Abstract
BACKGROUND
Stage II and III rectal cancers have been effectively treated with neoadjuvant chemoradiotherapy (NCRT) followed by definitive resection. Advancements in surgical technique and systemic therapy have prompted investigation of neoadjuvant multi-agent chemotherapy (NMAC) regimens with the elimination of radiation (RT). The purpose of our study was to investigate factors predicting for the use of NCRT versus NMAC and compare outcomes using the National Cancer Data Base (NCDB) for select Stage II/III rectal cancers.
METHODS
In the NCDB, 21,707 patients from 2004-2012 with clinical T2N1, T3N0, or T3N1 rectal cancers were identified treated with NCRT or NMAC followed by low anterior resection (LAR). Kaplan-Meier method, log-rank test, and Cox-proportional hazard regression analyses were conducted along with propensity-score-matching (PSM) analysis to reduce treatment selection bias.
RESULTS
5-year actuarial overall survival (OS) was 75.0% for patients receiving NCRT versus 67.2% for patients receiving NMAC (p<0.01). On MVA, NCRT patients had improved OS (HR=0.77, p<0.01); this effect was confirmed on PSM analysis (HR=0.72, p=0.01). In the same model, age <65 years old, having private insurance, treatment at an academic center, living in an affluent zip code, low co-morbidity score, receipt of adjuvant chemotherapy, and shorter duration to surgery had improved OS (all p<0.05). African-American race, male gender, high grade tumors, cT3N1 tumors, and R1 resections had worse OS (all p<0.05).
CONCLUSIONS
In this series, elimination of neoadjuvant RT for select Stage II/III rectal adenocarcinoma was associated with worse OS and should not be recommended outside of a clinical trial.
Keywords: Rectal cancer, neoadjuvant chemoradiation, neoadjuvant chemotherapy, National Cancer Database (NCDB), health disparities
INTRODUCTION
An estimated 40,000 new cases of rectal cancer are diagnosed in the United States annually1. Since 1990, the standard of care for Stage II (T3 and T4 tumors as defined by the AJCC staging system2) and Stage III (N1 or N2 disease as defined by the AJCC staging system2) is tri-modality therapy of radiation, chemotherapy, and surgery3. Historical studies showed that pelvic radiation (RT) reduced local rectal cancer recurrence and increased the chance for sphincter preservation with surgery4-6. The sequencing of chemoradiotherapy (CRT) for Stage II and III tumors was defined based on a landmark German randomized trial showing that neoadjuvant CRT had reduced local recurrence, improved sphincter preservation, and improved side effect profile7, 8. These results were confirmed in the United States with NSABP R-039, and as a result the standard of care for locally advanced rectal cancers is preoperative CRT with 5-FU followed by surgical resection and additional adjuvant chemotherapy.
Recently, with the incorporation of total mesorectal excision (TME) surgical principles10, 11 and improved systemic therapy12, 13, the benefit of pelvic radiation is being challenged. CRT is associated with short term toxicity in 50% of patients as well as long term effects including fibrosis, fecal incontinence, bladder and sexual dysfunction, and myelosuppression14-17. From a chemotherapy perspective, less toxicity has been reported with preoperative chemotherapy versus postoperative18. This rationale prompted investigational use of neoadjuvant multi-agent chemotherapy (NMAC) followed by excision, eliminating pelvic radiation, with a pilot trial showing promising results19. The accruing PROSPECT Trial is implementing this paradigm nationally for clinically staged T3N0, T3N1, and T2N1 rectal cancer20. Given the small number of patients studied with this evolving regimen, we used the National Cancer Data Base (NCDB) to identify patients who appear to meet eligibility criteria for the PROSPECT trial to determine the impact of NMAC versus neoadjuvant chemoradiotherapy (NCRT) on overall survival (OS), as well as determine variables that predicted for patients receiving NMAC versus NCRT.
METHODS
NCDB Criteria
The NCDB is a large, prospectively acquired database, drawn from cases from Commission on Cancer (CoC) accredited institutions across the United States, capturing approximately 70% of all newly diagnosed malignancies in the United States. The dataset includes detailed information on patient, disease, and treatment characteristics as well as survival outcomes. This database includes detailed radiotherapy (RT) information regarding treatment site, radiation source and dose, as well as detailed surgical and pathological information including type of surgery, margin status, and number of lymph nodes removed.
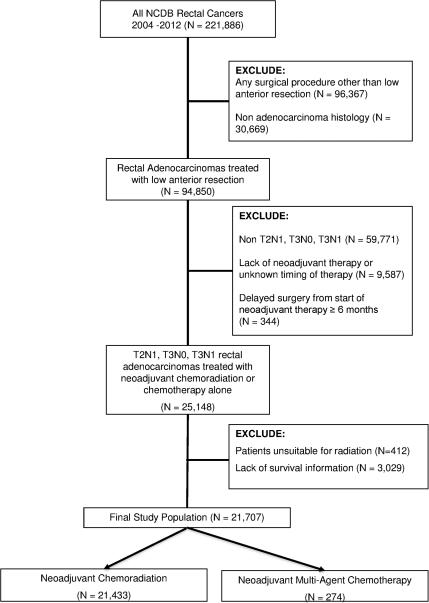
The rectal NCDB was queried for patients over the age of 18 diagnosed from 2004 to 2012. Patients with clinically staged T2N1, T3N0, and T3N1 rectal adenocarcinomas treated with NCRT or NMAC followed by a low anterior resection (LAR) were included. Patients treated with abdominoperineal resection, did not receive neoadjuvant therapy, treated with nonmegavoltage photon beam radiation, received single agent chemotherapy alone and patients who had medical reasons to not receive radiation or lack of survival information were excluded (Figure 1). The following patient characteristics were examined: age (<65, ≥65 years old), race (white, African-American, other), sex, treatment facility type (community cancer center, comprehensive community cancer center, or academic/research program), treatment facility geographical region, insurance coverage (not insured, private insurance, government insurance [including Medicaid and VA], and Medicare insurance), patient county of residence (urban, rural, or metro), distance from patient residence zip code to treatment facility (<10 miles, 10-50 miles, or >50 miles), year of diagnosis (2004-2006, 2007-2009, and 2010-2012), patient education and income based on county of residence (determined by 2012 American Community Survey administered by the U.S. Census), and co-morbidities as quantified by the Charlson-Deyo score21, 22. The following disease characteristics were evaluated: tumor grade (low, moderately, or poorly differentiated), lymphovascular invasion, serum carcinoembryonic antigen (positive or negative as defined by values above the upper limit of normal), tumor stage, pathological downstaging (complete response defined as ypT0N0, partial response defined as any T or N regression, and no response), and tumor regression grade (based on pathological assessment, ranging from complete response [no viable cancer cells] to poor response [extensive residual cancer cells]). Finally, the following treatment characteristics were also evaluated: receipt of NMAC or NCRT, time from end of neoadjuvant therapy to surgery (<1 month, 1-3 months, and >3 months), extent of resection (R0 or R1), number of lymph nodes surgically examined (0-7, 8-12, 12-17, and ≥18), and use of adjuvant chemotherapy (yes or no).
Figure 1.
CONSORT diagram of the study population.
Statistical Methods
Statistical analysis was conducted using SAS Version 9.4, and SAS macros developed by Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute23. Descriptive statistics for each variable were determined. Follow up was determined by the reverse Kaplan-Meier method24. The univariate association of each covariate with receipt of NCRT versus NAMC was assessed using the Chi-square test for categorical covariates and ANOVA for numerical covariates. The primary endpoint of OS was defined as months from LAR to death from any cause or last follow up if alive. The univariate association (UVA) of each variable with OS was tested by Cox proportional hazards models and log-rank tests. A multivariable Cox proportional hazard model (MVA) was fit by a backward elimination method applying p = 0.20 removal criteria. The effect of CRT vs. NAMC on OS stratified by different variables was estimated by testing the interaction effect between each modality and each relevant variable. Of note, lymphovascular space invasion, serum carcinoembryonic antigen, and tumor regression grade were eliminated from MVA due to the high number of patients missing this data. Kaplan-Meier plots were produced to compare the survival curves by subgroups along with log-rank p-value, and <0.05 was considered statistically significant.
Propensity score matching (PSM) method was then conducted to address a potential treatment selection bias. A logistic regression model predicting use of CRT versus NAMC was carried out to predict the propensity score by age, race, facility type and location, insurance status, county of residence, distance to treatment facility from home zip code, year of diagnosis, education and income level of home zip code, Charlson-Deyo score, lymph node positivity, T2N1 vs. T3N0 vs. T3N1 tumors, tumor grade, surgical margin, number of regional nodes examined, time from start of neoadjuvant therapy to surgery, and use of adjuvant chemotherapy. Patients from NMAC were matched to NCRT patients at a ratio of 1:5 based on the propensity score using a greedy algorithm and using a SAS macro provided by the Mayo Clinic25. The matching caliper was set at 0.2 standard deviation of logit of the propensity score. After matching, the balance of covariate between two cohorts was evaluated by the standardized differences and a value of < 0.1 was considered as negligible imbalance26. The effects were estimated in the matched sample by a Cox model with a robust variance estimator27 for OS.
RESULTS
Patient Characteristics
A total of 21,707 patients met study entry criteria (see Figure 1); of these 274 (1.3%) were treated with NMAC. The median age at diagnosis was 60 years (range 18-90 years old). Overall, the median follow-up was 123.3 months. The median distance from a patient's zip code to treatment facility was 11.2 miles. The median time from the end of neoadjuvant therapy to low anterior resection was 62 days. For patients in the NCRT group, the median dose of RT was 50.4 Gy (range 45 to 60 Gy). The median daily dose of RT was 1.8 Gy. Table 1 summarizes the study population.
Table 1.
Summary of patient demographic, socioeconomic, tumor, and treatment characteristics.
| Variable | N = 21,707 (%) |
|---|---|
|
Patient Characteristics
| |
| Age at Diagnosis (Years) | |
| <65 | 13,508 (62.2) |
| ≥65 | 8,199 (37.8) |
| Sex | |
| Male | 13,616 (62.7) |
| Female | 8,091 (37.3) |
| Race | |
| White | 18,932 (87.2) |
| African American | 1,669 (7.7) |
| Other/Missing | 1,106 (5.1) |
| Facility Type | |
| Community Cancer Program | 3,728 (17.2) |
| Comprehensive Community Cancer Program | 9,583 (44.1) |
| Academic/Research Program | 7,452 (34.3) |
| Unknown | 944 (4.3) |
| Facility Location | |
| Northeast | 3,996 (18.4) |
| South | 7,234 (33.3) |
| Midwest | 6,582 (30.3) |
| West | 2,951 (13.6) |
| Unknown | 944 (4.3) |
| Distance from Home Zip Code to Treatment Facility | |
| <10 Miles | 9,871 (46.0) |
| 10-50 Miles | 8,954 (41.7) |
| >50 Miles | 2,638 (12.3) |
| Missing | 244 |
| Insurance Status | |
| Not Insured | 959 (4.4) |
| Private Insurance | 11,250 (51.8) |
| Government Insurance (Medicaid & VA) | 1,464 (6.7) |
| Medicare | 7,618 (35.1) |
| Missing | 416 (1.9) |
| County of Residence | |
| Rural | 527 (2.4) |
| Urban | 3,958 (18.2) |
| Metro | 16,617 (76.6) |
| Missing | 605 (2.8) |
| Median Income Quartile of County of Residence | |
| <$30,000 | 2,605 (12.0) |
| $30,000 - $35,999 | 3,994 (18.4) |
| $36,000 - $45,999 | 6,018 (27.7) |
| $46,000+ | 8,335 (38.4) |
| Missing | 755 (3.5) |
| Percent No High School Degree in Patient County of Residence | |
| ≥29% | 3,275 (15.1) |
| 20-28.9% | 4,912 (22.6) |
| 14-19.9% | 5,328 (24.5) |
| <14% | 7,435 (34.3) |
| Missing | 757 (3.5) |
| Year of Diagnosis | |
| 2004-2006 | 5,299 (24.4) |
| 2007-2009 | 7,247 (33.4) |
| 2010-2012 | 9,161 (42.2) |
| Charlson-Deyo Score | |
| 0 | 17,480 (80.5) |
| 1 | 3,432 (15.8) |
| 2+ | 795 (3.7) |
|
Tumor Characteristics | |
| Stage | |
| T2N1 | 1,056 (4.9) |
| T3N0 | 11,366 (52.4) |
| T3N1 | 9,285 (42.8) |
| Grade | |
| Well Differentiated | 1,522 (7.0) |
| Moderately Differentiated | 14,969 (69.0) |
| Poorly Differentiated | 2,265 (10.4) |
| Not Determined/Missing | 2,951 (13.6) |
| Lymphovascular Invasion | |
| Yes | 891 (4.1) |
| No | 6,059 (86.8) |
| Missing | 14,726 |
| Carcinoembryonic Antigen | |
| Positive | 5,517 (40.9) |
| Negative | 7,985 (59.1) |
| Missing | 8,205 |
|
Treatment Characteristics | |
| Type of Neoadjuvant Therapy | |
| Multi-agent Chemotherapy (NMAC) | 274 (1.3) |
| Concurrent Chemoradiotherapy (NCRT) | 21,433 (98.7) |
| Tumor Regression Grade Based on Pathological Response | |
| Complete Response (no viable cancer cells) | 1,793 (52.8) |
| Moderate Response (single cells or small groups) | 1,051 (31.0) |
| Minimal Response (residual cancer) | 550 (16.2) |
| Poor Response (extensive residual cancer) | 43 |
| Missing | 16,866 |
| Pathological Downstaging | |
| Complete (ypT0N0) | 2,236 (13.2) |
| Partial Response | 6,176 (36.5) |
| No Response | 8,517 (50.3) |
| Missing | 4,778 |
| Number of Regional Lymph Nodes Examined | |
| 0-7 | 6,063 (28.7) |
| 8-11 | 5,238 (24.8) |
| 12-17 | 5,224 (24.7) |
| 17+ | 4,617 (21.8) |
| Missing | 565 |
| Extent of Resection | |
| R0 | 20,061 (92.4) |
| R1 | 891 (4.1) |
| Missing | 755 (3.5) |
| Time from End of Neoadjuvant Therapy to Surgery | |
| <1 month | 226 (1.0) |
| 1-3 months | 10,512 (48.4) |
| 3-6 months | 10,969 (50.5) |
| Adjuvant Chemotherapy | |
| Yes | 6,047 (33.0) |
| No | 12,251 (67.0) |
| Missing | 3,409 |
Predictors of Receipt of Neoadjuvant Treatment Type
On UVA, age less than 65 years, northeast and midwest practice locations, having government insurance or lack of insurance, patient's zip code within 50 miles of treatment facility and treatment at community based programs were demographic factors predictive of receiving NCRT. Node positive tumors (T2N1 or T3N1), having a complete pathological response, and time from the end of neoadjuvant therapy to surgery of 1-3 months were tumor and treatment factors associated with receipt of NCRT versus NMAC (all p <0.05; see Supplemental Table 1). On MVA, distance from home zip code to treatment facility of less than 50 miles, lack of insurance, and T3N1 tumors, were all more likely to receive NCRT versus NMAC (all p≤0.01; see Table 2).
Table 2.
Multivariable Analysis* of factors predicting for the use of neoadjuvant chemoradiotherapy (NCRT) over neoadjuvant multi-agent chemotherapy (NMAC).
| Variable | Odds Ratio (95% CI) | OR P-value | P-value |
|---|---|---|---|
|
Patient Characteristics
| |||
| Insurance Status | |||
| Unknown | 0.29 (0.11-0.80) | 0.02 | |
| Medicare | 0.46 (0.21-1.00) | 0.05 | <0.01 |
| Government Insurance (Medicaid & VA) | 0.60 (0.25-1.44) | 0.25 | |
| Private Insurance | 0.68 (0.32-1.47) | 0.33 | |
| Not Insured | -- | -- | |
| Distance from Home Zip Code to Treatment Facility | |||
| <10 Miles | 2.44 (1.65-3.60) | <0.01 | |
| 10-50 Miles | 1.65 (1.16-2.36) | 0.01 | <0.01 |
| >50 Miles | -- | -- | |
| Facility Location | |||
| Unknown | 1.11 (0.53-2.30) | 0.79 | |
| West | 0.68 (0.45-1.03) | 0.07 | 0.06 |
| Midwest | 1.17 (0.80-1.73) | 0.42 | |
| South | 0.85 (0.60-1.23) | 0.40 | |
| Northeast | -- | -- | |
| County of Residence | |||
| Unknown | 1.04 (0.43-2.56) | 0.93 | |
| Rural | 2.07 (0.83-5.17) | 0.12 | 0.11 |
| Urban | 1.46 (1.03-2.07) | 0.04 | |
| Metro | -- | -- | |
|
Tumor Characteristics | |||
| Grade | |||
| Well Differentiated | 0.74 (0.45-1.21) | 0.23 | |
| Moderately Differentiated | 1.17 (0.83-1.66) | 0.37 | 0.15 |
| Poorly Differentiated | 1.20 (0.72-1.97( | 0.49 | |
| Not Determined/Missing | -- | -- | |
| Stage | |||
| T2N1 | 1.21 (0.61-2.40) | 0.59 | |
| T3N0 | 0.70 (0.54-0.90) | 0.01 | 0.01 |
| T3N1 | -- | -- | |
|
Treatment Characteristics | |||
| Number of Regional Lymph Nodes Examined | |||
| 0-7 | 0.80 (0.35-1.87) | 0.61 | |
| 8-12 | 0.90 (0.38-2.10) | 0.80 | |
| 12-17 | 0.72 (0.31-1.68) | 0.46 | 0.15 |
| 17+ | 0.56 (0.14-1.29) | 0.18 | |
| Missing | -- | -- | |
| Time from End of Neoadjuvant Therapy to Surgery | |||
| <1 month | 1.21 (0.61-2.40) | 0.59 | |
| 1-3 months | 0.70 (0.54-0.90) | 0.01 | 0.01 |
| 3-6 months | -- | -- | |
Backward selection with an alpha level of removal of .2 was used. The following variables were removed from the model: Age at diagnosis, Charlson-Deyo Score, facility type, median income of county of residence, percent of county of residence without high school degree, race, surgical margins, sex, use of adjuvant chemotherapy, and year of diagnosis.
Impact of Neoadjuvant Treatment Type on Overall Survival
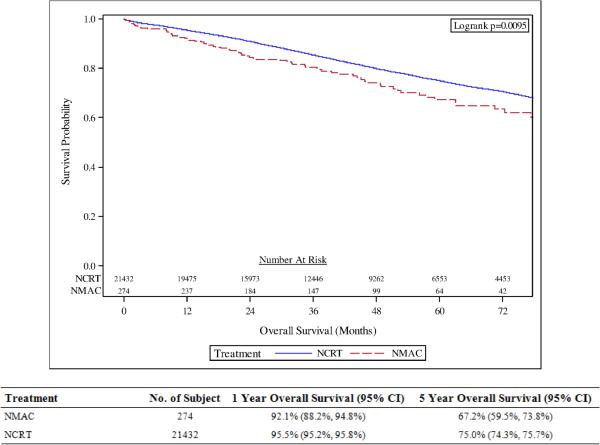
The actuarial 5-year OS was 75.0% for patients treated with NCRT compared with 67.2% for patients treated with NMAC (p<0.01; see Figure 2). NCRT was associated with improved OS on UVA (HR=0.74, 95% CI 0.58-0.93; p <0.01; see Supplemental Table 2) and MVA (HR=0.77, 95% CI 0.61-0.97; p=0.03). On MVA, additional variables associated with improved OS included age <65 years old, treatment at an academic cancer program, having private insurance, living in a zip code with median income greater than $46,000, Charlson-Deyo score of 0 or 1, having at least 12 lymph nodes examined during surgery, and LAR within 1-3 months from the end of neoadjuvant therapy (all p<0.05; see Table 3). Additional variables associated with worse OS on MVA included African-American race, male gender, diagnosis between 2010-2012, poorly differentiated tumors, cT3N1 tumors, R1 resections, and patients not receiving adjuvant chemotherapy (all p<0.05; see Table 3). Use of NCRT did not have significant interaction effect with tumor stage, facility type, and time from end of neoadjuvant therapy to surgery on overall survival (all p>0.10) indicating a benefit to NCRT across all levels of these variables.
Figure 2.
Overall survival amongst patients treated with neoadjuvant chemoradiotherapy (NCRT) compared with patients treated with neoadjuvant multi-agent chemotherapy (NMAC).
Table 3.
Multivariable Analysis* of patient, tumor, and treatment factors and their association with overall survival.
| Variable | Hazard Ratio (HR) (95% CI) | HR P-value | P-value |
|---|---|---|---|
|
Patient Characteristics
| |||
| Age at Diagnosis (Years) | |||
| ≥65 | 1.54 (1.38-1.71) | <0.01 | <0.01 |
| <65 | -- | -- | |
| Facility Type | |||
| Comprehensive Community Cancer Program | 1.02 (0.93-1.11) | 0.74 | <0.01 |
| Academic/Research Program | 0.85 (0.77-0.94) | 0.01 | |
| Unknown | 0.74 (0.60-0.93) | 0.01 | |
| Community Cancer Program | -- | -- | |
| Race | |||
| Other/Missing | 0.93 (0.78-1.10) | 0.38 | 0.01 |
| African American | 1.22 (1.08-1.37) | 0.01 | |
| White | -- | -- | |
| Sex | |||
| Male | 1.22 (1.14-1.31) | <0.01 | <0.01 |
| Female | -- | -- | |
| Insurance Status | |||
| Missing/Unknown | 0.77 (0.54-1.11) | 0.16 | |
| Private Insurance | 0.71 (0.60-0.84) | <0.01 | <0.01 |
| Government Insurance (Medicaid & VA) | 0.98 (0.80-1.20) | 0.83 | |
| Medicare | 0.98 (0.81-1.18) | 0.83 | |
| Not Insured | -- | -- | |
| Median Income Quartile of County of Residence | |||
| Missing/Unknown | 1.37 (1.13-1.64) | <0.01 | |
| <$30,000 | 1.32 (1.18-1.47) | <0.01 | <0.01 |
| $30,000 - $35,999 | 1.17 (1.06-1.28) | <0.01 | |
| $36,000 - $45,999 | 1.25 (1.15-1.35) | <0.01 | |
| $46,000+ | -- | -- | |
| Charlson-Deyo Score | |||
| 0 | 0.50 (0.44-0.57) | <0.01 | <0.01 |
| 1 | 0.65 (0.57-0.75) | <0.01 | |
| 2+ | -- | -- | |
| Year of Diagnosis | |||
| 2004-2006 | 0.87 (0.77-0.97) | 0.01 | 0.01 |
| 2007-2009 | 0.89 (0.82-0.96) | <0.01 | |
| 2010-2012 | -- | -- | |
| County of Residence | |||
| Missing/Unknown | 1.20 (1.01-1.43) | 0.04 | |
| Rural | 0.90 (0.75-1.09) | 0.28 | 0.11 |
| Urban | 0.97 (0.89-1.05) | 0.40 | |
| Metro | -- | -- | |
|
Tumor Characteristics | |||
| Grade | |||
| Well Differentiated | 1.15 (0.99-1.35) | 0.07 | <0.01 |
| Moderately Differentiated | 1.11 (1.00-1.23) | 0.06 | |
| Poorly Differentiated | 1.70 (1.49-1.94) | <0.01 | |
| Not Determined/Missing | |||
| Stage | |||
| T2N1 | 0.76 (0.63-0.91) | <0.01 | |
| T3N0 | 0.88 (0.83-0.95) | <0.01 | <0.01 |
| T3N1 | -- | ||
|
Treatment Characteristics | |||
| Type of Neoadjuvant Therapy | |||
| Concurrent Chemoradiotherapy (NCRT) | 0.72 (0.56-0.93) | 0.01 | 0.01 |
| Multi-agent Chemotherapy (NMAC) | -- | -- | |
| Surgical Margin | |||
| Missing/Unknown | 1.04 (0.85-1.28) | 0.68 | <0.01 |
| Positive | 2.53 (2.24-2.85) | <0.01 | |
| Negative | -- | -- | |
| Number of Regional Lymph Nodes Examined | |||
| Missing/Unknown | 0.84 (0.70-1.02) | 0.08 | 0.01 |
| 17+ | 0.89 (0.82-0.97) | 0.01 | |
| 12-17 | 0.87 (0.81-0.95) | <0.01 | |
| 8-12 | 0.94 (0.87-1.01) | 0.09 | |
| 0-7 | -- | -- | |
| Time from End of Neoadjuvant Therapy to Surgery | |||
| <1 month | 0.50 (0.32-0.79) | 0.01 | |
| 1-3 months | 0.86 (0.81-0.92) | <0.01 | <0.01 |
| 3-6 months | -- | -- | |
| Adjuvant Chemotherapy | |||
| No | 1.38 (1.28-1.49) | <0.01 | <0.01 |
| Yes | -- | -- | |
Backward selection with an alpha level of removal of .2 was used. The following variables were removed from the model: distance from patient zip code to treatment facility, facility location, and percent of zip code without a high school degree in patient's county of residence.
Impact of Neoadjuvant Therapy on Overall Survival with Propensity Score Matching Analysis
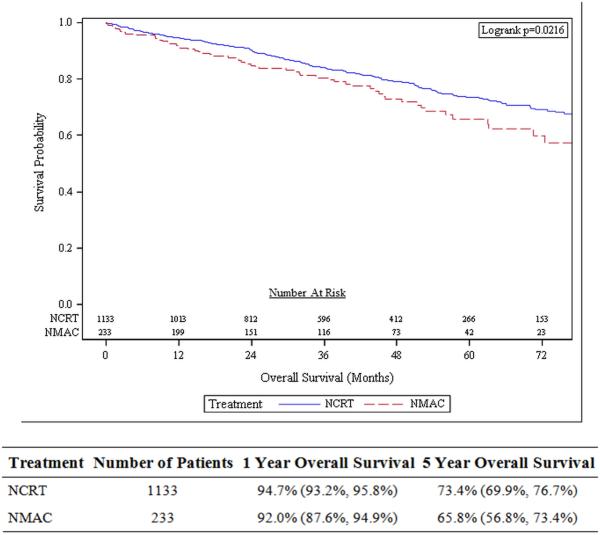
After matching, PSM analysis identified 1,343 patients receiving NCRT and 274 patients receiving NMAC, with the two groups being balanced in demographic, tumor, and treatment characteristics (see Supplemental Table 3). On PSM, the 5-year actuarial overall survival was 73.4 % in patients receiving NCRT compared with 65.8% in patients receiving NMAC (p=0.02; Figure 3). Cox analysis of the PSM patient cohorts, there was statistically significant improved OS with NCRT (HR=0.72, 95% CI 0.56-0.93; p=0.01).
Figure 3.
Overall survival on propensity score matched analysis comparing patients receiving neoadjuvant chemoradiotherapy (NCRT) to patients receiving neoadjuvant multi-agent chemotherapy (NMAC).
DISCUSSION
In this analysis of the NCDB, we found that use of NCRT in patients with clinical T2N1, T3N0, and T3N1 rectal adenocarcinomas treated with LAR, is associated with improved OS compared with NMAC on UVA, MVA, and interaction effect testing. This survival benefit persisted when propensity score matching was implemented to balance the two cohorts for patient, tumor, and treatment characteristics. Our study is the first in the literature to compare these two neoadjuvant treatment strategies head-to-head as well as to examine factors predicting for treatment choice. We found that patient's living in a zip code within 50 miles of treatment facility, lack of insurance, and T3N1 tumors were more likely to receive NCRT. Our study also confirmed known poor prognostic factors in rectal cancer including inadequate lymph node dissection, delay to surgery after neoadjuvant therapy28, incomplete resection, poorly differentiated tumors, and higher stage tumors.
There is a movement to eliminate or reduce the use of NCRT in favor of NMAC in select patients, as evidenced by the ongoing PROSPECT Trial, to decrease RT induced toxicity14-17, 29. The basis of this accruing trial is from a pilot single-arm phase II study from Memorial Sloan-Kettering Cancer Center that enrolled 32 patients19. These patients were clinically staged as T3 (N0 or N1) with MRI and endoscopic rectal ultrasound (ERUS), deemed resectable, and did not require temporary diverting ostomy or endorectal stent. Each patient received four cycles of mFOLFOX630 with bevacizumab followed by two cycles of mFOLFOX alone. If there was any progression, patients instead received concurrent 5FU and RT. Surgery with TME was performed 3 to 6 weeks from completion of neoadjuvant therapy. Adjuvant chemotherapy was personalized, with FOLFOX being recommended by the investigators. All patients had an R0 resection, with 25% having a pathological complete response. Only two patients required neoadjuvant radiation due to FOLFOX intolerance, with one requiring adjuvant radiation. The 4 year results were extremely promising, showing 0% local recurrence and 91% overall survival19, prompting the creation of the PROSPECT Trial. The PROSPECT trial randomizes cT2N1, cT3N0, and cT3N1 rectal adenocarcinomas to 6 cycles of FOLFOX versus the standard concurrent 5FU and RT to 50.4 Gy. Patients are clinically and radiographically (via MRI or ERUS) assessed in the FOLFOX group and if there is no progression and estimated regression of ≥20% they proceed to LAR with TME. Patients who progress or do have <20% regression received standard concurrent 5FU and RT. Systemic chemotherapy is delivered after surgery, with adjuvant CRT recommended for patients who do not achieve an R0 resection and did not receive radiation preoperatively. This trial is currently enrolling patients at multiple sites in the United States20.
There are several reasons for the interest in elimination of NCRT from the treatment paradigm in rectal cancer. Justification for the elimination of upfront radiation therapy in all Stage II and III rectal cancers patients comes from low local recurrence rates observed with modern TME techniques, with overall 5-year estimates of less than 10%10, 11, 31. Moreover, randomized evidence shows RT improves local control in the TME era with no improvement in OS32. Improved systemic therapies12, 13, 33, with increasing cases of pathological complete response and longer disease free survival intervals, have also been used to rationalize the elimination of radiation. However, beyond the above mentioned single arm study of 32 patients, there is lack of prospective evidence to eliminating radiation in Stage II/III rectal caners. There is a recently published Canadian review examining 566 patients who would meet eligibility for the PROSPECT trial which found that these patients had improved recurrence free survival (HR=0.75) compared to patients who did not meet enrollment criteria, suggesting that elimination of radiation could be considered29.
Our study has several strengths and limitations. The strengths include that our series serves as the largest assessment of the use of NMAC in cT2N1, cT3N0, and cT3N1 patients, and serves as the only comparison of these patients to the current standard of care of NCRT. Additionally, we are the first to summarize these practice patterns in the United States as well as report on factors that potentially influence clinician treatment modality choice. The limitations of our study are the lack of uncaptured variables by the NCDB, like other cancer registries, leading to a potential inherent imbalance between the NCRT and NMAC group. We performed stratified analysis, PSM, and interaction variable testing to account for these confounding variables, and there was a persistent OS benefit to NCRT, but even with these multiple statistical methods to balance the cohorts there may be confounders that cannot be accounted for in the NCDB. We excluded patients who were coded as not being able to receive radiation due to medical reasons, but there may be confounding uncaptured reasons why a patient did not get radiation that could impact OS. Additionally, we cannot determine specifically if all of the patients in the NMAC would meet eligibility criteria for the PROSPECT Trial. We can infer they are a similar cohort based on clinical stage, surgery modality, and their OS outcomes, but we do not have specific information on methods used to stage these patients or information on clinical response prior to surgery. Additionally, we cannot capture specific chemotherapy regimens in the NCDB, and can only assess that patients had multi-agent chemotherapy in the NMAC group and single-agent chemotherapy in the NCRT group. Toxicity information and patient reported outcomes are not reported, so we cannot comment on the morbidity of the treatment regimens. Lastly, the NCDB does not provide information on cause of death, and it is possible that the OS benefit to NCRT on stratified analysis is due to competing risks of death.
CONCLUSION
In summary, our series indicates that the use of NCRT is associated with improved OS compared with NMAC on stratified analysis for select Stage II/III rectal adenocarcinomas treated with LAR. This benefit persisted when demographic, tumor, and treatment variables are balanced. Patients that lived in a zip code within 50 miles of their treatment facility, had more advanced disease, and lacked insurance were more likely to receive NCRT over NMAC. Until the results of the accruing PROSPECT trial are published, our series suggests that cT2N1, cT3N0, and cT3N1 rectal adenocarcinomas should receive NCRT, with NMAC reserved for clinical trials.
Supplementary Material
Acknowledgements/Funding
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Conflicts of Interest/Disclosures: None
Contributions: Study design (RJC, YL, TWG, JCL), data collection/analysis (RJC, KP, JZ, YL, TG), manuscript writing-initial (RJC), validation (all).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 3.NIH consensus conference Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 4.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 6.Tepper JE, O'Connell MJ, Petroni GR, et al. Adjuvant postoperative fluorouracil-modulated chemotherapy combined with pelvic radiation therapy for rectal cancer: initial results of intergroup 0114. J Clin Oncol. 1997;15:2030–2039. doi: 10.1200/JCO.1997.15.5.2030. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 9.Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant NB, Guillem JG, Paty PB, et al. T3N0 rectal cancer: results following sharp mesorectal excision and no adjuvant therapy. J Gastrointest Surg. 1999;3:642–647. doi: 10.1016/s1091-255x(99)80087-0. [DOI] [PubMed] [Google Scholar]
- 11.Carne PW, Nelson H. Are we overtreating rectal cancer: time for another trial? Ann Surg Oncol. 2004;11:124–126. doi: 10.1245/aso.2004.11.905. [DOI] [PubMed] [Google Scholar]
- 12.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Gill S. Recent phase III trials of fluorouracil, irinotecan, and oxaliplatin as chemotherapy for metastatic colorectal cancer. Cancer Chemother Pharmacol. 2004;54(Suppl 1):S57–64. doi: 10.1007/s00280-004-0888-9. [DOI] [PubMed] [Google Scholar]
- 14.Minsky BD, Conti JA, Huang Y, Knopf K. Relationship of acute gastrointestinal toxicity and the volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol. 1995;13:1409–1416. doi: 10.1200/JCO.1995.13.6.1409. [DOI] [PubMed] [Google Scholar]
- 15.Matzel KE, Bittorf B, Gunther K, Stadelmaier U, Hohenberger W. Rectal resection with low anastomosis: functional outcome. Colorectal Dis. 2003;5:458–464. doi: 10.1046/j.1463-1318.2003.t01-1-00503.x. [DOI] [PubMed] [Google Scholar]
- 16.Temple LK, Wong WD, Minsky B. The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation. Semin Radiat Oncol. 2003;13:469–477. doi: 10.1016/S1053-4296(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 17.Prabhu RS, Cassidy RJ, Landry JC. Radiation therapy and neutropenia. Curr Probl Cancer. 2015;39:292–296. doi: 10.1016/j.currproblcancer.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–865. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 19.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PROSPECT: chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery. Available from URL: https://clinicaltrials.gov/ct2/show/NCT01515787?term=prospect+rectal+cancer&rank=1.
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Nickleach D, Liu Y, Shrewsberry A, Ogan K, Kim S, Wang Z. The Proceeding of the SouthEast SAS User Group. SESUG 2013; SAS® Macros to Conduct Common Biostatistical Analyses and Generate Reports. http://analytics.ncsu.edu/sesug/2013/PO-05.pdf. [Google Scholar]
- 24.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SAS SUGI. 2001;26:214–226. [Google Scholar]
- 26.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 27.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 28.Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56:921–930. doi: 10.1097/DCR.0b013e31828aedcb. [DOI] [PubMed] [Google Scholar]
- 29.Bosse D, Mercer J, Raissouni S, et al. PROSPECT Eligibility and Clinical Outcomes: Results From the Pan-Canadian Rectal Cancer Consortium. Clin Colorectal Cancer. 2016 doi: 10.1016/j.clcc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 33.Rodel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.