Abstract
Purpose
Bladder cancer is a common malignancy often diagnosed in older adults. Previous studies have reported racial/ethnic disparities in bladder cancer survival outcomes, but have not focused on younger patients. We sought to identify whether factors influencing cause-specific survival in adolescents and young adults (ages 15-39) differed from older adults, and to define prognostic factors specifically in adolescents and young adults using the California Cancer Registry.
Materials and Methods
Patients diagnosed with bladder cancer between 1988 through 2012 were included. The primary outcome measure was cause specific survival. A multivariable Cox proportional hazards regression model was used to evaluate predictors of cause-specific survival in patients of all ages and in adolescents/young adults. Interactions of age and other variables between younger and older adult patients were assessed.
Results
Of 104,974 bladder cancer patients, we identified 1,688 adolescent and young adult patients (1.6%). When compared to older patients, these patients had a 58% reduced risk of bladder cancer death (Hazard Ratio 0.42; p<0.001). Significant age interactions were identified involving race/ethnicity and histology. Among AYAs, non-Hispanic African Americans with low socioeconomic status had poor cause- specific (Hazard Ratio 7.1, p<0.001) and overall (Hazard Ratio 5.02, p<0.001) survival.
Conclusions
Racial/ethnic and socioeconomic disparities exist in adolescent and young adult patients with bladder cancer in California. Further studies are warranted to identify the underlying causes in order to overcome these disparities.
Keywords: Adolescents and young adults, urothelial cancer, disparities, epidemiology
BACKGROUND
Bladder (urothelial) cancer will be diagnosed in 76,960 Americans in 2016 and is expected to be responsible for approximately 16,000 deaths. (1) It predominantly affects males and subsequently accounts for 7% of all new cases of cancer in men. Transitional cell cancer is the most common histologic subtype, accounting for approximately 85% of patients. Soft tissue sarcoma, specifically rhabdomyosarcoma, is the most common malignancy of the bladder in the pediatric population. (2)
Bladder cancer is generally a disease of older patients. (3) The median age at diagnosis is approximately 72 years. (4) Its incidence in patients younger than 40 years of age is reported to be as low as 0.8%. (5) As a result, much of the published literature has been in older patients. Reports on younger patients - often defined as age less than 40 years - have consisted mostly of small single institution series or case reports. (6-10) For example, in 56 bladder cancer patients less than 40 years of age, higher grade and larger tumors were seen, potentially portending worse oncologic outcomes. (6) In contrast, a paper on 152 young bladder cancer patients reported that in those less than 30 years of age, 40.3% had a papillary urothelial neoplasia of low malignant potential.(11) In addition, the field is complicated by the variability of definitions of what constitutes a “young patient”. Most published series define “young” as less than 40 years of age. However, some investigators have focused on even younger subsets: i.e., less than 30 years, and have reported variable results. (7) Thus, there remains controversy, uncertainty, and knowledge gaps about whether the clinical behavior of bladder cancer in younger patients differs to that of older patients, and which baseline characteristics of young patients are prognostic of survival.
Survival disparities in bladder cancer patients also have been widely reported, and have been ascribed to imbalances in detection and treatment strategies due to social, economic, and community disadvantages. (12) However, these reports have typically focused on a broader group of patients, again mostly involving older individuals. There is no contemporary published study that has specifically investigated potential survival disparities in younger patients with this malignancy, highlighting the need for additional studies.
MATERIALS AND METHODS
To better understand outcomes in adolescent and young adults (AYA) patients, we utilized data from the large and diverse, population-based California Cancer Registry (CCR) to identify factors associated with survival in this patient subset compared to older patients. For the purposes of this analysis, the definition of AYA, age 15-39, was based on the age range specified by the National Cancer Institute's 2006 Adolescent and Young Adult Oncology Progress Review Group. (13)
Data were obtained from the CCR, a cancer surveillance system collecting cancer incidence and mortality information since 1988. (14) Cases are reported to the Cancer Surveillance Section of the California Department of Public Health from hospitals and any other facilities providing care or therapy to cancer patients residing in California. Cases included in these analyses were those > 15 years at diagnosis with any stage of bladder cancer diagnosed between January 1, 1988 and December 31, 2012 and reported to the Cancer Surveillance Section as of October 2013. Bladder cancer was defined using relevant SEER site recode. The Committee for the Protection of Human Subjects of the California Health and Welfare Agency, and the University of California, Davis Institutional Review Board approved this study..
We sought to describe the demographic characteristics of the bladder cancer population including the following variables: histologic subtype (transitional cell versus other); year of diagnosis (5 year categories); patient age; sex; race/ethnicity; stage (categorized as in situ, localized [Ta/T1], regional [T2-T4a], and distant [T4b or N+ or M1]); grade; initial treatment (surgery, radiation, chemotherapy); rural vs. urban residence; and neighborhood socioeconomic status (SES). Race/ethnicity was based on information obtained from the registry, which was derived from patient self-identification, assumptions based on personal appearance, or inferences based on the race/ethnicity of the parents, birthplace, surname, or maiden name. Hispanic ethnicity was based on information from the medical record and computerized comparisons to the 1980 U.S. census list of Hispanic surnames. Patients identified as Hispanic on the medical record, or patients identified as White, Black (African American), or of unknown race with a Hispanic surname were classified as Hispanic.
Neighborhood SES and rural/urban designation were assigned at the Census block group level (2000 U.S. Census) and based on patient address at the time of initial diagnosis as reported in the medical record. This SES variable is an index that uses education, employment characteristics, median household income, proportion of the population living 200% below the federal poverty level, median rent, and median housing value at the census tract level. (15) A principal components analysis was used to identify quintiles, based on the distribution of census tracts in California of neighborhood SES ranging from 1 (lowest) to 5 (highest), with quintiles collapsed into low (quintiles 1-3) and high (quintiles 4-5) categories for the analyses. Rural/urban designation was defined by Rural Urban Commuting Areas (RUCA) codes developed and categorized by the University of Washington's Rural Health Research Center. Principles of the Helsinki Declaration were followed as this was an IRB-exempt study.
Statistical Considerations
The primary outcome was cause-specific survival (CSS). Overall survival (OS) was a secondary endpoint. For deceased patients, survival time was measured in months from the date of diagnosis to the date of death from bladder cancer (CSS) or all causes (OS). Patients alive at the study end date (12/31/2011) were censored at this time or at date of last follow-up (i.e., last known contact). Patients diagnosed after 12/31/2011 were excluded from the survival models. A multivariable cox proportional hazard model was used to identify survival differences between AYA and older adults. Interactions between age (AYA vs. older adults) and gender, race/ethnicity, histology, stage, grade, and neighborhood SES were assessed. An interaction was considered significant for p-values < 0.05 in the multivariable model. Among AYAs, multivariable cox proportional hazard models were used to identify factors associated with CSS and OS. The proportionality assumption was assessed using log-negative-log plots. Kaplan Meier curves were constructed to display CSS for each relevant covariate. (16) A p-value of <0.05 was considered statistically significant. All analyses were performed using SAS 9.4 (SAS, Cary, NC). All p-values were for two-sided tests.
RESULTS
Patient demographics are summarized in Table 1. A total of 104,974 patients with bladder cancer were identified from the CCR. Of these, 1,688 patients (1.6%) were less than 40 years of age. X Among AYAs, the proportion of cases increased with age, with 2.6% of patients 15-19 years of age and 78.7% of patients 30-39 years of age. Most AYA patients were non-Hispanic white (66.4%) followed by Hispanic (20.1%), Asian (6.6%), and African-American (4.0%). The vast majority of AYA patients had transitional cell cancer as the primary histology (92.2%). Most patients had localized Ta/T1 stage (71.6%). Only 9.7% had in situ disease. Expectedly, the majority of AYA patients (94.7%) had undergone surgery – either a transurethral resection or cystectomy. Only 9.5% received chemotherapy as part of first course of treatment. The majority of patients (95%) lived in an urban location, while neighborhood SES was distributed as follows: Low SES (52.5%) and High SES (46.7%).
Table 1.
Bladder Cancer Cohort Characteristics, California 1988-2012
| Variables | All | AYA | NON-AYA | P-Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| All | 104,974 | 100.0% | 1,688 | 100.0% | 103,286 | 100.0% | . |
| Gender | |||||||
| Male | 78,702 | 75.0% | 1,229 | 72.8% | 77,473 | 75.0% | 0.0385 |
| Female | 26,272 | 25.0% | 459 | 27.2% | 25,813 | 25.0% | 0.0385 |
| Age at Diagnosis | |||||||
| 15-19 | 44 | 0.0% | 44 | 2.6% | . | . | . |
| 20-29 | 316 | 0.3% | 316 | 18.7% | . | . | . |
| 30-39 | 1,328 | 1.3% | 1,328 | 78.7% | . | . | . |
| 40-49 | 4,942 | 4.7% | . | . | 4,942 | 4.8% | . |
| 50-59 | 13,471 | 12.8% | . | . | 13,471 | 13.0% | . |
| 60-69 | 26,535 | 25.3% | . | . | 26,535 | 25.7% | . |
| 70-79 | 33,683 | 32.1% | . | . | 33,683 | 32.6% | . |
| Age 80+ | 24,655 | 23.5% | . | . | 24,655 | 23.9% | . |
| Race/Ethnicity | |||||||
| NH White | 84,882 | 80.9% | 1,120 | 66.4% | 83,762 | 81.1% | <.0001 |
| African American | 3,663 | 3.5% | 67 | 4.0% | 3,596 | 3.5% | 0.2789 |
| Hispanic | 9,264 | 8.8% | 340 | 20.1% | 8,924 | 8.6% | <.0001 |
| Asian/PI | 5,561 | 5.3% | 111 | 6.6% | 5,450 | 5.3% | 0.0181 |
| Other/Unknown | 1,604 | 1.5% | 50 | 3.0% | 1,554 | 1.5% | <.0001 |
| Year of Diagnosis | |||||||
| 1988-1992 | 19,872 | 18.9% | 374 | 22.2% | 19,498 | 18.9% | 0.0006 |
| 1993-1997 | 20,110 | 19.2% | 380 | 22.5% | 19,730 | 19.1% | 0.0004 |
| 1998-2002 | 20,798 | 19.8% | 356 | 21.1% | 20,442 | 19.8% | 0.1843 |
| 2003-2007 | 22,013 | 21.0% | 288 | 17.1% | 21,725 | 21.0% | <.0001 |
| 2008-2012 | 22,181 | 21.1% | 290 | 17.2% | 21,891 | 21.2% | <.0001 |
| Histology | |||||||
| Transitional Cell | 98,383 | 93.7% | 1,556 | 92.2% | 96,827 | 93.7% | 0.0085 |
| Other | 6,591 | 6.3% | 132 | 7.8% | 6,459 | 6.3% | 0.0085 |
| Histologic Grade | |||||||
| Low Grade | 51,521 | 49.1% | 1,273 | 75.4% | 50,248 | 48.6% | <.0001 |
| High Grade | 44,807 | 42.7% | 285 | 16.9% | 44,522 | 43.1% | <.0001 |
| Unknown Grade | 8,646 | 8.2% | 130 | 7.7% | 8,516 | 8.2% | 0.4203 |
| Stage at Diagnosis* | |||||||
| In Situ | 8,604 | 8.2% | 163 | 9.7% | 8,441 | 8.2% | 0.0275 |
| Localized | 66,896 | 63.7% | 1,208 | 71.6% | 65,688 | 63.6% | <.0001 |
| Regional | 18,768 | 17.9% | 166 | 9.8% | 18,602 | 18.0% | <.0001 |
| Distant | 6,704 | 6.4% | 81 | 4.8% | 6,623 | 6.4% | 0.0072 |
| Unknown Stage | 4,002 | 3.8% | 70 | 4.1% | 3,932 | 3.8% | 0.4693 |
| Treatment: Chemotherapy | |||||||
| No | 91,842 | 87.5% | 1,517 | 89.9% | 90,325 | 87.5% | 0.0029 |
| Yes | 12,352 | 11.8% | 161 | 9.5% | 12,191 | 11.8% | 0.0042 |
| Unknown | 780 | 0.7% | 10 | 0.6% | 770 | 0.7% | 0.4676 |
| Treatment: Radiation | |||||||
| No | 100,312 | 95.6% | 1,656 | 98.1% | 98,656 | 95.5% | <.0001 |
| Yes | 4,614 | 4.4% | 31 | 1.8% | 4,583 | 4.4% | <.0001 |
| Unknown | 48 | 0.0% | 1 | 0.1% | 47 | 0.0% | 0.7934 |
| Treatment: Surgery | |||||||
| No Surgery | 6,270 | 6.0% | 88 | 5.2% | 6,182 | 6.0% | 0.1843 |
| Local therapy | 86,104 | 82.0% | 1,454 | 86.1% | 84,650 | 82.0% | <.0001 |
| Cystectomy | 11,914 | 11.3% | 146 | 8.6% | 11,768 | 11.4% | 0.0004 |
| Unknown | 686 | 0.7% | . | . | 686 | 0.7% | 0.0008 |
| Neighborhood SES | |||||||
| Low SES | 54,706 | 52.1% | 886 | 52.5% | 53,820 | 52.1% | 0.7563 |
| High SES | 48,942 | 46.6% | 788 | 46.7% | 48,154 | 46.6% | 0.9606 |
| Unknown | 1,326 | 1.3% | 14 | 0.8% | 1,312 | 1.3% | 0.1077 |
| Location of Residence | |||||||
| Urban | 98,241 | 93.6% | 1,611 | 95.4% | 96,630 | 93.6% | 0.0017 |
| Rural | 6,506 | 6.2% | 76 | 4.5% | 6,430 | 6.2% | 0.0036 |
| Unknown | 227 | 0.2% | 1 | 0.1% | 226 | 0.2% | 0.1615 |
NH- Non-Hispanic
SES- Socioeconomic Status
Stage at diagnosis is created from a combination of tumor extension, lymphnode involvement, and metastatic disease
Low SES includes quintile 1, 2, 3
High SES includes quintile 4, 5
Overall, AYA patients were found to have a 58% reduced risk of bladder cancer death when compared to older patients (HR 0.42; 95% CI 0.35, 0.50; p<0.001). Similarly, AYA patients had better overall survival compared to older patients (HR 0.16; 95% CI 0.14, 0.18; p<0.001). Significant age interactions included race/ethnicity and histology (Table 2). All AYA patients had significant reduction in bladder cancer death compared to non-AYA patients in each race/ethnicity group except NH African Americans. AYA NH African Americans had a 36% reduced risk of bladder cancer death but this did not reach significance (HR 0.64; 95% CI 0.36, 1.16; p=0.144).
Table 2.
Risk of death comparing younger patients (15-39 years of age) with older patients 40 years of age and older, California, 1988-2011
| Cause-Specific Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-Value | HR | 95% CI | P-Value |
| Gender | ||||||
| Males | REF | - | - | |||
| Female | 1.27 | (1.23, 1.31) | <.0001 | 1.03 | (1.01, 1.05) | 0.0012 |
| Race/Ethnicity (AYA vs Older adults) | ||||||
| NH White | 0.34 | (0.26, 0.44) | <.0001 | 0.12 | (0.10, 0.14) | <.0001 |
| NH African American | 0.64 | (0.36, 1.16) | 0.1437 | 0.34 | (0.22, 0.52) | <.0001 |
| Hispanic | 0.29 | (0.18, 0.47) | <.0001 | 0.12 | (0.08, 0.17) | <.0001 |
| NH Asian/Pacific Islander | 0.36 | (0.16, 0.83) | 0.0165 | 0.13 | (0.07, 0.26) | <.0001 |
| Year of Diagnosis | ||||||
| 1988-1992 | 1.08 | (1.02, 1.14) | 0.0102 | 1.12 | (1.07, 1.16) | <.0001 |
| 1993-1997 | 0.98 | (0.92, 1.04) | 0.4488 | 1.03 | (0.99, 1.08) | 0.1004 |
| 1998-2002 | 1.05 | (1.00, 1.12) | 0.0670 | 1.06 | (1.02, 1.10) | 0.0037 |
| 2003-2007 | 1.05 | (0.99, 1.11) | 0.0957 | 1.04 | (0.99, 1.08) | 0.0902 |
| 2008-2011 | REF | - | - | |||
| Histology (AYA vs. Non-AYA) | ||||||
| Transitional Cell | 0.34 | (0.26, 0.44) | <.0001 | 0.12 | (0.10, 0.14) | <.0001 |
| Other | 0.59 | (0.42, 0.83) | 0.0025 | 0.40 | (0.30, 0.53) | <.0001 |
| Stage at Diagnosis | ||||||
| In Situ | REF | - | - | |||
| Localized | 1.21 | (1.11, 1.31) | <.0001 | 1.05 | (1.02, 1.08) | 0.0026 |
| Regional | 5.03 | (4.61, 5.49) | <.0001 | 2.12 | (2.05, 2.20) | <.0001 |
| Distant | 15.83 | (14.41, 17.39) | <.0001 | 6.28 | (5.99, 6.58) | <.0001 |
| Unknown | 3.61 | (3.26, 4.00) | <.0001 | 1.58 | (1.50, 1.65) | <.0001 |
| Histologic Grade | ||||||
| Low Grade | REF | - | - | |||
| High Grade | 2.92 | (2.81, 3.03) | <.0001 | 1.62 | (1.59, 1.66) | <.0001 |
| Surgery | ||||||
| No Surgery | REF | - | - | |||
| Local therapy | 0.48 | (0.45, 0.51) | <.0001 | 0.56 | (0.54, 0.58) | <.0001 |
| Cystectomy | 0.29 | (0.27, 0.31) | <.0001 | 0.33 | (0.32, 0.35) | <.0001 |
| Radiation | ||||||
| No | REF | - | - | |||
| Yes | 1.38 | (1.31, 1.45) | <.0001 | 1.48 | (1.43, 1.54) | <.0001 |
| Chemotherapy | ||||||
| No | REF | - | - | |||
| Yes | 0.86 | (0.83, 0.90) | <.0001 | 0.79 | (0.76, 0.81) | <.0001 |
| Residence Location | ||||||
| Urban | REF | - | - | |||
| Rural | 0.96 | (0.90, 1.02) | 0.1944 | 0.97 | (0.94, 1.00) | 0.0796 |
| Neighborhood SES | ||||||
| Low SES | 1.21 | (1.17, 1.24) | <.0001 | 1.28 | (1.26, 1.30) | <.0001 |
| High SES | REF | - | - | |||
Models include cases through 2011, follow-up is completed through 2011
HR-Hazard Ratio
NH- Non-Hispanic
SES- Socioeconomic Status
*Stage at diagnosis is created from a combination of tumor extension, lymphnode involvement, and meta static disease
Low SES includes quintile 1, 2, 3
High SES includes quintile 4, 5
Multivariable analysis of AYA patients for both CSS and OS is summarized in Table 3. Sex, year of diagnosis, chemotherapy use, and rural/urban location were not independent predictors of CSS. Expectedly, higher disease stage provided the strongest association with worsening outcome. When compared to patients with in-situ, those with localized disease had a HR of 0.69 (p=0.4), those with regional disease had a HR of 2.72 (p=0.047), and those with distant disease had a HR of 20.62 (p<0.001). Receipt of chemotherapy was not associated with CSS (HR=1.20, p=0.51). Radiation therapy was associated with worse CSS (HR=3.04, p<0.001); however, there were very few patients in this subset. AYA patients who received a cystectomy had improved CSS (HR=0.41, p=0.008).
Table 3.
Risk of Death among Adolescent and Young Adults (15-39 Years of Age when Diagnosed) with Bladder Cancer, California, 1988-2011
| Cause-Specific Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-Value | HR | 95% CI | P-Value |
| Gender | ||||||
| Males | REF | - | - | REF | - | - |
| Females | 1.26 | (0.84, 1.91) | 0.2657 | 1.26 | (0.93, 1.70) | 0.1366 |
| Age (continuous 5 yr increments) | 1.21 | (0.98, 1.50) | 0.0727 | 1.21 | (1.04, 1.41) | 0.0127 |
| Year of Diagnosis | ||||||
| 1988-1992 | 1.52 | (0.60, 3.87) | 0.3774 | 1.15 | (0.52, 2.50) | 0.7323 |
| 1993-1997 | 1.05 | (0.42, 2.63) | 0.9160 | 1.14 | (0.53, 2.45) | 0.7367 |
| 1998-2002 | 0.92 | (0.36, 2.36) | 0.8553 | 0.97 | (0.44, 2.15) | 0.9450 |
| 2003-2007 | 0.83 | (0.32, 2.15) | 0.7036 | 1.03 | (0.47, 2.28) | 0.9387 |
| 2008-2011 | REF | - | - | REF | - | - |
| Histology | ||||||
| Transitional Cell | REF | - | - | REF | - | - |
| Other | 2.62 | (1.66, 4.13) | <.0001 | 2.21 | (1.52, 3.21) | <.0001 |
| Stage at Diagnosis | ||||||
| In Situ | REF | - | - | REF | - | - |
| Localized | 0.69 | (0.28, 1.73) | 0.4301 | 1.05 | (0.59, 1.90) | 0.8610 |
| Regional | 2.72 | (1.01, 7.31) | 0.0474 | 2.88 | (1.49, 5.59) | 0.0017 |
| Distant | 20.62 | (7.03, 60.49) | <.0001 | 14.69 | (6.79, 31.78) | <.0001 |
| Unknown | 1.40 | (0.44, 4.51) | 0.5721 | 1.45 | (0.67, 3.14) | 0.3419 |
| Histologic Grade | ||||||
| Low Grade | REF | - | - | REF | - | - |
| High Grade | 4.49 | (2.80, 7.19) | <.0001 | 2.96 | (2.12, 4.12) | <.0001 |
| Surgery | ||||||
| No Surgery | REF | - | - | REF | - | - |
| Local therapy | 0.70 | (0.35, 1.40) | 0.3099 | 0.36 | (0.22, 0.61) | 0.0001 |
| Cystectomy | 0.41 | (0.21, 0.80) | 0.0086 | 0.29 | (0.18, 0.49) | <.0001 |
| Radiation | ||||||
| No | REF | - | - | REF | - | - |
| Yes | 3.04 | (1.64, 5.64) | 0.0004 | 2.83 | (1.65, 4.83) | 0.0001 |
| Chemotherapy | ||||||
| No | REF | - | - | REF | - | - |
| Yes | 1.20 | (0.69, 2.09) | 0.5138 | 1.05 | (0.67, 1.63) | 0.8338 |
| Residence Location | ||||||
| Urban | REF | - | - | REF | - | - |
| Rural | 0.94 | (0.37, 2.35) | 0.8883 | 1.77 | (1.01, 3.10) | 0.0453 |
| Neighborhood SES (vs. NH White) | ||||||
| Low SES (vs. NH White) | ||||||
| NH African American | 7.10 | (3.32, 15.21) | <.0001 | 5.02 | (2.84, 8.87) | <.0001 |
| Hispanic | 1.10 | (0.59, 2.07) | 0.7623 | 0.96 | (0.61, 1.50) | 0.8484 |
| Asian/Pacific Islander | 1.23 | (0.35, 4.26) | 0.7473 | 1.22 | (0.48, 3.10) | 0.6803 |
| High SES (vs. NH White) | ||||||
| NH African American | 1.22 | (0.28, 5.27) | 0.7898 | 2.05 | (0.92, 4.58) | 0.0784 |
| Hispanic | 1.23 | (0.48, 3.16) | 0.6646 | 1.12 | (0.55, 2.28) | 0.7650 |
| Asian/Pacific Islander | 1.13 | (0.33, 3.82) | 0.8501 | 0.78 | (0.28, 2.17) | 0.6282 |
Models include cases through 2011, follow-up is completed through 2011
* Age is continuous and HR is given for 5 year increment in age
NH- Non-Hispanic
SES- Socioeconomic Status
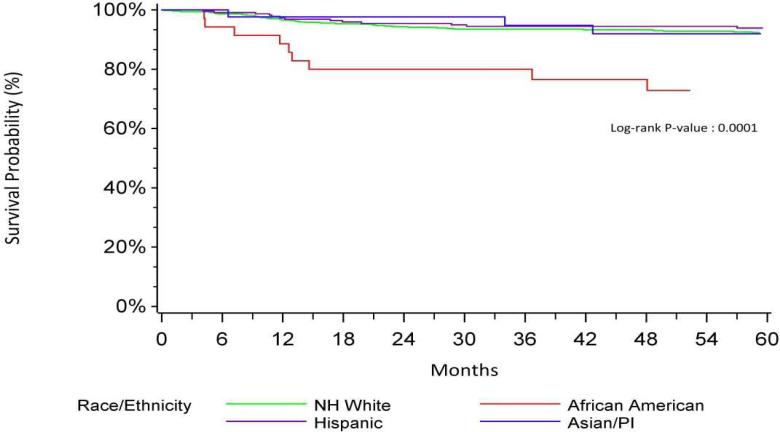
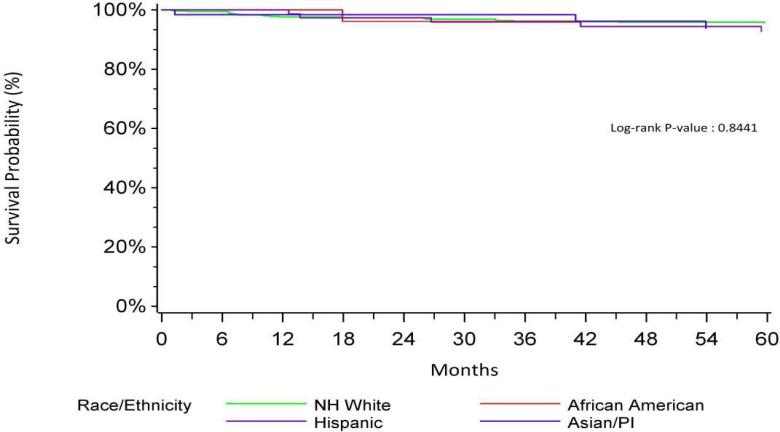
NH African Americans with a low neighborhood SES (1-3) had worse CSS (HR=7.10, p<0.001) when compared to non-Hispanic Whites with low neighborhood SES. In contrast, NH African Americans with high neighborhood SES had CSS that was no different from non-Hispanic Whites in the same SES group (HR=1.22, p=0.79). Kaplan Meier curves for CSS of neighborhood SES by race/ethnicity are shown in Figures 1 and 2. This interaction was not found in older patients.
Figure 1.
Bladder Specific Survival-Low SES by Race/Ethnicity
Figure 2.
Bladder Specific Survival-High SES by Race/Ethnicity
Additional exploratory models were developed to evaluate the relevance of histologic subtypes and muscle invasion (in lieu of summary stage) on the results reported above. In a multivariable model excluding sarcoma histology (n=229) results were similar to the primary analyses: AYA patients who were NH African American and resided in the lowest SES neighborhoods had worse CSS (HR 7.97, 95% CI 3.72, 17.10, p=<0.001). In contrast, NH African Americans residing in the highest SES neighborhoods had an HR of 1.21, 95% CI 0.28, 5.23, p=0.80.
DISCUSSION
This current study provides contemporary data on the largest group of AYA patients with bladder cancer ever reported. Using the CCR, we identified 1,688 AYA patients with annotated baseline clinical information as well as robust survival statistics. We also showed that AYA patients had better CSS and OS compared to older patients. This contrasts with a previous study (n=56) from Turkey that reported no difference in 5 year overall survival, recurrence-free survival, and progression free survival rates between young (≤ 40 years) and old (>40) patients. (6) In our multivariable models of AYAs, we found that NH African American race/ethnicity, high grade, distant stage, and low neighborhood SES were independent predictors of poor CSS and OS. Our interaction analysis showed that NH African Americans residing in the low SES neighborhoods had significantly worse CSS and OS than non-Hispanic white patients in these same neighborhoods and no racial/ethnic differences in CSS and OS were observed in AYAs residing in high SES neighborhoods. Our study appears to be the first to have specifically identified potential health disparities in the uncommon group of young patients with bladder cancer using a comprehensive cancer registry. It must be emphasized that prior studies in this age group were single institution series that mostly focused on pathologic features rather than clinical outcome, and did not specifically address socioeconomic or racial disparities. (6-10)
Racial/ethnic disparities in bladder cancer diagnosis, treatment, and outcome are well known, albeit in older patients. NH African Americans are reported to have a 70% greater risk of cancer-related death when compared to Whites. (17) In a comprehensive review, Jacobs et al reported that worse survival in African Americans was attributable to delayed presentation, advanced stage, and higher grade disease.(12) In our study, we found that AYA patients with more advanced stage had inferior CSS compared to older patients, suggesting a more aggressive biologic phenotype in the former cohort. Presumably, the presence of non-transitional cell histology such as sarcoma in AYA patients may have influenced this interaction. However, a subsequent analysis excluding 299 patients with sarcoma did not alter the overall finding.
Efforts to overcome the observed disparities in the outcome of young bladder cancer patients will require a more comprehensive understanding of the potential genetic underpinnings of the disease. It is hypothesized that molecular phenotypes unique to AYA populations – operating in the context of environmental and societal influences - are in part responsible for the differential outcomes reported here. Ascertaining the etiology of bladder cancer in AYA patients is beyond the scope of this current work; however, these results help identify a starting point for a focused future study of biologic differences underlying AYA versus non-AYA patients.
There are limitations to this study. Since the CCR is considered a state registry, the results emanating from it may not be readily applicable to other states that have different demographic features. Furthermore, SES is derived from neighborhood-level data and not available at the individual level. While neighborhood and individual SES are correlated, neighborhood SES may underestimate the impact of individual SES (18) or measure other neighborhood attributes, including access to resources and health care (19). Finally, the CCR does not collect all relevant clinical information such as more detailed treatment data (e.g., chemotherapy dose and number of cycles), known risk factors such as smoking, laboratory and molecular data, as well other important prognostic variables such as comorbidities, weight loss, and performance status. Nevertheless, we believe that our work serves as a new foundational reference for future studies of AYA bladder cancer patients.
In conclusion, we found that racial/ethnic and socioeconomic disparities exist in AYA patients with bladder cancer diagnosed in California, with African Americans residing in lower SES neighborhoods experiencing much worse survival than non-Hispanic Whites residing in the same low SES neighborhoods. Further studies are warranted to identify the underlying causes in order to overcome these disparities.
Supplementary Material
Acknowledgements
This work was supported in part by NCI P30 CA093373 to the UC Davis Comprehensive Cancer Center.
Key of Definitions for Abbreviations
- AYA
Adolescents and young adults
- CCR
California Cancer Registry
- CI
Confidence intervakl
- CSS
Cause-specific survival
- HR
Hazard ratio
- NH
Non-Hispanic
- SES
Socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Castagnetti M, Angelini L, Alaggio R, et al. Oncologic outcome and urinary function after radical cystectomy for rhabdomyosarcoma in children: role of the orthotopic ileal neobladder based on 15-year experience at a single center. J Urol. 2014;191(6):1850–5. doi: 10.1016/j.juro.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 3.Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939–49. doi: 10.2147/CIA.S74322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen ME, Smith AB, Meyer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer. 2014;120(1):86–95. doi: 10.1002/cncr.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paner GP, Zehnder P, Amin AM, et al. Urothelial neoplasms of the urinary bladder occurring in young adult and pediatric patients: a comprehensive review of literature and implication for patient management. Adv Anat Pathol. 2011;18:79–89. doi: 10.1097/PAP.0b013e318204c0cf. [DOI] [PubMed] [Google Scholar]
- 6.Telli O, Sarici H, Ozgur BC, et al. Urothelial cancer of bladder in young versus older adults: Clinical and pathological characteristics and outcomes. Kaohsiung Journal of Medical Sciences. 2014;30:466–470. doi: 10.1016/j.kjms.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanton M, Xiao L, Czerniak BA, et al. Urothelial tumors of the urinary bladder in young patients: a clinicopathologic study of 59 cases. Arch Pathol Lab Med. 2013;137(10):1337–1341. doi: 10.5858/arpa.2012-0322-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na SW, Yu SH, Kim KH, et al. The prognosis of patients less than 40 years with bladder cancer. Journal of Cancer Research and Therapeutics. 2014;10(3):710–14. doi: 10.4103/0973-1482.137928. [DOI] [PubMed] [Google Scholar]
- 9.Wen TC, Kuo JY, Chen KK, et al. Urothelial carcinoma of the urinary bladder in young adults – clinical experience at the Taipei Veterans General Hospital. J Chin Med Assoc. 2005;68(6):272–275. doi: 10.1016/S1726-4901(09)70149-2. [DOI] [PubMed] [Google Scholar]
- 10.Yossepowitch O, Dalbagni G. Transitional cell carcinoma of the bladder in young adults: presentation, natural history, and outcome. J Urol. 2002;168:61–66. [PubMed] [Google Scholar]
- 11.Comperat E, Larre S, Roupret M, et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015;466:589–594. doi: 10.1007/s00428-015-1739-2. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs BL, Montgomery JS, Zhang Y, et al. Disparities in bladder cancer. Urologic Oncology: Seminars and Original Investigations. 2012;30:81–88. doi: 10.1016/j.urolonc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Adolescent and Young Adult Oncology Progress Review Group, National Cancer Institute and LIVESTRONG Young Adult Alliance . Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. National Cancer Institute; Bethesda, MD: 2006. NIH publication 06-6067. [Google Scholar]
- 14.Harris DHA, Snipes KP. In: Research Utilizing the California Cancer Registry. California Department of Public Health CSS, editor. Sacramento, CA: 2007. [Google Scholar]
- 15.Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EMP. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 17.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 18.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am JPublic Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez SL, Shariff-Marco S, DeRouen MC, et al. The Impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodologic considerations, and future directions. Cancer. 2015;121:2314–30. doi: 10.1002/cncr.29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.