Abstract
The role of the thalamus in complex cognitive behavior is a topic of increasing interest. Here we demonstrate that lesions of the nucleus reuniens (NRe), a midline thalamic nucleus interconnected with both hippocampal and prefrontal circuitry, lead to enhancement of executive behaviors typically associated with the prefrontal cortex. Rats were tested on four behavioral tasks: (1) the combined attention-memory (CAM) task, which simultaneously assessed attention to a visual target and memory for that target over a variable delay; (2) spatial memory using a radial arm maze, (3) discrimination and reversal learning using a touchscreen operant platform, and (4) decision-making with delayed outcomes. Following NRe lesions, the animals became more efficient in their performance, responding with shorter reaction times but also less impulsively than controls. This change, combined with a decrease in perseverative responses, led to focused attention in the CAM task and accelerated learning in the visual discrimination task. There were no observed changes in tasks involving either spatial memory or value-based decision making. These data complement ongoing efforts to understand the role of midline thalamic structures in human cognition, including the development of thalamic stimulation as a therapeutic strategy for acquired cognitive disabilities (Schiff, 2008; Mair et al., 2011), and point to the NRe as a potential target for clinical intervention.
Keywords: cognition, thalamocortical, hippocampus, prefrontal, arousal
The contribution of the prefrontal cortex to aspects of executive control is well established. Recent work has emphasized the critical role of other brain structures to which the prefrontal cortex is connected. One of these structures, the nucleus reuniens (NRe) of the midline thalamus, exchanges projections with ventral and orbital regions of the prefrontal cortex (Ohtake and Yamada, 1989; McKenna and Vertes, 2004; Vertes et al., 2006; Hoover and Vertes, 2007; Prasad and Chudasama, 2013). Another is the ventral hippocampus, which in addition to receiving NRe input (Herkenham, 1978; Su and Bentivoglio, 1990; Wouterlood et al., 1990; Vertes et al., 2006) also projects strongly to the prefrontal cortex (Jay and Witter, 1991; Verwer et al., 1997; Ishikawa and Nakamura, 2006). Lesion studies in the rat have been critical for delineating the functional contribution of brain regions underlying so-called executive behaviors. Damage to the NRe, for example, can affect behavioral flexibility (Flämig and Klingberg, 1978; Dolleman-van der Weel et al., 2009; Cholvin et al., 2013; Prasad et al., 2013), in a way that under some conditions mimics ventral prefrontal (Ragozzino et al., 1999; Chudasama et al., 2003; Kim and Ragozzino, 2005; see also Murphy et al., 2005, 2012) or ventral hippocampal lesions (Abela et al., 2013). These anatomical connections, together with the overlap in behavioral deficits, have led to the conception that the NRe occupies a node in a larger network whose disruption leads to deficits in cognitive function (Cassel et al., 2013).
At the same time, the NRe receives abundant noradrenergic, serotonergic and cholinergic innervation from the brainstem (Kolmac and Mitrofanis, 1999; Vertes et al., 1999; Krout et al., 2002; Jones, 2003), and may therefore have a role in regulating cortical arousal levels (Vanderwolf and Stewart, 1988; McCormick, 1992; Van der Werf et al., 2002). This prospect gives the NRe a potentially unique role in thalamocortical control over executive behavior by adjusting the arousal level of the prefrontal cortex (Groenewegen and Berendse, 1994; Schiff, 2008; Mair et al., 2011).
Importantly, while some behavioral effects of NRe lesions match those of prefrontal and ventral hippocampal lesions, others do not. In fact, previous reports suggest that certain aspects of behavior are improved or enhanced following NRe lesions. We recently reported one such improvement following NRe lesions, expressed as an attenuation of compulsive behavior (i.e., perseverative responses) compared with control rats (Prasad et al., 2013). This finding contrasted sharply with the effects of lesions to the prefrontal cortex and ventral hippocampus, which resulted in the exaggeration of compulsive responses (Passetti et al., 2002; Chudasama et al., 2003; Abela et al., 2013). Moreover, the performance of rats with NRe lesions was marked by unusually high motivation and good accuracy, as if the NRe lesion made rats more attentive to their environment and focused on their task (Prasad et al., 2013).
In the present study, we investigate whether a similar improvement of cognitive function occurs in NRe-lesioned rats when tested in a variety of prefrontal-dependent tasks. We compared experimental and control rats on operant behavioral tasks assessing visual attention and working memory, associative learning and decision-making. We also tested rats on a radial arm maze as several studies indicate a role for the NRe in spatial memory (Hembrook and Mair, 2011; Loureiro et al., 2012; Layfield et al., 2015; Ito et al., 2015). We report several features that distinguish NRe lesions from those of its projection targets. Most notably, rats with NRe lesions showed enhanced cognitive performance along several dimensions that would normally be impaired following prefrontal or ventral hippocampal lesions. There were no obvious effects on behaviors associated with hippocampal lesions such as spatial memory, or decision-making with delayed outcomes. We conclude that the NRe contributes to cognitive-executive behavior through a modulation of the prefrontal cortex, perhaps via influence of projections from the ascending brainstem arousal systems.
EXPERIMENTAL PROCEDURES
Animals
Data were collected from 62 male Long Evans rats (Charles River, LaSalle, CA) weighing 200–225 g at the start of behavioral testing. All rats were maintained at 85% of their free-feeding weight with water available ad libitum. The rats were housed in pairs in a temperature-controlled room (21–22 °C) with a 12-h light/dark cycle. The animals were cared for under experimental procedures approved by the McGill University Animal Care and Use Committee, in accordance with the guidelines of the Canadian Council on Animal Care.
Surgery
Rats were anesthetized with isoflurane gas. Excitotoxic lesions were made by injecting 0.09 M N-methyl-D-aspartic acid (NMDA; Sigma-Aldrich, Canada) dissolved in 0.9% saline (pH 7.0–7.2) with a 0.5 μL SGE precision microsyringe (Canadian Life Science, Peterborough, CA). Lesions of the NRe are especially challenging because it is located beneath the midline sagittal sinus. To ensure that the lesion occupied as much of the rostro-caudal extent of the nucleus as possible, rats received three injections of 0.18 μL of 0.09 M NMDA which alternated across the midline (i.e., two injections in the left hemisphere and one in the right hemisphere, or vice versa). In each animal these injections were made at the following anterior–posterior (AP) and dorsoventral (DV) coordinates: AP: −1.3 mm, DV: −7.8 mm; AP: −1.9 mm, DV: −7.8 mm; and AP: −2.5 mm, DV: −7.8 mm. Due to the midline location of the NRe, the mediolateral (ML) reading was taken from either side of the sagittal sinus, which approximated to 0.2 mm from the bregma. Each injection was made over 1 min and the syringe remained in place for 90 s to permit dispersion of the toxin before the needle was retracted. Rats that served as sham controls received the same surgical treatment but received injections of saline. Rats were assigned to two cohorts. Rats in cohort 1 (n=28) were trained on the combined attention-memory (CAM) task before receiving the NRe lesion. Rats in cohort 2 (n=34) were trained on all other tasks after receiving the lesion.
Behavioral procedures
Four behavioral tasks were employed to systematically investigate the behavioral consequences of NRe lesions. They are described within the following section.
Combined attention-memory task
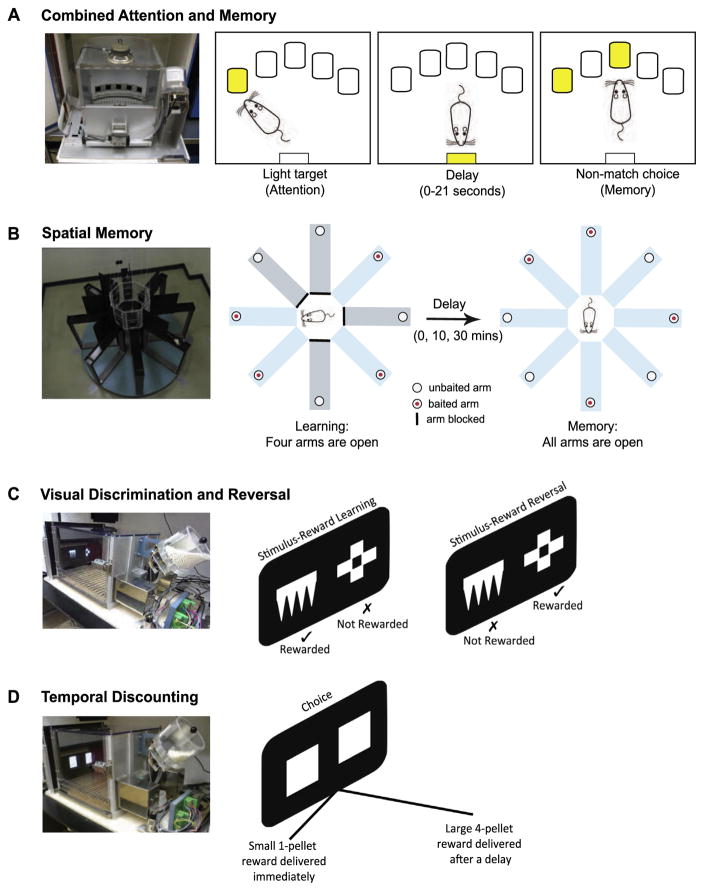
The data for this task were collected while rats performed a task that assessed visual attention and working memory in the same setting. The task, known as the CAM task, was initially developed to establish how overlapping mechanisms of attention and working memory were differentially affected by fluctuations in prefrontal catecholamine transmission (Chudasama and Robbins, 2004a). The CAM task is similar in principle to the delayed non-match to position task (Dunnett, 1985; Goldman- Rakic, 1987). To minimize the contribution of spatial cues, the test was conducted in operant testing chambers (Lafayette, Indiana, USA) measuring only 25 × 25 cm equipped with five nosepoke apertures or holes (see Fig. 1A). In the present study, holes in non-adjacent locations (positions 1, 3 and 5) were used. Each trial comprised a target (attention) phase and a choice (memory) phase. In the target phase, the rat was required to respond to a light stimulus (3-s duration) presented pseudorandomly in one of the three holes. Impulsive premature responses in the holes before the onset of the light target were without consequence. Following a correct response to the visual target, a variable delay (0, 7, 14 or 21 s) was signified by the illumination of the food magazine. A nosepoke entry into the food magazine after the programed delay presented the rat with a choice of two lights (3-s duration). One light (the matching stimulus) was presented in the hole identical to the target light. The second light (the non-matching stimulus) was presented in one of the remaining two holes. A correct response to the non-matching stimulus was rewarded with two sucrose pellets (Dustless Precision Pellets, Ren’s Pets Depot, ON, Canada). An incorrect response to the matching stimulus, a response in the non-illuminated hole, or a failure to respond within 5 s terminated the trial, and all lights were extinguished for 5 s. Each session consisted of 80 trials. Each delay was presented for 20 trials although the final number of trials depended on the number of ‘correct’ trials in the target phase. During initial training, the duration of the target was set to 3 s. When rats in cohort 1 completed ≥75% correct target responses at this duration, and ≥65% correct choice responses ~30 sessions), they received a NRe lesion. Following two weeks of postoperative recovery, rats were re-stabilized on the preoperative schedule of the task, and were subsequently challenged by reducing the duration of the target stimulus to 1 s and 0.7 s in separate sessions. The apparatus and online data collection were controlled by the Whisker control system for research (Cardinal and Aitken, 2010).
Fig. 1.
Rats were tested on four behavioral tasks: (A) The combined attention and memory (CAM) task was conducted in an operant chamber with an arc of five holes, three of which were active during the task. Each trial comprised two phases. In the target phase (attention), a brief light was presented pseudorandomly in one of three holes. A correct target response was followed by a variable delay signified by the illumination of the food magazine. Rats nose poked the food magazine during the delay. After the delay, the rats were presented with the choice phase (memory) in which two lights were presented simultaneously. A correct response to the non-matching stimulus was rewarded with two pellets. (B) Spatial memory was tested in a standard radial arm maze. First, rats learned which four arms were baited with food. After the delay, the remaining four arms were opened and the rat was required to enter and retrieve pellets from the new arms avoiding the arms from which pellets had already been collected. (C) The visual discrimination and reversal task was conducted in an automated operant touchscreen apparatus. Two different computer graphic stimuli were presented on the touchscreen. Only one stimulus was associated with reward (stimulus-reward learning). On reaching criterion performance, the stimulus-reward contingencies were reversed such that the previously non-rewarded stimulus was now rewarded (stimulus-reward reversal), and vice versa. (D) Delay discounting was also conducted in the touchscreen apparatus. This time the animal was presented with two identical white squares and the animal faced a choice. Responding on the left square resulted in the immediate delivery of a small 1-pellet reward, whereas a response to the right square delivered a large 4-pellet reward after a delay.
Spatial memory task
Rats with thalamic lesions or inactivations that include the NRe appear to be deficient in their memory for spatial locations when tested in a variety of maze paradigms (Davoodi et al., 2009; Hembrook and Mair, 2011; Loureiro et al., 2012). Consequently, we compared the animals’ working memory for a visual stimulus (CAM task, described above) with their working memory for spatial location. The spatial memory test used a standard radial eight-arm maze illustrated in Fig. 1B. In each trial, rats were placed in a central octagonal arena and allowed to explore and retrieve a single sucrose pellet from each of four randomly selected ‘open’ arms (learning phase). Upon collection of the fourth pellet, the remaining four arms were immediately opened (‘0’ min delay) allowing the animal access to all eight arms (memory phase). As the animal had already collected the pellets from the open arms, only the four arms that had been closed in the learning phase were baited. A reentry into an arm from which a pellet had been retrieved during the learning phase was recorded as a perseverative error. Criterion performance was set to ≤1 error over two consecutive sessions. The test was then repeated with increasing delays of 10 and 30 min between the learning and memory phases.
Visual discrimination and reversal task
A reversal learning task was conducted in touchscreen automated chambers (Lafayette, Indiana, USA) to assess control of responding with changing stimulus-reward contingencies. Following habituation to the apparatus, the rats were trained to make a nosepoke touch response to a white square (2″ × 2″) that was presented on the left or right side of the screen. A nosepoke touch response to the white square was rewarded with a single sucrose pellet. When rats were able to obtain 50 reward pellets within 20 min (~4 sessions), they were ready for surgery. After the rats had recovered from surgery, they were shaped to touch the screen until they achieved the same criterion before surgery (~2 sessions). The rats were then tested on their ability to acquire a visual discrimination by learning a stimulus– reward association.
Two white geometric computer graphic stimuli were presented on a black background on the touchscreen (see Fig. 1C). The left and right position of each stimulus was determined pseudorandomly. These stimuli remained on the screen until the rat made a nosepoke touch response to either stimulus. A correct response to one stimulus (designated A+) was associated with a sucrose pellet. An incorrect response to the other stimulus (designated B−) was not rewarded and instead resulted in the disappearance of both stimuli from the screen, and a 5-s timeout period during which all of the lights were extinguished. An incorrect response to B− resulted in a correction trial in which the same trial was repeated (i.e., the A+ and B− stimuli remained in the same left/right positions) until the rat responded correctly. Thus, each session could have an infinite number of correction trials, but was limited to a total of 60 non-correction trials. Criterion performance was set to 85% accuracy on two consecutive sessions after which the stimulus-reward contingencies were reversed so that the previously non-rewarded stimulus (B−) became the rewarded stimulus (B+), and vice versa. The rat was now required to reverse its response by inhibiting its response to the previously rewarded stimulus, and respond to the new rewarded stimulus. On reaching the 85% criterion on two consecutive sessions the reward contingencies were reversed again. A total of two reversals were given. The apparatus and online data collection were controlled by the Whisker control system for research (Cardinal and Aitken, 2010).
Decision-making task with delayed outcomes
The delay discounting task was conducted in the same touchscreen apparatus described above. In this case, the animal’s choice responses were used to assess behavioral decisions that involved a trade-off between reward size and delay (Fig. 1D). A detailed description of the task is provided in Abela and Chudasama (2013). In brief, rats chose between two identical white squares located on the left and right sides of a touchscreen. Responses to the left stimulus resulted in the immediate delivery of a small, one-pellet reward. Responses to the right stimulus resulted in a large four-pellet reward that was delivered after a delay. The side on which the large reward stimulus was presented (left or right) was counter-balanced between subjects, and remained in the same location throughout the entire experiment for each rat. Each session consisted of four blocks of 12 trials. In each block, two ‘forced choice’ trials in which the rat was forced to respond to either the left or the right stimulus demonstrated the outcome associated with the stimulus. The remaining 10 trials were ‘free choice’ trials in which the rats could choose between both stimuli. Rats were initially trained to discriminate between the two reward sizes when there were no delays until they were choosing the large reward >80% of the time (~3 days). Thereafter, the delay to delivery of the large reward was progressively increased in each block within a session (0, 8, 16, and 32 s). Each trial lasted for 70 s regardless of the rat’s choice of stimulus. The apparatus and online data collection were controlled by the Whisker control system for research (Cardinal and Aitken, 2010).
Data analyses
Data were analyzed using SPSS Statistical Software, v.20.0. (SPSS Inc., Illinois, USA). Data for each variable were subjected to a repeated measures analysis of variance. The between-subject factor (lesion) was at two levels: Sham and NRe. For the CAM task, the within-subject factor was delay at 4 levels (0, 7, 14, 21 s) and target duration at three levels (3, 1, and 0.7 s). For the delay discounting task, delay was a within-subject factor at 4 levels (0, 8, 16, 32 s). Homogeneity of variance was assessed using Mauchly’s sphericity test, and if this requirement was violated for a repeated measures design, the F-term was tested against degrees of freedom corrected by Greenhouse-Geisser to provide a more conservative P-value for each F-ratio.
For all other variables, the data were subjected to independent samples t-tests. This includes the number of errors committed for each delay in the spatial memory task and the number of errors committed during acquisition and reversal for the visual discrimination task. Levene’s test for equality of variance was used to determine homogeneity of variance for these tests. If the requirement for homogeneity of variance was violated, the t-term was tested against degrees of freedom corrected for a more conservative P-value.
RESULTS
The general approach in this study was to test rats in multiple tasks in order to comprehensively assess the behavioral effects of NRe lesions. Rats in cohort 1 were tested on the CAM task only. Rats in cohort 2 were tested in all other tasks. We found that some aspects of cognition were affected, whereas other aspects were equivalent to control levels. The most obvious change was improved performance on tasks that required focused attention. The following sections describe, in turn, the extent of the anatomical lesions and a comparison between lesion and control groups in the behavioral tasks.
Histology
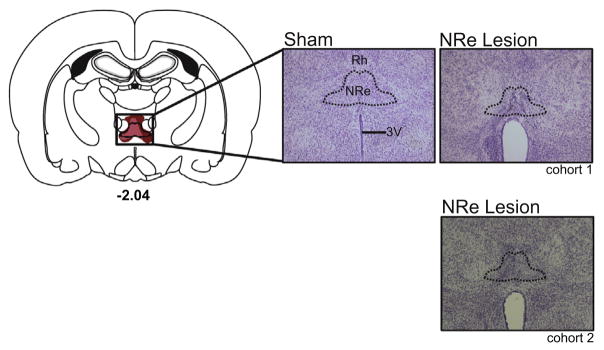
Fig. 2 provides a diagrammatic reconstruction of the lesion with accompanying high magnification photomicrographs of the NRe in a representative sham-operated rat and NRe-lesioned rat from cohorts 1 and 2. The tissue analyzed from each animal (shams, as well as lesions) consisted of sections collected between −0.84 and −4.36 mm posterior to the bregma according to the atlas of Paxinos and Watson (2005). This range encompassed the NRe and adjacent regions, which may have been inadvertently damaged by the excitotoxin. Histological analyses were performed using light microscopy, in which the cellular morphology of NRe neurons from sham controls provided a standard of healthy, unaffected tissue, which was compared with the lesioned tissue. Cells that were shrunken, striated and/or surrounded by gliosis were considered damaged by the excitotoxicity of the NMDA infusion. The completeness of the lesion was based on the ratio of damage within each section of the NRe (see Fig. 2 for an example of one such section). Sections in which the lesion encompassed the NRe proper as well as the ventral reuniens (i.e., the lateral, winglike adjacent subregions of the nucleus referred to as vRe in Paxinos and Watson, 2005) were used to define a “complete” lesion. We used stringent inclusion criteria to identify animals in which the lesion was complete. We first calculated the complete extent of the NRe from −1.08 mm to −3.96 mm posterior to bregma. For each animal, this comprised 25 sections. To be included in the final group for behavioral analyses, the lesion had to occupy at least 64% of the entire nucleus within the range of −1.72 to −3.48 mm posterior to the bregma, which comprised a minimum of 16 sections.
Fig. 2.
Left panel is a coronal section of the rat brain showing largest (dark red) and smallest (light red) extent of the NRe lesion. Right panels provide representative photomicrographs of Nissl-stained coronal sections providing a magnified view of an intact NRe within a sham control (Sham), and a lesioned NRe from a rat in each cohort (NRe lesion). For the lesion, the area outlined in black shows a characteristic excitotoxic reaction within the NRe accompanied by shrinkage of the tissue and expansion of the third ventricle. Number represents the anterior–posterior location of sections relative to bregma (in mm) according to Paxinos and Watson (2005).
In cohort 1, a total of 10 animals were excluded because the lesion was small and incomplete, there was extensive damage to the centromedial and/or rhomboid nuclei or the lesion was too lateralized. In addition, one NRe-sham animal from cohort 1 was removed due to inadvertent damage to the rostral midline, anterior and reticular thalamus in the right hemisphere. In cohort 2, eleven animals had minimal damage to the NRe area sparing the rostral and central aspect of the nucleus. In two animals, there was extensive damage to regions ventral to the NRe (i.e., the paraxiphoid nucleus, paraventricular hypothalamic nucleus). In addition one NRe-sham animal was excluded due to extensive pre-existing damage to the fimbria/fornix. These animals were excluded from the study. In cohort 1, the final group numbers were: Shams, 8, and NRe, 9. In cohort 2, the final group numbers were: Shams 10, and NRe, 10.
Behavioral results
NRe lesions enhance visual attention
We first trained a cohort of rats on a task that simultaneously measures attention to a visual stimulus and working memory for that stimulus (CAM task, see Experimental procedures). Briefly, a target light first appeared in one of three holes, into which the animal was required to poke its nose. Following a variable delay, the rat was given a choice of two lights. One light was presented in the same hole as the target, and a second light was presented in a different hole. The rat was required to “non-match” by poking its nose in the newly lit hole. The attentional requirement during this initial training was low, with the animal allowed 3 s to view and encode the initially illuminated target stimulus. Animals designated for the lesion and control groups were matched on all behavioral measures including target accuracy (t(15)=0.75, P>0.05), premature responses (t(15)=−1.22, P>0.05), choice accuracy (F(1,15)=0.01, P>0.05) and choice latency (F(1,15)=0.71, P>0.05). Upon reaching criterion, half of the animals received excitotoxic lesions of the NRe, whereas the other half underwent sham control surgeries. The animals were tested again, approximately two weeks later.
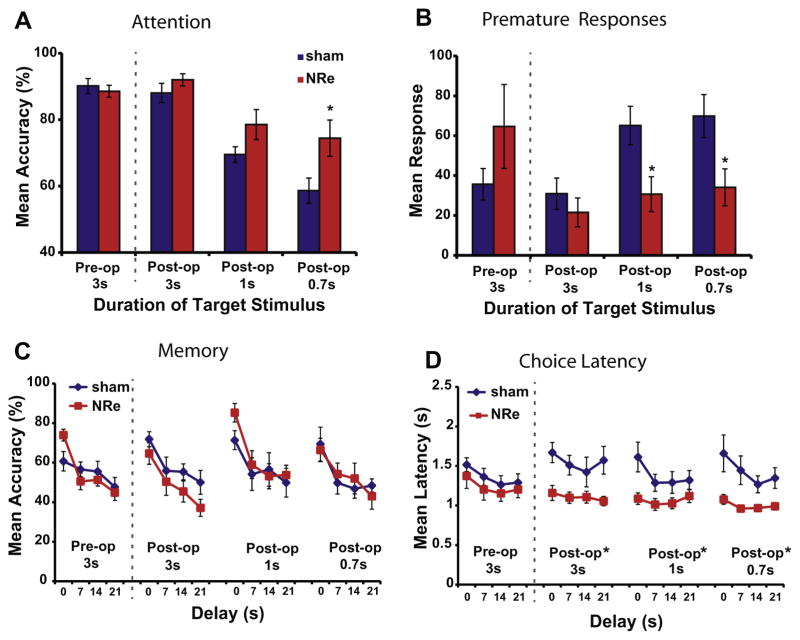
The NRe lesion group showed improved performance on the CAM task relative to controls, with the most obvious changes in aspects of the task related to attention. Following surgery, the animals were retested with the 3-s target presentation (“easy” schedule), to which both groups responded with a high level of accuracy that reflected their previous training (3A, Post-op 3 s). The animals were then challenged with shorter target presentations (≤1 s, “difficult” schedule), which led naturally to a decline in response accuracy (F(2,30)=36.9, P<0.001). This manipulation is frequently used to assess the capacity for attentional control, since inattentive animals are less likely to notice and encode a brief presentation (Bari and Robbins, 2011). Rats with NRe lesions outperformed sham controls in this attention phase of the task (F(1,15)=4.58, P=0.05), particularly when the duration of the visual target was very short (1 s, t(15)=−1.84, P=0.08; 0.7 s, t(15)=−2.37, P<0.05). These results demonstrate that the NRe lesion group was more likely to focus their attention during the task relative to the sham control group.
In addition to improved attentional focus, the lesioned animals were more controlled and less impulsive in their actions (Fig. 3B), as indicated by the marked reduction in the number of premature responses (F(1,15)=7.68, P<0.01) when the stimulus duration was 1 s (t(15)=2.64, P<0.05) and 0.7 s (t(15)=2.53, P<0.05). In the latter part of the task, which required choosing a non-matching stimulus after a delay, the lesioned rats showed a normal delay-dependent decline in their accuracy (F(3,45)=15.9, P<0.001) that did not differ from the control group (F(1,15)=0.05, P>0.05; Fig. 3C). However, the choices made by rats in the NRe group differed in one respect, in that they were significantly faster than those of the sham controls (F(1,15)=8.90, P<0.01; Fig 3D). This speed of response was irrespective of target duration (F(2,30)=2.94, P>0.05); the animals were fast in their response regardless of whether the target duration was 3 s (F(1,15)=8.2, P<0.01), 1 s (F(1,15)=5.24, P<0.05) or 0.7 s (F(1,15)=8.99, P<0.01). All other aspects of performance, including latency to collect food reward, were in the normal range (P>0.05). Thus, the NRe lesion enhanced attentional capacities for visually occurring stimuli, leading to more focused and quicker responses, but had little effect on other aspects of the task, such as the accuracy of delayed non-match responses, commonly associated with working memory.
Fig. 3.
Impact of reduced target duration on performance of the CAM task in animals with NRe lesions (red shading) compared with sham controls (blue shading) during pre-operative (Pre-op) and post-operative (Post-op) stages of testing. All graphs show mean performance (±S.E.M.): (A) accuracy in detecting the target stimulus; (B) the number of anticipatory premature responses committed before the appearance of the light target; (C) accuracy for correctly responding to the non-match stimulus after the variable delay, and (D) latency to respond to the correct non-match stimulus after the variable delay. *P<0.05 relative to shams.
NRe lesions disrupt spatial searching but not spatial memory
We next asked whether NRe lesions disrupt spatial memory, as might be expected given its projections to the hippocampus. To assess this, we tested animals in their capacity to acquire memory for a spatial location in a radial arm maze. In each trial, the rat first learned which arms were baited with food. Then, after the rat had collected the pellets from each of the baited arms, and following a delay, the other arms were opened. The animal was required to enter and retrieve the pellets from the new arms, logically avoiding the old arms from which the pellets had already been collected. Since the baiting pattern in each trial was independent from the previous one, correct performance required that the animal hold ‘on-line’ in memory which of the four arms had been previously visited so as to not re-enter these arms incorrectly, which would constitute a perseverative error.
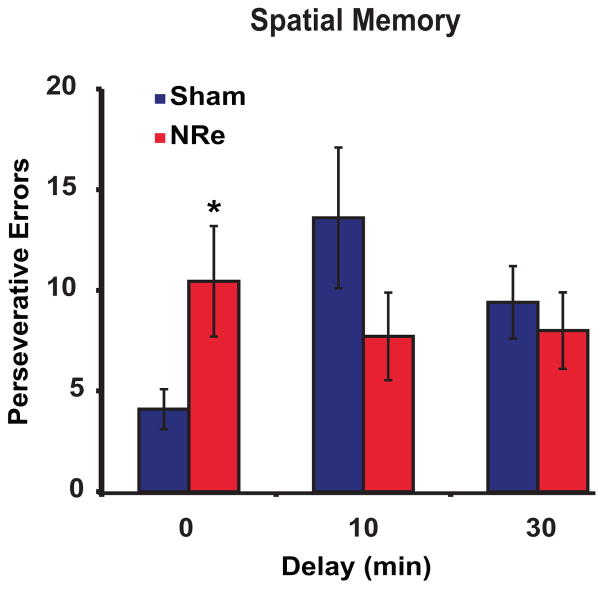
The influence of the NRe lesion depended critically on whether a delay was present between the first portion of the trial (the initial collection of four pellets), and the second portion of the trial (the opening of the new arms). In the no-delay condition, the rats with NRe lesions required almost twice as many sessions to reach criterion as controls (mean sessions ± S.E.M.: NRe group, 5.7 ± 1.0; sham controls, 3.4 ± 0.4; t(10)=−2.15, P=0.05), and made several errors during the second portion of the trial when all arms were open (mean errors ± S.E.M.: NRe, 12.7 ± 3.7; sham, 4.6 ± 1.0; t(17)=−2.20, P<0.05). This pattern of choices could not easily be attributed to a failure of working memory, as it was expressed as a selective perseverative reentry into the old arms (t(17)=−2.27, P<0.05; see Fig. 4), with few re-entries into the new arms (mean errors ± S.E.M.: NRe, 2.3 ± 1.2; sham, 0.5 ± 0.2; t(9)=−1.52, P>0.05). However, this deficit was transient and no longer apparent when the animals were tested when a delay was interposed between the two portions of the trial. When that delay was relatively long (10 min), the NRe-lesioned rats were normal (mean sessions ± S.E.M.: NRe, 4.6 ± 0.8; shams, 7.2 ± 1.4) and the two groups did not differ from each other (t(14)=1.57, P>0.05). Fig. 4 shows that rats with NRe lesions also outperformed the sham controls by committing fewer perseverative re-entries into the previously baited arms, even though this effect did not reach statistical significance (t(15)=1.28, P>0.05). With a 30-min delay, the two groups showed equivalent performance (sessions: t(15)=0.74, P>0.05; perseverative errors: t(15)=0.52, P>0.05). Thus, there was no obvious deficit in spatial memory.
Fig. 4.
Impact of delay on spatial memory in animals with NRe lesions (red shading) compared with sham controls (blue shading). Mean number of repeat entries (perseverative errors) into previously baited arms. *P<0.05 relative to shams. All error bars indicate S.E.M.
NRe lesions improve visual associative learning
We next asked whether the enhanced attentional capacities of animals with NRe lesions might affect their ability to discriminate perceptually different visual stimuli. We tested this by training them to form stimulus–reward associations to shapes presented on a touchscreen. In this task, a pair of shapes was presented on a touchscreen and the rat received a sucrose pellet reward upon pressing its nose against the correct one. For each trial, the left/right position of the correct shape was pseudorandomized. When the rat made an incorrect response, the trial was repeated (i.e., a correction trial) until the rat responded correctly. Errors on correction trials were distinguished from those on non-correction trials, with only the latter being a measure of stimulus-reward performance unrelated to spatial or side biases.
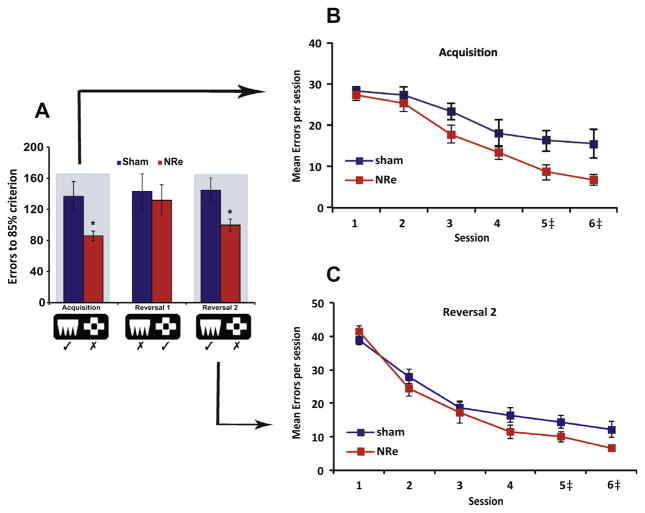
Rats with NRe lesions showed a faster than normal rate of learning than controls, requiring fewer sessions to reach criterion (mean sessions ± S.E.M.: NRe, 3.9 ± 0.3; shams, 6.0 ± 0.9; t(12)=2.16, P=0.05). Moreover, the lesioned animals successfully discriminated the perceptual features of the stimuli better than the controls, committing fewer non-correction trial errors (t(11)=2.50, P<0.05; Fig. 5A Acquisition). We looked at the temporal dynamics of this improvement by plotting the errors committed in noncorrection trials for the first 6 sessions (Fig. 5B). This confirmed that the NRe lesion accelerated learning by attenuating perseverative responses to the incorrect stimulus as early as session 3, with many of the lesioned rats reaching criterion by session 4. Moreover, consistent with their rapid learning, they needed few repeat trials to correct their errors (mean correction trial errors ± S.E.M.: NRe, 69 ± 8.4; sham, 106 ± 12.2; t(18)=2.5, P<0.05).
Fig. 5.
Mean performance (±S.E.M.) of animals with NRe lesions (red shading) compared with sham controls (blue shading) on the visual discrimination and reversal task. (A) Number of non-correction trial errors to reach 85% criterion for Acquisition, Reversal 1 and Reversal 2. (B) Mean number of errors committed in non-correction trials for first 6 sessions when learning the stimulus–reward association (Acquisition). (C) Mean number of errors committed in non-correction trials for first 6 sessions when stimulus reward contingencies were reversed the second time (Reversal 2). *P<0.05 relative to shams. ‡Some animals reached criterion by session 4. Therefore in sessions 5 and 6, the data are presented from a different number of animals. For acquisition session 5, Sham, n=10, NRe 9; session 6, Sham, n=7, NRe 6. For reversal 2 session 5, Sham, n=10, NRe 9; session 6, Sham, n=10, NRe 8.
Surprisingly, the animals’ ability to learn rapidly did not extend to the reversal of the stimulus–reward association, at least for the first reversal, where their performance overlapped with the shams in the number of sessions to criterion (t(18)=0.99, P>0.05), non-correction trial errors (t(18)=0.37, P>0.05) and correction trial errors (t(18)=1.19, P>0.05). However, when the stimulusreward contingency was reversed again, such that it returned to its original configuration as in the acquisition phase (see Fig. 5A, Reversal 2), the NRe-lesioned rats, again, outperformed the sham controls in terms of sessions (t(18)=2.85, P<0.05) and non-correction trial errors (t(13)=2.52, P<0.05), with error rates declining rapidly by session 4 (Fig. 5C). Thus while the rats appeared to be normal in their first reversal, they reverted quickly to their better than average performance on the initial stimulus-reward configuration. Other aspects of performance including speed of response and latency to collect food were all in the normal range (all P>0.05).
NRe lesions do not affect decision-making with delayed outcomes
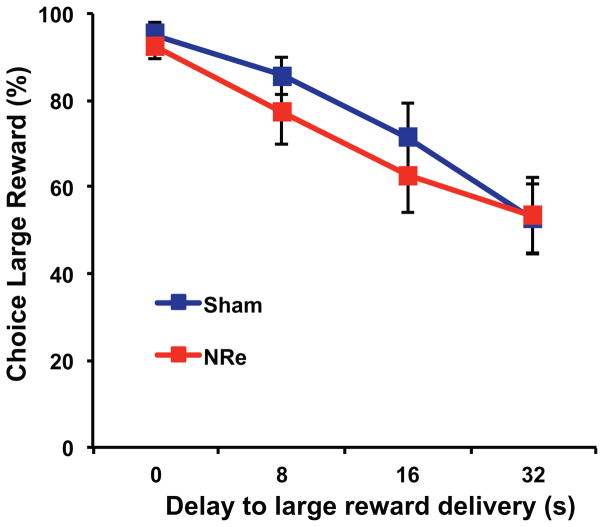
Finally, we tested rats on a decision-making task in which rats made choices between pairs of visual stimuli that traded off a small immediate reward for a large delayed reward. We were interested in whether the enhanced stimulus-reward learning, as shown above, would extend to learning associations involving time in which the reward followed several seconds after the response. Moreover, this behavior relies on an intact ventral hippocampus (Abela and Chudasama, 2013; Abela et al., 2015), a structure that receives a strong, direct input from the NRe (Herkenham, 1978; Wouterlood et al., 1990; Vertes et al., 2006; Prasad and Chudasama, 2013). We found, however, that NRe lesions did not impact this type of learning, which involves encoding the value of future outcomes. In the absence of delay, both lesioned and control rats consistently chose the large reward, indicating that they were capable of discriminating the reward size and making a choice based on this criterion. As the delay to the large reward increased, animals from both groups shifted their preference to the small, more immediate reward (F(2,27)=54.53, P<0.001; Fig. 6), and did not differ at the rate at which they chose the large, delayed reward (F(1,16)=0.29, P>0.05). Nor did they differ in their latencies to make their choice (F(1,16)=0.18, P>0.05) or collect food reward (F(1,16)=0.29, P>0.05). Together, these results indicate that an intact NRe is not necessary for decisions with delayed outcomes.
Fig. 6.
Impact of delay on choice of large reward stimulus in temporal discounting task in animals with NRe lesions (red squares) compared with sham controls (blue squares). Graph shows average percentage choice of large reward for each delay to reward delivery. All error bars indicate S.E.M.
DISCUSSION
The midline thalamic nuclei project to both prefrontal cortical and hippocampal sites and are thus in a position to influence activity related to multiple aspects of cognition. Here we demonstrate for the first time that under high attention demanding conditions, a lesion centered on the NRe primarily improves aspects of cognitive-executive performance. In contrast to our expectations based on previous studies, NRe lesions led to minimal disruption in tests of visual and spatial memory as well as decision-making. We discuss these findings in the context of thalamocortical circuitry and the influence of the brainstem arousal system.
Improved cognition following a focal lesion?
A small lesion within the NRe prompted animals to behave as if they were highly aroused and focused on the task at hand. Effective performance in the CAM task, which taps into aspects of both visual attention and visual working memory, requires the integration of multiple cognitive capacities for optimal behavior. The animals need to monitor the visual array, inhibit premature or impulsive urges to respond, selectively detect the target stimulus, and then hold on-line its location for a variable delay before using that information to guide its response (Chudasama and Robbins, 2004b). The improvement in attention following NRe lesions was most obvious when the task was made difficult by reducing the duration of the visual target. Under these conditions, the lesioned animals exhibited a higher than normal level of performance and a marked reduction in premature, impulsive responding. Thus, the NRe lesion led not only to heightened attention, but also to enhanced behavioral control, motor preparation, and quite possibly motivation. In the working memory aspect of the task, the lesioned animals were normal, with the exception that their responses were unusually fast. Thus, enhanced attentional performance does not necessarily lead to improved memory. The lesion also resulted in a general decrease in the frequency of perseverative errors, which in the case of the visual discrimination task, appears to have accelerated the rate of associative learning. This improvement did not appear to extend into the domain of cognitive flexibility, as a reversed stimulus–reward association was learned at a normal rate. However, the improvement returned when the stimulus-reward configuration was returned to its original (i.e., Reversal 2). One possibility is that rats with NRe lesions developed a learning set, thereby facilitating performance in the second reversal (see Jang et al., 2015). This hypothesis needs to be tested directly by administering serial reversals.
It is notable that the behavioral improvements following lesions to the NRe contrast sharply with the behavioral effects of damage to related structures, most notably the prefrontal cortex and hippocampus, both of which exchange projections with the NRe (Herkenham, 1978; Berendse and Groenewegen, 1991; Vertes, 2001; Prasad and Chudasama, 2013). In general, damage to these structures lead to deficits in behavioral control. Specifically, bilateral lesions placed in the prefrontal cortex (e.g., Muir et al., 1996; Passetti et al., 2002; Chudasama and Robbins, 2003; Chudasama et al., 2003) or ventral hippocampus (Bannerman et al., 1999; Mariano et al., 2009; Abela et al., 2013) cause rats to act impulsively or perseverate in their incorrect responses.
In some ways, the observed improvements in this study are most reminiscent of previous pharmacological findings involving direct infusions of certain drugs into the prefrontal cortex. For example, the local delivery of dopamine D1 receptor agonists has been shown to improve attention under similar conditions as the present study (Chudasama and Robbins, 2004a; see also Granon et al., 2000; Floresco and Phillips, 2001). This together with the known behavioral modulation of monaminergic and cholinergic inputs to the prefrontal cortex (for review, see (Chudasama and Robbins, 2006) suggests that the improvements may bear some relationship to ascending neuromodulation and cortical arousal, which we address next.
Modulating behavioral performance through cortical arousal
One interpretation of our results is that the NRe projections to the prefrontal cortex and hippocampus contribute to the balance of a circuit that regulates arousal and alertness (Steriade et al., 1990, 1997; Robbins and Everitt, 1995; Jones, 2003). The midline thalamus, like the prefrontal cortex and hippocampus, receives neuromodulatory input from the brainstem and basal forebrain and thus may participate in the neuromodulatory control over cortical arousal (Van der Werf et al., 2002; Vertes et al., 2015). However, the neuromodulatory input to the thalamus may have a fundamentally different role than that to the cortex and hippocampus. Previous studies have shown that the basal forebrain is foremost in the overall maintenance of cortical arousal (Steriade et al., 1990, 1997; Buzsáki et al., 1988; Vanderwolf and Stewart, 1988). The influence of ascending neurotransmitter systems through projections to the thalamus may be more nuanced. For example, modulation of the NRe may influence the excitatory state of hippocampal or prefrontal regions, which may in turn affect certain aspects of behavior. The anatomical features of NRe projections may provide some hints as to how this influence may be expressed. For example, NRe neurons terminate onto GABAergic interneurons within area CA1 of the hippocampus (Dolleman-van der Weel et al., 1997; Dolleman-van-der Weel and Witter, 2000). As tonic NRe activity would thus have the net effect of inhibiting CA1, a lesion to this structure may remove this inhibition, resulting in a net stimulation of the hippocampal circuit. While the cell-type specificity of NRe targets in the prefrontal cortex are less well explored, the hippocampal anatomy is suggestive that the effects observed in the present study may reflect a shift in the balance within an area toward excitation, although experiments involving direct microstimulation of the midline thalamus suggest that the influence of the NRe on the prefrontal cortex is excitatory (Di Prisco and Vertes, 2006).
As mentioned earlier, pharmacological intervention can lead to performance increases that closely follow those observed with the NRe lesions. For example, direct stimulation of dopamine D1 receptors in the prefrontal cortex leads to an attentional enhancement in the CAM task (Chudasama and Robbins, 2004b). Systemic injections of dopamine agents such as amphetamine can remediate attentional performance in rats with dorsal prefrontal lesions (Chudasama et al., 2005; see also Castner, 2003). Likewise, serotonergic reuptake inhibitors such as escitalopram counteract impulsive deficits induced by ventral hippocampal lesions (Abela et al., 2013) presumably through enhancement of extracellular 5-HT in the prefrontal cortex. Thus, the hippocampus and prefrontal cortex also draw upon neuromodulation to govern their interaction and steer executive function, perhaps also in part through the modulation of cortical arousal. Regarding the contribution of the NRe in this circuit, it is notable that deep brain stimulation of central thalamic regions has been shown to lead to restoration of cognitive behavior (Schiff et al., 2007). This effect, which is closely associated with cortical arousal, has been linked to activation of the prefrontal cortex, as stimulation of the thalamus leads to an upregulation of immediate early gene expression in this region (Shirvalkar et al., 2006).
Spatial searching or spatial memory?
The only hint of a cognitive deficit following the NRe lesion was in the radial arm maze where rats made numerous repeat entries into previously rewarded locations. This deficit occurred only when there was no delay interposed between the learning and memory phase of the task. In that sense, the deficits from NRe lesions resemble the disruption of prefrontal lesions on working memory tasks (Seamans et al., 1995; Kesner et al., 1996; Floresco et al., 1997; Ragozzino et al., 1998, 2002). Nonetheless, in contrast to the prefrontal effects on working memory, the deficit of the NRe-lesioned animals was relatively minor being expressed only when the delay constituted ‘zero’ seconds (see also Layfield et al., 2015), but recovered very quickly when the delays extended into several minutes. Thus, consistent with previous studies (Dolleman-van der Weel et al., 2009; Hembrook and Mair, 2011; Cholvin et al., 2013), the NRe lesions may function to disrupt optimal searching of spatial contexts rather than impact spatial memory. The absence of any spatial memory deficit seems at odds with the recent discovery of head direction cells (Jankowski et al., 2014) and trajectory specific firing patterns in the NRe (Ito et al., 2015). One possibility is that in the current study, the NRe lesion prevented the animal from establishing head directionality causing the animal to make many errors revisiting old, unfruitful locations. It is notable however, that although NRe lesions alter spatial coding specifically in the dorsal hippocampus (Ito et al., 2015), which is known to be critical for spatial memory (Moser and Moser, 1998), NRe lesions do not disrupt memory for alternating spatial direction (Ito et al., 2015). While it is feasible that a select group of neurons in the NRe participate in spatial navigation through its modulation of dorsal hippocampal CA1 fields (see Loureiro et al., 2012), there is minimal evidence to suggest that the NRe plays a substantial role in spatial memory, although this topic is presently an active area of research.
Importantly, the midline thalamus appears to contribute substantially to a range of cognitive behaviors. Unlike most other regions within the brain, damage to this structure leads to measured improvements. Previous work applied electrical stimulation to midline thalamic structures and reported enhanced memory-guided responding (Shirvalkar et al., 2006; Mair and Hembrook, 2008). In this study, we demonstrate that destruction of a specific midline thalamic structure, the NRe, can enhance executive function by improving several cognitive operations including attention, response control and some aspects of learning. The role of the midline thalamus as a relay, a mediator of cortical arousal, and a regulator of executive behaviors, will likely continue to be a topic of great interest, not only in the context of understanding thalamocortical interactions, but also in the search for potential neural targets for intervention in human patients with cognitive disabilities.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (No: 341600) and the Canadian Foundation for Innovation Leaders Opportunity Award (No: 14033). We thank Priya Patel, Cynthia Berthiaume, Benjamin Hatch and Elizabeth Waring for their help with behavioral testing, and Hyun Choong Yong for help with data input. Judy Prasad is now at The University of Chicago, Department of Neurobiology, Illinois, USA. Andrew Abela is now at the Centre for Addiction and Mental Health (CAMH), Toronto, Canada. Yogita Chudasama is now at the National Institute of Mental Health, Bethesda, MD, USA.
Abbreviations
- AP
anterior–posterior
- CAM
combined attention-memory
- DV
dorsoventral
- NMDA
N-methyl-D-aspartic acid
- NRe
nucleus reuniens
References
- Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci. 2013;37:640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- Abela AR, Dougherty SD, Fagen ED, Hill CJR, Chudasama Y. Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex. 2013;23:1396–1409. doi: 10.1093/cercor/bhs121. [DOI] [PubMed] [Google Scholar]
- Abela AR, Duan Y, Chudasama Y. Hippocampal interplay with nucleus accumbens is critical for decisions about time. Eur J Neurosci. 2015;42(5):2224–2233. doi: 10.1111/ejn.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Animal models of ADHD. Curr Top Behav Neurosci. 2011;7:149–185. doi: 10.1007/7854_2010_102. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bickford RG, Ponomareff G. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MRF. Whisker: a client-server high-performance multimedia research control system. Behav Res Methods. 2010;42:1059–1071. doi: 10.3758/BRM.42.4.1059. [DOI] [PubMed] [Google Scholar]
- Cassel J-C, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner S. Amphetamine sensitization of hallucinatory-like behaviors is dependent on prefrontal cortex in nonhuman primates. Biol Psychiatry. 2003;54:105–110. doi: 10.1016/s0006-3223(03)00292-0. [DOI] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A, Cassel J-C. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci. 2013;33:8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Nathwani F, Robbins TW. D-Amphetamine remediates attentional performance in rats with dorsal prefrontal lesions. Behav Brain Res. 2005;158:97–107. doi: 10.1016/j.bbr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004a;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004b;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, Akbari E. Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav Brain Res. 2009;198:130–135. doi: 10.1016/j.bbr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Morris RGM, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, da Silva FHL, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van derWeel MJ, Witter MP. Nucleus reuniens thalami innervates γ aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci Lett. 2000 doi: 10.1016/s0304-3940(99)00935-0. [DOI] [PubMed] [Google Scholar]
- Dunnett SB. Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology. 1985;87:357–363. doi: 10.1007/BF00432721. [DOI] [PubMed] [Google Scholar]
- Flämig R, Klingberg F. Participation of thalamic nuclei in the elaboration of conditioned avoidance reflexes of rats. IV. Lesions of the nucleus reuniens. Acta Biol Med Ger. 1978;37:1779–1782. [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. Hoboken, NJ, USA: John Wiley & Sons Inc; 1987. [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. The specificity of the “nonspecific” midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang S-J, Witter MP, Moser EI, Moser M-B. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522:50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Jang AI, Costa VD, Rudebeck PH, Chudasama Y, Murray EA, Averbeck BB. The role of frontal cortical and medial-temporal lobe brain areas in learning a bayesian prior belief on reversals. J Neurosci. 2015;35:11751–11760. doi: 10.1523/JNEUROSCI.1594-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP, O’Mara SM. Nucleus reuniens of the thalamus contains head direction cells. eLife Sci. 2014;3:e03075. doi: 10.7554/eLife.03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgarisl-eucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:s438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmac C, Mitrofanis J. Organization of the basal forebrain projection to the thalamus in rats. Neurosci Lett. 1999;272:151–154. doi: 10.1016/s0304-3940(99)00614-x. [DOI] [PubMed] [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, Griffin AL. Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiol Learn Mem. 2015;125:163–167. doi: 10.1016/j.nlm.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel J-C, Pereira de Vasconcelos A. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci. 2012;32:9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Hembrook JR. Memory enhancement with event-related stimulation of the rostral intralaminar thalamic nuclei. J Neurosci. 2008;28:14293–14300. doi: 10.1523/JNEUROSCI.3301-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Onos KD, Hembrook JR. Cognitive activation by central thalamic stimulation: the yerkes-dodson law revisited. Dose Response. 2011;9:313–331. doi: 10.2203/dose-response.10-017.Mair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JNP, Walton ME, Rushworth MFS, Baxter MG, Campbell TG. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology. 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando ABP, Urcelay GP, Robinson ESJ, Mar AC, Theobald DEH, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake T, Yamada H. Efferent connections of the nucleus reuniens and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci Res. 1989;6:556–568. doi: 10.1016/0168-0102(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier Academic Press; 2005. [Google Scholar]
- Prasad JA, Chudasama Y. Viral tracing identifies parallel disynaptic pathways to the hippocampus. J Neurosci. 2013;33:8494–8503. doi: 10.1523/JNEUROSCI.5072-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Vertes RP. Excitatory actions of the ventral midline thalamus (rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse. 2006;60:45–55. doi: 10.1002/syn.20271. [DOI] [PubMed] [Google Scholar]
- Ragozzino M, Detrick S, Kesner RP. Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol Learn Mem. 2002;77:29–43. doi: 10.1006/nlme.2001.4003. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga M, editor. The cognitive sciences. Cambridge, MA, US: The MIT Press; 1995. pp. 703–720. [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, O’Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci. 2006;103:17007–17012. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Datta S, Paré D, Oakson G, Curró Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Diffuse regulatory systems of the thalamus. In: Steriade M, Jones EG, McCormick DA, editors. Thalamus Vol 1: organisation and function. Amsterdam: Elsevier; 1997. pp. 269–338. [Google Scholar]
- Su H-S, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol. 1990;297:582–593. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Stewart DJ. Thalamic control of neocortical activation: a critical re-evaluation. Brain Res Bull. 1988;20:529–538. doi: 10.1016/0361-9230(88)90143-8. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2001;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Meijer RJ, Van Uum HF, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7:397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]