Abstract
The extracellular matrix (ECM) consists of diverse components that work bidirectionally with surrounding cells to create a responsive microenvironment. In some contexts (e.g., hepatic fibrosis), changes to the ECM are well recognized and understood. However, it is becoming increasingly accepted that the hepatic ECM proteome (i.e., matrisome) responds dynamically to stress well before fibrosis. The term “transitional tissue remodeling” describes qualitative and quantitative ECM changes in response to injury that do not alter the overall architecture of the organ; these changes in ECM may contribute to early disease initiation and/or progression. The nature and magnitude of these changes to the ECM in liver injury are poorly understood. The goals of this work were to validate analysis of the ECM proteome and compare the impact of 6 weeks of ethanol diet and/or acute lipopolysaccharide (LPS). Liver sections were processed in a series of increasingly rigorous extraction buffers to separate proteins by solubility. Extracted proteins were identified using liquid chromatography/tandem mass spectrometry (LC-MS/MS). Both ethanol and LPS dramatically increased the number of matrisome proteins ~25%. The enhancement of LPS-induced liver damage by ethanol preexposure was associated with unique protein changes.
Conclusion
An extraction method to enrich the hepatic ECM was characterized. The results demonstrate that the hepatic matrisome responds dynamically to both acute (LPS) and chronic (ethanol) stresses, long before more-dramatic fibrotic changes to the liver occur. The changes to the mastrisome may contribute, at least in part, to the pathological responses to these stresses. It is also interesting that several ECM proteins responded similarly to both stresses, suggesting a common mechanism in both models. Nevertheless, there were responses that were unique to the individual and combined exposures.
Although the structural role of the extracellular matrix (ECM) is well known, this compartment contains a diverse range of components that work bidirectionally with surrounding cells to create a dynamic and responsive microenvironment. This microenvironment, in turn, regulates cell and tissue homeostasis. The ECM components play a key role in signaling through interactions with cell-surface receptors. The ECM also indirectly impacts cell-to-cell communication by binding and retaining soluble mediators, including cytokines, chemokines, and growth factors.(1) Proteases and protease inhibitors associated with the ECM also contribute to maintaining its homeostasis, as well as mediating its changes in response to stress or injury.(2) A broader definition of the ECM proteome (i.e., “‘matrisome”‘) has been established to encompass not only fibrillar ECM proteins, but also the proteins that contribute to the homeostasis of the ECM proteome.(1)
In some contexts, changes to the ECM are well recognized and understood; for example, the formation of collagenous scars in tissue is almost a canonical response to unresolved chronic injury. Hepatic fibrosis (HF) is a well-known example of this scarring process,(3) given that fibrotic livers develop easily detectable collagenous scars. Given the dominance of these changes in the hepatic ECM during fibrogenesis, many studies have focused on the mechanisms that underlie the increases in collagen deposition. However, the alterations of the hepatic ECM during fibrosis are much more diverse than simply an increase in collagen. Indeed, fibrosis is characterized by changes in the deposition and distribution of a myriad of other ECM proteins (e.g., laminin and vitronectin).(4) Whereas many of these changes are described, there are still gaps in our understanding. Namely, the magnitude and impact of these changes on overall liver (dys)function are incompletely understood at this time.
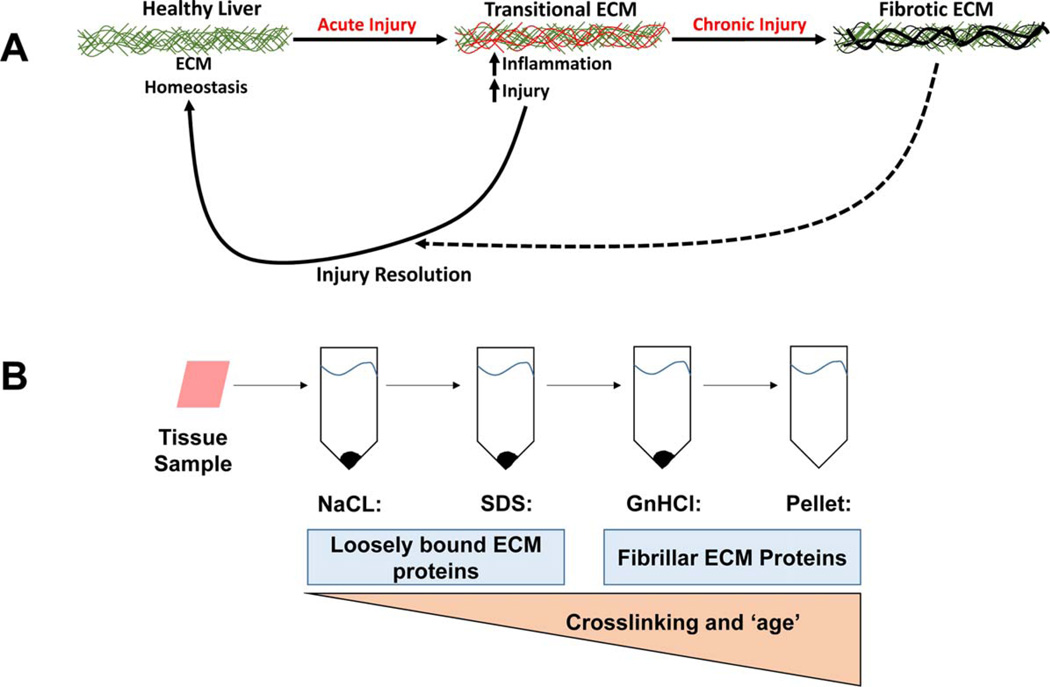
ECM remodeling is not solely relegated to chronic fibrogenesis, but also contributes to early injury responses in tissue; a well-known example of the latter is the wound-healing response. The term “transitional tissue remodeling” describes changes to the ECM that do not alter the overall architecture of the organ (Fig. 1A). For example, changes in the expression of ECM proteins, such as fibronectin(5) and fibrin,(6,7) have been observed in models of hepatic inflammation. Importantly, blocking these ECM changes blunts, at least in part, hepatic injury in these models. Therefore, transitional changes to the ECM may contribute to early disease initiation and/or progression before the onset of fibrogenesis (Fig. 1A). The nature and magnitude of these changes to the ECM are currently poorly understood. A better understanding could elucidate new mechanisms and/or biomarkers for diseases.
FIG. 1.
Scheme of transitional ECM changes and extraction methodology. Remodeling in response to chronic injury (i.e., fibrosis) is well known; however the hepatic ECM also responses dynamically to acute stress. These acute responses can be viewed as an arm of the wound-healing response and facilitate recovery from damage, which resolves once the damage is repaired. However, under conditions of chronic injury, these changes contribute to activation of a significant remodeling response that leads to scar formation (i.e., fibrosis).
Although previous studies have shown that subtle changes in the ECM may contribute to the development of inflammatory liver injury, the research in this area has generally been restricted to study of single ECM proteins (e.g., fibrin).(6,7) A more ‘omic approach has been previously hampered by the difficulties associated with the low abundance and insolubility of many ECM proteins. Others have recently reported on a sequential extraction method using increasingly rigorous solubilization buffers, coupled to liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis, specifically designed to enrich and characterize ECM proteins in solid organs.(8–10) This approach also potentially accounts for proteins that can exist in different solubility states, based on posttranslational modifications (e.g., cleavage, cross-linking, and degradation). The goals of the current study were 2-fold: (1) to characterize the sequential extraction method for hepatic matrisome and (2) to compare the impact of inflammatory liver injury (before fibrosis) on the hepatic matrisome.
Materials and Methods
ANIMALS AND EXPOSURES
Mice were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and procedures were approved by the University of Louisville’s Institutional Animal Care and Use Committee. For sacrifice, animals were anesthetized with ketamine/ xylazine (100/15 mg/kg, intraperitoneally) and blood was collected from the vena cava just before sacrifice. Paraffin-embedded, formalin-fixed samples were stained with Sirius Red/Fast Green to visualize fibrosis (CCl4 study).(11)
CCl4 Exposure
Male (4–6 weeks old) C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were administered CCl4 (1 mL/kg intraperitoneally; diluted 1:4 in olive oil; Sigma-Aldrich, St. Louis, MO) twice a week for 4 weeks.(11)
Ethanol Exposure
Male (8 weeks old) C57BL6/J mice were purchased from The Jackson Laboratory. Animals were given alcohol-containing Lieber DeCarli diet ad libitum or pair-fed isocaloric (maltose-dextrin) control diet.(12) After 6 weeks of feeding, animals were injected with either lipopolysaccharide (LPS; Escherichia coli; 10 mg/kg intraperitoneally; Sigma-Aldrich) or vehicle (saline) and euthanized 24 hours later.(12)
THREE-STEP ECM EXTRACTION SAMPLE CLEANUP AND PREPARATION FOR LIQUID CHROMATOGRAPHY
Sequential extraction of the hepatic ECM and preparation for LC-MS/MS was performed, as described by de Castro Bras et al., for heart tissue,(9) with minor modifications for liver (Fig. 1; see the Supporting Materials for more details).
LIQUID CHROMATOGRAPHY AND MASS SPECTROMETRY
Samples were separated on Dionex Acclaim PepMap 100 75 uM × 2 cm nanoViper (C18, 3 µm, 100 Å) trap and Dionex Acclaim PepMap RSLC 50 uM × 15 cm nanoViper (C18, 2 µm, 100 Å) separating columns (Sunnyvale, CA). An EASY n-LC (Thermo Fisher Scientific, Waltham, MA) ultra-high-performance liquid chromatography system was used with buffer A = 2% (v/v) acetonitrile/0.1% (v/v) formic acid and buffer B = 80% (v/v) acetonitrile/0.1% (v/v) formic acid as mobile phases. A Nanospray Flex source (Thermo Fisher Scientific) was used to position the end of the emitter near the ion transfer capillary of the mass spectrometer. An Orbitrap Elite–ETD mass spectrometer (Thermo Fisher Scientific) was used to collect data from the LC eluate. An Nth Order Double Play with ETD Decision Tree method was created in Xcalibur v2.2 (Thermo Fisher Scientific; see the Supporting Materials for more details).
DATA ANALYSIS
Proteome Discoverer v1.4.0.288 was used to analyze the data collected by the mass spectrometer. The database used in Mascot v2.4 and SequestHT searches was the 6/2/2015 version of the UniprotKB Mus musculus reference proteome canonical and isoform sequences. Carbamidomethylation (+57 on C) was selected as a fixed modification, and Asn->Asp (+1 on N) and Met or Pro oxidation (+16) were selected as variable modifications. A maximum of two missed cleavages were allowed. In order to estimate the false discovery rate, a Target Decoy PSM Validator node was included in the Proteome Discoverer workflow. The results were annotated with mouse gene ontology (GO) information from the Gene Ontology Annotations Database. Additional analysis of extracellular proteins were further categorized into four classes based on their role in the extracellular space as determined by a comprehensive literature search. These four ECM groups include: (1) glycoproteins and proteoglycans; (2) other ECM-associated proteins; (3) proteases and protease inhibitors; and (4) collagens. If no record could be found supporting the hypothesis that a protein was associated with the ECM, the protein was placed into (5), other proteins. Supporting Tables S1 and S2 show a list of all the proteins from this study that were identified by GO annotation as extracellular, their functional classification, and supporting references (see the Supporting Materials for more details).
Results
ANALYSIS OF CHANGES TO THE ECM PROTEOME CAUSED BY CCl4-INDUCED HF
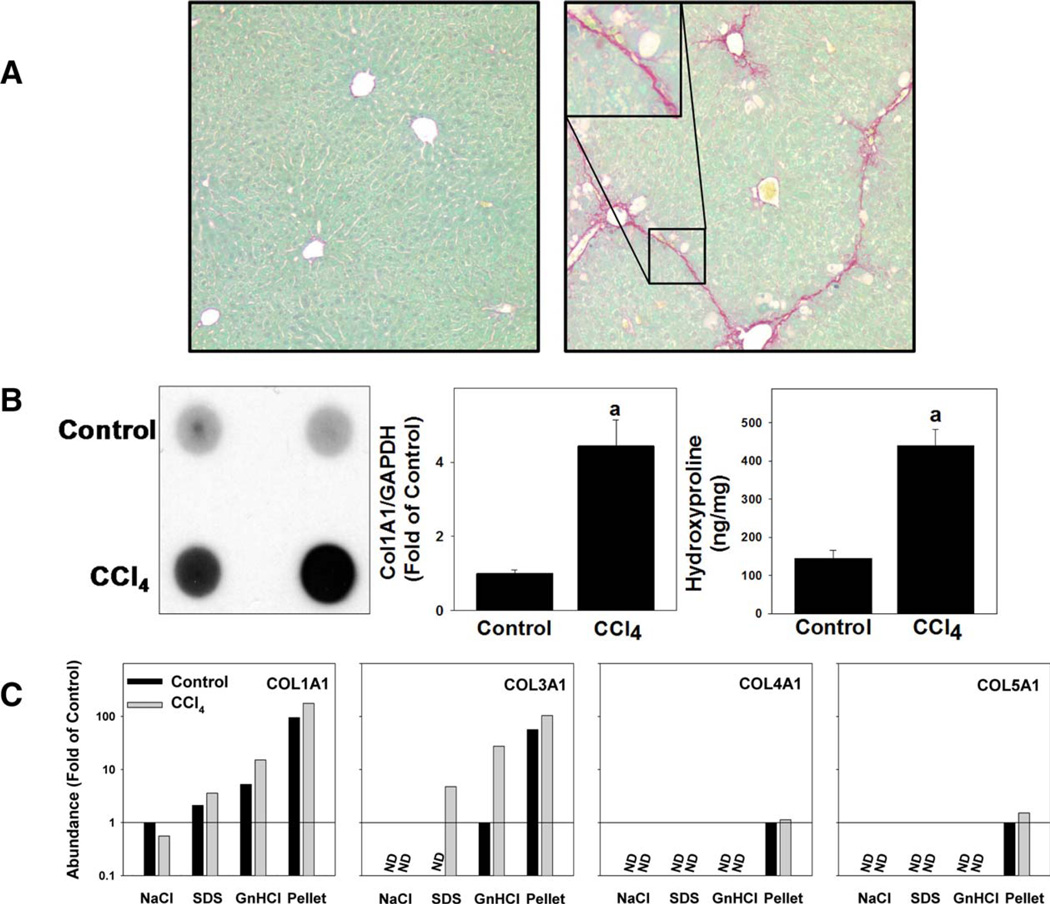
The first objective of this work was to validate LC-MS/MS analysis of the hepatic matrisome; toward this end, a model that causes robust changes to the ECM (i.e., fibrosis) was used. Specifically, the impact of 4 weeks of CCl4 exposure (see Materials and Methods) on the ECM proteome was determined (Fig. 2). As expected,(11) CCl4 exposure caused robust ECM deposition that was easily detected with standard collagen stains (Sirius Red/Fast Green; Fig. 2A), immunoblotting, and hydroxyproline content (Fig. 2B). An increase in collagen 1α1 was also detected by LC-MS/ MS (Fig. 2C). Exposure to CCl4 also increased levels of collagens type III, IV, and V (Fig. 2C).
FIG. 2.
Validation of extraction technique with CCl4 model of fibrosis. Animals were administered CCl4 or vehicle for 4 weeks. Collagen type I accumulation was determined by Sirius Red staining (A) and by dot blot and hydroxyproline content (B). Collagen 1 mRNA expression (B) was determined by real-time rtPCR. Quantitative changes in other collagens were also determined in the ECM extraction fractions (C). Abbreviations: COL, collagen; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; rtPCR, reverse-transcription PCR.
Standard proteomic analysis generally uses a direct analysis of total protein in the absence of additional fractionation steps. For this initial analysis, qualitative analysis of the proteomic data across the extractions (see Materials and Methods) were pooled to mimic this approach. The majority of ECM proteins were present, at least to some extent, in extracts of livers of both naïve and CCl4-challenged mice. Specifically, CCl4 exposure increased the number of proteins associated with the ECM by 7, and only one protein was lost compared to the control group (data not shown). These data indicate that when analyzed as a whole, there are few qualitative changes (i.e., disappearance or appearance of proteins) in the hepatic matrisome, even under conditions of significant histological ECM changes (e.g., fibrosis; Fig. 2A).
ETHANOL AND LPS EXPOSURE CAUSE GLOBAL CHANGES TO THE HEPATIC MATRISOME
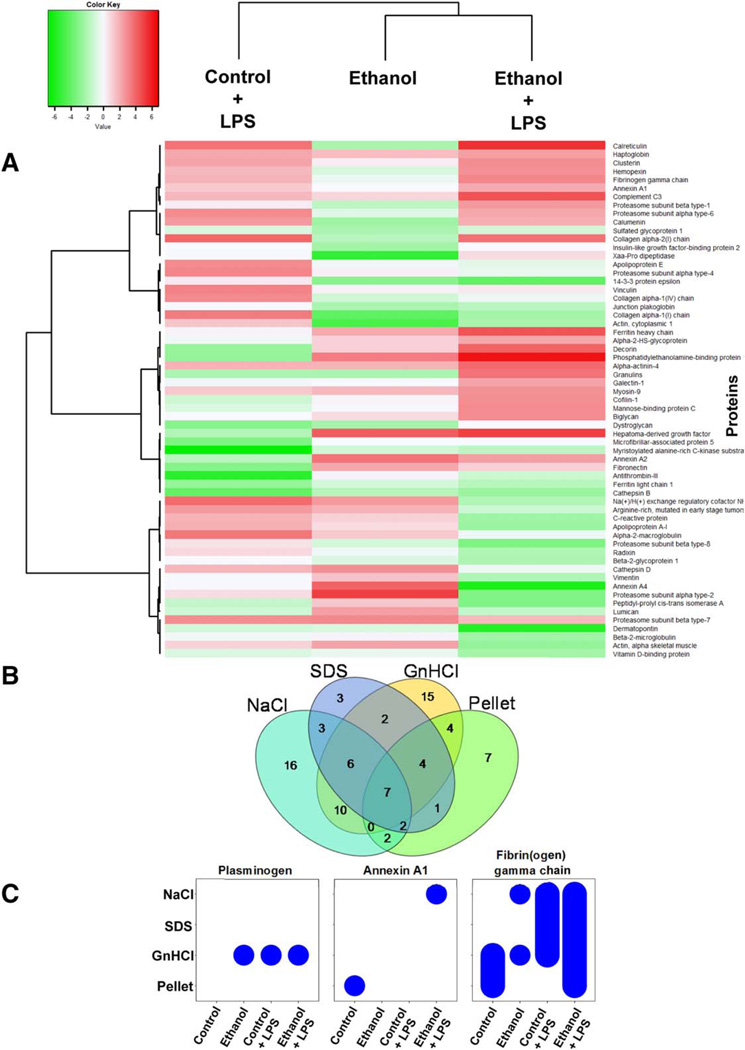
Based on the results with CCl4-challenged livers (Fig. 2), a more-detailed analysis of the effects of ethanol and LPS were used in subsequent studies. Figure 3A shows a heatmap comparing the quantitative changes in the ECM proteome between the four exposure groups (±ethanol diet, ±LPS injection) after fractions were artificially collapsed; the most up-regulated or down-regulated ECM proteins were used to compare the groups. Heatmap visualization of the ECM proteomic spectra showed distinct segregation between the control group and exposure groups, with ethanol and LPS exposure groups producing varying patterns; the combination of ethanol/LPS shared patterns similar to both exposures alone, but also demonstrated unique patterns (Fig. 3A). However, similar to the results with samples from CCl4 (see above and Fig. 2), there were few proteins that changed qualitatively in response to ethanol and/or LPS.
FIG. 3.
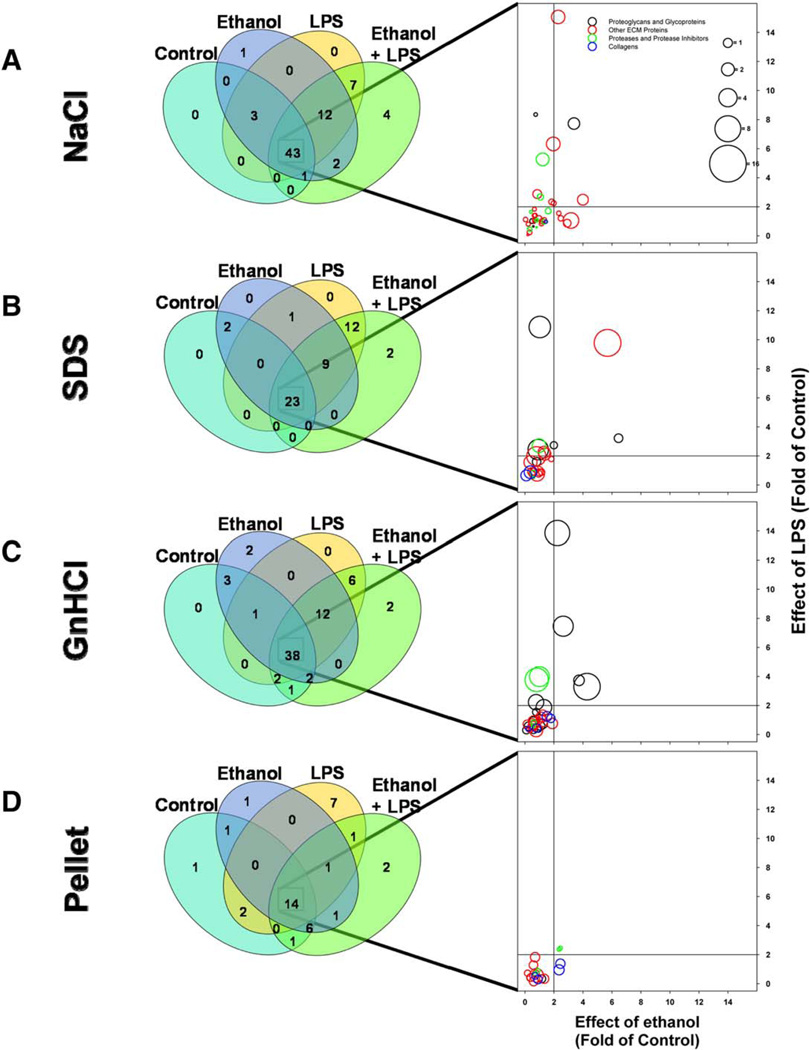
Liver extracts have unique protein profiles based on fraction type and experimental group. A heatmap depicting quantitative changes to the total ECM proteome in response to ethanol or LPS is shown in (A). The number of proteins unique to, or shared by, all four extractions of pair-fed animals is shown (B). Bubble graphs are used to show presence of plasminogen, Annexin A1, and fibrin(ogen) gamma chain across the four extracts (x-axis) and the four experimental groups (y-axis; C).
THREE-STEP SERIAL EXTRACTION CREATES DISTINCT PROTEIN PROFILES
ECM proteins are not only regulated at the level of de novo synthesis, but also at the level of enzymatic activation, degradation, and crosslinking. As mentioned in the Introduction, previous studies have used a three-step ECM purification method (Fig. 1) in other organs and that approach was adapted for use with liver tissue here.(9,13) The NaCl extraction displaces polyionic interactions between proteins, thereby solubilizing loosely bound proteins.(14) Following NaCl extraction, the remaining liver tissue was decellularized using 1% sodium dodecyl sulfate (SDS). SDS solubilizes cytoplasmic and nuclear membranes, allowing for release of cellular proteins. Decellularization was followed by a denaturing step with 4 M of GnHCl buffer.(15) Following the denaturing extraction, an insoluble pellet enriched in heavily cross-linked matrix proteins remained.(13) The four extracts of ECM proteins from control animals were qualitatively compared (Fig. 3B). Each extract yielded a distinct ECM protein profile consisting of not only ECM proteins that were shared among all four extracts, but also proteins unique to particular extracts (Fig. 3B). Analysis of the extractions of the CCl4 samples for collagen isoforms (Fig. 2C) indicated that, as expected, these proteins tended to accumulate in the later extraction fractions. These results indicate that the extraction approach was effective at separating ECM proteins.
When ECM proteins were compared between experimental groups, interesting patterns of accumulation in the extracts were revealed (Supporting Table S1); representative examples of some of these patterns are shown in Fig. 3C. For example, some proteins (e.g., plasminogen) changed in overall “presence” or “absence” in response to exposures, but localized consistently to the same fraction when present. Other proteins exhibited more-complex responses, also changing in fractionation pattern. For example, whereas Annexin A1 was found in the insoluble pellet from control animals, it was not detected in any fraction from animals exposed to ethanol or LPS alone; however, the combination of ethanol and LPS caused this protein to accumulate in the NaCl fraction. Similarly, fibrin(ogen) gamma chain was found in all treatment groups, but its fractionation pattern was unique to each exposure condition. These patterns likely represent differences in the synthesis, degradation, and/or maturity of the ECM proteins.
QUALITATIVE CHANGES TO THE ECM PROTEOME IN RESPONSE TO STRESS
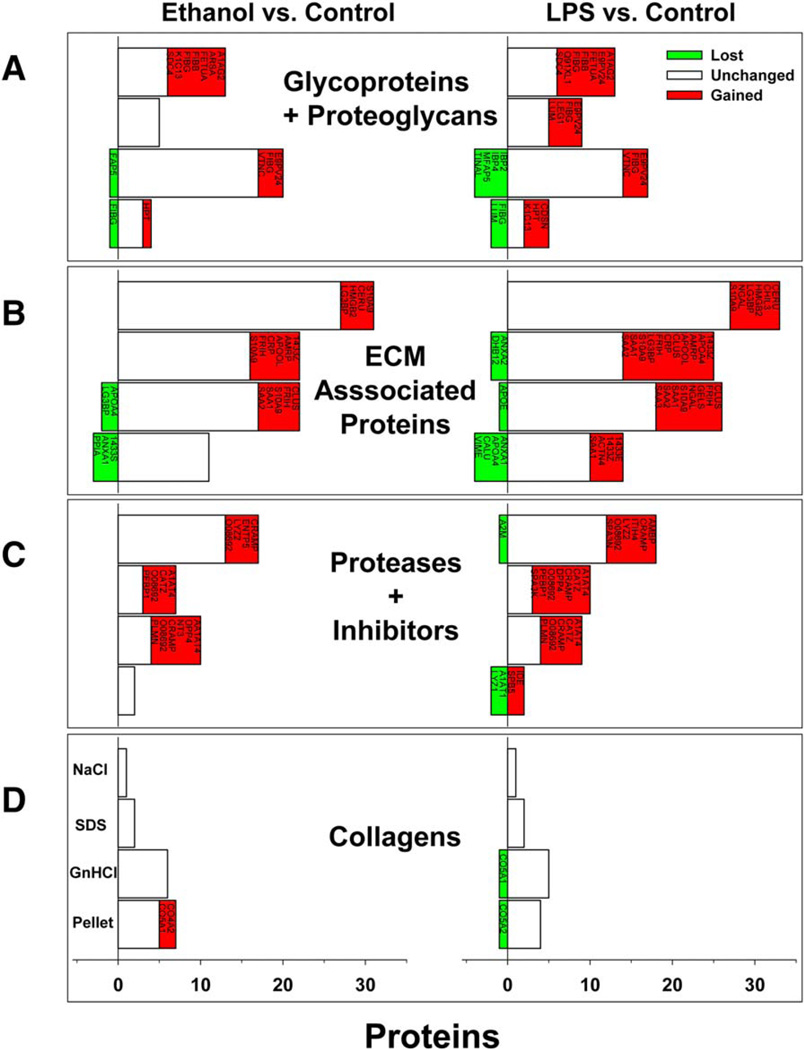
Figure 4 summarizes the abundance of proteins, organized by category (i.e., glycoproteins and proteoglycans, ECM-associated proteins, proteases, and inhibitors and collagens) across the four extraction conditions. The majority of the proteins in the NaCl and SDS extracts were ECM-associated proteins (class 2; Fig. 4A); this was expected, given that these are generally loosely associated with the ECM and are easily solubilized. Additionally, the low abundance of collagens in the NaCl and SDS fractions (Fig. 4D) was not surprising, given that collagens are often tightly cross-linked and require denaturing conditions for solubilization. As expected, the denaturing conditions created by GnHCl more than doubled the number of proteoglycans and glycoproteins in that extract compared to the NaCl and SDS fractions. The number of collagens in the GnHCl extract was also dramatically increased compared to the NaCl and SDS fractions. The pellet fraction contained the fewest proteins of all four extracts; this fraction has the greatest number of collagens.
FIG. 4.
Ethanol and LPS cause dynamic changes in the matrisome. The impact of ethanol diet (left panels) and 24-hour LPS (right panels) on the types of proteins found in the ECM proteome are shown. Proteins are categorized by class (A–D) and organized by extraction fraction (NaCl, SDS, GnHCl, and pellet). Red proteins indicate those that appeared with exposure, whereas green proteins indicate those that were lost with exposure, compared to control.
Exposure to ethanol and LPS, alone or combined, did not change the general pattern of proteins found in the various extracts (Fig. 4), but both tended to increase the total number of ECM proteins in the fractions combined. For example, ethanol exposure caused a net increase in the number of ECM proteins by ~25%. These changes were predominantly spread across the first three protein classes, with the NaCl, SDS, and GnHCl extracts all increasing to a similar extent. The least responsive protein class was the collagens (Fig. 4D). Likewise, the pellet fraction responded the least dynamically to ethanol or LPS exposure and actually showed a net loss in total proteins. Figure 5 (left panels) shows the distribution of the proteins in the various extracts between all four exposure groups.
FIG. 5.
Shared and unique changes to the hepatic matrisome. Venn diagrams (left column) show all proteins within an extract and indicate the number that are shared between, or that are unique to, the four experimental groups. Bubble plots (right column) show quantitative changes in protein expression of proteins that were shared by all four experimental groups. The bubble plots show fold change in protein expression caused by LPS (y-axis), ethanol (x-axis), and the combination of ethanol+LPS (bubble size). Each bubble represents a single extracellular protein; bubble color indicates the protein’s class.
In addition to proteins that changed in their extraction pattern in response to ethanol (e.g., see Fig. 3C), there were several proteins whose presence was unique to ethanol exposure compared to control (Figs. 4 and 5); these include fibrin(ogen) α and β chains, cytokeratin 13, vitronectin, plasminogen, high mobility group protein B2, and collagens IVα2 and Vα2 (see Supporting Table S1). Likewise, LPS exposure caused the appearance of several proteins that were unique compared to control (Figs. 4 and 5). Several of these proteins were shared with ethanol exposure, but there were also several that were unique, including serpine B5 (maspin), serine protease inhibitor A3N, and CXC motif chemokine ligand 9 (see Fig. 5; Supporting Table S1).
Previous work has shown that ethanol preexposure sensitizes the liver to inflammatory injury caused by a second insult (i.e., LPS).(16,17) Furthermore, previous studies have suggested that changes in ECM composition can contribute to the sensitizing effect of ethanol preexposure.(6) In this study, the combination of chronic ethanol exposure and a second hit of LPS caused unique changes in the ECM protein profile of the liver. The combination of EtOH+LPS resulted in the appearance of four unique proteins that were not present in livers from animals exposed to either ethanol or LPS alone, including serum amyloid P and serpine B9.
QUANTITATIVE CHANGES TO THE EXTRACELLULAR PROTEOME CAUSED BY STRESS
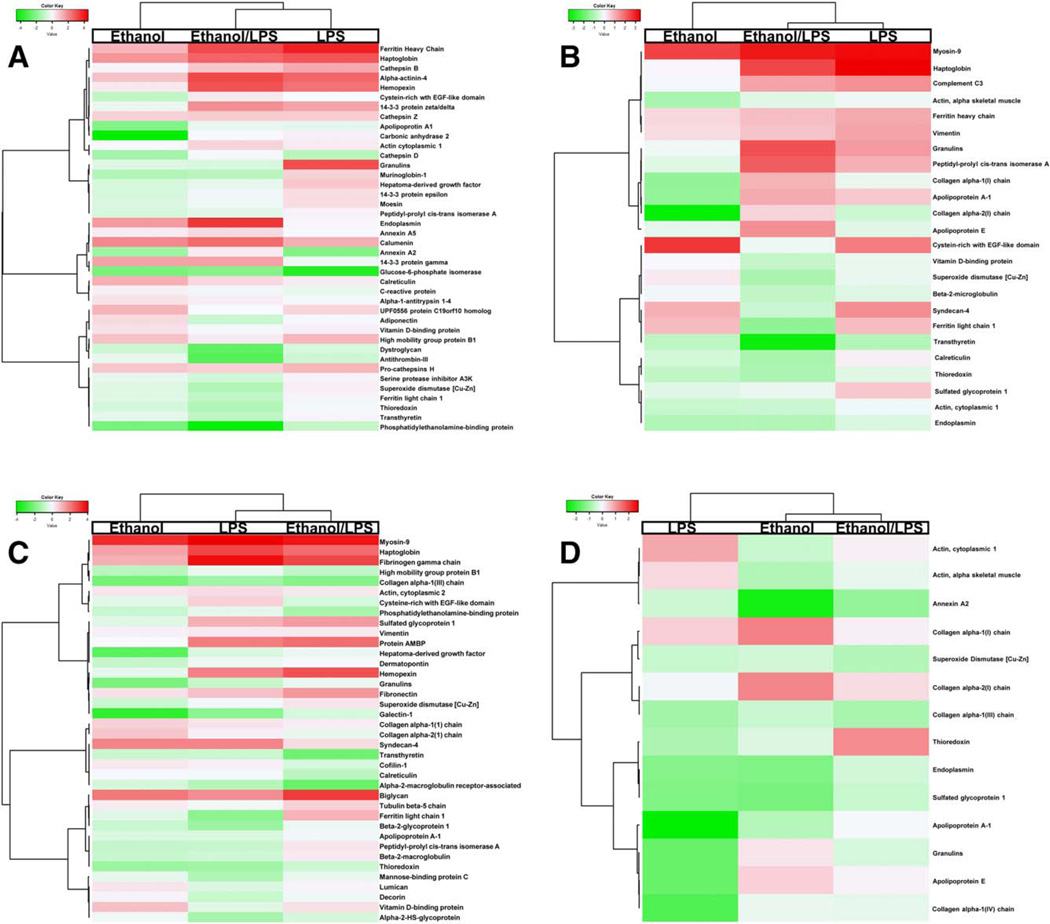
Dramatic changes to the ECM (i.e., “lost” or “gained”) can significantly impact overall organ function; it is not surprising that the majority of the matrisome did not change at the qualitative level. However, several of these proteins did change in relative abundance in response to ethanol and/or LPS (Figs. 5 and 6). Supporting Figs. S2–S5 provide results of the clustering analysis of the protein concentrations for each fraction. Based on this analysis, six clusters were identified as the best visual representation of the data. Supporting Table S3 identifies the membership of each protein within each cluster. Notable (>4-fold increase) changes caused by LPS included ferritin heavy chain (15-fold increase) in the NaCl fraction, haptoglobin and myosin-9 (11- and 9-fold, respectively) in the SDS fraction, and fibrinogen γ chain and haptoglobin (14-and 7-fold, respectively) in the GnHCl fraction. Likewise, ethanol exposure changed the abundance of several proteins, including myosin-9 (6-fold) and cysteine-rich with EGF-like domain protein 2 (CREDL2; 6-fold) in the SDS fraction. As was observed for qualitative analysis (Fig. 4), proteins in the pellet fraction responded the least to ethanol and/or LPS.
FIG. 6.
Quantitative changes to the matrisome. Heatmap analysis of the quantitative changes in protein expression of proteins that were shared by all four experimental groups (see Fig. 5) are shown for the NaCl (A), SDS (B), GnHCl (C), and pellet (D) extraction fractions.
In addition to the effects of ethanol and LPS, there were also proteins that responded uniquely to the combination of the two exposures (see Supporting Figs. S2–S5). For example, although LPS alone did not change endoplasmin levels and ethanol only increased it 3-fold, the combination of ethanol and LPS increased this protein 11-fold in the NaCl fraction (Figs. 5 and 6). A similar effect was observed on granulin expression in the SDS fraction, which was increased by 5-fold by ethanol and LPS despite no effect by ethanol alone and only a 2-fold increase by LPS (Figs. 5 and 6). Additionally, although both LPS and ethanol alone increased CRELD2, the combination of ethanol + LPS actually decreased the levels in the SDS fraction. In the GnHCl fraction, proteins that were differentially regulated by the combination of ethanol and LPS, including hemopexin and alpha-1-microglobulin/bikunin precursor.
Discussion
The goals of this work were 2-fold: (1) to characterize and validate a method of hepatic ECM protein extraction and analysis that would provide both the sensitivity to identify low-abundance proteins and the power to observe global changes in the ECM and (2) use this method to explore transitional (i.e., prefibrotic) changes to the hepatic matrisome caused by ethanol diet and/or LPS. As mentioned in the Introduction, the study of hepatic ECM proteins has largely been “collagenocentric” and “fibrosocentric”—that is, centered on the dramatic increase in collagen deposition during fibrosis, a quasi-permanent scarring of the organ. However, the matrisome of the healthy and diseased liver is significantly more diverse than collagen ECM. Indeed, studies have shown that in addition to collagen, laminin(18) and vitronectin(19,20) are also increased during fibrogenesis. Furthermore, proteomic-based studies in other organs have demonstrated that the matrisome responds dynamically in composition after insult well before fibrotic changes to the organ.(9,13,21) Previously, this group has shown that fibrin ECM accumulation correlates with inflammatory liver injury in several models and may well play a causal role in hepatic damage.(6) Additionally, Gillis et al.(5) have shown a similar role for fibronectin ECM in experimental alcoholic liver disease. However, little is known about global changes in the hepatic ECM during inflammatory liver injury. The models used (Lieber-DeCarli ethanol diet and acute LPS) are well known to cause significant liver damage, but do not result in histologically detectable changes to the ECM.
Global changes in the ECM may mediate tissue function through mechanisms that fall generally into three categories, including physical, biochemical, and signaling. Physical properties of the ECM include the matrix topography, organization, and cross-linking.(2) These physical properties support the structural role of the ECM, but can also regulate cell migration both by serving as a physical barrier or facilitator to that function.(22) Fibrin matrices have been shown to be permissive to chemotaxis and activation of monocytes and leukocytes.(23,24) Physical changes in the ECM can cause tissue rigidity, resulting in reduced organ function. Although such physical changes to the ECM can indirectly influence the biochemistry of the liver (e.g., hemostasis-induced hypoxia), changes to the matrisome can also directly cause biochemical changes that are independent of structural changes. For example, ECM components can facilitate interactions between ligands and receptors,(25) bind and retain chemokines,(1) and regulate activation of growth factors (i.e., transforming growth factor beta).(1) ECM molecules can also directly serve as signaling molecules through interactions with cell-surface receptors, including integrins.(26) Because of the multifaceted roles of many ECM molecules, any single change in the ECM can, in principle, trigger a cascade of dependent changes that influence the composition and properties of the ECM. For example, biglycan serves as a structural component that regulates collagen fiber assembly, but, upon release from the matrix, can serve as a signaling molecule binding to Toll-like receptor 4 (TLR4) receptors.(27)
In the current study, the individual and combined effects of two experimental exposures (ethanol and LPS) on the hepatic matrisome were determined. The rationale for selecting LPS is that it induces a robust inflammatory response in the liver. The liver is often exposed to LPS during several pathophysiologic states, including after alcohol consumption.(28) Whereas inflammatory responses triggered by small doses of LPS are typically noninjurious, other stresses can synergistically enhance the hepatotoxic response to LPS. Indeed, in addition to increasing circulating LPS, ethanol also enhances inflammation and liver damage caused by acute LPS exposure.(16) This “two-hit” paradigm is a common factor in fatty liver diseases.(29) The results here demonstrate that the hepatic matrisome responded dynamically to both stresses not only increasing the net amount of protein associated with the matrisome, but also differentially changing the amounts (Fig. 5) and likely the composition (Figs. 4 and 5) of the proteins found.
Several of the changes in proteins reported here reiterate results of previous hypothesis-driven studies. For example, this work validated that the fibrin(ogen), ECM, is dramatically altered by LPS and enhanced by the combination of ethanol and LPS. In the current study, LPS exposure increased the amount of fibrinogen gamma chain in the GnHCl extract. The fibrin(ogen) gamma chain is a major component of fibrin clots, given that it is polymerized into insoluble fibrin fibers.(30) Therefore, the localization of this robust increase in fibrinogen gamma chain in the GnHCl fractions suggests that there was an increase in fibrin(-ogen) gamma chain polymerization into a less-soluble, more highly cross-linked form. Furthermore, the combination of ethanol and LPS resulted in presence of the fibrin(ogen) gamma chain in the insoluble pellet, which suggests additional modifications that increased insolubility (e.g., cross-linking). The appearance of serum amyloid A-1 and A-2 proteins in response to LPS was also confirmed here.(31,32) In fact, LPS administration here increased the detection of several other acute-phase proteins, including haptoglobin, complement C, and ceruloplasmin, which are all known to be increased by LPS exposure.(33–35) This work validates previous studies with ethanol, which demonstrated increased fibronectin deposition before the onset of fibrosis.(5)
This technique also identified novel changes caused by ethanol and LPS. For example, vitronectin accumulation has previously been linked to HF and end-stage liver disease.(19,20) The findings of the current study suggest that more-subtle changes in vitronectin expression occur before the onset of fibrosis and hepatic decompensation. Ethanol exposure also resulted in the appearance of galectin-1. Galectin-1 is a glycosaminoglycan-binding lectin associated with cell proliferation and adhesion through modulation of glycoprotein cross-linking. Galectin-1 may also play a role in hepatic inflammation and fibrinogenesis.(36) These data suggest that ethanol and/or LPS is likely contributing to a myriad of changes in the ECM composition, many of which have not yet been fully investigated.
Changes in expression of protease and protease inhibitors can also contribute to inflammatory liver injury and fibrogenesis. In the current study, several ECM-associated proteases were increased in response to stress, including plasmin(ogen), antithrombin III, dipeptidyl peptidase, and alpha-1-antitrypsin. Stress also resulted in the presence of protease inhibitors, such as serpine B5 (maspin) and plasminogen activator inhibitor-1 (PAI-1). Several other proteases and protease inhibitors that may be critical for ECM homeostasis (e.g., transthyretin, phosphatidylethanolamin-binding protein-1 [PEBP1], and serine protease inhibitor A2K) increased in response to ethanol and/or LPS. These data support the notion that transitional remodeling of the hepatic matrisome is likely bidirectional and driven by both increased secretion of matrix proteins as well as changes in ECM degradation.
As mentioned above, ethanol is well known to synergize liver damage caused by LPS exposure. In the current study, the combination of ethanol and LPS resulted in unique changes to the hepatic matrisome compared to either ethanol or LPS alone. Indeed, fibronectin and biglycan expression were all differentially increase by coexposure (Fig. 3; Supporting Table S1). Fibronectin accumulation caused by ethanol may contribute to hepatic inflammation through stimulation of Kupffer cells (KCs).(37) Biglycan is a small proteoglycan was first recognized as a structural component and signaling molecule in the ECM,(38) but has also been implicated in inflammation,(39) potentially by retaining proinflammatory cytokines,(40) and/or by activating TLR4 signaling.(41) These data suggest that bigly-can expression may be increased in prefibrotic stages of liver disease. In contrast, the combination of ethanol and LPS synergistically decreased PEBP1; this enzyme has been shown to inhibit trypsin-like serine proteases, including thrombin, but not trypsin or tissue-type plasminogen activator.(42) Multiple studies have identified PEBP1 as a critical player in metastasis(43) and have defined it as a metastasis suppressor gene.(44) These changes represent dynamic (and potentially unique) responses of hepatic ECM to stress that may serve as a basis for future biomarker and/or mechanistic studies.
Whereas it is well understood that the primary source of fibrillar ECM during HF is myofibroblast-like cells (e.g., hepatic stellate cells), most, if not all, hepatic cells contribute to overall matrisome homeostasis. For example, hepatic sinusoidal endothelial cells are almost exclusively responsible for the metabolism and degradation of hyaluronic acid. Furthermore, inflammatory cells (e.g., KCs) and hepatocytes are well known to release proteases and protease inhibitors (e.g., PAI-1) in response to stress that can influence the ECM. Extrahepatic sources (e.g., the coagulation and complement cascades) can also influence the hepatic matrisome. The ECM not only serves as a physical structure, but also binds/ interacts with several biomolecules that can directly or indirectly alter responses. For example, ECM/integrin interactions mediate rapid and flexible responses to changes in the environment. It is known that fibrotic ECM is known to impact cell phenotype, inflammation, and metastasis in the liver.(45–47) The impact of the ECM changes observed here on earlier stages of liver injury should be determined.
In summary, the results demonstrate that the hepatic matrisome responds dynamically to both acute (LPS) and chronic (ethanol) stresses, preceding more-dramatic fibrotic changes to the liver. It is likely that these transitional changes to the hepatic ECM contribute to the pathological responses to these stresses. It is also interesting that several ECM proteins responded similarly to both stresses, suggesting a common mechanism in both models. The changes in proteins that were unique to either exposure alone (or their combination) also represents potential new biomarkers or targets. These results therefore also serve as a foundation for future analyses in hepatic models of liver disease.
Supplementary Material
Acknowledgments
Supported, in part, by grants from NIAAA (R01 AA021978; P50 AA024337, and R01 AA021978S1). V.L.M. was supported by a predoctoral training grant (T32 ES011564).
Abbreviations
- CREDL2
cysteine-rich with EGF-like protein domain 2
- ECM
extracellular matrix
- GO
gene ontology
- HF
hepatic fibrosis
- KCs
Kupffer cells
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- LPS
lipopolysaccharide
- PAI-1
plasminogen activator inhibitor-1
- PEBP1
phosphatidylethanolamin-binding protein-1
- SDS
sodium dodecyl sulfate
- TLR4
Toll-like receptor 4
Footnotes
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28918/suppinfo.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Stellate cell activation in alcoholic fibrosis—an overview. Alcohol Clin Exp Res. 1999;23:904–910. [PubMed] [Google Scholar]
- 4.Gressner OA, Weiskirchen R, Gressner AM. Evolving concepts of liver fibrogenesis provide new diagnostic and therapeutic options. Comp Hepatol. 2007;6:7. doi: 10.1186/1476-5926-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillis SE, Nagy LE. Deposition of cellular fibronectin increases before stellate cell activation in rat liver during ethanol feeding. Alcohol Clin Exp Res. 1997;21:857–861. [PubMed] [Google Scholar]
- 6.Beier JI, Luyendyk JP, Guo L, von Montfort C, Staunton DE, Arteel GE. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009;49:1545–1553. doi: 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, et al. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Castro Bras LE, Ramirez TA, DeLeon-Pennell KY, Chiao YA, Ma Y, Dai Q, et al. Texas 3-step decellularization protocol: looking at the cardiac extracellular matrix. J Proteomics. 2013;86:43–52. doi: 10.1016/j.jprot.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naba A, Clauser KR, Hynes RO. Enrichment of extracellular matrix proteins from tissues and digestion into peptides for mass spectrometry analysis. J Vis Exp. 2015;(101):e53057. doi: 10.3791/53057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Montfort C, Beier JI, Kaiser JP, Guo L, Joshi-Barve S, Pritchard MT, et al. PAI-1 plays a protective role in CCl4-induced hepatic fibrosis in mice: role of hepatocyte division. Am J Physiol Gastrointest Liver Physiol. 2010;298:G657–G666. doi: 10.1152/ajpgi.00107.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massey VL, Poole LG, Siow DL, Torres E, Warner NL, Schmidt RH, et al. Chronic alcohol exposure enhances lipopolysaccharide-induced lung injury in mice: potential role of systemic tumor necrosis factor-alpha. Alcohol Clin Exp Res. 2015;39:1978–1988. doi: 10.1111/acer.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics. 2010;9:2048–2062. doi: 10.1074/mcp.M110.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason RM, Mayes RW. Extraction of cartilage proteinpolysaccharides with inorganic salt solutions. Biochem J. 1973;131:535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sajdera SW, Hascall VC. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969;244:77–87. [PubMed] [Google Scholar]
- 16.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Brenner DA, et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, Hill D, Schmidt J, et al. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):171S–181S. doi: 10.1097/00000374-200105051-00029. [DOI] [PubMed] [Google Scholar]
- 18.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi J, Yamada S, Kawasaki H. Distribution of vitronectin in plasma and liver tissue: relationship to chronic liver disease. Hepatology. 1994;20:1412–1417. doi: 10.1002/hep.1840200606. [DOI] [PubMed] [Google Scholar]
- 20.Koukoulis GK, Shen J, Virtanen I, Gould VE. Vitronectin in the cirrhotic liver: an immunomarker of mature fibrosis. Hum Pathol. 2001;32:1356–1362. doi: 10.1053/hupa.2001.29675. [DOI] [PubMed] [Google Scholar]
- 21.Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, et al. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.008128. M111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holdsworth SR, Thomson NM, Glasgow EF, Atkins RC. The effect of defibrination on macrophage participation in rabbit nephrotoxic nephritis: studies using glomerular culture and electronmicroscopy. Clin Exp Immunol. 1979;37:38–43. [PMC free article] [PubMed] [Google Scholar]
- 24.Loike JD, el Khoury J, Cao L, Richards CP, Rascoff H, Mandeville JT, et al. Fibrin regulates neutrophil migration in response to interleukin 8, leukotriene B4, tumor necrosis factor, and formyl-methionyl-leucyl-phenylalanine. J Exp Med. 1995;181:1763–1772. doi: 10.1084/jem.181.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonai P. Epithelial mesenchymal interactions, the ECM and limb development. J Anat. 2003;202:43–50. doi: 10.1046/j.1469-7580.2003.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 27.Nastase MV, Young MF, Schaefer L. Biglycan: a multivalent proteoglycan providing structure and signals. J Histochem Cytochem. 2012;60:963–975. doi: 10.1369/0022155412456380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bode CH, Kugler V, Bode JCH. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 29.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 30.Mosesson MW. Fibrinogen gamma chain functions. J Thromb Haemost. 2003;1:231–238. doi: 10.1046/j.1538-7836.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 31.Migita K, Abiru S, Nakamura M, Komori A, Yoshida Y, Yokoyama T, et al. Lipopolysaccharide signaling induces serum amyloid A (SAA) synthesis in human hepatocytes in vitro. FEBS Lett. 2004;569:235–239. doi: 10.1016/j.febslet.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 32.Pruett BS, Pruett SB. An explanation for the paradoxical induction and suppression of an acute phase response by ethanol. Alcohol. 2006;39:105–110. doi: 10.1016/j.alcohol.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bopst M, Haas C, Car B, Eugster HP. The combined inactivation of tumor necrosis factor and interleukin-6 prevents induction of the major acute phase proteins by endotoxin. Eur J Immunol. 1998;28:4130–4137. doi: 10.1002/(SICI)1521-4141(199812)28:12<4130::AID-IMMU4130>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 34.Haziot A, Lin XY, Zhang F, Goyert SM. The induction of acute phase proteins by lipopolysaccharide uses a novel pathway that is CD14-independent. J Immunol. 1998;160:2570–2572. [PubMed] [Google Scholar]
- 35.Sun S, Guo Y, Zhao G, Zhou X, Li J, Hu J, et al. Complement and the alternative pathway play an important role in LPS/D-GalN-induced fulminant hepatic failure. PLoS One. 2011;6:e26838. doi: 10.1371/journal.pone.0026838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–8849. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aziz-Seible RS, Lee SM, Kharbanda KK, McVicker BL, Casey CA. Ethanol feeding potentiates the pro-inflammatory response of Kupffer cells to cellular fibronectin. Alcohol Clin Exp Res. 2011;35:717–725. doi: 10.1111/j.1530-0277.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- 38.Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- 39.Mohan H, Krumbholz M, Sharma R, Eisele S, Junker A, Sixt M, et al. Extracellular matrix in multiple sclerosis lesions: Fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 2010;20:966–975. doi: 10.1111/j.1750-3639.2010.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hengst U, Albrecht H, Hess D, Monard D. The phosphatidylethanolamine-binding protein is the prototype of a novel family of serine protease inhibitors. J Biol Chem. 2001;276:535–540. doi: 10.1074/jbc.M002524200. [DOI] [PubMed] [Google Scholar]
- 43.Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX, et al. PEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infection. J Hepatol. 2010;53:872–879. doi: 10.1016/j.jhep.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Keller ET, Fu Z, Brennan M. The biology of a prostate cancer metastasis suppressor protein: Raf kinase inhibitor protein. J Cell Biochem. 2005;94:273–278. doi: 10.1002/jcb.20169. [DOI] [PubMed] [Google Scholar]
- 45.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 46.Ford AJ, Jain G, Rajagopalan P. Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomater. 2015;24:220–227. doi: 10.1016/j.actbio.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Zeisberg M, Lively JC, Nyberg P, Afdhal N, Kalluri R. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003;63:8312–8317. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.