Abstract
Research suggests an important role of the eyes and mouth for discriminating facial expressions of emotion. A gaze-contingent procedure was used to test the impact of fixation to facial features on the neural response to fearful, happy and neutral facial expressions in an emotion discrimination (Exp.1) and an oddball detection (Exp.2) task. The N170 was the only eye-sensitive ERP component, and this sensitivity did not vary across facial expressions. In both tasks, compared to neutral faces, responses to happy expressions were seen as early as 100–120ms occipitally, while responses to fearful expressions started around 150ms, on or after the N170, at both occipital and lateral-posterior sites. Analyses of scalp topographies revealed different distributions of these two emotion effects across most of the epoch. Emotion processing interacted with fixation location at different times between tasks. Results suggest a role of both the eyes and mouth in the neural processing of fearful expressions and of the mouth in the processing of happy expressions, before 350ms.
1. Introduction
Facial expressions of emotion (hereafter facial emotions or facial expressions) are particularly salient stimuli and are direct indicators of others’ affective dispositions and intentions (Adolphs, 2003). The ability to quickly extract facial information and discriminate between facial emotions is crucial for proper social communication (e.g., discerning a friend from foe; Mehrabian, 1968) and the neural correlates of these cognitive processes have been studied extensively using various neuroimaging techniques. Scalp Event Related Potentials (ERPs) are well suited to study the temporal dynamics of neuro-cognitive events and have been used to examine the time course of facial expression processing. However, results remain inconsistent (Rellecke, Sommer, & Schact, 2013; and see Vuilleumier & Pourtois, 2007, for a review).
1.1 Early Event-Related Potentials in facial expression research
The first visual ERP investigated in facial emotion research is the visual P1, (~80–120ms post-stimulus onset at occipital sites), a component known to be sensitive to attention (Luck, 1995; Luck, Woodman, & Vogek, 2000; Mangun, 1995) and low-level stimulus properties such as colour, contrast and luminance (Johannes, Münte, Heinze & Mangun, 1995; Rossion & Jacques, 2008, 2012). A growing number of studies have now reported enhanced P1 amplitude for fearful relative to neutral faces (e.g., Batty & Taylor, 2003; Pourtois, Grandjean, Sander, & Vuilleumier, 2004; Sato, Kochiyama, Yoshikawa & Matsumura, 2001; Smith, Weinberg, Moran, & Jajcak, 2013; Wijers & Banis, 2012). It has been suggested that early occipito-temporal visual areas could be activated to a larger extent by intrinsically salient, threat-related stimuli, via possible projections from a subcortical route involving the amygdala (Vuilleumier & Pourtois, 2007). Fearful faces would automatically engage this subcortical structure which, in turn, would modulate and enhance cortical processing of the face stimuli (Morris et al., 1998; Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004; Whalen et al., 1998). Because of P1 early timing, which corresponds to the activation of early extrastriate visual areas (e.g. V2, V3, posterior fusiform gyrus, e.g. Clark, Fan, & Hillyard, 1995), this P1 fear effect is thought to reflect a coarse emotion extraction, the “threat gist” (e.g., Luo, Feng, He, Wang, & Lu, 2010; Vuilleumier & Poutois, 2007), that might rely on fast extraction of low spatial frequencies (Vuilleumier, Armony, Driver, & Dolan, 2003). Actual processing of the visual threat would occur later, around or after the N170 (e.g., Luo et al., 2010), the second ERP component studied in facial expression research. However, let’s note that this early P1 modulation by emotion is debated as many studies also failed to report modulations of the P1 by facial expressions of emotion (see Vuilleumier & Pourtois, 2007 for a review).
The N170 is a negative-going face-sensitive component measured at lateral occipito-temporal electrodes ~130–200ms post stimulus onset, and is considered to index the structural processing of the face, i.e. a stage where features are integrated into the whole percept of a face (Bentin & Deouell, 2000; Bentin, Allison, Puce, Perez, & McCarthy, 1996; Itier & Taylor, 2002; Rossion et al., 2000). Studies have suggested the involvement of the fusiform gyrus (e.g. Itier & Taylor, 2002; Rossion et al., 1999), the Superior Temporal Sulcus (e.g. Itier & Taylor, 2004) and the Inferior Occipital Gyrus, or their combination, as potential generators of the N170 (for a review see Rossion & Jacques, 2012). Reports of the N170 sensitivity to facial emotions have been inconsistent. A number of studies have reported emotion effects with larger N170 recorded in response to emotional faces, especially fearful expressions, compared to neutral faces (e.g., Batty & Taylor, 2003; Blau, Maurer, Tottenham, & McCandliss, 2007; Caharel, Courtay, Bernard, Lalonde, 2005; Leppänen, Hietanen, & Koskinen, 2008; Leppänen, Moulson, Vogel-Farley, & Nelson, 2007; also see Hinojosa, Mercado, & Carretié, 2015). However, as seen for the P1, a lack of sensitivity to facial expressions of emotion has also been reported for the N170 component in many studies (e.g., Ashley, Vuilleumier, & Swick, 2004; Balconi & Lucchiari, 2005; Herrmann et al., 2002; Krolak-Salmon, Fischer, Vighetto, & Mauguière, 2001; Münte et al., 1998; Pourtois, Dan, Granjean, Sander, & Vuilleumier, 2005; Shupp, Junghöfer, Weike, & Hamm, 2004; Smith et al., 2013). Therefore it remains unclear whether facial expression processing, in particular that of fearful faces, interacts with the processing of the face structure, as indexed by the N170.
Another well studied ERP in facial expression research is the well-known marker of emotion processing Early Posterior Negativity (EPN), a negative deflection measured over temporo-occipital sites ~150–350ms post-stimulus onset. The EPN is enhanced for emotional relative to neutral stimuli, for both verbal and non-verbal material including faces (Schacht & Sommer, 2009; Schupp Markus, Weike, & Hamm, 2003; Schupp et al., 2004; Rellecke et al., 2013). Like the N170, the EPN is commonly reported to be most pronounced for threat-related expressions (i.e., fearful and angry) compared to neutral and happy expressions (e.g., Schupp et al., 2004; Rellecke, Palazova, Sommer, & Schacht, 2011), although there are reports of a general emotion effect with more negative amplitudes for both threatening and happy expressions compared to neutral expressions (Sato et al., 2001; Schupp, Flaisch, Stockburger, & Junghöfer, 2006). Therefore this effect has been suggested to reflect enhanced processing of emotionally salient faces in general or of threatening faces in particular (i.e., fearful and angry) in temporo-occipital areas possibly including occipital gyrus, fusiform gyrus and Superior Temporal Sulcus regions (Schupp et al., 2004). The current view is that the EPN reflects more in depth appraisal of the emotion, some form of semantic stage where the meaning of the emotion is extracted (Luo et al., 2010; Vuilleumier & Pourtois, 2007). Some studies have suggested that the EPN reflects the neural activity related to the processing of the emotion that is superimposed onto the normal processing of the face. This superimposed activity would sometimes start around the N170 and be responsible for the emotional effects reported for the N170 (Leppänen et al., 2008; Rellecke et al., 2011; 2013; Schupp et al., 2004), although it seems largest after the N170 peak and around the visual P2 (see Neath & Itier, 2015, for a recent example). In other words, the emotion effect on the N170 would actually reflect superimposed EPN activity (Rellecke et al., 2011; Rellecke, Sommer, & Schacht, 2012; Schacht & Sommer, 2009). According to this interpretation, face structural encoding, as indexed by the N170, and facial emotion encoding, do not really interact and are separate processes that occur independently and in parallel, as proposed by classic cognitive and neural models of face processing (Bruce & Young, 1986; Haxby, Hoffman, & Gobbini, 2000).
1.2 Role of facial features in the processing of facial expression
One factor possibly contributing to these inconsistent early ERP effects of emotion is the differing amount of attention to facial features. Some features characterize particular facial expressions better than others, like the smiling mouth for happy faces and the wide open eyes for fearful faces (e.g., Kohler et al., 2004; Leppänen & Hietanen, 2007; Nusseck, Cunningham, Wallraven, & Bülthoff, 2008; Smith, Cottrell, Gosselin, & Schyns, 2005). Behavioural research presenting face parts (e.g., Calder, Young, Keane, & Dean,2000) or using response classification techniques such as Bubbles (e.g., Blais, Roy, Fiset, Arguin, & Gosselin, 2012; Smith et al., 2005) has highlighted the importance of these so-called “diagnostic features” for the discrimination and categorization of these facial emotions. Eye-tracking research also supports the idea that attention is drawn to these features early on, as revealed by spontaneous saccades towards the eyes of fearful faces or the mouth of happy faces presented for as short as 150ms (Gamer, Schmitz, Tittgemeyer, & Schilbach, 2013; Scheller, Büchel, & Gamer, 2012).
The role of these diagnostic features in the neural response to facial expressions has recently been investigated in ERP research but remains unclear. Research using the Bubbles technique in combination with ERP recordings has suggested that the eye region provides the most useful diagnostic information for the discrimination of fearful facial expressions and the mouth for the discrimination of happy facial expressions, and that the N170 peaks when these diagnostic features are encoded (Schyns, Petro, & Smith,2007, 2009). Leppänen et al. (2008) reported that an early fear effect, seen as more negative amplitudes for fearful compared to neutral faces from the peak of the N170 (~160ms in that study) until 260ms (encompassing the visual P2 and EPN), was eliminated when the eye region was covered, demonstrating the importance of this facial area in the neural response to fearful expressions. Calvo and Beltrán (2014) reported hemispheric differences in the processing of facial expressions using face parts and whole faces. An enhanced N170 in the left hemisphere was seen for happy compared to angry, surprised and neutral faces for the bottom face region presented in isolation (including the mouth), but not for the top face region presented in isolation (including the eyes), or for the presentation of the whole face. In the right hemisphere in contrast, the N170 was enhanced for angry compared to happy, surprised and neutral faces for whole faces only.
Taken together these studies suggest that the expression-specific diagnostic features modulate the neural response to facial expression at the level of the N170 or later. Importantly, all these ERP studies have employed techniques that forced feature-based processing by revealing facial information through apertures of various sizes and spatial frequencies (e.g. Bubbles, Schyns et al., 2007, 2009), by presenting isolated face parts (Calvo & Beltrán, 2014; Leppänen et al., 2008) or by covering portions of the face (Leppänen et al., 2008). However the bulk of the literature on face perception supports the idea that faces are processed holistically, i.e. as a whole, whether the focus is on identity (McKone, 2008; Rossion & Jacques, 2008) or emotion (Calder & Jansen, 2005; Calder et al., 2000) recognition. Moreover, components such as the N170 are very sensitive to disruption of this holistic processing (Rossion & Jacques, 2012; Itier, 2015, for reviews). A systematic investigation of the impact of facial features on the neural processing of facial emotion in the context of the whole face is lacking. This is important given we almost invariably encounter whole faces in our daily social interactions, and eye tracking studies suggest that faces are explored and that fixation moves across facial features, with a larger exploration of the eyes (see Itier, 2015, for a review).
Using an eye-tracker and a gaze-contingent procedure to ensure fixation on specific facial features of fearful, happy, and neutral expressions, Neath and Itier (2015) recently reported different spatio-temporal emotion effects for happy and fearful faces. Smaller amplitudes for happy than neutral faces were seen mainly at occipital sites, starting on the P1 and most pronounced around 120ms, followed by a sustained effect until 350ms post-stimulus onset. Fearful faces elicited smaller amplitudes than neutral faces mainly at lateral-posterior sites, first transiently around 80ms on the left hemisphere only, and then bilaterally starting at 150ms, i.e. right after the N170 peak, until 300ms. The N170 peak itself was not significantly modulated by emotion. These main effects of emotion interacted with fixation location at occipital sites only during 150–200ms, with smaller amplitudes for both happy and fearful faces compared to neutral faces, seen only when fixation was on the mouth (no emotion effects were seen for fixation on the eyes or the nose). Although limited temporally, these interactions between emotion and fixation location suggested a possibly important role of the mouth in processing happy but perhaps also fearful faces, while fixation to the eyes did not yield specific effects for the processing of fearful faces.
The present study
The Neath and Itier (2015) study employed a gender discrimination task, which was emotion-irrelevant. However, the diagnostic feature hypothesis suggests that different features might be used depending on task demands ((Schyns, Jentzsch, Johnson, Schweinberger, & Gosselin, 2003; Schyns et al., 2007, 2009;; Smith & Merlusca, 2014). The goal of the current study was to follow-up on the Neath and Itier’s study to investigate the impact of fixation to facial features on the neural processing of facial emotions in the context of the whole face, during an explicit emotion discrimination (ED) task (Experiment 1) and during another emotion-irrelevant task (oddball detection (ODD) task, Experiment 2).
In the present study, fearful, happy and neutral faces were presented with fixation to the left eye, right eye, nose and mouth using the same gaze-contingent design as Neath and Itier (2015). That is, the stimulus was presented only when an eye–tracker detected that the fixation cross was fixated for a certain amount of time. A face stimulus was then presented offset so as to put the desired feature in fovea, at the location of the fixation cross. We expected to replicate the findings of Neath and Itier (2015) regarding low-level stimulus position effects on the P1, as well as the larger N170 amplitude for fixation to the eyes compared to the nose and mouth (see also de Lissa et al., 2014; Nemrodov, Anderson, Preston, & Itier, 2014). We also hoped to reproduce their distinct spatio-temporal pattern of fear and happiness effects. We expected that the different task demands would impact the fixation and emotion interactions such that, for the explicit emotion discrimination task, an enhanced fear effect would be seen for fixation to the eyes compared to the nose or mouth and a larger happiness effect would be seen for fixation to the mouth compared to the eyes or nose, given the respective diagnosticity of these features for the two emotions. If diagnostic features are tied to explicit emotion discrimination, this interaction should not be seen, or should be different, in the oddball task. The interactions were expected around the timing of the N170 or later, during the timing coinciding with the EPN.
2. Experiment 1- Explicit emotion discrimination (ED) task
2.1 Methods
2.1.1. Participants
Forty-seven undergraduate students from the University of Waterloo (UW) were recruited and received course credit for their participation. All participants lived in North America for at least 10 years and they all reported normal or corrected-to-normal vision, no history of head-injury or neurological disease, and were not taking any medication. They all signed informed written consent and the study was approved by the Research Ethics Board at UW. At the start of the study, calibration of the eye tracker failed for 8 participants who were not further tested. The remaining 39 participants were tested but 19 were rejected for the following reasons. One participant completed less than half of the study; four were removed due to artefacts resulting in many conditions with fewer than our 40 trials per condition cut-off (50% of the initial trials per condition); 13 had too few trials after removing trials with eye movements outside of our defined fixation location interest area of 1.4° of visual angle (see below)1; one participant was rejected due to problems during EEG recordings. The results from 20 participants were kept in the final analysis (21 ± 3.1 years, all right-handed, 10 females).
2.1.2. Stimuli
Images were fearful, happy and neutral facial expressions of 4 males and 4 females from the MacBrain Face Stimulus Set2 (“NimStim faces”, Tottenham et al., 2009) (see Fig. 1A for examples of expressions). Images were converted to greyscale in Adobe™ Photoshop CS5 and an elliptical mask was applied so hair, ears, shoulders and other non-face items (e.g., earrings) were not visible. The faces subtended 6.30° horizontally and 10.44° vertically when viewed from a distance of 70cm and were presented on a grey background for an image visual angle of 9.32° horizontally and 13.68° vertically. The grey images were presented on a white computer monitor. No significant differences in mean RMS contrast and mean normalized pixel intensity were seen between the three emotion categories (RMS: Mfearful = .33 (.01 S.D); Mhappy= .34 (.01 S.D); Mneutral = .34 (.01 S.D); pixel intensity: Mfearful = .58 (.01 S.D); Mhappy= .57 (.01 S.D); Mneutral = .57 (.01 S.D); all t-tests at p > 0.05 using Bonferroni correction for multiple comparisons).
Figure 1.
A Left panel: Examples of fearful and happy expressions (and flowers for Exp. 2 only), with fixation crosses overlaid on the face to indicate where the fixation would occur (note that fixation crosses were never presented on faces in the actual experiment). Right panel: Participants fixated in the center of the screen represented here by the white rectangle (neutral expression example) and the face was offset in such a way that gaze fixated four possible fixation locations: left eye, right eye, nose and mouth. Note that eye positions are from a viewer perspective (i.e., left eye is on the left of the picture). B. Trial example with right eye fixation and fearful expression. First a fixation point was displayed on the screen for a jittered amount of time (0–107ms) with an additional fixation trigger of 307ms. A grayscale picture was then flashed for 257ms. In Exp. 1 (Explicit Discrimination – ED), a response screen immediately followed the stimulus and displayed a vertical list of emotions; participants selected, using a mouse, the correct emotion label that the face was expressing. The response screen remained until the participant’s response, followed by a blink screen for 307ms. In Exp. 2 (Oddball –ODD-task) the stimulus was followed by a fixation cross that was presented for 747ms for face trials or until response for flower trials.
To ensure that participants fixated on specific facial features, fixation locations corresponding to the left eye, right eye, nose and mouth for each face stimulus were calculated. Left and right sides are from a participant’s perspective such that the left eye means the eye situated on the left side of the participant and the right eye, the eye situated on the right side of the participant. The locations of the nose and mouth fixations were determined by aligning them along an axis passing through the middle of the nose and face, and the locations of the eye fixations were on the center of the pupil. A fixation-cross at the centre of the screen was always used followed by the face presented offset so the predetermined center of each feature would land on the center of that fixation-cross (see Fig. 1A). Due to variations in the coordinates for each identity and the three expressions used, all pictures were presented in slightly different locations3.
For each picture, the mean normalized pixel intensity (PI) and RMS contrast were also calculated for the pre-defined Interest Areas (IA) of 1.4° diameter centered on fixation locations that ensured foveal vision on each facial feature with no overlap. Mean PI and RMS contrast were analyzed using a 3 (emotion) X 4 (IA) repeated measure analysis of variance (ANOVA). The highest RMS contrast was seen for the left and right eyes IA (which did not significantly differ), followed by the mouth and then the nose IA (which did not significantly differ; effect of fixation location, F(1.34, 9.40) = 16.34, p < .001, ηp2 = .70, all paired comparisons at p-values < .05; see Table 1). However, the emotion by fixation location interaction (F(2.74, 19.18) = 11.48, p < .001, ηp2 = .62) revealed different emotion differences for each fixation location analyzed separately. For the left eye IA, a larger contrast was seen for neutral compared to happy faces (F = 9.70, p < .01) and contrast was also larger for both fearful and happy compared to neutral faces for the mouth IA (F = 11.52, p < .01). No emotion differences were seen for the nose IA (p = .12) and an effect of emotion was seen for the right eye IA (F = 4.97, p < .05) but no significant pairwise comparisons were found.
Table 1.
Pixel intensity and RMS contrast values within 1.4° radius centered on the left eye, right eye, nose and mouth for the fearful, happy and neutral expressions used in both experiments (standard errors to the means in parenthesis). Values for whole pictures can be found in the text.
| Mean pixel intensity (std error) | Mean RMS Contrast (std error) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Left Eye | Right Eye | Nose | Mouth | Left Eye | Right Eye | Nose | Mouth | |
| Fear | .43 (.02) | .44 (.04) | .52 (.02) | .49 (.03) | .14 (.01) | .14 (.01) | .12 (.01) | .13 (.02) |
| Happy | .44 (.03) | .44 (.03) | .51 (.02) | .55 (.02) | .13 (.01) | .14 (.02) | .11 (.01) | .13 (.01) |
| Neutral | .43 (.03) | .43 (.02) | .51 (.03) | .50 (.02) | .14 (.03) | .14 (.01) | .11 (.01) | .10 (.01) |
The lowest PI was seen for the left and right eyes IA (which did not significantly differ), followed by the mouth and the nose IA (which did not significantly differ; effect of fixation location, F(1.77, 12.36) = 42.29, p < .001, ηp2 = .86, all paired comparisons at p-values < .01). The emotion by IA interaction was also significant (F(2.54,17.81) = 6.79, p < .05, ηp2 = .49). No differences between emotions were seen for the left eye (p = .76) and right eye (p = .54) IA. For the nose IA, larger PI was seen for fearful compared to happy and neutral faces (F = 11.38, p < .05) and for the mouth IA larger PI was seen for happy compared to fearful and neutral faces (F = 20.99, p < .001).
2.1.3. Apparatus and Procedure
Participants sat in a sound-attenuated Faraday-cage protected booth 70cm away from a ViewSonic P95f+ CRT 19-inch monitor (Intel Quad CPU Q6700; 75Hz refresh rate) while performing an explicit emotion discrimination (ED) task. Participants were first given an 8 trial practice session (repeated if necessary), followed by 960 experimental trials. Each trial began with a 1–107ms jittered fixation-cross (see Fig.1B for a trial example). Participants were instructed to fixate on the black fixation-cross in the center of the screen in order to initiate each trial and to not move their eyes until the response screen appeared. To ensure that participants’ fixation was on the cross, a fixation contingent trigger enforced the fixation on the cross for 307ms. Due to sensitivity of the eye-tracker, on average participants took 728ms (S.D. = 926.02) between the onset of the fixation-cross and the stimulus presentation. The face stimulus was then presented for 257ms, immediately followed by the response screen displaying a vertical list of the three emotions (emotion word order counterbalanced between participants). The response screen remained until the response. Participants were instructed to categorize faces by their emotion as quickly and accurately as possible using a mouse by clicking on the appropriate emotion label. They were instructed to keep their hand on the mouse during the entire experiment to avoid unnecessary delays. On average, it took participants 1293ms (S.D. = 256.6ms) to respond. After their response, a screen appeared that read “BLINK” for 307ms. Participants were instructed to blink during this time to prevent eye movement artifacts during the first 500ms of trial recording.
The block of 96 face trials (3 emotions X 4 fixation locations X 8 identities) was repeated 10 times in a randomized trial order, for a total of 80 trials per condition. Following the computer task, participants completed the 21- item trait test from the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA; Ree, French, MacLeod, & Locke, 2008). The STICSA is a Likert-scale assessing cognitive and somatic symptoms of anxiety as they pertain to one’s mood in general. All participants scored 44 or below on the STICSA, reflecting low to mild anxiety traits. Anxiety was monitored as it is knowns to impact the processing of fear (e.g. Bar-Haim et al., 2007).
2.1.4. Electrophysiological Recordings
The EEG recordings were collected continuously at 516Hz by an Active-two Biosemi system at 72 recording sites: 66 channels4 in an electrode-cap under the 10/20 system-extended and three pairs of additional electrodes. Two pairs of electrodes, situated on the outer canthi and infra-orbital ridges, monitored eye movements; one pair was placed over the mastoids. A Common Mode Sense (CMS) active-electrode and a Driven Right Leg (DRL) passive-electrode acted as ground during recordings.
2.1.5. Eye-Tracking Recordings
Eye movements were monitored using a remote SR Research Eyelink 1000 eye-tracker with a sampling rate of 1000Hz. The eye-tracker was calibrated to each participant’s dominant eye, but viewing was binocular. If participants spent over 10s before successfully fixating on the cross at the start of the trial, a drift correction was used. After two drift corrections, a mid-block recalibration was performed. Calibration was done using a nine-point automated calibration accuracy test. Calibration was repeated if the error at any point was more than 1°, or if the average for all points was greater than 0.5°. The participants’ head positions were stabilized with a head and chin rest to maintain viewing position and distance constant.
2.1.6. Data Processing and Analyses
Each trial was categorized as correct or incorrect based on the emotion categorization and only correct response trials were used for further analysis. In addition, for each participant we also kept trials in which RTs were within 2.5 S.D. from the mean of each condition (Van Selst & Jolicoeur, 1994) as a way to eliminate anticipatory responses (which could overlap with EPN component) or late responses, which excluded 7.05% of the total number of trials across the 20 participants. To ensure foveation to the predefined fixation location areas (left eye, right eye, nose and mouth), trials in which a saccadic eye movement was recorded beyond 1.4° visual angle (70 pixels) around the fixation-location were removed from further analysis. An average of 3.29% of trials were removed during this step across the 20 participants included in the final sample.
The ERP data were processed offline using the EEGLab (Delorme & Makeig, 2004) and ERPLab (http://erpinfor.org/erplab) toolboxes implemented in Matlab (Mathworks, Inc.). The electrodes were average-referenced offline. Average-waveform epochs of 500ms were generated with a 100ms pre-stimulus baseline and digitally band-pass filtered using a two-way least squares FIR filter (0.01–30Hz). Trials containing artifacts >±70μV were rejected using an automated procedure. Trials were then visually inspected and those still containing artefacts were manually rejected. After this two-step cleaning procedure, participants with less than 40 trials in any condition (out of 80 initial trials) were rejected (the average number of trials per condition (M = 61, S.D = 9) did not significantly differ across emotions (p = .35) or fixation location (p = .20)).
Using automatic peak detection, the P1 component was measured between 80 and 130ms post-stimulus-onset (peak around 100ms) at electrodes O1, O2 and Oz where it was maximal (see also Neath & Itier, 2015). The N170 component was maximal at different electrodes across participants, and within a given participant the N170 was often maximal at different electrodes across the two hemispheres (but maximal at the same electrodes across conditions). Thus, the N170 peak was measured between 120–200ms at the electrode where it was maximal for each subject and for each hemisphere (see Table 2; also see Neath & Itier, 2015). This approach, although still infrequently used, has been recommended by some to maximize sensitivity (e.g. Rousselet & Pernet, 2011). To better capture the time course of the fixation and emotion effects, mean amplitudes were also calculated within 50ms windows starting from 50 to 350ms. As P1 peaked on average around 100ms and N170 around 150ms, this approach allowed us to monitor the scalp activity in between these prominent ERP markers, which is important given reports that information integration starts at the transition between these peaks (e.g. Rousselet, Husk, Bennett, Sekuler, 2008; Schyns et al., 2007; see also Itier, Taylor, & Lobaugh, 2004). This approach also allowed to monitor the entire waveform as recommended (e.g. Rousselet & Pernet, 2011), an especially important step to accurately track the emotion-sensitive Early Posterior Negativity (EPN) previously analyzed at very different time windows depending on studies (e.g. Leppänen et al., 2008; Schacht & Sommer, 2009; Schupp et al., 2004).
Table 2.
Number of subjects for whom the N170 was maximal at left (P9, CB1, PO7, P7, O1, TP9) and right hemisphere (P10, CB2, P08, P8, O2, TP10) electrodes. LH: left hemisphere; RH: right hemisphere.
| Explicit Emotion Discrimination (Exp.1) | Oddball Detection (Exp.2) | |||
|---|---|---|---|---|
|
| ||||
| LH | RH | LH | RH | |
| P9/P10 | 11 | 14 | 10 | 12 |
| CB1/CB2 | 6 | 4 | 11 | 6 |
| PO7/PO8 | 2 | 1 | 3 | 4 |
| P7/P8 | - | - | - | 3 |
| O1/O2 | 1 | 1 | 1 | 1 |
| TP9/TP10 | - | - | 1 | - |
|
| ||||
| Total n | 20 | 20 | 26 | 26 |
Inspection of the data revealed different effects over occipital sites and lateral-posterior sites. Thus for each time window, separate analyses were conducted using mean amplitudes calculated across three clusters: an occipital cluster (Occ, averaging O1, O2 and Oz), a left lateral cluster (Llat, averaging CB1, P9, P7 and PO7) and a right lateral cluster (Rlat, averaging CB2, P10, P8 and PO8). Note that the lateral-posterior electrodes included the electrodes where the N170 was measured across participants and also included the visual P2 component (peaking around 200ms post-face onset) as well as the EPN. Finally, to test the idea that the “fear effect” (the amplitude difference between fearful and neutral faces) and the “happiness effect” (the amplitude difference between happy and neutral faces) occurred at different sites, we also analyzed scalp distributions. We first created difference waveforms for each subject and each fixation location, by subtracting ERPs to neutral faces from ERPs to fearful faces (F-N) and ERPs to neutral faces from ERPs to happy faces (H-N). We then calculated the mean amplitude at each electrode across each of the 6 time windows (50ms to 350ms in 50ms bins) and analyzed them statistically. Although they have been criticized (Luck, 2005; Urbach & Kutas, 2002), normalized amplitudes are also still quite often used so we also normalized all mean amplitudes according to the method described in McCarthy and Wood (1985).
Repeated-measure ANOVAs were conducted separately for correct categorization and ERP amplitudes using SPSS Statistics 22. Within-subject factors included emotion (3: fear, happiness, neutral) and fixation location (4: left eye, right eye, nose, mouth) for all analyses, as well as hemisphere for N170 (2: left, right), electrode for P1 (3: O1, O2, Oz), and cluster for mean amplitudes (3: Occ, Llat, Rlat). If necessary, further analyses of the interactions found were completed with separate ANOVAs for each cluster, each fixation location or each emotion. For scalp distribution analyses, mean amplitude difference scores at each time window were analyzed using a repeated measure ANOVA with emotion effect (2: fear effect, happiness effect), fixation location (4: Left Eye, Right Eye, Nose, Mouth) and electrode (72) as within-subject factors. Interactions between electrode and emotion effect would reveal a significant difference in scalp distribution between the two emotional effects.
All ANOVAs used Greenhouse-Geisser adjusted degrees of freedom whenever the Mauchly’s test of sphericity was significant (i.e. when sphericity was violated) and pair-wise comparisons used Bonferroni corrections for multiple comparisons.
2.2 Results
2.2.1. Correct responses
The overall categorization rate was very good (≥80%, Table 3). Overall, participants made fewer correct responses for neutral than happy faces (main effect of emotion, F(1.61, 30.67)=3.98, p< .05, ηp2= .17; significant neutral-happy paired comparison at p< .05). Correct responses were also slightly better for nose and mouth fixations compared to eye fixations (main effect of fixation location, F(2.35, 44.67)=18.01, p< .005, ηp2 =.24; left eye-nose and left eye-mouth paired comparisons at p< .05). No emotion by fixation location interaction was seen.
Table 3.
Mean correct responses for fearful, happy and neutral expressions presented during the emotion discrimination task in Exp. 1 (standard errors to the means in parenthesis).
| Mean (%) Correct in ED task (std error) | ||||
|---|---|---|---|---|
|
| ||||
| Left Eye | Right Eye | Nose | Mouth | |
| Fear | 87.4 (1.2) | 90.8 (1.2) | 90.0 (1.0) | 97.0 (1.2) |
| Happy | 90.7 (0.8) | 91.4 (1.1) | 91.6 (0.9) | 91.7 (1.1) |
| Neutral | 85.9 (1.9) | 87.5 (1.8) | 88.8 (1.2) | 91.4 (1.0) |
2.2.2. P1 Peak Amplitude
Overall largest P1 amplitude was found for fixation to the mouth (main effect of fixation, F(2.18, 41.39) = 31.9, p < .0001, ηp2 = .63) (see Fig. 2A). Fixation location also interacted with electrode (F(2.58, 48.97) = 9.9, p < .0001, ηp2 = .34) due to opposite hemispheric effects for fixation to each eye. On the left hemisphere (O1), P1 was larger for the mouth and left eye (which did not differ significantly) compared to the right eye and the nose fixations (which did not differ) (main effect of fixation location, F(1.95, 37.13)=27.75, p< .0001, significant paired comparisons at p< .0001). On the right hemisphere (O2), P1 was larger for the mouth and right eye (which did not differ significantly) compared to the left eye and nose fixations which did not differ (main effect of fixation location, F(2.34, 44.5)=14.45, p< .0001; significant paired comparisons p< .001). At Oz electrode, P1 was also larger for fixation to the mouth compared to the left eye, right eye and nose which did not differ significantly from each other (main effect of fixation location, F(2.04, 38.71) = 34.62, p < .0001, ηp2 = .65; significant paired comparisons with mouth fixation at p < .001) (Fig. 2A).
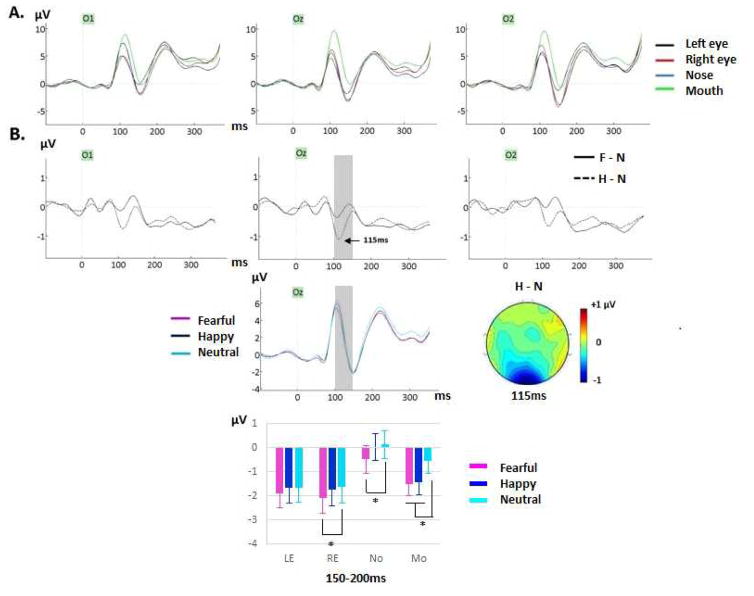
Figure 2.
(A) Grand-averages featuring the P1 component for Exp. 1 (ED) for neutral faces at O1, Oz, and O2 electrodes, showing effects of fixation location with larger amplitudes for mouth fixation and opposite hemispheric effects for eye fixations. (B) Grand-average difference waveforms generated by subtracting ERPs to neutral faces from ERPs to fearful faces (F-N, solid line) and ERPs to neutral faces from ERPs to happy faces (H-N, dashed line) (across fixation locations). A clear difference peak for H-N was seen between 100–150ms (light gray band on Oz, peak of the “happiness effect” around 115ms, see topographic map – view from above) and was confirmed by mean amplitude analysis during that time window (see main text and Table 4). The grand-averaged waveforms for fearful, happy and neutral faces (across fixation locations) at Oz clearly show that this “happiness effect” started on the P1 peak. (C) Between 150–200ms, the happiness effect was seen for the mouth fixation condition only (smaller amplitudes for happy than neutral faces) as shown by the bar graph (computed across clusters). The fear effect was also seen mainly for mouth fixation and to a lesser extent for nose and right eye fixations.
An effect of emotion was also found (F(1.86, 35.47) = 3.37, p = .049, ηp2 = .15) due to a reduced positivity for happy expressions, but the Bonferroni corrected happy-neutral paired comparison only approached significance (p=.088). Although the emotion by electrode interaction also only approached significance (F(2.61, 49.69) = 2.72, p = .062, ηp2 = .125), we analyzed each electrode separately given our previous study where a similar emotion effect was found only at Oz site (Neath & Itier, 2015). This happiness effect was indeed significant at Oz (main effect of emotion, F(1.98, 37.65) = 5.74, p <.01, ηp2 = .23; happy-neutral paired comparison p=.013) as best seen by difference waveforms (happy-neutral), and was largest right after the P1, around 115ms (Fig. 2B). In contrast, no emotion effect was seen at O1 (F(1.81, 34.5)=2.75, p=.083) or O2 (F(1.96, 37.31)=.22, p=.79) electrodes. This occipital happiness effect was also confirmed statistically with mean amplitude analyzes during the 100–150ms window as an interaction between cluster and emotion (Table 4, discussed below). Please note that in the remainder of the paper, the “happiness effect” will denote significantly smaller amplitudes for happy than neutral faces and the “fear effect” will denote significantly smaller amplitudes for fearful than neutral faces.
Table 4.
Exp. 1 (Emotion discrimination –ED- task) statistical effects on mean amplitudes analyzed over six 50ms time windows at 3 clusters (occipital cluster - Occ: O1, Oz, O2-, Left lateral cluster – Llat: CB1, P7, PO7, P9- and Right lateral cluster – Rlat: CB2, P8, PO8, P10), with F, p and ηp2 values. LE, left eye; RE, right eye; No, nose; Mo, mouth; F, fear; H, happiness; N, neutral. Significant Bonferroni-corrected paired comparison tests are also reported with their p values (e.g., H < F + N means that mean amplitude for happy was significantly smaller compared to both fearful and neutral expressions, while H + F < N means that mean amplitudes for both happy and fearful faces were significantly smaller than to neutral expressions). Effects reported in italics in parenthesis are effects that were weak and not clear and thus were treated as non-significant and not discussed in the text.
| Statistical effects | 50–100ms | 100–150ms | 150–200ms | 200–250ms | 250–300ms | 300–350ms |
|---|---|---|---|---|---|---|
| Fixation location | - |
F (3,57)= 22.41, p<.0001, ηp2 =.54 Mo > LE > RE + No |
F(1.78,33.87)= 12.46, p<.0001, ηp2=.4 LE + RE + Mo < No |
F(3,57)= 3.78, p=.024, ηp2=.17, RE + Mo < No | - | - |
| Emotion | - | - |
F(2,38)= 22.32, p<.0001, ηp2=.54 F< H+N |
F(2,38)= 31.26, p<.0001, ηp2=.62, F<H<N |
F(2,38)= 23.33, p<.0001, ηp2=.55 F<H<N |
F(2,38)= 21.32, p<.0001, ηp2=.53 F<H<N |
| Cluster X Emotion | - |
F(4,76)= 7.23, p<.0001, ηp2=.28 *Occ: F (2,38)= 7.09, p=.003, ηp2= .27 H<N (p=.009); H<F (p=.025); *Llat: no emotion effect *Rlat: F (2,38)= 5.16, p=.014, ηp2=.21, F>N (p=.013); |
- |
F(4,76)= 2.95, p=.037, ηp2=.13 *Occ: F (2,38)= 11.6, p<.0001, ηp2=.38 H<N (p=.037) *Llat: F (2,38)= 29.39, p<.0001, ηp2=.61 F<H ; F<N (ps<.001) *Rlat: F (2,38)= 29.34, p<.0001, ηp2=.61 F<H; F<N; H<N (ps<.005) |
- | - |
| Cluster X Fixation location | - (F(3.47,66.02)= 5.27, p=.002, ηp2 =.22, *Occ: no fixation effect *Llat: F(1.96,37.33)= 4.2, p=.023, ηp2=.18, Paired Comparisons ns *Rlat: F(3,57)=3.48, p=.031; ηp2=.16 Paired Comparisons ns) |
F(6,114)= 21.89, p<.0001, ηp2=.54 *Occ: F(3,57)= 35.25, p<.0001, ηp2=.65 Mo > all (ps<.0001); LE > RE (p=.015) *Llat: F(3,57)= 12.54, p<.0001, ηp2=.4 LE > RE + No (ps<.001) *Rlat: F(3,57) =5.19, p=.004; ηp2 =.22 Mo > No (p=.005) |
F(3.28,62.34)= 3.34, p=.021, ηp2=.15 *Occ: F(1.91, 36.34)= 4.2, p=.024, ηp2=.18; No + Mo > eyes but paired comparisons ns *Llat: F(3,57)= 11.31, p<.0001, ηp2 =.37 RE < LE < No *Rlat: F(3,57) =15.33, p<.0001; ηp2=.45 LE + RE < Mo < No |
- | - |
- (F(2.88,54.67)= 2.82, p=.05, ηp2=.13 *Occ: no fixation effect *Llat: no fixation effect *Rlat: no fixation effect) |
| Emotion X Fixation location | - | - |
F (6, 114) = 3.03, p=.009, ηp2=.14 *LE: no emotion effect *RE: F(2,38) = 4.85, p=.013, ηp2=.2; F<N (p=.028) *No: F(2,38) = 6.85, p=.003, ηp2=.26; F<H (p=.016); F<N (p=.01) *Mo: F(2,38) = 15.38, p<.0001, ηp2=.45; F<N ; H<N (ps<.001) |
- |
F (6,114) = 2.47, p=.048, ηp2=.12, *LE: F(2,38) = 14.72, p<.001, ηp2=.44; F<H; F<N (ps<.005) *RE: F(2,38) = 16.23, p<.001, ηp2=.46; F<H (p=.017); F<N (p<.0001) *No: no emotion effect *Mo: F(2,38) = 7.99, p=.001, ηp2=.30; F<N (p=.007); H<N (p=.015); |
F (6, 114) = 3.27, p=.012, ηp2=.15 *LE: F(2,38) = 9.81, p=.001, ηp2=.34; F<N (p=.001) *RE: F(2,38) = 11.03, p<.001, ηp2=.37; F<N (p<.001) *No: no emotion effect *Mo: F(2,38) = 16.56, p<.0001, ηp2=.47; F<N (p=.001); H<N (p<.001); |
| Cluster X Emotion X Fixation location |
- | - (F(6.45,122.5)= 2.5, p=.023, ηp2=.12 *Occ: no emo x fix *Llat: no emo x fix *Rlat: emo x fix, F(6,114)= 3.35, p=.011, ηp2 =.15 but no emotion effect for any fixation location) |
- | - | - | - |
2.2.3. N170 Peak Amplitude
The N170 amplitude was larger for fixation to the left and right eyes (which did not differ) compared to fixation to the mouth and nose which did not differ significantly (main effect of fixation location, F(1.49, 28.29)=12.63, p<.0001, ηp2=.40; all paired comparisons at p-values <.01) (Fig. 3A). This fixation effect was more pronounced on the right than on the left hemisphere (hemisphere by fixation location, F(1.57, 29.84)=3.61, p<.05, ηp2=.56). The N170 amplitude was also larger in the right compared to the left hemisphere (main effect of hemisphere, F(1, 19)=8.52, p<.01, ηp2=.31). No effects of emotion or emotion by fixation location interaction were seen.
Figure 3.
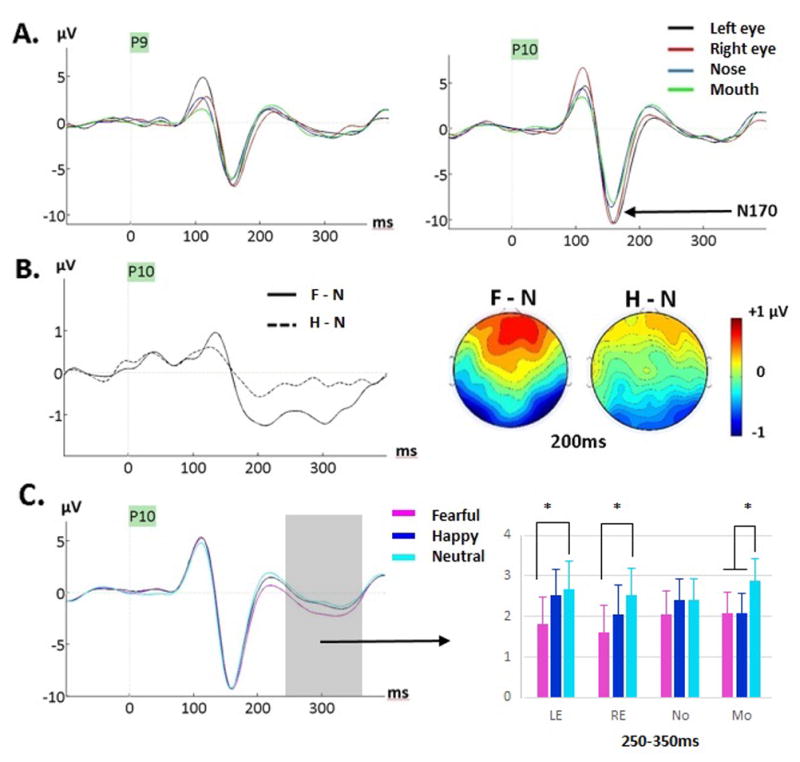
(A) Grand-averages featuring the N170 component for neutral faces at P9 and P10 as a function of fixation location during Exp. 1 (ED task). A clearly larger N170 for eyes compared to nose and mouth fixations, is seen. The opposite hemispheric effects for eye fixations are also seen on the preceding P1. (B) Grand-average difference waveforms generated by subtracting ERPs to neutral from ERPs to fearful faces (F-N, solid line) and ERPs to neutral from ERPs to happy faces (H-N, dashed line) at P10, showing the emergence of the fear effect around 200ms with lack of emotion effect on the N170. The maps show the voltage difference between fearful and neutral faces (F-N) and happy and neutral faces (H-N) at 200ms post-stimulus, when the fear effect was largest. (C) Grand-averages for fearful, happy and neutral faces (across fixation locations) at P10 site where the effect was clearly seen. The gray interval over 250–350ms is where an emotion by fixation interaction was seen (see Table 5); the bar graph depicts the mean amplitudes averaged across the 250–300 and 300–350ms intervals (across the three clusters). The fear effect was seen for both eyes and mouth fixations while the happiness effect was seen for mouth fixation only.
2.2.4. Mean Amplitudes over Six Time Windows (Occipital, Left lateral and Right lateral clusters)
Statistical results for these analyses (50–350ms) are reported in Table 4 and visually depicted in Figures 2, 3 and 4.
Figure 4.
Mean voltage distribution maps of the grand-average difference waveforms between fear and neutral and happy and neutral faces across six 50ms time intervals from 50ms to 350ms (averaged across fixation location) in Exp. 1 (ED task, left panels) and in Exp. 2 (ODD task, right panels). The early occipital effect for happy faces is clearly seen between 100–150ms while the fear effect starts at 150–200ms. Different topographies are seen for the two emotions. The grand-average difference waveforms (F-N and H-N, across fixation locations) are shown at lateral-posterior sites (left and right clusters, bottom panels) and show the stronger fear than happiness effect across most of the epoch. The gray zones indicates the time during which significant emotion effects were seen at these sites (150–350ms, see Tables 4–5).
Fixation location interacted with clusters between 100–200ms (Table 4). More positive amplitudes were seen when fixation was to the mouth compared to the other facial features between 100 and 150ms, and this effect was strongest at occipital sites, as found for P1 peak (Table 4, Fig.2). During that time window, amplitudes were also larger for the left than right eye fixation at left lateral sites, reminiscent of the fixation effect seen on the P1 peak. Between 150 and 200ms, overall more positive amplitudes were seen during fixation to the nose and mouth at occipital sites. A lateral sites, mean amplitudes became more negative for the eyes than the nose and mouth, paralleling effects seen on the N170 component (Fig.3). Between 200 and 250ms, the mean amplitudes were overall larger for nose and mouth fixations than for eye fixations. After 250ms, no more fixation location effect was seen.
An emotion effect was first seen during the 100–150ms time window with smaller amplitudes for happy compared to neutral (and fearful) expressions (Fig. 2B) at occipital sites only (cluster x emotion, Table 4), confirming the happiness effect found on P1 reported previously. At 150–200ms, a significant emotion by fixation location interaction revealed that the happiness effect was only seen for the mouth fixation condition (Fig.2B, Table 4). During that time window, a fear effect (more negative amplitudes for fearful than neutral faces) was seen for the right eye, nose and mouth fixations, but not for the left eye fixation. Between 200–250ms, the happiness effect was seen at occipital and right lateral sites but not at left lateral sites, while the fear effect was seen at both left and right lateral sites but not at occipital sites (cluster x emotion interaction, Table 4). Between 250 and 350ms, emotion interacted again with fixation location (Table 4, Fig. 3C) as the happiness effect was seen only at the mouth fixation while the fear effect was seen when fixation was on the eyes and mouth, but not when fixation was on the nose.
To summarize, a happiness effect was seen from ~100ms until 350ms, as clearly seen on the difference waveforms and their topographic maps (Fig. 4, see also Fig. 2B). This happiness effect was overall less pronounced than the fear effect, and was only seen at the mouth fixation location between 150–200ms, and between 250–350ms. A fear effect was seen a bit later, starting at 150ms until 350ms, best captured by difference waves and their topographies as a bilateral posterior negativity with positive counterpart at frontal sites (Fig. 3–4); this fear effect peaked around 200ms (Fig. 3B).
2.2.5. Scalp Topographies
The analysis of the mean amplitude differences (fear-neutral and happy-neutral) revealed a significant interaction between the emotion effect and electrode factors for all but one time windows: 50–100ms (F(6.55, 124.54)=2.12, p=.05, ηp2=.1); 100–150ms (F(5.52, 105.05)=2.03, p=.073, ηp2=.097); 150–200ms (F(7.64, 145.15)=4.51, p<.0001, ηp2=.192); 200–250ms (F(7.01, 133.24)=5.45, p<.0001, ηp2=.223); 250–300ms (F(5.47, 103.88)=3.69, p=.003, ηp2=.163); 300–350ms (F(7.87, 149.5)=3.06, p=.003, ηp2=.139). When normalized amplitudes were used, the interaction between emotion effect and electrode factors was significant for the same time windows: 50–100ms (F(7.05, 134.01)=2.87, p=.008, ηp2=.13); 100–150ms (F(6.29, 119.52)=1.68, p=.127, ηp2 =.081); 150–200ms (F(7.79, 147.96)=4.01, p<.001, ηp2 =.174); 200–250ms (F(7.32, 139.08)=3.94, p<.0005, ηp2=.172); 250–300ms (F(6.75, 128.25)=3.02, p=.006, ηp2=.137); 300–350ms (F(8.06, 153.05)=2.34, p=.021, ηp2=.11). These results confirm that scalp topographies of the fear and happiness effects were different during most of the epoch analyzed (see Figure 4), and based on the effect sizes, the difference was maximal between 150ms and 250ms.
2.3 Discussion
Using the same gaze-contingent procedure as Neath and Itier (2015), we investigated the effects of fixation to different facial features on the neural processing of fearful, happy and neutral facial expressions in an explicit emotion discrimination task. Overall emotion categorization performance was very good and in line with the ratings originally reported in the validation study of the NimStim database when using faces with open mouths (Tottenham et al., 2009), with better discrimination for happy relative to neutral expressions. A categorization performance advantage was also seen during fixation to the nose and mouth compared to the eyes, supporting the idea of an emotion recognition advantage from facial information in the bottom half of the face (e.g., Blais et al., 2012).
As predicted, a clear fixation effect was seen between 100 and 150ms at occipital sites (Figure 2A, Table 4) with larger amplitude when fixation was on the mouth compared to the eyes and nose. This effect was also seen on P1 peak and likely reflected sensitivity to the face position on the screen, given that most facial information was in the upper visual field during fixation to the mouth. P1 amplitude was also larger when fixation was on the right than on the left eye on the right hemisphere and larger for fixation on the left eye compared to the right eye on the left hemisphere (Fig.2A). The larger amplitude for the left than the right eye fixation was also captured by mean amplitude analyses between 100–150ms (Table 4). This fixation effect reflects hemifield presentation effects as most of the facial information was in the left visual field when fixation was on the right eye and the right visual field when fixation was on the left eye (Fig.1A). This effect mirrors fixation effects reported by recent studies using similar gaze-contingent procedures (de Lissa et al., 2014; Neath & Itier, 2015; Nemrodov et al., 2014; Zerouali, Lena, & Jemel, 2013).
As also expected, we found larger N170 amplitudes for both eye fixations (Fig. 3A) compared to the nose and mouth fixations (de Lissa et al., 2014; Neath & Itier, 2015; Nemrodov et al., 2014). This larger amplitude for the eyes was also found with the mean amplitude analysis at lateral-posterior sites between 150–200ms and supports the idea of a special role for the processing of eyes at the level of the face structural encoding. This N170 eye sensitivity was seen to the same extent for the three facial expressions, as also reported by Neath and Itier (2015), and there was no effect of emotion on this component, consistent with previous ERP studies requiring discrimination of facial expressions (e.g., Kerestes et al., 2009; Leppänen et al., 2008; Schupp et al., 2004; however see Hinojosa et al., 2015).
Like Neath and Itier (2015), we also found smaller amplitudes for happy relative to neutral expressions (happiness effect) starting on P1, ~100–350ms post-stimulus, and smaller amplitudes for fearful relative to neutral expressions (fear effect) starting later, right after the N170, ~150–350ms post-stimulus. The overall scalp distribution and timing of these happiness and fear effects were remarkably similar to the Neath and Itier (2015) study and topography analyses confirmed the different scalp distribution of these effects during most of the epoch, especially during 150–250ms. The mean amplitude analysis also revealed differences at posterior sites, with the happiness effect being uniquely occipital early on (100–150ms). Between 200–250ms, the happiness effect was distributed over occipital and right lateral sites (but not left lateral sites), while the fear effect was found at both right and left lateral sites but not at occipital sites. Together these findings suggest that these emotion effects have a distinct time course and that their underlying generators are distinct or work differently.
Emotion also interacted with fixation location between 150 and 200ms post-stimulus onset (Table 4), with the happiness effect seen only during fixation to the mouth while the fear effect was seen when fixation was on the right eye, the nose and the mouth (Fig. 2B). Neath and Itier (2015) reported a similar interaction during that same time period except only at occipital sites with both fear and happiness effects seen for the mouth fixation only. This lsight difference is likely due to the separate measure of occipital and posterior lateral sites in that study, compared to a cluster approach in the current study. Novel to the current explicit emotion discrimination task was an emotion by fixation location interaction between 250 and 350ms (coinciding with EPN), with a fear effect seen when fixation was on either the eyes or the mouth, but not on the nose. During that time window, a happiness effect was only seen during fixation on the mouth, but not during fixation on the nose or eyes. In other words, fixation on the eyes impacted processing of fearful faces but not happy faces while fixation on the mouth impacted processing of both happy and fearful expressions.
Overall, the present results support the importance of diagnostic features at the neural level. In line with eye movement monitoring studies (Bombardi et al., 2013; Eisenbarth & Alpers, 2011) the current study suggests that both the mouth and eyes are important for the processing of fearful faces, not just the eyes as suggested by others (e.g., Schyns et al., 2007, 2009; Smith et al., 2005). It is important to note, however, that these results might be specific to the current emotion discrimination task. Whether these features play an important role in the processing of fearful and happy expressions during tasks where less attention to the face is required was tested in Experiment 2 (oddball detection task).
3. Experiment 2 – Oddball (ODD) detection task
3.1 Method
3.1.1. Participants
Forty-five undergraduate students were tested at UW and received course credit. All participants lived in North America for at least 10 years and reported normal or corrected-to-normal vision, no history of head-injury or neurological disease, and were not taking any medication. They all signed informed written consent and the study was approved by the Research Ethics Board at UW. A total of 19 participants were rejected: 2 for completing less than half of the experiment thus rendering too few trials per condition; 5 for too many trials with artefacts resulting in too few trials per condition; 10 due to too few trials remaining after removing trials with eye movements greater than 1.4° of visual angle from the fixation location (see Exp. 1 method); and 2 due to high anxiety (scores equal or higher than 44 on the STICSA, Van Dam, Gros, Earleywine, & Antony, 2013). The results from 26 participants were kept in the final analysis (20.8 ± 1.7 years, 15 female, 22 right-handed). None of the participants took part in Exp.1.
3.1.2. Stimuli
The exact same faces as those in Exp. 1 were used. In addition, 6 flower images were used as oddball stimuli. To be consistent with the face images, all flower stimuli were converted to greyscale in Adobe™ Photoshop CS5 and an elliptical mask was applied (see Fig. 1A bottom left). As in Exp. 1, a unique central fixation-cross was used and each face was presented offset so the pre-determined center of each feature would land on the center of the fixation-cross. To keep in line with the experimental paradigm, coordinates corresponding to the left eye, right eye, nose and mouth of a randomly selected neutral face identity were used for all flower stimuli (see Fig. 1A).
3.1.3. Apparatus and Procedure
Participants completed an oddball-detection task where they were instructed to press the space bar as quickly and accurately as possible to the target stimuli (flowers) which occurred infrequently (20% of the time) amongst non-target stimuli (fearful, happy and neutral faces). Participants were given 8 practice trials to introduce them to the experimental procedure. The experimental session used the same gaze-contingent procedure as in Exp. 1 except for the response screen (Fig. 1B). On average participants took 880ms (S. D. = 781) between the onset of the fixation cross and the stimulus presentation. The stimulus was immediately followed by a fixation cross that was presented until response after a flower stimulus, and for 747ms after a face stimulus or if no response was recorded to the flower picture. Participants were instructed to blink during this time.
Each block contained 96 face trials (3 emotions X 4 fixation locations X 8 identities) and 24 flower trials (4 fixation locations X 6 flowers), and was repeated 10 times in a randomized order, yielding 80 trials per face condition. Participants then completed the 21 item trait anxiety test from the STICSA.
3.1.4. Electrophysiological and eye-tracking recordings
Identical to Exp. 1.
3.1.5. Data processing and analyses
Identical to Exp. 1. In this task 6.8% of trials across the final 26 participants were removed due to eye movements recorded beyond 1.4° visual angle (70px) around the fixation location. The average trial number (M= 55, S.D = 10) did not differ significantly by emotion (p = .17) or fixation location (p = .33).
3.2 Results
Overall detection of flower stimuli was excellent (~98%) demonstrating that participants were attending to the task. In addition, participants correctly withheld their responses when they detected a facial stimulus (~99%) and this did not differ by emotion (p = .13) or fixation location (p = .17).
3.2.1. P1 Peak Amplitude
P1 amplitude was largest at O2 (main effect of electrode, F(2, 50) = 7.29, p = .002, ηp2 = .23; O2-Oz comparison p =.002, O2-O1 comparison p =.09) and overall largest for fixation to the mouth (main effect of fixation, F(2.21, 55.19) = 14.39, p < .0001, ηp2 =.37) (see Fig. 5A). As seen in Exp. 1, an interaction between fixation location and electrode (F(3.45, 86.25) = 15.77, p <.0001, ηp2 =.39) was due to eye fixations yielding opposite effects on each hemisphere. On the left hemisphere (O1), P1 was larger for the mouth and left eye (which did not differ significantly) compared to the right eye and the nose fixations (which did not differ) (main effect of fixation location, F(2.42, 60.72) = 20.32, p < .0001; ηp2 = .45). On the right hemisphere (O2), P1 was larger for the mouth and right eye (which did not differ significantly) compared to the left eye and nose fixations which did not differ (main effect of fixation location, F(1.85, 46.27) = 11.57, p < .001; ηp2 = .32; significant paired comparisons p < .01). P1 at Oz was also larger for fixation to the mouth compared to all other fixation locations which did not differ significantly from each other (main effect of fixation location, F(2.42, 60.53) = 14.69, p < .0001, ηp2 = .37; significant paired comparisons with mouth fixation at p < .05) (Fig. 5A).
Figure 5.

(A) Grand-averages featuring the P1 component for Exp. 2 (ODD task) for neutral faces at O1, Oz, and O2 electrodes, showing effects of fixations with larger amplitudes for mouth fixation and opposite hemispheric effects for eye fixations. (B) Top row: Grand-average difference waveforms generated by subtracting ERPs to neutral from ERPs to fearful faces (F-N, solid line) and ERPs to neutral from ERPs to happy faces (H-N, dashed line) at O1, Oz and O2 (across fixation locations). A clear peak for the happy-neutral difference was seen between 100–150ms (gray band, peak of the effect around 120ms) and was confirmed by mean amplitude analysis at occipital sites during that time window (see main text and Table 5). Bottom row: (middle) Grand-averaged waveforms for fearful, happy and neutral faces (across fixation locations) at Oz showing the “happiness effect” starting at P1, although only for the mouth fixation condition (left, bar graph). (Right) The topographic map depicting the happy-neutral difference is shown at 120ms, clearly revealing an occipital distribution.
An effect of emotion was also found, with reduced positivity for happy compared to neutral (and fearful) faces (main effect of emotion, F(1.98, 49.56) = 5.67, p =.006, ηp2 = .19; significant paired comparisons happy-neutral, p=.014 and happy-fearful, p=.03). This happiness effect also interacted with fixation location (F(4.96, 123.99) = 2.49, p =.035, ηp2 =.09) as the effect of emotion was only significant at mouth fixation (F(1.63, 40.75) =9.9, p=.001; significant comparisons: happy-neutral p =.002, and happy-fearful, p =.023). No emotion effect was seen for the left eye (p =.99) or the right eye (p =.42); although an effect of emotion was seen for the nose fixation (p =.038), paired comparisons did not reach significance (see Fig 5B P1 bar graph). Difference waveforms (fearful-neutral and happy-neutral, across fixation locations) clearly revealed this happiness effect at occipital sites that was largest around 120ms (Fig. 5B). This early effect was confirmed with mean amplitude analyzes during the 100–150ms window (Fig.4, see below).
3.2.2. N170 Peak Amplitude
The N170 amplitude was larger in the right compared to the left hemisphere (F(1,25)=7.12, p=.013, ηp2=.22) and for fixation to the left and right eye (which did not differ) compared to fixation to the mouth and nose which did not differ significantly (main effect of fixation location, F(2.66, 66.41)=23.52, p<.0001, ηp2=.49; all paired comparisons at p-values <.001) (Fig. 6A). In contrast to Exp.1, the N170 was larger for fearful compared to neutral (and happy) faces (Fig. 6B) (main effect of emotion, F(1.93, 48.33)=10.34, p<.001, ηp2=.29; significant fearful-happy and fearful-neutral paired comparisons p<.01). There was also an emotion by hemisphere interaction (F(1.94, 48.37)=4.33, p=.02, ηp2=.15) such that N170 amplitudes were larger for fearful compared to both neutral and happy faces in the left hemisphere (emotion effect, F=11.28, p<.001; significant fearful-neutral paired comparison p=.028 and fearful-happy p=.001), while N170 was larger for fearful only compared to neutral faces in the right hemisphere (emotion effect, F=6.61, p<.01; significant fearful-neutral comparison p=.003).
Figure 6.
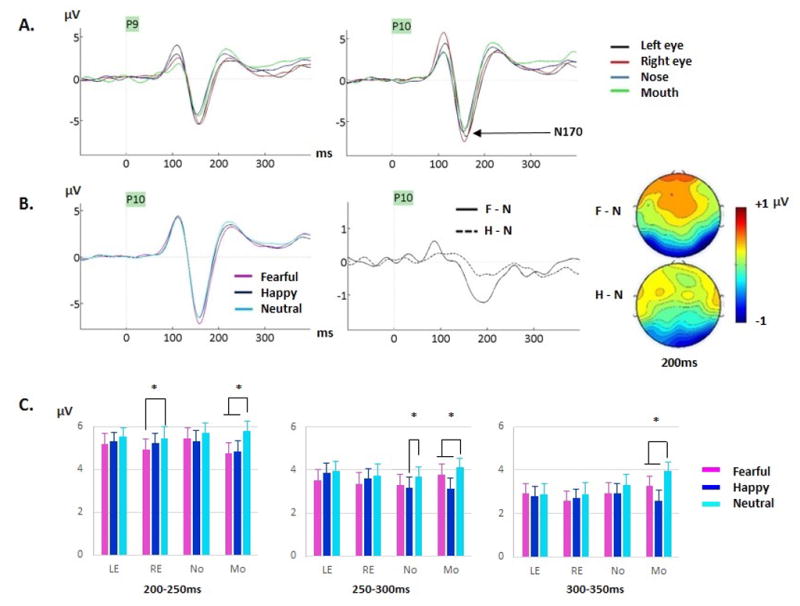
(A) Grand-averages featuring the N170 component for neutral faces at P9 and P10 as a function of fixation location during Exp.2 (ODD task). A clearly larger N170 for eyes compared to nose and mouth fixations, is seen. The opposite hemispheric effects for eye fixations are also seen on the preceding P1. (B) Left. Grand-averages for fearful, happy and neutral faces (across fixation locations) at P10 site featuring a larger N170 peak for fearful than neutral and happy faces. Right. Grand-average difference waveforms generated by subtracting ERPs to neutral from ERPs to fearful faces (F-N, solid line) and ERPs to neutral from ERPs to happy faces (H-N, dashed line) at P10. The maps show the F-N and H-N voltage differences across the scalp at a latency of 200ms where the effects were largest. (C) Emotion by fixation location interactions are displayed in 3 bar graphs, between 200–250ms, 250–300ms and 300–350ms. Note the clear fear and happiness effects at mouth fixation (also see Table 5).
3.2.3. Mean Amplitudes over Six Time Windows (Occipital, Left lateral and Right lateral clusters)
Statistical results for these analyses (50–350ms) are reported in Table 5 and visually depicted in Figures 4, 5 and 6.
Table 5.
Exp. 2 (Oddball –ODD- task) statistical effects on mean amplitudes analyzed over six 50ms time windows at 3 clusters (occipital cluster - Occ: O1, Oz, O2-, Left lateral cluster – Llat: CB1, P7, PO7, P9- and Right lateral cluster – Rlat: CB2, P8, PO8, P10), with F, p and ηp2 values. LE, left eye; RE, right eye; No, nose; Mo, mouth; F, fear; H, happiness; N, neutral. Bonferroni-corrected significant paired comparison tests are also reported with their p values (e.g., H < F + N means that mean amplitude for happy was significantly smaller compared to both fearful and neutral expressions, while H + F < N means that mean amplitudes for both happy and fearful faces were significantly smaller than to neutral expressions). Effects reported in italics in parenthesis are effects that were weak and not clear and thus were treated as non-significant and not discussed in the text.
| Statistical effects | 50–100ms | 100–150ms | 150–200ms | 200–250ms | 250–300ms | 300–350ms |
|---|---|---|---|---|---|---|
| Fixation location | - |
F (2.25,56.43)= 21.43, p<.0001, ηp2 = .46 Mo > LE > RE + No |
F(1.78,44.39)= 20.74, p<.0001, ηp2=.45 LE + RE + Mo < No |
- | - | F(2.37,59.29)= 3.42, p=.032, ηp2=.12, Mo > RE |
| Emotion | - | - |
F(1.59,39.88)= 17.22, p<.0001, ηp2=.41 F<H<N |
F(2,50)= 17.13, p<.0001, ηp2=.41 F+H<N |
F(2,50)= 8.92, p<.001, ηp2=.26 F+H<N |
F(2,50)= 13.43, p<.0001, ηp2=.35, F+H<N |
| Cluster X Emotion | - |
F(4,100)= 15.72, p<.0001, ηp2=.39 *Occ: F (2,50)= 16.32, p=.0001, ηp2 = .39 H<F; H<N (ps<.0001) *Llat: no emotion effect *Rlat: no emotion effect |
F(4,100)= 6.79, p<.0001, ηp2=.21 *Occ: F (2,50)= 13.72, p=.0001, ηp2=.35 F<N; H<N (ps=.001) *Llat: F (1.51,37.85)= 11.18, p<.001, ηp2=.31 F<H; F<N (ps<.005) *Rlat: F (2,50)= 19.23, p<.0001, ηp2=.44 F<N (p<.001); H<N (p=.02) |
F(4,100)= 3.78, p=.009, ηp2=.13 *Occ: F (2,50)= 16.65, p<.0001, ηp2=.4 F<N; H<N (ps<.001) *Llat: F (2,50)= 10.3, p<.001, ηp2=.29 F<N (p=.001) *Rlat: F (2,50)= 15.65, p<.0001, ηp2=.38 F<N (p<.001); H<N (p=.02) |
F(4,100)= 4.74, p=.002, ηp2=.16 *Occ: F (2,50)= 9.5, p<.0001, ηp2=.27 H<N (p<.001) *Llat: F (2,50)= 5.53, p=.007, ηp2=.18 F<N (p=.024) *Rlat: F (2,50)= 8.33, p=.001, ηp2=.25 F<N (p=.017); H<N (p<.001) |
- |
| Cluster X Fixation location |
F(6,150)= 5.42, p<.001, ηp2 =.18 *Occ: no fixation effect *Llat: F(3,75)= 4.71, p=.007, ηp2 = .16 LE > RE *Rlat: F(3,75)=5.46, p=.003; ηp2 = .18; RE + No > LE + Mo |
F(6,150)= 21.46, p<.0001, ηp2=.46 *Occ: F(2.23, 55.74)= 40.98, p=.007, ηp2 = .62 Mo > all (ps<.0001); LE > RE (p=.016) *Llat: F(3,75)= 6.39, p=.001, ηp2 =.2 LE + Mo > RE + No *Rlat: F(2.08,52.21) =6.92, p=.002; ηp2 =.22 RE + LE + Mo > No |
F(3.87,96.7)= 4.41, p=.003, ηp2=.15, *Occ:
F(1.71, 42.7)= 7.48, p=.003, ηp2=.23 RE + Mo < No (ps<.05) *Llat: F(1.79,44.93)= 20.94, p<.0001, ηp2 =.46 RE + Mo < No (ps<.05) *Rlat: F(3,75) =18.87, p<.0001; ηp2=.43 LE + RE + Mo < No (ps<.001) |
F(6,150)= 7.5, p<.0001, ηp2=.23 *Occ: F(2.19, 54.83)= 6.67, p=.002, ηp2=.21 Mo<all (ps<.05) *Llat: no fixation effect *Rlat: F(2.03,50.73) =3.23, p=.047; ηp2=.11 paired comparisons ns |
F(4.01,100.27)= 4.86, p=.001, ηp2=.16 *Occ: F(3, 75)= 4.55, p=.007, ηp2=.15 No<LE (p=.009) *Llat: F(3,75)=6.47, p=.001, ηp2=.21; RE<LE+Mo *Rlat: no fixation effect |
F(4.22,105.37)= 2.48, p=.045, ηp2=.09 *Occ: no fixation effect *Llat: F(2.29,57.45)= 5.25, p=.006, ηp2=.17, RE<Mo (p=.001) *Rlat: F(3,75)= 3.86, p=.019, ηp2=.13 RE<Mo (p=.045) |
| Emotion X Fixation location |
(F(6, 150) = 2.87, p=.023, ηp2=.10 *LE: no emotion effect *RE: no emotion effect *No: F(2,50) = 8.31, p=.001, ηp2 = .25; H<F (p<.001) *Mo: no emotion effect) |
- |
F (6, 150) = 2.62, p=.031, ηp2=.095 *LE: F(2,50) = 5.31, p=.009, ηp2=.175; F<N (p=.009) *RE: F(2,50) = 5.44, p=.01, ηp2=.179; F<H (p=.023); F<N (p=.01) *No: F(2,50) = 7.24, p=.003, ηp2=.225; F<H (p=.026); F<N (p=.005) *Mo: F(2,50) = 16.01, p<.0001, ηp2=.39; F<N; H<N (ps<.001) |
F (4.23, 105.72) = 3.06, p=.018, ηp2=.11 *LE: no emotion effect *RE: F(2,50)=4.21, p=.026, ηp2=.14; F<N (p=.038) *No: no emotion effect *Mo: F(2,50) = 16.81, p<.0001, ηp2=.4; F<N; H<N (ps<.001) |
F (4.32, 107.99) = 3.95, p=.004, ηp2=.14 *LE: no emotion effect *RE: no emotion effect *No: F(1.53,38.43) = 4.69, p=.022, ηp2=.16; H<N (p=.034) *Mo: F(2,50) = 12.58, p<.0001, ηp2=.34; H<F; H<N (ps<.005) |
F (6, 150) = 5.42, p<.001, ηp2=.18 *LE: no emotion effect *RE: no emotion effect *No: no emotion effect *Mo: F(2,50)=26.31, p<.0001, ηp2=.51 H<F; H<N; F<N (ps<.005) |
| Cluster X Emotion X Fixation location |
- |
F(6.5,162.57)= 2.82, p=.01, ηp2=.102 *Occ: emo x fix, F(6,150)= 4.38, p=.001, ηp2 = .15 -LE & RE: no emotion effect -No: H<F (p=.001); H<N (p=.037) -Mo: H<F (p=.001); H<N (p=.001) *Llat: no emo x fix *Rlat: no emo x fix |
- | - | - | - |
Between 50 and 100ms, fixation location interacted with cluster such that a different effect of fixation to the eyes was seen on each hemisphere at lateral clusters, while no fixation effect was seen at occipital sites (Table 5). This different effect of eye fixation depending on the hemisphere was carried across the 100–150ms window (although less clearly) while at occipital sites, amplitudes were most positive when fixation was on the mouth, reminiscent of the P1 effects (Fig.5A). From 150 to 300ms various fixation effects were seen with no clear stable pattern other than more negative amplitudes for the eyes between 150–200ms, paralleling the N170 results.
Small amplitude differences were seen between happy and fearful expressions between 50 and 100ms for nose fixation (emotion by fixation interaction, Table 5). However, as no difference was seen between any emotion and neutral expressions, this sporadic effect is treated as meaningless. As seen in Exp.1, a true emotion effect was first seen during the 100–150ms time window at occipital sites with smaller amplitudes for happy compared to neutral (and fearful) expressions (Fig. 5B), confirming the happiness effect found on P1 peak reported previously. However, in contrast to Exp.1, this effect was seen for the nose and mouth fixations, but not for the eye fixations (cluster by emotion by fixation location interaction, Table 5). The happiness effect was also seen at occipital and right (but not left) lateral sites from 150–300ms (cluster by emotion interactions, Table 5, Fig. 4–6). During that time, the happiness effect was seen only for mouth fixation, as well as for nose fixation during 250–300ms (emotion by fixation interactions, Table 5, Fig.6C).
The fear effect started at 150ms, i.e. after the happiness effect, and lasted until 350ms (Fig.4). This fear effect was seen at left and right lateral sites from 150–300ms and at occipital sites from 150–250ms (cluster by emotion interactions, Table 5, Fig. 4–6). It was seen maximally around 200ms (Fig.6B). From 150–200ms, the fear effect was seen at all fixation locations. From 200–250ms, it was seen at all but the left eye fixation. By 250ms, the effect was no longer seen when fixation was on the eyes, but was still seen for nose and mouth fixations. Finally, from 300–350ms, the fear effect was seen only for mouth fixation (Fig.6C).
3.2.4. Scalp Topographies
The analysis of the mean amplitude differences (fear-neutral and happy-neutral) revealed a significant interaction between the emotion effect and electrode factors for 4 of the 6 time windows. In the 50–100ms window, the emotion effect by electrode interaction was not significant (F(6.43, 160.95)=1.26, p=.27, ηp2=.048). However, the emotion effect by electrode interaction was significant during 100–150ms (F(5.88, 147.21)=9.58, p<.0001, ηp2=.227) and 150–200ms (F(7.1, 177.63)=4.98, p<.0001, ηp2=.166); trending during 200–250ms (F(6.63, 165.84)=1.79, p=.096, ηp2=.067); and significant again during 250–300ms (F(7.26, 181.56)=2.26, p=.03, ηp2=.083) and 300–350ms (F(9.28, 232.22)=1.96, p=.043, ηp2=.073). When normalized amplitudes were used, similar results were found: the emotion effect by electrode interaction was not significant between 50–100ms (F(7.3, 182.48) =1.69, p=.109, ηp2 =.064); was significant between 100–150ms (F(6.48, 162.11)=8.32, p<.0001, ηp2 =.25) and 150–200ms (F(7.73, 193.29)=5.1, p<.0001, ηp2 =.17); was trending during 200–250ms (F(7.85, 196.32)=1.84, p=.073, ηp2=.068) and 250–300ms (F(8.49, 212.47)=1.81, p=.073, ηp2 =.067) and was not significant between 300–350ms (F(10.01, 250.35)=1.48, p=.146, ηp2=.056). Overall, scalp topographies of the fear and happiness effects were similar to those seen in Exp.1 and were different from each other from 100–300ms, but maximally so between 100ms and 200ms (Fig.4).
3.3 Discussion
Using the same gaze-contingent procedure as Exp.1 we investigated the effects of fixation to different facial features on the neural processing of fearful, happy and neutral facial expressions during an oddball detection task that required less attention to the face compared to the emotion discrimination task. Overall, behavioural performance was excellent demonstrating that participants were attending well to the task.
Consistent with Exp. 1, P1 and N170 components were sensitive to fixation location. Fixation effects on the P1 reflected differences in face position on the screen (Fig.1A) whereas effects on the N170 reflected an eye-sensitivity during encoding of the structure of the face (Nemrodov et al., 2014), as discussed in section 2.3. We come back to these effects in the general discussion.
General emotion effects were also consistent with Exp. 1, reproducing the distinct distributions of the effects for fearful and happy expressions. An early happiness effect began on P1 ~100ms and was seen only at occipital sites between 100–150ms during which no fear effect was found. The happiness effect was also seen at occipital and right (but not left) lateral sites between 150–300ms. The fear effect, in contrast, was seen at both occipital and lateral sites between 150–250ms after which time it was seen at lateral sites only (until 300ms). Despite no modulation of the N170 by emotion in Exp. 1, the N170 amplitude was larger for fearful compared to neutral (and happy) expressions in this oddball task. Inspection of the difference waves and topographical maps (Figures 4–6) revealed that the fear effect was extremely similar between the two experiments, starting around or right after the N170 and continuing until 350ms, encompassing the Early Posterior Negativity (EPN; Leppänen et al., 2008; Rellecke et al., 2013; Schupp et al., 2004; see Hinojosa et al., 2015). The reason why this effect starts slightly earlier in some studies (e.g., in the present ODD task) so as to impact the N170, but not in other studies (Exp.1) remains unknown, but could be related to attentional task demands (Hinojosa et al., 2015). However, two recent studies directly comparing tasks reported a lack of significant task by emotion interaction (Rellecke et al., 2012), or an emotional modulation of the N170 in a discrimination task and not in a gender task but only on the right hemisphere (Wronka & Walentoska, 2011). The present study cannot directly address this point given task was a between-subjects factor. More within-subject task comparisons are needed to illuminate this point. Consistent with Exp.1, there was no early effect of fear on the P1. Previous reports of early fear effects in oddball detection tasks (e.g., Batty & Taylor, 2003) may have been driven by uncontrolled low-level stimulus properties.
The emotion effects for happy and fearful expressions also interacted with fixation to facial features. The happiness effect was seen only during fixation to the mouth between 100–350ms (except during 250–300ms where it was also seen for nose fixation). The mouth thus seems to provide important information for the processing of happy expressions, both early, during a stage that is most likely reflecting sensitivity to the low-level cues of the smile, and later, during the processing of the emotional content of the face (EPN). We come back to these effects in the general discussion.
The fear effect interacted with fixation location during several time windows. Between 150–200ms, the fear effect was seen for all fixation locations. By 250ms, the effect was no longer seen for eye fixations but was still seen for nose and mouth fixations and remained seen only for mouth fixation from 300–350ms (Fig. 6C). Thus, information provided by the mouth and the eyes appears to be critical to process the emotional content of fearful expressions even when attention is not directed to the emotional content of the face, as in this oddball task.
4. General Discussion
Combining ERP and eye-tracking recordings using a gaze-contingent procedure, the current study tested the impact of fixation to the eyes and mouth on the neural processing of whole fearful and happy expressions, and whether this differed between an emotion-relevant (Exp. 1) and an emotion-irrelevant (Exp. 2) tasks. Effects of fixation location were seen for the P1 and N170 components, with an eye-sensitivity specific to the N170. Remarkably similar emotion effects were seen in both experiments, with only a few differences between the two tasks. Importantly, these emotion effects interacted with fixation location, revealing an important role of the mouth for processing happy expressions and for both the mouth and the eyes for processing fearful expressions.
4.1. P1 sensitivity to face position
Faces were moved around a central fixation location in order to achieve fixation on the desired facial features (Fig.1). This resulted in different amounts of facial information presented in the visual fields and in fixation location effects on the P1 component. The P1 is a well-known early visual response generated within the extrastriate cortex ~80–120ms post-stimulus onset at occipital sites. The P1 is sensitive to the low-level stimulus characteristics including contrast, luminance, color and spatial frequencies (Rossion & Jacques, 2008) and is also sensitive to attentional effects (Luck et al., 2000; Mangun, 1995). A clear hemifield effect was seen on the P1 amplitude, with larger P1 for fixation on the right than on the left eye on the right hemisphere and vice versa for the left hemisphere. This hemifield effect was virtually identical between Exp.1 and 2 and reproduced Neath and Itier’s finding (2015). A similar effect was also reported in the first studies using the gaze-contingent procedure with expressionless faces (de Lissa et al., 2014; Nemrodov et al., 2014 –supplementary information; Zerouali et al., 2013). This P1 sensitivity to face position was also revealed by a larger P1 response when fixation was on the mouth compared to each of the other locations, as also found in our previous gender discrimination task (Neath & Itier, 2015). As most of the face is in the upper visual field when fixation falls on the mouth, this effect might reflect an early sensitivity of the visual system for that region of space when meaningful stimuli such as faces are presented, possibly related to experience given faces are most often seen in that area in the real world. Whether similar or different P1 variations could be found for fixation on specific features of non-face objects remains to be empirically tested.
4.2. N170 is sensitive to eye fixation
The N170 was sensitive to fixation location and was larger for fixation to the left and right eyes compared to the nose and the mouth. This effect was remarkably similar between the two experiments, and across emotions, again reproducing Neath and Itier (2015)’s finding. This eye sensitivity occurred to the same extent for neutral, fearful and happy faces and thus appears facial-expression-invariant. In addition, while this effect was not directly compared between tasks, the current studies, in addition to our previous report, also speak to task-invariance such that the eye sensitivity was seen to the same extent for three facial expressions in three separate tasks varying in degrees of attention required to the face: a gender discrimination task (Neath and Itier, 2015), an explicit emotion discrimination task (Exp.1-ED) and an oddball task (Exp.2-ODD). These findings are in line with a recent within-subject design study reporting no interaction between emotion and task for the N170 component (Rellecke et al., 2012).
This effect of fixation reflects a true eye sensitivity rather than a simple face position effect as seen for the P1. In contrast to P1, the N170 reliably differs between object categories, supporting the commonly accepted view that both components reflect distinct stages of visual processing with only the N170 reflecting high level vision and face categorization (e.g., Desjardin & Segalowitz, 2013; Ganis, Smith, 7 Schendan, 2012; Jemel et al., 2003; Rossion & Caharel, 2011; Rousselet et al., 2008; Tarkiainen, Cornelissen, & Salmelin, 2002). Additionally, research has shown that the N170 decreases with face eccentricity (Rousselet, Husk, Bennett, & Sekuler, 2005). Given the more lateral position of the face for the eye fixation locations compared to the midline fixation locations (nose and mouth), we would expect to see smaller, rather than larger, N170 amplitude for fixation to the eyes if this N170 eye sensitivity reflected a mere effect of face position. Nemrodov et al. (2014) also showed that the same eye fixation locations did not yield these larger N170 amplitudes when the eyes were not present in fovea (in eyeless faces), despite the same positions of those faces on the screen, thus ruling out a mere effect of face position and demonstrating the dependence of this sensitivity to the presence of eyes in fovea.
This eye sensitivity is in agreement with sensitivity of the N170 to eye regions presented in isolation (Bentin et al., 1996; Itier, Latinus, & Taylor, 2006; Itier, Alain, Sedore, & McIntosh, 2007; Itier, Van Roon, & Alain, 2011; Taylor, Edmonds, McCarthy, & Allison, 2001) and confirms a special role for eyes in the early processing of the face structure, as also suggested by others (e.g., Rousselet, Ince, van Rijsbergen, & Schyns, 2014; Schyns et al., 2003, 2007, 2009). Importantly however, our studies demonstrate the sensitivity of the N170 to eyes within full faces when the face configuration is not altered (configuration is altered with presentation of isolated eyes or when portions of faces are revealed as in the Bubbles technique). Overall the present data provide further support for the hypothesis of an eye detector at play during the early stages of face structural encoding (e.g. Nemrodov et al, 2014), an idea reinforced by the lack of clear fixation location effect after 200ms, as also found by Neath and Itier (2015). Mean pixel intensity (PI) and contrast did not differ between pictures; however, higher contrast and lower PI were seen for the eyes compared to the nose and mouth interest areas. Therefore the hypothesized eye detector might rely on these low-level cues, a possibility that will have to be tested by future studies.
4.3. Early and later “happiness effects” and importance of the mouth
In both Exp1–2, an early happiness effect started on the P1 but was maximal after the peak (115–120ms), as also reported by Neath and Itier (2015). As no such effect was seen for fearful faces, this early effect seems specific to the processing of happy faces and unlikely reflects a general emotion effect. Using an intact/smeared face decision task, a previous study reported a similar effect, with more negative amplitudes for happy than neutral (and angry) faces at occipito-parietal sites during a 128–144ms windows, which corresponded to the transition between P1 and N170 (Schacht & Sommer, 2009). These results suggest possibly faster processing of happy than fearful expressions (the latter starting around the N170 or later, as discussed next), which is in line with behavioural reports of faster discrimination of happy faces compared to the other basic emotions (e.g., Calvo & Lundqvist, 2008; Palermo & Colheart, 2004; Tottenham et al., 2009).
This early happiness effect is possibly due to rapid discrimination of smiling mouth cues. Indeed, between 150–200ms the happiness effect was seen only for fixation on the mouth in Exp.1 and 2, and also, for Exp.2, between 100–150ms at occipital sites and on the P1. This early happiness effect was also reported in Neath and Itier (2015) and thus seems to reflect a general, possibly task-invariant effect, although more studies are needed to confirm this idea. Halgren, Raij, Marinkovic, Jousmäki, and Hari (2000) recorded magnetic fields in response to happy and sad faces and found a midline occipital source (around areas V1–V2) that discriminated happy from neutral expressions between 100–120ms post-stimulus onset. That source was sensitive to stimulus sensory cues and was different from the source corresponding to the M170, the magnetic equivalent of the N170. The authors proposed that a fast discrimination of the smile could occur during this time frame in those early visual areas, based on luminance and contrast information that could be relayed rapidly to the amygdala by direct V2-amygdala connections. Such an explanation is possible given the higher pixel intensity and RMS contrast of the mouth area for our happy compared to neutral face stimuli. As the exact same stimuli were used in both tasks and in our previous study (Neath & Itier, 2015), this early effect might be driven by the specific faces used here, in which the smile was prominent, an idea that will need to be tested by future studies.
The current findings further suggest that the information provided by the mouth region is used during the processing of happy expressions not just early, but also during later stages of processing. Indeed, the happiness effect was seen only when fixation was on the mouth during most of the epoch in both experiments. The later time frame coincides with the EPN that reflects deeper appraisal of the emotional content, suggesting that the mouth also provides cues for the semantic processing of the emotional content of the face.
4.4. Fearful expression processing: It’s not that early and it’s not just about the eyes
In contrast to several previous studies, we did not find the “threat gist” effect on P1 that is thought to reflect the coarse extraction of fearful information (e.g., Luo et al., 2010; see Vuilleumier & Poutois, 2007 for a review). We also did not reproduce the early left-lateralized fear effect around 80ms reported by Neath and Itier (2015), although the exact reason why remains unknown. The N170 peak was larger for fearful than neutral faces in Exp.2 but not in Exp.1 (nor in Neath & Itier, 2015). In contrast, in both experiments, as well as in Neath and Itier’s study, the fear effect was seen reliably and maximally after the N170 peak, around 180–200ms, and lasted until 300ms. This fear effect was seen at lateral-posterior and occipital sites, and encompassed the visual P2 (~200ms) component and the EPN (Rellecke et al., 2011, 2013; Schupp et al., 2004). A similar fear effect was reported by previous studies (Eimer & Kiss, 2007; Eimer, Holmes, & McGlone,2003; Leppänen et al., 2008; Schupp et al., 2004; Sprengelmeyer & Jentzsch, 2006) and likely reflects activity linked to the processing of fear superimposed onto the normal activity related to the processing of neutral faces in occipito-temporal visual areas, as proposed by other groups (e.g. Rellecke et al., 2013; Schacht & Sommer, 2009; Schupp et al., 2004;). That is, the emotion effect on the N170 would actually reflect superimposed EPN activity (Rellecke et al., 2011; 2012; Schacht & Sommer, 2009). This superimposed neural activity could be the result of additional activity in a network of perceptual visual areas including the face-sensitive fusiform gyrus, triggered by projections from the amygdala, as reported in intracranial (Pourtois, Spinelli, Seeck, & Vuilleumier, 2010a) and MEG (e.g., Dumas et al., 2013) studies around the same time. Intracranial ERP studies have indeed reported amygdala activation in response to fearful faces starting ~150–200ms post-stimulus (Krolak-Salmon, Hénaff, Vighetto, Bertrand, & Mauguière, 2004; Meletti et al., 2012; Pourtois, Spinelli, Seeck, & Vuilleumier, 2010b). As different scalp distributions between the actual N170 component and the superimposed fear-related EPN effect have been found, some have proposed that fear processing does not interact with the structural encoding of the face but rather occurs in parallel to (or in addition to) face processing (e.g. Rellecke et al., 2011, 2013; Schacht & Sommer, 2009).
The wide open eyes are particularly salient for fearful expressions and are used most prominently when discriminating fear from other expressions (e.g., Calder et al., 2000; Smith et al., 2005). Recent ERP research that forced feature-based processing (by presenting portions of faces) has suggested the importance of the eye region in the neural response to fearful expressions at the level of the N170 or later (Leppänen et al., 2008; Schyns et al., 2007, 2009). When presenting whole facial expressions, as seen in everyday life, results from the current study showed that both the eyes and mouth are important for processing fearful expressions. First, between 150 and 200ms, a fear effect was seen at almost all fixation locations in both experiments, including mouth fixation. Mouth cues seem important also later with the fear effect being seen during mouth fixation between 250–350ms in Exp.1 and between 200–350ms in Exp.2. The fear effect was also seen during fixation to the eyes between 150–350ms in Exp.1 and until 250ms in Exp.2. Thus, fearful cues in the eyes and mouth both seem to impact neural activity after the N170 and during the semantic processing of the emotional content of the face (i.e., timing of the EPN). The importance of the eyes during that time frame is in line with Leppänen et al. (2008) who demonstrated that the added negativity in response to fearful expressions (between 160–240ms) was eliminated when the eye region was covered. Novel to the current study is the finding that the fearful mouth also provides an important cue during processing of fearful expressions, a finding especially striking in Exp.2 at later stages of processing where the fear effect was found only for mouth fixation. This result supports recent behavioural studies that have demonstrated the importance of the mouth region in the discrimination of fearful expressions (Blais et al., 2012) and visual scanning studies where participants made saccades equally towards the eyes and mouth of fearful faces (e.g. Eisenbarth & Alpers, 2011; see also Bombardi et al., 2013). These modulations of the neural activity with fixation to eyes and mouth seen in the present studies for fearful and happy expressions contrast with a lack of such effects in our previous gender discrimination task (Neath & Itier, 2015), pointing at possible effects of task demands on the use of featural information that future studies will have to elucidate further using task as a within-subject factor.
The gaze-contingent approach we took allowed us to probe the effect of fixation to facial features on the neural processing of intact full faces, presumably allowing some form of holistic processing to take place. Although the intent was to be a bit closer to real life situations in which faces are explored and fixations go from one feature to another while the entire face remains present, it is worth acknowledging that our design still does not completely reflects face processing under natural conditions. Faces were presented fast and the quite large proportion of trials eliminated due to contamination by some eye movement, even tiny, suggests this mode of stimulus presentation was not optimal for participants. However, the artificial presentation of face photographs on a computer screen for very short durations is not uncommon in the face ERP literature (especially studies focusing on the N170). We just additionally manipulated face position and prevented micro eye movements from contaminating the neural activity recorded. It is unclear how this specific mode of presentation impacted the results although the fact that our emotional effects are largely in line with previous literature, and that main effects of fixation were no longer seen past 200–250ms, suggest this approach is sound. A more important question for future studies might be the role of these micro eye movements in vision, which ERP researchers view as noise and attempt to systematically eliminate, despite suggestion that they are an integral part of real life visual perception.
4.5. Conclusions
The current study is the first to test the impact of fixation to facial features on the neural processing of whole fearful and happy expressions during an emotion discrimination (Exp.1) and an oddball detection (Exp.2) tasks. Different effects of fixation location were seen for the P1 and N170, with a general sensitivity of the P1 to face position, followed by an eye sensitivity for the N170 component, and no more effect of fixation beyond 200ms. The N170 eye sensitivity possibly reflects the activity of an eye-detector in the processing of the face structure that seems facial expression invariant. The scalp topographies associated with these emotion effects were significantly different, and were predominantly seen at occipital sites for happy expressions at early stages. However, scalp topographies cannot reveal whether this topography difference is the result of different underlying generators or the same generators with one or two nodes more active for one emotion compared to the other (see Luck, 2005; Urbach & kutas, 2002). Results also suggest that cues from the mouth might have been used for early processing of happy expressions (i.e., happy gist), likely driven by low-level information, and during the later semantic processing of the emotional content of the face. Cues from both the mouth and the eyes impacted the semantic processing of the fearful content of the face. This study highlights the need for monitoring gaze fixation in ERP emotion research and the importance of quantifying neural activity around P1 and N170 peaks, as emotion effects may be missed by simply measuring these commonly studied ERP markers. Results also help elucidate the debated early emotion effects and extend our current understanding of the role of facial features during neural processing of facial expressions of emotion.
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant #418431), the Ontario government (Early Researcher Award, ER11-08-172), the Canada Foundation for Innovation (CFI, #213322), and the Canada Research Chair (CRC, #213322 and #230407) program to RJI, as well as by a doctoral NSERC grant to KNN (PGS D Grant # 210825634). The funding agencies did not have any involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the article for publication. We would like to warmly thank Frank Preston for all his technical help, as well as Casey Oliver for testing most of the participants in Exp.2.
Footnotes
This high attrition rate indirectly shows that, even with 257ms presentation times, many participants make many eye movements that put fixation on another facial feature given the size of the stimuli.
Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set. The models used in the present study were models # 2, 3, 6, 8, 20, 24, 33, 34
The average angular distance between features was 3.12° from the center of the left eye to the center of the right eye, 2.43° from each eye to the nose tip and 2.26° from the nose tip to the center of the mouth.
Note that the custom-made montage used by our lab includes CB1 and CB2 electrodes that are usually not part of the Biosemi 64 channel system.
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Ashley V, Vuilleumier P, Swick D. Time course and specificity of event-related potentials to emotional expressions. Neuroreport. 2004;15(1):211–216. doi: 10.1097/00001756-200401190-00041. [DOI] [PubMed] [Google Scholar]
- Balconi M, Lucchiari C. Event-related potentials related to normal and morphed emotional faces. The Journal of Psychology. 2005;139(2):176–192. doi: 10.3200/JRLP.139.2.176-192. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Batty M, Taylor M. Early processing of the six basic facial emotional expressions. Cognitive Brain Research. 2003;17(3):613–620. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Bentin S, Deouell LY. Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology. 2000;17(1–3):35–55. doi: 10.1080/026432900380472. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais C, Roy C, Fiset D, Arguin M, Gosselin F. The eyes are not the window to basic emotions. Neuropsychologia. 2012;50(12):2830–2838. doi: 10.1016/j.neuropsychologia.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behavioral and Brain Functions. 2007;3(7):1–13. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardi D, Schmid PC, Schmid Mast M, Birri S, Mast FW, Lobmaier JS. Emotion recognition: the role of featural and configural information. Q J Exp Psychol. 2013;66(12):2426–42. doi: 10.1080/17470218.2013.789065. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Caharel S, Courtay N, Bernard C, Lalonde R. Familiarity and emotional expression influence an early stage of face processing: An electrophysiological study. Brain and Cognition. 2005;59(1):96–100. doi: 10.1016/j.bandc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Jansen J. Configural coding of facial expressions: The impact of inversion and photographic negative. Visual Cognition. 2005;12(3):495–518. [Google Scholar]
- Calder AJ, Young AW, Keane J, Dean M. Configural information in facial expression perception. Journal of Experimental Psychology: Human perception and performance. 2000;26(2):527. doi: 10.1037//0096-1523.26.2.527. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Beltrán D. Brain lateralization of holistic versus analytic processing of emotional facial expressions. Neuroimage. 2014;92:237–247. doi: 10.1016/j.neuroimage.2014.01.048. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Lundqvist D. Facial expressions of emotion (KDEF): Identification under different display-duration conditions. Behavior research methods. 2008;40(1):109–115. doi: 10.3758/brm.40.1.109. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Human Brain Mapping. 1995;2(3):170–187. [Google Scholar]
- de Lissa P, McArthur G, Hawelka S, Palermo R, Mahajan Y, Hutzler F. Fixation location on upright and inverted faces modulates the N170. Neuropsychologia. 2014;57:1–11. doi: 10.1016/j.neuropsychologia.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desjardins JA, Segalowitz SJ. Deconstructing early visual electrocortical responses to face and house stimuli. Journal of Vision. 2013;13(5):22, 1–18. doi: 10.1167/13.5.22. [DOI] [PubMed] [Google Scholar]
- Dumas T, Dubal S, Attal Y, Chupin M, Jouvent R, Morel S, George N. MEG evidence for dynamic amygdala modulations by gaze and facial emotions. PLoS One. 2013;8(9):e74145. doi: 10.1371/journal.pone.0074145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biological Psychology. 2007;74(1):108–112. doi: 10.1016/j.biopsycho.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone FP. The role of spatial attention in the processing of facial expression: An ERP study of rapid brain responses to six basic emotions. Cognitive, Affective, & Behavioural Neuroscience. 2003;3(2):97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Eisenbarth H, Alpers GW. Happy mouth and sad eyes: Scanning emotional facial expressions. Emotion. 2011;11:860–865. doi: 10.1037/a0022758. [DOI] [PubMed] [Google Scholar]
- Gamer M, Schmitz AK, Tittgemeyer M, Schilbach L. The human amygdala drives reflexive orienting towards facial features. Current Biology. 2013;23(20):R917–R918. doi: 10.1016/j.cub.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Ganis G, Smith D, Schendan HE. The N170, not the P1, indexes the earliest time for categorical perception of faces, regardless of interstimulus variance. Neuroimage. 2012;62(3):1563–1574. doi: 10.1016/j.neuroimage.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmäki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Aranda D, Ellgring H, Mueller TJ, Strik WK, Heidrich A, Fallgatter AJ. Face-specific event-related potential in humans is independent from facial expression. International Journal of Psychophysiology. 2002;45(3):241–244. doi: 10.1016/s0167-8760(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Hinojosa JA, Mercado F, Carretié L. N170 sensitivity to facial expression: A meta-analysis. Neuroscience and Biobehavioural Reviews. 2015;55:498–509. doi: 10.1016/j.neubiorev.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: A repetition study using ERPs. NeuroImage. 2002;15(2):353–372. doi: 10.1006/nimg.2001.0982. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15(8):1261–1265. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ, Lobaugh Spatiotemporal analysis of event-related potentials to upright, inverted and contrast-reversed faces: effects on encoding and recognition. Psychophysiology. 2004;41:643–653. doi: 10.1111/j.1469-8986.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Latinus M, Taylor MJ. Face, eye and object early processing: What is the face specificity? NeuroImage. 2006;29(2):667–676. doi: 10.1016/j.neuroimage.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Alain C, Sedore K, McIntosh AR. Early face processing specificity: It’s in the eyes! Journal of Cognitive Neuroscience. 2007;19(11):1815–1826. doi: 10.1162/jocn.2007.19.11.1815. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Van Roon P, Alain C. Species sensitivity of early face and eye processing. NeuroImage. 2011;54(1):705–713. doi: 10.1016/j.neuroimage.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ. Attention to eyes in face perception. In: Fawcett JM, Risko EF, Kingstone A, editors. The Handbook of Attention. MIT Press; 2015. pp. 369–387. [Google Scholar]
- Jemel B, Schuller A, Cheref-Khan Y, Goffaux V, Crommelinck M, Bruyer R. Stepwise emergence of the face-sensitive N170 event-related potential component. Neuroreport. 2003;14(16):2035–2039. doi: 10.1097/00001756-200311140-00006. [DOI] [PubMed] [Google Scholar]
- Johannes S, Münte TF, Heinze HJ, Mangun GR. Luminance and spatial attention effects on early visual processing. Cognitive Brain Research. 1995;2(3):189–205. doi: 10.1016/0926-6410(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Labuschagne I, Croft RJ, O’Neill BV, Bhagwagar Z, Phan KL, Nathan PJ. Evidence for modulation of facial emotional processing bias during emotional expression decoding by serotonergic and noradrenergicantic depressants: an event-related potential (ERP) study. Psychopharmacology(Berl) 2009;202:621–634. doi: 10.1007/s00213-008-1340-3. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Labuschagne I, Croft RJ, O’Neill BV, Bhagwagar Z, Phan KL, Nathan PJ. Evidence for modulation of facial emotional processing bias during emotional expression decoding by serotonergic and noradrenergic antidepressants: An event-related potential (ERP) study. Psychopharmacology. 2009;202:621–634. doi: 10.1007/s00213-008-1340-3. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner T, Stolar NM, Bilker WB, Brensinger CM, Gur RE, Gur RC. Differences in facial expressions of four universal emotions. Psychiatry research. 2004;128(3):235–244. doi: 10.1016/j.psychres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Fischer C, Vighetto A, Mauguiere F. Processing of facial emotional expression: Spatio-temporal data as assessed by scalp event-related potentials. European Journal of Neuroscience. 2001;13(5):987–994. doi: 10.1046/j.0953-816x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Hénaff M, Vighetto A, Bertrand O, Mauguière F. Early amygdala reaction to fear spreading in occipital, temporal, and frontal cortex: A depth electrode ERP study in human. Neuron. 2004;42:665–676. doi: 10.1016/s0896-6273(04)00264-8. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, Hietanen JK. Is there more in a happy face than just a big smile? Visual Cognition. 2007;15(4):468–490. [Google Scholar]
- Leppänen JM, Moulson MC, Vogel-Farley VK, Nelson CA. An ERP study of emotional face processing in the adult and infant brain. Child Development. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Hietanen JK, Koskinen K. Differential early ERPs to fearful versus neutral facial expressions: a response to the salience of the eyes? Biological Psychology. 2008;78:150–158. doi: 10.1016/j.biopsycho.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4(11):432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Multiple mechanisms of visual-spatial attention: recent evidence from human electrophysiology. Behavioural brain research. 1995;71(1):113–123. doi: 10.1016/0166-4328(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Luck S. An introduction to the event-related potential technique. The MIT Press; Cambridge: 2005. [Google Scholar]
- Luo W, Feng W, He W, Wang NY, Luo YJ. Three stages of facial expression processing: ERP study with rapid serial visual presentation. Neuroimage. 2010;49(2):1857–1867. doi: 10.1016/j.neuroimage.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32(1):4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62(3):203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- McKone E. Configural processing and face viewpoint. Journal of Experimental Psychology: Human Perception and Performance. 2008;34(2):310. doi: 10.1037/0096-1523.34.2.310. [DOI] [PubMed] [Google Scholar]
- Mehrabian A. Communication without words. Psychology Today. 1968;2:53–56. [Google Scholar]
- Meletti S, Cantalupo G, Benuzzi F, Mai R, Tassi L, Gasparini E, Tassinari CA, Nichelli P. Fear and happiness in the eyes: An intra-cerebral event-related potential study from the human amygdala. Neuropsychologia. 2012;50:44–54. doi: 10.1016/j.neuropsychologia.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Münte TF, Brack M, Grootheer O, Wieringa BM, Matzke M, Johannes S. Brain potentials reveal the timing of face identity and expression judgments. Neuroscience Research. 1998;36(1):25–34. doi: 10.1016/s0168-0102(97)00118-1. [DOI] [PubMed] [Google Scholar]
- Neath KN, Itier RJ. Fixation to features and neural processing of facial expressions in a gender discrimination task. Brain and Cognition. 2015;99:97–111. doi: 10.1016/j.bandc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemrodov D, Anderson T, Preston FF, Itier RJ. Early sensitivity for eyes within faces: A new neuronal account of holistic and featural processing. NeuroImage. 2014;97:81–94. doi: 10.1016/j.neuroimage.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusseck M, Cunningham DW, Wallraven C, Bülthoff HH. The contribution of different facial regions to the recognition of conversational expressions. Journal of vision. 2008;8(8):1. doi: 10.1167/8.8.1. [DOI] [PubMed] [Google Scholar]
- Palermo R, Coltheart M. Photographs of facial expression: Accuracy, response times, and ratings of intensity. Behavior Research Methods, Instruments, & Computers. 2004;36(4):634–638. doi: 10.3758/bf03206544. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral cortex. 2004;14(6):619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass: Time course and topographic evoked-potentials mapping. Human Bran Mapping. 2005;26:65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Modulation of face processing by emotional expression and gaze direction during intracranial recordings in right fusiform cortex. Journal of Cognitive Neuroscience. 2010a;22:90–107. doi: 10.1162/jocn.2009.21404. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Temporal precedence of emotion over attention modulations in the lateral amygdala: Intracranial ERP evidence from a patient with temporal lobe epilepsy. Cognitive, Affective, & Behavioural Neuroscience. 2010b;10(1):83–93. doi: 10.3758/CABN.10.1.83. [DOI] [PubMed] [Google Scholar]
- Ree MJ, French D, MacLeod C, Locke V. Distinguishing cognitive and somatic dimensions of state and trait anxiety: Development and validation of the state-trait inventory for cognitive and somatic anxiety (STICSA) Behavioural and Cognitive Psychotherapy. 2008;36(3):313–332. [Google Scholar]
- Rellecke J, Palazova M, Sommer W, Schacht A. On the automaticity of emotion processing in words and faces: Event-related brain potentials evidence from a superficial task. Brain and Cognition. 2011;77(1):23–32. doi: 10.1016/j.bandc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Rellecke J, Sommer W, Schacht A. Does processing of emotional facial expressions depend on intention? Time-resolved evidence from event-related brain potentials. Biological Psychology. 2012;90(1):23–32. doi: 10.1016/j.biopsycho.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Rellecke J, Sommer W, Schacht A. Emotion effects on the N170: A question of reference? Brain Topography. 2013;26(1):62–71. doi: 10.1007/s10548-012-0261-y. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caharel S. ERP evidence for the speed of face categorization in the human brain: Disentangling the contribution of low-level visual cues from face perception. Vision Research. 2011;51(12):1297–1311. doi: 10.1016/j.visres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysioloigcal face sensitive responses in the human brain? Ten lessons on the N170. NeuroImage. 2008;39(4):1959–1979. doi: 10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Rossion B, Jacques C. The N170: Understanding the time course of face perception in the human brain. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of Event-Related Potential Components. Oxford: Oxford University Press; 2012. pp. 115–141. [Google Scholar]
- Rossion B, Campanella S, Gomez CM, Delinte A, Debatisse D, Liard L, Guerit JM. Task modulation of brain activity related to familiar and unfamiliar face processing: An ERP study. Clinical Neurophysiology. 1999;110(3):449–462. doi: 10.1016/s1388-2457(98)00037-6. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I, Tarr MJ, Despland P, Bruyer R, Linotte S, Crommelinck M. The N170 occipito-temporal component is delayed and enhanced to inverted faces but not to inverted objects: An electrophysiological account of face-specific processes in the human brain. Neuroreport. 2000;11(1):69–72. doi: 10.1097/00001756-200001170-00014. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Pernet CR. Quantifying the time course of visual object processing using ERPs: it’s time to up the game. Front Psych. 2011;23(2):107. doi: 10.3389/fpsyg.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Bennett PJ, Sekuler AB. Spatial scaling factors explain eccentricity effects on face ERPs. Journal of Vision. 2005;5(10):755–763. doi: 10.1167/5.10.1. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Bennett PJ, Sekuler AB. Time course and robustness of ERP object and face differences. Journal of Vision. 2008;8(12):3, 1–18. doi: 10.1167/8.12.3. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Ince RAA, van Rijsbergen NJ, Schyns PG. Eye coding mechanisms in early human face event-related potentials. Journal of Vision. 2014;14(13):7, 1–24. doi: 10.1167/14.13.7. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Matsumura M. Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. Cognitive Neuroscience and Neuropsychology. 2001;12(4):709–714. doi: 10.1097/00001756-200103260-00019. [DOI] [PubMed] [Google Scholar]
- Schacht A, Sommer W. Emotions in word and face processing: early and late cortical responses. Brain and Cognition. 2009;69:538–550. doi: 10.1016/j.bandc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Scheller E, Büchel C, Gamer M. Diagnostic features of emotional expressions are processed preferentially. PloS one. 2012;7(7):e41792. doi: 10.1371/journal.pone.0041792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Markus J, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological science. 2003;14(1):7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004;41(3):441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: event-related brain potential studies. Progress in brain research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Jentzsch I, Johnson M, Schweinberger SR, Gosselin F. A principled method for determining the functionality of brain responses. Neuroreport. 2003;14(13):1665–1669. doi: 10.1097/00001756-200309150-00002. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Petro LS, Smith ML. Dynamics of visual information integration in the brain for categorizing facial expressions. Curr Biol. 2007;17(18):1580–1585. doi: 10.1016/j.cub.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Petro LS, Smith ML. Transmission of facial expressions of emotion co-evolved with their efficient decoding in the brain: Behavioral and brain evidence. PLoS One. 2009;4(5):e5625. doi: 10.1371/journal.pone.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Merlusca C. How task shapes the use of information during facial expression categorizations. Emotion. 2014;14(3):478–487. doi: 10.1037/a0035588. [DOI] [PubMed] [Google Scholar]
- Smith E, Weinberg A, Moran T, Hajcak G. Electrocortical responses to NIMSTIM facial expressions of emotion. International Journal of Psychophysiology. 2013;88(1):17–25. doi: 10.1016/j.ijpsycho.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Smith ML, Cottrell GW, Gosselin F, Schyns PG. Transmitting and decoding facial expressions. Psychological Science. 2005;16(3):184–189. doi: 10.1111/j.0956-7976.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Jentzsch I. Event related potentials and the perception of intensity in facial expressions. Neuropsychologia. 2006;44(14):2899–2906. doi: 10.1016/j.neuropsychologia.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Cornelissen PL, Salmelin R. Dynamics of visual feature analysis and object-level processing in face versus letter-string perception. Brain: A Journal of Neurology. 2002;125:1125–1136. doi: 10.1093/brain/awf112. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Edmonds GE, McCarthy G, Allison T. Eyes first! Eye processing develops before face processing in children. Neuro Report. 2001;12:1671–1676. doi: 10.1097/00001756-200106130-00031. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, ücCarry T, Nurse, Hare TA, … Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39(6):791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Gros DF, Earleywine M, Antony MM. Establishing a trait anxiety threshold that signals likelihood of anxiety disorders. Anxiety, Stress & Coping. 2013;26(1):70–86. doi: 10.1080/10615806.2011.631525. [DOI] [PubMed] [Google Scholar]
- Van Selst M, Jolicoeur P. A solution to the effect of sample size on outlier elimination. The quarterly journal of experimental psychology. 1994;47(3):631–650. [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience. 2003;6(6):624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of neuroscience. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers AA, Banis S. Foveal and parafoveal spatial attention and its impact on the processing of facial expressions: An ERP study. Clinical Neurophysiology. 2012;123:513–526. doi: 10.1016/j.clinph.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Wronka E, Walentowska W. Attention modulates emotional expression processing. Psychophysiology. 2011;48(8):1047–1056. doi: 10.1111/j.1469-8986.2011.01180.x. [DOI] [PubMed] [Google Scholar]
- Zerouali Y, Lina J, Jemel B. Optimal eye-gaze fixation position for face-related neural responses. PloS One. 2013;8(6):e60128. doi: 10.1371/journal.pone.0060128. [DOI] [PMC free article] [PubMed] [Google Scholar]