Abstract
Objective
To assess whether maintenance of labor epidural analgesia using programmed intermittent epidural bolus (PIEB) is associated with reduced local anesthetic (LA) consumption, patient-controlled epidural analgesia (PCEA) use, and rescue analgesia requirements compared to continuous epidural infusion (CEI).
Research Design and Methods
This is a retrospective study at an academic university medical center. Women receiving epidural labor analgesia from March-July of 2015 were identified and categorized into three groups: 1) CEI 5 mL/hr, 2) PIEB 5 mL/60 minutes, 3) PIEB 3 mL/30 minutes. The LA consisted of bupivacaine 0.125 mg/mL and fentanyl 2 µg/mL. All patients had similar PCEA settings. Data were collected on pattern of LA usage, obstetric outcomes and Bromage scores.
Main Outcome Measures
The primary endpoint was total volume of LA consumed/hour. Secondary outcomes included need for clinician boluses, pattern of PCEA use, degree of motor blockade and delivery mode.
Results
We included 528 patients (262 had CEI, 162 had PIEB 5 mL/60 minutes, and 104 had PIEB 3 mL/30 minutes). Median LA consumed was 10.3, 9.5, and 9.7 mL/hr, respectively (p=0.10). There were no differences in PCEA attempts or rescue clinician boluses, but PCEA volume (p=0.03) and ratio of PCEA attempts/given (p<0.01) were significantly different among the groups. Patients receiving PIEB 3 mL/30 minutes used lower PCEA volume than patients receiving CEI (p=0.04). Patients with PIEB 5 mL/60 minutes and PIEB 3 mL/30 minutes had higher ratio of PCEA attempts/given than CEI patients (p=0.01 and p<0.01, respectively). There were no differences in Bromage scores (p=0.14) or delivery mode (p=0.55) among the groups.
Conclusions
The epidural maintenance regimen used (CEI vs. PIEB) was not associated with differences in LA consumption, motor blockade or delivery mode. Main limitations of the study include its single center retrospective design and the fact that patients were not randomized to treatment groups.
Keywords: programmed intermittent epidural bolus, continuous epidural infusion, labor analgesia
Introduction
In contemporary practice, maintenance of epidural analgesia is achieved with a local anesthetic (LA) in combination with an opioid administered via continuous epidural infusion (CEI) and patient-controlled epidural analgesia (PCEA) for breakthrough pain. CEI is associated with greater LA consumption compared to intermittent boluses, which may increase the degree of maternal motor blockade1. This may contribute to increased rates of dystocia and instrumental deliveries due to reduced pelvic muscle tone and a decreased ability to “bear down” during the second stage of labor2. Despite these limitations, CEI is commonly used due to lack of pump technology capable of administering programmed intermittent epidural boluses (PIEB) along with PCEA. Previous studies have demonstrated that, compared with CEI, PIEB reduces LA usage and improves patient satisfaction3, and is associated with a lower incidence of motor blockade and instrumental delivery4. However, many of these studies were conducted with two separate pumps, one to administer CEI or PIEB and another to administer PCEA.
Recently, the CADD®-Solis v3.0 pump system (Smiths Medical, St. Paul, MN) upgraded its software to support the automated administration of LA via PIEB. This pump can be used to administer epidural analgesia via CEI or PIEB with PCEA. The upgraded software has been available at Duke University Medical Center since March of 2015 and is currently used in routine practice on the Labor and Delivery Unit. Since the new pump is able to administer boluses at a higher flow rate and has different interactions between the PIEB interval and the PCEA lockout interval, outcomes may be different than previous studies. For instance, the old software allowed a maximum infusion rate of 175 mL/hr, whereas the new software allows rates of up to 250 mL/hr with standard tubing and 500 mL/hr with special high flow tubing. In addition, when two pumps were used, the lockout period of the PIEB was independent of that of the PCEA, whereas when one pump is used, there is the option to choose either the PCEA or the PIEB lockout periods, as the interval between a PIEB and a PCEA dose. Furthermore, while it has been shown that increasing bolus volumes and time intervals results in decreased LA consumption without affecting patient satisfaction5, optimal PIEB settings have not yet been determined6. We therefore performed this retrospective study to assess whether PIEB is associated with reduced LA consumption, PCEA use, and rescue analgesia requirements compared to CEI in women receiving labor epidural analgesia, and whether PIEB is associated with reduced motor blockade and incidence of instrumental deliveries.
Patients and Methods
After obtaining Duke University Institutional Review Board approval, a retrospective analysis was conducted using the procedures log to identify all women who received labor epidural analgesia with an epidural or combined spinal epidural (CSE) technique between March and July 2015. Patients with asymmetric or unilateral blocks requiring catheter manipulation or replacement, spinal catheterization following inadvertent dural puncture, no documentation of LA volumes or PIEB settings, changes in epidural infusion settings, or patients with PIEB or CEI settings different from standard settings described below were excluded from the study.
Patients were categorized into three groups based on the modality of maintenance labor epidural analgesia: 1) CEI of 5 mL/hr immediately following initiation dose, 2) PIEB of 5 mL every 60 minutes, 3) PIEB of 3 mL every 30 minutes. In the PIEB groups, the first bolus was administered 45 minutes after initiation of labor analgesia. The regimens used were chosen at the discretion of the supervising anesthesia provider. The epidural analgesic solution used at our institution consists of bupivacaine 0.125 mg/mL and fentanyl 2 µg/mL, and all patients were provided with PCEA set to 5 mL boluses with an 8-minute lockout interval and a 1-hour maximum of 35 mL. Analgesia was initiated in all patients with epidural bupivacaine 15–20 mg with fentanyl 50 µg or with a combined spinal epidural (CSE) technique using intrathecal bupivacaine 1.25–2.5 mg with fentanyl 10–15 µg. The PIEB delivery rate was set at 250 mL/hr and the lockout interval between PIEB and PCEA doses was set at the PCEA lockout interval of 8 minutes. Data were collected on patient demographics, obstetric data, mode of delivery, length of epidural usage, pattern of LA usage, and degree of motor blockade as indicated by the lowest documented modified Bromage scale score7.
Statistical Analysis
The primary outcome was the total volume of LA consumed per hour. Secondary outcomes included the volume of clinician boluses required per hour, the proportion of patients requiring clinician boluses, PCEA attempts per hour, PCEA boluses given per hour, unsuccessful PCEA attempts per hour, ratio of attempts/given per hour, the degree of motor blockade as measured by the lowest recorded modified Bromage scale score and mode of delivery. Data about the pattern of LA use was collected from the pump following delivery and documented in the medical record. Duration of labor analgesia was calculated from the time of initiating the pump to the time of delivery or time of transferring the patient to the operating room for cesarean delivery.
All summary data are represented as median and interquartile ranges. Comparisons among the groups were made using the chi-square test and Kruskal-Wallis one-way ANOVA test for nonparametric data. We performed a subgroup analysis of the total LA consumed per hour according to parity (separate analysis for primiparous and multiparous women) and for those who had labor analgesia initiated by a CSE technique. Post-hoc pairwise comparisons among the groups were performed using the Dwass-Steel test. We also performed a multivariable regression analysis with LA consumption per hour as the outcome and parity, epidural maintenance regimen, attending anesthesiologist, body mass index and mode of initiating analgesia (epidural or combined spinal epidural) as predictors. All data were analyzed using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC), and p<0.05 was considered statistically significant. We also performed a post-hoc power analysis using PASS software (PASS 13. NCSS, LLC. Kaysville, Utah, USA).
Results
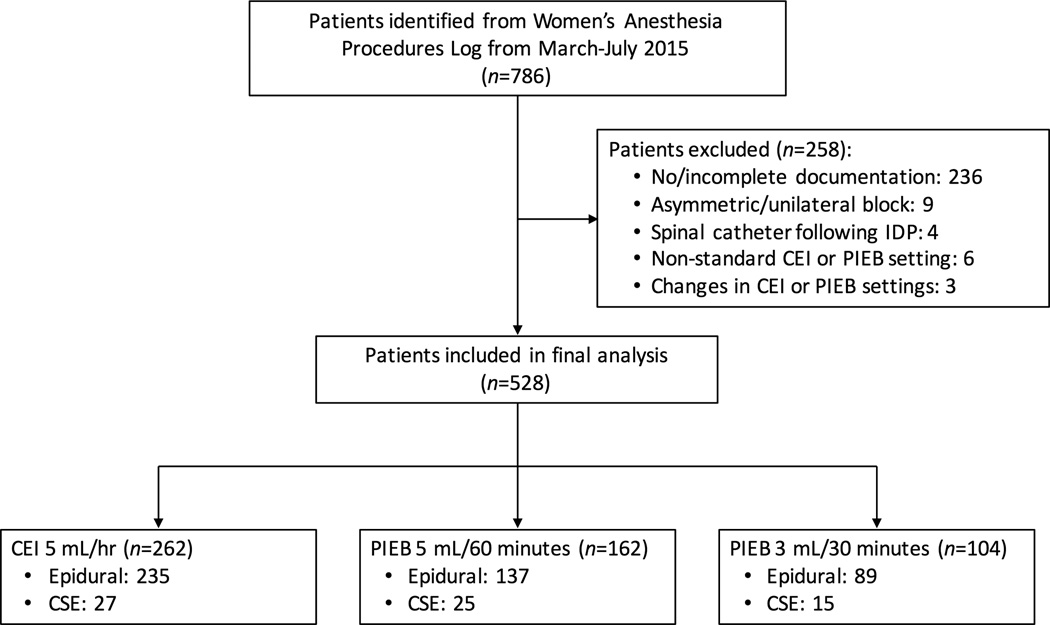
A total of 786 patients were identified who received epidural labor analgesia in the study period. Of those, 258 patients were excluded because of incomplete documentation (n=236), asymmetric or unilateral block requiring catheter manipulation (n=9), spinal catheterization following an inadvertent dural puncture (n=4), use of a CEI or PIEB regimen different than the three aforementioned groups (n=6), or changes in CEI or PIEB settings during the clinical course (n=3) (Figure 1). Thus, a total of 528 were included in the final analysis, with 262 patients receiving 5 mL/hr CEI, 162 patients receiving PIEB of 5 mL every 60 minutes, and 104 patients receiving PIEB of 3 mL every 30 minutes. Patient characteristics, obstetric data, and neuraxial techniques were not different among the groups (Table 1). The choice of the maintenance regimen differed significantly among the nine attending anesthesiologists in our group (p<0.0001) (Table 2).
Figure 1.
Flow diagram of patients included in the study
Table 1.
Patient characteristics and obstetric data
| CEI 5 mL/hr (n=262) |
PIEB 5 mL/60 min (n=162) |
PIEB 3 mL/30 min (n=104) |
p-value | |
|---|---|---|---|---|
| Height (cm) | 162.6 [157.0–167.6] | 162.6 [157.0–167.6] | 162.6 [157.5–167.6] | 0.99 |
| Weight (kg) | 78.0 [67.5–92.6] | 79.7 [67.0–90.6] | 78.6 [68.5–93.7] | 0.90 |
| Gravidity | 2.0 [1.0–4.0] | 2.0 [1.0–4.0] | 2.0 [1.0–3.0] | 0.27 |
| Parity | 1.0 [0.0–1.0] | 1.0 [0.0–2.0] | 1.0 [0.0–1.0] | 0.09 |
| Ethnicity | 0.74 | |||
| White/Caucasian | 97 (37.0%) | 70 (43.2%) | 44(42.3%) | |
| African American | 76 (29.0%) | 51 (31.5%) | 31 (29.8%) | |
| American Indian | 3 (1.2%) | 1 (0.6%) | 0 (0.0%) | |
| Asian/Indian | 19 (7.3%) | 8 (4.9%) | 7 (6.73%) | |
| Hispanic/Other | 67 (25.6%) | 32 (19.8%) | 22 (21.2%) | |
| Neuraxial technique | 0.26 | |||
| Epidural | 235 (89.7%) | 137 (84.6%) | 89 (85.6%) | |
| CSE | 27 (10.3%) | 25 (15.4%) | 15 (14.4%) | |
Data presented as median [interquartile range] or n (% of column). CEI = continuous epidural infusion, PIEB = programmed intermittent epidural bolus, CSE= combined spinal epidural.
Table 2.
Epidural maintenance regimen by attending anesthesiologist
| Attending | CEI 5 mL/hr (n=262) |
PIEB 5 mL/60 min (n=162) |
PIEB 3 mL/30 min (n=104) |
Total (n = 528) | p-value |
|---|---|---|---|---|---|
| 1 | 9 (3.4%) | 32 (19.8%) | 7 (6.73%) | 48 (9.1%) | <0.0001 |
| 2 | 19 (7.3%) | 2 (1.2%) | 1 (1.0%) | 22 (4.2%) | |
| 3 | 36 (13.7%) | 35 (21.6%) | 8 (7.7%) | 79 (15.0%) | |
| 4 | 13 (5.0%) | 14 (8.6%) | 40 (38.5%) | 67 (12.7%) | |
| 5 | 62 (23.7%) | 32 (19.8%) | 23 (22.1%) | 117 (22.2%) | |
| 6 | 15 (5.7%) | 16 (9.9%) | 3 (2.9%) | 34 (6.4%) | |
| 7 | 36 (13.7%) | 18 (11.1%) | 12 (11.5%) | 66 (12.5%) | |
| 8 | 46 (17.6%) | 8 (4.9%) | 6 (5.8%) | 60 (11.4%) | |
| 9 | 26 (9.9%) | 5 (3.1%) | 4 (3.9%) | 35 (6.6%) |
Data presented as n (% of column). CEI = continuous epidural infusion, PIEB = programmed intermittent epidural bolus
Overall, there was no significant difference in patterns of LA use among the three groups. The median total volume of LA consumed for patients receiving 5 mL/hr CEI, PIEB of 5 mL every 60 minutes, and PIEB of 3 mL every 30 minutes was 10.3 mL/hr, 9.5 mL/hr, and 9.7 mL/hr, respectively (p=0.10). In a subgroup analysis according to parity, there was also no difference among the three groups in the total volume of LA consumed among primiparous (10.1 mL/hr, 8.9 mL/hr and 9.4 mL/hr respectively, p=0.15) or multiparous women (10.8 mL/hr, 10.1 mL/hr and 9.8 mL/hr respectively, p=0.29). In subgroup analysis in patients who had a CSE, there was also no difference among the three regimens in median LA consumption per hour (10.8 mL/hr, 11.6 mL/hr and 9.8 ml/hr respectively, p=0.61). Additionally, there was no difference in the volume of clinician boluses required per hour, the proportion of patients requiring clinician boluses, PCEA attempts per hour, or number of PCEA boluses given per hour among all three groups (Table 3). However, there was a significant difference in total PCEA volume per hour (p=0.03), with pairwise comparisons showing that patients receiving PIEB 3 mL/30 minutes used a lower volume than patients receiving CEI (p=0.04). There were no differences in PCEA volume per hour between the other groups. There was also a significant difference among the groups in the ratio of PCEA attempts to PCEA boluses given per hour (p<0.01), with patients receiving PIEB 5 mL/60 minutes and PIEB 3 mL/30 minutes having a higher ratio compared to patients receiving CEI (p=0.01 and p<0.01, respectively). However, there was no difference in the ratio between the two PIEB regimens (p=0.06). There was also a significant difference among the groups in the number of unsuccessful PCEA attempts per hour (p<0.01), with those receiving PIEB 3 mL/30 minutes having more unsuccessful attempts than those receiving CEI (p<0.01), but no difference between the other groups. In the multivariable model, none of the tested variables (parity, attending anesthesiologist, epidural maintenance regimen, body mass index, or mode of initiating labor analgesia) was significantly associated with the volume of LA consumed per hour (Table 4). Lastly, there was no significant difference in the median lowest Bromage scale scores (p=0.14) or delivery mode (p=0.55) among the groups (Table 5).
Table 3.
Patterns of local anesthetic use
| CEI 5 mL/hr (n=262) |
PIEB 5 mL/60 min (n=162) |
PIEB 3 mL/30 min (n=104) |
p-value | |
|---|---|---|---|---|
| Total LA volume (mL/hr) | 10.3 [8.1–13.1] | 9.5 [7.2–12.8] | 9.7 [7.2–13.8] | 0.10 |
| PCEA volume (mL/hr)§ | 4.8 [3.0–7.3] | 4.5 [2.1–6.8] | 4.0 [1.8–6.7] | 0.03 |
| PCEA attempts per hr | 1.1 [0.6–2.0] | 1.3 [0.6–2.4] | 1.2 [0.5–2.8] | 0.66 |
| PCEA boluses given per hr | 1.0 [0.6–1.5] | 0.9 [0.4–1.4] | 0.8 [0.4–1.9] | 0.07 |
| PCEA attempts/given# | 1.0 [1.0–1.5] | 1.2 [1.0–1.9] | 1.4 [1.0–2.2] | <0.0001 |
| PCEA unsuccessful attempts per hr‡ | 0 [0–0.5] | 0.2 [0–0.7] | 0.3 [0–1.2] | 0.003 |
| Patients requiring clinician bolus | 65 (24.8%) | 48 (29.6%) | 31 (29.8%) | 0.45 |
| Clinician bolus volume (mL/hr) | 0.0 [0.0–0.0] | 0.0 [0.0–0.5] | 0.0 [0.0–0.6] | 0.33 |
| Duration of labor analgesia (hr) | 6.7 [3.8–11.8] | 6.5 [3.2–11.0] | 6.5 [3.7–10.5] | 0.47 |
Data presented as median [interquartile range] or n (% of column). CEI = continuous epidural infusion, PIEB = programmed intermittent epidural bolus.
PCEA volume (mL/hr) Dwass-Steel test for pair-wise comparison: CEI vs. PIEB 5 mL/60 minutes (p=0.17), CEI vs. PIEB 3 mL/30 minutes (p=0.04), PIEB 5 mL/60 minutes vs. PIEB 3 mL/30 minutes (p=0.63)
PCEA attempts/given Dwass-Steel test for pair-wise comparison: CEI vs. PIEB 60 (p=0.01), CEI vs. PIEB 30 (p<0.01), PIEB 60 vs. PIEB 30 (p=0.06)
PCEA unsuccessful attempts per hr Dwass-Steel test for pair-wise comparison: CEI vs. PIEB 5 mL/60 minutes (p=0.14), CEI vs. PIEB 3 mL/30 minutes (p=0.003), PIEB 5 mL/60 minutes vs. PIEB 3 mL/30 minutes (p=0.18)
Table 4.
Multivariable model for the outcome of local anesthetic consumption per hour
| β Coefficient | Standard error | p-value | |
|---|---|---|---|
| Intercept | 11.3776 | 1.4096 | <0.0001 |
| Parity | 0.3138 | 0.1996 | 0.11 |
| Epidural regimen: CEI vs. PIEB 5 ml/60 min | −0.2120 | 0.7384 | 0.77 |
| Epidural regimen: CEI vs. PIB 3 ml/ 30 min | −0.7420 | 0.6264 | 0.24 |
| Block type: Epidural vs. CSE | −0.8080 | 0.7798 | 0.30 |
| Body mass index | −0.0128 | 0.0208 | 0.54 |
| Attending anesthesiologist: 1 vs. 9 | 2.0666 | 1.3388 | 0.12 |
| Attending anesthesiologist: 2 vs. 9 | 1.6152 | 1.5820 | 0.31 |
| Attending anesthesiologist: 3 vs. 9 | 0.0078 | 1.1992 | 0.10 |
| Attending anesthesiologist: 4 vs. 9 | −0.0069 | 1.2709 | 0.10 |
| Attending anesthesiologist: 5 vs. 9 | 0.7851 | 1.1278 | 0.49 |
| Attending anesthesiologist: 6 vs. 9 | 1.4520 | 1.4126 | 0.30 |
| Attending anesthesiologist: 7 vs. 9 | 2.2291 | 1.2202 | 0.07 |
| Attending anesthesiologist: 8 vs. 9 | 0.9632 | 1.2367 | 0.44 |
CEI = continuous epidural infusion, PIEB = programmed intermittent epidural bolus, CSE = combined spinal epidural
Table 5.
Secondary outcomes: motor block and delivery mode.
| CEI 5 mL/hr (n=262) |
PIEB 5 mL/60 min (n=162) |
PIEB 3 mL/30 min (n=104) |
p-value | |
|---|---|---|---|---|
| Lowest Bromage scale score* | 4.0 [3.0–5.0] | 5.0 [3.0–5.0] | 4.0 [3.0–5.0] | 0.14 |
| Delivery mode | 0.55 | |||
| Spontaneous vaginal | 212 (80.9%) | 136 (84.0%) | 83 (79.8%) | |
| Assisted vaginal | 18 (6.9%) | 8 (4.9%) | 4 (3.9%) | |
| Cesarean | 32 (12.2%) | 18 (11.1%) | 17 (16.4%) | |
Data presented as median [interquartile range] or n (% of column). CEI = continuous epidural infusion, PIEB = programmed intermittent epidural bolus.
Not all patients had Bromage scale scores documented: CEI (n=176), PIEB 60 (n=116), PIEB 30 (n=63)
A post-hoc power analysis based on 5000 Monte Carlo samples with the same properties as our observed data (sample size and distribution), indicated that a similar study would have 90% power at the 0.05 alpha level in a three group Kruskal-Wallis test to detect a 1.5 mL/hr difference in local anesthetic consumption between the observed median of the CEI group and the two PIEB groups.
Discussion
This study found no difference in total LA consumption, degree of motor block, or delivery mode for patients receiving 5 mL/hr CEI, PIEB 5 mL/60 minutes, and PIEB 3 mL/30 minutes when utilizing the CADD®-Solis v3.0 pump system. However, we did find that patients receiving the PIEB regimen of 3 mL/30 minutes used a lower PCEA volume than patients receiving the CEI regimen, that patients with PIEB 5 mL/60 minutes and PIEB 3 mL/30 minutes had a higher ratio of PCEA attempts/given than patients with CEI, and that patients with PIEB 3 mL/30 minutes had more unsuccessful PCEA attempts her hour than patients with CEI.
The technology for PIEB is relatively new and has received limited investigation over the last decade. Prior to the advent of PIEB, labor epidural analgesia was maintained with intermittent manual boluses, continuous infusion, PCEA alone or PCEA combined with a continuous background infusion. Although a previous study had demonstrated that a demand-only PCEA regimen alone provided satisfactory maintenance analgesia8, there has been no consensus on an optimal analgesic regimen. In 2004, Chua and Sia randomized patients to receive a solution of 0.1% ropivacaine and fentanyl 2 µg/mL via PIEB (5 mL bolus every 60 minutes) or CEI (5 mL/hr infusion rate) and demonstrated that PIEB could improve the quality and duration of labor analgesia compared to CEI9. In subsequent years, studies reported that PIEB decreased the incidence of breakthrough pain, increased patient satisfaction10, and reduced LA consumption with similar pain scores, sensory, and motor block11 when compared to CEI. In these early studies, however, patients were allocated to receive either intermittent epidural boluses or continuous infusion without PCEA for supplementary analgesia.
More recent studies have incorporated the use of PCEA in addition to PIEB and CEI administered using two separate pumps. In 2006, Wong et al. compared the use of PIEB with CEI for labor analgesia in healthy, parous women with singleton pregnancies. Women were randomized in a double-blinded fashion to receive PIEB or CEI with PCEA for maintenance of labor analgesia, with those receiving PIEB having lower bupivacaine usage, similar analgesia, and improved patient satisfaction compared to patients receiving CEI3. Further investigation by Wong et al. demonstrated that increasing the PIEB bolus interval and volume resulted in decreased bupivacaine consumption without impacting pain scores, PCEA requests or administrations, need for rescue boluses, or patient satisfaction5. In another study, nulliparous term women in spontaneous labor who were randomized to receive PIEB of 10 mL every 60 minutes demonstrated a significantly reduced incidence of motor block and instrumental delivery compared to those randomized to receive CEI of 10 mL/hr4. Similar findings of reduced motor block and increased satisfaction have been reported in a prospective randomized controlled trial of women receiving PIEB vs. CEI for pain relief during termination of pregnancy12. A recent systematic review and meta-analysis of nine randomized controlled trials reported that despite a slight decrease in LA consumption and increase in maternal satisfaction scores, there were no differences in cesarean delivery rate, duration of labor, or need for anesthetic intervention between PIEB and CEI13.
Many of these previous studies employed two separate pumps to administer anesthetic boluses or infusions in an investigational set-up that was not used as part of standard clinical practice. This current study differs from most previous studies in that it investigated the use of a single pump to administer both the PIEB or CEI and the PCEA for labor analgesia. Because the pump has different flow rates and settings compared to older pumps, there is good reason to believe that the outcomes may not be identical. Another group has studied a single-pump system to administer automated LA boluses in addition to PCEA boluses, but they focused on the development of a computer software to deliver boluses at varying frequencies based on the patient’s demand in the previous hour using a computer and infusion pump14.
Additionally, previous studies investigating PIEB versus CEI with PCEA used epidural analgesic solutions consisting of half the concentration and double the volume of bupivacaine compared to what was used at our institution3–5. This may have some influence on labor analgesic outcomes, since larger boluses of more dilute LA may have improved spread in the epidural space, resulting in enhanced analgesia of longer duration and increased patient satisfaction15,16. Thus, while our current results differed from what has been shown in previous reports, the conclusions must be interpreted in the context of these potential differences. Future prospective studies with more rigorous controls are necessary.
Interestingly, we found a significant difference among the groups in the ratio of PCEA attempts/given and the number of unsuccessful PCEA attempts per hour. These measurements can be thought of as surrogate indicators of patient discomfort17, and using them can be helpful in developing optimal regimens for PCEA dosing. As patients experience more pain, they are likely to increase PCEA attempts in an effort to achieve pain relief. Because the number of PCEA boluses delivered is restricted by pre-set lockout intervals and a 1-hour maximum limit, patients may end up with more attempts than actual boluses delivered. Thus, a higher ratio and more unsuccessful attempts could be interpreted as more pain or discomfort. Alternatively, the ratio differences that we found in this study could simply be an artifact of the increased frequency in which patients receiving PIEB were locked out from receiving PCEA boluses. The fact that patients receiving the PIEB regimen of 3 mL/30 minutes received reduced PCEA volumes per hour may also be a result of the frequent lockout periods. Furthermore, PCEA attempts per hour were not significantly different among the three groups, suggesting that the higher ratios in the PIEB groups were a result of more frequent lockout intervals. However, without data on patient satisfaction scores, there is no indication of whether analgesia overall was satisfactory despite more unsuccessful PCEA attempts.
This study has limitations that must be acknowledged. Because this was a retrospective analysis, the study data is dependent on accurate and complete documentation in the electronic medical records. For instance, the modified Bromage scale scores were not routinely checked at regular intervals, and some of the data were not recorded. Another limitation is that patients were not randomized to treatment groups, and the choice of the regimen used was at the discretion of the providers, who may have had their inherent biases. The total volume of local anesthetic delivered per hour was higher by 1 mL in the PIEB regimen of 3 mL/30 minutes compared to the other two groups. Additionally, our patient population consisted of both primiparous and multiparous women. However, a subgroup analysis and multivariable model did not suggest that parity affected the outcome of LA consumption. Furthermore, the instructions provided to the patients on how to use the pump were not standardized, which might have impacted the way our parturients used PCEA. Information about patient satisfaction or pain scores would have provided useful information about the level of analgesia, but this data was not available given the retrospective nature of the study. However, our unit is staffed by a dedicated group of obstetric anesthesiologists who provide round the clock coverage, and all patients with labor epidurals are followed up at least once every two hours to assess for the adequacy of analgesia. Our analysis did not suggest a difference in the need for clinician boluses between the groups. Finally, data might not be generalizable to regimens using different LA concentrations.
Conclusion
In conclusion, the different epidural regimens used for labor analgesia in this study were not associated with any differences in LA consumption, degree of motor block, or delivery mode when utilizing the CADD®-Solis v3.0 pump system. While this study must be interpreted in the context of its limitations, it provides insight in designing future prospective studies to optimize PIEB settings and improve patient outcomes.
Acknowledgments
Michael Tien was supported by the Medical Student Anesthesia Research Fellowship offered by the Foundation of Anesthesia Education and Research.
Terrence K. Allen was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001115 TKA. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of funding: This study was supported solely by departmental funds.
Footnotes
Declaration of financial/other relationships: None of the other authors have any financial disclosures or relevant conflicts of interest.
Contributor Information
Michael Tien, Mayo Medical School, Mayo Clinic College of Medicine, Rochester, Minnesota 55902, USA.
Terrence K. Allen, Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina 27710, USA
Amy Mauritz, Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina 27710, USA.
Ashraf S. Habib, Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina 27710, USA
References
- 1.Leighton BL, Halpern SH. The effects of epidural analgesia on labor, maternal, and neonatal outcomes: a systematic review. Am J Obstet Gynecol. 2002;186:S69–S77. doi: 10.1067/mob.2002.121813. [DOI] [PubMed] [Google Scholar]
- 2.Thornton JG, Capogna G. Reducing likelihood of instrumental delivery with epidural anaesthesia. Lancet. 2001;358:2. doi: 10.1016/s0140-6736(00)05295-8. [DOI] [PubMed] [Google Scholar]
- 3.Wong CA, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, McCarthy RJ. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg. 2006;102:904–909. doi: 10.1213/01.ane.0000197778.57615.1a. [DOI] [PubMed] [Google Scholar]
- 4.Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113:826–831. doi: 10.1213/ANE.0b013e31822827b8. [DOI] [PubMed] [Google Scholar]
- 5.Wong CA, McCarthy RJ, Hewlett B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesth Analgesia. 2011;112:904–911. doi: 10.1213/ANE.0b013e31820e7c2f. [DOI] [PubMed] [Google Scholar]
- 6.Sng BL, Kwok SC, Sia AT. Modern neuraxial labour analgesia. Curr Opin Anaesthesiol. 2015;28:285–289. doi: 10.1097/ACO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 7.Breen TW, Shapiro T, Glass B, Foster-Payne D, Oriol NE. Epidural anesthesia for labor in an ambulatory patient. Anesth Analg. 1993;77:919–924. doi: 10.1213/00000539-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Sezer OA, Gunaydin B. Efficacy of patient-controlled epidural analgesia after initiation with epidural or combined spinal-epidural analgesia. Int J Obstet Anesth. 2007;16:226–230. doi: 10.1016/j.ijoa.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Chua SM, Sia AT. Automated intermittent epidural boluses improve analgesia induced by intrathecal fentanyl during labour. Can J Anaesth. 2004;51:581–585. doi: 10.1007/BF03018402. [DOI] [PubMed] [Google Scholar]
- 10.Lim Y, Sia AT, Ocampo C. Automated regular boluses for epidural analgesia: a comparison with continuous infusion. Int J Obstet Anesth. 2005;14:305–309. doi: 10.1016/j.ijoa.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Fettes PD, Moore CS, Whiteside JB, McLeod GA, Wildsmith JA. Intermittent vs continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. Br J Anaesth. 2006;97:359–364. doi: 10.1093/bja/ael157. [DOI] [PubMed] [Google Scholar]
- 12.Leone Roberti Maggiore U, Silanos R, Carlevaro S, Gratarola A, Venturini PL, Ferrero S, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for pain relief during termination of pregnancy: a prospective, double-blind, randomized trial. Int J Obstet Anesth. 2016;25:37–44. doi: 10.1016/j.ijoa.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 13.George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg. 2013;116:133–144. doi: 10.1213/ANE.0b013e3182713b26. [DOI] [PubMed] [Google Scholar]
- 14.Sia AT, Leo S, Ocampo CE. A randomised comparison of variable-frequency automated mandatory boluses with a basal infusion for patient-controlled epidural analgesia during labour and delivery. Anaesthesia. 2013;68:267–275. doi: 10.1111/anae.12093. [DOI] [PubMed] [Google Scholar]
- 15.Lyons GR, Kocarev MG, Wilson RC, Columb MO. A comparison of minimum local anesthetic volumes and doses of epidural bupivacaine (0.125% w/v and 0.25% w/v) for analgesia in labor. Anesth Analg. 2007;104:412–415. doi: 10.1213/01.ane.0000252458.20912.ef. [DOI] [PubMed] [Google Scholar]
- 16.Bernard JM, Le Roux D, Vizquel L, Barthe A, Gonnet JM, Aldebert A, et al. Patient-controlled epidural analgesia during labor: the effects of the increase in bolus and lockout interval. Anesth Analg. 2000;90:328–332. doi: 10.1097/00000539-200002000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Badner NH, Doyle JA, Smith MH, Herrick IA. Effect of varying intravenous patient-controlled analgesia dose and lockout interval while maintaining a constant hourly maximum dose. J Clin Anesth. 1996;8:382–385. doi: 10.1016/0952-8180(96)00077-3. [DOI] [PubMed] [Google Scholar]