Abstract
Previous event-related potential (ERP) and neuroimaging evidence suggests that directing attention toward single item-context associations compared to intra-item features at encoding improves context memory performance and reduces demands on strategic retrieval operations in young and older adults. In everyday situations, however, there are multiple event features competing for our attention. It is not currently known how selectively attending to one contextual feature while attempting to ignore another influences context memory performance and the processes that support successful retrieval in the young and old. We investigated this issue in the current ERP study. Young and older participants studied pictures of objects in the presence of two contextual features: a color and a scene, and their attention was directed to the object’s relationship with one of those contexts. Participants made context memory decisions for both attended and unattended contexts and rated their confidence in those decisions. Behavioral results showed that while both groups were generally successful in applying selective attention during context encoding, older adults were less confident in their context memory decisions for attended features and showed greater dependence in context memory accuracy for attended and unattended contextual features (i.e., hyper-binding). ERP results were largely consistent between age groups but older adults showed a more pronounced late posterior negativity (LPN) implicated in episodic reconstruction processes. We conclude that age-related suppression deficits during encoding result in reduced selectivity in context memory, thereby increasing subsequent demands on episodic reconstruction processes when sought after details are not readily retrieved.
Keywords: Attention, Context memory, Retrieval, Aging, ERPs, LPN
1. Introduction
Healthy aging is typically accompanied by episodic memory decline. This decline is disproportionately greater for context memory than item memory (Mitchell and Johnson, 2009; Spencer and Raz, 1995). Memory for contextual details of encoded events is believed to rely on frontally-mediated cognitive control processes to a greater extent than item memory (Mitchell and Johnson, 2009). These processes include elaboration of relational information during encoding and monitoring of retrieved information during retrieval. As cognitive control processes are widely believed to be disrupted by normal aging, we and others have argued that memory tasks placing high demands on cognitive control (e.g., context memory) are more likely to reveal age-related impairments (Cohn et al., 2008; Duarte et al., 2008).
Emerging evidence suggests that context memory accuracy improves for both young and older adults when their attention is directed toward task-relevant associations during encoding (Dulas and Duarte, 2013, 2014; Glisky and Kong, 2008; Glisky et al., 2001; Hashtroudi et al., 1994; Naveh-Benjamin et al., 2007). For example, when participants are directed to attend to the item-context associations during encoding (i.e., “Does this chair (item) suit the room (context)?”), context memory improves for both age groups, relative to attending to item-only features (i.e., “How comfortable is this chair likely to be?”) (Glisky et al., 2001). While the mechanisms supporting this benefit are not entirely clear, it is likely that focusing attention on a specific relationship between an item and its context allows for the formation of a stronger association. Because the item and context are tightly bound in memory, they are easier to recover during a memory test. Consequently, demands on cognitive control operations, which are engaged when sought after contextual details are difficult to recover, should be reduced (Cohn et al., 2008).
Event-related potentials (ERPs) are useful for investigating the time-course of neural activity associated with processes that aid in the recovery of contextual details. During retrieval, previously studied items correctly recognized as old (i.e., hits) typically show more positive-going activity than new items correctly identified as new (i.e., correct rejections). Several “old-new” effects have been linked with different aspects of memory retrieval. An early (~300–500 ms post-stimulus) effect, the “FN400,” or “mid-frontal” old-new is maximal over frontal regions and is thought to reflect familiarity-based processes (for reviews Curran, 2000; Friedman and Johnson, 2000; Rugg and Curran, 2007). A later occurring (~500–800 ms post-stimulus) “parietal old-new effect” is maximal over left parietal electrodes, greater for correct than incorrect context judgments, and thought to reflect recollection-based processing (Friedman and Johnson, 2000; Rugg and Curran, 2007 for reviews). A late onsetting (~1000 ms post-stimulus) “late-frontal old-new effect” is often right lateralized, maximal over frontal channels, and sustained for several hundred milliseconds or until the end of the trial (Cruse and Wilding, 2009; Friedman and Johnson, 2000; Senkfor and Van Petten, 1998; Wilding and Rugg, 1996). This effect is particularly evident in tasks, like context memory tasks, in which participants must evaluate retrieved information in order to make a specific memory decision. The effect is larger when judgments of memory confidence are low and when memory details are difficult to recover (Cruse and Wilding, 2009; Senkfor and Van Petten, 1998). Given its onset after ERPs of item familiarity and recognition, the late-frontal old-new effect has been associated with post-retrieval monitoring (Swick, Senkfor, and Van Petten, 2006). Finally, a late posterior-maximal negativity (new > old) “LPN” effect has additionally been observed in some context retrieval studies (see Johansson and Mecklinger, 2003 for review). The LPN is suggested to reflect processes that act to reconstruct the original episode associated with recognized items. These processes are engaged when contextual attributes are not readily recovered or require continued evaluation until or even after response.
Several studies have investigated the effects of aging on old-new effects during context retrieval with the most common observation being later onsetting and/or smaller magnitude effects in the old (Duarte et al., 2006; Dulas and Duarte, 2011; Mark and Rugg, 1998; Trott et al., 1997; Wang et al., 2012a; Wegesin et al., 2002). Interestingly, some evidence shows that even when FN400 and parietal old-new effects are relatively intact, late frontal old-new effects are reduced in older adults (Gutchess et al., 2007; Wegesin et al., 2002). This suggests that cognitive control operations such as post-retrieval monitoring may be impaired even when recollection and familiarity processes are intact. In these studies, however, no means were taken to control large group differences in performance. Consequently, the neural activity differences between age groups may have been due, at least in part, to differences in performance rather than aging, per se (reviewed in Rugg and Morcom, 2005).
Recent findings from our lab (Dulas and Duarte, 2013) and others’ (Kuo and Van Petten, 2006) have shown that context memory accuracy is enhanced and frontal old-new ERP effects are reduced when participants are explicitly directed to attend to item-context relationships during encoding. In our study, we directed young and older adults to attend to either objects only or to object-color (context) relations during encoding and measured late right frontal old-new ERPs during retrieval. Importantly, we attempted to match overall memory performance between groups by halving the memory load for older adults. We found context memory improvements and reduced right late frontal old-new effects following directed attention for both age groups, albeit with a smaller benefit in the old. In a parallel fMRI study, we identified a similar pattern of attenuation in right lateral PFC for both age groups (Dulas and Duarte, 2014). From these studies we concluded that when attention is directed toward task relevant features during encoding, context memory improves in both young and older adults. Furthermore, older adults can engage in right PFC mediated post-retrieval monitoring like young adults when performance levels are roughly similar and object - context associations are difficult to recover. Interestingly, only older adults showed a large LPN in our ERP study (Dulas and Duarte, 2013). Given the hypothesized relationship between the LPN and sensory search or episodic reconstruction processes (Cycowicz et al., 2001; Johansson and Mecklinger, 2003), we reasoned that older adults additionally engaged in these operations to support context memory performance.
Directing attention toward single item-context associations compared to non-contextual feature at encoding improves context memory performance and reduces demands on strategic retrieval operations in young and older adults (Dulas and Duarte, 2013). In everyday situations, however, we likely have multiple event features competing for our attention and our ability to successfully recover some features may vary depending on where we focused our attention during encoding. Older adults are prone to failures of selective attention originating from reduced inhibitory control (Hasher and Zacks, 1988). These failures can lead to increased binding of task-irrelevant distractors. For example, findings from paired associate learning tasks show that older adults have greater memory for picture-word pairs that are re-presented despite the words having been previously presented as task irrelevant distractors (Campbell et al., 2010). This ‘hyper-binding’ effect in which older adults are more likely to bind together irrelevant distractors and targets presented in close spatial or temporal proximity has implications for context memory tasks. In a context memory task, optimal performance is likely dependent on the ability to limit attention toward the relevant item-context relationship while ignoring and consequently not encoding irrelevant event details. Hyper-binding can adversely affect performance in traditional tests of associative memory (Campbell et al., 2010).
It is not currently known how selectively attending to one contextual feature while attempting to ignore another influences context memory performance and the processes that support successful retrieval in the young and old. The current study seeks to address this issue. During study, participants were presented with black and white objects flanked by two contextual features: a color and a scene. They were asked to attend to one of the object - context relationships (attended context) while ignoring the other (unattended context). During test, they were asked to judge each object as old or new and to determine if the color and scene contexts matched those with which the object was presented during encoding. Participants were asked to judge the confidence with which they recognized each feature. Importantly, the memory load was halved for older adults in order to more closely match performance between age groups (Rugg and Morcom, 2005).
If subjects successfully restrict their attention to the target object-context relationship, then memory accuracy and confidence for the attended context will be higher than for the unattended context. Prior research documents that the parietal old-new effect varies in magnitude with the number of episodic details retrieved (Vilberg et al., 2006). If participants successfully encode both attended and unattended contexts, the magnitude of the parietal old-new effect should be larger for those “both correct” trials. In contrast, if unattended context accuracy is very low, and participants are effectively guessing, we should not find differences in parietal old-new effect magnitude between the Attended only context correct and Both contexts correct trials. If older adults bind too many event details due to a limited ability to suppress distraction, they may show reduced selectivity in their context memory performance manifesting in greater co-dependence in accuracy for attended and unattended contexts compared to young adults. The consequence of this ‘hyper’ encoding for older adults may be reduced recollection and increased post-retrieval monitoring (right frontal) and episodic reconstruction (LPN) processes that are needed when sought after associations are not readily retrieved.
2. Materials and methods
2.1. Participants
Participants were 22 young adults, ages 18–35 and 21 older adults, ages 60–80, recruited from the Georgia Institute of Technology and the Atlanta community and compensated with $10 per hour or class credit. All participants were right-handed, native English speakers, had normal or corrected to normal vision, with no reports of psychiatric or neurological disorders, vascular disease, use of psychiatric drugs, or any drugs affecting the central nervous system. All participants signed consent forms approved by the Georgia Institute of Technology Institutional Review Board.
2.2. Neuropsychological assessment
After completing the EEG component of the study, participants were administered a battery of standardized neuropsychological tests to rule out cognitive impairments, such as mild cognitive impairment. The tasks consists of subtests from the Memory Assessment Scale (Williams, 1991) including list learning, recognition, immediate and delayed recall, verbal span forward and backwards, visual recognition, recall, reproduction, and delayed recognition. Participants also completed Trails A and B, a subtest of the Halstead-Reitan Neuropsychological Test Battery (Reitan and Wolfson, 1985), and the Controlled Oral Word Association Test (“FAS”) (Benton et al., 1994), older adults were administered the Montreal Cognitive Assessment (Nasreddine et al., 2005) to further test for mild cognitive impairments. Only participants with scores within 2 standard deviations of the group mean were included. One older adult participant was excluded from all analyses for this reason, leaving 21 older adults.
2.3. Materials
The study used a pool of 432 grayscale images obtained from Hemera Technologies Photo-Objects DVDs and from Google. Each image displayed a single, namable object presented on a white background. Two hundred and eighty-eight objects were presented during study and 144 objects were presented as new items during retrieval. Old/new status for objects was counterbalanced across the experiment. Furthermore, the attended contextual feature (color or scene) for each object was counterbalanced across the experiment. Each object was flanked with 1 of 3 possible color squares (red, green, or brown) and 1 of 3 possible scene images (studio, island, or city), acting as the context for the grayscale image. Each object and each context image subtended a maximum horizontal and vertical visual angle of approximately 3°.
2.4. Procedure
The procedure consisted of four blocks of study trials and four blocks of test trials. Young adults completed all four study blocks followed by all four test blocks. The memory load was halved for the older adults such that they studied and were tested on half of the blocks before repeating this sequence for the second half of the blocks (study-study-test-test-study-study-test-test). Participants were given a short practice of both study and test blocks before beginning the experiment. The practice was repeated until understanding of the procedure was demonstrated. EEG data was collected for both the study and test blocks, although only the test/retrieval data is presented here.
The left side of Fig. 1 illustrates the study design including trial timing. Participants were instructed that they would be shown an image of a grayscale object flanked by a color and a scene and would be asked to make a subjective judgment about the relationship between the object and either the color (i.e., “Is this color likely for this object?”) or the scene (i.e., “Is this object likely to appear in this scene?”). Written directions made it clear that participants should orient their attention to one context but not the other and verbal instructions reinforced that participants should attend to one context while ignoring the other. Participants responded by pressing one of two keys on the response pad with the first two fingers of their right hand to indicate their answer of “yes” or “no.” Button mapping was counterbalanced across participants. Each study block was divided into four mini blocks, each of which contained 18 trials. Piloting determined that this blocking procedure, as opposed to a randomized trial procedure, was necessary to ensure suitable levels of performance for both age groups. Before beginning each mini block, participants were prompted with the question “Likely color?” or “Likely scene?” to inform them of which judgment they should make. These prompts were also presented on the screen during each trial, underneath the images. After the completion of one mini block, they were prompted with the other question. Half of the participants began by making object-color judgments, while the other half began by making object-scene judgments. Location of color or scene was blocked such that two study blocks had a one spatial orientation (e.g. color on the left and scene on the right) while the other two study blocks had the opposite orientation (e.g. scene on the left and color on the right).
Fig. 1.
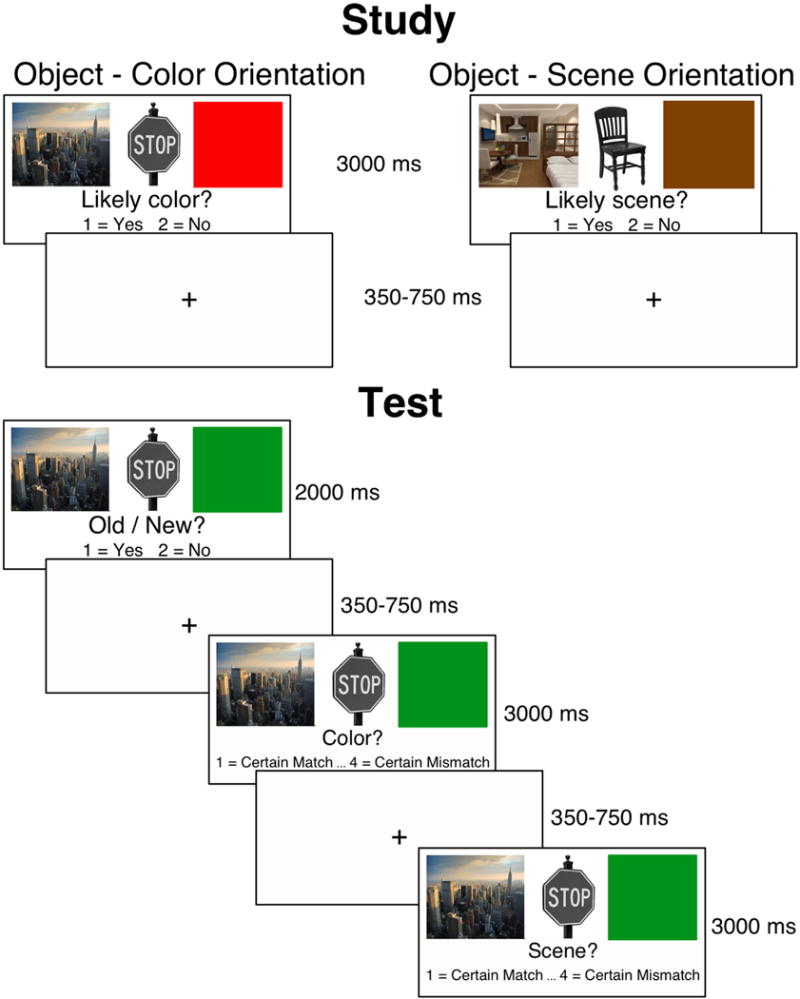
Experimental design.
The structure of test trials including trial timing can be seen in Fig. 1. For all test trials, objects were presented centrally on the computer monitor along with one color and one scene. As with study trials, a color and a scene were presented on each side of the object’s picture. For each old object, the color and scene contexts were located on the same side of the object as they were during study. Participants first decided if objects were old or new by pressing one of two keys on the response pad with the first two fingers of their right hand to indicate “old” or “new.” If they responded that the object was new, the next trial would begin after 2000 ms. If they responded that the object was old, they were asked to make two context judgments - one judgment indicating whether the color that was presented with the test object matched the color that was presented with the object during encoding, and one judgment indicating whether the scene that was presented with the test object matched the scene that was presented with the object during encoding. Context match judgments were made on a scale ranging from 1 (certain match) to 4 (certain mismatch) by pressing 1 of 4 keys on a response box. Half of the participants first judged color context match, while the other half of the participants first judged scene context match. Trials were designed such that for one quarter of the objects, the color and scene matched those presented during encoding, for another quarter of the objects the color matched but the scene did not, one quarter had a matching scene but nonmatching color, and the final quarter had neither matching scene nor color.
2.5. EEG acquisition and analysis
Electrophysiological signals were recorded from 32 Ag-AgCl electrodes using an ActiveTwo amplifier system (Biosemi, Amsterdam, Netherlands). Electrodes were positioned according to the extended 10–20 system (Nuwer et al., 1998). Electrodes were located at left/right hemisphere locations (FP1/FP2, AF3/AF4, F3/F4, F7/F8, FC1/FC2, FC5/FC6, C3/C4, T7/T8, CP1/CP2, CP5/CP6, P3/P4, P7/P8, PO3/PO4, O1/O2) as well as midline sites (Fz, Cz, Pz, Oz). Two electrodes were placed on the left and right mastoids to be used for offline referencing. Vertical electrooculogram (VEOG) and horizontal electrooculogram (HEOG) were monitored by four additional electrodes placed above and below the right eye and on the outer canthus of each eye, respectively. The ActiveTwo system replaces traditional reference and ground electrodes with common mode sense (CMS) and driven right leg (DRL) electrodes, respectively. EEG was acquired with 24-bit resolution at a sampling rate of 512 Hz.
EEGLAB (Delorme and Makeig, 2004) and ERPLAB (Lopez-Calderon and Luck, 2014) were used for all offline data analysis. EEG data were re-referenced to the average of the left and right mastoid electrodes and were digitally band-pass filtered between 0.01 Hz and 40 Hz. The EEG continuous data was epoched into time windows from 200 ms before to 1800 ms after the onset of the first retrieval question (old/new). Each epoch was baselined corrected using the 200 ms prior to object onset. Artifacts were removed in 2 steps. First, epochs containing non-ocular artifacts (e.g. large drift, electrode spikes, saturation) were removed. Second, independent component analysis was used to remove ocular artifacts components from the remaining epochs (Delorme and Makeig, 2004). Epochs containing uncorrected artifacts (+ 150 μV)were removed. Epochs were averaged separately for each participant, electrode, and condition. Lastly, individual waveforms were smoothed with a low-pass filter of 12 Hz before averaging across participants and statistical analysis.
2.6. ERP analysis
EEG was recorded for both the study and test phases of the experiment, analyses focused solely on retrieval trials in order to evaluate our hypotheses regarding context memory accuracy and retrieval. In order to limit the number of comparisons, data were selected from 9 electrode sites (AF3, AF4, Fz, C3, C4, Cz, P3, P4, Pz). Electrodes were chosen where ERP effects of interest were most evident and for consistency with similar previous studies (Cruse and Wilding, 2009; Cycowicz et al., 2001; Dulas and Duarte, 2013; Mark and Rugg, 1998; Trott et al., 1997). ERPs were locked to stimulus onset and were averaged separately for test trials on which participants (1) correctly judged studied items as “old” and correctly judged both contexts (Both correct ERPs hereafter), (2) correctly judged studied items as “old” and correctly judged only the context to which they attended during encoding (Attended only correct ERPs hereafter), and (3) correctly judged new test objects as unstudied (Correct rejections). There were insufficient numbers of trials for other conditions (i.e., false alarms, Unattended only context correct, high vs. low confidence for each context) to form reliable ERPs for all participants. Statistical analyses were performed on mean ERP amplitudes for conditions of interest over latency windows described below. These data were submitted to the following within and between group analyses.
First, in order to establish the reliability of old-new effects across the recording epoch for each age group, 3 Condition [Both correct, Attended only correct, Correct rejection] × 3 Sagittal [Left, Middle, Right] × 3 Coronal [Frontal (AF3/FZ/AF4), Central (C3/CZ/C4), Posterior (P3/PZ/P4)] omnibus ANOVAs were conducted separately for 250–500 ms and 500–800 ms time windows, based on similar previous studies (Cruse and Wilding, 2009; Cycowicz et al., 2001; Dulas and Duarte, 2013; Mark and Rugg, 1998; Trott et al., 1997), and for the 1000–1600 ms window in order to better evaluate the centroposterior maximal LPN effect. Second, between group analyses were conducted with a Sagittal × Coronal × Group ANOVA on the reliable old-new difference scores of each group within each time window. If the ANOVA revealed significant effects involving Sagittal or Coronal factors, the ANOVA was run again with vector-length rescaled difference scores (McCarthy and Wood, 1985). The vector-length rescaling method removes the overall amplitude differences between electrodes while preserving topographical differences. Significant effects involving Sagittal or Coronal factors between groups are indicative of differences in the underlying neural generators.
For all analyses, significant effects at an alpha level of 0.05 were followed up with subsidiary ANOVAs to determine the source of the effects. P-values reflect Huynh-Feldt corrections, where appropriate. The behavioral averages and the ERP averages are based on the same data (i.e., the data for the 22 young adults and 21 older adults were used in all statistical tests).
3. Results
3.1. Neuropsychological assessment results
Group characteristics and results for neuropsychological tests are shown in Table 1. Older adults exhibited significantly poorer performance as compared to the young on several tasks, including Trails A and B, Visual Recognition, Visual Reproduction, and Delayed Visual Recognition [t(41)’s > 2.16, p’s < 0.04, d’s > 0.72]. There were no other significant group differences [t(41)’s < 1.48, p’s > 0.15, d’s < 0.49].
Table 1.
Group characteristics.
| Measure | Young (n=22) | Old (n=21) |
|---|---|---|
| Age | 21.33 (19.41, 23.25) | 67.86 (66.06, 69.66) |
| Gender (F/M) | 9/13 | 14/7 |
| Education | 14.21 (13.51, 14.91) | 15.21 (14.22, 16.20) |
| Letter Fluency | 46.39 (40.30, 52.48) | 50.61 (40.93, 60.29) |
| List Recall (Immediate) | 10.28 (9.43, 11.13) | 9.16 (7.74, 10.58) |
| List Recall (Immediate, Cued) | 10.28 (9.56, 11.00) | 10.26 (9.37, 11.16) |
| List Recall (Delayed) | 11.28 (10.64, 11.91) | 10.05 (8.45, 11.66) |
| List Recall (Delayed, Cued) | 11.17 (10.44, 11.90) | 10.79 (9.99, 11.59) |
| List Recognition | 12.00 (12.00, 12.00) | 11.61 (11.31, 11.91) |
| MAS Digit Span Forward | 7.61 (6.95, 8.27) | 7.0 (6.38, 7.62) |
| MAS Digit Span Backward | 5.50 (4.77, 6.23) | 4.78 (4.04, 5.51) |
| Trails A (in seconds) | 23.89 (20.62, 27.16) | 36.48 (25.94, 47.03)** |
| Trails B (in seconds) | 47.45 (41.08, 53.83) | 84.81 (67.30, 102.31)** |
| Visual Recognition | 18.17 (17.26, 19.07) | 16.68 (15.57, 17.80)** |
| Delayed Visual Recognition | 19.11 (18.41, 19.81) | 16.47 (15.20,17.74)** |
| Visual Reproduction | 8.89 (8.33, 9.45) | 5.58 (4.43, 6.73)** |
| MOCA (older adults only) | – | 27.06 (26.02, 28.10) |
Note: The 95% confidence interval for the mean is in parentheses. All test scores reported as raw scores.
Significant group difference (p < 0.05).
3.2. Behavioral results
Table 2 presents the mean proportion of hits, false alarms, and correct context judgments for attended and unattended contexts. Item recognition accuracy was estimated using the Pr measure of discriminability: p(hits)-p(false alarms) (Snodgrass and Corwin, 1988). Pr estimates were 0.67 (SD = 0.15) and 0.61 (SD = 0.15) for young and older adults, respectively. An independent t-test revealed no significant group difference in item recognition [t(41) = 0.78, p = 0.44, d = 0.24]. Given that people judged contexts as matching or mismatching the context that was studied with a picture, chance performance for context accuracy in Table 2 was 0.5. For both age groups, context accuracy was above chance for attended features, [t(21) = 14.19, p < 0.001, d = 3.03] for the young and [t(20) = 8.42, p < 0.001, d = 1.84] for the old. Context accuracy was above chance for unattended features for the young [t(21) = 3.93, p = 0.001, d = 1.72] but not the old [t(20) = 1.72, p = 0.10, d = 0.77]. A Context (Attended, Unattended) × Group (Young, Old) ANOVA revealed main effects of Context [F(1, 41) = 139.25, p < 0.001, ] and Group [F(1, 41) = 25.68, p < 0.001, ] that was modified by an interaction between these factors [F(1, 41) = 15.83, p < 0.001, ]. The main effect of Context reflects the fact that participants recognized attended features better than unattended features, suggesting that our manipulation of attention during encoding was effective at enhancing context memory accuracy. The interaction reflects the fact that older adults’ context memory accuracy was particularly impaired for attended contextual features. Young adults were better able to correctly identify attended contextual features than older adults [t(41) = 5.18, p < 0.001, d = 1.62], but the two groups did not differ in ability to correctly identify unattended features [t(41) = 0.74, p = 0.463, d = 0.23].
Table 2.
Mean proportion of hits, false alarms to unstudied items, and correct context judgments for attended and unattended context.
| Hits | False alarms | Attended context accuracy | Unattended context accuracy | |
|---|---|---|---|---|
| Young | 0.73 (0.67, 0.80) |
0.06 (0.04, 0.08) |
0.74 (0.71, 0.78) |
0.53 (0.51, 0.54) |
| Old | 0.70 (0.64, 0.76) |
0.10 (0.07, 0.12) |
0.62 (0.59, 0.65) |
0.52 (0.50, 0.54) |
Note: Proportion correct context accuracy represents the percentage of trials on which participants both judged a studied item old (hits) and judged a context (attended or unattended) accurately. The 95% confidence interval for the mean is in parentheses. All values have been rounded to the nearest hundredth
Fig. 2 presents the mean proportions of hits for which participants correctly judged both contexts (Both correct trials), only the attended context (Attended only correct), only the unattended context (Unattended only correct), or only the item (i.e., neither context was correctly judged; Neither correct).2 Consistent with the analyses presented above, young adults performed significantly better than older adults, as can be seen in their higher proportion of Both correct and Attended only correct trials [t’s > 3.844, p’s < 0.001, d’s > 1.24].
Fig. 2.
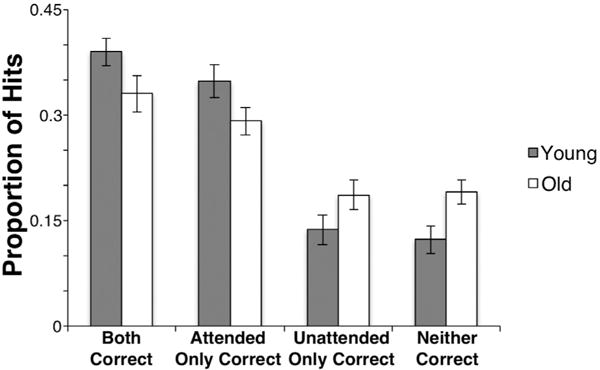
Proportions of item hits associated with correct and incorrect judgments for attended and unattended context features. Error bars represent the 95% confidence interval for the mean.
The reduced proportion of Attended only correct trials in older adults compared to young adults is potentially consistent with reduced selective attention during encoding in the old. In order to determine whether older adults show evidence of ‘hyper-binding’ of attended and unattended contextual features, we calculated the conditional probabilities of correct judgments for attended and unattended contextual features using the proportions in Fig. 2. Specifically, the probability of correctly endorsing the attended context given that the unattended context was correct was calculated as: p(Both correct)/[p(Both correct)+p(Unattended only correct)]. The probability of correctly endorsing the attended context, given that the unattended context was incorrect, was calculated as: p(Attended only correct)/[p(Attended only correct) + p(Neither correct)]. Similarly, the probability of correctly endorsing the unattended context given that the attended context was correct was calculated as: p(Both correct)/[p(Both correct) + p (Attended only correct)]. The probability of correctly endorsing the unattended context given that the attended context was incorrect was calculated as: p(Unattended only correct)/[p(Unattended only correct) + p(Neither correct)]. Similar formulas have been used to assess conditional context accuracy previously (Uncapher et al., 2006). These conditional context accuracy probabilities are shown in Table 3.
Table 3.
Correct context probabilities for attended and unattended context features conditionalized on context accuracy for the other context feature.
| Attended correct if unattended correct | Unattended correct if attended correct | Attended correct if unattended incorrect | Unattended correct if attended incorrect | |
|---|---|---|---|---|
| Young | 0.74 (0.70, 0.78) |
0.53 (0.51, 0.55) |
0.74 (0.70, 0.78) |
0.53 (0.49, 0.56) |
| Old | 0.64 (0.60, 0.68) |
0.53 (0.50, 0.56) |
0.60 (0.57, 0.64) |
0.49 (0.46, 0.52) |
Note: The 95% confidence interval for the mean is in parentheses. All values have been rounded to the nearest hundredth.
If older adults are more likely than young adults to show evidence of hyper-binding because of reduced selective attention, they should show greater conditional dependence between attended and unattended context accuracy. To examine this possibility, we conducted a Context (Attended, Unattended) × Accuracy of the other feature (Correct, Incorrect) × Group (Young, Old) ANOVA. This revealed a main effect of Context [F(1, 41) = 133.71, p < 0.001, ], a main effect of Group [F(1, 41) = 28.93, p < 0.001, ], a Context × Group interaction [F(1, 41) = 13.36, p = 0.001, ], and a marginal Accuracy × Group interaction [F(1, 41) = 2.73, p = 0.11, ]. Subsidiary ANOVAs for each age group revealed a main effect of Context for the young [F(1, 21) = 108.60, p < 0.001, ], but no other significant effects [F(1, 21)’s < 0.049, p’s > 0.83, ]. Older adults showed a main effect of Context [F(1, 20) = 33.81, p < 0.001, ] as well as a main effect of Accuracy [F(1, 20) = 4.84, p = 0.04, ]. These results suggest that for older adults only, context accuracy was more likely for one feature (attended or unattended) if accuracy for the other feature was correct as opposed to incorrect.
The mean proportions of high and low confidence judgments for attended and unattended contexts for Both correct, Attended only correct, and Neither correct conditions can be seen in Table 4. First, both age groups were more likely to judge attended and unattended contextual features with low confidence when neither context was correct (item only hits) than for either of the correct context conditions, [t(21)’s > 7.29, p’s < 0.001, d’s = > 3.18] for the young and [t(20)’s > 1.75, p’s < 0.095, d’s = > 0.78] for the old. We conducted a Condition (Both correct, Attended only correct) × Confidence (Attended high/Unattended high, Attended high/Unattended low, Attended low/Unattended high, Attended low/Unattended low) × Group (Young, Old) ANOVA. The analysis revealed a main effect of Confidence [F(3, 123) = 14.28, p < 0.001, ] and an interaction between Confidence and Group [F (3,123) = 9.42, p < 0.001, ]. As can be seen in the table, the lack of Condition effect reflects the fact that there were no differences in confidence ratings between Both correct and Attended only correct conditions. Young participants were more likely to judge attended features with high confidence and unattended features with low confidence than any of the other confidence combinations [t(21)’s > 2.27, p’s < 0.034, d’s > 0.99]. By contrast, for the majority of trials, older adults judged both attended and unattended features with low confidence [t (20)’s > 2.38, p’s < 0.027, d’s > 1.07]. These results suggest that older adults were more likely than the young to judge attended object - context associations with low confidence.
Table 4.
Mean proportion of high or low confidence judgments for attended and unattended contexts as a function of whether participants judged both contexts correct or only the attended context correctly.
| Both correct | Attended only correct | Neither correct | |
|---|---|---|---|
| Young | |||
| Attended High, Unattended High | 0.25 (0.13, 0.37) | 0.26 (0.14, 0.0.39) | 0.16 (0.05, 0.27) |
| Attended High, Unattended Low | 0.49 (0.39, 0.58) | 0.49 (0.39, 0.58) | 0.23 (0.16, 0.30) |
| Attended Low, Unattended Low | 0.05 (0.03, 0.07) | 0.04 (0.03, 0.06) | 0.11 (0.07, 0.15) |
| Attended Low, Unattended Low | 0.21 (0.13, 0.29) | 0.21 (0.14, 0.28) | 0.50 (0.39, 0.61) |
| Old | |||
| Attended High, Unattended High | 0.21 (0.11, 0.31) | 0.19 (0.10, 0.28) | 0.15 (0.07, 0.23) |
| Attended High, Unattended Low | 0.23 (0.16, 0.30) | 0.23 (0.16, 0.31) | 0.18 (0.12, 0.23) |
| Attended Low, Unattended High | 0.11 (0.06, 0.15) | 0.11 (0.06, 0.15) | 0.15 (0.10, 0.20) |
| Attended Low, Unattended Low | 0.45 (0.32, 0.58) | 0.47 (0.33, 0.60) | 0.53 (0.39, 0.66) |
Note: The 95% confidence interval for the mean is in parentheses. All values have been rounded to the nearest hundredth.
Finally, as a parallel to the ERP results, we computed reaction times (RTs) for Both correct, Attended only correct, and Correct rejection trials relative to the onset of the initial old-new response prompt. These RTs (in milliseconds) were 1256.25, 1257.29, and 1157.49, respectively, for young adults and 1417.05, 1426.15, and 1478.49 for older adults. A Condition (Both correct, Attended only correct, and Correct rejection) × Group (Young, Old) ANOVA revealed a main effect of Group [F(1, 41) = 17.01, p < 0.001, ], and a significant Condition × Group interaction [F(2, 82) = 5.819, p = 0.015, ]. Follow-up tests (using a Bonferroni corrected α = 0.0167) revealed that response times were faster for Correct rejections than Both correct and Attended only correct trials for young adults [t(21)’s > 3.25, p’s < 0.004, d’s> 0.98]. Both correct and Attended only correct trial response times did not differ [t(21) = 0.071, p = 0.944, d = 0.02]. No differences were significant for older adults [t(20)’s < 1.12, p’s > 0.28, d’s < 0.50].
3.3. ERP results
ERPs to studied objects associated with correct context judgments for only the attended context or for both the attended and unattended contextual features, along with ERPs for correctly rejected new objects, are shown for young adults in Fig. 3 and older adults in Fig. 4. Both groups showed evidence of old-new effects reported in previous studies, where correct context ERPs elicited more positive-going activity than correct rejection ERPs starting around 250 ms post-stimulus onset. A sustained negative-going effect was particularly evident in older adults beginning around 1000 ms post-stimulus onset. It has been suggested that early old-new effects (i.e., FN400, parietal old-new) may be obscured by sustained effects like the LPN, potentially confounding the interpretation of differences between the young and old for these effects (Dulas and Duarte, 2013; Li et al., 2004). To account for this possible confound, we applied a first-order polynomial detrend temporal filter to remove the sustained components from the ERPs for both young and older adults. Results for the early time windows (250–500 ms, 500–800 ms) are reported for this detrended data, while results for the late time window (1000–1600 ms) are reported for the non-detrended data. Results for the ANOVAs for each time window are shown for young adults in Table 5 and for older adults in Table 6.
Fig. 3.
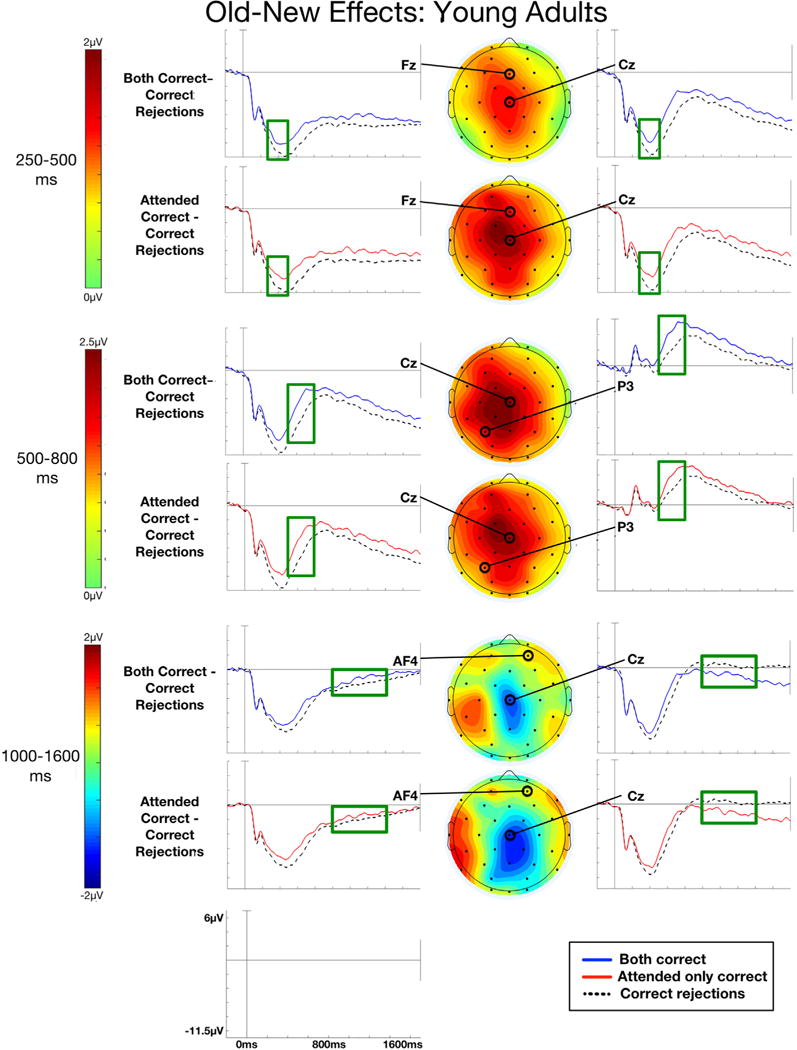
Young participants. Grand average ERPs for objects recognized and associated with correct context judgments for the attended context only (Attended only correct), both attended and unattended contexts (Both correct), and for objects correctly rejected (Correct rejections) as new are shown for exemplar electrodes. Scalp topographies of old-new effects for Both correct and Attended only correct conditions are also shown. Time windows 250–500 ms and 500–800 ms depict polynomial detrended data while 1000–1600 ms depicts non-detrended data. An average number of 125.41 (range: 73–139) Correct rejection trials, 74.95 (range: 34–126) Both correct trials, and 67.59 (range: 25–126) Attended only correct trials are included in the ERP averages depicted in this figure.
Fig. 4.
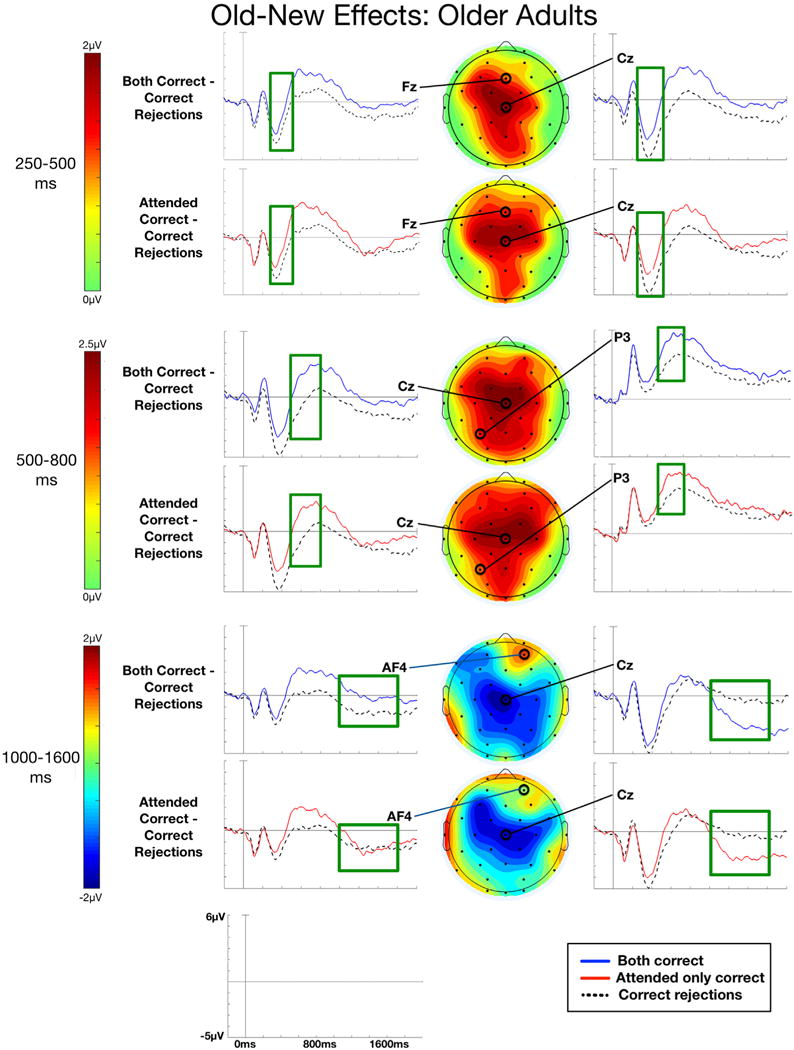
Older participants. Grand average ERPs for objects recognized and associated with correct context judgments for the attended context only, both attended and unattended contexts, and for objects correctly rejected as new are shown for exemplar electrodes. Scalp topographies of old-new effects for Both correct and Attended only correct conditions are also shown. Time windows 250–500 ms and 500–800 ms depict polynomial detrended data while 1000–1600 ms depicts non-detrended data. An average number of 117.57 (range: 69–142) Correct rejection trials, 56.19 (range: 28–100) Both correct trials, and 49.81 (range: 15–78) Attended only correct trials are included in the ERP averages depicted in this figure.
Table 5.
ANOVA results for all ERP time windows for young adults.
| 250–500 ms | 500–800 ms | 1000–1600 ms | |
|---|---|---|---|
| Omnibus | |||
| Cond (2, 42) | 6.87 p = 0.003 η2 = 0.25 |
13.14 p <0.001 η2=0.39 |
– |
| Cond × Sagittal (4.84) | 2.64 p = 0.04 η2 = 0.11 |
3.20 p = 0.02 η2 = 0.13 |
4.07 p = 0.005 η2 = 0.16 |
| Cond × Coronal (4,84) | 3.31 p = 0.02 η2 = 0.14 |
– | – |
| Cond × Sagittal × Coronal (8,168) | – | – | – |
| BC vs. CR | |||
| Cond (1,21) | 9.41 p = 0.006 η2 = 0.31 |
17.13 p < 0.001 η2 = 0.45 |
– |
| Cond × Sagittal (2,42) | – | 4.19 p = 0.02 η2 = 0.17 |
7.43 p = 0.002 η2 = 0.26 |
| Cond × Coronal (2,42) | – | – | – |
| Cond × Sagittal × Coronal (4,84) | – | – | – |
| AC vs. CR | |||
| Cond (1,21) | 8.59 p = 0.008 η2 = 0.29 |
14.80 p = 0.001 η2 = 0.41 |
– |
| Cond × Sagittal (2,42) | 4.45 p = 0.02 η2 = 0.18 |
4.89 p = 0.01 η2 = 0.19 |
4.38 p = 0.02 η2 = 0.17 |
| Cond × Coronal (2,42) | 5.32 p = 0.02 η2 = 0.20 |
– | – |
| Cond × Sagittal × Coronal (4,84) | 2.48 p = 0.05 η2 = 0.11 |
– | – |
| BC vs. AC | |||
| Cond (1,21) | – | – | – |
| Cond × Sagittal (2,42) | – | – | – |
| Cond × Coronal (2,42) | 4.42 p = 0.03 η2 = 0.17 |
– | – |
| Cond × Sagittal × Coronal (4,84) | – | – | – |
Note: CR = Correct rejection; Cond = Condition (Both correct, Attended only correct, Correct rejection); Sagittal = (Left, top, Right); Coronal (Frontal, Central, Posterior). – = No significant effect (α> 0.05). All reported η2 are values. All values have been rounded to the nearest hundredth.
Table 6.
ANOVA results for all ERP time windows for older adults.
| 250–500 ms | 500–800 ms | 1000–1600 ms | |
|---|---|---|---|
| Omnibus | |||
| Cond (2,40) | 5.77 p = 0.006 η2 = 0.22 |
12.57 p < 0.001 η2 = 0.39 |
– |
| Cond × Sagittal (4,80) | 3.20 p = 0.02 η2 = 0.14 |
– | – |
| Cond × Coronal (4,80) | – | – | 4.30 p = 0.01 η2 = 0.18 |
| Cond × Sagittal × Coronal (8,160) | – | – | 2.37 p = 0.03 η2 = 0.11 |
| BC vs. CR | |||
| Cond (1,20) | 7.50 p = 0.01 η2 = 0.27 |
18.14 p < 0.001 η2 = 0.48 |
– |
| Cond × Sagittal (2,40) | 6.85 p = 0.003 η2 = 0.26 |
3.86 p = 0.03 η2 = 0.16 |
– |
| Cond × Coronal (2,40) | – | – | 8.68 p = 0.002 η2 = 0.30 |
| Cond × Sagittal × Coronal (4,80) | – | – | 4.66 p = 0.002 η2 = 0.19 |
| AC vs. CR | |||
| Cond (1,20) | 12.02 p = 0.002 η2 = 0.38 |
15.95 p = 0.001 η2 = 0.44 |
– |
| Cond × Sagittal (2,40) | – | – | – |
| Cond × Coronal (2,40) | 3.71 p = 0.03 η2 = 0.16 |
– | 4.45 p = 0.02 η2 = 0.18 |
| Cond × Sagittal × Coronal (4,80) | – | – | – |
| BC vs. AC | |||
| Cond (1,20) | – | – | – |
| Cond × Sagittal (2,40) | – | – | – |
| Cond × Coronal (2,40) | – | – | – |
| Cond × Sagittal × Coronal (4,80) | – | – | – |
Note: CR = Correct rejection; Cond = Condition (Both correct, Attended only correct, Correct rejection); Sagittal = (Left, top, Right); Coronal (Frontal, Central, Posterior). – = No significant effect (α> 0.05). All reported η2 are values. All values have been rounded to the nearest hundredth.
3.3.1. 250–500 ms
3.3.1.1. Young adults
The ANOVAs for the 250–500 ms time window revealed significant old-new effects for Both correct and Attended only correct trial types, as seen in Fig. 3 and Table 5. Follow-up ANOVAs (using a Bonferroni corrected α = 0.0167) for Attended only correct trials revealed reliable effects of condition at frontal and central electrodes [F(1, 21)’s > 10.10, p’s < 0.005, ] and at left and middle locations [F(1, 21)’s > 7.27, p’s < 0.015, ], but effects at right and posterior electrodes were marginal [F(1, 21)’s o 1.55, p’s > 0.03, ]. Follow-up tests revealed no differences between Both correct and Attended only correct old-new effects at any location [F(1, 21)’s < 1.73, p’s > 0.20, ].
3.3.1.2. Oder adults
Similar to the young, ANOVAs for older adults revealed reliable old-new effects for both conditions, as seen in Table 6 and Fig. 4. Follow-up analyses for Both correct trials indicated that these effects were significant at the corrected α level (0.0167) for middle electrodes [F(l, 20) = 11.48, p = 0.003, ] and marginal at right and left locations [F(1, 20)’s < 6.10, p’s > 0.02, ]. For Attended only correct trials, follow-up ANOVAs revealed a significant old-new effect for central [F(1, 20) = 20.61, p < 0.001, ] and posterior electrodes [F(2, 40) = 5.67, p = 0.007, ], and a marginal effect at frontal electrodes [F(1, 20) = 6.42, p = 0.02, ].
3.3.1.3. Between-group analyses
Because there were no differences between Both correct and Attended only correct conditions for either group, we collapsed across these old-new effects before comparing them between groups. Between-group ANOVAs indicated no differences in old-new effects exhibited by young and older adults in this early time window (all effects involving Group: F’s < 1). As there were no reliable group effects in the raw difference scores, vector-length rescaled analyses were not performed.
3.3.2. 500–800 ms
3.3.2.1. Young adults
The ANOVAs for the 500–800 ms time window revealed robust old-new effects across the scalp in both conditions, as can be seen in Fig. 3 and Table 5. Follow-up analyses for Both correct and Attended only correct trials revealed these effects of condition were reliable (using a corrected α = 0.0167) at left, middle, and right locations [F(1, 21)’s > 11.88, p’s < 0.002, ].
3.3.2.2. Older adults
ANOVAs for this window showed similar robust old-new effects as seen in the young. Follow-up analyses for Both correct trials revealed significant effects at left, middle, and right locations [F(1, 20)’s > 12.34, p’s < 0.002, ].
3.3.2.3. Between-group analyses
Because there were no differences between Both correct and Attended only correct conditions for either group, we collapsed across these old-new effects before comparing them between groups. Between-group ANOVAs indicated no differences in old-new effects exhibited by young and older adults in this time window (all effects involving Group: Fs< 1.20). As there were no reliable group effects in the raw difference scores, vector-length rescaled analyses were not performed.
3.3.3. 1000–1600 ms
3.3.3.1. Young adults
A negativity was observed in the 1000–1600 ms time window across centroposterior midline sites for Both correct and Attended only correct conditions, as can be seen in Fig. 3. The ANOVAs for the 1000–1600 ms time window revealed significant old-new effects for both conditions as seen in Table 5. However, follow-up analyses were not significant [F(1,21)’s < 2.56, p’s > 0.101, ], owing to the fact that the LPN was fairly weak for the young.
3.3.3.2. Older adults
The ANOVAs for this time window revealed significant negativities for Both correct and Attended only correct trial types, as can be seen in Table 6 and Fig. 4. Follow-up tests for Both correct trials revealed significant effects at the corrected alpha level (0.0167) for middle and right electrodes [F(2,40)’s > 7.56, p’s < 0.002, ], but not left electrodes [F(2,40) = 0.46, p = 0.96, ]. Follow-up ANOVAs for Attended only correct trials were marginal at middle electrodes [F(1,20)’s < 3.27, p’s > 0.09, ]. Because the widespread LPN might swamp positive effects, we tested specifically for a right frontal positivity over electrode AF4, which did not quite reach significance in either the Both correct or the Attended only correct condition [t(20)’s < 1.97, p’s > 0.06, d’s < 0.43].
3.3.3.3. Between-group analyses
Because there were no differences between Both correct and Attended only correct conditions for either group, we collapsed across these old-new effects before comparing them between groups. The ANOVAs comparing difference scores in this time window revealed a reliable Sagittal × Group interaction [F(2, 82) = 4.01, p = 0.02, ]. The vector-length rescaled ANOVAs also showed a reliable Sagittal × Group interaction [F(2, 82) = 5.05, p = 0.009, ]. As can be seen in Figs. 3 and 4, while LPN effects were localized to centroposterior midline locations for the young, they were more widespread for older adults.
4. Discussion
In the current study participants selectively attended to a target contextual feature while ignoring a co-occurring distractor feature during encoding. Thus, we investigated the effect of selective attention with competition at encoding on the processes supporting successful context retrieval in the young and old. Both young and older adults were generally successful in selectively attending to the context during encoding, as evidenced by greater memory accuracy and confidence for attended than unattended contextual features. Older adults, compared to young adults, were less confident in their memory decisions for attended features and showed greater conditional dependence in memory accuracy for attended and unattended features (i.e., hyper-binding). While early old-new ERP results were largely consistent between young and older adults, older adults showed a pronounced late posterior negativity (LPN) consistent with enhanced engagement of episodic reconstruction processes. These results and their implications are discussed below.
4.1. Behavioral results
As is commonly observed in aging studies, context memory but not item recognition was diminished in older adults compared to the young. Importantly, our manipulation of halving the memory load for older adults allowed us to examine the interactions between aging, selective attention, and context memory performance and related ERPs without the confounding influence of large group differences in memory performance (Rugg and Morcom, 2005). With regard to the selective attention manipulation, both young and older adults showed much greater context memory accuracy for previously attended than previously unattended contextual features. This suggests that young and older adults alike were able to selectively attend to and encode task relevant contextual features. This finding builds upon previous findings from our lab (Dulas and Duarte, 2013) and others’ (Glisky and Kong, 2008; Glisky et al., 2001; Hashtroudi et al., 1994; Naveh-Benjamin et al., 2007) showing that young and older adults can direct their attention toward relevant event details in a manner that supports subsequent context or associative memory performance. Here we extend these findings to show that participants can successfully focus their attention on these relevant details even in the presence of a contextual distractor.
In our previous study, participants were directed to attend to the color of studied objects or to the relative size of the objects, and subsequent context memory judgments referenced the objects’ prior encoding color (Dulas and Duarte, 2013). Color context accuracy was still well above chance even when participants did not attend to object-color associations during encoding. This differs from the present findings in which context memory accuracy for the unattended context was only barely above chance in each age group. One important difference between the design of this study and our previous one is that in the current study, color and scene contexts were presented as extra-item features, flanking the centrally presented objects. Associative memory accuracy is greater for intra-item features, like the color in which objects are presented, than for extra-item features (Moscovitch, 1992). Intraitem features are believed to be bound into memory automatically even when they are incidental to encoding task demands (Ecker et al., 2013). Thus, the most likely explanation for the relatively poor performance for unattended contextual features in the current study is that presenting them extrinsically to the objects made them less likely to be obligatorily encoded.
Context accuracy performance indicated that young and older adults were able to selectively attend to and encode task-relevant contextual features during encoding. There were also some important group differences in patterns of context memory performance that point to older adults’ limited success in ignoring distracting contextual features. First, young adults remembered the majority of attended object-context relationships with high confidence (75%) and of unattended object-context relationships with low confidence (70%). By contrast, older adults remembered the majority of attended (56%) and unattended (68%) context features with low confidence. Importantly, both groups showed similar levels of low confidence when they failed to recover any contextual details suggesting that older adults were not simply less confident, or conversely young adults very confident, across all conditions. The confidence responses for young adults are consistent with their accuracy data showing very low/chance level accuracy for unattended contextual features. That is, if participants were very successful in selectively attending to and binding targets during encoding, their context decisions about unattended features would be based largely on guesses and confidence is typically low for guess-based judgments (Dunn, 2004).
Why might older adults show primarily low confidence for the attended object-context relationships that they correctly recovered? One possible explanation is that different neurocognitive processes support high and low confidence context memory judgments. Specifically, high confidence responses may be supported by a threshold recollection signal while low confidence judgments are supported by a continuous familiarity signal (see Yonelinas and Parks (2007) for review of process models of source memory). We think this possibility is unlikely for two reasons. First, numerous behavioral studies have shown that correct context memory decisions can be based on signals that vary continuously in memory strength (Qin et al., 2001; Slotnick, 2010; Slotnick and Dodson, 2005). Second, as discussed below, the ERP data do not indicate age-related differences in the degree of familiarity or recollection supporting context memory retrieval. An alternative possibility that we favor is that both young and older adults based attended context memory decisions on recollected context-specifying details but that the quality of this information may have been less robust or complete for the old. For example, participants can sometimes recollect partial contextual information such as the gender of a previously heard speaker but not the specific voice (Dodson et al., 1998). The presence of a contextual distractor during context encoding in the current study may have increased the proportion of correct judgments based on partial contextual details, particularly for older adults. As we did not measure the amount of specific features recollected (e.g. that scene was indoor but not the particular features of the room), future studies will be necessary to determine the kinds of specifying information upon which participants based their context memory judgments.
The second major difference in performance between age groups was the finding that the probability of correctly retrieving one contextual feature, particularly the unattended, was dependent on successfully retrieving the other, attended feature for the older adults only. The conditional dependence in context accuracy for the old is consistent with the ‘hyper-binding’ phenomenon in which older adults form associations between targets and distractors occurring simultaneously (Campbell et al., 2010) or near in time (Campbell et al., 2014). While some evidence suggests dependence in accuracy for co-occurring contextual features in young adults, these studies investigated intrinsic contextual features (color, location, font size), which as we discussed above, are more likely to be bound together automatically than are extrinsic ones (Meiser and Broder, 2002; Uncapher et al., 2006). Numerous studies show that young adults are better at suppressing task-irrelevant distractors than are older adults in various kinds of tasks, including memory (reviewed in Hasher and Zacks, 1988; Healey et al., 2008). Thus, the most likely explanation for the dependence in context accuracy for older adults is that they were less able than the young to suppress the to-be-ignored features during encoding, and consequently formed associations between these distractors and the target contextual features. It should be noted that in previous studies the associations formed between targets and distractors improve associative memory performance in older participants, although they are not able to explicitly recognize these associations (Campbell et al., 2010). In the current study context accuracy for unattended features did not exceed the level of chance in older adults. Thus, these results are consistent with the idea that hyper-binding can affect explicit memory performance even if the associations are only known implicitly.
Collectively, these behavioral results support the inhibitory deficit hypothesis of aging and suggest that inhibitory dysfunction may interfere with selective item-context encoding thereby contributing to age-related contextual memory impairments. The question of whether the current results can better be explained by dysfunction in limiting memory access to the distractors and/or to deleting them from working memory during encoding (Hasher and Zacks, 1988) will require further investigation.
4.2. ERP results
Before discussing the individual ERP effects, it is important to note that there were virtually no differences between ERPs for trials for which both contexts or only the attended context only was correctly retrieved. Taken alone, this may seem somewhat surprising given that some ERP effects like the parietal old-new effect have been shown to vary with the number of recollected event details (Vilberg and Rugg, 2008). However, the lack of difference between these trial types is highly consistent with the behavioral findings that indicate the majority of the trials on which people correctly judged both contexts might have simply reflected accurate guessing of the unattended context.
4.2.1. Early effects: FN400 and parietal old-new
The FN400, which has been tied to familiarity-based recognition (Duarte et al., 2004; Friedman and Johnson, 2000; Rugg and Curran, 2007) or conceptual priming (Voss, Lucas, and Paller, 2009) was equivalent in magnitude for young and older adults. These results stand in contrast to previous findings showing reduced behavioral estimates of familiarity (Davidson and Glisky, 2002; Duarte et al., 2006; Parks, 2007; Wang et al., 2012a) and attenuated FN400 effects (Duarte et al., 2006; Dulas and Duarte, 2013; Dulas et al., 2011; Trott et al., 1999; Wang et al., 2012a; Wolk et al., 2009) in older adults. However, there is also evidence of age-invariance in the FN400 (Ally et al., 2008; Gutchess et al., 2007; Mark and Rugg, 1998; Nessler et al., 2008; Wegesin et al., 2002). The discrepancies between studies cannot be obviously explained by the presence or absence of group differences in memory accuracy or the procedure used to assess recognition (i.e., remember/know, context memory). The most likely explanation for the current results is that older adults were able to make use of the same familiarity signal as young adults during context recognition decisions. Why might this be? As previously discussed, the poor accuracy for unattended compared to attended contextual features indicates that both groups were able to selectively attend to and encode target contextual features during encoding. The context memory decisions for unattended contexts were likely based primarily on weak familiarity signals. Thus, selective attention demands may have increased the degree of familiarity-based recognition for both age groups. It is also possible that the perceptually rich stimuli used here (objects, scenes, and colors) enhanced familiarity signals (see Ally et al., 2008; Wang et al., 2012b for similar explanations). It would be important to directly test these possibilities in future work by varying the nature of the stimuli, and including a direct measure of familiarity.
The parietal old-new effect has typically been associated with recollection in many studies using multiple response methods (remember/know, context memory) (Curran, 2000; Friedman and Johnson, 2000; Rugg and Curran, 2007; Wilding, 2000) and the magnitude of this effect is proportional to the amount of information recollected (Vilberg et al., 2006). If accuracy for unattended contextual features had been greater, it is probable that parietal old-new effects would have been larger for trials for which both contexts were judged correctly compared to those for which only the attended context was retrieved. The lack of magnitude difference between these trial types in either age group is consistent with the behavioral findings showing chance level performance for the unattended context. Thus, the amount of information recollected for these trial types was similar and parietal old-new effects were very likely driven by memory for the attended context.
There was no difference in the magnitude of the parietal old-new effect between young and older adults. Age-related reductions (Ally et al., 2008; Wang et al., 2012a; Wegesin et al. 2002) as well as age-invariance (Duarte et al., 2006; Li et al., 2004; Mark and Rugg, 1998; Trott et al., 1997, 1999) in parietal old-new effects have been observed in previous studies. As discussed for the FN400, the discrepancies do not necessarily seem related to whether group differences in context memory accuracy or recollection estimates exist. The current results suggest that the amount of information successfully recollected was similar for the young and old. This conclusion might seem at odds with the fact that context memory accuracy was reliably lower for older adults. However, this is not surprising if one considers a few factors. First, context judgments are not solely reliant on recollective processing, as discussed earlier (Mollison and Curran, 2012; Quamme et al., 2002; Slotnick and Dodson, 2005). Second, we did not interrogate all the possible event details that participants recollected, some of which may have been ‘non-criterial’ such as thoughts and feelings experienced during encoding (e.g. Mollison and Curran, 2012; Yonelinas and Jacoby, 1996). Thus, young and older adults may have recollected the same number of details but only some of them (i.e., color and scene details) would likely have supported context memory decisions (Duarte et al., 2008, 2006). In support of this view, previous behavioral evidence suggests that young and older adults report recollecting a similar amount but qualitatively different kinds of event details (Leshikar et al., 2014). Finally, lower quality perceptual details may have accompanied recollection for older adults thereby contributing to their reduced accuracy for scene and color context. Recent fMRI evidence showing reduced perceptual reactivation in visual association cortex for older adults (McDonough et al., 2014) as well as our LPN ERP results discussed below offer support for this hypothesis.
4.2.2. Late old-new effects
We had predicted that a consequence of impaired suppression of distractors during encoding would be weaker object – context associations for attended/target features, and demands on post-retrieval monitoring and/or episodic reconstruction operations would be greater for older adults than young adults. Our ERP results were partially consistent with this hypothesis. Both young and older adults demonstrated a robust centroposterior maximal LPN, which was more widespread for older adults. Previous evidence suggests that the LPN reflects processes that serve to reconstruct the original encoding episode through reactivation of context-specifying information (Cycowicz et al., 2001; Friedman et al., 2005; Johansson and Mecklinger, 2003; Mecklinger et al., 2007). Because of the spatial distribution of the LPN, it has been hypothesized to reflect reactivation in visual cortical areas (Cycowicz et al., 2001), which would be consistent with nature of the scene and color contextual features in the current study. However, it is more likely that the LPN reflects processes that attempt to reconstruct the encoding episode by retrieving contextual attributes, which may be but do not necessarily need to be visual (Johansson and Mecklinger, 2003; Mecklinger et al., 2007). Importantly, these reconstruction processes are disproportionately engaged when these attributes are not readily available and also when multiple possible contextual associations can be retrieved (Mecklinger et al., 2007), as in the current study.
The episodic reconstruction hypothesis intimates that the LPN should be initiated prior to the participant’s response. The LPN in the current study was observed over the period of 1000–1600 ms while old-new responses occurred between ~100–500 ms later and color and scene context memory decisions followed old-new responses. These data are consistent with our view that the LPN observed in the current study reflects episodic reconstruction processes that contribute to the memory decision. It is important to note that prior research has decomposed the LPN into temporally and functionally dissociable components (Herron, 2007). While an early LPN occurring between 600 and 1200 ms and prior to response is sensitive to demands on searches for context-specifying information, a late LPN occurring between 1200 and 1900 ms and after response may reflect post-retrieval evaluation of this information. Participants in this previous study made only one response that combined old-new and context memory decisions. As the LPN in the current study temporally overlaps these early and late components, it is possible that the later portion of the LPN may reflect post-retrieval evaluation. However, the fact that contextual response decisions occurred more than a second after the old-new responses could suggest that the late “post-retrieval” LPN may not substantially contribute to the LPN measured here. Unfortunately, preliminary analyses of response-locked ERPs and later portions of the test trials revealed that the data became too noisy to obtain reliable ERPs for both age groups. An interesting question for future studies would be to assess the effects of age on different components of the LPN with experiments designed to disentangle these components, as has been conducted in young adults (Herron, 2007).
Several previous studies have shown stronger LPN effects in older than young adults (Cansino et al., 2012; Dulas and Duarte, 2013; Li et al., 2004). This group difference may suggest that older adults, to a greater extent than the young, rely on episodic reconstruction processes that help support context memory decisions. For example, remembering an object from the original encoding episode may lead participants to visualize the spatial configuration of the object with the flanking color and scene or some finer detail about features within the scene. Assuming that the quality of retrieved attended contextual information was inferior for older adults, their enhanced LPN is consistent with the idea that reconstruction processes are engaged when context-specifying details are not readily retrieved. Some theories of aging suggest that older adults may recruit additional processes relative to the young in a compensatory manner (Reuter-Lorenz and Cappell, 2008). It is possible that the enhanced recruitment of these reconstruction operations may serve a compensatory function for older adults but this is insufficient to raise context memory performance to the level of the young. Future studies incorporating simultaneous ERP and brain stimulation techniques such as transcranial magnetic stimulation (TMS) could be an interesting approach for testing this possibility.
Another late ERP effect that was evident but not particularly robust in the current study was the right frontal old-new effect for older adults. This effect is often observed in context retrieval studies (e.g. Cruse and Wilding, 2009; Senkfor and Van Petten, 1998; Wilding, 1999; Wilding and Rugg, 1997). As it is typically larger when item-context associations are relatively weak (Dulas and Duarte, 2013; Kuo and Van Petten, 2006), it is suggested to reflect post-retrieval monitoring operations that are engaged when sought after information is difficult to recover and one is close to his or her decision criterion (i.e., “Am I certain this is the same scene?”). By this logic, we had predicted that the right frontal old-new effect, like the LPN, would be larger for older adults than the young. Indeed, we found evidence of this effect in the Both correct condition for older adults, as would be expected during the recovery of weaker item-context associations, although it was not reliable at an alpha level of 0.05. Nonetheless, the fact that the right frontal old-new effect was not absent in older adults is consistent with the idea that older adults are able to recruit frontally-mediated control operations to support memory performance when encouraged by the demands of the task (Dulas and Duarte, 2013, 2014; Logan et al., 2002).
4.3. Conclusion
In conclusion, results from the present study show that both young and older adults are generally successful in selectively attending to target contextual features during encoding. Consistent with the inhibition deficit hypothesis of aging, however, older adults are less able to suppress co-occurring distracting contextual features leading to less selective context memory accuracy (i.e., hyper-binding) and poorer memory quality compared to that of the young. ERPs indexing successful context memory retrieval were largely similar for young and older adults. However, suppression deficits in older adults may have led to greater demands on processes that act to reconstruct prior learning episodes when sought after contextual details are not readily retrieved. These results have important implications for the ability of older adults to navigate through real world scenarios in which multiple event features compete for attention, but only a subset is relevant to current memory goals.
Supplementary Material
Acknowledgments
This study was supported by National Science Foundation Grant # 1125683 awarded to Audrey Duarte. We thank all of our research participants.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.neuropsychologia.2016.04.009.
Footnotes
We analyzed these proportions for attend color and attend scene trials separately. These results are presented in Supplementary material. Importantly, the results reveal a similar pattern of response type across conditions suggesting that the results are not driven by greater difficulty ignoring one context or the other. However, context memory performance was somewhat better for attend color than attend scene trials across groups.
References
- Ally BA, Waring JD, Beth EH, McKeever JD, Milberg WP, Budson AE. Aging memory for pictures: using high-density event-related potentials to understand the effect of aging on the picture superiority effect. Neuropsychologia. 2008;46:679–689. doi: 10.1016/j.neuropsychologia.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination 1994 [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-binding: a unique age effect. Psychol Sci. 2010;21:399–405. doi: 10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Trelle A, Hasher L. Hyper-binding across time: age differences in the effect of temporal proximity on paired-associate learning. J Exp Psychol Learn Mem Cognit. 2014;40:293–299. doi: 10.1037/a0034109. [DOI] [PubMed] [Google Scholar]
- Cansino S, Hernandez-Ramos E, Trejo-Morales P. Neural correlates of source memory retrieval in young, middle-aged and elderly adults. Biol Psychol. 2012;90:33–49. doi: 10.1016/j.biopsycho.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychol Aging. 2008;23:93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Cruse D, Wilding EL. Prefrontal cortex contributions to episodic retrieval monitoring and evaluation. Neuropsychologia. 2009;47:2779–2789. doi: 10.1016/j.neuropsychologia.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Mem Cognit. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG. Remembering the color of objects: an ERP investigation of source memory. Cereb Cortex. 2001;11:322–334. doi: 10.1093/cercor/11.4.322. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Holland PW, Shimamura AP. On the recollection of specific- and partial-source information. J Exp Psychol Learn Mem Cognit. 1998;24:1121–1136. doi: 10.1037//0278-7393.24.5.1121. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J Cognit Neurosci. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Brain Res Cogn Brain Res. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage. 2011;57:1192–1204. doi: 10.1016/j.neuroimage.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The influence of directed attention at encoding on source memory retrieval in the young and old: an ERP study. Brain Res. 2013;1500:55–71. doi: 10.1016/j.brainres.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. Aging affects the interaction between attentional control and source memory: an fMRI study. J Cogn Neurosci. 2014;26:2653–2669. doi: 10.1162/jocn_a_00663. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Newsome RN, Duarte A. The effects of aging on ERP correlates of source memory retrieval for self-referential information. Brain Res. 2011;1377:84–100. doi: 10.1016/j.brainres.2010.12.087. [DOI] [PubMed] [Google Scholar]
- Dunn JC. Remember-know: a matter of confidence. Psychol Rev. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- Ecker UK, Maybery M, Zimmer HD. Binding of intrinsic and extrinsic features in working memory. J Exp Psychol: Gen. 2013;142:218–234. doi: 10.1037/a0028732. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Bersick M. The late negative episodic memory effect: the effect of recapitulating study details at test. Brain Res Cogn Brain Res. 2005;23:185–198. doi: 10.1016/j.cogbrainres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc Res Tech. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. J Exp Psychol Learn Mem Cognit. 2008;34:809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: an encoding or retrieval problem? J Exp Psychol: Learn Mem Cognit. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Ieuji Y, Federmeier KD. Event-related potentials reveal age differences in the encoding and recognition of scenes. J Cogn Neurosci. 2007;19:1089–1103. doi: 10.1162/jocn.2007.19.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension, and aging: a review and a new view. In: Bower G, editor. The Psychology of Learning and Motivation. Academic Press; San Diego, CA: 1988. [Google Scholar]
- Hashtroudi S, Johnson MK, Vnek N, Ferguson SA. Aging and the effects of affective and factual focus on source monitoring and recall. Psychol Aging. 1994;9:160–170. doi: 10.1037//0882-7974.9.1.160. [DOI] [PubMed] [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. Prog Brain Res. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- Herron JE. Decomposition of the ERP late posterior negativity: effects of retrieval and response fluency. Psychophysiology. 2007;44:233–244. doi: 10.1111/j.1469-8986.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mecklinger A. The late posterior negativity in ERP studies of episodic memory: action monitoring and retrieval of attribute conjunctions. Biol Psychol. 2003;64:91–117. doi: 10.1016/s0301-0511(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Kuo TY, Van Petten C. Prefrontal engagement during source memory retrieval depends on the prior encoding task. J Cogn Neurosci. 2006;18:1133–1146. doi: 10.1162/jocn.2006.18.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Dulas MR, Duarte A. Self-referencing enhances recollection in both young and older adults. Neuropsychol Dev Cogn B: Aging Neuropsychol Cognit. 2014:1–25. doi: 10.1080/13825585.2014.957150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Morcom AM, Rugg MD. The effects of age on the neural correlates of successful episodic retrieval: an ERP study. Cogn Affect Behav Neurosci. 2004;4:279–293. doi: 10.3758/cabn.4.3.279. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study Brain. 1998;121:861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- McDonough IM, Cervantes SN, Gray SJ, Gallo DA. Memory’s aging echo: age-related decline in neural reactivation of perceptual details during recollection. Neuroimage. 2014;98:346–358. doi: 10.1016/j.neuroimage.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A, Johansson M, Parra M, Hanslmayr S. Source-retrieval requirements influence late ERP and EEG memory effects. Brain Res. 2007;1172:110–123. doi: 10.1016/j.brainres.2007.07.070. [DOI] [PubMed] [Google Scholar]
- Meiser T, Broder A. Memory for multidimensional source information. J Exp Psychol Learn Mem Cognit. 2002;28:116–137. doi: 10.1037/0278-7393.28.1.116. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollison MV, Curran T. Familiarity in source memory. Neuropsychologia. 2012;50:2546–2565. doi: 10.1016/j.neuropsychologia.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J Cogn Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychol Aging. 2007;22:202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Jr, Bersick M, Friedman D. Age-related ERP differences at retrieval persist despite age-invariant performance and left-frontal negativity during encoding. Neurosci Lett. 2008;432:151–156. doi: 10.1016/j.neulet.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guerit JM, Hinrichs H, Ikeda A, Luccas FJ, Rappelsburger P. IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1998;106:259–261. doi: 10.1016/s0013-4694(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Parks CM. The role of noncriterial recollection in estimating recollection and familiarity. J Mem Lang. 2007;57:81–1000. doi: 10.1016/j.jml.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Raye CL, Johnson MK, Mitchell KJ. Source ROCs are (typically) curvilinear: comment on Yonelinas (1999) J Exp Psychol Learn Mem Cognit. 2001;27:1110–1115. [PubMed] [Google Scholar]
- Quamme JR, Frederick C, Kroll NE, Yonelinas AP, Dobbins IG. Recognition memory for source and occurrence: the importance of recollection. Mem Cognit. 2002;30:893–907. doi: 10.3758/bf03195775. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical assessment. Neuropsychological Press; Tucson, AZ: 1985. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Morcom AM. The relationship between brain activity, cognitive performance and aging: the case of memory. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging. Oxford University Press; Oxford: 2005. pp. 132–154. [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. J Exp Psychol Learn Mem Cogn. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. “Remember” source memory ROCs indicate recollection is a continuous process. Memory. 2010;18:27–39. doi: 10.1080/09658210903390061. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Dodson CS. Support for a continuous (single-process) model of recognition memory and source memory. Mem Cogn. 2005;33:151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol. 1988;116:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Swick D, Senkfor AJ, Van Petten C. Source memory retrieval is affected by aging and prefrontal lesions: behavioral and ERP evidence. Brain Res. 2006;1107:161–176. doi: 10.1016/j.brainres.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M. Item and source memory: differential age effects revealed by event-related potentials. Neuroreport. 1997;8:3373–3378. doi: 10.1097/00001756-199710200-00036. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: event-related potentials reveal age- related differences in prefrontal functioning. Psychol Aging. 1999;14:390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. Conceptual priming and familiarity: different expressions of memory during recognition testing with distinct neurophysiological correlates. J Cogn Neurosci. 2009;22:2638–2651. doi: 10.1162/jocn.2009.21341. [DOI] [PubMed] [Google Scholar]
- Wang TH, de Chastelaine M, Minton B, Rugg MD. Effects of age on the neural correlates of familiarity as indexed by ERPs. J Cogn Neurosci. 2012;24:1055–1068. doi: 10.1162/jocn_a_00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, Pare N, Arfanakis K, Saykin AJ. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta. 2012;1822:423–430. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegesin DJ, Friedman D, Varughese N, Stern Y. Age-related changes in source memory retrieval: an ERP replication and extension. Brain Res Cogn Brain Res. 2002;13:323–338. doi: 10.1016/s0926-6410(01)00126-4. [DOI] [PubMed] [Google Scholar]
- Wilding EL. Separating retrieval strategies from retrieval success: an event-related potential study of source memory. Neuropsychologia. 1999;37:441–454. doi: 10.1016/s0028-3932(98)00100-6. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? Int J Psychophysiol. 2000;35:81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of memory for words spoken aloud or heard. Neuropsychologia. 1997;35:1185–1195. doi: 10.1016/s0028-3932(97)00048-1. [DOI] [PubMed] [Google Scholar]
- Williams J. Memory Assessment Scales professional Manual 1991 [Google Scholar]
- Wolk DA, Sen NM, Chong H, Riis JL, McGinnis SM, Holcomb PJ, Daffner KR. ERP correlates of item recognition memory: effects of age and performance. Brain Res. 2009;1250:218–231. doi: 10.1016/j.brainres.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. Noncriterial recollection: familiarity as automatic, irrelevant recollection. Conscious Cognit. 1996;5:131–141. [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


