO represents the cause of the tissue injury (e.g., Campos et al. 1989; Gao and Zhou 2005; Hsu et al. 2008; Küpeli et al. 2006; Wu et al. 2008; Yuan et al. 2010). However, behind these conclusions are a number of issues that are rarely addressed. First, tissue damage may cause oxidant stress and LPO. Therefore, LPO can be a consequence of tissue injury rather than the cause. Second, the compound may protect through different mechanisms and the reduced LPO is a consequence of this reduced tissue damage. Third, can the drug really get to the target cell in sufficient quantities that it can effectively strengthen the already present antioxidant levels in the cell? With respect to this latter issue, it is important to keep in mind that even an extreme, 90% reduction of hepatic GSH levels still leaves the hepatocyte with about 1 mM GSH. Based on these considerations, the conclusion that LPO is the main mechanism of liver injury induced by a certain drug may not be justified.
The most important argument against LPO as a relevant mechanism of cell death is quantitative considerations. How much LPO is necessary to kill a liver cell? When massive amounts of tert-butyl hydroperoxide (tBHP) were infused into rat liver for 45 min, there was no liver injury despite an increase in LPO products by 2-to-3-fold compared to baseline (Mathews et al., 1994). However, when tBHP was infused for 90 min, LPO products increased by >50-fold and liver injury developed (Mathews et al., 1994). These data suggest that if LPO is the direct mechanism of liver cell killing, LPO parameters should increase 50-to-100-fold. In other words, the generally measured increase of LPO products in almost all studies (2-to-3-fold above baseline) appears insufficient to directly cause cell injury. Similar findings were obtained in drug toxicity studies. For example, acetaminophen (APAP) overdose causes severe liver injury but at best a minor increase in LPO in normal animals (Knight et al., 2003). However, if animals are fed a diet high in polyunsaturated fatty acids and deficient in vitamin E, APAP overdose triggers massive LPO (> 50-fold increase of ethane exhalation and TBARS) and liver injury (Wendel and Feuerstein, 1981; Wendel et al., 1982). In these deficient animals, but not in normal animals, APAP-induced liver injury can be reduced with vitamin E pretreatment (Knight et al., 2003; Werner and Wendel, 1990). These data demonstrate that an animal fed a regular diet has generally sufficient vitamin E in cell membranes to limit drug-induced LPO. Thus, for LPO to be a relevant mechanism of cell damage requires not only oxidant formation but also impairment of antioxidant defense systems. However, LPO products are biomarkers for oxidant stress (Guéraud et al., 2010; Jaeschke, 2011) and they can act as signaling molecules by modifying proteins or DNA within the cell or can act as chemotactic agents (Dianzani, 2003; Jaeschke, 2011; Marnett, 2002; Negre-Salvayre et al., 2010).
ACETAMINOPHEN HEPATOTOXICITY
Acetaminophen (APAP) is a safe drug at therapeutic levels but an overdose can cause severe liver injury in animals and man (Larson, 2007). Currently, APAP hepatotoxicity is the most frequent cause of acute liver failure in the US (Larson et al., 2005) and APAP is one of the most studied hepatotoxic drugs. Despite substantial progress in understanding the mechanism of APAP-induced liver injury during the last four decades, many details are still unknown.
Oxidant stress versus protein binding
The role of ROS in APAP hepatotoxicity was controversial for many years due to the competing hypotheses of protein binding (Mitchell et al., 1973) and oxidant stress/lipid peroxidation (Wendel et al., 1979). However, it was soon recognized that the P450-dependent metabolism of APAP, as originally assumed (Wendel and Feuerstein, 1981), was not the main source of ROS formation (Lauterburg et al., 1984; Smith and Jaeschke, 1989). In contrast, ROS formation occurs selectively in mitochondria after the initial metabolism of APAP (Jaeschke, 1990; Tirmenstein and Nelson, 1990; Bajt et al., 2004) (Figure 2). Formation of protein adducts has been implicated in the toxicity of a number of compounds (Park et al., 2011). In the case of APAP, earlier studies comparing protein binding induced by APAP versus its regioisomer 3'-hydroxyacetanilide (AMAP) indicated that although both drugs caused the same overall protein binding in the liver, the hepatotoxic drug APAP forms mitochondrial adducts while the nontoxic compound AMAP does not (Tirmenstein and Nelson, 1989; Myers et al., 1995; Matthews et al., 1997). More recent studies using 2D gel electrophoresis and mass spectrometry have resulted in identification of a number of the APAP adducted proteins in various cellular compartments. Using fasted B6C3F1 mice given 0.1% phenobarbital in drinking water for 5 days, Qiu et al. (1998) identified 30 proteins modified by APAP at a dose of 350 mg/kg for 2 hours. Of these, 6 were mitochondrial proteins (Table 1), including glutathione peroxidase and the ATP synthase α subunit. Modification of glutathione peroxidase has a functional consequence, since APAP administration results in a 60% reduction in glutathione peroxidase activity (Tirmenstein and Nelson, 1990). Though compromised activity of glutathione peroxidase could presumably have significant consequences due to lack of its anti-oxidant function, this does not seem to ultimately affect the extent of liver injury, since GPx−/− animals showed no increase in injury after APAP treatment (Knight et al., 2002). A potential explanation for the limited relevance of GPx could be that peroxynitrite may be the more important oxidant generated during APAP hepatotoxicity compared to the GPx substrate hydrogen peroxide (Knight et al., 2002). Modification of the ATP synthase α subunit, which is a catalytically critical part of the complex, would be expected to abolish function of ATP synthase and result in depletion of ATP, which is evident in APAP hepatotoxicity (Jaeschke, 1990; Saito et al., 2010b; Tirmenstein and Nelson, 1990). As ATP depletion is partially reversible with supplying mitochondrial energy substrates (Saito et al., 2010b), inactivation of ATP synthase by protein adduct formation may only be partially responsible for the declining ATP levels during APAP toxicity. Similar studies using AMAP resulted in identification of 12 proteins including cytosolic proteins such as methionine adenosyl transferase and selenium binding liver protein, but did not include any mitochondrial proteins (Qiu et al., 2001). These studies illustrate the fact that protein adducts formation after APAP administration seems to be a targeted phenomenon with specific proteins being affected, which could impact mitochondrial function, especially in a milieu of depleted glutathione. A caveat to these studies using 2D gel electrophoresis however is the fact that highly hydrophobic mitochondrial inner membrane proteins such as components of the respiratory chain would not enter the gels easily and hence would be excluded from detection.
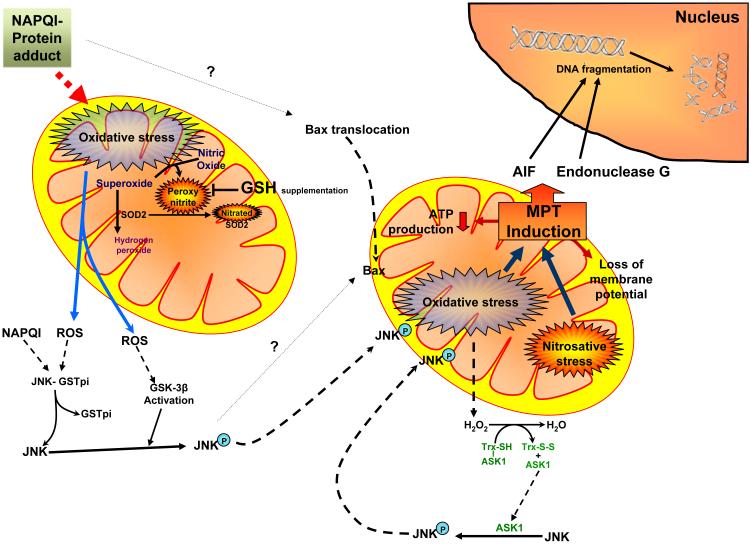
Figure 2. Acetaminophen-induced mitochondrial oxidant stress and its influence on cellular signaling.
Metabolism of APAP results in generation of the reactive intermediate NAPQI, which forms protein adducts and induces mitochondrial oxidative stress. The increased generation of superoxide, especially from the mitochondrial electron transport chain and its reaction with nitric oxide results in production of peroxynitrite. The superoxide can be scavenged by superoxide dismutase 2 (SOD2) and converted to hydrogen peroxide, though the generation of peroxynitrite can interfere in this process by nitration of SOD2. Mitochondrial oxidative stress and hydrogen peroxide can also activate the MAP kinase JNK by multiple pathways, resulting in its phosphorylation and translocation to the mitochondria. This then amplifies the mitochondrial oxidant stress, which subsequently leads to activation of the mitochondrial permeability transition, translocation of mitochondrial proteins such as apoptosis inducing factor (AIF) and endonuclease G to the nucleus. This then results in DNA fragmentation and finally oncotic necrosis.
Mitochondrial proteins identified as forming acetaminophen-adducts (From Qui et al., 1998)
| Protein | Function | |
|---|---|---|
| 1 | Glutathione peroxidase | Anti-oxidant |
| 2 | Housekeeping protein | - |
| 3 | Aldehyde dehydrogenase | Alcohol metabolism |
| 4 | ATP synthase alpha subunit | ATP synthesis |
| 5 | Homologous to 2,4-dienoyl-CoA reductase precursor from rat |
? Beta oxidation |
| 6 | Glutathione S transferase pi | Detoxification |
Together these observations support the hypothesis that the metabolic activation of APAP leads to the formation of N-acetyl-p-benzoquinone imine (NAPQI), which binds to a large number of cellular proteins, especially mitochondrial proteins (Cohen et al., 1997; Qiu et al., 1998). Importantly, this binding to mitochondrial proteins correlates with the mitochondrial oxidant stress and toxicity (Jaeschke et al., 2003). This suggests that protein binding is a critical initiating event which, though insufficient to cause cell death by itself, triggers a mitochondrial oxidant stress and other downstream events that result in oncotic necrosis of the cell (Jaeschke and Bajt, 2006; Hinson et al., 2004). However, the molecular events that trigger the oxidant stress are still unknown. Recently, we demonstrated that apoptosis-inducing factor (AIF), which functions as NADH oxidase in mitochondria and regulates complex I activity (Miramar et al., 2001; Vahsen et al., 2004), can promote APAP-induced oxidant stress (Bajt et al., 2011).
Mitochondrial superoxide and peroxynitrite formation
Superoxide can react with nitric oxide (NO) to form peroxynitrite (ONOO −), a highly reactive and potent oxidant and nitrating species (Radi et al., 2001). During APAP hepatotoxicity, peroxynitrite is generated in hepatocytes and sinusoidal endothelial cells as indicated by formation of nitrotyrosine protein adducts (Hinson et al., 1998; Knight et al., 2001) (Figure 2). Not unexpected, nitrotyrosine adducts are found mainly in mitochondria suggesting that peroxynitrite is formed inside of mitochondria in hepatocytes (Cover et al., 2005b) not by macrophages on the outside (James et al., 2003a). In contrast to the source of superoxide formation, the source of NO is controversial. All three forms of nitric oxide synthase (NOS), inducible NOS (Gardner et al., 2002), endothelial NOS (Salhanick et al., 2006) and neuronal NOS (Burke et al., 2010) have been implicated in contributing to peroxynitrite formation under certain conditions. However, others found no effect of iNOS on APAP hepatotoxicity (Burke et al., 2010; Michael et al., 2001; Saito et al., 2010a). Nevertheless, evidence for peroxynitrite formation was reported in cultured hepatocytes exposed to APAP (Burke et al., 2010; Yan et al., 2010) indicating that NO can be generated within hepatocytes. This suggests that, depending on the experimental systems and the specific conditions, different NOS enzymes located in different cell types may contribute the NO that is used to form peroxynitrite during APAP hepatotoxicity. Independent of the actual source of NO, the peroxynitrite formed in mitochondria leads to nitration of mitochondrial proteins, e.g. SOD2 (Agarwal et al., 2011) and damage to mitochondrial DNA (Cover et al., 2005b). The pathophysiological significance of peroxynitrite for APAP-induced liver injury was demonstrated by protection against APAP overdose when recovery of mitochondrial GSH levels was accelerated after delayed treatment with GSH precursors (Bajt et al., 2003; James et al., 2003b; Knight et al., 2002; Saito et al., 2010b). Under these conditions, the newly synthesized GSH scavenged peroxynitrite and enhanced the detoxification of hydrogen peroxide (Knight et al., 2002). This not only results in short-term protection but also attenuated long-term injury and enhanced regeneration (Bajt et al., 2003). However, scavenging reactive oxygen and peroxynitrite is not the only mechanism of protection after treatment with GSH or NAC after APAP. The generally used high dose of NAC in the clinic and the laboratory exceeds the amount of cysteine needed for GSH synthesis. The surplus cysteine is degraded and ends up as energy substrate in the Krebs cycle to support mitochondrial energy production (Saito et al., 2010b). The fact that hepatic ATP levels can be enhanced by excess amino acids indicates that the depletion of hepatic ATP levels after APAP overdose is not a completely irreversible effect but is at least in part caused by a relative shortage of Krebs cycle substrates (Saito et al., 2010b). Supply of mitochondrial energy substrates and partial restoration of cellular ATP levels adds to the protective effect of GSH and GSH precursors against APAP toxicity as scavengers of ROS and peroxynitrite (Saito et al., 2010b). However, simply promoting mitochondrial ATP production without scavenging ROS does not protect, suggesting that the support of the impaired mitochondrial energy metabolism alone is ineffective in preventing cell death (Saito et al., 2010b).
The critical role of the mitochondrial oxidant stress in APAP hepatotoxicity was also demonstrated in animals heterozygous for MnSOD-deficiency (MnSOD+/-). These animals had increased liver injury after APAP together with increased peroxynitrite and protein carbonyl formation (Fujimoto et al., 2009; Ramachandran et al., 2011b). These data indicate the importance of mitochondrial MnSOD in removing superoxide to limit peroxynitrite formation. On the other hand, MnSOD is nitrated and partially inactivated by peroxynitrite after APAP treatment (Agarwal et al., 2011). This impairment of the mitochondrial antioxidant defense may enhance the hepatocyte’s susceptibility to ROS formation. Because the formation of superoxide in mitochondria can depend on the oxygen tension, it is important to keep in mind that the oxygen levels of standard cell culture conditions (21% oxygen) are much higher than what hepatocytes experience in vivo, i.e. 3 - 9% oxygen (Kietzmann and Jungermann, 1997). In fact, when primary mouse hepatocytes were incubated at 10% oxygen instead of the standard 21% oxygen, mitochondrial oxidant stress and peroxynitrite formation and cell death were reduced (Yan et al., 2010). These data suggest that under conditions of a drug-induced mitochondrial stress, ROS formation and cell injury may be amplified under hyperoxic cell culture conditions.
Amplification of mitochondrial oxidant stress
There are several consequences of the initial mitochondrial oxidant stress that is triggered by protein binding (Figure 2). At first, during the very early time period after APAP, there is evidence of c-jun-N-terminal kinase (JNK) activation, which can be attenuated by antioxidants (Hanawa et al., 2008; Saito et al., 2010a). However, ROS do not seem to affect JNK directly. The redox-sensitive step appears to be the dissociation of thioredoxin and apoptosis signal-regulating kinase 1 (ASK1), which triggers the activation of ASK1 (Saitoh et al., 1998). Nakagawa et al. (2008) demonstrated that APAP treatment induced ASK1 activation after dissociation from thioredoxin. In addition, JNK activation and liver injury are attenuated in ASK1-deficient mice suggesting that ASK1 activation is upstream of JNK (Nakagawa et al., 2008). However, only late (≥ 3 h after APAP) JNK activation was attenuated. There was no effect at an earlier time point (1.5 h) (Nakagawa et al., 2008). This indicates that additional mechanisms of JNK activation are involved after APAP overdose. This is also supported by the fact that a JNK inhibitor is more effective in protecting against APAP hepatotoxicity than ASK1 deficiency (Nakagawa et al., 2008). Another possible mechanism of JNK activation is its release from glutathione-S-transferase Pi (GSTPi), which was shown to bind JNK (Elsby et al., 2003). The release of JNK from GSTPi could be triggered by oxidant stress or direct NAPQI binding. Alternatively, it was shown that glycogen synthase kinase-3β (GSK-3β) is activated very early during APAP hepatotoxicity (Shinohara et al., 2010). Down-regulation of GSK-3β protein levels attenuated the early JNK activation and protected against APAP-induced liver injury (Shinohara et al., 2010). Independent of the activation mechanism, activation of JNK leads to mitochondrial translocation of both bax (discussed below) and P-JNK (Hanawa et al., 2008) and further promotes the mitochondrial oxidant stress and peroxynitrite formation (Saito et al., 2010a). Overall, inhibition of JNK has proved to be a highly effective intervention against APAP hepatotoxicity (Gunawan et al., 2006; Henderson et al., 2007, Latchoumycandane et al., 2007; Saito et al., 2010a).
Further consequences of this amplified mitochondrial formation of ROS and peroxynitrite includes mitochondrial DNA damage (Cover et al., 2005b) and selective, partial loss of mitochondrial enzyme activities through thiol oxidation (Andringa et al., 2008) and nitration (Agarwal et al., 2011). However, the most critical effect of the oxidant stress is the opening of the mitochondrial membrane permeability transition (MPT) pore leading to collapse of the membrane potential and cessation of ATP synthesis (Kon et al., 2004; Masubuchi et al., 2005; Ramachandran et al., 2011a; Reid et al., 2005). Pharmacological inhibition of cyclophilin D, a critical regulator of the MPT pore (Baines et al., 2005), with cyclosporin A provides only temporary protection in vitro (Kon et al., 2004) but appears to more fully protect in mice treated with 350 mg/kg APAP in vivo (Masubuchi et al., 2005). However, the non-immunesuppressive cyclosporin A analogue Debio 025 did not protect in vivo when using a dose of 600 mg/kg APAP (Loguidice and Boelsterli, 2011). Similarly, cyclophilin D-deficient mice were protected against a low overdose (200 mg/kg) of APAP (Ramachandran et al., 2011a) but not against the higher dose (Loguidice and Boelsterli, 2011). An explanation could be that the cyclophilin D-regulated MPT occurs mainly at moderate stress levels, while a more severe stress may trigger an unregulated MPT, which is no longer affected by cyclophilin D inhibition (He and Lemasters, 2002). In the case of APAP, this may apply to higher, more toxic doses of APAP in vivo (Loguidice and Boelsterli, 2011) or in the presence of additional stress when keeping cells in culture (Kon et al., 2004). A higher dose of APAP leads to more severe stress due to prolonged depletion of GSH, which means a longer period where no scavengers for mitochondrial ROS and peroxynitrite are available (Saito et al., 2010b). Similarly, the higher oxygen concentrations generally used for cell culture experiments cause more severe APAP-induced oxidant stress and peroxynitrite formation in hepatocytes (Yan et al., 2010). In addition, more recent data also suggested an involvement of lysosomal iron in the MPT that translocated into the mitochondria through the calcium uniporter (Kon et al., 2010).
Nuclear DNA fragmentation
One of the consequences of the MPT is swelling of the matrix, which leads to the rupture of the outer mitochondrial membrane. As a result, there is extensive release of proteins from the intermembrane space. Proteins released after APAP overdose include endonuclease G and apoptosis-inducing factor (AIF) both of which can translocate to the nucleus (Bajt et al., 2006) due to their nuclear localization sequences (Norberg et al., 2010). AIF is involved in chromatin condensation and large scale DNA fragmentation (Boujrad et al., 2007). Endonuclease G cleaves DNA at the level of 50-300 kb and then at the level of internucleosomes (Widlak and Garrard, 2005). This is the reason that both large and small mono- and oligonucleosomal length DNA fragments are generated during APAP hepatotoxicity (Jahr et al., 2001). This gives a typical DNA ladder on an agarose gel and makes the fragments indistinguishable from classical apoptotic DNA fragments (Cover et al., 2005b; Ray et al., 1990; Shen et al., 1992). In addition to unregulated release after the MPT, AIF and endonuclease G can also be released earlier through a bax pore (Bajt et al., 2008). Mitochondrial bax translocation is a very early event after APAP overdose (Adams et al., 2001; Bajt et al., 2008; El-Hassan et al., 2003; Jaeschke and Bajt, 2006) leading to the mitochondrial outer membrane permeabilization (MOMP) and release of intermembrane proteins (Bajt et al., 2008). The importance of this event for APAP-induced cell injury has been demonstrated in bax-deficient mice. At 6 h after APAP overdose, there was inhibition of intermembrane protein release, reduced DNA fragmentation and attenuated liver injury in bax-deficient compared to wild type mice (Bajt et al., 2008). However, the absence of bax had no effect on the mitochondrial oxidant stress and peroxynitrite formation. As a consequence, the MPT was not prevented and the subsequent release of intermembrane proteins led to delayed DNA fragmentation and cell injury with no difference between wild type and bax-deficient mice at 12 h (Bajt et al., 2008). In contrast, scavenging the mitochondrial oxidant stress strongly attenuated nuclear DNA fragmentation and cell injury and promoted survival and regeneration (Bajt et al., 2003, Cover et al., 2005b; Knight et al., 2002). Furthermore, partial AIF-deficient mice showed significantly reduced DNA damage and liver injury after APAP (Bajt et al., 2011). These observations support the conclusions that nuclear DNA damage contributes to the cell injury mechanism during APAP hepatotoxicity. However, mitochondrial dysfunction and oxidant stress are the central regulators of APAP-induced cell death.
The Ca2+/Mg2+-dependent endonuclease deoxyribonuclease-1 (DNase1) translocates to the endoplasmic reticulum after translation and is then secreted by hepatocytes into the blood (Napirei et al., 2005). It was shown that extracellular DNase1 can gain access into cells undergoing necrosis and contribute to nuclear fragmentation (Napirei et al., 2004). After APAP overdose, DNase1-deficient mice had less nuclear DNA damage and less injury (Napirei et al., 2006). The temporary pattern of DNA strand breaks and injury indicated that cells towards the periportal area were more susceptible to DNase1, which did not affect the initial damage in the centrilobular hepatocytes (Napirei et al., 2006). This suggested that DNase1 released by centrilobular hepatocytes may be involved in the progression of the injury. This expansion of the area of necrosis may not only involve DNase1 but also calpains and phospholipases (Limaye et al., 2003; Bhave et al., 2011). Regarding the mechanisms of DNase1-induced cell death, it was hypothesized that the DNA damage induced by DNase1 may lead to activation of repair mechanisms including poly-adenosine diphosphate-ribose polymerase-1 (PARP-1) (Napirei et al., 2006). The excessive consumption of NAD+ by activated PARP-1 can contribute to cell necrosis (Ha and Snyder, 1999). Although hepatic NAD+ levels decline at 6 -12 h (Napirei et al., 2006) parallel to PARP-1 activation (Cover et al., 2005a), PARP-1-deficient mice were not protected against APAP hepatotoxicity (Cover et al., 2005a). In fact, PARP-1-deficient mice showed a moderate increase in liver injury suggesting that activation of DNA repair mechanisms after APAP overdose is beneficial (Cover et al., 2005a).
Apoptosis versus necrosis
It has been proposed that oxidant stress causes apoptotic cell death involving JNK activation in cultured hepatocytes (Czaja, 2002; Conde de la Rosa et al., 2006). However, oxidant stress in vivo generally leads to oncotic necrosis (Hong et al., 2009). Consistent with this observation, the extensive oxidant stress and JNK activation after APAP overdose causes mainly oncotic necrosis (Gujral et al., 2002; Jaeschke et al., 2004). APAP-induced cell death in vivo and in vitro is characterized by cell and organelle swelling, extensive cell contents release, karyolysis and inflammation (Gujral et al., 2002; Jacob et al., 2007; Jaeschke et al., 2004). In general, there is no caspase activation after APAP overdose (Lawson et al., 1999) despite the release of mitochondrial intermembrane proteins (cytochrome c, second mitochondrial activator of caspases [smac]) (Bajt et al., 2006). In addition, caspase inhibitors do not protect (Gujral et al., 2002; Jaeschke et al., 2006; Lawson et al., 1999; Williams et al., 2010b). A study reported protection against APAP hepatotoxicity with a pan-caspase inhibitor in the absence of caspase activation (El-Hassan et al., 2003). However, the protection was caused by the solvent (dimethyl sulfoxide, DMSO) (Jaeschke et al., 2006), which is an effective inhibitor of P450 enzymes (Yoon et al., 2006). On the other hand, in cases of Fas- or TNF-induced hepatocellular apoptosis, caspase inhibitors are highly effective and completely eliminate cell death (Bajt et al., 2000; Jaeschke et al., 1998). Interestingly, APAP toxicity even prevents Fas-induced apoptosis mainly due to the APAP-induced mitochondrial damage, which interrupts the apoptotic signaling through the mitochondria (Lawson et al., 1999; Knight and Jaeschke, 2002). Although both apoptosis and APAP-induced necrosis cause DNA fragmentation, there is a clear distinction in the staining patterns with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay between the two forms of cell death (Jaeschke and Lemasters, 2003), which is caused by the generally larger DNA fragments generated by AIF and endonuclease G compared to a caspase-activated DNase (Jahr et al., 2001). Nevertheless, in other assays of DNA damage, i.e. DNA ladder or anti-histone ELISA to measure DNA fragments in the cytosol, apoptosis and APAP-induced necrosis are indistinguishable (Cover et al., 2005b). Thus, conclusions regarding the mode of cell death should never be based on only one parameter but should include morphological and several biochemical parameters (Jaeschke et al., 2004).
Although the conclusions of most papers suggesting a role for apoptotic cell death can be questioned due to methodological concerns, a recent study indicated that APAP overdose can cause a temporary moderate caspase activation and generation of caspase-specific cleavage products of cytokeratin 18 in fed CD-1 mice (Antoine et al., 2009). A caspase inhibitor prevented these effects but had no impact on the overall liver injury (Antoine et al., 2009). The authors suggested that this transient caspase activation may be characteristic of APAP toxicity in fed mice and is missed in starved animals due to the lower ATP levels, which prevents caspase activation (Antoine et al., 2009, 2010). Our own studies confirmed this minor (compared to TNF-induced apoptosis) caspase activation in fed Swiss-Webster mice but not in fed C57Bl/6 mice (Jaeschke et al., 2011a) or in fed C3Heb/HeJ mice (Gujral et al., 2002). However, this effect appears to be more related to the use of outbred strains rather than fed animals. In either case, no protection was found with caspase inhibitor treatment and no morphological evidence for apoptosis was detected. This suggests that while modest caspase activation due to release of mitochondrial proteins may occur, it is insufficient to actually cause significant apoptotic cell death (Jaeschke et al., 2011a).
APAP toxicity in human cells and humans
The discussed mechanisms of acetaminophen-induced liver injury are based mainly on the mouse model and primary cultured mouse hepatocytes. The relevance of these findings for the human pathophysiology needs to be addressed. The most obvious approach to get at this question would be to use human hepatocytes. However, primary human hepatocytes have several disadvantages including limited availability, high cost and highly variable drug metabolism. On the other hand, hepatoma cell lines, e.g. HepG2 cells, are readily available but have a very poor capacity to metabolically activate APAP and therefore can not produce data that are relevant for animals or humans. HepaRG is a stem cell-derived human hepatocyte cell line that has retained the metabolic capacity of human hepatocytes (Guillouzo et al., 2007). Exposure of these cells to APAP demonstrated dose- and time-dependent depletion of glutathione, protein adduct formation, mitochondrial superoxide and peroxynitrite formation, mitochondrial dysfunction (loss of the membrane potential) and eventually cell necrosis (McGill et al., 2011b). In contrast, APAP did not change any of these parameters in HepG2 cells and did not cause cell death. A very minor formation of protein adducts (<10% of HepaRG cell levels) confirmed the limited capacity of HepG2 cells to metabolically activate APAP in these cells at less than pathophysiologically relevant levels (McGill et al., 2011b). APAP toxicity was also shown in a different, spontaneously immortalized human hepatocyte cell line (HC-04). This cell line is less well characterized and not commercially available. However, it also appears to retain its metabolic capacity (Lim et al., 2007). In the HC-04 cell line, APAP-induced cell death was prevented by the MPT inhibitor cyclosporin A and by the JNK inhibitor leflunamide (Latchoumycandane et al., 2006). In both cell lines, necrosis was the dominant form of cell death (Latchoumycandane et al., 2006; McGill et al., 2011b).
Earlier studies showing the formation of a reactive metabolite with GSH depletion and protein binding in mice (Mitchell et al., 1973) led to the introduction of NAC as an antidote against APAP overdose in patients (Prescott et al., 1977). Even today, NAC is the only approved treatment for APAP hepatotoxicity in the clinic (Larson, 2007). Subsequent studies in humans confirmed the initial assumption that there are similar mechanisms of toxicity in humans. Indirect evidence for a stress on the hepatic glutathione pool after therapeutic doses of APAP was provided by analysis of plasma GSH and cysteine turnover in healthy volunteers (Burgunder et al., 1989; Lauterburg and Mitchell, 1987). Later measurement of APAP protein adducts, which correlated with the severity of liver injury, in plasma of overdose patients provided direct support for the formation of a reactive metabolite and protein binding in humans (Davern et al., 2006; James et al., 2009; Muldrew et al., 2002). In addition, recent preliminary data demonstrating markers for mitochondrial injury (glutamate dehydrogenase activity; mitochondrial DNA) in plasma of APAP overdose patients with substantial liver injury suggest that APAP toxicity in patients correlates with mitochondrial damage (McGill et al., 2011a). In terms of necrosis and apoptosis, the massive cell contents release including liver enzymes observed in patients support the conclusion that necrosis is the dominant form of cell death in humans (McGill et al., 2011a). Although no increase in caspase activities were detected in our patient samples (McGill et al., 2011a), low and variable levels of a caspase-dependent cleavage product of cytokeratin-18 have been detected (Bechmann et al., 2008; Rutherford et al., 2007; Volkmann et al., 2008). However, even if this would reflect actual apoptotic cell death and not just a minor activation of caspases, the consensus is that this represents only a minor fraction of cell death. Thus, together these data support the conclusion that mechanisms of APAP-induced liver injury in mice and in primary mouse hepatocytes can also be observed in human hepatocyte cell lines and in patients.
DRUG-INDUCED MITOCHONDRIAL INJURY AND OXIDANT STRESS
While acetaminophen toxicity is the most thoroughly investigated and well established example of mitochondria mediated drug-induced hepatic injury, there are other liver toxicants which appear to have similar mechanisms. Among the most interesting of these are drugs with idiosyncratic toxicity in humans. Idiosyncratic toxicity is toxicity that occurs at pharmacological doses and is characterized by low incidence (< 1 in 10,000 patients on the drug) and delayed onset. The non-steroidal antiinflammatory drugs (NSAIDs) diclofenac and nimesulide are well studied examples. While hepatotoxicity at therapeutic doses is rare, at supra-therapeutic doses both of these drugs can cause mitochondrial membrane permeabilization and mitochondrial dysfunction in both rodent models and in vitro (Berson et al., 2006; Lim et al., 2006). The ability of these somewhat acidic and lipophilic compounds to decrease the mitochondrial transmembrane potential is partially dependent upon their protonophoric properties. However, there is also evidence that both of these drugs can induce the MPT (Masubuchi et al., 2002; Berson et al., 2006; Lim et al., 2006). Masubuchi et al. (2002) found swelling and loss of Ca2+ retention in isolated rat liver mitochondria and loss of fluorescence of the mitochondria-targeted cationic fluorophore rhodamine 123 in cultured rat hepatocytes treated with diclofenac. Importantly, all of these effects were cyclosporin A (CsA)-sensitive. Follow-up studies by Lim et al. (2006) revealed that the human hepatocyte cell line HC-04 cotreated with diclofenac and the CYP2C inhibitor sulfaphenazole had reduced injury, despite a reduction in the mitochondrial transmembrane potential similar to cells treated with diclofenac alone. It was suggested that the uncoupling caused by the parent compound could dissipate the proton gradient across the inner mitochondrial membrane, but that true MPT was caused by reactive metabolites of the drug. This highlights the importance of studying drug toxicity in intact cells or animals, as the effect of the metabolite would have been missed in experiments using isolated mitochondria. Interestingly, later work by the same group found complete protection with CsA in HC-04 cells even in the absence of the CsA target cyclophilin D (Siu et al., 2008). Coupled with the observation that bax translocated into the mitochondria after diclofenac treatment and that siRNA knockdown of bax could partially protect against diclofenac toxicity in this model, these data suggest that the diclofenac-induced MPT is independent of the traditional MPT pore (composed of cyclophilin D, the adenine nucleotide transporter [ANT], and the voltage-dependent anion channel [VDAC]) and involves Bcl-2 family member proteins. It remains to be seen whether or not these findings are specific to HC-04 cells. It is also somewhat puzzling that nearly complete knockdown of bax only afforded about 50% protection based on enzyme release, while CsA completely protected in this model. In either case, mitochondrial depolarization plays an important role in diclofenac toxicity. Similar data are available as evidence of the MPT after nimesulide (Berson et al., 2006).
The observation that these drugs can induce the MPT at high doses led to the hypothesis that their idiosyncratic toxicity may be the result of some deficiency in mitochondrial function or antioxidant defenses in affected patients (Masubuchi et al, 2002; Ong et al., 2007). Consistent with this, it was reported that delayed-onset troglitazone hepatotoxicity can be induced in partial SOD2-deficient mice (Ong et al., 2007). In addition to the implications for idiosyncratic toxicity, these results suggest a role for mitochondrial oxidative stress in the mechanism of troglitazone toxicity. Unfortunately, Fujimoto et al. (2009) were unable to repeat these data, despite finding that partial SOD2-deficient mice were more susceptible to APAP toxicity. Whether or not the difference in toxicity is related to the different vehicles and routes of administration used in the two studies has yet to be determined. Several other drugs with idiosyncratic toxicity have since been found to induce similar injury in this model of mitochondrial genetic insufficiency, including the NSAID nimesulide and the anti-androgen flutamide (Ong et al, 2006; Kashimshetty et al., 2009). Unfortunately, these studies suffer from a lack of complete time course information. Nevertheless, if these data can be confirmed, this would suggest that mitochondrial oxidative stress is important in many drug toxicities and that impairment of mitochondrial antioxidant defenses may be part of the mechanism of many idiosyncratic adverse drug reactions. These results indicate that subclinical abnormalities in mitochondria may accelerate mitochondrial aging and lead to accumulation of mitochondrial damage with chronic drug dosing, which becomes apparent upon development of idiosyncratic toxicity (Boelsterli and Lim, 2007).
Interestingly, associations between drug-induced liver injury and genotypic variation in SOD2 and glutathione peroxidase 1 (GPX1) in humans have been reported (Huang et al., 2007; Lucena et al., 2010). These data suggest that mutant SOD2 and/or GPX1 protein expression correlates with greater susceptibility to drug hepatotoxicity. It has been suggested that these polymorphisms result in greater H2O2 production or reduced detoxification within mitochondria upon drug stimulation, leading to increased mitochondrial damage (Huang et al., 2007; Lucena et al., 2010). In another recent study, evidence was provided for an association between idiosyncratic liver injury caused by the anti-epileptic valproate (VPA) and allelic variants of the mitochondrial DNA polymerase γ (POLG) gene in humans (Stewart et al., 2010). Based on these data, it is tempting to speculate that gradual accumulation of mitochondrial DNA (mtDNA) damage caused by drug-induced oxidative stress in patients with impaired mtDNA repair mechanisms may be a factor in some cases of idiosyncratic drug toxicity, especially if these changes affect expression of antioxidant defense genes. Evidence has been reported for the development of oxidative stress prior to toxicity in rats and primary rat hepatocytes after VPA administration (Tong et al., 2005a,b). Daily treatment with VPA up to two weeks resulted in a significant increase in 15-F2t-isoprostane, a marker of oxidative stress, in both plasma and tissue prior to an increase in serum levels of the hepatocyte-specific enzyme α glutathione S-transferase (Tong et al., 2005a). Similar data were obtained in vitro using 15-F2t-isoprostane and 2’,7’-dichlorodihydrofluorescein diacetate fluorescence as markers of ROS (Tong et al., 2005b). In the latter model, depletion of GSH prior to VPA treatment resulted in greater oxidative stress. The lack of GSH also facilitated the loss of the mitochondrial transmembrane potential and cell death. Importantly, several studies in humans taking VPA at therapeutic doses have revealed increases in plasma and urine markers of oxidative stress (Chang and Abbott, 2006). Based on these data, it is possible that VPA-induced oxidative stress in patients with an underlying impairment in antioxidant defenses or with silent mitochondrial dysfunction could lead to toxicity over time. Additional evidence for oxidant stress in idiosyncratic liver injury comes from a study which found an association between a combined glutathione-S-transferase GSTT1-GSTM1 deficiency and troglitazone hepatotoxicity in humans (Watanabe et al., 2003).
It has also been suggested that VPA can exert toxic effects through inhibition of mitochondrial β oxidation, leading to liver injury and microvesicular steatosis. VPA has been shown to cause carnitine depletion in some patients and a decrease in mitochondrial carnitine levels. There appear to be multiple mechanisms and possibly multiple metabolites involved (Lheureux and Hanston, 2009), with recent data showing inhibition of carnitine palmitoyl transferase I (CPT1) by the VPA metabolite valproyl-CoA (Aires et al., 2010). While glucuronidation eliminates most of the drug, β-oxidation is one of the major routes of metabolism for the remaining parent compound. Though not addressed by the authors, it is possible that inhibition of this pathway may result in shunting through other metabolic pathways, possibly enhancing the other hepatic effects of VPA including hepatocellular necrosis. VPA may also inhibit mitochondrial respiration (Jimenez-Rodriguezvila et al., 1985).
Other drugs which may be direct inhibitors of mitochondrial respiration or β oxidation and which can cause liver injury include the non-nucleoside reverse transcriptase inhibitor efavirenz. Recent data in Hep3b cells suggest that this drug inhibits complex I in the electron transport chain, leading to dose-dependent ATP depletion, ROS formation, and altered lipid metabolism possibly as a result of increased AMPK activation (Blas-Garcia et al., 2010). The authors obtained similar, though less convincing, results with human liver tissues. Interestingly, these effects were exacerbated in Hep3b cells by treatment with a commonly prescribed combination of drugs for HIV treatment. Other drugs thought to cause liver injury through altered respiration or lipid metabolism include aspirin, tetracycline antibiotics (Fréneaux et al., 1988), and many more. Curiously, minocycline has been shown to inhibit mitochondrial respiration and cause mitochondrial dysfunction but has also been used as an MPT inhibitor in some conditions (Gieseler et al., 2009; Kupsch et al., 2009; Theruvath et al., 2008).
The antiarrhythmic amiodarone and the structurally similar drugs benzbromarone and benzarone have been shown to induce the MPT in hepatocytes (Kaufman, et al., 2005; Waldhauser et al., 2006). Treatment of rat hepatocytes for 1 hour with each of these drugs and loaded with [3H]-tetraphenylphosphonium bromide resulted in accumulation of radioactivity within mitochondria (Kaufman et al., 2005). They also showed an increase in mitochondrial swelling and reported that this effect could be partially inhibited by the MPT-inhibitor CsA. At 8 hours, there was a significant increase in propidium iodide labeling of necrotic cells. The authors also found a small increase in the number of apoptotic rat hepatocytes and the release of cytochrome c in HepG2 cells treated with these drugs. Unfortunately, interpretation of these data is complicated by the use of the different hepatocyte models. Based on the results from rat hepatocytes, it seems likely that these drugs cause cell necrosis through a mechanism involving the MPT. Other xenobiotics which have been suggested to induce the MPT in liver mitochondria include the alcohol abuse deterrent disulfiram (Balakirev and Zimmer, 2001), the peripheral benzodiazepine receptor/translocator protein agonist alpidem (Berson et al., 2001), and aspirin (Battaglia et al., 2005; Trost and Lemasters, 1997).
A large number of drugs are thought to cause liver injury through mitochondrial dysfunction and oxidative stress (Pessayre et al., 2010). While acetaminophen is currently the best characterized example, future investigation will reveal in greater detail the mechanisms of toxicity of these other compounds. A possible involvement of mitochondria and oxidative stress in idiosyncratic drug hepatotoxicity is particularly exciting. (Boelsterli and Lim, 2007). However, it is likely that idiosyncratic toxicity can occur through a number of different mechanisms, depending upon the drug. The delayed onset characteristic of many idiosyncratic reactions suggests a role for the adaptive immune system (Uetrecht, 2009; Adams et al., 2010; Zhang et al., 2011). Variation in drug metabolizing enzymes may also explain some cases of idiosyncratic toxicity (Andrade et al., 2009). The emerging evidence for the involvement of mitochondria and oxidative stress among these other mechanisms is an interesting contribution to our understanding of this phenomenon.
EXTRACELLULAR OXIDANT STRESS DURING DRUG-INDUCED INFLAMMATION
ROS generation by inflammatory cells
Resident macrophages (Kupffer cells) and infiltrating phagocytes (neutrophils, monocytes) can be extracellular sources of ROS formation during an inflammatory response. Kupffer cells are in a fixed position in sinusoids. Therefore, any ROS generated by Kupffer cells (NOX2-derived superoxide and hydrogen peroxide) is released into the vascular space and space of Disse before it can affect hepatocytes (Figure 3). Thus, GSH released by hepatocytes can in part neutralize this oxidant stress in the vascular space (Bilzer et al., 2002; Jaeschke, 1992; Liu et al., 1994). In contrast, neutrophils have to extravasate and adhere to the target cell in order to be fully activated and produce a long-lasting adherence-dependent oxidant stress (Jaeschke and Smith 1997). In this case, the ROS (hydrogen peroxide, hypochlorous acid) are generated in very close proximity to the target and can easily diffuse into the target cell (Entman et al., 1992). Thus, both KC and PMN generate ROS outside hepatocytes but the ROS diffuse into hepatocytes and trigger an intracellular oxidant stress as indicated by increased GSSG levels (Jaeschke et al., 1999) or increased chlorotyrosine protein adducts (Gujral et al., 2004a,b; Hasegawa et al., 2005). In addition, enhancement or deficiency of intracellular antioxidant systems protected or enhanced the injury, respectively (Bilzer et al., 1999; Jaeschke et al., 1999; Schauer et al., 2003). Because inflammatory liver injury is generally a self-aggravating process once it begins, strengthening of intracellular oxidant defenses attenuates the initial injury and, through limited further release of cell contents (damage-associated molecular patterns, DAMPs), also prevents the propagation of the inflammatory response (Jaeschke, 2006).
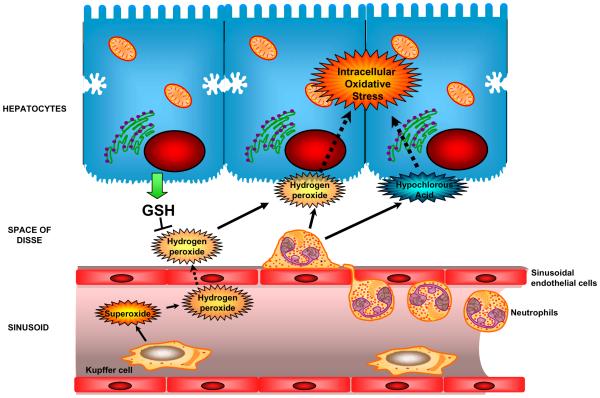
Figure 3. Extracellular generation of ROS generation by inflammatory cells.
Though both Kupffer cells as well as neutrophils can generate free radicals in the vicinity of hepatocytes, the location of generation influences the effect of oxidant generation. Radicals generated in the sinusoids by Kupffer cells need to diffuse through the vascular space as well as the space of Disse before reaching hepatocytes, and hence can be scavenged by GSH released from hepatocytes. The formation of reactive oxygen by extravasated neutrophils in close proximity to hepatocytes results in the diffusion of the oxidants into the target cell. The oxidant stress derived from neutrophils or Kupffer cells results mitochondrial dysfunction, mitochondrial oxidant stress and ultimately cell death.
Extracellular oxidant stress-induced cell death
Investigations of the mechanisms of hepatocyte cell death induced by externally added ROS, mainly hydrogen peroxide and tert-butyl hydroperoxide (tBHP), focused initially on protein thiol oxidation (Bellomo and Orrenius, 1985) and LPO (Rubin and Farber 1984). However, protein thiol oxidation could not be observed during oxidant stress-induced liver injury in vivo and the extent of LPO was insufficient to explain cell death (Smith et al., 1985). An important reason for some of the discrepancies was certainly the fairly high doses of ROS used in the early cell culture studies. Despite some of the concerns, two important observations were made (Farber, 1994). First, oxidant stress-induced cell death involves an endogenous source of ferric iron (Starke et al., 1985). Second, toxicity by hydrogen peroxide and tBHP requires formation of superoxide (Nakae et al., 1990; Starke and Farber, 1985). Subsequent experiments exposing cultured hepatocytes to moderate concentrations of tBHP demonstrated that the toxicity is caused by the mitochondrial MPT pore opening, which involves mitochondrial superoxide formation (Nieminen et al., 1995,1997). Furthermore, chelation of iron also reduced cell death (Nieminen et al., 1997). The lysosomal compartment was identified as the source of chelatable iron contributing to cell death (Starke et al., 1985; Uchiyama et al., 2008). Thus, moderate amounts of external ROS diffusing into hepatocytes can lead to lysosomal instability and release of iron into the cytosol where it is taken up into mitochondria by the calcium uniporter and contributes to the MPT (Uchiyama et al., 2008). These experiments documented that lysosomal iron mobilization can substantially sensitize hepatocytes to oxidant stress-mediated cell killing through a mitochondria-dependent mechanism (Uchiyama et al., 2008). A role for chelatable iron has also been shown for oxidant stress-induced liver injury in vivo (Smith, 1987).
ROS formation during drug-induced inflammation
There is no question that Kupffer cells, infiltrating monocytes/macrophages and recruited neutrophils can be activated and produce ROS and directly, through ROS-mediated mechanisms, can cause liver injury in hepatic ischemia-reperfusion injury, obstructive cholestasis and various models of endotoxemia (Jaeschke, 2003; 2011; Jaeschke and Hasegawa, 2006) (Figure 3). However, the role of inflammatory cells in producing ROS and causing liver injury during drug-induced liver injury is mechanistically much less defined and in some cases controversial. An involvement of Kupffer cells in the hepatotoxicity of various drugs and chemicals has been shown mainly by reducing the injury with gadolinium chloride (GdCl3), an intervention that inactivates Kupffer cells. Reduced liver injury with GdCl3 was shown after treatment with carbon tetrachloride (Edwards et al., 1993; elSisi et al., 1993), acetaminophen (Laskin et al., 1995; Michael et al., 1999), allyl alcohol (Przybocki et al., 1992) and cadmium (Sauer et al., 1997). Although GdCl3 can attenuate the capacity of Kupffer cells to produce ROS (Liu et al., 1995), it can also modulate the formation of cytokines and other mediators (Kono et al., 2002). Thus, it remains unclear in most cases how exactly Kupffer cells contribute to the injury mechanism of drug hepatotoxicity. In hepatic ischemia-reperfusion injury, there is direct evidence for Kupffer cell-induced ROS formation selectively during reperfusion (Jaeschke et al., 1991), consistent documentation that reduction in Kupffer-induced oxidant stress reduces reperfusion injury and activation of Kupffer cells enhances both the oxidant stress and the injury (Jaeschke and Farhood, 1991; Liu et al., 1995) and it was demonstrated that selective interventions against Kupffer cell-derived ROS in the hepatic vasculature can reduce liver injury (Liu et al., 1994; Bilzer et al., 1999; Schauer et al., 2004). In contrast, a role for Kupffer cell-derived ROS has rarely been conclusively demonstrated in the pathophysiology of drug-induced liver injury. In the case of APAP, it was reported that GdCl3 pretreatment attenuated liver injury in the rat (Laskin et al., 1995) and mouse (Michael et al., 1999) and oxidant stress/peroxynitrite formation in mice (Michael et al., 1999). However, these results could not be confirmed by others in terms of both injury and oxidant stress reduction (Ito et al., 2003; Ju et al., 2002; Knight and Jaeschke, 2004). Most importantly, complete elimination of Kupffer cells by clodronate liposomes actually aggravated liver injury (Ju et al., 2002). The enhanced injury was caused by the elimination of IL-10 formation, which has a critical role in limiting the induction of intracellular iNOS (Bourdi et al., 2002). In addition, mice with deficiency of functional NADPH oxidase, which is the enzyme responsible for ROS formation in phagocytes, showed neither a reduction in ROS and peroxynitrite formation nor reduced liver injury (James et al., 2003a). In addition, the most active Kupffer cells are located in the periportal area (Bautista et al., 1990), which is away from the centrilobular area where necrosis develops after APAP treatment. Thus, these observations are inconsistent and incompatible with a role for Kupffer cell-induced oxidant stress in the pathophysiology of APAP hepatotoxicity. However, cytokine formation by Kupffer cells may modulate intracellular events and therefore Kupffer cells may play a role in the overall pathophysiology. Unfortunately, for most other drugs or chemicals where Kupffer cells have been implicated, there is little support for this hypothesis beyond reduced injury after GdCl3 treatment and limited information regarding the actual mechanism.
Recruitment of neutrophils is a common response after tissue injury. However, this does not necessarily mean that these phagocytes aggravate the injury. Only if appropriately stimulated to extravasate and attack a stressed target cell, which would survive in the absence of neutrophils, are these phagocytes able to contribute to liver injury (Jaeschke, 2006). In general, neutrophils kill by formation of ROS, especially hypochlorous acid (Jaeschke, 2006). In support of this concept, it was demonstrated that neutrophil cytotoxicity during obstructive cholestasis (bile duct ligation) involves formation of chlorotyrosine adducts in hepatocytes suggesting the diffusion of neutrophil-derived oxidants into hepatocytes (Gujral et al., 2003, 2004b). The fact that a MPT inhibitor protected against inflammatory liver injury after bile duct ligation supports the concept that the neutrophil-induced oxidant stress kills hepatocytes through mitochondrial depolarization (Rehman et al., 2008). Similar findings were obtained in hepatic ischemia-reperfusion injury. From the massive accumulation of neutrophils at the site of injury (Jaeschke et al., 1990), the direct evidence for hepatic neutrophil activation and neutrophil-specific ROS production during the late phase of reperfusion injury (Jaeschke et al., 1992; Hasegawa et al., 2005), the fact that various antibodies against β2 integrins on neutrophils reduce the postischemic oxidant stress and injury (Jaeschke et al., 1990; 1993; Liu et al., 1995) and the protection by NADPH oxidase inhibitors (Liu et al., 2008), there is solid experimental support for the importance of a neutrophil-mediated oxidant stress as the central mechanism of reperfusion injury. In contrast, there is much more limited support for a neutrophil component in drug- or chemical-induced hepatotoxicity. Halothane hepatitis (You et al., 2006), alpha-naphthylisothiocyanate (ANIT) toxicity (Kodali et al., 2006; Roth and Dahm 1997) and carbon tetrachloride-induced liver injury (Ohta et al., 2006) are examples for drug toxicity with evidence for neutrophil involvement. The conclusion that neutrophils contribute to APAP hepatotoxicity (Liu et al., 2006; Ishida et al., 2006) has been questioned due to off-target effects of the neutropenia-inducing antibody used in these studies (Jaeschke and Liu, 2007) and extensive experimental evidence that hepatic or peripheral neutrophils are not activated for ROS formation after APAP (Williams et al., 2010a). This is consistent with the absence of hypochlorite-modified proteins in the liver indicating that the accumulating neutrophils during APAP overdose do not produce ROS (Cover et al., 2006) and the fact that NADPH oxidase-deficient mice are not protected against APAP hepatotoxicity (James et al., 2003a). Furthermore, a battery of interventions against neutrophils including NADPH oxidase inhibitors, antibodies against β2 integrins or use of mice deficient in these adhesion molecules failed to protect against APAP hepatotoxicity (Cover et al., 2006; James et al., 2003a; Lawson et al., 2000; Williams et al., 2010a). Thus, despite the cytotoxic potential of neutrophils, an involvement of neutrophils in drug hepatotoxicity needs to be carefully assessed using multiple experimental approaches. In particular, it has to be kept in mind that many immunological interventions may also affect drug metabolism or cause gene expression changes that protect independent of the intended immune cell target, which makes these types of experiments especially vulnerable to misinterpretations. However, if an involvement of neutrophils can be established, the mechanism of toxicity most likely depends on the neutrophil-derived ROS affecting target cell mitochondria (Figure 3).
ACKNOWLEDGMENT
Work discussed in this review was supported in part by the National Institutes of Health R01 DK070195 and R01 AA12916, and by P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Footnotes
DECLARATION OF INTEREST
The authors report no declarations of interest.
REFERENCES
- Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–21. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. 2001;60:907–15. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Macmillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337:110–8. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires CC, Iijst L, Stet F, Prip-Buus C, de Almeida IT, Duran M, Wanders RJ, Silva MF. Inhibition of hepatic carnitine palmitoyl-transferase I (CPT IA) by valproyl-CoA as a possible mechanism of valproate-induced steatosis. Biochem Pharamacol. 2010;79:792–9. doi: 10.1016/j.bcp.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Andrade RJ, Agúndez JA, Lucena MI, Martinez C, Cueto R, García-Martín E. Pharmacogenomics in drug induced liver injury. Curr Drug Metab. 2009;10:956–70. doi: 10.2174/138920009790711805. [DOI] [PubMed] [Google Scholar]
- Andringa KK, Bajt ML, Jaeschke H, Bailey SM. Mitochondrial protein thiol modifications in acetaminophen hepatotoxicity: effect on HMG-CoA synthase. Toxicol Lett. 2008;177:188–97. doi: 10.1016/j.toxlet.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–31. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–90. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–25. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2003;307:67–73. doi: 10.1124/jpet.103.052506. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–9. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–17. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakirev MY, Zimmer G. Mitochondrial injury by disulfiram: two different mechanisms of the mitochondrial permeability transition. Chem Biol Interact. 2001;138:299–311. doi: 10.1016/s0009-2797(01)00283-6. [DOI] [PubMed] [Google Scholar]
- Battaglia V, Salvi M, Toninello A. Oxidative stress is responsible for mitochondrial permeability transition induction by salicylate in liver mitochondria. J Biol Chem. 2005;280:33864–72. doi: 10.1074/jbc.M502391200. [DOI] [PubMed] [Google Scholar]
- Bautista AP, Mészáros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990;48:123–8. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28:713–6. doi: 10.1111/j.1478-3231.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bellomo G, Orrenius S. Altered thiol and calcium homeostasis in oxidative hepatocellular injury. Hepatology. 1985;5:876–82. doi: 10.1002/hep.1840050529. [DOI] [PubMed] [Google Scholar]
- Berson A, Cazanave S, Descatoire V, Tinel M, Grodet A, Wolf C, Feldmann G, Pessayre D. The anti-inflammatory drug, nimesulide (4-nitro-2-phenoxymethane-sulfoanilide), uncouples mitochondria and induces mitochondrial permeability transition in human hepatoma cells: protection by albumin. J Pharmacol Exp Ther. 2006;318:444–54. doi: 10.1124/jpet.106.104125. [DOI] [PubMed] [Google Scholar]
- Berson A, Descatoire V, Sutton A, Fau D, Maulny B, Vadrot N, Feldmann G, Berthron B, Tordjmann T, Pessayre D. Toxicity of alpidem, a peripheral benzodiazepine receptor ligand, but not zolpidem, in rat hepatocytes: role of mitochondrial permeability transition and metabolic activation. J Pharmacol Exp Ther. 2001;299:793–800. [PubMed] [Google Scholar]
- Bhave VS, Donthamsetty S, Latendresse JR, Cunningham ML, Mehendale HM. Secretory phospholipase A(2)-mediated progression of hepatotoxicity initiated by acetaminophen is exacerbated in the absence of hepatic COX-2. Toxicol Appl Pharmacol. 2011;251:173–80. doi: 10.1016/j.taap.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Bilzer M, Baron A, Schauer R, Steib C, Ebensberger S, Gerbes AL. Glutathione treatment protects the rat liver against injury after warm ischemia and Kupffer cell activation. Digestion. 2002;66:49–57. doi: 10.1159/000064415. [DOI] [PubMed] [Google Scholar]
- Bilzer M, Jaeschke H, Vollmar AM, Paumgartner G, Gerbes AL. Prevention of Kupffer cell-induced oxidant injury in rat liver by atrial natriuretic peptide. Am J Physiol. 1999;276:G1137–44. doi: 10.1152/ajpgi.1999.276.5.G1137. [DOI] [PubMed] [Google Scholar]
- Blas-García A, Apostolova N, Ballesteros D, Monleón D, Morales JM, Rocha M, Victor VM, Esplugues JV. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology. 2010;52:115–25. doi: 10.1002/hep.23647. [DOI] [PubMed] [Google Scholar]
- Boelsterli UA, Lim PL. Mitochondrial abnormalities--a link to idiosyncratic drug hepatotoxicity? Toxicol Appl Pharmacol. 2007;220:92–107. doi: 10.1016/j.taap.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–9. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Masubuchi Y, Reilly T, Amouzadeh H, Martin J, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289–98. doi: 10.1053/jhep.2002.30956. [DOI] [PubMed] [Google Scholar]
- Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36:127–31. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Burke AS, MacMillan-Crow LA, Hinson JA. Reactive nitrogen species in acetaminophen-induced mitochondrial damage and toxicity in mouse hepatocytes. Chem Res Toxicol. 2010;23:1286–92. doi: 10.1021/tx1001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Canada AT. Catalase: its role in xenobiotic detoxification. Pharmacol. Ther. 1989;44:297–307. doi: 10.1016/0163-7258(89)90069-7. [DOI] [PubMed] [Google Scholar]
- Campos R, Garrido A, Guerra R, Valenzuela A. Silybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetaminophen on rat liver. Planta Med. 1989;55:417–9. doi: 10.1055/s-2006-962055. [DOI] [PubMed] [Google Scholar]
- Chang TK, Abbott FS. Oxidative stress as a mechanism of valproic acid-associated hepatotoxicity. Drug Metab Rev. 2006;38:627–39. doi: 10.1080/03602530600959433. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918–29. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Cover C, Fickert P, Knight TR, Fuchsbichler A, Farhood A, Trauner M, Jaeschke H. Pathophysiological role of poly(ADP-ribose) polymerase (PARP) activation during acetaminophen-induced liver cell necrosis in mice. Toxicol Sci. 2005a;84:201–8. doi: 10.1093/toxsci/kfi065. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005b;315:879–87. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal. 2002;4:759–67. doi: 10.1089/152308602760598909. [DOI] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM, Acute Liver Failure Study Group Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–94. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Dianzani MU. 4-hydroxynonenal from pathology to physiology. Mol Aspects Med. 2003;24:263–72. doi: 10.1016/s0098-2997(03)00021-9. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275–9. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–29. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem. 2003;278:22243–9. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- elSisi AE, Earnest DL, Sipes IG. Vitamin A potentiation of carbon tetrachloride hepatotoxicity: role of liver macrophages and active oxygen species. Toxicol Appl Pharmacol. 1993;119:295–301. doi: 10.1006/taap.1993.1072. [DOI] [PubMed] [Google Scholar]
- Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335–45. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber JL. Mechanisms of cell injury by activated oxygen species. Environ Health Perspect. 1994;102(Suppl 10):17–24. doi: 10.1289/ehp.94102s1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréneaux E, Labbe G, Letteron P, Le Dinh The, Degott C, Genève J, Larrey D, Pessayre D. Inhibition of the mitochondrial oxidation of fatty acids by tetracycline in mice and in man: possible role in microvesicular steatosis induced by this antibiotic. Hepatology. 1988;8:1056–62. doi: 10.1002/hep.1840080513. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- Gao H, Zhou YW. Anti-lipid peroxidation and protection of liver mitochondria against injuries by picroside II. World J Gastroenterol. 2005;11:3671–4. doi: 10.3748/wjg.v11.i24.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P, Laskin DL. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol Appl Pharmacol. 2002;84:27–36. [PubMed] [Google Scholar]
- Gieseler A, Schultze AT, Kupsch K, Haroon MF, Wolf G, Siemen D, Kreutzmann P. Inhibitory modulation of the mitochondrial permeability transition by minocycline. Biochem Pharmacol. 2009;77:888–96. doi: 10.1016/j.bcp.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos. 2007;35:1–8. doi: 10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44:1098–124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: A highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–63. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004a;287:G243–52. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–8. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004b;286:G499–507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–77. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760–7. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–90. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11:604–7. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–22. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- Hong JY, Lebofsky M, Farhood A, Jaeschke H. Oxidant stress-induced liver injury in vivo: role of apoptosis, oncotic necrosis, and c-Jun NH2-terminal kinase activation. Am J Physiol Gastrointest Liver Physiol. 2009;296:G572–81. doi: 10.1152/ajpgi.90435.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Lin KY, Wang ZH, Lin WL, Yin MC. Preventive effect of Ganoderma amboinense on acetaminophen-induced acute liver injury. Phytomedicine. 2008;15:946–50. doi: 10.1016/j.phymed.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Huang YS, Su WJ, Huang YH, Chen CY, Chang FY, Lin HC, Lee SD. Genetic polymorphisms of manganese superoxide dismutase, NAD(P)H:quinone oxidoreductase, glutathione S-transferase M1 and T1, and the susceptibility to drug-induced liver injury. J Hepatol. 2007;47:128–34. doi: 10.1016/j.jhep.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol. 2006;36:1028–38. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bethea NW, Abril ER, McCuskey RS. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10:391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- Jacob M, Mannherz HG, Napirei M. Chromatin breakdown by deoxyribonuclease1 promotes acetaminophen-induced liver necrosis: an ultrastructural and histochemical study on male CD-1 mice. Histochem Cell Biol. 2007;128:19–33. doi: 10.1007/s00418-007-0289-3. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–41. [PubMed] [Google Scholar]
- Jaeschke H. Enhanced sinusoidal glutathione efflux during endotoxin-induced oxidant stress in vivo. Am J Physiol. 1992;263:G60–8. doi: 10.1152/ajpgi.1992.263.1.G60. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Mechanisms of Liver Injury II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–8. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Antioxidant defense mechanisms. In: McQueen CA, editor. Comprehensive Toxicology. Vol. 9. Academic Press; Oxford: 2010. pp. 319–37. [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–9. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–84. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–82. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–6. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–62. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–23. [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–9. [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–9. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–9. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ho YS, Fisher MA, Lawson JA, Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–50. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–88. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Koerner M, Williams CD. Activation of caspases during acetaminophen toxicity is a strain dependent phenomenon (abstract) Toxicol Sci. 2011a;120(Suppl 2):96. [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–57. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45:1588–9. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity-A clinically relevant model to test the efficacy of natural products. Life Sci. 2011b;88:737–45. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–53. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–84. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003a;37:1289–97. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003b;75:458–67. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- Jimenez-Rodriguezvila M, Caro-Patón A, Dueña-Laita A, Conde M, Coca MC, Martin-Lorente JL, Velasco A, Marañon A. Histological, ultrastructural and mitochondrial oxidative phosphorylation studies in liver of rats chronically treated with oral valproic acid. J Hepatol. 1985;1:453–65. doi: 10.1016/s0168-8278(85)80744-3. [DOI] [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–13. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- Kashimshetty R, Desai VG, Kale VM, Lee T, Moland CL, Branham WS, New LS, Chan EC, Younis H, Boelsterli UA. Underlying mitochondrial dysfunction triggers flutamide-induced oxidative liver injury in a mouse model of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. 2009;238:150–9. doi: 10.1016/j.taap.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Török M, Hänni A, Roberts P, Gasser R, Krähenbühl S. Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology. 2005;41:925–35. doi: 10.1002/hep.20634. [DOI] [PubMed] [Google Scholar]
- Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Jungermann K. Modulation by oxygen of zonal gene expression in liver studied in primary rat hepatocyte cultures. Cell Biol Toxicol. 1997;13:243–55. doi: 10.1023/a:1007427206391. [DOI] [PubMed] [Google Scholar]
- Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229–36. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181:133–41. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H. Peroxynitrite formation and sinusoidal endothelial cell injury during acetaminophen-induced hepatotoxicity in mice. Comp Hepatol. 2004;3(Suppl 1):S46. doi: 10.1186/1476-5926-2-S1-S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–20. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–75. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355–63. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci. 2010;117:101–8. doi: 10.1093/toxsci/kfq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Fujii H, Asakawa M, Yamamoto M, Maki A, Matsuda M, Rusyn I, Matsumoto Y. Functional heterogeneity of the kupffer cell population is involved in the mechanism of gadolinium chloride in rats administered endotoxin. J Surg Res. 2002;106:179–87. doi: 10.1006/jsre.2002.6434. [DOI] [PubMed] [Google Scholar]
- Küpeli E, Orhan DD, Yesilada E. Effect of Cistus laurifolius L. leaf extracts and flavonoids on acetaminophen-induced hepatotoxicity in mice. J Ethnopharmacol. 2006;103:455–60. doi: 10.1016/j.jep.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Kupsch K, Hertel S, Kreutzmann P, Wolf G, Wallesch CW, Siemen D, Schonfeld P. Impairment of mitochondrial function by minocycline. FEBS J. 2009;276:1729–38. doi: 10.1111/j.1742-4658.2009.06904.x. [DOI] [PubMed] [Google Scholar]
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–48. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–50. [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–21. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Seah QM, Tan RC, Sattabongkot J, Beerheide W, Boelsterli UA. Leflunomide or A77 1726 protect from acetaminophen-induced cell injury through inhibition of JNK-mediated mitochondrial permeability transition in immortalized human hepatocytes. Toxicol Appl Pharmacol. 2006;217:125–33. doi: 10.1016/j.taap.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Mitchell JR. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol. 1987;4:206–11. doi: 10.1016/s0168-8278(87)80081-8. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Smith CV, Hughes H, Mitchell JR. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984;73:124–33. doi: 10.1172/JCI111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–16. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–86. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Lheureux PE, Hanston P. Carnitine in the treatment of valproic-acid induced toxicity. Clin Toxicol (Phila) 2009;47:101–11. doi: 10.1080/15563650902752376. [DOI] [PubMed] [Google Scholar]
- Lim MS, Lim PL, Gupta R, Boelsterli UA. Critical role of free cytosolic calcium, but not uncoupling, in mitochondrial permeability transition and cell death induced by diclofenac oxidative metabolites in immortalized human hepatocytes. Toxicol Appl Pharmacol. 2006;217:322–31. doi: 10.1016/j.taap.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Lim PL, Tan W, Latchoumycandane C, Mok WC, Khoo YM, Lee HS, Sattabongkot J, Beerheide W, Lim SG, Tan TM, Boelsterli UA. Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol In Vitro. 2007;21:1390–401. doi: 10.1016/j.tiv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Limaye PB, Apte UM, Shankar K, Bucci TJ, Warbritton A, Mehendale HM. Calpain released from dying hepatocytes mediates progression of acute liver injury induced by model hepatotoxicants. Toxicol Appl Pharmacol. 2003;191:211–26. doi: 10.1016/s0041-008x(03)00250-3. [DOI] [PubMed] [Google Scholar]
- Liu P, Fisher MA, Farhood A, Smith CW, Jaeschke H. Beneficial effects of extracellular glutathione against endotoxin-induced liver injury during ischemia and reperfusion. Circ Shock. 1994;43:64–70. [PubMed] [Google Scholar]
- Liu P, McGuire GM, Fisher MA, Farhood A, Smith CW, Jaeschke H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol. 2008;14:2832–7. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–30. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Loguidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011;54:969–78. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- Lucena MI, García-Martín E, Andrade RJ, Martinez C, Stephens C, Ruiz JD, Ulzurrun E, Fernandez MC, Romero-Gomez M, Castiella A, Planas R, Durán JA, De Dios AM, Guarner C, Soriano G, Borraz Y, Agundez JA. Mitochondrial superoxide dismutase and glutathione peroxidase in idiosyncratic drug-induced liver injury. Hepatology. 2010;52:303–12. doi: 10.1002/hep.23668. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181-182:219–22. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Nakayama S, Horie T. Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology. 2002;35:544–51. doi: 10.1053/jhep.2002.31871. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–6. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mathews WR, Guido DM, Fisher MA, Jaeschke H. Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med. 1994;16:763–70. doi: 10.1016/0891-5849(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Matthews AM, Hinson JA, Roberts DW, Pumford NR. Comparison of covalent binding of acetaminophen and the regioisomer 3’-hydroxyacetanilide to mouse liver protein. Tox Lett. 1997;90:77–82. doi: 10.1016/s0378-4274(96)03831-3. [DOI] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Jaeschke H. Acetaminophen hepatotoxicity in humans: Mitochondrial injury and DNA fragmentation in overdose patients (abstract) Toxicol Sci. 2011a;120(Suppl. 2):97. [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011b;53:974–82. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SL, Mayeux PR, Bucci TJ, Warbritton AR, Irwin LK, Pumford NR, Hinson JA. Acetaminophen-induced hepatotoxicity in mice lacking inducible nitric oxide synthase activity. Nitric Oxide. 2001;5:432–41. doi: 10.1006/niox.2001.0385. [DOI] [PubMed] [Google Scholar]
- Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–95. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- Miramar MD, Costantini P, Ravagnan L, Saraiva LM, Haouzi D, Brothers G, Penninger JM, Peleato ML, Kroemer G, Susin SA. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J Biol Chem. 2001;276:16391–8. doi: 10.1074/jbc.M010498200. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–7. [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–51. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TG, Dietz EC, Anderson NL, Khairallah EA, Cohen SD, Nelson SD. A comparative study of mouse liver proteins arylated by reactive metabolites of acetaminophen and its nonhepatotoxic regioisomer, 3’-hydroxyacetanilide. Chem Res Toxicol. 1995;8:403–13. doi: 10.1021/tx00045a012. [DOI] [PubMed] [Google Scholar]
- Nakae D, Yoshiji H, Amanuma T, Kinugasa T, Farber JL, Konishi Y. Endocytosis-independent uptake of liposome-encapsulated superoxide dismutase prevents the killing of cultured hepatocytes by tert-butyl hydroperoxide. Arch Biochem Biophys. 1990;279:315–9. doi: 10.1016/0003-9861(90)90497-m. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–21. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Napirei M, Basnakian AG, Apostolov EO, Mannherz HG. Deoxyribonuclease 1 aggravates acetaminophen-induced liver necrosis in male CD-1 mice. Hepatology. 2006;43:297–305. doi: 10.1002/hep.21034. [DOI] [PubMed] [Google Scholar]
- Napirei M, Wulf S, Eulitz D, Mannherz HG, Kloeckl T. Comparative characterization of rat deoxyribonuclease 1 (Dnase1) and murine deoxyribonuclease 1-like 3 (Dnase1l3) Biochem J. 2005;389:355–64. doi: 10.1042/BJ20042124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napirei M, Wulf S, Mannherz HG. Chromatin breakdown during necrosis by serum Dnase1 and the plasminogen system. Arthritis Rheum. 2004;50:1873–83. doi: 10.1002/art.20267. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RC, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125–71. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol. 1997;272:C1286–94. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J. 1995;307:99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg E, Orrenius S, Zhivotovsky B. Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF) Biochem Biophys Res Commun. 2010;396:95–100. doi: 10.1016/j.bbrc.2010.02.163. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Imai Y, Matsura T, Kitagawa A, Yamada K. Preventive effect of neutropenia on carbon tetrachloride-induced hepatotoxicity in rats. J Appl Toxicol. 2006;26:178–86. doi: 10.1002/jat.1122. [DOI] [PubMed] [Google Scholar]
- Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97:205–13. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- Ong MM, Wang AS, Leow KY, Khoo YM, Boelsterli UA. Nimesulide-induced hepatic mitochondrial injury in heterozygous Sod2 (+/-) mice. Free Radic Biol Med. 2006;40:420–9. doi: 10.1016/j.freeradbiomed.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Park BK, Laverty H, Srivastava A, Antoine DJ, Naisbitt D, Williams DP. Drug bioactivation and protein adduct formation in the pathogenesis of drug-induced toxicity. Chem Biol Interact. 2011;192:30–36. doi: 10.1016/j.cbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Mansouri A, Berson A, Fromenty B. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010;196:311–65. doi: 10.1007/978-3-642-00663-0_11. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–4. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Przybocki JM, Reuhl KR, Thurman RG, Kauffman FC. Involvement of nonparenchymal cells in oxygen-dependent hepatic injury by allyl alcohol. Toxicol Appl Pharmacol. 1992;115:57–63. doi: 10.1016/0041-008x(92)90367-2. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–53. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3'-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv Exp Med Biol. 2001;500:663–73. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–88. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011a;45:156–64. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011b;251:226–33. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 1990;106:346–51. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- Rehman H, Ramshesh VK, Theruvath TP, Kim I, Currin RT, Giri S, Lemasters JJ, Zhong Z. NIM811 (N-methyl-4-isoleucine cyclosporine), a mitochondrial permeability transition inhibitor, attenuates cholestatic liver injury but not fibrosis in mice. J Pharmacol Exp Ther. 2008;327:699–706. doi: 10.1124/jpet.108.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 2005;312:509–16. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Roth RA, Dahm LJ. Neutrophil- and glutathione-mediated hepatotoxicity of alpha-naphthylisothiocyanate. Drug Metab Rev. 1997;29:153–65. doi: 10.3109/03602539709037578. [DOI] [PubMed] [Google Scholar]
- Rubin R, Farber JL. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984;228:450–9. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Rutherford AE, Hynan LS, Borges CB, Forcione DG, Blackard JT, Lin W, Gorman AR, Shaikh OS, Reuben A, Harrison E, Reddy KR, Le WM, Chung RT, ALF Study Group Serum apoptosis markers in acute liver failure: a pilot study. Clin Gastroenterol Hepatol. 2007;5:1477–83. doi: 10.1016/j.cgh.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010a;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–54. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhanick SD, Orlow D, Holt DE, Pavlides S, Reenstra W, Buras JA. Endothelially derived nitric oxide affects the severity of early acetaminophen-induced hepatic injury in mice. Acad Emerg Med. 2006;13:479–85. doi: 10.1197/j.aem.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Sauer JM, Waalkes MP, Hooser SB, Kuester RK, McQueen CA, Sipes IG. Suppression of Kupffer cell function prevents cadmium induced hepatocellular necrosis in the male Sprague-Dawley rat. Toxicology. 1997;121:155–64. doi: 10.1016/s0300-483x(97)00062-0. [DOI] [PubMed] [Google Scholar]
- Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R, Schildberg FW, Messmer K, Bilzer M. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg. 2004;239:220–31. doi: 10.1097/01.sla.0000110321.64275.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer RJ, Gerbes AL, Vonier D, op den Winkel M, Fraunberger P, Bilzer M. Induction of cellular resistance against Kupffer cell-derived oxidant stress: a novel concept of hepatoprotection by ischemic preconditioning. Hepatology. 2003;37:286–95. doi: 10.1053/jhep.2003.50064. [DOI] [PubMed] [Google Scholar]
- Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr. 1985;5:365–90. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca(2+)-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol Appl Pharmacol. 1992;112:32–40. doi: 10.1016/0041-008x(92)90276-x. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–55. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu WP, Pun PB, Latchoumycandane C, Boelsterli UA. Bax-mediated mitochondrial outer membrane permeabilization (MOMP), distinct from the mitochondrial permeability transition, is a key mechanism in diclofenac-induced hepatocyte injury: multiple protective roles of cyclosprin A. Toxicol Appl Pharmacol. 2008;227:451–61. doi: 10.1016/j.taap.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Smith CV. Evidence for participation of lipid peroxidation and iron in diquat-induced hepatic necrosis in vivo. Mol Pharmacol. 1987;32:417–22. [PubMed] [Google Scholar]
- Smith CV, Hughes H, Lauterburg BH, Mitchell JR. Oxidant stress and hepatic necrosis in rats treated with diquat. J Pharmacol Exp Ther. 1985;235:172–7. [PubMed] [Google Scholar]
- Smith CV, Jaeschke H. Effect of acetaminophen on hepatic content and biliary efflux of glutathione disulfide in mice. Chem Biol Interact. 1989;70:241–48. doi: 10.1016/0009-2797(89)90047-1. [DOI] [PubMed] [Google Scholar]
- Starke PE, Farber JL. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985;260:10099–104. [PubMed] [Google Scholar]
- Starke PE, Gilbertson JD, Farber JL. Lysosomal origin of the ferric iron required for cell killing by hydrogen peroxide. Biochem Biophys Res Commun. 1985;133:371–9. doi: 10.1016/0006-291x(85)90916-7. [DOI] [PubMed] [Google Scholar]
- Stewart JD, Horvath R, Baruffini E, Ferrero I, Bulst S, Watkins PB, Fontana RJ, Day CP, Chinnery PF. Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52:1791–6. doi: 10.1002/hep.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporine (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–46. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9. [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem. 1990;265:3059–65. [PubMed] [Google Scholar]
- Tong V, Teng XW, Chang TK, Abbott FS. Valproic acid I: time course of lipid peroxidation in biomarkers, liver toxicity, and valproic acid metabolite levels in rats. Toxicol Sci. 2005a;86:427–35. doi: 10.1093/toxsci/kfi184. [DOI] [PubMed] [Google Scholar]
- Tong V, Teng XW, Chang TK, Abbott FS. Valproic acid II: effects on oxidative stress, mitochondrial membrane potential, and cytotoxicity in glutathione-depleted rat hepatocytes. Toxicol Sci. 2005b;86:436–43. doi: 10.1093/toxsci/kfi185. [DOI] [PubMed] [Google Scholar]
- Toppo S, Flohé L, Ursini F, Vanin S, Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta. 2009;1790:1486–500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Trost LC, Lemasters JJ. Role of the mitochondrial permeability transition in salicylate toxicity to cultured rat hepatocytes: implications for the pathogenesis of Reye's syndrome. Toxicol Appl Pharmacol. 1997;147:431–41. doi: 10.1006/taap.1997.8313. [DOI] [PubMed] [Google Scholar]
- Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48:1644–54. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. Immunoallergic drug-induced liver injury in humans. Semin Liver Dis. 2009;29:383–92. doi: 10.1055/s-0029-1240007. [DOI] [PubMed] [Google Scholar]
- Vahsen N, Candé C, Brière JJ, Bénit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, Feraud O, Debili N, Wissing S, Engelhardt S, Madeo F, Piacentini M, Penninger JM, Schägger H, Rustin P, Kroemer G. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–89. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann X, Anstaett M, Hadem J, Stiefel P, Bahr MJ, Lehner F, Manns MP, Schulze-Osthoff K, Bantel H. Caspase activation is associated with spontaneous recovery from acute liver failure. Hepatology. 2008;47:1624–33. doi: 10.1002/hep.22237. [DOI] [PubMed] [Google Scholar]
- Waldhauser KM, Turuk M, Ha HR, Thomet U, Konrad D, Brecht K, Follath F, Krähenbühl S. Hepatocellular toxicity and pharmacological effect of amiodarone and amiodarone derivatives. J Pharmacol Exp Ther. 2006;319:1413–23. doi: 10.1124/jpet.106.108993. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Tomita A, Shimizu M, Sugawara M, Yasumo H, Koishi R, Takahashi T, Miyoshi K, Nakamura K, Izumi T, Matsushita Y, Furukawa H, Haruyama H, Koga T. A study to survey susceptible genetic factors responsible for troglitazone-associated hepatotoxicity in Japanese patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2003;73:435–55. doi: 10.1016/s0009-9236(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78:3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- Wendel A, Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981;30:2513–20. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- Wendel A, Feuerstein S, Konz KH. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979;28:2051–5. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- Wendel A, Jaeschke H, Gloger M. Drug-induced lipid peroxidation in mice--II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol. 1982;31:3601–5. doi: 10.1016/0006-2952(82)90582-2. [DOI] [PubMed] [Google Scholar]
- Werner C, Wendel A. Hepatic uptake and antihepatotoxic properties of vitamin E and liposomes in the mouse. Chem Biol Interact. 1990;75:83–92. doi: 10.1016/0009-2797(90)90024-h. [DOI] [PubMed] [Google Scholar]
- Widlak P, Garrard WT. Discovery, regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J Cell Biochem. 2005;94:1078–87. doi: 10.1002/jcb.20409. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010a;30:1280–92. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010b;247:169–78. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YL, Piao DM, Han XH, Nan JX. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol Pharm Bull. 2008;31:1523–9. doi: 10.1248/bpb.31.1523. [DOI] [PubMed] [Google Scholar]
- Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117:515–23. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MY, Kim SJ, Lee BH, Chung JH, Kim YC. Effects of dimethylsulfoxide on metabolism and toxicity of acetaminophen in mice. Biol Pharm Bull. 2006;29:1618–24. doi: 10.1248/bpb.29.1618. [DOI] [PubMed] [Google Scholar]
- You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–31. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- Yuan HD, Jin GZ, Piao GC. Hepatoprotective effects of an active part from Artemisia sacrorum Ledeb. against acetaminophen-induced toxicity in mice. J Ethnopharmacol. 2010;127:528–33. doi: 10.1016/j.jep.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu F, Chen X, Zhu X, Uetrecht J. Involvement of the immune system in idiosyncratic drug reactions. Drug Metab Pharmacokinet. 2011;26:47–59. doi: 10.2133/dmpk.dmpk-10-rv-085. [DOI] [PubMed] [Google Scholar]