Summary
Objective
The clinical benefit of newborn screening (NBS) for cystic fibrosis (CF) has been primarily nutritional, with less overt respiratory impact. Identification of risk factors for infant CF lung disease could facilitate targeted interventions to improve pulmonary outcomes.
Methods
This retrospective study evaluated socioeconomic information, clinical data, and results from routine infant pulmonary function testing (iPFT) of infants diagnosed with CF through NBS (N = 43) at a single CF center over a 4-year period (2008–2012). A five-item composite clinical score was developed and combined with socioeconomic indicators to facilitate identification of CF infants at increased risk of early-onset respiratory impairment.
Results
Paternal education was positively associated with lung function (P = 0.02). Clinical score <7 (on a scale of 0–10) predicted diminished pulmonary measure (P < 0.005). Retrospective risk stratification by clinical score and paternal education identified CF infants at low, intermediate, or high risk of pulmonary disease. Forced expiratory volume (FEV0.5%, mean ± SD) averaged 115 ± 19% in the low-risk group, 97 ± 17% in the intermediate-risk group, and 90 ± 8% in the high-risk group (P < 0.005). Results were similar for mid-expiratory flows (FEF25–75%). Multiple regression analysis confirmed the predictive value of this risk stratification model of CF infant pulmonary health.
Conclusion
We combined socioeconomic and clinical data to risk-stratify CF infants for early-onset lung disease as quantified by iPFT. Our model showed significant differences in infant pulmonary function across risk groups. The developed tool offers an easily available, inexpensive, and non-invasive way to assess risk of respiratory decline in CF infants and identify those meriting targeted therapeutic attention.
Keywords: newborn screening, cystic fibrosis, respiratory outcomes, infant lung function, paternal education
INTRODUCTION
Newborn screening (NBS) for cystic fibrosis (CF) has been linked primarily to improved nutritional outcomes.1–3 The impact of NBS on lung function measures in CF infants and toddlers is less established.2–8
Lung disease continues to be common in CF infants as assessed by chest computed tomography (CT),7 lung physiology,9 and airway infection.7–12 Currently, there is a lack of risk-prediction tools, and it is difficult to identify infants at increased risk for pulmonary decline who may benefit from intensified therapeutic intervention.13 An association of Pseudomonas aeruginosa (PsA) infection with accelerated disease progression has been reported in older children and adults with CF,14,15 and early acquisition of PsA may be a risk factor for infant pulmonary disease.7,8 Socioeconomic, biological, genetic, and environmental factors may also influence pulmonary outcomes.16–19
This single-center retrospective study sought to identify the subpopulation with significant pulmonary compromise and evaluate factors contributing to infant lung disease in CF. We hypothesized that both clinical (nutritional status, microbiology, cough, and pulmonary exacerbations) and socioeconomic (maternal and paternal education, household income, health insurance, and second-hand smoke) determinants are associated with infant pulmonary function. Our findings introduce the clinical utility of a five-item non-invasive clinical score for detecting pulmonary disease, emphasize paternal education as a prominent socioeconomic predictor of CF health, and combine these two variables to identify CF infants in need of increased therapeutic intervention.
METHODS
Study Population
The study population comprised infants diagnosed with CF by newborn screening (NBS) between 2008 and 2012 and treated at the University of Alabama at Birmingham/Children’s of Alabama (UAB/COA) pediatric CF Center. These infants were seen at least monthly through 6 months of age, and at least every 2 months through their first year of life in a separate NBS clinic.
Newborn screening for CF in Alabama began in 2008. The UAB/COA CF Center coordinates the state program in partnership with the Alabama Department of Public Health. The initial screen for CF in Alabama is an immunoreactive trypsinogen/DNA (IRT/DNA) regimen.20 Elevated IRT in a newborn blood spot (Guthrie) card prompts genetic analysis of the specimen for a CF mutation. Infants with a positive IRT/DNA screen are referred for sweat chloride testing to establish the diagnosis. Sweat chloride testing typically occurs before 4 weeks of age.
Infant Pulmonary Function
In the UAB/COA CF Center, routine infant pulmonary function testing (iPFT) is performed in all CF infants near 12 months of age. iPFT measurements are obtained using the nSpire IPL device.21–23 During this test, forced expiratory flows are monitored using raised volume rapid thoracoabdominal compression (RVRTC) in infants sedated with chloral hydrate at a dosage of 85 mg/kg. The patient is given 2–3 inflated breaths to a pressure of 30 cm H20 (V30) to induce respiratory pause, which is followed by thoracoabdominal compression and measurement of forced expiratory flow. Forced vital capacity (FVC) is the volume of air expired between V30 and residual volume (RV). FEV0.5 is the volume of air expired in the initial 0.5 sec of forced expiration. The midexpiratory flow (FEF25–75) is the calculation of the forced expiratory flow between 25% and 75% of FVC. Lung function data are expressed as absolute data, then converted to z-scores compared to historical controls (normalized to length and age)21,23 and represented as percent-predicted normative values (e.g., FVC%, FEV0.5%, and FEF25–75%).
Data Collection
Socioeconomic and clinical data were obtained from the patient registry maintained by the UAB/COA CF Center through an ongoing protocol approved by theUAB Institutional Review Board for Human Use (X080225007). Socioeconomic data are updated annually through a patient survey and include maternal and paternal level of educational attainment, annual household income, type of health insurance, ZIP-code of residence, and exposure to second-hand smoke. Exposure to second-hand smoke is determined with the questions, “Does anyone in the patient’s household smoke cigarettes?” (Yes/No/Unknown) and “During the reporting year, how often was this patient exposed to secondhand smoke?” (Daily/Several times per week/Several times per month, or less/Never/Declined to Answer/Unknown). Clinical data include cough frequency, hospitalizations, pulmonary exacerbations, microbiology, weight-forlength percentile, chronic therapies, CF genotype, sweat chloride result, and stool pancreatic elastase.
Composite Clinical Score
From the clinical data, we developed a composite clinical score to summarize the extent of CF lung disease, focusing on major CF care considerations during infancy. The composite clinical score included five indicators summarizing the overall health of CF infants: (i) nutritional status (weight-for-length percentile at 12 months of age); (ii) microbiology (history of PsApositive respiratory culture [yes/no] during infancy); (iii) presence of cough at 12 months of age; (iv) outpatient pulmonary exacerbations (defined as a respiratory flare prompting oral antibiotic prescription by the clinical pediatric pulmonologist) in the first year; and (v) hospitalization (yes/no) in the first 12 months of life. Each of the five clinical indicators was scored on a scale of 0–2 and weighted equally. A higher composite score (on a scale of 0–10) indicated better health (Table 1).
TABLE 1.
CF Infant Clinical Score
| Component | Score | |
|---|---|---|
| 1 | Nutrition: weight-for-length % | 0 = <25%; 1 = 25–49%; 2 = ≥50% |
| 2 | Microbiology: PsA | 0 = colonization; 1 = successful eradication; 2 = never |
| 3 | Cough | 0 = daily; 1 = occasional (any cough in the last week, but less than daily); 2 = none |
| 4 | Pulmonary exacerbations | 0 = >3 in first year of life; 1 = 1–2 in the first year of life; 2 = none |
| 5 | Hospitalizations | 0 = any hospitalization for pulmonary exacerbation in the first year of life; 2 = none |
Five components measures at 12 months of life scored 0–2 points each, for a total maximum of 10 points (higher score indicates better health).
Statistical Analysis
We analyzed retrospectively the socioeconomic and clinical characteristics of infants diagnosed with CF through NBS. Descriptive statistics, including means, standard deviations, frequencies, and proportions, were calculated for all lung function, clinical, and sociodemographic variables. Distributions of lung function were examined using stem-and-leaf plots, normal probability plots, and the Kolmogorov–Smirnov test; each of these variables was determined to follow a normal distribution. For statistical study of the relationships between categorical variables (e.g., presence/absence of risk factors), Fisher’s exact test was performed. Relationships between continuous variables were examined with the comparisons of means and with linear regression analysis. Means of two individual groups, such as the clinical score and parental education groups, were compared using the unpaired t-test. Means of several groups, such as for stratified risk, were compared using analysis of variance (ANOVA). The Tukey–Kramer multiple comparisons test determined which pairs of means were significantly different.
Simple linear regression was used to examine associations between outcome variables and clinical and socio-demographic data. Multivariate analyses of factors associated with the outcome variables were performed using stepwise multiple regression. Backward multiple linear regression was performed as well, and yielded results that were similar to the stepwise protocol. Variables significant at α = 0.20 in the simple regression model were considered as possible predictor variables for multiple linear regression analyses. Criterion for entry into the multiple linear regression model was significance at α = 0.20, whereas the criterion for remaining in the model was significance at α = 0.05. A multiple linear regression model containing the best predictor variables from the stepwise analysis was then run using all available data in order to obtain more robust estimates of the model parameters.
All statistical tests were two-sided and results deemed significant at a 5%significance level (α = 0.05). Analyses and graphical interpretations were performed using SAS (Version 9.4, SAS Institute, Inc., Cary, NC) and GraphPad Prism (Version 6.05, GraphPad, La Jolla, CA) software.
RESULTS
Forty-three CF infants (58% male) underwent iPFT evaluation during the study period. Their clinical characteristics are presented in Table 2. Most infants (91%) had at least one copy of the F508del gene, and approximately half (56%) were homozygous for the F508del mutation. Infant PFT values were near normal, and weight-for-length percentile at 12 months was greater than 50% for 74% of the infants (range 19–99%). Infants averaged nearly 2 (range 1–5) outpatient pulmonary exacerbations in their first year. Forty-four percent of infants had at least one positive culture for PsA by 12 months of age. The mean composite clinical score of CF health at 12 months of age was 7.6 ± 2.1 (mean ± SD; range 0–10). A clinical score of 7 was thus chosen as a cut-off point in the risk stratification models, allowing comparison of infants with below-average clinical score to the normative group.
TABLE 2.
Clinical Characteristics of CF Infants (N = 43)
| Variable | Mean (SD) or % |
|---|---|
| Genotype (F508del homozygous) | 56% |
| CFTR function (mean sweat chloride, mmol/L) | 98 (13.1) |
| iPFT | |
| FVC% | 108 (17.2) |
| FEV0.5% | 102 (19.9) |
| FEF25–75% | 96 (28.5) |
| RV/TLC% | 144 (24.6) |
| Nutritional status (weight/length percentile at 12 months) |
65% (24%) |
| Microbiology (history of PsA) | 44% |
| Cough (occasional/daily) | 42% |
| Pulmonary exacerbations (in first 12 months of life; range 1–5) |
1.9 (1.5) |
| Hospitalizations (in first 12 months of life) | 47% |
| Clinical score (range 0–10) | 7.6 (2.1) |
| Prescribed chronic pulmonary therapies | 67% |
| Inhaled corticosteroid | 42% |
| Dornase alpha | 40% |
| Azithromycin | 23% |
The socio-demographic characteristics of caregivers are presented in Table 3. The mothers were better educated than the fathers, with 71% versus 50% having more than a high-school education (P < 0.05). Most caregivers were married or cohabiting, 25% were single or divorced, and 5% were foster parents. More than half (59%) of households had annual income less than $50,000, and 38% included a smoker.
TABLE 3.
Socio-Demographic Characteristics of Caregivers
| Variable | Mean (SD) or % |
|---|---|
| Age (n = 32) | |
| Maternal | 27 (5) |
| Paternal | 32 (6) |
| Maternal education (n = 41) | |
| ≥Some college | 71% |
| ≤High school | 29% |
| Paternal education (n = 36) | |
| ≥Some college | 50% |
| ≤High school | 50% |
| Marital status (n = 43) | |
| Married | 60% |
| Single | 16% |
| Divorced | 9% |
| Cohabiting | 9% |
| Foster care | 5% |
| Annual household income (n = 39) | |
| ≥$50,000 | 41% |
| <$50,000 | 59% |
| Health insurance (n = 42) | |
| Private | 52% |
| Public | 48% |
| Household smoke exposure (n = 42) | |
| No | 62% |
| Yes | 38% |
| Missed appointments (n = 42) | |
| None | 57% |
| 1 | 21% |
| ≥2 | 21% |
Paternal education of high school or less was associated with other socioeconomic risk factors, including low income (<$50,000, P < 0.001), second-hand smoke exposure (P < 0.02), Medicaid coverage (P < 0.001), and single-parent home environment (P < 0.05). Maternal education, on the other hand, while correlated with paternal education (r = 0.42, P = 0.01), did not significantly impact household income (P = 0.29), smoking (P = 0.16), or marital status (P = 0.08).
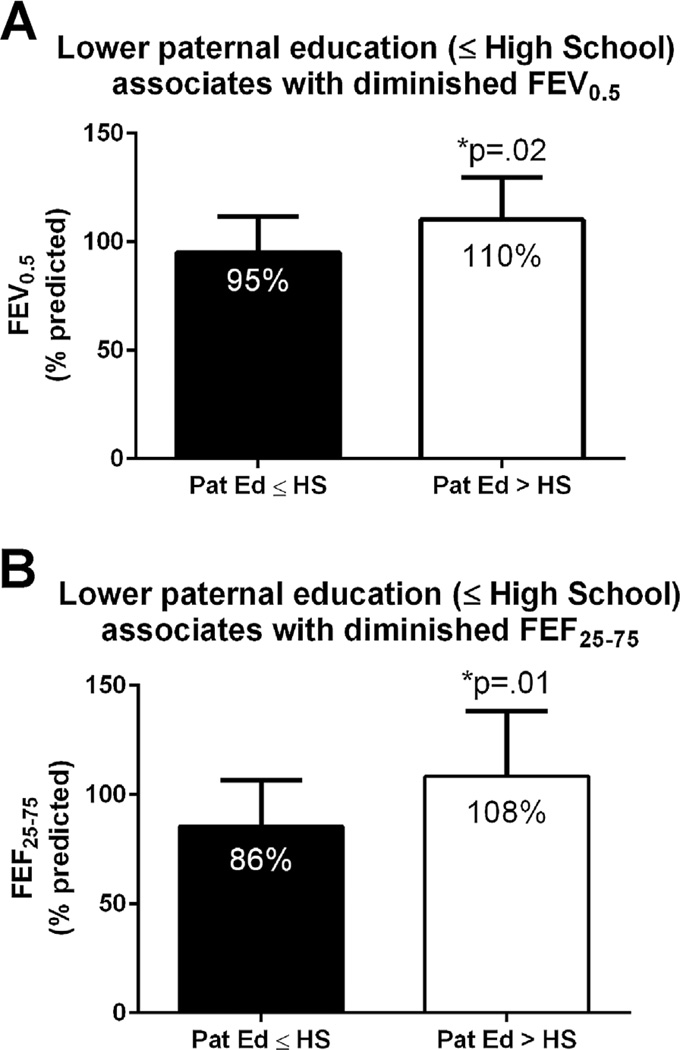
Regression analysis indicated that paternal education is the leading socioeconomic predictor of lung function, both for FEV0.5% (P = 0.018) and FEF25–75% (P = 0.012). Other socioeconomic factors were not statistically significant for either FEV0.5% or FEF25–75%: e.g., maternal education (P = 0.69 and P = 0.59), household income (P = 0.11 and P = 0.12), marital status (P = 0.82 and P = 0.59), insurance status (P = 0.32 and P = 0.12), and smoke exposure (P = 0.38 and P = 0.12). As illustrated in Figure 1, infants whose fathers had attended college had an FEV0.5% of 110 ± 20% (mean ± SD), while infants whose fathers had only high-school education or less had an FEV0.5%of 95 ± 17%, P = 0.02. Similar results were obtained for FEF25–75%: infants whose fathers had attended college had an FEF25–75% of 108 ± 30%, while infants whose father received a high-school education or less had an FEF25–75% of 86 ± 21%, P = 0.01.
Fig. 1.
Relationship between paternal education and infant pulmonary function: (A) FEV0.5 and (B) FEF25–75 are significantly decreased in CF infants whose father did not attend college. Error bars are mean ± SD.
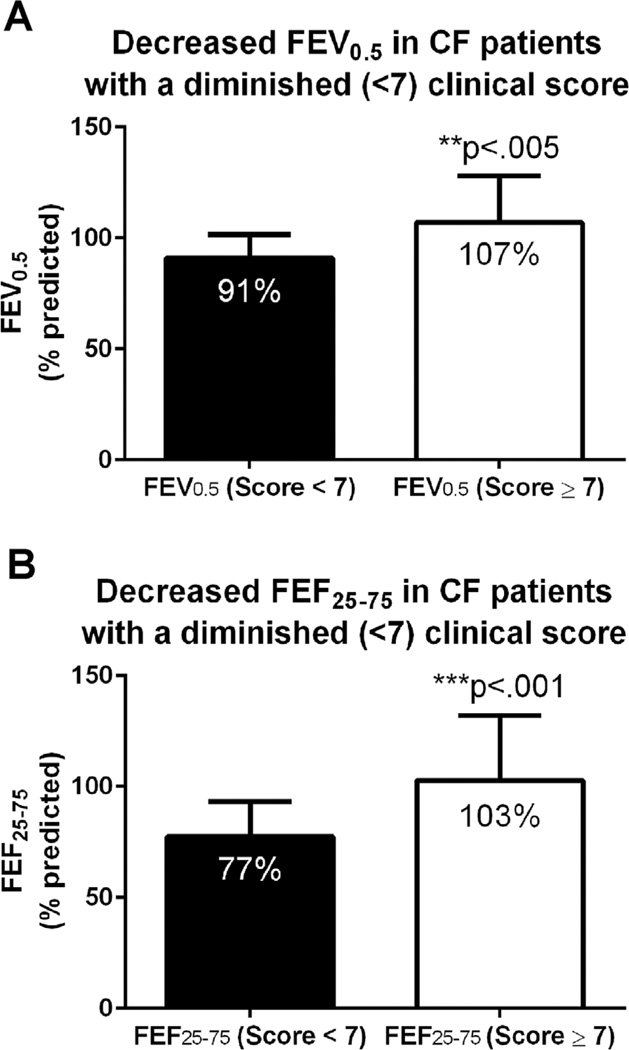
Clinical score <7 was also strongly associated with diminished lung function (both FEV0.5% and FEF25–75%), as seen in Figure 2. Infants with a higher clinical score (≥7) averaged FEV0.5% of 107 ± 21% (mean ± SD), while those with lower clinical score (<7) averaged FEV0.5% of 91 ± 11%, P < 0.005. An even greater difference was observed in FEF25–75%: those with the higher clinical score averaged FEF25–75% of 103 ± 29%, while those with the lower clinical score averaged FEF25–75% of 77 ± 16%, P < 0.001. A smaller but statistically significant difference in FVC% was also recorded between the two groups: 111 ± 16% versus 99 ± 13%, P < 0.05.
Fig. 2.
Relationship between clinical score and infant pulmonary function: (A) FEV0.5 and (B) FEF25–75 are significantly decreased in CF infants with a clinical score of <7 compared to infants with a higher score. Error bars are mean ± SD.
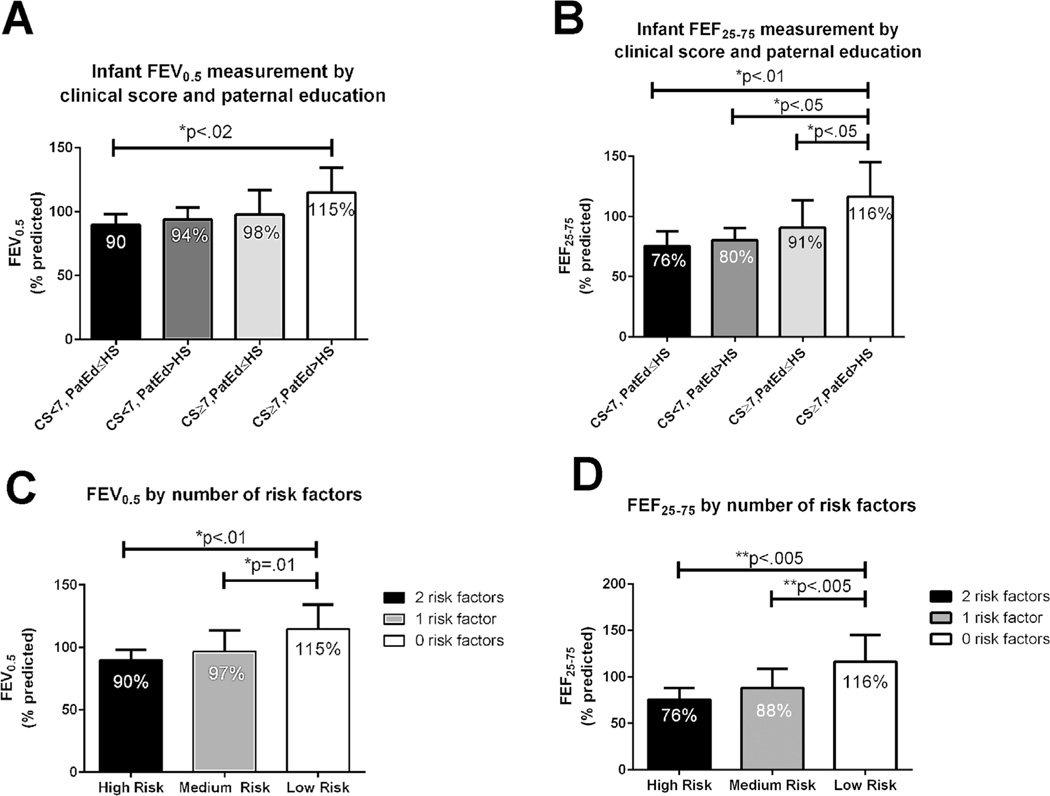
Because both paternal education and composite clinical score were independent risk factors for pulmonary outcome, we stratified infants into three risk groups (Fig. 3A and B). The high-risk group was defined as presence of two risk factors: low clinical score (<7) and paternal education of high school or less. The intermediate-risk group was defined as presence of one risk factor: either low clinical score (<7) or low paternal education, but not both. The low-risk group was defined as no risk factors (clinical score ≥7 and paternal education greater than high school). Stratification of risk between these groups was confirmed by ANOVA. FEV0.5% (Fig. 3C) was 90 ± 8% (mean ± SD) in the high-risk group, 97 ± 17% in the intermediate-risk group, and 115 ± 19% in the low-risk group (P < 0.005), with significant pair-wise differences between the high- and low-risk groups (P < 0.01) and the intermediate- and low-risk groups (P = 0.01). A 3 × 3 contingency table (0, 1, 2 risk factors vs. FEV0.5 of > 105%, 95–105%, and <95%) indicated the significance of this risk profile for the identification of mild, intermediate, and suboptimal pulmonary involvement (P = 0.007). To assess the predictive clinical utility of these variables, a simpler 2 × 2 contingency table examining the relationship between risk factors and extremes of lung involvement (0 vs. 2 risk factors, FEV0.5 > 105% vs. FEV0.5 < 95%, no intermediate group) was analyzed. In this test of predictors for suboptimal versus mild disease, the presence of two risk factors had a sensitivity of 83%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 91% (P = 0.001). No patients with two risk factors had an FEV0.5 of > 105% predicted.
Fig. 3.
Relationship between risk stratification and infant lung function: (A) FEV0.5 and (B) FEF25–75 by clinical score (CS) and paternal education (PatEd). (C) FEV0.5 by number of risk factors (0, 1, 2). (D) FEF25–75 by number of risk factors (0, 1, 2). Error bars are mean ± SD. Significance of lung function difference between groups measured by ANOVA.
A similar pattern was found for FEF25–75% (Fig. 3D): the mean (± SD) was 76 ± 12% in the high-risk group, 88 ± 21% in the intermediate-risk group, and 116 ± 29% in the low-risk group (P = 0.001), with significant pairwise differences between the high- and low-risk groups (P < 0.005) and the intermediate- and low-risk groups (P < 0.005). Again, the 3 × 3 contingency table of risk factors and lung impairment (0, 1, 2 risk factors versus mild [FEF25–75 > 105%], moderate [FEF25–75 95–105%], and suboptimal [FEF25 75 < 95%] disease) demonstrated a significant relationship between risk factors and iPFT results. As with FEV0.5, in using 0 versus 2 risk factors to screen for mild versus suboptimal disease (FEF25–75 > 105% or FEF25–75 <95%), the presence of two risk factors had a sensitivity of 75%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 78%. Again, no patients with two risk factors had an FEF25–75 of > 105%.
The independent contribution of study variables to lung function was ascertained with multiple regression analysis. Variables at α = 0.20 in the simple regression model were incorporated into multiple regression. These variables included: clinical score, education (≤high school, >high school), household income (<$50,000, ≥$50,000), second-hand smoke exposure (yes/no), genotype (F508del homozygous, other), previous hospitalizations, number of pulmonary therapies (none, one or more), and missed appointments (yes/no). Criterion for remaining in the model was significant at α = 0.05.
The multiple regression model identified the following as the best predictive equation of FEV0.5 in CF infants:
where paternal education is 0 if ≤high school and 1 if >high school (model P = 0.014, model R2 = 0.229). By this predictive model, paternal college experience improves infant lung function by 13%, and each 1 point increase in clinical score advances lung function by 3%.
The multiple regression model identified the following as the best predictive equation of FEF25–75, in CF infants:
where paternal education is as defined above (model P = 0.013, model R2 = 0.317). By this predictive model, paternal college experience improves infant FEF25–75 by 19%, and each 1 point increase in clinical score advances FEF25–75 by 6%.
DISCUSSION
To identify CF infants at risk for pulmonary decline, we analyzed retrospectively socioeconomic, clinical, and iPFT data of infants diagnosed with CF through NBS in a single CF center. We developed a five-item composite clinical score that predicted pulmonary function measured with a routine iPFT at 12 months of age. In multiple regression analysis, the predictive value of the composite clinical score for infant pulmonary function at 12 months of age was statistically superior to any single clinical measure. This score offers to pediatricians an easily available, inexpensive, and non-invasive tool to estimate the risks for pulmonary disease in CF infants.
We also found that paternal (rather than maternal) educational attainment is significantly associated with lung function at 12 months of age and is the best socioeconomic predictor of lung health in this population. We therefore combined the composite clinical evaluation measure with the paternal educational attainment measure to classify CF infants into low-, intermediate-, and high-risk groups for respiratory impairment. Our model showed significant differences in infant pulmonary function across risk groups.
The study has important implications both for clinical practice and research. The simple stratification tool combining socioeconomic and clinical data allows clinicians to assess the level of risk—increased (2+ risk factors) versus low (0 risk factors)—for significant respiratory impairment in CF infants. Its application to long-term disease trajectory in a larger cohort of CF patients, and the mechanisms by which paternal education alters the care environment offer future directions for investigation.
Assessing the extent of pulmonary involvement in infancy continues to be a highly sought-after but difficult to achieve goal of CF care.24 Although iPFT is an appealing solution,25,26 the limited availability and high cost of the equipment as well as the need for sedation are obstacles to its wide-spread use. Currently, iPFT is limited to specialized CF care centers. This study suggests that in the absence of such resources, pulmonary involvement (particularly at the poles of mild vs. severe) can be assessed by readily available information from the patient’s clinical and social history. This screening tool may be of particular utility to general pediatricians who lack specialty resources but provide routine care to CF infants.
The finding that paternal educational attainment is strongly associated with infant pulmonary function deserves special attention. Literature regarding the impact of paternal education on CF health is limited, as paternal education seldom is assessed separately from maternal education. The strong association of low paternal education with low household income and exposure to second-hand smoke suggests that in our patient cohort, paternal education may be a marker of multiple factors in the CF care environment that are significant for lung health. While second-hand smoke has been reported as a primary mechanism through which socioeconomic status affects CF lung health,27,28 paternal education has an additional impact on CF lung health, likely by determining access to material resources and thereby affecting living conditions,29,30 nutrition,31 stress exposure,32 family functioning,33 and other aspects of the care environment. For example, a study of older children in our CF center found that CF patients whose fathers had college experience were eight times more likely to have medium-high than low lung function compared to CF patients whose fathers had high-school education or less (P < 0.05) after controlling for household income and exposure to second-hand smoke.34
Paternal college attendance has been shown to be an influential marker of child health in general.35–38 Lower paternal education has been associated with substandard prenatal care, prematurity, and low birth weight.37,39 Paternal education influences measles immunization independently of maternal education,36 and fathers of children with diabetes contribute to health outcomes even when they are not primary caregivers.35 Whether through being the default provider of socioeconomic means or through other mechanisms, such as response to stress, coping strategies, and health behavior attitudes, the father plays an important role for CF lung health. We therefore suggest that paternal education, identified in our study as the most robust socioeconomic determinant of infant pulmonary health, should be routinely included in patient socio-demographic assessments.
While a number of studies demonstrate the adverse effect of socioeconomic limitations (low education, low income, or single caregiver) on CF health,16,34,40–43 our data show that these processes are at work from the very first months of life. Attempts to interrupt socioeconomic mechanisms affecting CF lung health need to be applied in very early infancy, before disease trajectory is established.44 Our findings augment the literature concerning the role of social and physical environment for CF lung health, and emphasize the need to add paternal education to the list of impactful socioeconomic variables.
The study has some obvious limitations. The CF infant population was relatively small, and the impact of socioeconomic factors in this single-center study may not be generalizable to the CF population in the United States or internationally. Multicenter studies with a larger patient cohort are necessary to validate our findings. Finally, we have not examined the mechanism of the relationship between paternal education and care environment, or its interpretation when paternal education is unknown. Despite these acknowledged shortcomings, our study is among the first to suggest an important relationship between paternal education and lung function in CF infants. Additionally, we have introduced a simple clinical score to detect pulmonary impairment. Our data suggest that, combined, these two variables (clinical score and paternal education) can serve as a simple risk-assessment tool to predict level of pulmonary disease in CF infants. We anticipate that these findings may be of benefit to general pediatricians and pulmonary subspecialists without access to infant pulmonary function measurement tools.
CONCLUSION
Identifying risk factors for infant lung disease is essential for interrupting disease progression. We developed a five-item composite clinical measure that predicts CF infant lung health, and identified paternal education as the most significant socioeconomic predictor of CF infant lung health. We combined these two findings to risk-stratify CF infants for early-onset lung disease and identify those meriting targeted therapeutic attention. Our model showed significant differences in infant pulmonary function across risk groups. The developed tool offers an easily available, inexpensive, and non-invasive way to assess risk of respiratory decline in CF infants. Future studies will determine the predictive value of this stratification model on long-term respiratory outcomes and its application to older CF populations.
Acknowledgments
Funding source: Cystic Fibrosis Foundation, Numbers: HARRIS12Q0, GUTIER14QIO, CC032-14; NIH, Numbers: UL1TR001417, P30DK72482; Children’s of Alabama.
ABBREVIATIONS
- ANOVA
Analysis of variance
- CF
Cystic fibrosis
- COA
Children’s of Alabama
- CS
Clinical score
- Ed
Education
- FEF25–75
Forced expiratory flow at 25–75% of the forced vital capacity
- FEV0.5
Forced expiratory volume at 0.5 sec
- FVC
Forced vital capacity
- iPFT
Infant pulmonary function test
- IRT
Immunoreactive trypsinogen
- NBS
Newborn screening
- NS
Not significant
- PsA
Pseudomonas aeruginosa
- RV/TLC
Residual volume divided by total lung capacity, a measure of hyperexpansion
- RVRTC
Raised volume rapid thoracoabdominal compression
- SD
Standard deviation
- SEM
Standard error of the mean
- UAB
University of Alabama at Birmingham
- V30
Lung volume at a pressure of a 30 cm water.
Footnotes
Conflicts of interest: None.
The authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Dankert-Roelse JE, Merelle ME. Review of outcomes of neonatal screening for cystic fibrosis versus non-screening in Europe. J Pediatr. 2005;147:S15–S20. doi: 10.1016/j.jpeds.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Merelle ME, Nagelkerke AF, Lees CM, Dezateux C. Newborn screening for cystic fibrosis. Cochrane Database Syst Rev. 2001:CD001402. doi: 10.1002/14651858.CD001402. [DOI] [PubMed] [Google Scholar]
- 3.Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A United Kingdom Cystic Fibrosis Database Steering C. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics. 2007;119:19–28. doi: 10.1542/peds.2006-1498. [DOI] [PubMed] [Google Scholar]
- 4.Baussano I, Tardivo I, Bellezza-Fontana R, Forneris MP, Lezo A, Anfossi L, Castello M, Aleksandar V, Bignamini E. Neonatal screening for cystic fibrosis does not affect time to first infection with Pseudomonas aeruginosa. Pediatrics. 2006;118:888–895. doi: 10.1542/peds.2004-2599. [DOI] [PubMed] [Google Scholar]
- 5.Farrell PM, Li Z, Kosorok MR, Laxova A, Green CG, Collins J, Lai HC, Rock MJ, Splaingard ML. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med. 2003;168:1100–1108. doi: 10.1164/rccm.200303-434OC. [DOI] [PubMed] [Google Scholar]
- 6.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 7.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155:623 e1–628 e1. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 9.Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, et al. Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med. 2008;178:1238–1244. doi: 10.1164/rccm.200804-551OC. [DOI] [PubMed] [Google Scholar]
- 10.Brennan S, Hall GL, Horak F, Moeller A, Pitrez PM, Franzmann A, Turner S, de Klerk N, Franklin P, Winfield KR, et al. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax. 2005;60:159–163. doi: 10.1136/thx.2004.026419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 12.Hilliard TN, Sukhani S, Francis J, Madden N, Rosenthal M, Balfour-Lynn I, Bush A, Davies JC. Bronchoscopy following diagnosis with cystic fibrosis. Arch Dis Child. 2007;92:898–899. doi: 10.1136/adc.2006.105825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahiri T, Hempstead SE, Brady C, Cannon CL, Clark K, Condren ME, Guill MF, Guillerman RP, Leone CG, Maguiness K, et al. Clinical practice guidelines from the cystic fibrosis foundation for preschoolers with cystic fibrosis. Pediatrics. 2016;137:pii: e20151784. doi: 10.1542/peds.2015-1784. [DOI] [PubMed] [Google Scholar]
- 14.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, Green CG, Collins J, Farrell PM. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 15.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23:330–335. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163:1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 17.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ Investigators, Coordinators of the Epidemiologic Study of Cystic F. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142:624–630. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 18.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 19.Sanders DB, Li Z, Laxova A, Rock MJ, Levy H, Collins J, Ferec C, Farrell PM. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann Am Thorac Soc. 2014;11:63–72. doi: 10.1513/AnnalsATS.201309-303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comeau AM, Accurso FJ, White TB, Campbell PW, 3rd, Hoffman G, Parad RB, Wilfond BS, Rosenfeld M, Sontag MK, Massie J, et al. Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation workshop report. Pediatrics. 2007;119:e495–e518. doi: 10.1542/peds.2006-1993. [DOI] [PubMed] [Google Scholar]
- 21.Castile R, Filbrun D, Flucke R, Franklin W, McCoy K. Adult-type pulmonary function tests in infants without respiratory disease. Pediatr Pulmonol. 2000;30:215–227. doi: 10.1002/1099-0496(200009)30:3<215::aid-ppul6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Stocks J, Godfrey S, Beardsmore C, Bar-Yishay E, Castile R. Plethysmographic measurements of lung volume and airway resistance. ERS/ATS task force on standards for infant respiratory function testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2001;17:302–312. doi: 10.1183/09031936.01.17203020. [DOI] [PubMed] [Google Scholar]
- 23.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161:353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld M, Allen J, Arets BH, Aurora P, Beydon N, Calogero C, Castile RG, Davis SD, Fuchs S, Gappa M, et al. An official American Thoracic Society workshop report: optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Ann Am Thorac Soc. 2013;10:S1–S11. doi: 10.1513/AnnalsATS.201301-017ST. [DOI] [PubMed] [Google Scholar]
- 25.Gappa M, Ranganathan SC, Stocks J. Lung function testing in infants with cystic fibrosis: lessons from the past and future directions. Pediatr Pulmonol. 2001;32:228–245. doi: 10.1002/ppul.1113. [DOI] [PubMed] [Google Scholar]
- 26.Ranganathan SC, Bush A, Dezateux C, Carr SB, Hoo AF, Lum S, Madge S, Price J, Stroobant J, Wade A, et al. Relative ability of full and partial forced expiratory maneuvers to identify diminished airway function in infants with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:1350–1357. doi: 10.1164/rccm.2202041. [DOI] [PubMed] [Google Scholar]
- 27.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, Cutting GR. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299:417–424. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schechter MS. Nongenetic influences on cystic fibrosis outcomes. Curr Opin Pulm Med. 2011;17:448–454. doi: 10.1097/MCP.0b013e32834ba899. [DOI] [PubMed] [Google Scholar]
- 29.Goeminne PC, Kicinski M, Vermeulen F, Fierens F, De Boeck K, Nemery B, Nawrot TS, Dupont LJ. Impact of air pollution on cystic fibrosis pulmonary exacerbations: a case-crossover analysis. Chest. 2013;143:946–954. doi: 10.1378/chest.12-1005. [DOI] [PubMed] [Google Scholar]
- 30.Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc. 2015;12:385–391. doi: 10.1513/AnnalsATS.201408-400OC. [DOI] [PubMed] [Google Scholar]
- 31.Taylor-Robinson D, Whitehead M, Diggle P, Smyth R. The effect of social deprivation on weight in the UK cystic fibrosis population. J Epidemiol Community Health. 2011;65:A389. [Google Scholar]
- 32.Thoits PA. Stress and health: major findings and policy implications. J Health Soc Behav. 2010;51:S41–S53. doi: 10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- 33.Everhart RS, Fiese BH, Smyth JM, Borschuk A, Anbar RD. Family functioning and treatment adherence in children and adolescents with cystic fibrosis. Pediatr Allergy Immunol Pulmonol. 2014;27:82–86. doi: 10.1089/ped.2014.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to airway clearance therapy in pediatric cystic fibrosis: socioeconomic factors and respiratory outcomes. Pediatr Pulmonol. 2015;50:1244–1252. doi: 10.1002/ppul.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dashiff C, Morrison S, Rowe J. Fathers of children and adolescents with diabetes: what do we know? J Pediatr Nurs. 2008;23:101–119. doi: 10.1016/j.pedn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Rammohan A, Awofeso N, Fernandez RC. Paternal education status significantly influences infants’ measles vaccination uptake, independent of maternal education status. BMC Public Health. 2012;12:336. doi: 10.1186/1471-2458-12-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumenshine PM, Egerter SA, Libet ML, Braveman PA. Father’s education: an independent marker of risk for preterm birth. Matern Child Health J. 2011;15:60–67. doi: 10.1007/s10995-009-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartley M, Martikainen P, Shipley M, Marmot M. Gender differences in the relationship of partner’s social class to behavioural risk factors and social support in the Whitehall II study. Soc Sci Med. 2004;59:1925–1936. doi: 10.1016/j.socscimed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Nicolaidis C, Ko CW, Saha S, Koepsell TD. Racial discrepancies in the association between paternal vs. maternal educational level and risk of low birthweight in Washington State. BMC Pregnancy Childbirth. 2004;4:10. doi: 10.1186/1471-2393-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balmer DF, Schall JI, Stallings VA. Social disadvantage predicts growth outcomes in preadolescent children with cystic fibrosis. J Cyst Fibros. 2008;7:543–550. doi: 10.1016/j.jcf.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: a longitudinal study. Lancet Respir Med. 2013;1:121–128. doi: 10.1016/S2213-2600(13)70002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor GT, Quinton HB, Kneeland T, Kahn R, Lever T, Maddock J, Robichaud P, Detzer M, Swartz DR. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111:e333–e339. doi: 10.1542/peds.111.4.e333. [DOI] [PubMed] [Google Scholar]
- 43.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr. 1998;132:260–264. doi: 10.1016/s0022-3476(98)70442-1. [DOI] [PubMed] [Google Scholar]
- 44.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]