Abstract
Background
Current guidelines for imaging surveillance after proximal aortic repair are not evidence based. This study sought to characterize the incidence and causes of reintervention after proximal aortic operations to provide data to guide the frequency and duration of postoperative surveillance.
Methods
Data on all patients undergoing proximal aortic operations (ascending, with or without root, with or without aortic valve replacement, or with or without arch) during a 9-year period (n = 869) at a single institution were prospectively collected. Patients who required reintervention on the proximal or distal aorta were identified and causes for reintervention determined. Planned two-stage repairs and index procedures done at other hospitals were excluded. The primary end point was the time to the first reintervention, and competing-risk Cox regression was used to model reintervention risk.
Results
Reinterventions occurred in 4.3% of patients (n = 37), with 48.6% (n = 18) involving the proximal aorta and 51.4% (n = 19) the distal. Median time to reintervention was 2.8 years (interquartile range, 1.5 to 3.6 years). For index aneurysm cases, reintervention for aneurysm of the descending/thoracoabdominal aorta and root were most common. Of the 6 root aneurysms/pseudoaneurysms, 5 (83%) were due to degeneration of a stentless porcine aortic root. For index type A dissections, reintervention for aneurysm of the descending/thoracoabdominal aorta and arch were most common. The mean duration of follow up was 4.2 ± 2.5 years. The 9-year actuarial freedom from reintervention was 92.9%. Cox regression showed index type A dissection was a significant predictor of time to aortic reintervention (hazard ratio, 2.01; 95% confidence interval, 1.04 to 3.9; p = 0.038).
Conclusions
Reinterventions after proximal aortic operations are uncommon; most occur within 3 years of the index operation and involve the proximal and distal aorta nearly equally. Patients with type A dissection or stentless porcine roots require aggressive surveillance, whereas a more liberal approach is suitable for patients without such risk factors. This strategy may reduce the lifetime radiation burden and health care costs.
Despite advances in anesthesia, postoperative care, circulatory management, and cerebral protection strategies, reoperative cardiac operations are associated with an increased risk of morbidity. The subgroup of reoperative thoracic aortic surgical patients presents in itself a unique challenge, with perioperative mortality ranging from 7% to 15% compared with 3% to 4% in the elective setting [1–10]. Thoracic aortic reoperations may be required for a multitude of reasons, including chronic/residual dissection, progression of aortic disease, anastomotic pseudoaneurysm, endocarditis or deterioration of bioprosthetic devices, graft infection, or valvular insufficiency, among others [6, 9, 11–14].
The published literature on reoperative thoracic aortic procedures, however, is limited to single institutional series, and criteria for “reoperative aortic operations” are heterogeneous. Although data exist regarding the inherent morbidity and the potential for death resulting from reoperation on the thoracic aorta, there remains only Level C evidence on the use of imaging surveillance with computed tomography (CT), magnetic resonance imaging (MRI), or transthoracic echocardiography (TTE) after aortic repair. Further, it is on this low-quality evidence that current postoperative aortic surveillance guidelines are based [15]. Unfortunately, owing to the long-term time frame in which an aortic reintervention may become a necessity—in some series with a mean range of 7 to 11 years [12, 16–18]—and the radiation and cost burden placed on patients as a result of aortic surveillance imaging, more concrete data are required for guideline recommendations.
Given this lack of clinical evidence for postoperative imaging surveillance, the goal of the current study was to describe the incidence and causes for reintervention after proximal aortic operations to provide guidance regarding the needed frequency and duration of postoperative surveillance and address the knowledge gap in the current literature and guidelines.
Patients and Methods
Study Design and Patients
An institutional database was used to identify all patients who underwent proximal aortic operations at a single referral aortic center (Duke University Medical Center, Durham, NC) from June 2005 to March 2014. Proximal aortic operations were defined as ascending aortic replacement with or without aortic root replacement, with or without aortic valve repair or replacement, or with or without aortic arch replacement. Patients who underwent reoperations after having undergone an index proximal aortic operation at another institution were excluded from the reintervention cohort because the surveillance screening interval, if any, was unknown. In addition, patients with planned two-stage procedures were not considered as a reintervention; specifically, the second stage of a planned two-stage procedure was not considered a reintervention because the decision to return to the operating room was prespecified.
Demographic, operative, and postoperative outcome data were extracted from the Duke Thoracic Aortic Surgery Database, a prospectively maintained clinical registry of all patients undergoing thoracic aortic surgery at Duke University Medical Center since June 2005. Patients underwent lifelong follow-up examination at the Duke Center for Aortic Disease, with clinical assessment and CT angiography (CTA) plus TTE or cardiac MRI/aortic magnetic resonance angiography. Investigational Review Board approval was obtained for this study, and the need for individual patient consent was waived.
End Points
The primary end point of the analysis was the need for operative reintervention on the thoracoabdominal aorta. Reinterventions on the proximal as well as the distal (descending, thoracoabdominal, abdominal) aorta were included in this analysis. Secondary end points included time to reintervention, reason for reintervention, reintervention performed, and postoperative morbidity and death. Indications for reintervention were in strict accordance with The Society of Thoracic Surgeons (STS)/American Heart Association (AHA) published guidelines for management of aortic disease, assuming the patient was otherwise a suitable operative candidate based on medical comorbidities [15].
Data Analysis
Categoric variables are reported as numbers and percentages and continuous variables as means and SDs. Continuous and categoric variables were compared between groups using the Mann-Whitney rank sum test and the Fisher exact test, respectively. Overall estimates of freedom from reintervention were calculated for all patients using the Kaplan-Meier method. In instances where a patient underwent more than one reintervention, the time to the first aortic reintervention was considered. Competing-risk regression using a Cox model was used to model the competing risk of time to the first aortic reintervention vs death, while controlling for differences in baseline characteristics. Rather than reporting the cumulative incidence estimator, the point estimate from the competing risk model, the subhazard ratio, is reported. Stata 11.0 software (StataCorp LP, College Station, TX) was used for all statistical analyses.
Results
Patient Characteristics
From June 1, 2005, to March 15, 2014, 869 patients at our institution underwent proximal aortic operations. Reinterventions occurred in 4.3% of patients (n = 37). Baseline characteristics of all patients at the time of the index proximal aortic operation are reported in Table 1, stratified by whether patients ultimately went on to have a reintervention. Among patients who subsequently required reinterventions, there was a higher frequency of baseline hypertension, history of stroke or transient ischemic attack, and creatinine exceeding 1.5 mg/dL at the time of the index operation (Table 1). There was no significant difference in prevalence of connective tissue disease between groups.
Table 1.
Baseline Patient Characteristics
| Variable | No Reintervention (n = 832) No. (%) |
Reintervention (n = 37) No. (%) |
p Value |
|---|---|---|---|
| Male gender | 579 (70) | 22 (60) | 0.205 |
| White race | 628 (76) | 23 (62) | 0.08 |
| Hypertension | 649 (78) | 35 (95) | 0.013 |
| Hyperlipidemia | 445 (54) | 22 (60) | 0.505 |
| Tobacco abuse history | 366 (44) | 20 (54) | 0.241 |
| Diabetes | 78 (9) | 1 (3) | 0.243 |
| CAD | 206 (25) | 10 (27) | 0.702 |
| History of stroke or TIA | 68 (8) | 7 (19) | 0.033 |
| COPD | 108 (13) | 9 (24) | 0.079 |
| Baseline creatinine >1.5 mg/dL | 93 (11) | 11 (30) | 0.002 |
| PVD | 62 (8) | 1 (3) | 0.511 |
| Connective tissue disorder | 65 (8) | 2 (5) | 1.0 |
| Bicuspid aortic valve disease | 305 (36.7) | 3 (8.1) | <0.001 |
CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; PVD = peripheral vascular disease; TIA = transient ischemic attack.
Aortic Reintervention: Indications and Surgical Management
Among the 37 patients in the study cohort who required reintervention, 18 (48.6%) involved reinterventions on the proximal aorta, and 19 (51.4%) involved the distal aorta. Moreover, 21 patients (56.8%) had an index procedure for a proximal aneurysm, 15 (40.5%) had an index procedure for type A dissection, and 1 (2.7%) had an index root abscess.
Table 2 summarizes the indication for reintervention and the reintervention performed among the 21 patients who had an operation for an index proximal aneurysm and subsequently required reintervention. The most common indication for reintervention was metachronous development of an aneurysm of the descending or thoracoabdominal aorta, which occurred in 10 patients (47.6%) in this group. All but 1 patient in this group was able to be treated with thoracic endovascular aortic repair. The second most common indication for reintervention was development of a root aneurysm or pseudoaneurysm, which occurred in 6 patients (28.6%) and necessitated reoperative root replacement. Of these 6 root aneurysms/pseudoaneurysms, 5 (83%) were due to degeneration of a Freestyle (Medtronic, Minneapolis, MN) stentless porcine aortic root, a recently described mode of failure unique to this particular prosthesis [14]. If patients with a Freestyle root are excluded, the risk of reintervention among the elective proximal aneurysm group drops significantly to 1.8% (n = 16).
Table 2.
Among Patients With Index Proximal Aortic Repair for Aneurysm, Subsequent Reason for Reintervention and Type of Reintervention Performed
| Variable | % (No.) (n = 21) |
|---|---|
| Indication for reintervention | |
| Descending/thoracoabdominal aneurysm | 47.6 (10) |
| Root aneurysm/pseudoaneurysm | 28.6 (6) |
| Severe AI | 14.3 (3) |
| Endocarditis | 4.8 (1) |
| Transverse arch aneurysm | 4.8 (1) |
| Reintervention performed | |
| TEVAR | 42.9 (9) |
| Redo root replacement/ascending | 28.6 (6) |
| AVR | 19.1 (4) |
| Open thoracoabdominal | 4.8 (1) |
| Arch | 4.8 (1) |
AI = aortic insufficiency; AVR = aortic valve replacement; TEVAR = thoracic endovascular aortic repair.
Table 3 reports the indication for reintervention and the reintervention performed among the 15 patients who had operations for an index type A dissection and required reintervention. Among 60% of patients in this group, the reason for reintervention was the development of a descending or thoracoabdominal aneurysm (n = 9). Most required an open thoracoabdominal approach (46.7% [n = 7]) vs thoracic endovascular aortic repair (13.3% [n = 2]). The next most common indication for reintervention was the development of a transverse arch aneurysm (26% [n = 4]).
Table 3.
Among Patients With Index Proximal Aortic Repair for Dissection Subsequent Reason for Reintervention and Type of Reintervention Performed
| Variable | % (No.) (n = 15) |
|---|---|
| Indication for reintervention | |
| Descending/thoracoabdominal aneurysm | 60 (9) |
| Transverse arch aneurysm | 26.7 (4) |
| Root aneurysm/ pseudoaneurysm | 13.3 (2) |
| Reintervention performed | |
| Open thoracoabdominal | 46.7 (7) |
| Arch | 26.7 (4) |
| Redo root replacement/ascending | 13.3 (2) |
| TEVAR | 13.3 (2) |
TEVAR = thoracic endovascular aortic repair.
One patient in the series presented with an index aortic root abscess from endocarditis that was repaired with a pericardial composite root. Recurrent aortic root endocarditis with valved conduit dehiscence subsequently developed and required redo aortic root replacement 3.5 years later.
Reintervention: Timing, Risk Factors, and Postoperative Outcomes
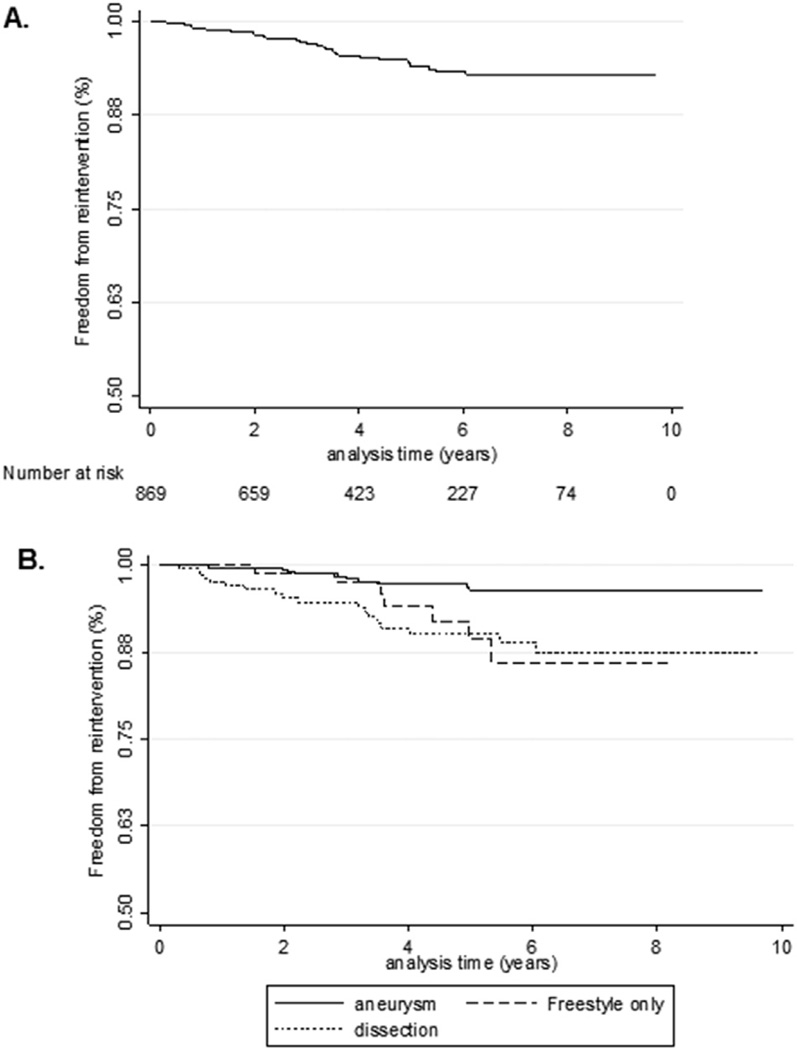
Overall freedom from reintervention is shown in Figure 1. The mean duration of follow up was 4.1 ± 2.5 years. The incidence rate of reintervention was 0.0102, or 1 reintervention per 100 patient-years. At 9 years, the actuarial freedom from reintervention was 92.9%. The probability of reintervention was 0.0426 or 4.3% for the series.
Fig 1.
Kaplan-Meier estimate of (A) overall freedom from aortic reintervention and (B) freedom from aortic reintervention stratified by aneurysm (Freestyle [Medtronic, Minneapolis, MN] excluded; solid line), Freestyle only (dashed line), and dissection (dotted line).
The overall median time to reintervention was 2.8 years (interquartile range [IQR], 1.5 to 3.6 years). Patients who underwent an index operation for a type A dissection had a higher risk of reintervention than those who underwent an index operation for a proximal aneurysm (p = 0.001). The median time to reintervention was 2.0 years (IQR, 0.8 to 3.2 years) for type A dissection patients and 3.4 years (IQR, 2.2 to 4.4 years) for proximal aneurysm patients. The median time to reintervention was 2.8 years (IQR, 2.1 to 3.5 years) when patients receiving a Freestyle root were excluded and was 3.6 years (IQR, 2.9 to 5.0 years) for only Freestyle roots.
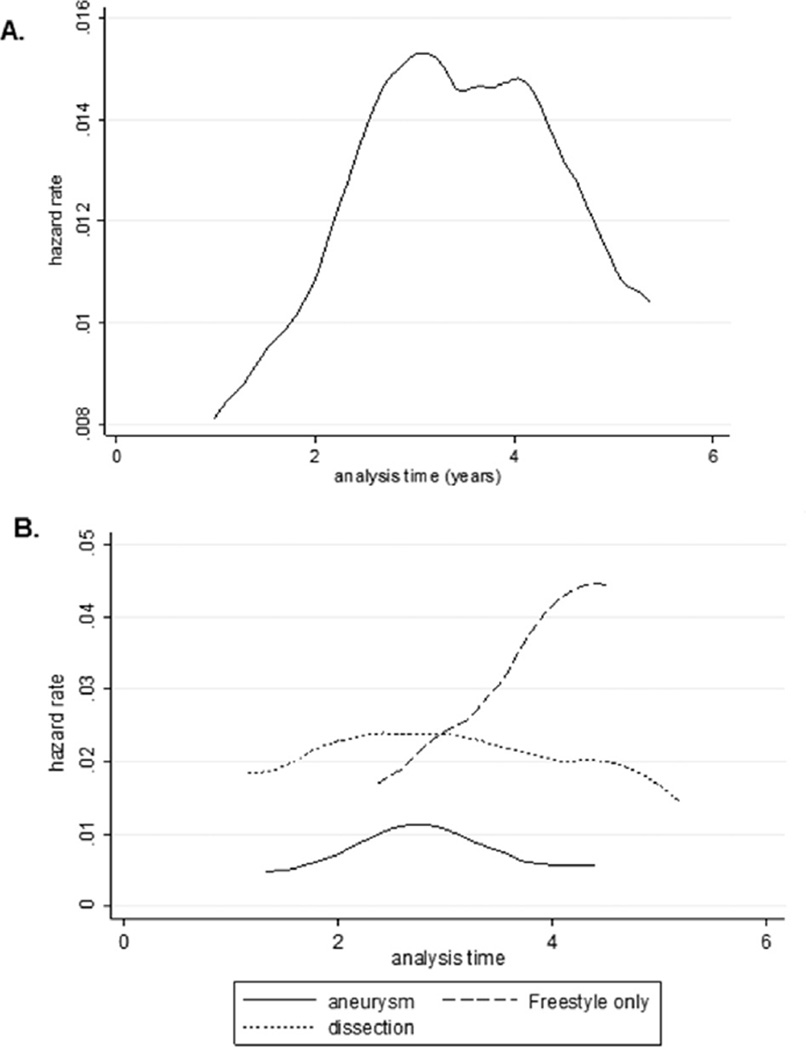
Figure 2 demonstrates the hazard rate as a function of time, stratified by indication for the index operation. Although the risk of reintervention was persistently higher throughout the study period for dissection patients than for aneurysm patients (excluding Freestyle bioroots), aneurysm and dissection patients both had peak risks of reintervention at approximately 3 years, with risk then beginning to plateau or decline after that point. For patients with Freestyle bioroots, the risk of reintervention was progressive and occurred later than for dissection or aneurysm patients. Multivariable Cox competing-risk regression showed the indication for the index operation (aortic dissection vs proximal aneurysm) was the only significant risk factor for reintervention (hazard ratio, 2.01; 95% confidence interval, 1.04 to 3.9; p = 0.038).
Fig 2.
Smoothed hazard estimate for (A) the overall study cohort and (B) stratified by aneurysm (Freestyle [Medtronic, Minneapolis, MN] excluded; solid line), Freestyle only (dashed line), and dissection (dotted line).
For patients who underwent a reintervention operation, the overall mean postoperative length of stay was 6.7 ± 4.8 days and the median length of stay was 5 days. The mean cross-clamp time for applicable cases was 124.1 × 74.3 minutes. There were no reoperations for bleeding. The frequency of neurologic complications, prolonged mechanical ventilation, and acute renal failure were each 3.3% (n = 1). The 30-day mortality rate was 6.7% (n = 2), and 1 death occurred beyond 30 days.
Comment
In the current analysis we demonstrate that thoracic aortic surgical reinterventions among patients who undergo proximal aortic operations are uncommon, occurring at a frequency of 4.3% over a 9-year study period. For both patients with an index proximal aneurysm operation or type A dissection repair, the most common indication for surgical reintervention was the metachronous development of an aneurysm of the descending or thoracoabdominal aorta, which occurred at an overall median time of 2.8 years postoperatively.
Current guidelines from the STS/AHA writing group on the diagnosis and management of thoracic aortic disease contain recommendations for surveillance of various aortic pathologies after repair. However, the recommendations are Class IIa and based on Level C evidence, and the authors note that, “the frequency of surveillance imaging is not clear as there are no data to accurately dictate surveillance intervals” [15]. European Society of Cardiology guidelines on postoperative imaging surveillance after proximal aortic operations are similarly based on Level C evidence [19].
For patients who have undergone repair of a type A dissection, current STS/AHA guidelines recommend imaging by CT or MRI at 1, 3, 6, and 12 months after dissection and then annually. No recommendations are provided regarding the duration of surveillance beyond 1 year. The most common indication for reintervention in our analysis was the development of a descending or thoracoabdominal aneurysm, occurring in 60% of reinterventions after aortic dissection repair. Halstead and colleagues [20] examined distal reoperations among 179 consecutive acute type A dissection repairs, and similar to the results of the current study, found that distal reoperations were more prevalent (8.9%) than proximal reinterventions (2.8%). The median time to reintervention after type A dissection repair in the current study was 2.0 years, with an observed hazard that began to decline at 3 years. Thus, current guidelines for surveillance imaging at 1, 3, 6, and 12 months would seem more frequent than necessary, and a more liberal approach to annual screening beyond postoperative years 3 or 4 would likewise seem reasonable, assuming continued stable imaging findings on prior studies.
Recommendations for imaging surveillance after a proximal aortic operation for aneurysm are less well defined. STS/AHA guidelines, for example, recommend only TTE before discharge and yearly for aortic root repair and aortic valve replacement plus ascending aortic repair (Wheat procedure). Few studies have examined the incidence of reintervention in proximal aortic aneurysm operations. Strauch and colleagues [21] analyzed 2,281 anastomoses among 1,475 patients and found that only 34 patients (2.3%) required reoperation for suture-line disruptions after graft-to-aorta anastomosis using Teflon (DuPont, Wilmington, DE) felt for an incidence of 0.052 per patient-year. Our analysis found descending or thoracoabdominal aneurysm was the most common indication for reintervention after a proximal aortic operation for aneurysm, occurring at a median interval of 3.4 years. Therefore, the recommended isolated TTE would not be an effective postoperative screening modality because this study would not capture the most common indication for reintervention.
We previously reported the frequency of pseudoaneurysm formation after Medtronic Freestyle porcine aortic full root bioprosthesis implantation, a complication that appears to be potentially immune mediated and necessitates redo aortic root replacement for correction [14]. In our previous analysis, the frequency of this complication was 4.7% [14]. In the current study, for proximal reinterventions secondary to pseudoaneurysm formation, 83% patients had a history of implantation of a Freestyle root at the index operation. Thus, patients in this group would require more aggressive surveillance imaging than other proximal aortic aneurysm surgical patients.
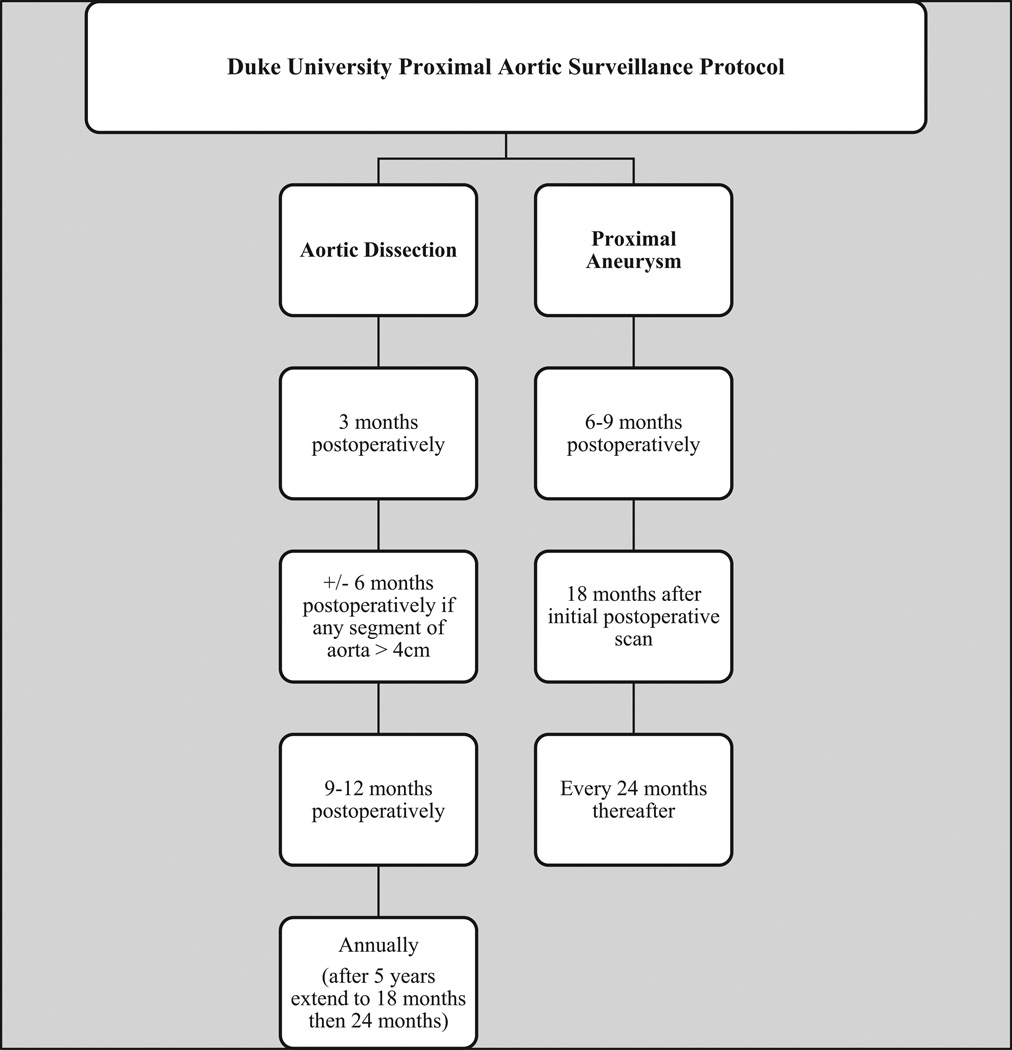
Figure 3 demonstrates our current institutional protocol for postoperative aortic surveillance based on the current analysis of reinterventions after a proximal aortic operation. In addition to imaging, all office visits include optimization of medical care, such as blood pressure medication titration, compliance with taking a statin, and use of an angiotensin receptor blocker if the patient has connective tissue disease. Among patients with a DeBakey type I dissection, connective tissue disorder, or vasculitis/aortitis, at each surveillance time point there is either a CT angiography (CTA) of the chest, abdomen, and pelvis plus TTE, or cardiac MRI/aortic magnetic resonance angiography. Among patients with a DeBakey type II dissection, degenerative (atherosclerotic) aneurysm, or bicuspid aortic valve, there is an initial scan of the chest, abdomen, and pelvis, but subsequent scans are limited to the chest if no abdominal pathology is present. With regard to surveillance interval, the initial postoperative surveillance in proximal aneurysm patients occurs between 6 and 9 months, then 18 months later, and then every 2 years thereafter if imaging shows the repair is stable. This less aggressive surveillance protocol reflects the lower early rate of reinterventions in this group and the decreased likelihood of reintervention beyond 3 to 4 years. The exception is patients with a Freestyle aortic bioprosthesis who require annual surveillance imaging, as noted above.
Fig 3.
Duke aortic surveillance protocol. DeBakey type I dissection, connective tissue disorder, or vasculitis/aortitis: computed tomography angiography of the chest, abdomen, and pelvis plus transthoracic echocardiography or cardiac magnetic resonance imaging/aortic magnetic resonance angiography. DeBakey type II dissection, degenerative (atherosclerotic) aneurysm, or bicuspid aortic valve: initial scan of the chest, abdomen, and pelvis but subsequent scans are of the chest only if no abdominal pathology is noted.
Our evidence-based surveillance protocol attempts to maximize screening during the period of highest risk and minimize screening among low-risk patients, a strategy that has the potential for significant reductions in lifetime radiation exposure and overall health care costs. We also recommend that for patients who have had a previous proximal aortic operation elsewhere and are establishing care at our institution, that a baseline TTE and CTA or cardiovascular MRI be performed to fully evaluate the aorta and arterial tree as well as cardiac and valvular function. Although we acknowledge that not all patients may be able to return to the tertiary care center where their aortic operation was performed for ongoing surveillance, we believe that other providers can still follow an evidence-based approach and work collaboratively with aortic surgeons at tertiary care centers to evaluate any new findings by electronically transferring images.
Fazel and colleagues [22] recently published a consensus statement from the AHA on approaches to enhancing radiation safety in cardiovascular imaging. The proximal aortic surveillance protocol outlined in this study is consistent with their recommendation as well as United States Food and Drug Administration recommendations for patient-centered imaging, whereby the decision to use imaging is individualized according to patient characteristics rather than generalized routine annual surveillance [23].
The patients undergoing reinterventions in the current analysis were a mean age of 61 years. Data on lifetime cancer risk for CT imaging is sparse; however, the estimated lifetime risk of cancer attributable to coronary CTA imaging at age 60 is estimated at 0.10% for men and 0.20% for women [24]. Routine annual surveillance imaging over a 20-year period would therefore potentially result in a lifetime cumulative cancer risk of 2% among men and 4% among women [24]. Thus, for a 60-year-old man, a patient-centered approach with surveillance at 2-year intervals after aggressive early screening has the potential to reduce lifetime cancer risk by half, to approximately 1%, and reduce individual health care cost by approximately $10,000, assuming an average Medicare cost of $1,000 per CTA. Of note, this cost estimate does not include the additive cost of TTE. In addition, we acknowledge that coronary CTA has higher radiation exposure and is thus higher risk than routine thoracic CTA, that coronary CTA data do not account for abdominal and pelvic radiation exposure risk, and that radiation doses have continued to decrease with changing technology [25]. In addition to patient-centered imaging approaches, the routine use of magnetic resonance angiography has the potential to reduce radiation burden and is frequently used in our institution for the postoperative surveillance of younger patients.
The major potential limitation of the current analysis is the possibility that a patient might have undergone reintervention at another institution, such that the reported incidence rates underestimate the true risk of reintervention. Our follow-up with patients, however, was very close, and patients were individually contacted if they did not return for scheduled follow-up. We have a dedicated aortic surgery nurse practitioner, and estimate that less than 1% of patients do not return for an initial follow-up imaging study and less than 3% do not return for further surveillance imaging.
Another potential limitation is the relatively short duration of follow-up, especially given the presumed lifetime risk that metachronous aortic pathology will develop after the index repair, with the analysis covering only a 9-year period and with a mean follow-up duration of only 4.5 years. However, although reinterventions in this cohort will continue to occur in the future, the rate of such interventions is not likely to increase because the observed hazard for reinterventions began to plateau or decline during the study period.
A final limitation is that the study excluded patients if their index operation was performed at an another institution because the surveillance frequency was unknown, as was the number of patients undergoing proximal aortic repair at other institutions who did not require reintervention. Therefore, our analysis may either overestimate or underestimate the true prevalence of reinterventions among all proximal aortic repair patients and the importance of serial imaging.
In summary, the current analysis demonstrates that reinterventions after a proximal aortic operation are uncommon and that the timing of reintervention differs by the indication for the index procedure, with peak risk occurring at approximately 3 to 4 years postoperatively. Developing an evidence-based surveillance algorithm has the potential for significant reductions in health care costs and lifetime radiation exposure.
Footnotes
Presented at the Sixty-second Annual Meeting of the Southern Thoracic Surgical Association, Orlando, FL, Nov 4–7, 2015.
Author Interview: The Author Interview can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2016.06.085] or from The Annals YouTube channel [https://youtu.be/A0uKCIQ9sgE].
References
- 1.Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol. 2012;60:1156–1162. doi: 10.1016/j.jacc.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Bartolomeo R, Berretta P, Petridis FD, et al. Reoperative surgery on the thoracic aorta. J Thorac Cardiovasc Surg. 2013;145:S78–S84. doi: 10.1016/j.jtcvs.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 3.Estrera AL, Miller CC, 3rd, Porat E, et al. Determinants of early and late outcome for reoperations of the proximal aorta. Ann Thorac Surg. 2004;78:837–845. doi: 10.1016/j.athoracsur.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 4.Etz CD, Plestis KA, Homann TM, et al. Reoperative aortic root and transverse arch procedures: a comparison with contemporaneous primary operations. J Thorac Cardiovasc Surg. 2008;136:860–867. doi: 10.1016/j.jtcvs.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 5.Fukunaga N, Koyama T, Konishi Y, Murashita T, Kanemitsu H, Okada Y. Clinical outcome of redo operation on aortic root. Gen Thorac Cardiovasc Surg. 2014;62:215–220. doi: 10.1007/s11748-013-0332-3. [DOI] [PubMed] [Google Scholar]
- 6.LeMaire SA, DiBardino DJ, Koksoy C, Coselli JS. Proximal aortic reoperations in patients with composite valve grafts. Ann Thorac Surg. 2002;74:S1777–S1780. doi: 10.1016/s0003-4975(02)04152-8. [DOI] [PubMed] [Google Scholar]
- 7.Luciani N, De Geest R, Anselmi A, Glieca F, De Paulis S, Possati G. Results of reoperation on the aortic root and the ascending aorta. Ann Thorac Surg. 2011;92:898–903. doi: 10.1016/j.athoracsur.2011.04.116. [DOI] [PubMed] [Google Scholar]
- 8.Malvindi PG, van Putte BP, Heijmen RH, Schepens MA, Morshuis WJ. Reoperations on the aortic root: experience in 46 patients. Ann Thorac Surg. 2010;89:81–86. doi: 10.1016/j.athoracsur.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Schepens MA, Dossche KM, Morshuis WJ. Reoperations on the ascending aorta and aortic root: pitfalls and results in 134 patients. Ann Thorac Surg. 1999;68:1676–1680. doi: 10.1016/s0003-4975(99)00760-2. [DOI] [PubMed] [Google Scholar]
- 10.Shrestha M, Khaladj N, Baraki H, et al. Aortic root reoperation: a technical challenge. J Heart Valve Dis. 2010;19:177–181. [PubMed] [Google Scholar]
- 11.Crawford ES, Crawford JL, Safi HJ, Coselli JS. Redo operations for recurrent aneurysmal disease of the ascending aorta and transverse aortic arch. Ann Thorac Surg. 1985;40:439–455. doi: 10.1016/s0003-4975(10)60099-9. [DOI] [PubMed] [Google Scholar]
- 12.Szeto WY, Bavaria JE, Bowen FW, et al. Reoperative aortic root replacement in patients with previous aortic surgery. Ann Thorac Surg. 2007;84:1592–1598. doi: 10.1016/j.athoracsur.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 13.Estrera AL, Miller CC, 3rd, Villa MA, et al. Proximal reoperations after repaired acute type a aortic dissection. Ann Thorac Surg. 2007;83:1603–1608. doi: 10.1016/j.athoracsur.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Englum BR, Pavlisko EN, Mack MC, et al. Pseudoaneurysm formation after Medtronic Freestyle porcine aortic bioprosthesis implantation: a word of caution. Ann Thorac Surg. 2014;98:2061–2067. doi: 10.1016/j.athoracsur.2014.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch EW, Radu NC, Mekontso-Dessap A, Hillion ML, Loisance D. Aortic root replacement after previous surgical intervention on the aortic valve, aortic root, or ascending aorta. J Thorac Cardiovasc Surg. 2006;131:601–608. doi: 10.1016/j.jtcvs.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Silva J, Maroto LC, Carnero M, et al. Ascending aorta and aortic root reoperations: are outcomes worse than first time surgery? Ann Thorac Surg. 2010;90:555–560. doi: 10.1016/j.athoracsur.2010.03.092. [DOI] [PubMed] [Google Scholar]
- 18.Hughes GC, Daneshmand MA, Swaminathan M, et al. “Real world” thoracic endografting: results with the Gore Tag device 2 years after U.S. FDA approval. Ann Thorac Surg. 2008;86:1530–1537. doi: 10.1016/j.athoracsur.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 19.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 20.Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007;133:127–135. doi: 10.1016/j.jtcvs.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 21.Strauch JT, Spielvogel D, Lansman SL, et al. Long-term integrity of Teflon felt-supported suture lines in aortic surgery. Ann Thorac Surg. 2005;79:796–800. doi: 10.1016/j.athoracsur.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Fazel R, Gerber TC, Balter S, et al. Approaches to enhancing radiation safety in cardiovascular imaging: a scientific statement from the American Heart Association. Circulation. 2014;130:1730–1748. doi: 10.1161/CIR.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. Performance standards for ionizing radiation emitting products. Code of Federal Regulations, Title 21, Chapter I—Food and Drug Administration, Department of Health and Human Services (US), Subchapter J—Radiologic health, Part 1020. [Accessed March 5, 2016]; Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart = 1020.
- 24.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 25.Lungren MP, Yoshizumi TT, Brady SM, et al. Radiation dose estimations to the thorax using organ-based dose modulation. AJR Am J Roentgenol. 2012;199:W65–W73. doi: 10.2214/AJR.11.7798. [DOI] [PubMed] [Google Scholar]