Abstract
Purpose
The aim of this phase I/II study was to assess the long-term clinical benefits and toxicities of proton beam therapy for medically inoperable early-stage non-small cell lung cancer (NSCLC).
Patients and methods
From June 2006 to September 2011, 35 patients with medically inoperable T1N0M0 (central or superior location, 12 patients) or T2-3N0M0 (any location, 23 patients) NSCLC were treated with 87.5 Gy at 2.5 Gy/fraction of proton therapy. Toxicities were scored according to the Common Terminology Criteria for Adverse Events, version 4.0.
Results
The median follow-up time was 83.1 months (95% CI: 69.2-97.1 months). For all 35 patients, the 1, 3, and 5-year overall survival rates were 85.7%, 42.9%, and 28.1%, respectively. The 5-year local recurrence-free, regional recurrence-free, and distant metastasis-free survival rates were 85.0%, 89.2%, and 54.4%, respectively. Different T stages had no effect on local and regional recurrence (p=0.499, p=1.00). However, with the increase in T stages, the distant metastasis rate increased significantly (p=0.006). The most common adverse effects were dermatitis (grade 2, 51.4%; grade 3, 2.9%) and radiation pneumonitis (grade 2, 11.4%; grade 3, 2.9%). Other grade 2 toxicities included esophagitis (2.9%), rib fracture (2.9%), heart toxicities (5.7%), and chest wall pain (2.9%).
Conclusions
According to our long-term follow-up data, proton therapy with ablative doses is well tolerated and effective in medically inoperable early-stage NSCLC. Systemic therapy should be considered to reduce the rate of distant metastasis in cases of T2 and T3 lesions.
Keywords: proton therapy, non-small cell lung cancer, early stage, toxicity, survival, phase I/II study
Introduction
Lung cancer is the leading cause of cancer related-death in US [1]. For early-stage non-small cell lung cancer (NSCLC) patients with no regional or distant metastasis, surgical resection is the standard treatment, with 5-year overall survival rates of 50%-70% [2]. However, many patients are not good candidates for surgery for medical reasons, such as poor pulmonary function, poor performance status, and medical comorbidities. For medically inoperable, peripherally located stage I NSCLC, stereotactic ablative radiotherapy (SABR)—also called stereotactic body radiation therapy (SBRT)—is the standard therapy [3-7]; outstanding local control has been reported, with some data indicating that it results in survival rates comparable to those of surgical resection [8, 9]. However, lesions that are located centrally or superiorly in the thorax, that invade the chest wall or hilum, or that are larger than 3 cm carry the risk of high-grade radiation pneumonitis (RP) and other severe radiation therapy (RT)-induced complications [10-13].
Proton therapy, with its sharp distal fall-off of the Bragg peaks, has superior dose distribution properties to those of conventional photon RT. Proton therapy for locally advanced NSCLC can deliver a higher dose to the primary tumor, leading to improved local tumor control and simultaneously decreasing the irradiated volume and dose delivered to the surrounding critical organs [14-19]. For centrally located early stage NSCLC, proton therapy was shown a dosimetric benefit and thus resulting in less toxicities compared to SABR photon plan [20].
The aim of this phase I/II prospective study was to assess the long-term clinical benefits and toxicities of proton beam therapy for patients with medically inoperable early-stage NSCLC and not suitable for SABR/SBRT. In this study, we used dose-escalated proton therapy, with a relative biological effectiveness (RBE)-equivalent total dose of 87.5 Gy (RBE) at 2.5 Gy (RBE)/fraction. All patients had centrally or superiorly located stage IA (T1N0M0) NSCLC, any stage IB (T2N0M0) NSCLC, or selected stage II (T3N0M0) NSCLC [21].
Patients and Methods
Study design, patient eligibility, and disease staging
This phase I/II study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center (Houston, Texas) [21]. From June 2006 to September 2011, 35 patients were enrolled with proton therapy. Each patient provided written informed consent before receiving therapy on the protocol. The eligibility criteria for the clinical studies were as follows (all required): 1) medically inoperable, evaluated and judged by multidisciplinary oncology team including thoracic surgeon, histologically or cytologically documented NSCLC; 2) T1N0M0 (stage IA) tumor that was centrally or superiorly located within 2 cm in all directions of any critical mediastinal structure, including the bronchial tree, esophagus, heart, brachial plexus, major vessels, spinal cord, phrenic nerve, and recurrent laryngeal nerve [22], T2N0M0 disease in any location (stage IB, tumor size >3 cm, with no upper size limit), or selected T3N0M0 (stage II) disease (chest wall, mediastinal pleura, or parietal pericardium involvement) in any location (according to the American Joint Committee on Cancer, 7th edition); and 3) an Eastern Cooperative Oncology Group performance status of 2 or less. In all cases, disease was staged by MRI or CT of the brain, CT of the chest, and PET within 3 months before RT. The median interval between PET-CT to the beginning date of RT was 1.25 months (range 0.0-2.7). The median interval between brain MRI and RT was 0.75 months (range 0.0-2.6). Mediastinoscopy or endobronchial ultrasound-guided biopsy was used to examine mediastinal or hilar lymph nodes that were suspected of harboring disease.
Simulation, target volume delineations, and radiation doses
Our simulation protocols were described in detail previously [21]. In brief, all patients underwent treatment simulation with 4-dimensional (4D) CT. The internal gross tumor volume was defined as the envelope of motion of the gross tumor volume on a reconstructed maximal intensity projection image at the lung window level and was verified across all phases of the 4D-CT dataset. The internal clinical target volume (iCTV) was defined the internal gross tumor volume, isotropically expanding 8 mm to encompass microextensions of the tumor. Two to 3 beams were used in each case. Proton plans were evaluated beam-by-beam to determine whether the iCTV plus the lateral, distal, and proximal margins were adequately covered for each beam. The total radiation dose for the tumor target was 87.5 Gy (RBE) at 2.5 Gy (RBE) per fraction, with fractions given once a day, 5 days per week. The biological effective dose (BED) of the prescribed regimen was 109.4 Gy using a / of 10. The minimum iCTV + margin dose was not allowed to fall below 95% of the prescribed dose. Dose volume constraints for critical structures have been reported previously [21].
Passive scattering proton therapy planning and adaptive proton delivery
Proton therapy planning and delivery with passive scattering was described in detail previously [17, 21, 23]. The individualized proton planning target volume margin, range uncertainties and treatment planning parameters were calculated on the basis of published formulae that were modified locally and adopted as the standard of practice at MD Anderson [17]. In brief, for each beam, the iCTV projection in the beam-eye view is expanded laterally by a margin that is the same as the one for photon planning (5 mm) to account for day-to-day setup uncertainties. In addition, beam-specific distal and proximal margins to the iCTV were assigned along the longitudinal axis of the beam to account for uncertainties in the range of protons. The range uncertainties was estimated using the smearing formula [17]. On the first day of treatment, orthogonal kilovoltage (KV) images were performed to confirm isocenter. In addition, each treatment position with and without aperture was verified using KV images. Subsequently, daily on-board KV orthogonal radiographic images were used to confirm the isocenter. 4D-CT scans were obtained for each patient during week 3 or 4 of treatment to evaluate tumor motion and anatomy changes. If the changes were found to significantly alter the proton dose distribution for target coverage or normal tissue toxicity, as judged by the treating physician [24], a new adaptive treatment plan was designed for the remainder of the treatments (Figure 1 and 2).
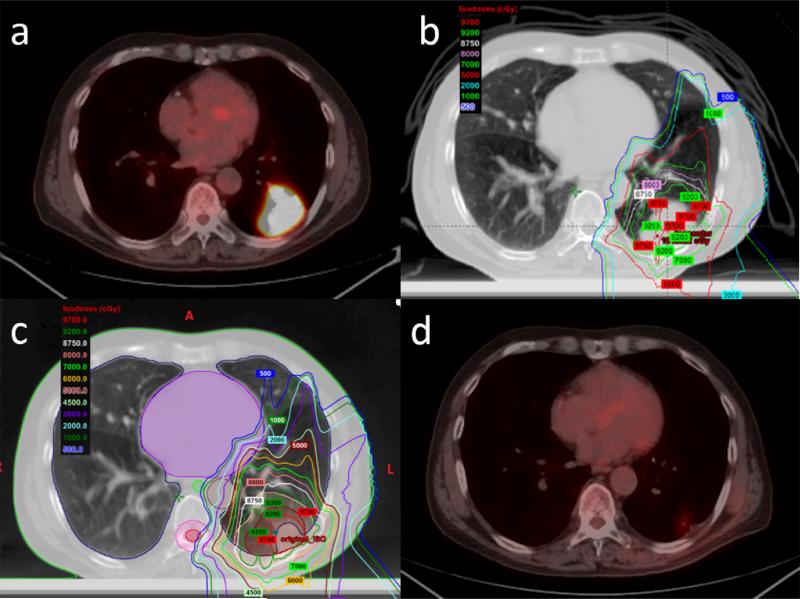
Figure 1.
a: PET-CT images obtained before proton therapy show T2N0M0 peripheral non-small cell lung cancer and a tumor located in the left lower lobe. b: Isodose distribution of proton therapy using 3-beam passive scattering proton therapy. c: After 3 weeks, resimulation was performed and significant tumor shrinkage was found. The treatment plan was redesigned. A high dose to the chest wall led to an acute grade 2 skin reaction. d: PET-CT images obtained after 3 months of proton therapy show a complete clinical response.
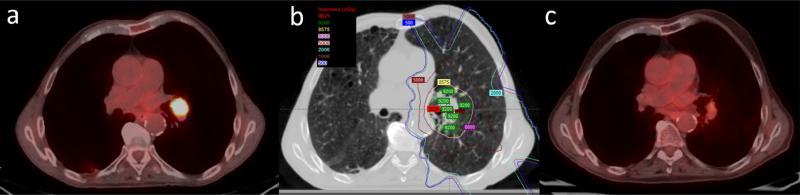
Figure 2.
a: PET-CT images obtained before proton therapy show T1N0M0 central non-small cell lung cancer and a tumor located in the left lung. b: Isodose distribution of proton therapy using 3-beam passive scattering proton therapy. c: PET-CT images obtained after 3 months of proton therapy show a complete clinical response.
Treatment evaluation and follow-up
After the completion of proton therapy, the patients were followed up with physical examinations, imaging (chest CT or PET-CT), and blood tests. The follow-up studies and the evaluation of tumor recurrence were described in detail previously [21]. In brief, patients were followed up at 6 weeks after the completion of RT, every 3 months (± 1 month) for 2 years, every 6 months (± 1 month) for 3 years, and then annually thereafter. Local recurrence at the primary tumor site must have been confirmed by PET-CT or biopsy. Recurrences were scored from the time at which the first image (PET or CT) showed abnormalities.
Radiation reactions, including esophagitis, pneumonitis, and chest wall pain, were evaluated during the course of treatment and follow-up. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0, but were reclassified according to version 4.0.
Statistical analysis
We calculated Kaplan-Meier curves for overall survival, progression-free survival, local recurrence-free survival, regional recurrence-free survival, and distant metastasis-free survival. Differences between pairs of Kaplan-Meier curves were examined using the log-rank test. The Fisher's exact test was used to compare local, regional, and distant recurrence rates. A p value of ≤0.05 was considered statistically significant. All statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) software v.21.0 (SPSS, Inc., Chicago, IL).
Results
Thirty-five patients (16 male and 19 female) were prospectively enrolled and treated between June 2006 and September 2011. The patient and tumor characteristics are summarized in Table 1. The median age was 73 years, and the median Karnofsky performance status score was 80. Twelve patients had T1 lesions, 20 patients had T2 lesions, and 3 patents had T3 lesions, while 25 patients had central or superior lesions and 10 patients had peripheral lesions. Using current SABR indications, among all 35 patients, 25 (71.4%) patients with either very large tumors (≥5cm) (Figure 1) or tumors abutting critical structures cannot be treated safely with SABR; eight patients (22.9%) with large (≥4cm, but <5cm) and/or central tumors but not abutting critical structures were not ideal candidates for SABR; only 2 patients with small (<4cm) and peripheral tumors could be treated potentially by SABR safely. The median gross tumor volume was 42.9 cm3 (range 4.2-435.0), and the median iCTV was 123 cm3 (range 14.3-786.6). Most patients had severe chronic obstructive pulmonary disease, with median pretreatment DLCO and FEV1 at 49% and 48% of predicted values, respectively. Only 2 patients underwent consolidation chemotherapy after proton therapy.
Table 1.
Characteristics of patients with NSCLC treated with proton therapy
| Characteristic | No. (%) |
|---|---|
| Age (years) | |
| Median | 73 |
| Range | 66-83 |
| ≤70 (%) | 13 (37.1) |
| >70 (%) | 22 (62.9) |
| Sex (%) | |
| Male | 16 (45.7) |
| Female | 19 (54.3) |
| Karnofsky performance score | |
| Median | 80 |
| Range | 60-90 |
| Smoking history (%) | |
| Yes | 31 (91.2) |
| No | 3 (8.8) |
| Chronic pulmonary disease (%) | |
| COPD | 16 (45.7) |
| Emphysema | 3 (8.6) |
| Pulmonary fibrosis | 1 (2.9) |
| Normal | 15 (42.8) |
| Tumor histological type (%) | |
| Squamous cell carcinoma | 17 (48.5) |
| Adenocarcinoma | 11 (31.4) |
| Squamous cell carcinoma + adenocarcinoma | 1 (2.9) |
| Neuroendocrine carcinoma | 1 (2.9) |
| Non-small cell carcinoma | 5 (14.3) |
| Tumor size (%) | |
| ≤3 cm | 13 (37.2) |
| >3 cm ≤5 cm | 16 (45.7) |
| >5 cm | 6 (17.1) |
| Tumor location (%) | |
| Central or superior | 25 (71.4) |
| Peripheral | 10 (28.6) |
| Charlson Index (%) | |
| 0 | 3 (8.6) |
| 1-2 | 17 (48.5) |
| 3-4 | 15 (42.9) |
| T stage (%) | |
| T1 | 12 (34.3) |
| T2 | 20 (57.1) |
| T3 | 3 (8.6) |
| AJCC 7th stage (%) | |
| IA | 12 (34.3) |
| IB | 16 (45.7) |
| IIA | 4 (11.4) |
| IIB | 3 (8.6) |
| GTV (cm3) | |
| Median | 42.9 |
| Range | 4.2-435.0 |
| iCTV (cm3) | |
| Median | 123 |
| Range | 14.3-786.6 |
| Pretreatment pulmonary function | |
| FEV1 | |
| Median | 48% |
| Range | 18%-100% |
| DLCO | |
| Median | 49% |
| Range | 18%-98% |
| Post-treatment pulmonary function | |
| FEV1 | |
| Median | 44% |
| Range | 16%-100% |
| DLCO | |
| Median | 49% |
| Range | 26%-98% |
FEV1, forced expiratory volume in 1 second; DLCO, carbon monoxide lung diffusion capacity
The last patient was enrolled on September 1, 2011. All patients were observed for a minimum of 49 months or until death. The median duration of follow-up was 83.1 months (95% CI: 69.2-97.1 months) using reverse Kaplan-Meier method. Verification 4D-CT scans were obtained during week 3 or 4 of proton therapy. Of 8 patients underwent adaptive replanning using new contours, 7 patients with central tumors or tumors abutting critical structures developed tumor shrinkage and underwent adaptive replan to avoid high dose to organs at risk (Figure 1 and 2). One patient felt uncomfortable in treatment position due to kyphosis and a pressure ulcer, and underwent repositioning and replanning.
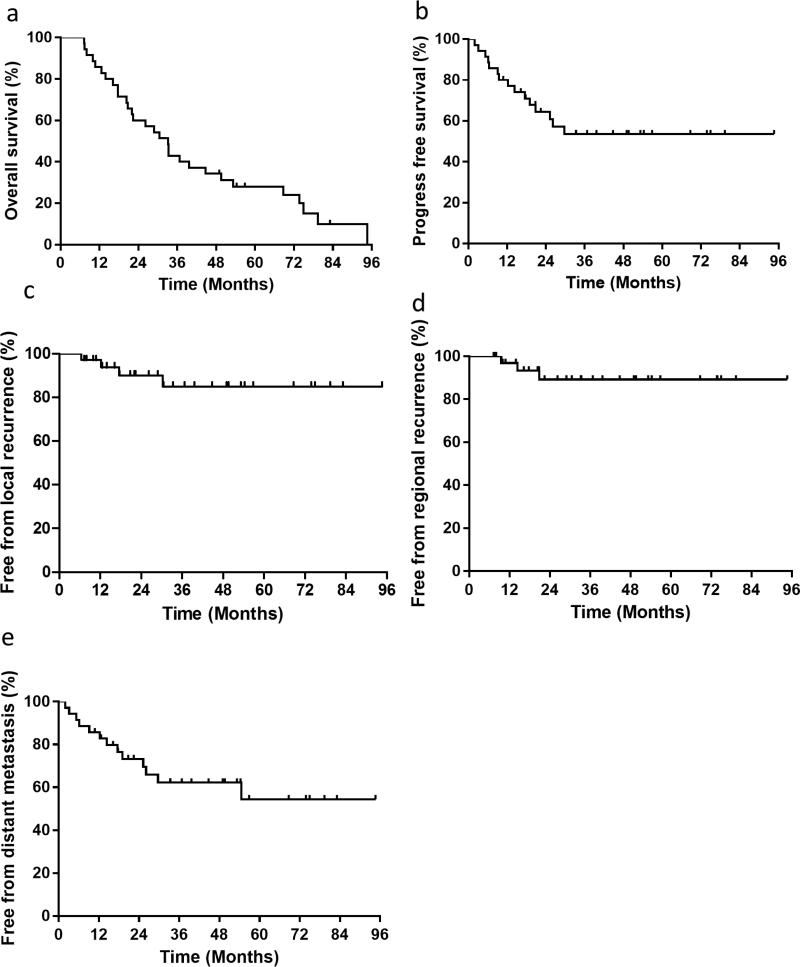
For all 35 patients, the 1-, 2-, 3-, and 5-year overall survival rates were 85.7%, 60.0%, 42.9%, and 28.1%, respectively, and the median overall survival duration was 33.2 months (Figure 3a). The 1-, 2-, 3-, and 5-year progression-free survival rates were 80.0%, 64.4%, 53.6%, and 53.6%, respectively, and the median progression-free survival duration was not reached (Figure 3b). The 5-year free from local recurrence, regional recurrence, and distant metastasis rates were 85.0%, 89.2%, and 56.0%, respectively (Figure 3c, 3d and 3f).
Figure 3.
Kaplan-Meier analyses of overall survival (a), progression-free survival (b), local recurrence (c), regional recurrence (d) and distant metastasis (e) rate. The 1-, 3-, and 5-year overall survival rates were 85.7%, 42.9%, and 28.1%, respectively, and the median overall survival duration was 33.2 months. The 1-, 3-, and 5-year progression-free survival rates were 80.0%, 53.6%, and 53.6%, respectively. The median progression-free survival was not reached. The 1-, 3-, and 5-year free from local recurrence rates were 97.1%, 85.0%, and 85.0%, respectively; the 1-, 3-, and 5-year free from regional rates were 96.9%, 89.2%, and 89.2%; and the 1-, 3-, and 5-year free from distant metastasis rates were 85.7%, 62.2%, and 54.4%.
Fifteen patients experienced relapse, including 4 with simultaneous local and distant recurrences. No patients had a local recurrence only, 1 had regional and distant recurrences, 2 had regional recurrences only, and 8 had distant recurrences only. Local recurrences occurred 6.4-30.4 months after proton therapy. One local recurrence was confirmed by both PET-CT and biopsy, and the other 3 patients were diagnosed on the basis of PET-CT imaging only. The rate of accumulated local recurrences was 2.9% at 1 year and 15.0% at 3 and 5 years. Three patients had regional recurrences, and the rates of accumulated regional recurrences were 3.1% at 1 year and 10.8% at 3 years and 5 years. Thirteen patients developed distant metastases; the rates of accumulated distant metastases were 14.3% at 1 year, 37.8% at 3 years, and 45.6% at 5 years. The metastatic sites included the liver (5 patients), brain (3 patients), lungs (3 patients), adrenal grand (2 patients), bone (2 patient), and pleura (1 patient). The patterns of failure are shown in Table 2. Different T stages had no effect on local and regional recurrence, with local recurrences in 1 of 12 T1 tumors, 2 of 20 T2 tumors, and 1 of 3 T3 tumors (p=0.499), and regional recurrences in 1 of 12 T1 tumors, 2 of 20 T2 tumors, and 0 of 3 T3 tumors (p=1.00). However, with the increase in T stage, the distant metastasis rate increased significantly, with rates in T1, T2, and T3 tumors of 8.3% (1), 45.0% (9), and 100% (3), respectively (p=0.006). Salvage therapy for 7 patients with local and/or regional recurrence included chemotherapy (1 patient), targeted therapy (1 patient), chemoradiotherapy with photon (2 patients) and unknown/no treatment (3 patients).
Table 2.
Pattern of first recurrence sites by stage T
| Recurrence type | T1 (n=12) | T2 (n=20) | T3 (n=3) | P value | |
|---|---|---|---|---|---|
| Relapse | Yes | 2 | 10 | 3 | 0.017 |
| No | 10 | 10 | 0 | ||
| Local recurrence | Yes | 1 | 2 | 1 | 0.499 |
| No | 11 | 18 | 2 | ||
| Regional recurrence | Yes | 1 | 2 | 0 | 1.000 |
| No | 11 | 18 | 3 | ||
| Distant metastasis | Yes | 1 | 9 | 3 | 0.006 |
| No | 11 | 11 | 0 |
All patients completed the prescribed treatment, and no patient experienced grade 4 or 5 toxicity. The most common adverse effect was dermatitis: grade 3 was observed in 1 patient (2.9%), and grade 2 was scored in 18 patients (51.4%). Grade 3 RP was noted in 1 patient (2.9%) who had chronic obstructive pulmonary disease and very poor respiratory function before RT, grade 2 pneumonitis was observed in 4 patients (11.4%). Most symptomatic grade 2 or 3 RP occurred within 3 to 6 months after the start of RT. The FEV1 and DLCO did not change before and after therapy. Grade 2 esophagitis occurred in 1 patient (2.9%), and there was no grade 3 or higher esophagitis. Possible grade 2 cardiac toxicity (atrial fibrillation) were noted in 2 patients (5.7%) at 4 and 8 months after the end of radiotherapy respectively. In addition, grade 2 rib fracture, without the presence of cancer, was found in 1 patient (2.9%). Grade 2 and 1 chest wall pain occurred in 1 (2.9%) and 4 patients (11.4%) respectively.
Discussion
With long-term follow-up (83.1 months), the current study demonstrates that proton beam therapy with an ablative dose (biologically effective dose [BED] =109.4 Gy with α/β of 10 [BED10]) was effective in patients with early-stage NSCLC, with an 85.0% 5-year local control rate and no grade 4 or 5 toxicities. The 5-year free from local and regional recurrence rates were 85.0% and 89.2%, respectively, with a dominant pattern of failure of distant.
For patients with medically inoperable disease and those who decline surgery, stereotactic ablative RT is a standard treatment; it achieves a >90% local control rate and promising survival rates [3-6]. However, this technique delivers an extremely high dose per fraction that can lead to severe toxicity if critical organs are within or next to the ablative dose [10, 11, 13, 25]. Proton therapy allows improved dose conformity, particularly to organs distal to the proton beam range. Thus, we hypothesized that it might be of benefit patients with large tumors (>5 cm) or tumors next to or abutting critical structures, such as the heart, spinal cord, large vessels, bronchial tree, and esophagus [26, 27]. Some planning studies suggested that passive scattering plans may deliver a lower radiation dose to normal tissues including the lungs, esophagus, bronchial tree and spinal cord than photon-based radiation therapy [14, 28], however may increase the dose to the skin and chest wall [14], so we performed a safety and efficacy study to formally test our hypothesis.
Several retrospective studies have shown the safety and efficacy of proton therapy in early-stage NSCLC, although the BED was lower than 100 Gy in most reports [29-33]. Bush et al. [29] reported 37 patients with early-stage NSCLC, including 27 stage I patients who underwent proton therapy or a combination of proton and photon therapy. With a short follow-up, the 2-year local control rate was 87%, and the overall survival rate was 31%. The BED10 was 77 Gy (5.1 Gy x 10 fractions). In other studies of proton therapy for early-stage NSCLC, the local control rate was 39%-74% at 3 years. In these studies, the total dose and fraction included 76 Gy (range, 49-93 Gy) with 3.0 Gy/fraction (range, 2-6 Gy) [30], 51 Gy in 10 fractions [31], and 70-94 Gy in 20 fractions (3.5-4.9 Gy per fraction) [32]; the BED10 was mostly less than 100 Gy except 70 Gy in 10 fractions for a subgroup in a large study [33]. A retrospective collaborative study showed that both local control and survival rates were higher in those who received more than 100 Gy than in those who received <100 Gy, according to BED calculations using tissue that responded early and an α/β of 10 [34]. Indeed, this conclusion was confirmed by several studies of proton therapy [35, 36].
In our study, 87.5 Gy (RBE) of proton therapy was given in 35 2.5-Gy fractions over 7 weeks. The BED10 (109 Gy) was higher than that in previous studies, and excellent local (85.0%) and regional lymph node control (89.2%) was achieved at 5 years that appeared to not be correlated with patients’ T stage. Chemotherapy, with or without RT, was given as salvage therapy after local, regional lymph node or distant recurrence. The dominant pattern of failure in all patients was distant metastasis (45.6%) that appeared to be correlated with T stage, indicating that effective chemotherapy is needed. However, most patients did not undergo chemotherapy because of poor performance status. The pattern of failure and survival was comparable to reported data of early stage NSCLC treated with other hypofractionated proton therapy [20]. Further hypofractionation using proton therapy in this group of patients should be explored to improve efficiency and reduce the cost.
The dose volume constraints for critical structures in this protocol was reported previously [21]. The dose limits for bronchial tree and large blood vessels was both 87.5 Gy (RBE) < 10cm3 and heart was 70 Gy <10%. As compared with a recent clinical trial EORTC 221133 (LungTech), our dose volume constraints appears to allow more dose delivered to esophagus, brachial plexus. However, we restricted dose to lung, heart and major vessels that was not included in LungTech trial [37].
Our results showed that the incidence of ≥grade 2 toxicity was relatively uncommon and no patient experienced grade 4 or 5 toxicities. The most common toxicities included dermatitis and RP. Grade 2 dermatitis occurred in 51.4% (18 of 35), and grade 3 occurred in 2.9% (1 of 35). The main reason for the dermatitis in this study was that 3 or less proton beam fields were used at early enrollment and therefore the proton beam entry dose was higher. We recommend the use of at least 3 to 4 beams when high doses are required. Although our patients had poor performance status and had a median age of 73 years, there was only 1 (2.9%) grade 3 RP, and it developed in a patient who had significant chronic obstructive pulmonary disease before proton therapy. Grade 2 RP was observed in 4 patients (11.4%). Interestingly, patients’ pulmonary function did not change after proton therapy compared with that before therapy. The data compared favorably with those of the RTOG 9311 >84 Gy cohorts using photon therapy, in which grade 2 RP was found in up to 45% of cases [38]. Similar results were shown in another study of early-stage NSCLC treated by proton therapy [37, 39]. The rate of rib fracture was also low (2.9%), similar to that in other reports [33, 35, 40].
Compared with most published studies of proton therapy in early-stage NSCLC, our patients had larger tumors and more challenging tumor locations, and most (94.3%) patients cannot be treated with SABR safely. We delivered an ablative dose (BED>100 Gy) to the target, which was higher than those in most previously published studies; on long-term follow-up, this had resulted in promising local control with minimal toxicity. Given these findings, dose escalated/hypofractionated proton therapy can be considered as safe and effective regimen in this group of patients. It is not clear, however, from this work that hypofractionated proton therapy is better than results that can be achieved with conventional SABR techniques using photon therapy although toxicity appears significantly lower. Based on the dosimetric advantages of sparing normal critical structures, the meaningfully clinical value of proton therapy deserves further research in randomized study. At the same time, technical challenges of proton therapy demand further technique optimization for mature application in clinical practice [41]. In this study, distant failure remained high, indicating better systemic control is needed. Many of these patients were unable to tolerate conventional chemotherapy, therefore, novel systemic treatment such as immunotherapy should be explored. Recent development of combined radiotherapy and immunotherapy provides unique opportunity to investigate how to reduce distant failure by radiation-induced immune modulation and immunotherapy [42]. There was hypothesis generating preliminary data indicated that immune stimulation could be higher using particle therapy as compared photon therapy (personal communication). More studies are needed to validate this hypothesis.
Acknowledgments
Funding sources: This research was supported in part by National Cancer Institute grant P01 CA021239, NCI Cancer Center Core Support Grant CA016672, and NCI Clinical and Translational Science Award UL1 RR024148 to MD Anderson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no actual or potential financial conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Su S, Scott WJ, Allen MS, Darling GE, Decker PA, McKenna RJ, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. The Journal of thoracic and cardiovascular surgery. 2014;147:747–52. doi: 10.1016/j.jtcvs.2013.10.001. Discussion 52-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solda F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;109:1–7. doi: 10.1016/j.radonc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Taremi M, Hope A, Dahele M, Pearson S, Fung S, Purdie T, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. International journal of radiation oncology, biology, physics. 2012;82:967–73. doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:5153–9. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 7.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;101:240–4. doi: 10.1016/j.radonc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Shirvani SM, Jiang J, Chang JY, Welsh J, Likhacheva A, Buchholz TA, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA surgery. 2014;149:1244–53. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. The Lancet Oncology. 2015;16:630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milano MT, Chen Y, Katz AW, Philip A, Schell MC, Okunieff P. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;91:301–6. doi: 10.1016/j.radonc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Nuyttens JJ, van der Voort van Zyp NC, Praag J, Aluwini S, van Klaveren RJ, Verhoef C, et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;102:383–7. doi: 10.1016/j.radonc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Chang JY, Li QQ, Xu QY, Allen PK, Rebueno N, Gomez DR, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a “no fly zone”. International journal of radiation oncology, biology, physics. 2014;88:1120–8. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald OK, Kruse JJ, Miller JM, Garces YI, Brown PD, Miller RC, et al. Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: a comparative dosimetric analysis. International journal of radiation oncology, biology, physics. 2009;75:950–8. doi: 10.1016/j.ijrobp.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2011;80:1015–22. doi: 10.1016/j.ijrobp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westover KD, Seco J, Adams JA, Lanuti M, Choi NC, Engelsman M, et al. Proton SBRT for medically inoperable stage I NSCLC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1021–5. doi: 10.1097/JTO.0b013e31824de0bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2006;65:1087–96. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Stuschke M, Kaiser A, Pottgen C, Lubcke W, Farr J. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;104:45–51. doi: 10.1016/j.radonc.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen QN, Ly NB, Komaki R, Levy LB, Gomez DR, Chang JY, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;115:367–72. doi: 10.1016/j.radonc.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wink KC, Roelofs E, Solberg T, Lin L, Simone CB, 2nd, Jakobi A, et al. Particle therapy for non-small cell lung tumors: where do we stand? A systematic review of the literature. Frontiers in oncology. 2014;4:292. doi: 10.3389/fonc.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang JY, Komaki R, Wen HY, De Gracia B, Bluett JB, McAleer MF, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2011;80:1350–7. doi: 10.1016/j.ijrobp.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JY, Bezjak A, Mornex F, Committee IART Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:577–85. doi: 10.1097/JTO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 23.Kang Y, Zhang X, Chang JY, Wang H, Wei X, Liao Z, et al. 4D Proton treatment planning strategy for mobile lung tumors. International journal of radiation oncology, biology, physics. 2007;67:906–14. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Koay EJ, Lege D, Mohan R, Komaki R, Cox JD, Chang JY. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2012;84:1093–100. doi: 10.1016/j.ijrobp.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. The New England journal of medicine. 2012;366:2327–9. doi: 10.1056/NEJMc1203770. [DOI] [PubMed] [Google Scholar]
- 26.Terasawa T, Dvorak T, Ip S, Raman G, Lau J, Trikalinos TA. Systematic review: charged-particle radiation therapy for cancer. Annals of internal medicine. 2009;151:556–65. doi: 10.7326/0003-4819-151-8-200910200-00145. [DOI] [PubMed] [Google Scholar]
- 27.Simone CB, 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer journal. 2014;20:427–32. doi: 10.1097/PPO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 28.Hoppe BS, Huh S, Flampouri S, Nichols RC, Oliver KR, Morris CG, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;97:425–30. doi: 10.1016/j.radonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Bush DA, Slater JD, Bonnet R, Cheek GA, Dunbar RD, Moyers M, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest. 1999;116:1313–9. doi: 10.1378/chest.116.5.1313. [DOI] [PubMed] [Google Scholar]
- 30.Shioyama Y, Tokuuye K, Okumura T, Kagei K, Sugahara S, Ohara K, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2003;56:7–13. doi: 10.1016/s0360-3016(02)04416-4. [DOI] [PubMed] [Google Scholar]
- 31.Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 32.Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2006;65:107–11. doi: 10.1016/j.ijrobp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Bush DA, Cheek G, Zaheer S, Wallen J, Mirshahidi H, Katerelos A, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. International journal of radiation oncology, biology, physics. 2013;86:964–8. doi: 10.1016/j.ijrobp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–31. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 35.Iwata H, Demizu Y, Fujii O, Terashima K, Mima M, Niwa Y, et al. Long-term outcome of proton therapy and carbon-ion therapy for large (T2a-T2bN0M0) non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:726–35. doi: 10.1097/JTO.0b013e318288ab02. [DOI] [PubMed] [Google Scholar]
- 36.Kanemoto A, Okumura T, Ishikawa H, Mizumoto M, Oshiro Y, Kurishima K, et al. Outcomes and prognostic factors for recurrence after high-dose proton beam therapy for centrally and peripherally located stage I non--small-cell lung cancer. Clinical lung cancer. 2014;15:e7–12. doi: 10.1016/j.cllc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Adebahr S, Collette S, Shash E, Lambrecht M, Le Pechoux C, Faivre-Finn C, et al. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. The British journal of radiology. 2015;88:20150036. doi: 10.1259/bjr.20150036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. International journal of radiation oncology, biology, physics. 2005;61:318–28. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 39.Hata M, Tokuuye K, Kagei K, Sugahara S, Nakayama H, Fukumitsu N, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. International journal of radiation oncology, biology, physics. 2007;68:786–93. doi: 10.1016/j.ijrobp.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the university of tsukuba. International journal of radiation oncology, biology, physics. 2010;78:467–71. doi: 10.1016/j.ijrobp.2009.07.1707. [DOI] [PubMed] [Google Scholar]
- 41.Chang JY, Jabbour SK, De Ruysscher D, Schild SE, Simone CB, 2nd, Rengan R, et al. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2016;95:505–16. doi: 10.1016/j.ijrobp.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nature reviews Clinical oncology. 2016;13:516–24. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]