Abstract
Introduction
This study investigates social determinants of systemic inflammation, focusing on race, SES, and perceived discrimination.
Methods
Data on 884 white and 170 black participants were obtained from the Survey of Midlife in the U.S., a cross-sectional observational study combining survey measures, anthropometry, and biomarker assay. Data, collected in 2004–2009, were analyzed in 2016. Main outcome measures were fasting blood concentrations of C-reactive protein, interleukin 6, fibrinogen, and E-selectin. For each biomarker, series of multivariate linear regression models were estimated for the pooled sample and separately for blacks and whites. Full models included social determinants; psychological, lifestyle, and health factors; and demographic covariates.
Results
Bivariate analyses indicated higher concentrations of all inflammation markers among blacks compared with whites (p<0.001). In fully adjusted models using the pooled sample, racial differences persisted for interleukin 6 (p<0.001) and fibrinogen (p<0.01). For E-selectin and C-reactive protein, racial differences were explained after adjusting for covariates. Education was linked to lower fibrinogen concentration (p<0.05) in the fully adjusted model and C-reactive protein concentration (p<0.01) after adjusting for demographic factors and income. Lifetime perceived discrimination was related to higher concentrations of fibrinogen (p<0.05) in the fully adjusted model, and higher concentrations of E-selectin and interleukin 6 (p<0.05) after adjusting for socioeconomic status (SES) and demographic factors.
Conclusions
This study clarifies the contributions of race, SES, and perceived discrimination to inflammation. It suggests that inflammation-reducing interventions should focus on blacks and individuals facing socioeconomic disadvantages, especially low education.
INTRODUCTION
Systemic inflammation has received attention as a preventable factor in chronic conditions such as hypertension,1 cardiovascular disease,2,3 insulin resistance,4 Type 2 diabetes,3,5–7 and cancer.8–10 Smoking,11 alcohol consumption,12 sedentary lifestyle,13,14 and obesity,4,15,16 are established factors in inflammation. Recent research, however, indicates that social determinants are as important—if not more important—as health behaviors for shaping health.17,18 In fact, social determinants affect both the biological processes and health lifestyles of individuals.
Key social determinants of health include SES and race/ethnicity.19–21 Higher inflammation levels among racial/ethnic minorities, especially blacks,22–24 and individuals with lower SES25,26 have been reported, but several investigations do not corroborate these findings.1,27 Others argue that the role of SES in inflammation varies with SES measures28 and racial/ethnic background.29
Perceived discrimination (PD) has been suggested as another social factor with relevance to inflammation. PD has been associated with inflammation among young adults,30 midlife adults,31 low-income black youths,32 and older blacks,33 although the Dallas Heart Study showed no relationship between PD and inflammation among blacks, Hispanics, and whites.34 In other studies, the link between PD and inflammation was limited to specific subpopulations, including women anticipating a racial threat35 and non-obese women.36
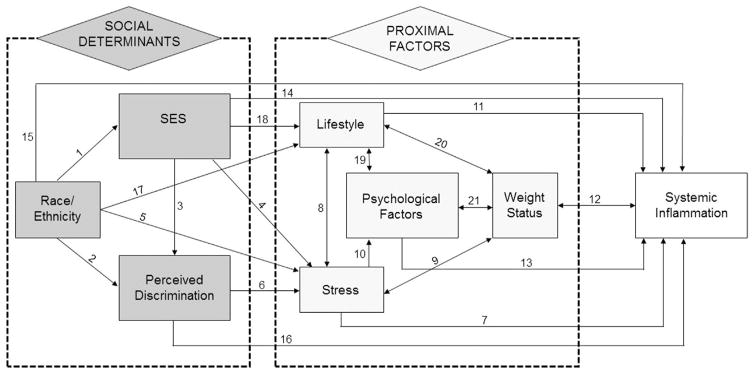
One important limitation of most prior research on inflammation is the lack of a theoretically grounded framework. To address this limitation, this study proposes a conceptual model of social determinants of inflammation (Figure 1) informed by fundamental cause theory (FCT), a sociological perspective. FCT postulates social determinants as key causes shaping health outcomes through multiple pathways that can evolve dynamically across life stages and historic periods in response to societal and technologic changes.37–39
Figure 1.
Social determinants of systemic inflammation: conceptual model.
Using the FCT, the proposed model specifies that SES, race, and PD act as key social determinants of inflammation. SES is a multidimensional construct consisting of income, education, and occupational prestige40; this study focuses more specifically on income and education as dimensions strongly associated with health outcomes in the U.S.41,42 In the conceptual model, minority race contributes to lower SES20 (Arrow 1) and to higher PD, which is also linked to lower SES43–47 (Arrows 2 and 3). Proximal factors are those through which social determinants influence inflammation. Stress, an established mechanism leading to poor health,48–50 is linked to all three social determinants specified in this model, with minorities, persons of lower SES, and those exposed to discrimination experiencing higher stress (Arrows 4–6). Stress harms health through overactivation of the biological stress response system, which may directly lead to increased inflammation (Arrow 7); furthermore, it contributes to unhealthy lifestyle and poorer psychological health (Arrows 8–10), which are also implicated in inflammation (Arrows 11–13).11–14,51–54
Figure 1 illustrates the complexity of relations among social determinants and inflammation. In addition to effects through proximal factors, the model allows for direct effects of unspecified mechanisms (Arrows 14–16). Reciprocal relationships reflect yet-unknown causal direction. Because of the model’s complexity, comprehensive evaluation is outside the scope of this study. Instead, the study focuses on evaluating select components using three hypotheses:
Inflammation levels are higher among blacks compared with whites.
Inflammation levels decrease with higher SES.
Inflammation levels are higher among persons reporting PD.
This study sequentially evaluates the contribution of each group of proximal factors as suggested by the conceptual model, using a hierarchy-of-effects approach.55 In addition to testing for the pooled sample, inflammation is modeled separately for blacks and whites because factors contributing to inflammation may vary by race/ethnicity.29,30,51–54
METHODS
Data Sample
Data were obtained from the Survey of Midlife in the U.S., an ongoing national survey using a random-digit-dial sample representative of non-institutionalized English-speaking residents of 48 contiguous U.S. states who are aged ≥35 years.56 The present study was limited to 1,054 participants in the biomarker sub-study that collected biological specimens (whites, n=884; blacks, n=170). Sub-study participants are similar to the national sample on age, sex, race, marital status, income, and health characteristics (subjective health, chronic conditions, activities of daily living, exercise, alcohol consumption, health insurance, physician visits) but are more educated and less likely to smoke.57 Data collection took place between 2004 and 2009. Biological specimens and anthropometry were collected by trained staff during an overnight clinic stay. Demographic, social, and psychological indicators were measured using mail surveys and telephone interviews.
Measures
Biomarkers of systemic inflammation included C-reactive protein (CRP), which is produced by hepatocytes in response to infection or injury58; interleukin 6 (IL-6), a proinflammatory cytokine; fibrinogen, a blood clotting factor involved in the coagulation response to vascular injury59; and soluble E-selectin, an endothelial adhesion molecule expressed as a result of endothelial damage.60 Fibrinogen concentrations (mg/dL) and CRP concentrations (ug/mL) were measured in citrated plasma using immunoturbidometric assay. Soluble E-selectin concentrations (ng/mL) and IL-6 concentrations (pg/mL) were measured in serum using enzyme-linked immunosorbent assay. Standardized procedures were used for fasting blood samples collection and processing.
Race was self-reported and categorized as black and white. Dimensions of SES were years of education and annual household income from all sources, measured in U.S. dollars and log-transformed. Age in years and gender (woman=1, man=0) were also included.
The Daily Discrimination and Lifetime Discrimination scales61 were used to measure PD. Consistent with the argument that PD is harmful to health regardless of the reason (race/ethnicity, gender, or others),47 these scales measure PD experiences of any type. The Daily Discrimination scale asks respondents how often they experience each of nine types of discrimination (e.g., being treated with less courtesy, less respect, or receiving poorer service at restaurants because of race/ethnicity, gender, age, religion, physical appearance, sexual orientation, or other characteristics [never=1, rarely=2, sometimes=3, often=4]). The Daily Discrimination scale totals the responses; higher values indicate higher levels of perceived discrimination. The Lifetime Discrimination scale measures experiences of major discriminatory events in life domains including employment, education, health care, and housing. Examples include not being hired for a job or being prevented from renting or buying a home. Respondents are asked how many times in their lifetime they have experienced each event. Lifetime discrimination is calculated as a total of items for which respondents indicate experiencing the event at least once.
Measures of generalized anxiety and depressed affect were based on Wang et al.62 The generalized anxiety scale consists of ten items, for example: How often over the past 12 months were you restless because of your worry?; the scale totals items for which most days was chosen. For depressed affect, respondents were asked: During the past 12 months, was there ever a time when you felt sad, blue, or depressed for 2 weeks or more in a row? (yes/no), and an additional seven items, for example, During two weeks in past 12 months, when you felt sad, blue, or depressed, did you feel more tired out or low on energy than is usual? (yes/no). The depressed affect scale totals yes answers on these seven items. This study also used three scales representing Negative Emotionality in Multidimensional Personality Questionnaire63: stress reactivity (three items; e.g., minor setbacks sometimes irritate me too much), aggression (four items; e.g., when I get angry I am often ready to hit someone), and alienation (three items; e.g., I would be more successful if people did not make things difficult for me). Items use a 1–4 response scale (false to true). A sum of responses is calculated for each scale.
Participants’ BMI, a measure of weight status (calculated as kg/m2), was based on anthropometric data. Two dichotomous indicators for weekly strenuous physical activity and weekly moderate physical activity were included (Appendix 1, available online), as well as two indicators of smoking capturing whether respondents ever smoked cigarettes regularly and whether they currently smoked cigarettes regularly (both yes/no). Because of potential effects on inflammation, current preventive use of aspirin was included. Finally, models controlled for chronic conditions during the past 12 months that had prevalence ≥5% in the sample and showed relationships with inflammation at p<0.10 in preliminary analyses. These conditions included high blood pressure/hypertension (henceforth hypertension), diabetes/high blood sugar (henceforth diabetes), joint/bone diseases, persistent skin trouble, teeth trouble, and sleep problems.
Statistical Analysis
First, descriptive statistics for the pooled sample and by race were obtained. T-tests were used to compare blacks with whites on continuous variables, and chi-square tests were performed for categorical variables. Next, series of multivariate linear regression models of each inflammation marker were estimated; robust estimators accounted for deviations from normality. Because CRP and IL-6 had skewed distributions, they were log-transformed for modeling purposes. Model 1 included race and demographic covariates (gender, age). Model 2 added income and education. Model 3 further added PD measures. Model 4 added psychological factors. Model 5 added lifestyle (smoking indicators, physical activity indicators, and BMI). Finally, Model 6 added health characteristics, including preventive use of aspirin and chronic conditions. After estimating multivariate models for the pooled sample, Models 2–6 were estimated separately by race.
RESULTS
As shown in Table 1, blacks had higher concentrations of all four biomarkers of inflammation compared with whites (p<0.001). They had lower SES as indicated by fewer years of education and lower income, and scored higher on both measures of PD, as well as generalized anxiety, alienation, and BMI (p-values<0.001). They were also more likely to smoke regularly (p<0.01) and less likely to engage in weekly physical activity (vigorous, p<0.05; moderate, p<0.001). They had higher rates of diabetes, teeth problems (p-values<0.001), joint/bone disease, and sleep problems (p-values<0.01), but lower rates of hypertension (p<0.001) and preventive aspirin use (p<0.05). Demographically, blacks were younger (p<0.001) and more commonly women (p<0.01).
Table 1.
Characteristics of the Pooled Sample and by Racial Background
| Variablea | All (n=1,054) | Blacks (n=170) | Whites (n=884) |
|---|---|---|---|
| Systemic inflammation markers | |||
| Fibrinogen, mg/dL (range, 94.0–857.0) | 348.36 (88.24) | 388.70 (101.67)*** | 340.61 (83.27) |
| E-selectin, ng/mL (range, 0.1–161.9) | 42.51 (22.01) | 49.19 (25.75)*** | 41.22 (20.99) |
| CRP, ug/mL (range, 0.1–59.3) | 3.11 (5.01) | 4.78 (6.90)*** | 2.79 (4.49) |
| IL-6, pg/mL (range, 0.2–21.8) | 2.97 (2.90) | 3.91 (3.05)*** | 2.79 (2.83) |
| SES | |||
| Education, years (range, 2–20) | 14.75 (2.56) | 13.58 (2.75)*** | 14.98 (2.46) |
| Income, log $ (range, 0–30) | 10.27 (3.77) | 9.81 (3.45)*** | 10.36 (3.83) |
| Perceived discrimination | |||
| Daily (range, 9–32) | 12.87 (4.60) | 14.65 (6.46)*** | 12.53 (4.07) |
| Lifetime (range, 0–11) | 1.23 (1.90) | 3.02 (2.82)*** | 0.88 (1.44) |
| Psychological factors | |||
| Depression (range, 0–7) | 0.72 (1.85) | 0.88 (2.06) | 0.69 (1.81) |
| Anxiety (range, 0–10) | 0.15 (.94) | 0.41 (1.64)*** | 0.10 (.73) |
| Stress reactivity (range, 3–12) | 6.15 (2.31) | 6.45 (2.61) | 6.09 (2.24) |
| Aggression (range, 4–14) | 5.31 (1.66) | 5.36 (1.65) | 5.30 (1.66) |
| Alienation (range, 3–12) | 5.14 (1.89) | 6.04 (2.39)*** | 4.96 (1.73) |
| Lifestyle factors | |||
| Ever regular smoker | 44.6 | 55.9** | 42.4 |
| Currently regular smoker | 12.9 | 25.9*** | 10.4 |
| Vigorous physical activity | 30.5 | 22.4* | 32.0 |
| Moderate physical activity | 44.1 | 30.6*** | 46.7 |
| BMI (range, 14.23–161.10) | 30.6 (13.99) | 34.59 (17.79)*** | 29.84 (13.01) |
| Health factors | |||
| Preventive aspirin | 31.2 | 24.7* | 32.5 |
| Chronic conditions | |||
| Hypertension | 72.0 | 58.8*** | 75.0 |
| Diabetes | 10.0 | 18.8*** | 8.3 |
| Joint/bone diseases | 27.0 | 37.6** | 25.0 |
| Persistent skin trouble | 9.1 | 7.1 | 9.5 |
| Teeth problems | 6.5 | 14.1*** | 5.1 |
| Sleep problems | 12.6 | 20.6** | 11.1 |
| Demographic factors | |||
| Age, y (range, 35–82) | 54.56 (11.62) | 51.27 (10.48)*** | 55.19 (11.73) |
| Woman | 56.6 | 67.6** | 54.5 |
Source: Survey of Midlife in the U.S. (MIDUS II).
Note: Values are M (SD) for continuous variables and percentages for dichotomous variables. T-tests were used to compare blacks to whites on continuous variables. χ2 tests were used to compare blacks to whites on categorical variables. Boldface indicates statistical significance of the differences between blacks and whites (*p<0.05, **p<0.01, ***p<0.001; two-tailed tests).
Ranges are given for continuous variables only.
CRP, C-reactive protein; IL-6, interleukin 6; y, years.
Table 2 summarizes results of multivariate models of inflammation markers for the pooled sample. Because of space limitations, only Models 2, 3, and 6 are displayed; Models 1, 4, and 5 appear in Appendix 2 (available online). In Model 3, which included race, SES, PD, and demographic covariates, blacks had higher concentrations of fibrinogen (p>0.001), IL-6 (p>0.001), CRP (p<0.01), and E-selectin (p<0.05), lending support to Hypothesis 1. In fully adjusted models (Model 6), higher levels of IL-6 (p<0.001) and fibrinogen (p<0.01) among blacks persisted, though the coefficients underwent attenuation. For E-selectin, the black–white difference was explained after including PD, SES, psychological characteristics, and lifestyle factors in Model 5 (Table 1); for CRP, the difference was explained when health factors were controlled in Model 6. Hypothesis 2, which argues that inflammation decreases with SES, was supported for education but not for income in the pooled sample. Individuals with higher education had lower concentrations of fibrinogen (p<0.05 in Model 6) and CRP (p<0.01 in Model 3); a trend toward lower E-selectin was evident as well (p<0.10 in Model 3). Lifetime perceived discrimination was related to higher concentrations of fibrinogen (p<0.05 in Model 6), E-selectin (p<0.05 in Model 3), and IL-6 (p<0.05 in Model 3). These results support Hypothesis 3, which predicts increased inflammation with PD.
Table 2.
Coefficients from Linear Regression Models for Systemic Inflammation Markers: Pooled Sample (n=1,054)
| Variable | Fibrinogena | E-selectinb | CRPc | IL-6d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | |
| Demographic factors | ||||||||||||
|
| ||||||||||||
| Age, y | 1.13*** (0.25) | 1.17*** (0.25) | 1.07*** (0.28) | −0.19** (0.05) | −0.17** (0.06) | −0.23** (0.07) | 0.005 (0.003) | 0.01 (0.003) | 0.001 (0.003) | 0.01*** (0.002) | 0.01*** (0.002) | 0.01*** (0.002) |
|
| ||||||||||||
| Woman | 29.51*** (5.15) | 28.33*** (5.14) | 30.18** (5.46) | −4.25** (1.35) | −4.54** (1.34) | −4.15** (1.40) | 0.37*** (0.07) | 0.36*** (0.07) | 0.07*** (0.39) | 0.06 (0.04) | 0.05 (0.05) | 0.07 (0.05) |
|
| ||||||||||||
| Social determinants | ||||||||||||
|
| ||||||||||||
| Race, black | 45.49*** (8.34) | 34.53*** (9.51) | 29.25** (9.71) | 7.25** (2.10) | 4.52* (2.28) | 2.52 (2.34) | 0.45*** (0.10) | 0.36** (0.11) | 0.16 (0.12) | 0.43*** (0.06) | 0.36*** (0.07) | 0.28*** (0.07) |
|
| ||||||||||||
| SES | ||||||||||||
|
| ||||||||||||
| Education, y | −2.59** (1.07) | −2.86** (1.09) | −2.34* (1.10) | −0.43 (0.027) | −0.46 (0.27) | −0.21 (0.28) | −0.04** (0.01) | −0.04** (0.02) | −0.03 (0.02) | −0.01 (0.01) | −0.02 (0.01) | −0.001 (0.01) |
|
| ||||||||||||
| Income, log $ | 0.82 (0.56) | 0.91 (0.56) | 0.82 (0.57) | 0.11 (0.014) | 0.13 (0.13) | 0.08 (0.15) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.0004 (0.01) | 0.001 (0.01) | −0.003 (0.01) |
|
| ||||||||||||
| Perceived discrimination | ||||||||||||
|
| ||||||||||||
| Daily | −0.06 (0.69) | −0.42 (0.73) | 0.18 (0.18) | 0.07 (0.19) | 0.01 (0.01) | 0.003 (0.01) | 0.002 (0.01) | −0.01 (0.01) | ||||
|
| ||||||||||||
| Lifetime | 5.18** (1.95) | 4.52* (1.90) | 1.14* (0.47) | 0.81 (0.48) | 0.04 (0.02) | 0.02 (0.02) | 0.03* (0.01) | 0.02 (0.01) | ||||
|
| ||||||||||||
| Psychological factors | ||||||||||||
|
| ||||||||||||
| Depressed affect | 0.46 (1.71) | 0.10 (0.43) | −0.01 (0.02) | 0.02 (0.02) | ||||||||
|
| ||||||||||||
| General anxiety | −2.33 (2.66) | −1.71** (0.62) | 0.05 (0.04) | 0.001 (0.03) | ||||||||
|
| ||||||||||||
| Stress reactivity | −0.88 (1.37) | −0.33 (0.38) | −0.01 (0.02) | −0.02 (0.01) | ||||||||
|
| ||||||||||||
| Aggression | 0.14 (1.89) | 0.32 (0.47) | −0.01 (0.02) | 0.02 (0.01) | ||||||||
|
| ||||||||||||
| Alienation | 1.31 (1.68) | 0.53 (0.47) | 0.02 (0.02) | 0.03 (0.01) | ||||||||
|
| ||||||||||||
| Lifestyle factors | ||||||||||||
|
| ||||||||||||
| Ever regular smoker | −3.30 (6.11) | 1.51 (1.52) | 0.04 (0.08) | 0.07 (0.05) | ||||||||
|
| ||||||||||||
| Currently regular smoker | 0.78 (9.05) | 2.18 (2.63) | 0.06 (0.21) | 0.01 (0.07) | ||||||||
|
| ||||||||||||
| Vigorous physical activity | 1.88 (6.63) | −1.98 (1.64) | −0.07 (0.09) | −0.12* (0.06) | ||||||||
|
| ||||||||||||
| Moderate physical activity | −9.65 (6.15) | −0.98 (1.55) | −0.16 (0.08) | −0.07 (0.05) | ||||||||
|
| ||||||||||||
| BMI | 0.63* (0.24) | 0.02 (0.04) | 0.01*** (0.003) | 0.01*** (0.0002) | ||||||||
|
| ||||||||||||
| Health factors | ||||||||||||
|
| ||||||||||||
| Preventive aspirin | 5.13 (6.51) | −1.21 (1.65) | 0.13 (0.08) | 0.02 (0.05) | ||||||||
|
| ||||||||||||
| Hypertension | −6.43 (6.72) | −2.34 (1.62) | −0.24** (0.08) | −0.18** (0.05) | ||||||||
|
| ||||||||||||
| Diabetes | 16.14 (9.75) | 8.75** (2.84) | 0.31** (0.11) | 0.06 (0.07) | ||||||||
|
| ||||||||||||
| Joint/bone diseases | −8.06 (6.72) | 1.68 (1.65) | 0.09 (0.08) | −0.01 (0.05) | ||||||||
|
| ||||||||||||
| Persistent skin trouble | 14.01 (10.17) | 0.94 (2.69) | −0.08 (0.12) | 0.12 (0.08) | ||||||||
|
| ||||||||||||
| Teeth problems | 17.93 (11.90) | 4.21 (3.81) | 0.26 (0.15) | 0.04 (0.09) | ||||||||
|
| ||||||||||||
| Sleep problems | 3.89 (9.00) | 0.18 (2.33) | 0.13 (0.12) | 0.07 (0.07) | ||||||||
|
| ||||||||||||
| Intercept | 292.40*** | 290.03*** | 284.89*** | 59.31*** | 55.32*** | 57.42*** | 0.38 | 0.24 | 0.46 | 0.15 | 0.09 | 0.12 |
|
| ||||||||||||
| Model F | 17.94*** | 15.08*** | 5.69*** | 8.24*** | 7.82*** | 3.33*** | 14.10*** | 11.66*** | 7.82*** | 23.16*** | 17.30*** | 8.77*** |
|
| ||||||||||||
| R2, % | 9.45 | 10.44 | 13.23 | 3.83 | 5.06 | 8.74 | 6.36 | 6.86 | 14.10 | 8.85 | 9.46 | 15.68 |
Source: Survey of Midlife in the U.S. (MIDUS II).
Note: Values are coefficients unless otherwise noted; standard errors appear in parentheses. Robust estimators are used. Boldface indicates statistical significance (*p<0.05, **p<0.01, ***p<0.001; two-tailed tests).
mg/dL.
ng/mL.
log ug/mL.
log pg/mL.
CRP, C-reactive protein; IL-6, interleukin 6; y, years.
Tables 3 and 4 shows multivariate model estimates separately for whites and blacks. Results for whites were similar to the pooled sample and generally supportive of Hypothesis 2; one exception concerns income, which showed a positive relation to E-selectin (p<0.05 in Model 6). Models for blacks supported the hypothesized inverse relationships between education and CRP (p<0.05, Model 3) and between income on E-selectin (p<0.01, Model 6). Hypothesis 3, however, was not supported for blacks, except for a marginally significant relationship between fibrinogen and lifetime perceived discrimination in Model 3 (p<0.10).
Table 3.
Coefficients from Linear Regression Models for Systemic Inflammation Markers by Racial Background
| Variable | Whites (n=884) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Fibrinogena | E-selectinb | CRPc | IL-6d | |||||||||
|
|
|
|
|
|||||||||
| Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | |
| Demographic factors | ||||||||||||
|
| ||||||||||||
| Age, y | 0.16*** (0.25) | 1.20*** (0.25) | 1.17*** (0.29) | −0.15** (0.06) | −0.12* (0.06) | −0.19** (0.07) | 0.01* (0.003) | 0.01* (0.004) | 0.002 (0.004) | 0.02*** (0.002) | 0.01*** (0.002) | 0.01*** (0.002) |
|
| ||||||||||||
| Woman | 24.93*** (5.43) | 23.11*** (5.37) | 26.91*** (4.65) | −4.66** (1.43) | −5.21**-* (1.43) | −4.56** (1.50) | 0.34*** (0.08) | 0.32*** (0.08) | 0.37*** (0.08) | 0.03 (0.05) | 0.004 (0.05) | 0.04 (0.05) |
|
| ||||||||||||
| Social determinants | ||||||||||||
|
| ||||||||||||
| SES | ||||||||||||
|
| ||||||||||||
| Education, y | −2.87* (1.20) | −3.06* (1.23) | −2.33 (1.22) | −0.54 (0.29) | −0.48 (0.29) | −0.23 (0.29) | −0.04* (0.02) | −0.04* (0.02) | −0.02 (0.02) | −0.013 (0.01) | −0.01 (0.01) | 0.0004 (0.01) |
|
| ||||||||||||
| Income, log $ | 0.71 (0.48) | 0.76 (0.50) | 0.62 (0.51) | 0.31** (0.10) | 0.33* (0.11) | 0.31* (0.12) | 0.02 (0.01) | 0.02 (0.01) | 0.01 (0.01) | −0.001 (0.01) | −0.0003 (0.01) | −0.003 (0.01) |
|
| ||||||||||||
| Perceived discrimination | ||||||||||||
|
| ||||||||||||
| Daily | −0.39 (0.78) | −0.59 (0.81) | 0.37 (0.20) | 0.23 (0.22) | 0.01 (0.01) | 0.002 (0.01) | 0.01 (0.01) | 0.001 (0.01) | ||||
|
| ||||||||||||
| Lifetime | 5.39* (2.47) | 4.47 (2.35) | 1.21* (0.61) | 0.70 (0.62) | 0.05 (0.03) | 0.03 (0.03) | 0.06** (0.02) | 0.04* (0.02) | ||||
|
| ||||||||||||
| Psychological factors | ||||||||||||
|
| ||||||||||||
| Depressed affect | −0.51 (1.82) | −0.27 (0.43) | −0.01 (0.02) | 0.02 (0.02) | ||||||||
|
| ||||||||||||
| General anxiety | −1.17 (2.99) | −1.37 (0.96) | 0.002 (0.05) | −0.02 (0.03) | ||||||||
|
| ||||||||||||
| Stress reactivity | −1.74 (1.46) | −0.66 (0.40) | −0.01 (0.02) | −0.03* (0.01) | ||||||||
|
| ||||||||||||
| Aggression | 1.13 (2.01) | 0.41 (0.49) | 0.003 (0.03) | 0.02 (0.02) | ||||||||
|
| ||||||||||||
| Alienation | 1.79 (1.93) | 1.14* (0.54) | 0.01 (0.03) | 0.02 (0.02) | ||||||||
|
| ||||||||||||
| Lifestyle factors | ||||||||||||
|
| ||||||||||||
| Ever regular smoker | −2.03 (6.31) | 2.29 (1.61) | 0.05 (0.09) | 0.07 (0.05) | ||||||||
|
| ||||||||||||
| Currently regular smoker | 2.58 (9.91) | −1.02 (2.83) | 0.03 (0.14) | −0.04 (0.08) | ||||||||
|
| ||||||||||||
| Vigorous physical activity | 0.07 (7.02) | −2.04 (1.70) | −0.09 (0.10) | −0.15* (0.06) | ||||||||
|
| ||||||||||||
| Moderate physical activity | −10.17 (6.44) | −0.71 (1.61) | −0.20* (0.09) | −0.08 (0.06) | ||||||||
|
| ||||||||||||
| BMI | 0.48 (0.31) | 0.09 (0.05) | 0.01** (0.004) | 0.01** (0.002) | ||||||||
|
| ||||||||||||
| Health factors | ||||||||||||
|
| ||||||||||||
| Preventive aspirin | 4.27 (6.95) | −1.45 (1.72) | 0.13 (0.09) | 0.03 (0.06) | ||||||||
|
| ||||||||||||
| Hypertension | −4.67 (7.48) | −1.18 (1.70) | −0.18 (0.10) | −0.19** (0.06) | ||||||||
|
| ||||||||||||
| Diabetes | 14.77 (11.18) | 6.76* (3.27) | 0.37** (0.13) | 0.04 (0.09) | ||||||||
|
| ||||||||||||
| Joint/bone diseases | 14.02 (7.31) | 2.92 (1.73) | 0.04 (0.09) | −0.01 (0.06) | ||||||||
|
| ||||||||||||
| Persistent skin trouble | 15.98 (10.65) | −0.54 (2.49) | −0.01 (0.12) | 0.13 (0.09) | ||||||||
|
| ||||||||||||
| Teeth problems | 20.87 (15.09) | 7.70 (4.57) | 0.19 (0.19) | 0.09 (0.11) | ||||||||
|
| ||||||||||||
| Sleep problems | 5.84 (10.29) | 0.29 (2.44) | 0.13 (0.14) | 0.05 (0.09) | ||||||||
|
| ||||||||||||
| Intercept | 298.34*** | 299.59*** | 286.24*** | 56.94*** | 48.50*** | 45.83*** | 0.18 | 0.00 | 0.09 | 0.11 | 0.06 | 0.15 |
|
| ||||||||||||
| Model F | 12.96*** | 9.14*** | 3.74*** | 7.23*** | 6.39*** | 2.99*** | 8.31*** | 6.72*** | 4.40 | 14.40*** | 11.75*** | 5.65*** |
|
| ||||||||||||
| R2, % | 5.63 | 6.37 | 9.38 | 2.53 | 4.13 | 8.44 | 3.43 | 4.00 | 10.54 | 5.75 | 7.27 | 13.86 |
Source: Survey of Midlife in the U.S. (MIDUS II).
Note: Values are coefficients unless otherwise noted; standard errors appear in parentheses. Robust estimators are used. Boldface indicates statistical significance (*p<0.05, **p<0.01, ***p<0.001; two-tailed tests).
mg/dL.
ng/mL.
log ug/mL.
log pg/mL.
CRP, C-reactive protein; IL-6, interleukin 6; y, years.
Table 4.
Coefficients from Linear Regression Models for Systemic Inflammation Markers by Racial Background
| Variable | Blacks (n=170) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Fibrinogena | E-selectinb | CRPc | IL-6d | |||||||||
|
|
|
|
|
|||||||||
| Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | Model 2 | Model 3 | Model 6 | |
| Demographic factors | ||||||||||||
|
| ||||||||||||
| Age, y | 0.75 (0.91) | 0.69 (0.94) | 0.33 (1.08) | −0.42* (0.16) | −0.44* (0.17 | −0.34 (0.24) | −0.01 (0.01) | −0.01 (0.01) | −0.03** (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) |
|
| ||||||||||||
| Woman | 57.45*** (15.02 | 62.13*** (14.51) | 49.25** (16.82) | −2.39 (4.13) | −1.67 (4.24) | −2.42 (4.58) | 0.64** (0.19) | 0.67*** (0.19) | 0.52** (0.19) | 0.29* (0.12) | 0.29* (0.12) | 0.23 (0.13) |
|
| ||||||||||||
| Social determinants | ||||||||||||
|
| ||||||||||||
| SES | ||||||||||||
|
| ||||||||||||
| Education, y | −1.96 (2.34) | −3.14 (2.40) | −2.85 (2.85) | 0.05 (0.68) | −0.18 (0.72) | 0.01 (0.75) | −0.06* (0.03) | −0.07* (0.03) | −0.05 (0.03) | −0.02 (0.02) | −0.02 (0.02) | −0.01 (0.02) |
|
| ||||||||||||
| Income, log $ | 1.98 (2.82) | 2.36 (2.49) | 1.75 (2.92) | −1.10* (0.54) | −0.99* (0.49) | −1.41** (0.45) | 0.01 (0.01) | 0.01 (0.01) | 0.03 (0.02) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) |
|
| ||||||||||||
| Perceived discrimination | ||||||||||||
|
| ||||||||||||
| Daily | 0.68 (1.50) | −0.11 (1.75) | −0.16 (0.40) | −0.08 (0.37) | 0.01 (0.02) | −0.01 (0.02) | 0.004 (0.01) | −0.01 (0.01) | ||||
|
| ||||||||||||
| Lifetime | 5.57 (3.28) | 4.91 (3.52) | 1.26 (0.83) | 1.04 (0.81) | 0.03 (0.04) | 0.02 (0.04) | 0.01 (0.02) | −0.001 (0.03) | ||||
|
| ||||||||||||
| Psychological factors | ||||||||||||
|
| ||||||||||||
| Depressed affect | 5.58 (5.49) | 2.39 (1.32) | 0.06 (0.05) | 0.04 (0.04) | ||||||||
|
| ||||||||||||
| General anxiety | −5.11 (5.83) | −3.20** (1.15) | 0.10* (0.05) | 0.03 (0.05) | ||||||||
|
| ||||||||||||
| Stress reactivity | 2.47 (3.81) | 1.29 (0.98) | −0.04 (0.04) | 0.02 (0.03) | ||||||||
|
| ||||||||||||
| Aggression | −6.04 (5.43) | −0.99 (1.59) | −0.10 (0.07) | −0.02 (0.04) | ||||||||
|
| ||||||||||||
| Alienation | −1.19 (3.76) | −1.53 (0.91) | 0.06 (0.04) | 0.02 (0.03) | ||||||||
|
| ||||||||||||
| Lifestyle factors | ||||||||||||
|
| ||||||||||||
| Ever regular smoker | −14.13 (18.83) | −3.80 (4.18) | −0.01 (0.20) | −0.02 (0.12) | ||||||||
|
| ||||||||||||
| Currently regular smoker | 1.95 (21.96) | 10.07 (6.51) | −0.02 (0.22) | 0.16 (0.14) | ||||||||
|
| ||||||||||||
| Vigorous physical activity | 7.39 (24.70) | −0.08 (5.25) | −0.13 (0.32) | −0.04 (0.18) | ||||||||
|
| ||||||||||||
| Moderate physical activity | 4.65 (22.01) | −2.42 (4.68) | 0.29 (0.29) | 0.06 (0.17) | ||||||||
|
| ||||||||||||
| BMI | 0.91 (0.50) | −0.11 (0.09) | 0.01** (0.01) | 0.01** (0.003) | ||||||||
|
| ||||||||||||
| Health factors | ||||||||||||
|
| ||||||||||||
| Preventive aspirin | 0.77 (21.91) | −3.76 (5.01) | 0.08 (0.24) | −0.11 (0.14) | ||||||||
|
| ||||||||||||
| Hypertension | −13.98 (17.33) | −6.60 (5.36) | −0.49** (0.18) | −0.12 (0.11) | ||||||||
|
| ||||||||||||
| Diabetes | 22.14 (19.16) | 14.10* (5.84) | 0.24 (0.19) | 0.12 (0.14) | ||||||||
|
| ||||||||||||
| Joint/bone diseases | 14.51 (16.54) | −5.42 (4.63) | 0.27 (0.18) | −0.05 (0.11) | ||||||||
|
| ||||||||||||
| Persistent skin trouble | −18.29 (38.72) | 4.16 (11.32) | −0.79 (0.43) | −0.01 (0.24) | ||||||||
|
| ||||||||||||
| Teeth problems | 13.93 (20.99) | −2.25 (7.00) | 0.40 (0.27) | −0.09 (0.16) | ||||||||
|
| ||||||||||||
| Sleep problems | 3.53 (23.98) | −0.29 (6.09) | 0.24 (0.24) | 0.19 (0.14) | ||||||||
|
| ||||||||||||
| Intercept | 318.50*** | 304.19*-** | 351.47*-** | 82.54*** | 83.52*** | 100.13*-** | 1.84** | 1.67* | 2.96** | 0.81* | 0.86* | 0.32 |
|
| ||||||||||||
| Model F | 3.85** | 5.00** | 1.65* | 3.26* | 3.44** | 2.26** | 4.00** | 3.46** | 4.88 | 2.10 | 1.45 | 1.94* |
|
| ||||||||||||
| R2, % | 7.90 | 11.07 | 17.87 | 4.81 | 6.16 | 22.65 | 8.52 | 9.80 | 29.92 | 5.34 | 5.48 | 17.50 |
Source: Survey of Midlife in the U.S. (MIDUS II).
Note: Values are coefficients unless otherwise noted; standard errors appear in parentheses. Robust estimators are used. Boldface indicates statistical significance (*p<0.05, **p<0.01, ***p<0.001; two-tailed tests).
mg/dL.
ng/mL.
log ug/mL.
log pg/mL.
CRP, C-reactive protein; IL-6, interleukin 6; y, years.
BMI showed a positive relationship with three inflammation markers in the pooled sample (Table 2, Model 6), including fibrinogen (p<0.05), CRP (p<0.001), and IL-6 (p<0.001). Among whites, CRP concentrations decreased with moderate physical activity, and IL-6 concentrations decreased with vigorous physical activity (p-values<0.05, Model 6, Table 3).
Among psychological factors, general anxiety was associated with higher CRP (p<0.05, Model 6, Table 4) and lower E-selectin (p<0.01) in blacks; there were also trends toward increased E-selectin with higher depressed affect and lower alienation in this racial group (p-values<0.10). Among whites, stress reactivity was linked to lower IL-6 (p<0.05, Model 6, Table 3), whereas alienation was linked to higher E-selectin (p<0.05, Model 6, Table 3). E-selectin concentrations further increased among blacks and whites who reported having diabetes (p<0.05, Model 6, Tables 3 and 4); among whites, diabetes was further linked to higher CRP (p<0.01, Model 6, Table 3). In whites, hypertension was inversely associated with IL-6 (p<0.01, Model 6, Table 3) and marginally with CRP (p<0.10); an inverse relationship between hypertension and CRP was also evident among blacks (p<0.01, Model 6, Table 4).
In models for the pooled sample (Table 2), older individuals had higher fibrinogen (p<0.001) and IL-6 (p<0.001), but lower E-selectin (p<0.01). Older whites had higher CRP (p<0.05, Model 3, Table 3), but the opposite was true for blacks (p<0.01 in Model 6, Table 4). Older blacks also had lower E-selectin (p<0.05, Model 3, Table 4). White women had higher fibrinogen and CRP (p<0.001, Model 6, Table 3), but lower E-selectin (p-values<0.01) compared with white men. Black women had higher fibrinogen and CRP (p-values<0.01, Model 6, Table 4), as well as higher IL-6 concentrations (p<0.05, Model 3, Table 4) compared with black men.
DISCUSSION
Among the examined social determinants (race, SES, and PD), race was most consistently linked to inflammation, with blacks showing higher levels of all examined biomarkers. For fibrinogen and IL-6, racial differences tended to persist in fully adjusted models, whereas for E-selectin and CRP, racial differences were explained after including covariates. Consistent with research highlighting the importance of education for health,41 inverse associations between inflammation markers and educational attainment were observed, especially among whites, and an inverse association between income and E-selectin was found for blacks. For whites, there was an increase—not a decrease—of E-selectin with income, suggesting that higher income may have protective effects for blacks but not for whites. PD, the third social determinant considered in this study, was related to increased concentrations of most biomarkers of inflammation, but only for lifetime discrimination, not daily discrimination. In supplementary analyses (data not shown), bivariate associations between daily discrimination and inflammation markers were statistically significant but dissipated after controlling for lifetime discrimination. The reasons for this are unclear. Daily discrimination represents relatively minor events, such as being given poor service in a restaurant. Nevertheless, chronic exposure to such experiences may influence responses to major lifetime discriminatory events and other race-related stressors once they occur, increasing physiologic and perceived stress and consequently harming health. More research is needed to disentangle these processes.
Importantly, the hypotheses proposed in this study received weaker support for blacks than for whites. This could be because of fewer blacks in the sample and lower statistical power, but the possibility that different mechanisms contribute to inflammation among different racial populations cannot be ruled out, especially in light of prior studies, in which education, weight status, and depressive symptoms showed weaker associations with inflammation among blacks compared with whites.29,51–53,64 By contrast, the results of this study were less consistent with prior research, suggesting that the effects of PD on health are mediated by psychological factors. Few statistically significant relationships between psychological factors and inflammation emerged. The absence of positive associations between stress reactivity and inflammation biomarkers was especially surprising, given the known role of stress in physical and mental health. One explanation is that the measure of stress reactivity does not capture the level of stress exposure; instead, it measures a relatively stable personality characteristic representing how a person responds emotionally once stress has occured.63 Future research should assess stress exposure more directly to clarify its influence on inflammation.
Limitations
This study has a cross-sectional, observational design, which prevents assessing changes over time and causally interpreting results, though specifically for race and education, it seems unlikely that they might change in response to changes in inflammation among midlife individuals. Racial identification tends to be stable over the life course, and education is typically completed during young adulthood. Nevertheless, inflammation may reduce SES by contributing to poorer health, which may limit earnings, productivity, and career advancement. This study addressed two dimensions of PD but it did not capture institutional, implicit, and covert discrimination. Finally, this investigation focused on blacks and whites; in future studies, it will be important to assess inflammation among Latinos and Native Americans, who have high prevalence of cardiovascular disease65 and diabetes66 and may be at risk of perceived discrimination.
CONCLUSIONS
As systemic inflammation is implicated in many chronic diseases, evidence of the role of social determinants in inflammation highlights the social origins of chronic disease during midlife and informs scholarship seeking to pinpoint the processes leading to health disparities. Better understanding is the first step toward preventive interventions to reduce the health risks among vulnerable populations, including racial/ethnic minorities, individuals at risk for discrimination, and those facing socioeconomic disadvantages. Notably, this work suggests the importance of systemic interventions that address large-scale social determinants of health, including system-level factors that underlie discrimination and lead to disparities in income and education.
Acknowledgments
Publication of this article was supported by the National Institutes of Health. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
This work was supported by a grant from the National Institute on Minority Health and Health Disparities (U54MD008176).
No financial disclosures were reported by the authors of this paper.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.amepre.2016.09.026.
This article is part of a supplement issue titled Social Determinants of Health: An Approach to Health Disparities Research.
References
- 1.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15(2):152–158. doi: 10.1097/01.mnh.0000203189.57513.76. http://dx.doi.org/10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 2.Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199–207. doi: 10.1016/j.ahj.2013.03.018. http://dx.doi.org/10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97(2):3–11. doi: 10.1016/j.amjcard.2005.11.010. http://dx.doi.org/10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. http://dx.doi.org/10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 5.Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia. 2010;53(1):10–20. doi: 10.1007/s00125-009-1573-7. http://dx.doi.org/10.1007/s00125-009-1573-7. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi FG, Stringuetta Belik F, e Silva O, et al. Inflammation, diabetes, and chronic kidney disease: role of aerobic capacity. Exp Diabetes Res. 2012 doi: 10.1155/2012/750286. http://dx.doi.org/10.1155/2012/750286. [DOI] [PMC free article] [PubMed]
- 7.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. http://dx.doi.org/10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. http://dx.doi.org/10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. http://dx.doi.org/10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 10.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology. 2011;25(5):400–410 413. [PubMed] [Google Scholar]
- 11.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. http://dx.doi.org/10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16(11):1304–1313. doi: 10.3748/wjg.v16.i11.1304. http://dx.doi.org/10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunol Allergy Clin North Am. 2009;29(2):381–393. doi: 10.1016/j.iac.2009.02.011. http://dx.doi.org/10.1016/j.iac.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11–12):785–793. doi: 10.1016/j.cca.2010.02.069. http://dx.doi.org/10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. http://dx.doi.org/10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 16.Pischon N, Heng N, Bernimoulin J-P, et al. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86(5):400–409. doi: 10.1177/154405910708600503. http://dx.doi.org/10.1177/154405910708600503. [DOI] [PubMed] [Google Scholar]
- 17.Marmot M, Allen JJ. Social determinants of health equity. Am J Public Health. 2014;104(S4):S517–S519. doi: 10.2105/AJPH.2014.302200. http://dx.doi.org/10.2105/AJPH.2014.302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braveman PA, Egerter SA, Mockenhaupt RE. Broadening the focus: the need to address the social determinants of health. Am J Prev Med. 2011;40(1):S4–S18. doi: 10.1016/j.amepre.2010.10.002. http://dx.doi.org/10.1016/j.amepre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082. http://dx.doi.org/10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. http://dx.doi.org/10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frieden TR. CDC Health Disparities and Inequalities Report-United States, 2013. Foreword. MMWR Surveill Summ. 2013;62:1–2. [PubMed] [Google Scholar]
- 22.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54(6):1027–1037. doi: 10.1373/clinchem.2007.098996. http://dx.doi.org/10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 23.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. http://dx.doi.org/10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 24.Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethn Dis. 2011;21(2):142. [PMC free article] [PubMed] [Google Scholar]
- 25.Pollitt RA, Kaufman JS, Rose KM, et al. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur J Epidemiol. 2007;22(1):55–66. doi: 10.1007/s10654-006-9082-1. http://dx.doi.org/10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- 26.Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. J Epidemiol Community Health. 2003;57(9):730–733. doi: 10.1136/jech.57.9.730. http://dx.doi.org/10.1136/jech.57.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford ES, Giles WH, Myers GL, et al. C-reactive protein concentration distribution among U.S. children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49(8):1353–1357. doi: 10.1373/49.8.1353. http://dx.doi.org/10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 28.Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (the MIDUS study) Psychosom Med. 2010;72(3):290. doi: 10.1097/PSY.0b013e3181cfe4c2. http://dx.doi.org/10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller-Rowell TE, Curtis DS, Doan SN, Coe CL. Racial disparities in the health benefits of educational attainment: a study of inflammatory trajectories among African American and white adults. Psychosom Med. 2015;77(1):33–40. doi: 10.1097/PSY.0000000000000128. http://dx.doi.org/10.1097/PSY.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham TJ, Seeman TE, Kawachi I, et al. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 U.S. communities. Soc Sci Med. 2012;75(5):922–931. doi: 10.1016/j.socscimed.2012.04.027. http://dx.doi.org/10.1016/j.socscimed.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman EM, Williams DR, Singer BH, Ryff CD. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: the MIDUS study. Brain Behav Immun. 2009;23(5):684–692. doi: 10.1016/j.bbi.2009.01.002. http://dx.doi.org/10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goosby BJ, Malone S, Richardson EA, Cheadle JE, Williams DT. Perceived discrimination and markers of cardiovascular risk among low-income African American youth. Am J Hum Biol. 2015;27(4):546–552. doi: 10.1002/ajhb.22683. http://dx.doi.org/10.1002/ajhb.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24(3):438–443. doi: 10.1016/j.bbi.2009.11.011. http://dx.doi.org/10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert MA, Ravenell J, Glynn RJ, et al. Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas Heart Study. Am Heart J. 2008;156(6):1103–1109. doi: 10.1016/j.ahj.2008.07.027. http://dx.doi.org/10.1016/j.ahj.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Nuru-Jeter A, Chae DH, Price M, et al. Anticipatory racism threat and superwoman schema: elucidating the relationship between racial discrimination and chronic inflammation. Circulation. 2013;128(22 suppl):A9550. [Google Scholar]
- 36.Beatty Moody DL, Brown C, Matthews KA, Bromberger JT. Everyday discrimination prospectively predicts inflammation across 7–years in racially diverse midlife women: Study of Women’s Health across the Nation. J Soc Issues. 2014;70(2):298–314. doi: 10.1111/josi.12061. http://dx.doi.org/10.1111/josi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;36:80–94. http://dx.doi.org/10.2307/2626958. [PubMed] [Google Scholar]
- 38.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol. 2015;41:311–330. http://dx.doi.org/10.1146/annurev-soc-073014-112305. [Google Scholar]
- 39.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities theory, evidence, and policy implications. J Health Soc Behav. 2010;51(1 suppl):S28–S40. doi: 10.1177/0022146510383498. http://dx.doi.org/10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 40.Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007:81–82. doi: 10.1093/bmb/ldm001. http://dx.doi.org/10.1093/bmb/ldm001. [DOI] [PubMed]
- 41.Mirowsky J, Ross CE. Education, Social Status, and Health. Livingston, NJ: Transaction Publishers; 2003. [Google Scholar]
- 42.Lynch J, Kaplan GA. Socioeconomic position. In: Berkman LF, Kawachi I, editors. Social Epidemiology. New York: Oxford University Press; 2000. pp. 13–35. [Google Scholar]
- 43.Fuller-Rowell TE, Evans GW, Ong AD. Poverty and health: the mediating role of perceived discrimination. Psychol Sci. 2012;23(7):734–739. doi: 10.1177/0956797612439720. http://dx.doi.org/10.1177/0956797612439720. [DOI] [PubMed] [Google Scholar]
- 44.Jackson JS, Williams D, Torres M. Perceptions of Discrimination: The Stress Process and Physical and Psychological Health. Washington, DC: National Institute of Mental Health; 1997. [Google Scholar]
- 45.Sigelman L, Welch S. Black Americans’ Views of Racial Inequality: The Dream Deferred. New York: Cambridge University Press; 1991. [Google Scholar]
- 46.Seaton EK, Caldwell CH, Sellers RM, Jackson JS. The prevalence of perceived discrimination among African American and Caribbean Black youth. Dev Psychol. 2008;44(5):1288. doi: 10.1037/a0012747. http://dx.doi.org/10.1037/a0012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, Brody G. Perceived discrimination and substance use in African American parents and their children: a panel study. J Pers Soc Psychol. 2004;86(4):517. doi: 10.1037/0022-3514.86.4.517. http://dx.doi.org/10.1037/0022-3514.86.4.517. [DOI] [PubMed] [Google Scholar]
- 48.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: some conceptual perspectives. J Health Soc Behav. 2005;46(2):205–219. doi: 10.1177/002214650504600206. http://dx.doi.org/10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- 49.Pearlin LI. The sociological study of stress. J Health Soc Behav. 1989;30(3):241–256. http://dx.doi.org/10.2307/2136956. [PubMed] [Google Scholar]
- 50.Thoits PA. Stress and health: major findings and policy implications. J Health Soc Behav. 2010;51(suppl):S41–S53. doi: 10.1177/0022146510383499. http://dx.doi.org/10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- 51.Vrany EA, Berntson JM, Khambaty T, Stewart JC. Depressive symptoms clusters and insulin resistance: race/ethnicity as a moderator in 2005–2010 NHANES data. Ann Behav Med. 2016;50(1):1–11. doi: 10.1007/s12160-015-9725-0. http://dx.doi.org/10.1007/s12160-015-9725-0. [DOI] [PubMed] [Google Scholar]
- 52.Case SM, Stewart JC. Race/ethnicity moderates the relationship between depressive symptom severity and C-reactive protein: 2005–2010 NHANES data. Brain Behav Immun. 2014;41:101–108. doi: 10.1016/j.bbi.2014.04.004. http://dx.doi.org/10.1016/j.bbi.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Stewart JC. One effect size does not fit all: is the depression-inflammation link missing in racial/ethnic minority individuals? JAMA Psychiatry. 2016;73(3):301–302. doi: 10.1001/jamapsychiatry.2015.3205. http://dx.doi.org/10.1001/jamapsychiatry.2015.3205. [DOI] [PubMed] [Google Scholar]
- 54.Deverts DJ, Cohen S, DiLillo VG, et al. Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2010;72(8):734. doi: 10.1097/PSY.0b013e3181ec4b98. http://dx.doi.org/10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray KB, Vogel CM. Using a hierarchy-of-effects approach to gauge the effectiveness of corporate social responsibility to generate goodwill toward the firm: financial versus nonfinancial impacts. J Bus Res. 1997;38(2):141–159. http://dx.doi.org/10.1016/S0148-2963(96)00061-6. [Google Scholar]
- 56.Ryff CD, Seeman T, Weinstein M. National Survey of Midlife Development in the United States (MIDUS II): Biomarker Project, 2004–2009. ICPSR29282-v6. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2013. 05-02. [Google Scholar]
- 57.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22(8):1059–1080. doi: 10.1177/0898264310374355. http://dx.doi.org/10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. http://dx.doi.org/10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 59.Toker S, Shirom A, Shapira I, Berliner S, Melamed S. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J Occup Health Psychol. 2005;10(4):344. doi: 10.1037/1076-8998.10.4.344. http://dx.doi.org/10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- 60.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. http://dx.doi.org/10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 61.Williams D, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socioeconomic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. http://dx.doi.org/10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 62.Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States: prevalence and conformance with evidence-based recommendations. J Gen Intern Med. 2000;15(5):284–292. doi: 10.1046/j.1525-1497.2000.9908044.x. http://dx.doi.org/10.1046/j.1525-1497.2000.9908044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. http://dx.doi.org/10.1037/1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- 64.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232–244. doi: 10.1111/obr.12003. http://dx.doi.org/10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 65.Daviglus ML, Talavera GA, Avilés-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–1784. doi: 10.1001/jama.2012.14517. http://dx.doi.org/10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Connell J, Yi R, Wilson C, Manson SM, Acton KJ. Racial disparities in health status: a comparison of the morbidity among American Indian and U.S. adults with diabetes. Diabetes Care. 2010;33(7):1463–1470. doi: 10.2337/dc09-1652. http://dx.doi.org/10.2337/dc09-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]