Abstract
Heart rate variability (HRV) is associated with positive physiological and psychological effects. HRV is affected by breathing parameters, yet, debate remains regarding the best breathing interventions for strengthening HRV. The objective of the current study was to test whether the inclusion of a post-exhalation rest period was effective at increasing HRV, while controlling for breathing rate. A within-subjects crossover design was used with 40 participants who were assigned randomly to a breathing pattern including a post-exhalation rest period or a breathing pattern that omitted the post exhalation rest period. Participants completed training on each breathing pattern, practiced for six minutes, and sat quietly during a five-minute washout period between practices. Participants were given instructions for diaphragmatic breathing (DB) at a pace of six breaths/minute with or without a post-exhalation rest period. Recordings of heart rate, breathing rate, HF-HRV, RMSSD, LF-HRV, SDNN were collected before and during each of the breathing trials. HRV indices were derived from Lead one ECG recordings. Pairwise contrasts showed inclusion of a post-exhalation rest period significantly decreased heart rate (p < .001) and increased HF-HRV (p < .05). No differences were found for breathing rates (p > .05), RMSSD (p > .05), and SDNN (p > .05). Results indicted omission of the post-exhalation rest period resulted in higher LF-HRV (p < .05). A post-exhalation rest period improves HF-HRV, commonly associated with self-regulatory control, yet a post-exhalation rest period’s importance requires further exploration.
Introduction
Slow-paced diaphragmatic breathing is an important component of behavioral interventions for a broad spectrum of symptoms including pain, anxiety, and motion sickness (Ferreira et al., 2013; Fried & Grimaldi, 1993; Lehrer & Gevirtz, 2014; Litchfield, 2003; Russell, Hoffman, Stromberg, & Carlson, 2014; Sauer, Burris, & Carlson, 2010; Zucker, Samuelson, Muench, Greenberg, & Gevirtz, 2009). Manipulation of breathing through behavioral interventions strengthens autonomic reflex efficiency and promotes appropriate behavioral or emotional responses (Appelhans & Luecken, 2006; Gevirtz, 2013; Porges, 2007). Additionally, breathing training increases self-regulation of physiological, affective, and cognitive processes associated with problematic behaviors such as impulsiveness or failure to plan (Brown, Gerbarg, & Muench, 2013; Litchfield, 2003; Porges, 2007; Thayer, Hansen, Saus-Rose, & Johnsen, 2009; Thayer & Lane, 2009). Taken together, these findings suggest breathing training is an important component of behavioral therapies.
The efficacy of breathing control in behavioral therapies may be partly due to its broad psychophysiological effects (Brown et al., 2013; Fried & Grimaldi, 1993; p. 193–227). When breathing treatments are employed, breathing frequency, respiration mechanics, tidal volume, and inhalation-exhalation ratios are important considerations (Porges, 2007). Each exerts significant effects on cardiac functioning quantified as high frequency heart rate variability (HF-HRV; 0.15-0.4 Hz). HF-HRV is a naturally occurring variation in the heartbeat-to-heartbeat time interval and is positively associated with cardiac health, autonomic balance, self-regulation, task performance, and negatively associated with chronic muscle pain, anxiety, depression, hypertension, and nausea (Lehrer et al., 2010; Lehrer & Gevirtz, 2014; Litchfield, 2003; Nazarewicz, Verdejo- Garcia, & Giummarra, 2015; Russell et al., 2014; Sargunaraj et al., 1996). Cumulatively, these studies indicate slow-paced diaphragmatic breathing may improve psychological functioning by altering HF-HRV.
Despite the benefits of HF-HRV, how to maximize HF-HRV during breathing training is not well understood. A series of studies by Lehrer and colleagues indicated breathing at a rate of 5.5 breaths/minute (or ~0.092 Hz) with diaphragmatic breathing mechanics might be optimal for increasing HF-HRV (Lehrer et al., 2000; Vaschillo, Lehrer, Rishe, & Konstantinov, 2002). The work of Lehrer and colleagues suggested HRV biofeedback allows individuals to synchronize respiration with the naturally corresponding increases and decreases of heart rate through the baroreflex. The baroreflex rapidly modulates blood pressure through control of heart rate and contractility of blood vessels to maintain a balanced blood pressure and is thought to occur at a frequency of 0.1 Hz, which translates to approximately six breaths/minute (Hall & Guyton, 2011, p. 205–208). Thus, evidence suggests that paced breathing at 5.5 breaths/minute produces the maximum effect on HF-HRV (Lehrer & Gevirtz, 2013).
Paced breathing of six or 5.5 breaths/minute, however, can be achieved through various methods. Inhalation for three seconds and exhalation for 2.5 seconds yields the same breathing frequency as an inhalation for two seconds and exhalation for 3.5 seconds. The optimal balance of inhalation and exhalation is the topic of scientific inquiry (Fried & Grimaldi, 1993, p. 129; Lin, Tai, & Fan, 2014; Strauss-Blasche, et al., 2000; Van Diest et al., 2014). Our data suggest a 4-2-4 paced diaphragmatic breathing cycle of inhale-exhale-rest, rather than a 5–5 (inhale-exhale), maximizes the effects of respiration on HF-HRV (Kniffin et al., 2014; Russell et al., 2014; Stromberg, Russell, & Carlson, 2015).
Although HF-HRV may be the most frequently examined quantification of HRV, there are at least three additional indices. The first is the root mean square of the successive differences between adjacent heartbeats (RMSSD; Task Force of the European Society of Cardiology, 1996). The second is low frequency HRV (LF-HRV; ~0.04–0.15 Hz). LF-HRV reflects the influence of the baroreflex on cardiac activity and is thought to reflect the balance between the sympathetic and parasympathetic systems (Berntson et al., 1997). LF-HRV becomes especially important if an individual’s breathing rate falls out of the HF-HRV range (9–24 breaths/minute) and into the LF-HRV range (2.4 to 9 breaths/minute). The third is the standard deviation of normal R-R intervals (SDNN; Berntson et al., 1997). SDNN reflects all cyclical components of HRV and is used in 24-hour recordings (Task Force, 1996). Yet, little data exist directly comparing the effectiveness of different respiratory cycles on increasing HF-HRV or the impact on other HRV indices such as RMSSD, SDNN, LF-HRV, or LF/HF ratios.
Using a within-subjects crossover design, the current study examined the potential advantages of a 4-2-4 breathing cycle (inhale-exhale-rest) above the commonly practiced inhale-exhale approach to strengthen HF-HRV and RMSSD. It was expected that the 4-2-4 breathing cycle would exert a greater effect on cardiac parameter than the 5-5 breathing cycle. The impact of breathing cycle on HRV indices of LF-HRV, LF/HF ratios, and SDNN were also explored, as were associations with respiration rate, heart rate, the number of interval differences of successive N to N intervals or heartbeat intervals greater than 50ms divided by the total number of NN intervals yielding a percentage (pNN50), inter-beat-interval (IBI), and abdominal respiration amplitude measured in volts (ARA).
Method
Participants
Forty undergraduate students from the University of Kentucky were recruited. Participants were ruled out if they reported a history of asthma, cardiovascular disease such as hypertension, gastrointestinal disorders, panic disorder, or anxiety disorders on a study pre-screening questionnaire. Participants were self-selected volunteers who were screened for eligibility through an online screening questionnaire. They were provided course credit for their participation in the study. The University of Kentucky Institutional Review Board for the Protection of Human Participants approved this research protocol.
Procedures
Participants were provided with general information about the study and invited to sign up through the University of Kentucky’s online system. Upon arrival at the lab, interested participants were scheduled and given informed consent. Participants were placed in a comfortable cushioned chair in an upright position for the duration of the experiment. Following consent, participants completed brief demographics questionnaires and baseline measurement of breathing rate and heart rate were taken. Electrodes to gather heart rate data were attached in a Lead One configuration with active leads on the right and left wrists and the ground lead on the right ankle. All HRV indices were calculated from heart rate recordings. Respiration was measured with a strain gauge placed around the abdomen just above the navel. Five minutes of baseline physiological variables were collected as the participant was asked to sit quietly without talking and minimal movement.
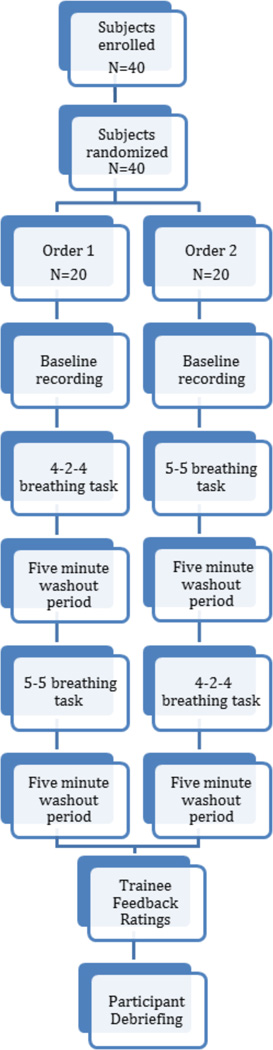
A within-subjects crossover design was used (see Figure 1). Participants were randomly assigned to receive training on the 4-2-4 breathing cycle first or the 5–5 breathing cycle. Before beginning their breathing practice both conditions were provided approximately six minutes of audio directions on diaphragmatic breathing and the condition specific breathing pattern. Participants in the 4-2-4 condition received training on inhaling for 4 seconds, exhaling for 2 seconds, and resting for 4 seconds. Participants in the 5–5 condition were trained to inhale for 5 seconds and exhale for 5 seconds without a rest period. Prior to each trial, participants were given the opportunity to ask questions.
Figure 1.
Study Flow Chart.
During both the 4-2-4 and 5-5 breathing tasks, participants were asked to follow a visual cue presented on a computer screen to aid with pacing their breathing rate. The visual cue consisted of an oval that expanded (inhale), contracted (exhale), or remained still (rest). The rest period was only presented in the 4-2-4 condition. The visual cue also included a soft tone corresponding with the initial moment of the inhalation orienting participants to the oval beginning to expand. Participants practiced the breathing cycle for six minutes as physiological recordings were collected. Following the six-minute practice period, they were asked to sit quietly without talking or moving for five minutes to serve as a washout period. Following the washout period, participants switched breathing cycles (4-2-4 to 5-5 or from 5-5 to 4-2-4).
Materials
Demographic information
Participants were asked age, year in school, and ethnicity.
Participant feedback
Participants were asked which of the two breathing cycles was easiest to follow and which of the two breathing methods made them feel the most relaxed. Items were dichotomous and forced choice.
High frequency heart rate variability (HF-HRV) and heart rate
High frequency HRV (HF-HRV) was operationalized as spectral power occurring between 0.15-0.40 Hz based on the recommendations of the 1997 HRV Committee Report (Berntson et al., 1997). Although there is a significant debate regarding the need to covary the effects of respiration, “In general, the literature does not provide evidence that findings change when the amplitude of respiratory sinus arrhythmia is adjusted for respiration parameters” (Denver, Reed, & Porges, 2007, p. 288). Thus, the effects of respiration termed respiratory sinus arrhythmia were not covaried for HF-HRV (0.15-0.4 Hz; 9-24 breaths/minute) or LF-HRV (0.04-0.15 Hz; 2.4-9 breaths/minute). Heart function was recorded with three Ag/AgCl electrodes using shielded leads connected to BioPac ECG100C electrocardiogram amplifier module. Sampling rate for heart function was set to 2000 samples/second. Data were stored using Acq-knowledge software (Biopac, Santa Barbara, CA) and analyzed with the MindWare data analysis program version 3.1 (MindWare, Gahana, OH).
Root mean square of the successive differences (RMSSD)
When examining the effects of breathing on cardiac functioning it is important to consider the frequency of breathing. As long as minute-by-minute breathing rates remain within the range of ~9 to 24 breaths, HF-HRV accurately assesses the effects of breathing on cardiac functioning. Within the range of 9 to 24 breaths/minute, HF-HRV values serve as an indicator of the parasympathetic tone within the body and index self-regulatory ability. However, if an individual’s breathing rate falls below or above the range of 9 to 24 breaths/minute, the time domain measure of RMSSD should be considered as an assessment of parasympathetic tone within the body. According to the published standards (Berntson, et al., 1997; Task Force, 1996) RMSSD is an appropriate measure of parasympathetic tone if there are at least five minutes of heart rate data.
Low frequency heart rate variability (LF-HRV)
Low frequency HRV occurs in the range of 0.04-0.15 Hz and is often associated with influence of the baroreflex that regulates blood pressure given changes in heart rate (Berntson et al., 1997; Task Force et al., 1996). It is thought that LF-HRV is influenced by both autonomic nervous system branches of the making its interpretation difficult. Although the interpretation is complicated, LF-HRV is important to consider when respiration rates are between 2.4 to 9 breaths/minute (Moak et al., 2007).
Additional HRV indices
The standard deviation of NN intervals (SDNN) is considered a measure of overall HRV and is linked to general health (Berntson et al., 1997). SDNN takes into account the effects of both sympathetic tone and parasympathetic tone and indicates fluctuations of the two systems change over the course of 24 hours. Although SDNN is suggested for 24-hour heart recordings it provides an index of parasympathetic versus sympathetic balance and was therefore included. In order to provide a complete picture of the effects of the breathing cycle, we have also included: heart rate, the inter-beat interval between R-peaks in milliseconds, very low frequency that ranges from 0.0033 to 0.04 Hz and is not well defined, pNN50 which is the number of interval differences of successive N to N intervals or heartbeat intervals greater than 50ms divided by the total number of NN intervals yielding a percentage. Although these HRV indices vary in the level of empirical evidence clarifying their utility, each provides a unique vantage point for the effects of respiration on cardiac functioning. Therefore, we believe it advantageous to present a complete picture.
Breathing rate
Breathing rate was recorded using the BSL-SS5LB respiratory effort transducer and amplifier module for the BioPac MP100 system. The sensor was placed around the abdomen below the rib cage and an inch above the navel. Sampling rate for breathing rate was set to 2000 samples/second. Breathing rates were recorded as mean breaths/minute.
Abdominal respiration amplitude (ARA)
Abdominal movement was measured as change in tension on the abdominal pressure gauge and recorded as volts. ARA was measured with the same equipment as breathing rate and had a sampling rate set to 2000 samples/second. ARA serves as a mean measurement of abdominal displacement during each of the experiment’s physiological recording stages. Therefore, ARA is used to indicate mean diaphragm muscle engagement and estimate mean volume displacement during a breathing cycle.
HF-HRV Cleaning Procedures
ECG data were visually examined in 60-second tachograms without stage identifiers to avoid experimenter bias during cleaning. Missing or erroneous heartbeats were cleaned according to the recommendations made by the Task Force and then expanded by the 1997 Committee Report (Berntson et al., 1997, p. 631; Task Force, 1996). Specifically, movement or aberrant heartbeats were visually examined and adjusted for by either measuring the actual R-R interval or interpolating the missing heartbeat. Movement artifacts where the heartbeats were not clearly indicated were corrected with a mid-beat replacement. Double marked R-peaks and erroneous R-peaks were removed. Based on recommendation from Mindware Technologies, epochs with over 10% erroneous beats or that did not have at least 30 consecutive seconds of measurable data were not included in calculations. After initial visual inspection of files, three baseline files were excluded and one participant’s breath cycle file were excluded from analysis due to the significant number of artifacts and the researcher’s inability to visually identify R-peaks within the ECG recordings.
Statistical Analyses Plan
In order to calculate the necessary sample size (Faul, Erdfelder, Buchner, & Lang, 2009), G*Power software was employed. Based upon previous research, it was determined that the effect size for this study was likely to be medium to large (Russell et al., 2014). It was determined that a sample size of 40 participants allowed for an 81% power with, α=.05. All measures met assumptions of normality except abdominal respiration amplitude. Failing to adjust for non-normal distribution with transformations, seven participants were determined to be significant outliers (greater than 2 SD) and were excluded from analyses. In addition, three baseline and one 5-5 breath cycle physiological recording were excluded due to significant movement artifacts. No other data were excluded and no transformations were made.
Data were analyzed with SPSS version 22. First, bivariate correlations among HF-HRV, breaths/minute, heart rate, LF-HRV, RMSSD, SDNN, inter-beat interval, abdominal respiration amplitude, age, and sex at baseline were computed. Because of expected correlations between these outcomes, repeated measures multivariate analyses of variance (MANOVAs) were conducted to investigate within-subject differences between the three breathing stages (baseline, 4-2-4, 5-5). For these analyses, order was treated as a between-subject effect and breathing stage was the repeated measure, or within-subject effect. Within-subjects designs maximize power because each person effectively serves as her/his own control; that is, participants are compared to themselves in each stage. Greenhouse-Geisser correction was used to control for violation of sphericity in all MANOVA analyses. Pairwise contrasts were used to investigate significant omnibus tests. To control for the large number of comparisons, the Holm-Bonferroni method was used. This method is more precise than the overly-conservative Bonferroni method, and is considered the most appropriate method to use when dependent variables are correlated with each other. For details of the Holm-Bonferroni method, see Aickin and Gensler (1996).
Results
Demographics, Mean Differences, and Bivariate Correlations
The participant sample was 70% Caucasian, 12.5% African-American, 10% Hispanic, and 7.5% mixed race or other. The average age of the sample was 18.9 years (SD = 1.00) and 32 participants were female. Bivariate correlations among outcome measures at baseline are presented in Table 1. Means and SDs of study outcomes by breathing condition are presented in Table 2.
Table 1.
Bivariate Correlations among Outcomes at Baseline
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. HF-HRV | - | −.53** | −.70** | .83** | .86** | .66** | −.72** | .62** | .84** | .67** | .40 | .01 | −.15 |
| 2. BPM | - | .30 | −.18 | −.29 | −.09 | .61** | −.10 | −.06 | −.29 | .16 | −.39* | .11 | |
| 3. HR | - | −.66** | −.64** | −.47** | .38* | −.42* | −.58** | −.97** | −.22 | .00 | −.05 | ||
| 4. RMSSD | - | .93** | .78** | −.50** | .70** | .93** | .70** | .02 | −.10 | .00 | |||
| 5. SDNN | - | .83** | −.45** | .83** | .86** | .66** | .07 | −.05 | .00 | ||||
| 6. LF-HRV | - | −.10 | .71** | .63** | .49** | .04 | −.09 | .02 | |||||
| 7. LF/HF Ratio | - | −.26 | −.47** | −.38* | −.04 | −.03 | .18 | ||||||
| 8. VLF-HRV | - | .62** | .43** | .12 | .08 | .13 | |||||||
| 9. pNN50 | - | .60** | −.06 | −.14 | .00 | ||||||||
| 10. IBI | - | .22 | .03 | .19 | |||||||||
| 11. ARA | - | −.12 | .30 | ||||||||||
| 12. Age | - | .11 | |||||||||||
| 13. Sex | - |
Note: High frequency heart rate variability (HF-HRV; 0.15 Hz to 0.4 Hz). Respiration rate was measured in breaths-per-minute (BPM). Heart rate measured as the number of beats in 60 seconds (HR). Root mean squared of the successive differences between adjacent N-to-N peaks measured in ms (RMSSD). Standard deviation in N-to-N interval measured in ms (SDNN). Low frequency heart rate variability measured in ms2 (LF-HRV). Low frequency to high frequency ratio measured in ms2 (LF/HF Ratio). Very low frequency heart rate variability measured in ms2 (VLF-HRV). The number of interval differences of successive N to N intervals or heartbeat intervals greater than 50ms divided by the total number of NN intervals yielding a percentage (pNN50). Inter-beat-interval measured in ms (IBI). Abdominal Respiration Amplitude measured in volts (ARA).
p<.01 two-tailed.
p<.05 two-tailed.
Table 2.
Assessment of Physiological Measures
| Variable | Baseline | 4-2-4 Stage | 5-5 Stage | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| HF-HRV | 6.68 | 1.19 | 6.98 | 0.99 | 6.67 | 1.29 |
| BPM | 15.25 | 3.19 | 6.61 | 1.61 | 6.57 | 1.44 |
| HR | 75.15 | 11.91 | 71.26 | 10.24 | 72.60 | 10.08 |
| RMSSD | 52.16 | 30.39 | 65.35 | 34.57 | 66.23 | 38.36 |
| SDNN | 61.10 | 26.70 | 94.07 | 38.76 | 99.40 | 45.44 |
| LF-HRV | 981.47 | 666.42 | 5573.39 | 4605.47 | 6593.45 | 5618.72 |
| LF/HF Ratio | 1.27 | 1.00 | 4.47 | 3.13 | 7.51 | 5.35 |
| VLF-HRV | 223.08 | 240.13 | 139.79 | 95.51 | 191.95 | 176.12 |
| pNN50 | 30.32 | 18.91 | 30.83 | 14.27 | 30.05 | 16.87 |
| IBI | 816.92 | 120.29 | 858.04 | 113.64 | 841.03 | 104.85 |
| ARA | 7.02 | 7.56 | 16.02 | 21.01 | 27.95 | 31.59 |
Note: High frequency heart rate variability (HF-HRV). Respiration rate was measured in breaths-per-minute (BPM). Heart rate measured as the number of beats in 60 seconds (HR). Root mean squared of the successive differences between adjacent N-to-N peaks measured in ms (RMSSD). Standard deviation in N-to-N interval measured in ms (SDNN). Low frequency heart rate variability measured in ms2 (LF-HRV). Low frequency to high frequency ratio measured in ms2 (LF/HF Ratio). Very low frequency heart rate variability measured in ms2 (VLF-HRV). The number of interval differences of successive N to N intervals or heartbeat intervals greater than 50ms divided by the total number of NN intervals yielding a percentage (pNN50). Inter-beatinterval measured in ms (IBI). Abdominal Respiration Amplitude measured in volts (ARA).
Training Feedback Ratings
Participants were asked which breathing pattern was easiest to follow and most relaxing. Sixty-eight percent of participants rated the 4-2-4 breath cycle as the easiest to follow. In addition, sixty-three percent of participants rated the 4-2-4 breath cycle as the most relaxing.
Effects of 4-2-4 vs. 5-5 Breathing Method on Physiological Functioning
A repeated measures MANOVA was used to test if breathing stage influenced physiological functioning; specifically, breaths/minute (BPM), heart rate (HR), HF-HRV, RMSSD, LF-HRV, SDNN, LF/HF ratio, VLF-HRV, pNN50, inter-beat interval (IBI), and abdominal respiration amplitude (ARA). Omnibus tests indicated no significant interactions between order (between subject) and breathing stage (within-subject) on any of the 11 physiological outcomes, p’s > .05. Additionally, there were no significant order effects on any physiological variables. However, as hypothesized, omnibus tests revealed a significant main effect of breathing stage on BPM, HR, HF-HRV, RMSSD, SDNN, LF-HRV, LF/HF ratio, IBI, and ARA. Omnibus test values are presented in Table 3.
Table 3.
Omnibus Tests of Within Subject Effects
| Dependent Measures | F (df) | P | n2 | Post-hoc Power |
|---|---|---|---|---|
| HF-HRV | 3.51 (2, 68) | < .05 | .09 | 0.57 |
| BPM | 218.23 (2, 68) | < .001 | .87 | 1.00 |
| HR | 21.66 (2, 68) | < .001 | .39 | 1.00 |
| RMSSD | 10.21 (2, 68) | < .001 | .23 | 0.98 |
| SDNN | 50.80 (2, 68) | < .001 | .60 | 1.00 |
| LF-HRV | 39.72 (2, 68) | < .001 | .54 | 1.00 |
| LF/HF Ratio | 36.87 (2, 68) | < .001 | .52 | 1.00 |
| VLF-HRV | 3.12 (2, 68) | > .05 | .08 | 0.53 |
| pNN50 | 0.69 (2, 64) | > .05 | .008 | 0.08 |
| IBI | 17.25 (2, 68) | < .001 | .34 | 1.00 |
| ARA | 13.02 (2, 54) | < .001 | .33 | 1.00 |
Note: Omnibus tests indicated no significant order effects, all p’s > .05. Omnibus tests indicated significant stage (baseline, 4-2-4, and 5-5) effects and are represented here. High frequency heart rate variability (HF-HRV). Respiration rate was measured in breaths-per-minute (BPM). Heart rate measured as the number of beats in 60 seconds (HR). Root mean squared of the successive differences between adjacent N-to-N peaks measured in ms (RMSSD). Standard deviation in N-to-N interval measured in ms (SDNN). Low frequency heart rate variability measured in ms2 (LF-HRV). Low frequency to high frequency ratio measured in ms2 (LF/HF Ratio). Very low frequency heart rate variability measured in ms2 (VLF-HRV). The number of interval differences of successive N to N intervals or heartbeat intervals greater than 50ms divided by the total number of NN intervals yielding a percentage (pNN50). Inter-beat-interval measured in ms (IBI). Abdominal Respiration Amplitude measured in volts (ARA).
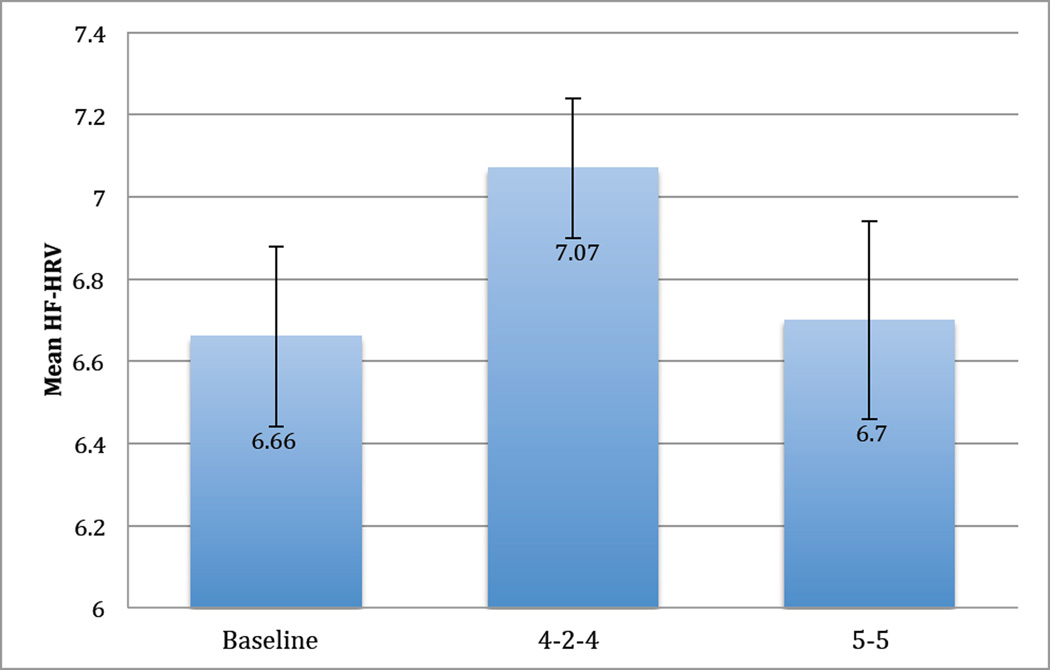
Pairwise contrasts further probed significant stage effects with results listed in Table 4. Multiple comparisons were controlled with the Holm-Bonferroni corrections. For breathing rate, no differences were found between the 4-2-4 or 5-5 stages but both stages breathing rates were lower than baseline. Heart rate decreased from baseline in both the 4-2-4 and 5-5 stages with the 4-2-4 stage being lower than the 5-5 stage. HF-HRV was higher for the 4-2-4 stage than the 5-5 stage, trending towards being higher than the baseline stage, and results indicated no difference between baseline and the 5-5 stage. RMSSD and SDNN results indicated increases from baseline for both stages but no differences between stages. Of note, RMSSD and SDNN were higher during the 5-5 stage than the 4-2-4 stage. LF-HRV increased from baseline to both stages with the 5-5 stage trending towards being significantly higher than the 4-2-4 stage. IBI increased from baseline to both stages with the 4-2-4 stage having higher IBI values than the 5-5 stage. Finally, pairwise contrasts indicated increases in ARA from baseline to both breathing stages with the 5-5 stage having significantly higher abdominal respiration amplitude values. For a summary of the primary results comparing the effects of each stage on HF-HRV, please see Figure 2.
Table 4.
Pairwise Contrasts Between Baseline, 4-2-4, and 5-5 Breathing Stages
| Variable | Stage | Comparison Stage |
Mean Difference (Stage -Comparison) |
S.E. | p± |
|---|---|---|---|---|---|
| HF-HRV | Baseline | 4-2-4 | −0.33 | 0.13 | .016 |
| Baseline | 5-5 | 0.04 | 0.18 | .843 | |
| 4-2-4 | 5-5 | 0.36 | 0.12 | .006 | |
| BPM | Baseline | 4-2-4 | 3.79 | 0.64 | < .001 |
| Baseline | 5-5 | 8.78 | 0.55 | < .001 | |
| 4-2-4 | 5-5 | 0.08 | 0.17 | .64 | |
| HR | Baseline | 4-2-4 | 3.79 | 0.64 | < .001 |
| Baseline | 5-5 | 2.14 | 0.67 | < .001 | |
| 4-2-4 | 5-5 | −1.65 | 0.28 | < .001 | |
| RMSSD | Baseline | 4-2-4 | −12.90 | 2.91 | < .001 |
| Baseline | 5-5 | −12.70 | 4.02 | .004 | |
| 4-2-4 | 5-5 | 0.20 | 2.87 | .945 | |
| SDNN | Baseline | 4-2-4 | −33.58 | 4.35 | < .001 |
| Baseline | 5-5 | −37.00 | 5.08 | < .001 | |
| 4-2-4 | 5-5 | −3.42 | 2.47 | .175 | |
| LF-HRV | Baseline | 4-2-4 | −4773.52 | 823.16 | < .001 |
| Baseline | 5-5 | −5547.25 | 1005.39 | < .001 | |
| 4-2-4 | 5-5 | −773.73 | 385.10 | .03 | |
| IBI | Baseline | 4-2-4 | −40.60 | 7.89 | < .001 |
| Baseline | 5-5 | −19.47 | 8.14 | .023 | |
| 4-2-4 | 5-5 | 21.13 | 3.73 | < .001 | |
| ARA | Baseline | 4-2-4 | −9.10 | 3.31 | .03 |
| Baseline | 5-5 | −20.98 | 5.16 | < .001 | |
| 4-2-4 | 5-5 | −11.88 | 2.68 | < .001 |
Note:
Using the Holm-Bonferroni correction for multiple comparisons, only p-values smaller than .011 are considered significant and are bolded. Pairwise contrasts were used to investigate between stage differences. High frequency heart rate variability (HF-HRV). Respiration rate was measured in breaths-per-minute (BPM). Heart rate measured as the number of beats in 60 seconds (HR). Root mean squared of the successive differences between adjacent N-to-N peaks measured in ms (RMSSD). Standard deviation in N-to-N interval measured in ms (SDNN). Low frequency heart rate variability measured in ms2 (LF-HRV). Low frequency to high frequency ratio measured in ms2 (LF/HF Ratio). Very low frequency heart rate variability measured in ms2 (VLF-HRV). The number of interval differences of successive N to N intervals or heartbeat intervals greater than 50ms divided by the total number of NN intervals yielding a percentage (pNN50). Inter-beat-interval measured in ms (IBI). Abdominal Respiration Amplitude measured in volts (ARA).
Figure 2.
Repeated Measures MANOVA Test of HF-HRV Differences. High frequency heart rate variability (HF-HRV)*. Cohen’s d value represents the difference between the 4-2-4 training and 5-5 training, d = .32
* HF-HRV stage means presented in Figure 2 are not consistent with HF-HRV stage means presented in Table 2 due to missing data for the Repeated Measures MANOVA analyses. For further detail, please see Statistical Analyses Plan.
Discussion
As predicted, results supported the inclusion of a post exhalation rest period for behavioral interventions targeting HF-HRV and improvement of self-regulatory ability. When including a post inhalation rest period (4-2-4), participants achieved higher HF-HRV tone than without (5-5). In addition, the majority of participants reported the 4-2-4 breathing pace was easier to follow and more relaxing than the 5-5 breathing pace. For self-regulation protocols targeting HF-HRV, the present results highlight the potential importance for breathing entrainment programs to include a post exhalation rest period in the breathing cycle.
Previous studies validated the use of slow-paced breathing to increase HF-HRV tone (Courtney, Cohen, & van Dixhoorn, 2011; Henriques, Keffer, Abrahamson, & Horst, 2011; Lehrer et al. 2000; Patron et al., 2013; Prinsloo, Derman, Lambert, & Rauch, 2013; Song & Lehrer, 2003; Strauss-Blasche, et al., 2000; Wang et al., 2010; Whited, 2014). Each program, however, uses a rhythmic breathing cycle limited to inhalation and exhalation. Yet, the present data suggest the gains in HF-HRV through manipulation of breathing can be exploited to a greater degree through inclusion of a rest period in the breathing cycle. The 4-2-4 pattern (inhalation-exhalation-rest) increased the length between inhalations associated with increased vagal influence on cardiac activity and greater parasympathetic tone without adverse effects. This is consistent with previous work demonstrating the current protocol is a reliable method to strengthen HF-HRV, parasympathetic tone, and improve performance on a variety of in lab stressor tasks (Kniffin et al., 2014; Russell et al., 2014; Stromberg et al., 2015). Furthermore, the use of rest periods avoids unnecessary strain on muscles of respiration accompanying breathing protocols advocating prolonged exhalations or potential hyperventilation from protocols advocating prolonged inhalations.
Although significant differences were found in HF-HRV between the 5-5 and the 4-2-4 conditions, no differences were found between breathing cycles for RMSSD, SDNN, and LF-HRV. Despite a within-subjects crossover design, interpretation is potentially complicated by the potential influence of age, physical conditioning, depth of respiration, possible cardiac medication use, and variable respiratory frequencies (Berntson, Lozano, & Chen, 2005). The impact of variable respiratory frequencies was explored by limiting analyses to participants whose breaths/minute remained within the LF range of 2.4-9 breaths/minute. No differences in results were found. Interpretation may be further complicated by participants’ depth of respiration, which was not controlled. Although participants with cardiovascular diseases were excluded from participation and potential effects are controlled for with the within-subjects design, it may be important to record the use of cardiovascular. In addition, as participants’ breathing slowed to 0.1 Hz or ~6 during both breathing cycles, LF-HRV interpretations become conflated by the combined influence of vagal and sympathetic systems (Berntson et al., 1997). Also, as RMSSD estimates can be biased by differences in heart rate and appreciable contributions from LF sympathetic cardiac control, especially when comparing across conditions, delineating the impact of breathing cycles becomes challenging (Berntson, Lozano, & Chen, 2005).
Of additional interest is the non-significant change in HF-HRV values from baseline to the 5-5 breathing cycle despite a significant drop in breathing rate from approximately 15 breaths/minute to approximately seven breaths/minute. The data were reviewed carefully to insure these results were accurately interpreted. Given the healthy nature of the participants, the 5-5 breathing cycle intervention in this study perhaps did not exert enough influence on short-term measurements of HF-HRV for this sample. Moreover, participants within the 5-5 breathing cycle had significantly higher abdominal respiration amplitude values than in the 4-2-4 breathing cycle; such an outcome may have altered arterial CO2 levels enough to stimulate sympathetic activity diminishing HF-HRV for that group. Without the use of a rest period in the breathing cycle, participants may have experienced slight over-breathing due to the 5-second inhalation period triggering an increase in sympathetic tone and a subsequent reduction in HF-HRV. However, without measurement of end tidal CO2 values to index arterial CO2 levels the outcomes observed are not fully explainable. Future studies should look at this issue more carefully.
Although the diaphragm is considered important when teaching slow-paced breathing, the influences of thoracic (chest) versus diaphragmatic (abdominal) muscles should be further explored. Because breathing data were collected from a single pressure belt placed around the abdomen, the use of thoracic muscles versus abdominal muscles and the importance of tidal volume can only be hypothesized in the present study. Notably, results indicated that participants found the 4-2-4 breathing cycle to be easier to follow and more relaxing than the 5-5 breathing cycle. These data suggest individuals may be more motivated to use the 4-2-4 breathing cycle because the physiological effects are more noticeable. The importance of “diaphragmatic” breathing remains speculative until validated within a more clinical population.
To date, the training protocol has only demonstrated effectiveness in predominately healthy samples of young female adults. Although the training protocol has significantly increased HF-HRV tone for over 300 participants in other published projects, future studies should test the training protocol with a wider array of individuals and in clinical settings characterized by low resting HF-HRV tone (Hallman & Lyskov, 2012; Kang, Chen, Chen, & Jaw, 2012; Solberg Nes, Carlson, Crofford, de Leeuw, & Segerstrom, 2010; Schmidt & Carlson, 2009; Zucker et al., 2009). Given that higher HF-HRV is considered a protective factor for a variety of health conditions including chronic pain, continued exploration of breathing entrainment programs for self-management of such symptoms is warranted. In addition, the long-term effectiveness of the training protocol on baseline levels of HF-HRV has yet to be established.
The role of breathing training in health interventions and the ability of behavioral health specialists to alter breathing is important. An individual’s ability to volitionally alter breathing parameters and within minutes also alter HF-HRV tone is a powerful tool for the behavioral health specialist to exploit. With breathing interventions being relatively rapid interventions to implement and also demonstrating a wide range of positive clinical outcomes, breathing interventions warrant closer consideration from healthcare professionals. For breathing interventions focused on increasing HF-HRV tone, the present results demonstrated the importance of including a rest period into the traditional breath cycle.
Acknowledgments
Financial support: Work reported in this publication was supported in part by the National Institute on Aging of the National Institutes of Health under Award Number F31AG048692. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data access and responsibility: The principal investigator, Matthew Russell, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Potential conflicts of interest: None
References
- Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. American Journal of Public Health. 1996;86(5):726–728. doi: 10.2105/ajph.86.5.726. doi: http://dx.doi.org/10.2105/AJPH.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of general psychology. 2006;10(3):229. doi: http://dx.doi.org/10.1037/1089-2680.10.3.229. [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. doi: http://dx.doi.org/10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology. 2005;42(2):246–252. doi: 10.1111/j.1469-8986.2005.00277.x. doi: http://dx.doi.org/10.1111/j.1469-8986.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL, Muench F. Breathing practices for treatment of psychiatric and stress-related medical conditions. Psychiatric Clinics of North America. 2013;36(1):121–140. doi: 10.1016/j.psc.2013.01.001. doi: http://dx.doi.org/10.1016/j.psc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Courtney R, Cohen M, van Dixhoorn J. Relationship between dysfunctional breathing patterns and ability to achieve target heart rate variability with features of" coherence" during biofeedback. Alternative Therapies in Health and Medicine. 2010;17(3):38–44. [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biological Psychology. 2007;74(2):286–294. doi: 10.1016/j.biopsycho.2005.09.00. doi: http://dx.doi.org/10.1016/j.biopsycho.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DR, Boggero IA, Segerstrom SC. The nature of self-regulatory fatigue and “ego depletion” lessons from physical fatigue. Personality and Social Psychology Review. 2015 doi: 10.1177/1088868315597841. Advance online publication. doi: http://dx.doi.org/10.1177/1088868315597841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JB, Plentz RDM, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: A randomized controlled trial. International Journal of Cardiology. 2013;166(1):61–67. doi: 10.1016/j.ijcard.2011.09.069. doi: http://dx.doi.org/10.1016/j.ijcard.2011.09.069. [DOI] [PubMed] [Google Scholar]
- Fried R, Grimaldi J. The psychology and physiology of breathing: In behavioral medicine, clinical psychology and psychiatry. New York, NY: Plenum Press; 1993. [Google Scholar]
- Gevirtz R. The promise of heart rate variability biofeedback: evidence based applications. Biofeedback. 2013;41:110–120. doi: http://dx.doi.org/10.5298/1081-5937-41.3.01. [Google Scholar]
- Gillie BL, Vasey MW, Thayer JF. Individual differences in resting heart rate variability moderate thought suppression success. Psychophysiology. 2015;52(9):1149–1160. doi: 10.1111/psyp.12443. doi: http://dx.doi.org/10.1111/psyp.12443. [DOI] [PubMed] [Google Scholar]
- Hall JE, Guyton AC. Textbook of medical physiology. Saunders; 2011. [Google Scholar]
- Hallman DM, Lyskov E. Autonomic regulation, physical activity and perceived stress in subjects with musculoskeletal pain: 24-hour ambulatory monitoring. International Journal of Psychophysiology. 2012;86(3):276–282. doi: 10.1016/j.ijpsycho.2012.09.017. doi: http://dx.doi.org/10.1016/j.ijpsycho.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Henriques G, Keffer S, Abrahamson C, Horst SJ. Exploring the effectiveness of a computer-based heart rate variability biofeedback program in reducing anxiety in college students. Applied Psychophysiology and Biofeedback. 2011;36(2):101–112. doi: 10.1007/s10484-011-9151-4. doi: http://dx.doi.org/10.1007/s10484-011-9151-4. [DOI] [PubMed] [Google Scholar]
- Kang JH, Chen HS, Chen SC, Jaw FS. Disability in patients with chronic neck pain: heart rate variability analysis and cluster analysis. The Clinical journal of pain. 2012;28(9):797–803. doi: 10.1097/AJP.0b013e3182442afd. doi: http://dx.doi.org/10.1097/AJP.0b013e3182442afd. [DOI] [PubMed] [Google Scholar]
- Kniffin TC, Carlson CR, Ellzey A, Eisenlohr-Moul T, Beck KB, McDonald R, Jouriles EN. Using virtual reality to explore self-regulation in high-risk settings. Trauma, Violence, & Abuse. Trauma, Violence, & Abuse. 2014;15(4):310–321. doi: 10.1177/1524838014521501. doi: http://dx.doi.org/10.1177/1524838014521501. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback. 2000;25(3):177–191. doi: 10.1023/a:1009554825745. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Frontiers in psychology. 2014;5 doi: 10.3389/fpsyg.2014.00756. doi: http://dx.doi.org/10.3389/fpsyg.2014.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin IM, Tai LY, Fan SY. Breathing at a rate of 5.5 breaths per minute with equal inhalation-to-exhalation ratio increases heart rate variability. International Journal of Psychophysiology. 2014;91(3):206–211. doi: 10.1016/j.ijpsycho.2013.12.006. doi: http://dx.doi.org/10.1016/j.ijpsycho.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Litchfield PM. A brief overview of the chemistry of respiration and the breathing heart wave. California Biofeedback. 2003;19(1):1–11. [Google Scholar]
- Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4(12):1523–1529. doi: 10.1016/j.hrthm.2007.07.019. doi: http://dx.doi.org/10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarewicz J, Verdejo-Garcia A, Giummarra MJ. Sympathetic pain? A role of poor parasympathetic nervous system engagement in vicarious pain states. Psychophysiology. 2015;52(11):1529–1537. doi: 10.1111/psyp.12516. doi: http://dx.doi.org/10.1111/psyp.12516. [DOI] [PubMed] [Google Scholar]
- Patron E, Benvenuti SM, Favretto G, Valfrè C, Bonfa C, Gasparotto R, Palomba D. Biofeedback assisted control of respiratory sinus arrhythmia as a biobehavioral intervention for depressive symptoms in patients after cardiac surgery: A preliminary study. Applied Psychophysiology and Biofeedback. 2013;38(1):1–9. doi: 10.1007/s10484-012-9202-5. doi: http://dx.doi.org/10.1007/s10484-012-9202-5. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. doi: http://dx.doi.org/10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsloo GE, Derman WE, Lambert MI, Rauch HL. The effect of a single session of short duration biofeedback-induced deep breathing on measures of heart rate variability during laboratory-induced cognitive stress: a pilot study. Applied psychophysiology and biofeedback. 2013;38(2):81–90. doi: 10.1007/s10484-013-9210-0. doi: http://dx.doi.org/10.1007/s10484-013-9210-0. [DOI] [PubMed] [Google Scholar]
- Russell MEB, Hoffman B, Stromberg S, Carlson CR. Use of controlled diaphragmatic breathing for the management of motion sickness in a virtual reality environment. Applied psychophysiology and biofeedback. 2014;39(4):1–9. doi: 10.1007/s10484-014-9265-6. doi: http://dx.doi.org/10.1007/s10484-014-9265-6. [DOI] [PubMed] [Google Scholar]
- Sargunaraj D, Lehrer PM, Hochron SM, Rausch L, Edelberg R, Porges SW. Cardiac rhythm effects of. 125-Hz paced breathing through a resistive load: implications for paced breathing therapy and the polyvagal theory. Biofeedback and self-regulation. 1996;21(2):131–147. doi: 10.1007/BF02284692. doi: http://dx.doi.org/10.1007/BF02284692. [DOI] [PubMed] [Google Scholar]
- Sauer SE, Burris JL, Carlson CR. New directions in the management of chronic pain: Self-regulation theory as a model for integrative clinical psychology practice. Clinical Psychology Review. 2010;30(6):805–814. doi: 10.1016/j.cpr.2010.06.008. doi: http://dx.doi.org/10.1016/j.cpr.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Schmidt JE, Carlson CR. A controlled comparison of emotional reactivity and physiological response in masticatory muscle pain patients. Journal of orofacial pain. 2009;23(3):230. [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. doi: http://dx.doi.org/10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Solberg Nes L, Carlson CR, Crofford LJ, Leeuw RD, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain. 2010;151(1):37–44. doi: 10.1016/j.pain.2010.05.009. doi: http://dx.doi.org/10.1016/j.pain.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Applied Psychophysiology and Biofeedback. 2003;28(1):13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- Strauss-Blasche G, Moser M, Voica M, McLeod DR, Klammer N, Marktl W. Relative timing of inspiration and expiration affects respiratory sinus arrhythmia. Clinical and Experimental Pharmacology and Physiology. 2000;27(8):601–606. doi: 10.1046/j.1440-1681.2000.03306.x. doi: http://dx.doi.org/10.1046/j.1440-1681.2000.03306.x. [DOI] [PubMed] [Google Scholar]
- Stromberg S, Russell MEB, Carlson CR. Diaphragmatic Breathing and its Effectiveness for the Management of Motion Sickness. Aerospace Medicine and Human Performance. 2014;86(5):452–457. doi: 10.3357/AMHP.4152.2015. doi: http://dx.doi.org/10.3357/AMHP.4152.2015. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. doi: http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a014868. [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. doi: http://dx.doi.org/10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Van Diest I, Verstappen K, Aubert AE, Widjaja D, Vansteenwegen D, Vlemincx E. Inhalation/Exhalation Ratio Modulates the Effect of Slow Breathing on Heart Rate Variability and Relaxation. Applied psychophysiology and biofeedback. 2014;39(3-4):171–180. doi: 10.1007/s10484-014-9253-x. doi: http://dx.doi.org/10.1007/s10484-014-9253-x. [DOI] [PubMed] [Google Scholar]
- Vaschillo E, Lehrer P, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback. 2002;27(1):1–27. doi: 10.1023/a:1014587304314. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Li S, Xu XY, Lin GP, Shao L, Zhao Y, Wang TH. Effect of slow abdominal breathing combined with biofeedback on blood pressure and heart rate variability in prehypertension. The Journal of Alternative and Complementary Medicine. 2010;16(10):1039–1045. doi: 10.1089/acm.2009.0577. doi: http://dx.doi.org/10.1089/acm.2009.0577. [DOI] [PubMed] [Google Scholar]
- Whited A, Larkin KT, Whited M. Effectiveness of emWave biofeedback in improving heart rate variability reactivity to and recovery from stress. Applied Psychophysiology and Biofeedback. 2014;39(2):75–88. doi: 10.1007/s10484-014-9243-z. doi: http://dx.doi.org/10.1007/s10484-014-9243-z. [DOI] [PubMed] [Google Scholar]
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Applied psychophysiology and biofeedback. 2009;34(2):135–143. doi: 10.1007/s10484-009-9085-2. doi: http://dx.doi.org/10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]