Abstract
The introduction of antiretroviral therapy (ART) 20 years ago has dramatically reduced morbidity and mortality associated with HIV-1. Initially there was hope that ART would be curative, but it quickly became clear that even though ART was able to restore CD4+ T cell counts and suppress viral loads below levels of detection, discontinuation of treatment resulted in a rapid rebound of infection. This is due to persistence of a small reservoir of latently infected cells with a long half-life, which necessitates life-long ART. Over the past few years, significant progress has been made in defining and characterizing the latent reservoir of HIV-1, and here we review how understanding the latent reservoir during suppressive therapy will lead to significant advances in curative approaches for HIV-1.
Keywords: HIV-1, Latent HIV-1, HIV-1 reservoirs, Long-term ART, Memory CD4+ T cells, Persistent HIV-1
Approaches to Cure HIV-1
To date, the only documented case of a patient cured of HIV-1 is the so-called Berlin Patient who was HIV-1 positive and subsequently diagnosed with acute myeloid leukemia. He then received an allogeneic hematopoietic cell transplantation (HCT) from a CCR5 Δ32/Δ32 donor, which is a genotype resulting in a mutated CCR5 receptor that leaves the CD4+ T cells resistant to most HIV-1 strains. He has now been off treatment for more than eight years and has not experienced viral rebound [1, 2]. Even though the treatment regimen the Berlin Patient underwent is associated with a very high risk of mortality and is not feasible for the general population of HIV-1 infected individuals, this case provides a proof-of-concept for a cure for HIV-1 and continues to drive and inspire the field of HIV-1 cure research. This review will provide an overview of some of the key strategies that are currently being pursued to cure HIV-1. We will then discuss why persistent HIV-1 is a challenge to the scientific community with specific emphasis on the complicated question of which cells and tissues contribute to persistence of HIV-1 during suppressive therapy.
There are a number of different strategies to cure HIV-1 currently being investigated. One of the strategies that has received the most attention is the ‘kick and kill’ approach. The kick comprises the administration of a small molecule to induce viral transcription in latently infected cells. The subsequent kill would theoretically happen after activation of viral production due to the cytopathic effects of the virus or through recognition and destruction of infected cells by the immune system. However, latent viruses contain cytotoxic T lymphocyte (CTL) escape mutations and despite successful reversion of latency the immune system may not be able to clear the viruses [3]. Therefore, in addition to latency reversal, CTLs may need specific stimulation to eliminate infected cells [3, 4]. This is in accordance with several clinical trials testing latency reversing agents that have demonstrated disruption of latency without a significant reduction in the size of the cellular reservoir (see Glossary) [5-7]. These findings have led to new approaches combining latency reversing agents with immune therapy to enhance the killing of latently infected cells.
Another approach that is more directly inspired by the Berlin Patient is utilization of hematopoietic cell transplantation (HCT) with cells that naturally harbor CCR5 Δ32/Δ32 mutations or are genetically modified to this genotype to generate an HIV-1 resistant immune system. Several attempts of HCTs with human cord or adult blood to HIV-1 positive patients who were also suffering from hematological cancer have shown promise initially but later resulted in viral rebound, viral escape, or poor outcomes for the patients due to the nature of the underlying cancer [8-11].
Many studies have shown a clear correlation between early initiation of antiretroviral therapy (ART) and a smaller and less genetically diverse reservoir [12-14]. It has been hypothesized that starting treatment during the acute phase of infection could prevent establishment of a latent reservoir, but several studies have made it evident that the HIV-1 reservoir is seeded very early in the course of infection [15-17]. As an alternative to a sterilizing cure, a treatment that reduces the overall number of latently infected cells could lead to remission. Statistical models predict that a 50-70-fold reduction in the latent reservoir may be sufficient to achieve HIV-1 remission for one year during which patients could discontinue ART without experiencing viral rebound [18]. Due to the cost and unpleasant side effects of many ART regimens, ART-free periods during viral remission may provide significant benefits to patients.
Latent HIV-1 and Measuring the Latent Reservoir
Studies into where persistent HIV-1 resides in the human body are identifying the barriers to curing HIV-1. The latent HIV-1 reservoir has been defined as infected cell populations containing replication-competent HIV-1 in patients on ART. New theories regarding the advantages of latency to HIV-1 survival are discussed in Box 1. The majority of latently infected cells contain a single copy of HIV-1 DNA that is stably integrated into the genome and is transcriptionally silent [19, 20]. The mechanisms that control and maintain latency have been well defined and discussed in great detail in previous reviews [21]. However, a comprehensive understanding of where HIV persists needs to be achieved to develop curative strategies targeted at the cell types and anatomical sites that contain replication-competent HIV (Figure 1, Key Figure). In addition, mapping reservoirs will help guide sample collection when assessing potential cures during clinical trials of eradication strategies.
Box 1. Latency May Cconfer A Transmission Advantage.
Activated CD4+ T cells are the preferred target cells for HIV-1 as they express high amounts of CD4, which is the primary receptor for HIV-1 binding. Most infected cells are eliminated due to the cytotoxic effects of the virus or because of the short life span of activated CD4+ T cells. It was previously thought that latency was established due to a small proportion of activated T cells reverting to a resting memory state in which integrated but transcriptionally silent HIV-1 proviruses can persist. However, recent studies have shown that latency can be established and persist in activated CD4+ T cells as well as resting CD4+ T cells [92]. Furthermore, modelling studies predict enhanced transmission of virus that has an innate propensity to establish latent infection, which confers a transmission advantage by permitting the virus to persist in the mucosal environment; whereas, productively infected cells are eliminated and fail to establish infection [93]. A related study suggests that regulation of tat may be more important for control of whether the virus establishes latent or active infection than the activation state of the infected cell [94]. Both of these models predict that latency is a stochastic event; however, cells in the activated state have a higher tendency toward productive infection rather than latency. Therefore, because the immune system is constantly exposed to antigens that activate memory T cells, it is expected that as cells are activated and start to produce virus they would be cleared and the number of latently infected cells would decline over time. However, the latent pool is relatively stable and does not decline [12]. To reconcile these findings, a recent study by Seu et al. demonstrated that some infected memory T cells are anergic and unresponsive to activation signals and may therefore remain in the resting state despite the presence of activating antigens [95].
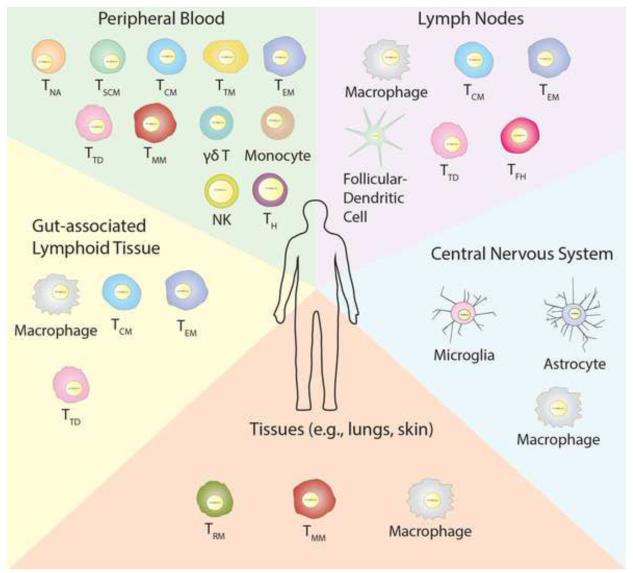
Figure 1. Latent HIV Is a Multifactorial Challenge.
In addition to the CD4+ memory T cell subsets found in the peripheral blood, the lymphoid tissue, gut-associated lymphoid tissue, and the central nervous system are significant anatomical focuses of latent HIV infection. Furthermore, additional tissues such as the lungs and skin may also contain cells harbouring latent HIV. Additionally, unknown potential reservoirs remain to be defined. Each of these tissues contains unique cell types that contribute to the persistence of HIV during effective ART. TH – CD4+ helper T cells, NK – natural killer cells.
The mechanisms that contribute to maintenance of the HIV-1 reservoir during ART have been widely discussed, and several contributing factors have been proposed. While it is well established that longevity of latently infected cells allows for persistence of the reservoir over time [22], the question of whether ongoing replication or cell proliferation is maintaining the HIV-1 reservoir is more controversial. On one hand, studies showing viral evolution during ART [23, 24] and increases in 2-LTR circles following integrase inhibitor intensification [25, 26] have pointed towards a role for ongoing replication. On the other hand, the lack of effect of ART intensification on the reservoir [27-29] and several phylogenetic studies showing lack of viral evolution along with evidence of expanding populations of identical sequences over time during long-term suppressive ART [12, 30-32] suggest that homeostatic and antigen-driven cell proliferation without new rounds of infection are responsible for replenishment of the HIV-1 reservoir (Box 2).
Box 2. T cell Proliferation Maintains the HIV-1 Reservoir.
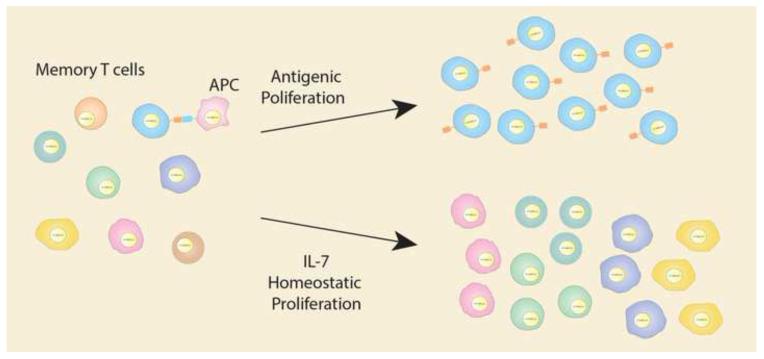
The stability of the CD4+ T memory cell reservoir may be naturally maintained by the immune system via T cell survival through homeostatic and antigenic driven proliferation, providing HIV-1 proviruses in infected cells with the ability to expand in the absence of viral replication [96]. Antigenic driven proliferation occurs when the T cell receptor of a memory T cell is stimulated by a specific antigen that drives clonal expansion. Homeostatic proliferation is induced by interleukin-2 (IL-2) when the levels of circulating lymphocytes declines to detectable levels. HIV-1 expansion through cell proliferation has been demonstrated by several different studies. The first showed that CD4+ memory T cells expressing high levels of PD-1, indicating recent proliferation, contained higher levels of HIV-1 DNA than those that were PD-1 negative. Furthermore, sequencing studies showing clonal expansion of defective viral genomes in memory T cell subsets provide concrete evidence that proliferation of cells can expand HIV-1 proviruses [12, 96]. Lastly, integration studies have also found expansions of HIV-1 proviruses integrated into a single site in CD4+ memory T cells, further indicating that these proviruses were propagated through clonal expansion as insertion of virus into exactly the same location by two different infection events is statistically unlikely [97]. Two patterns of clonal expansion have been identified. Multiple small expansions of HIV-1 integrants are likely due to homeostatic driven proliferation of memory T cell subsets while large single HIV-1 integrant expansions are likely due to antigenic stimulation through a specific T cell receptor (Figure 2) [31].
One of the key barriers to developing a cure for HIV-1 is a lack of a sensitive and accurate measure of replication-competent virus. Several assays have been developed with the gold standard being the viral outgrowth assay (VOA), which quantitates inducible replication-competent virus produced from CD4+ T cells. However, the VOA does not detect all replication-competent virus, is resource intensive, and can be variable [33]. Assays that measure cell-associated HIV DNA and RNA are very sensitive measures of the HIV reservoir [34-36]. However, a substantial proportion of intracellular HIV-1 DNA and RNA in individuals on effective ART is defective [35] resulting in an overestimation of HIV-1 with the potential to rebound. Another approach to measure persistent HIV is the use of immunological biomarkers, which have been shown to correlate with the levels of HIV-1 DNA [37-39]. In addition to the limitation of not being able to accurately quantitate replication-competent virus, these assays have largely been tested in samples from the peripheral blood although tissue reservoirs of latent HIV-1 are also important contributors to persistent HIV-1. Further details regarding these techniques can be found in previous reviews [40].
Until an accurate measure of replication-competent HIV-1 is developed, ART interruption may be the ultimate determination of whether all replication-competent virus has been eliminated. Closely monitored analytical treatment interruptions with strict protocols to resume ART at the first sign of viral rebound are being proposed to test curative strategies. However, in light of the SMART study, careful consideration is warranted when proposing and conducting ART interruption. Further analysis needs to be conducted to determine the effect of well-monitored and controlled ART interruptions on infected individuals and their latent reservoirs.
Memory T Cells are a Well-defined Reservoir of Latent HIV-1
The resting CD4+ T cell was first described as an HIV-1 reservoir 20 years ago and is still regarded as the largest reservoir and the primary barrier to a cure. Since then, HIV-1 DNA has been detected in a number of different CD4+ T cell subsets, and the characteristics and dynamics of these cells have been studied extensively (Figure 1). The exact lineage progression of CD4+ T cell subsets is not fully understood. However, several different subsets can be identified according to expression of cell surface markers. The earliest differentiation stage of memory cells is the naïve CD4+ T cell (TNA). These cells are produced in the bone marrow and then undergo selection in the thymus. When the T cell receptor of a TNA is stimulated by antigen presenting cells, it differentiates into a lineage of short-lived effector cells that include T helper and T regulatory cells. A small subset of these effector cells then differentiate into long-lived memory T cells that are thought to be the primary cells that harbour latent HIV-1. These memory cells can further be categorized into T stem cell memory (TSCM), central memory (TCM), transitional memory (TTM), effector memory (TEM), migratory memory (TMM), tissue resident memory (TRM), and terminally differentiated (TTD) cells [41, 42].
TNA, which are precursors to the memory subsets, are relatively constant over time and have a long half-life [43]. These cells are permissive to HIV-1 infection at very low frequencies. The total proportion of TNA cells relative to the total memory T cell population increases following ART initiation [12, 43-46]. Interestingly, very few expansions of clonal sequences have been detected in naïve cells indicating that cell proliferation only minimally contributes to the expansion of latent infection of these cells over time [31, 43].
The recently identified TSCM are considered the least differentiated stage of a memory T cell [47, 48]. This subset comprises 2-4% of circulating lymphocytes, is long-lived, and has the ability to differentiate into TCM and TEM [47]. TSCM are permissive to HIV-1 infection, and the initial study describing these cells found that the proportion containing cell-associated HIV DNA is higher than in TNA, TCM, TEM, and TTD [49]. TSCM are extremely stable over time and are maintained by homeostatic self-renewal, which although this cell population is relatively small in size, makes it a durable HIV-1 reservoir.
TCM are a significant reservoir for replication-competent HIV-1. These cells are very long-lived and persist through T cell survival and low-level antigen driven proliferation [44, 46]. Recently, a subset of TCM cells that are functionally similar to follicular T helper (Tfh) cells were identified. This subset, called peripheral T follicular helper (pTfh) cells, was shown to be permissive to HIV infection and to make up a substantial proportion of the HIV-infected TCM cells [50]. The TCM subset reservoir, due to its relatively large size, retention of proliferative ability, and long-life span is regarded as one of the most significant HIV-1 reservoirs.
TTM are an intermediate between the effector cells and the TCM and TEM reservoirs. They are a major reservoir for HIV-1 DNA and can expand by homeostatic proliferation (Box 2, Figure 2) [44]. It was recently shown that the HIV-1 DNA in TTM is less frequently replication-competent than in TCM, indicating that there is an excess of defective viruses that are possibly expanded through proliferation [46].
Figure 2. Latent HIV Reservoir Is Maintained Through Cell Proliferation.
CD4+ memory T cells undergo cellular proliferation through two mechanisms that can be detected individually. The first is due to specific antigenic stimulation by antigen presenting cells (APC), which results in a large expansion of a single cell. Second, the overall population of memory T cells is kept at a relatively constant number through homeostatic proliferation. When the total numbers of memory T cells decline, the remaining cells are stimulated to proliferate by cytokines such as interleukin-7 (IL-7). If these cells contain latent HIV, it is also expanded, which increases the size of the latent reservoir. As the latent reservoir is stable over time, it is likely that decline of virus due to cell death is somewhat balanced with proliferation.
TEM are characterized by a more differentiated phenotype, higher rates of proliferation, and a shorter life span than its precursor cells. Clonal sequences are present in several memory subsets but are more apparent in TEM, which is consistent with the higher proliferation rates and the differentiation level of these cells (Figure 2) [31].
Integrated HIV-1 DNA has also been detected in TTD, a subset which contributes in very small numbers and make up less than 0.3% of the CD4+ T cell reservoir [44].
Two types of tissue memory CD4+ T cell subsets have recently been described in human tissues [51, 52]. TMM recirculate into the blood, and TRM reside in and patrol a limited local tissue region [53]. These two tissue memory T cells provide local surveillance and protection against infection and are particularly important for pathogens that establish latent infection and reactivate in a predictable and specific region, such as herpes simplex virus [54]. However, the function of these tissue resident cells, proliferation, and contribution to the persistent HIV-1 reservoir need to be elucidated.
The relative contribution of the memory cell subsets has been the subject of several studies. Three studies have measured HIV-1 DNA in sorted CD4+ T cell subsets from the peripheral blood. Two studies found the highest level of HIV-1 DNA in TEM cells with similar levels in TTM cells [31, 55]. The third study found the highest level of HIV-1 DNA in TCM cells with the next highest levels in TTM and TEM [44]. A later study found that TCM contained the highest level of inducible replication-competent virus followed by TTM and TNA cells [46]. The discrepancies between these studies are mirrored by high levels of variability between patients within each study. Overall, TEM, TCM, and TTM consistently contain higher levels of HIV-1 than TNA. The relative contribution of each subset also varies depending on when the participant began ART and is different when the subsets are sorted from tissues. When the individual HIV-1 proviruses are sequenced from cell subsets, expansions of identical HIV-1 sequences are more commonly found in the TEM than in the other subsets indicating that cellular proliferation maintains the reservoir in TEM cells (Box 2). One study demonstrated that a large clonal expansion making up a significant proportion of the measurable HIV-1 DNA contained a defective provirus, indicating that some clonally expanded cells contain defective HIV-1 [12]. However, a recent study found that although rare, HIV-1 proviruses from clonally expanded cells can contribute to viremia following ART discontinuation [56]. This study is particularly important because it disproves a long-standing assumption in the field that cell-activation and proliferation are sufficient to activate and clear an infected cell. Overall, these studies provide evidence that all CD4+ T cell subsets contribute to the persistent reservoir of HIV-1 to some extent emphasizing the importance of addressing HIV-1 in each subset.
Non-memory T Cells Contribute to Persistence of HIV-1 During ART
In addition to the memory T cell subsets, some effector T cells have been demonstrated to harbour replication-competent HIV-1 in individuals on long-term effective ART. The T helper cells, which are an important part of the adaptive immune system, can also be infected with HIV-1. Furthermore, HIV-1 DNA has been detected in CD4+ T helper cells in HIV-infected individuals on long-term ART [57]. The majority of these cells die within 1-2 weeks. However, a subset of these cells persists for many years.
Hematopoietic precursor cells can be infected with HIV-1 ex vivo and have been implicated as cells that potentially carry latent HIV-1 proviruses in vivo [58, 59]. However, early studies detected only low levels of HIV-1 DNA that were similar to the frequency of contaminating CD4+ cells [60]. Later studies failed to find any HIV-1 sequences in these cells, and therefore, the contribution of hematopoietic precursor cells to the persistence of latent HIV-1 is undetermined [60, 61].
Innate Immune Cells Harbour Latent HIV-1
In addition to the T cells described above, γδ T cells are a relatively small subset of T cells that recognize phosphoantigens commonly found on bacterial pathogens. They are considered a link between innate and adaptive immunity as they are also able to develop a memory phenotype. γδ T cells isolated from HIV-1 infected individuals on long-term suppressive therapy have been shown to contain replication-competent HIV-1 DNA, indicating that they harbour latent HIV-1. The memory subset of γδ T cells was included in this study but not exclusively studied [62]. Due to the longevity of the memory subset, they should be considered in curative approaches for HIV-1.
As HIV infection progresses, a number of individuals develop virus that can use CXCR4 and/or CCR5 as a co-receptor. These viruses can infect cells with low levels of CD4, such as monocytes and macrophages. Monocytes and macrophages have been demonstrated to contain HIV-1 DNA in HIV-1 infected individuals on ART [63-65]. Monocytes originate in the bone marrow and are precursor cells to macrophages and dendritic cells. Macrophages are part of the innate immune system in which they clear infected and dead cells through phagocytosis. It is unclear whether the HIV-1 detected in macrophages is due to receptor-mediated infection, phagocytosis-mediated infection, or to residual DNA from engulfed cells that does not integrate or lead to productive infection [66]. Macrophages are largely resistant to the cytopathic effects of HIV-1 and have demonstrated long life spans in vitro. Therefore, if macrophages are permissive to productive infection following treatment interruption, they may be an important reservoir of HIV-1. A study in non-human primates however suggests a considerably shorter half life for this cell type in vivo [67]. Natural killer cells are cytotoxic lymphocytes that function as part of the innate immune system and they can be infected with HIV-1 ex vivo [68]. Valentin et al. found HIV-1 DNA in natural killer cells isolated from participants on ART and recovered replication-competent virus in an outgrowth assay [69].
Follicular-dendritic cells are not infected with HIV-1. However, these cells retain HIV-1 virions in immune complexes on their cell surface. These virions have the potential to remain infectious for at least nine months [70-77]. The full extent of the infectivity of HIV-1 in the immune complexes of follicular-dendritic cells and whether they contribute to new infections during effective therapy are ongoing areas of study. Memory follicular T helper cells are HIV-1 target cells and contain significant levels of HIV-1 DNA in HIV-1 infected individuals who are not on ART [77]. Due to their close proximity to the follicular-dendritic cells in B cell follicles of the lymph nodes, they may serve as a potential target population for new infection during ART. While the follicular-dendritic cells do not contain latent HIV-1 proviruses, they may serve as an important source of infectious virions during and after ART making them a critical contributor to HIV-1 persistence.
The Peripheral Blood Contains Well-defined Latent HIV-1 Populations
Studies of the HIV-1 reservoir have largely focused on the peripheral blood, as it contains most of the memory T cell subsets discussed above. Cell-associated HIV-1 sequences from the peripheral blood intermingle with sequences isolated from the gut-associated lymphoid tissue (GALT) and lymph nodes indicating that cells containing HIV-1 proviruses traffic between these tissues [78]. As the peripheral blood is the most accessible source of cells containing latent HIV-1, it is the best studied and understood reservoir. Many of the assays to measure biomarkers of latent HIV-1 and approaches to cure HIV-1 currently target the peripheral blood reservoir.
Lymphoid Tissues Harbor Latent HIV
In addition to the memory T cell subsets in the peripheral blood, the GALT also contains memory T cells with persistent HIV-1 in infected individuals on effective long-term therapy. The GALT is the largest lymphoid tissue in the body, and the memory T cell subsets from the peripheral blood and macrophages migrate into the GALT. The GALT contains 2-4 fold higher levels of HIV-1 DNA than cell populations from the peripheral blood [78]. However, when comparing intrapatient memory cell subsets from the peripheral blood and GALT, the amount of HIV recovered from individual cell subsets was less than 1.5 fold different between these two compartments [31]. The HIV-1 DNA integrants in cells from the GALT are stable and do not evolve during effective therapy, suggesting that ART penetration is sufficient to prevent ongoing viral replication [12, 31]. Studies are in progress to determine the extent to which the GALT contributes to rebound following treatment interruption.
The average adult human body contains between 500-700 lymph nodes through which the CD4+ T cell populations discussed above circulate. Consequently, persistent HIV-1 can also be isolated from these tissues. The lymph nodes also contain germinal centers with memory Tfh that have been demonstrated to contain inducible HIV-1. As discussed above, Tfh cells are in close proximity to follicular dendritic cells, which may harbor infectious virus [76, 77]. In further support of these findings, Rothenberger et al. demonstrated that multiple focal points of viral replication can be detected in lymph nodes from participants during a treatment interruption [79]. Tfh cells and HIV-1 in the lymph nodes may present a unique challenge to eradication of HIV-1 because of their anatomical location, which has been demonstrated to achieve lower doses of some drugs than the peripheral blood [80]. Furthermore, access and measurement of the latent HIV-1 population in the lymph nodes is more difficult due to the relative complexity of obtaining lymph node biopsies.
Latent HIV-1 in Tissue Reservoirs
HIV-1 associated neurocognitive disorder is a common complication of HIV-1 infection that is associated with replication of HIV-1 in the central nervous system (CNS). The CNS contains very few CD4+ T cells; however, it contains several different types of macrophages including microglial cells, perivascular macrophages, meningeal macrophages, and macrophages of the choroid plexus, which are the primary infected cell types in the CNS. Furthermore, HIV-1 DNA has been detected in CNS resident macrophages in individuals on long-term suppressive therapy [81]. Schnell et al. demonstrated that viral sequences detected in the CNS were R5 trophic, further supporting a role for macrophages in HIV-1 infection of the CNS [82-84]. HIV-1 in the CNS is well documented indicating that HIV-1 can cross the blood brain barrier. However, in some individuals, the HIV-1 population in the CNS is genetically distinct from that in the blood indicating that the blood brain barrier does not continually permit free exchange of virus between the CNS and peripheral blood in all infected individuals making this compartment a unique challenge for elimination of HIV-1 [83, 85]. Astrocytes are the most common cell type in the CNS, and HIV-1 DNA has been detected in astrocytes [86, 87]. However, because astrocytes have not been demonstrated to produce virus, infected astrocytes may not necessarily need to be cleared for individuals to be cured of HIV-1.
The anatomical compartments discussed above are some of the most well characterized reservoirs of latent HIV-1. However, many of the cell types, including the TMM, TRM, and macrophages, migrate into tissue compartments throughout the body including the skin, adipose tissue, liver, spleen, lungs, and genital tract [51]. Limited studies have demonstrated that HIV-1 DNA is present in some of these tissues [88-90] (http://www.croiconference.org/sessions/liver-macrophages-and-hiv-1-persistence). However, the extent of the contribution of each of these compartments to persistence of latent HIV-1 has not been fully characterized; yet, it is likely that infected immune cells migrate through and sometimes become permanent residents of these tissues. Non-human primate models using simian-human immunodeficiency virus (SHIV) have recovered virus from animals on effective ART from the intestine, lung, spleen, CNS, epithelium, and genital tract further supporting a role for these tissues in persistence of latent HIV-1 [91]. Therefore, the role of these potential anatomical reservoirs needs to be clearly defined to allow development of effective cure strategies.
Concluding Remarks
The latent HIV-1 reservoir is one of the most difficult and significant medical research problems of the modern era. The unique nature of latent HIV-1, which is indistinguishable from the host’s DNA, makes it a particularly challenging scientific problem. Each of the cell types and anatomical reservoirs discussed above should be considered when designing curative approaches as even a single cell containing a replication-competent virus could spark renewed infection. Furthermore, it is likely that undefined cell types and tissues that harbor latent HIV-1 exist and defining and characterizing these reservoirs should be a significant focus of HIV-1 cure research (see Outstanding Questions). New guidelines suggest that patients initiate ART during acute infection, which limits the size and diversity of the HIV reservoir. This could potentially make them more amenable to some approaches to cure HIV-1. Currently several approaches being tested are applying novel scientific advances to find a cure for HIV-1 including pharmacological and gene therapy approaches. The ‘kick and kill’ step is the furthest along in terms of clinical trials and has potential to be transferable to the developing world making it the most promising approach. As the field moves forward, the development of novel ideas to cure HIV focused on delivery in resource limited settings could have the strongest impact on the HIV infection worldwide. One of the most important changes in the HIV-1 field has been the introduction of large collaborations of key leaders, which have driven significant advances in the latent HIV-1 arena over the last few years and provide hope that a cure will be developed in our lifetime.
Outstanding questions.
Do latency-reversing agents penetrate into tissue compartments?
Do follicular-dendritic cell associated virions in the lymph nodes infect cells during and after ART?
Which cell types and tissue compartments contribute to viral rebound following treatment discontinuation?
What is the contribution of non-CD4 T cells to recrudescence following treatment interruption?
How do we best assess the effectiveness of clinical trials investigating curative strategies?
How do we determine when a treatment interruption is warranted?
Trends box.
-Pharmocological and gene therapy are leading approaches to developing a cure for HIV-1.
-Cells infected with latent HIV-1 that persist during antiretroviral therapy are the primary barrier to a cure.
-While latent HIV-1 persists primarily in CD4+ T cells in the peripheral blood, many additional cell types and tissues also contribute to the persistence of HIV.
-The stable latent HIV-1 reservoir is maintained by homeostatic and antigenic-driven proliferation of infected cells.
-New strategies to accurately measure latent HIV and guide clinical decisions need to be developed.
Acknowledgements
This work was primarily supported by the amfAR Research Consortium on HIV Eradication (ARCHE) (107857-48-RGRL), (108074-50-RGRL), The Foundation for AIDS Research (108021-49-RFRL), the Delaney AIDS Research Enterprise (U19 AI096109), and the Australian National Health and Medical Research Council (AAP1061681).
Glossary
- Cellular reservoir
cells that are infected with latent HIV-1
- Latent HIV-1
transcriptionally silent, but replication-competent HIV proviruses that persist during treatment with ART and are capable of resuming replication upon discontinuation of ART
- Tissue reservoir
anatomically defined structures that contain cells that harbour latent HIV-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hutter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 2.Yukl SA, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:9. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng K, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott JH, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen TA, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. The Lancet HIV. 2015;1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 7.Sogaard OS, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutter G. More on shift of HIV tropism in stem-cell transplantation with CCR5 delta32/delta32 mutation. N Engl J Med. 2014;371:2437–2438. doi: 10.1056/NEJMc1412279. [DOI] [PubMed] [Google Scholar]
- 9.Kordelas L, et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med. 2014;371:880–882. doi: 10.1056/NEJMc1405805. [DOI] [PubMed] [Google Scholar]
- 10.Henrich TJ, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Annals of internal medicine. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte RF, et al. CCR5 Delta32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. The lancet. HIV. 2015;2:e236–242. doi: 10.1016/S2352-3018(15)00083-1. [DOI] [PubMed] [Google Scholar]
- 12.Josefsson L, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A. 2013;110:E4987–4996. doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archin NM, et al. Immediate antiviral therapy appears to restrict resting CD4(+) cell HIV-1 infection without accelerating the decay of latent infection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain V, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. The Journal of infectious diseases. 2013;208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzuriaga K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun TW, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, et al. HIV Rebound Kinetics and CD4+ T-Cell Loss after Treatment Interruption: A Pooled Analysis of Six AIDS Clinical Trials Group (ACTG) Studies. Open forum infectious diseases. 2014;1:S23. [Google Scholar]
- 18.Pinkevych M, et al. HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days-Implications for HIV Remission. PLoS Pathog. 2015;11:e1005000. doi: 10.1371/journal.ppat.1005000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josefsson L, et al. Majority of CD4+ T cells from peripheral blood of HIV-1-infected individuals contain only one HIV DNA molecule. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11199–11204. doi: 10.1073/pnas.1107729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josefsson L, et al. Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. PLoS Pathog. 2013;9:e1003432. doi: 10.1371/journal.ppat.1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Archin NM, et al. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nature reviews. Microbiology. 2014;12:750–764. doi: 10.1038/nrmicro3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer S, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiu C, et al. Identification of ongoing human immunodeficiency virus type 1 (HIV-1) replication in residual viremia during recombinant HIV-1 poxvirus immunizations in patients with clinically undetectable viral loads on durable suppressive highly active antiretroviral therapy. Journal of virology. 2009;83:9731–9742. doi: 10.1128/JVI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi B, et al. Evolution and recombination of genes encoding HIV-1 drug resistance and tropism during antiretroviral therapy. Virology. 2010;404:5–20. doi: 10.1016/j.virol.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzon MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 26.Hatano H, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. The Journal of infectious diseases. 2013;208:1436–1442. doi: 10.1093/infdis/jit453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon D, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:912–919. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi RT, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney MF, et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004010. doi: 10.1371/journal.ppat.1004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Stockenstrom S, et al. Longitudinal Genetic Characterization Reveals That Cell Proliferation Maintains a Persistent HIV Type 1 DNA Pool During Effective HIV Therapy. J Infect Dis. 2015;212:596–607. doi: 10.1093/infdis/jiv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner TA, et al. An increasing proportion of monotypic HIV-1 DNA sequences during antiretroviral treatment suggests proliferation of HIV-infected cells. Journal of virology. 2013;87:1770–1778. doi: 10.1128/JVI.01985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan MR, et al. Comparison of standard PCR/cloning to single genome sequencing for analysis of HIV-1 populations. J Virol Methods. 2010;168:114–120. doi: 10.1016/j.jviromet.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eriksson S, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olesen R, et al. Innate Immune Activity Correlates with CD4 T Cell-Associated HIV-1 DNA Decline during Latency-Reversing Treatment with Panobinostat. J Virol. 2015;89:10176–10189. doi: 10.1128/JVI.01484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cockerham LR, et al. CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One. 2014;9:e110731. doi: 10.1371/journal.pone.0110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatano H, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruner KM, et al. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 2015;23:192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Current opinion in immunology. 2013;25:556–563. doi: 10.1016/j.coi.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed R, et al. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 43.Wightman F, et al. Both CD31(+) and CD31(−) naive CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. The Journal of infectious diseases. 2010;202:1738–1748. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- 44.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature medicine. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrowski MA, et al. Both memory and CD45RA+/CD62L+ naive CD4(+) T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soriano-Sarabia N, et al. Quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. Journal of virology. 2014;88:14070–14077. doi: 10.1128/JVI.01900-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cieri N, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 49.Buzon MJ, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pallikkuth S, et al. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir Within Central Memory CD4 T Cells in Peripheral Blood from chronic HIV infected individuals on cART. J Virol. 2015 doi: 10.1128/JVI.02883-02815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark RA, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe R, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7:279ra239. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. Journal of immunology. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 55.Yukl SA, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. The Journal of infectious diseases. 2013;208:1212–1220. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kearney MF, et al. Origin of Rebound Plasma HIV Includes Cells with Identical Proviruses that are Transcriptionally Active Before Stopping Antiretroviral Therapy. J Virol. 2015;90:1369–1376. doi: 10.1128/JVI.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun H, et al. Th1/17 Polarization of CD4 T Cells Supports HIV-1 Persistence during Antiretroviral Therapy. Journal of virology. 2015;89:11284–11293. doi: 10.1128/JVI.01595-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter CC, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nature medicine. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zauli G, et al. A subset of human CD34+ hematopoietic progenitors express low levels of CD4, the high-affinity receptor for human immunodeficiency virus-type 1. Blood. 1994;84:1896–1905. [PubMed] [Google Scholar]
- 60.Josefsson L, et al. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. The Journal of infectious diseases. 2012;206:28–34. doi: 10.1093/infdis/jis301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Durand CM, et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. The Journal of infectious diseases. 2012;205:1014–1018. doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soriano-Sarabia N, et al. Peripheral Vgamma9Vdelta2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015;11:e1005201. doi: 10.1371/journal.ppat.1005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambotte O, et al. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 64.Sonza S, et al. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. Aids. 2001;15:17–22. doi: 10.1097/00002030-200101050-00005. [DOI] [PubMed] [Google Scholar]
- 65.Zalar A, et al. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Res. 2010;87:269–271. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Baxter AE, et al. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe. 2014;16:711–721. doi: 10.1016/j.chom.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Micci L, et al. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014;10:e1004467. doi: 10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernstein HB, et al. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology. 2009;387:59–66. doi: 10.1016/j.virol.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valentin A, et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2002;99:7015–7020. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burton GF, et al. Follicular dendritic cell contributions to HIV pathogenesis. Seminars in immunology. 2002;14:275–284. doi: 10.1016/s1044-5323(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 71.Aguzzi A, et al. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends in immunology. 2014;35:105–113. doi: 10.1016/j.it.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Hlavacek WS, et al. Retention of antigen on follicular dendritic cells and B lymphocytes through complement-mediated multivalent ligand-receptor interactions: theory and application to HIV treatment. Mathematical biosciences. 2002;176:185–202. doi: 10.1016/s0025-5564(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 73.Hlavacek WS, et al. Influence of follicular dendritic cells on HIV dynamics. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2000;355:1051–1058. doi: 10.1098/rstb.2000.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith BA, et al. Persistence of infectious HIV on follicular dendritic cells. Journal of immunology. 2001;166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Perelson AS. Contribution of follicular dendritic cells to persistent HIV viremia. J Virol. 2013;87:7893–7901. doi: 10.1128/JVI.00556-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schacker T, et al. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J Infect Dis. 2001;183:555–562. doi: 10.1086/318524. [DOI] [PubMed] [Google Scholar]
- 77.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. The Journal of experimental medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chun TW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. The Journal of infectious diseases. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 79.Rothenberger MK, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A. 2015;112:E1126–1134. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Mascio M, et al. Antiretroviral tissue kinetics: in vivo imaging using positron emission tomography. Antimicrob Agents Chemother. 2009;53:4086–4095. doi: 10.1128/AAC.00419-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Churchill MJ, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. Journal of neurovirology. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 82.Schnell G, et al. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7:e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schnell G, et al. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–2407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schnell G, et al. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5:e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dahl V, et al. An example of genetically distinct HIV type 1 variants in cerebrospinal fluid and plasma during suppressive therapy. J Infect Dis. 2014;209:1618–1622. doi: 10.1093/infdis/jit805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Churchill MJ, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Annals of neurology. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 87.Nuovo GJ, Alfieri ML. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol Med. 1996;2:358–366. [PMC free article] [PubMed] [Google Scholar]
- 88.Couturier J, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. Aids. 2015;29:667–674. doi: 10.1097/QAD.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Damouche A, et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canaud G, et al. The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol. 2014;25:407–419. doi: 10.1681/ASN.2013050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kline C, et al. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One. 2013;8:e84275. doi: 10.1371/journal.pone.0084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chavez L, et al. HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells. PLoS Pathog. 2015;11:e1004955. doi: 10.1371/journal.ppat.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rouzine IM, et al. An evolutionary role for HIV latency in enhancing viral transmission. Cell. 2015;160:1002–1012. doi: 10.1016/j.cell.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Razooky BS, et al. A hardwired HIV latency program. Cell. 2015;160:990–1001. doi: 10.1016/j.cell.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seu L, et al. Stable Phenotypic Changes of the Host T Cells Are Essential to the Long-Term Stability of Latent HIV-1 Infection. J Virol. 2015;89:6656–6672. doi: 10.1128/JVI.00571-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? The Journal of experimental medicine. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohn LB, et al. HIV-1 integration landscape during latent and active infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]