SUMMARY
Center-surround antagonism has been used as the canonical model to describe receptive fields of retinal ganglion cells (RGCs) for decades. We describe a newly identified RGC type in mouse, called the ON delayed (OND) RGC, with receptive field properties that deviate from center-surround organization. Responding with an unusually long latency to light stimulation, OND RGCs respond earlier as the visual stimulus increases in size. Furthermore, OND RGCs are excited by light falling far beyond their dendrites. We unravel details of the circuit mechanisms behind these phenomena, revealing new roles for inhibition in controlling both temporal and spatial receptive field properties. The non-canonical receptive field properties of the OND RGC – integration of long temporal and large spatial scales – suggest that unlike typical RGCs, it may encode a slowly varying, global property of the visual scene.
Keywords: retina, ganglion cells, receptive-field, latency, center-surround, serial inhibition, extra-dendritic, mouse, image focus, circuit
INTRODUCTION
Center-surround spatial organization is one of the core features of receptive field structure in visual neurons. Beginning at the first synapse in the retina, lateral inhibition shapes signals, creating antagonism between local (center) and global (surround) regions of visual space. Retinal ganglion cells (RGCs) were the first visual neurons to be described in terms of center-surround receptive fields [1], and the circuit mechanisms of receptive field structure have been studied extensively in the retina [2–4].
Alongside this classical view of receptive field structure has been a growing appreciation for the diversity of RGC types and their specific roles in detecting specific visual features [5–12]. Some of these feature detectors, like object-motion-sensitive RGCs, rely on an antagonistic surround [7,13] while others, like M1 intrinsically photosensitive RGCs eschew center-surround antagonism to suit their role in reporting global luminance [14]. Despite recent progress, few RGC types have been characterized in terms of unique electrophysiological responses, and circuit mechanisms responsible for such specific responses have only been identified for a handful of RGC types among the ~40 types thought to exist in the mammalian retina [12].
Here we describe a new type of RGC in the mouse, named the ON delayed (OND) RGC, whose receptive field structure deviates from the center-surround paradigm. Rather than being antagonized by large light stimuli, the OND RGC responds earlier than it does to smaller stimuli. The range of possible response latencies for OND RGCs is large, and the response to a stimulus of a size comparable to the cell’s dendrites is unusually late. In addition, opposite to the usual antagonistic effect of the surround, firing in the OND RGC can be elicited by light patterns far outside its dendritic field. Our studies of the mechanisms responsible for these unusual receptive field characteristics yield new insights into the synaptic organization of retinal circuits and broaden our understanding of the varied roles of inhibition.
The canonical center-surround organization of RGCs serves to localize their spatial responses. The unusual characteristics of the receptive field of OND RGCs lead us to suggest that they report a more global property of the scene, requiring integration rather than precisely reporting an event in time and space.
RESULTS
Functional and morphological classification of the ON delayed RGC
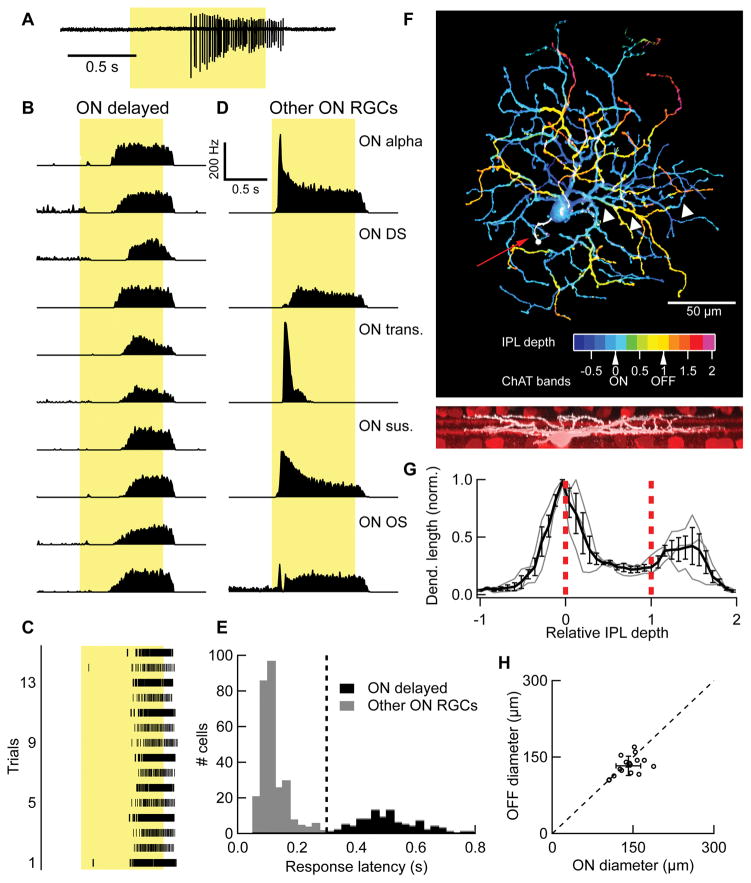
As part of an effort to catalogue the diversity of RGC light responses in the mouse, we encountered a set of cells with unusually long response latencies. Examples of ten of these “ON delayed” (OND) RGCs responding to a 200 μm spot of light (intensity 100 rod isomerizations (R)*/rod/s, presented from dark and centered on the receptive field) are shown in Figure 1A,B. Consistency of the response across trials (Figure 1C), suggested that the long latency was not a result of adaptation to repeated light flashes.
Figure 1. Light response and morphology of the OND RGC.
(A) Light response of an OND RGC to a spot of light 200 μm in diameter, at 100 R*/rod/s presented from darkness (“Light Step”). Cell-attached spiking response in a single trial. Duration of stimulus presentation denoted by yellow rectangle here and elsewhere. (B) Peri-stimulus time histogram (PSTH) for 10 representative OND RGCs responding to the light step stimulus, 10–20 trials each. (C) PSTH of spike activity in response to the light step stimulus in representative examples of 5 other RGC types computed from 10–20 trials each. ON DS, ON direction selective; ON OS, ON orientation selective; ON trans., ON transient; ON sus., ON sustained. (C) Spike raster showing individual trials of an OND RGC responding to light step. (E) Distribution of half-maximum latency in response to light step across a sample of 382 RGCs containing ON, non-direction selective cells. Vertical line, classifier of OND cells at latency = 0.3 s. Cells to the right of this line are OND cells (black), whereas cells to the left of this line are not (gray). (F) Top, maximum projection confocal image of an OND RGC. Color scale represents depth within the inner plexiform layer (IPL). Red arrow points to axon stub (white). White arrows point to a recursive dendrite. Arrows below color scale denote IPL depth of the ON and OFF ChAT bands. Bottom, side view of the same cell, same scale (white) along with starburst amacrine cells and ChAT bands (red). (G) Dendritic density profile. IPL depth is in normalized coordinates where 0 and 1 correspond to the ON and OFF ChAT bands, respectively (red dashed lines). Gray lines, individual cells; Black, mean density ± SEM (n = 3 cells). (H) Equivalent diameter of the dendritic fields of the ON and OFF strata of OND RGCs (see Experimental Procedures). Empty circles, OND cells; filled symbol and error bars, mean ± SD. (n = 16 cells). Dashed line is the line of equal ON and OFF dendritic diameters. See also Figures S1, S2.
The response pattern was highly similar across cells within this group (Figure 1B), compared to ON RGCs outside this group that responded differently to the light step (Figure 1D). While ON alpha, ON direction-selective, and ON orientation-selective are well characterized RGC types (independently confirmed by large somata, direction-selectivity, and orientation selectivity, respectively), ON transient and ON sustained RGCs were grouped according to the temporal profile of their light responses and likely each included multiple RGC types. The similarity in response profile among OND RGCs and their differences from other ON RGCs suggest that OND RGCs may consist a single functional RGC type.
We further investigated the statistical separation of OND RGCs from other RGC types based quantified properties of their light responses. OND RGCs were not direction selective when probed with moving bars (see Experimental Procedures, DSI = 0.05 ± .01. n = 14). ON direction selective RGCs cells also showed relatively long response latencies to a light step (0.28 ± .02 s, n = 54), but were easily distinguished from OND RGCs by their direction selectivity (p < 10−10, unpaired t-test; DSI = 0.39 ± .02, n = 56). Among non-direction-selective ON RGCs we recorded (DSI < 0.2), there was a significantly bimodal distribution of response latencies (p < 10−5, Hartigan’s dip test) with a clear separation at 0.3 s (Figure 1E). Thus, we established the threshold of 0.3 s latency to characterize ON non-direction-selective RGCs as OND.
A clear statistical separation of the OND RGCs from all other RGCs based on response latency provided evidence that OND RGCs may constitute a single RGC type. Results below show that cells classified by this functional criterion also shared characteristic receptive field properties not found in other RGCs, as well as other physiological and morphological characteristics, further supporting the claim that they represent a single RGC type. We found no evidence for heterogeneity within OND RGCs that would imply further splitting of this population into multiple types.
We filled and imaged OND RGCs to quantify their dendritic morphology (Figure 1F–H, Figure S1). OND RGCs stratified near the ON choline acetyl-transferase (ChAT) band and distal to the OFF ChAT band (Figure 1G). This represents another example, along with the M1 ipRGC [15] and the ON OS RGC [16] of a functionally pure ON RGC with an OFF dendritic stratification. We confirmed that OND RGCs retained pure ON polarity in photopic conditions (Figure S2).
OND RGCs had relatively small dendritic fields with an equivalent diameter of 141 ± 23 μm (mean ± SD) in the ON stratum and 133 ± 19 μm (mean ± SD, n = 16) in the OFF stratum (Figure 1H). We also measured the longest axis through a polygon outlining the dendritic field in each stratum (Figure S1A). The long axes were only ~40% longer than the equivalent diameter, a sign of symmetric morphology shared by most RGCs [16–18]. We found OND RGCs in locations throughout the retina with no apparent gradient in density, function or morphology.
The most distinctive morphological feature of OND RGCs was their recursive dendrites that extended to the OFF stratum before returning back to the ON stratum (Figure S1B). Recursive dendrites have been described in a different RGC type in rabbit and mouse, the suppressed-by-contrast RGC [19–22]. We found more and longer recursive dendrites in the OND RGC than in the mouse suppressed-by-contrast RGC or in two other bistratified RGC types (Figure S1C,D). The closest morphological match to the OND RGC in a public online database of electron-microscopic reconstructions is the “73” (museum.eyewire.org). More tentative matches exist in the published connectomics electron-microscopy data: gc31–56 from [23], and in a recent functional study of RGC calcium signals: cluster no. 37, “ON slow” from [12]. We note that a morphologically similar cell to the OND RGC, the recursive bistratified RGC, has been found in non-human primates [24].
Inverted size-latency tuning and its mechanism
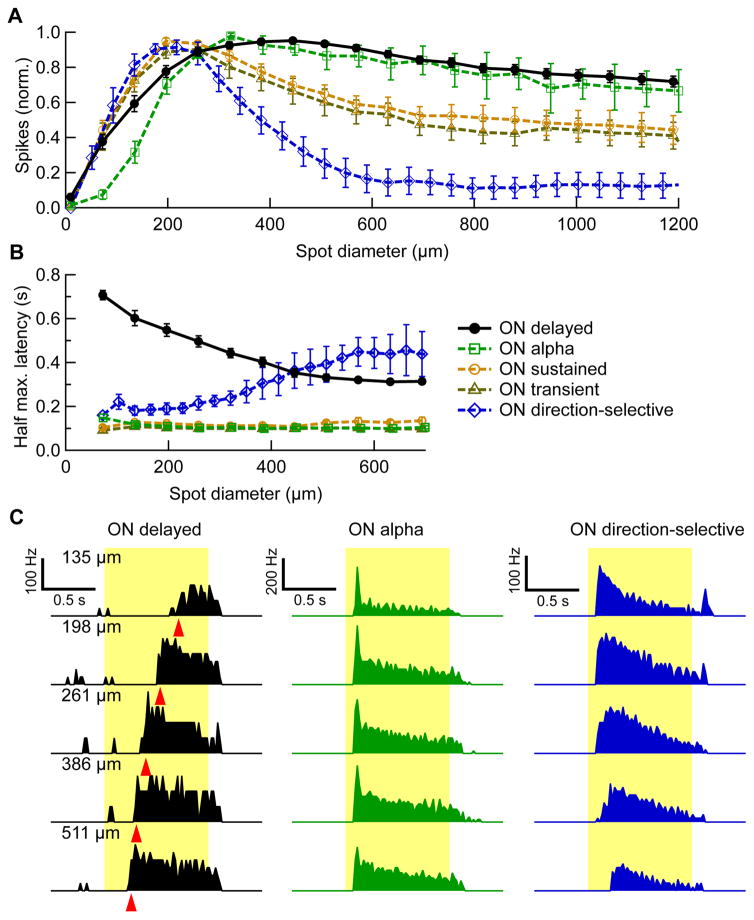
To measure the extent and strength of the OND RGC receptive field center and surround, we presented light spots of varying sizes from darkness (to 100 R*/rod/s). Given the small dendritic field of the OND RGC, we expected to find a small receptive field center. Figure 2A shows the spike count during stimulus presentation normalized to its maximum as a function of spot size for OND RGCs and four other ON RGC groups. In fact, OND RGCs had surprisingly large receptive fields (maximal response diameter, 386 ± 30 μm, n = 16) given their dendritic diameter. Unlike most RGC types ([7,16,25]; Figure 2A), OND RGCs showed very little surround suppression.
Figure 2. Response latency and spike count as a function of stimulus size.
(A) Spike count during stimulus presentation vs. spot diameter, normalized by its maximum (average ± SEM over cells within each group). Numbers of cells: OND n = 16, ON alpha, n = 4, ON direction-selective, n = 6, ON sustained, n = 13, ON transient, n = 9. (B) Half maximum latency vs. spot diameter (average ± SEM over cells within each group). (C) PSTH of an OND RGC (black), ON alpha RGC (green) and a ON direction-selective RGC (blue) in response to spots of different sizes. Spot sizes are denoted on the upper left of each row. From PSTHs, spike count and latency were extracted. Red triangles, half maximum latency for the OND PSTHs. See also Figure S3.
An unusual feature of the OND RGC’s response to the spot stimuli was its inverted size-latency tuning (Figure 2B). While other RGCs either increased response latency or showed no change in latency with increases in spot size, OND RGCs responded earlier to larger spots. The range over which the latency varied with spot diameter was large (0.3 ± 0.02 s, n = 20) compared to that observed in RGCs in mouse and other species [26]. Examples for the same cell responding at five spot sizes are shown in Figure 2C, together with examples of two other RGCs for comparison. We also measured inverted size-latency tuning in OND RGCs in photopic conditions indicating that this feature of the response was robust across luminance conditions (Figure S3).
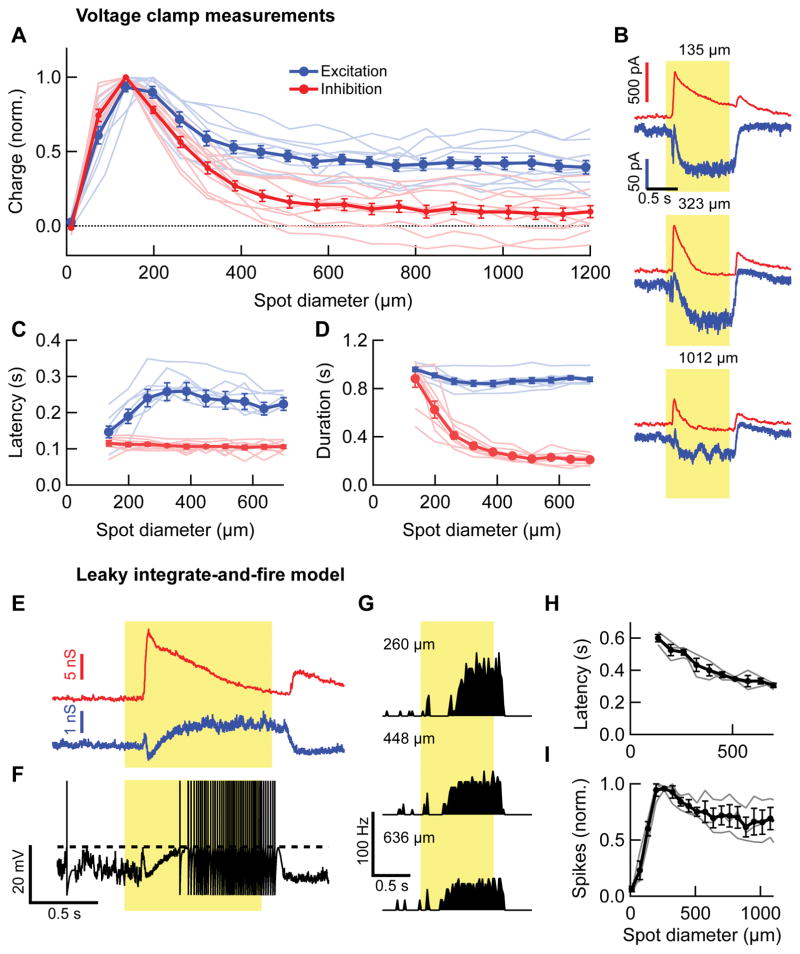
To determine the mechanisms responsible for inverted size-latency tuning, we performed whole-cell voltage clamp recordings from OND RGCs (Figure 3). Excitation to the OND RGC (peak current = −122 ±10 pA, n = 11) occurred only at light onset, was slow and sustained, and sometimes had a small very transient initial component (Figure 3B). Inhibition was large compared to excitation (peak current = 750 ± 70 pA, n = 13; maximum excitation-to-inhibition ratio of peak current across cells = 0.2 ± 0.02, n = 11), was present at light onset and offset and was more transient than excitation. Two features of the synaptic conductances were distinct from patterns typically observed in RGCs. First, inhibition had stronger surround suppression than excitation (Figure 3A). Second, inhibition had a shorter latency (Figure 3C) and shorter duration (Figure 3D) than excitation. The duration of the inhibitory conductance also showed a marked dependence on spot size, becoming briefer for larger spots (Figure 3D).
Figure 3. Stimulus size dependence of synaptic currents in OND RGCs and Leaky integrate-and-fire model.
(A) Total charge of excitatory (blue, n = 10 cells) and inhibitory (red, n = 11 cells) currents in OND RGCs, normalized by their maximum, as a function of spot diameter. Thin lines are individual cells, thick lines are mean ± SEM throughout figure. (B) Excitatory and inhibitory currents as a function of time, measured in an OND RGC for three different spot sizes, denoted above each panel (mean traces over 3 trials, same colors as in main figure). From current time traces, charge, latency and duration were extracted. (C) Latency of excitatory (blue) and inhibitory (red) response currents as a function of spot diameter. Inhibition was earlier than excitation: spot diameter ≥ 197 μm, p < 0.0013, one-tailed paired t-test. (D) Duration of excitatory (blue) and inhibitory (red) response as a function of spot diameter. Inhibition was more transient than excitation: spot diameter ≥ 197 μm, p < 0.0017, one-tailed paired t-test. (n = 8 cells in (C, D)). (E) Excitatory (blue) and inhibitory (red) synaptic conductances in an OND RGC in response to a 200 μm diameter spot, obtained directly from measured synaptic currents (such as those in (B)), and fed into the leaky integrate-and-fire model. (F) resulting simulated membrane potential, using conductances in (F). Dashed line is the spiking threshold. (G) Simulated PSTH for different spot diameters that are denoted on the left. Each PSTH was calculated from the spiking in 12 simulated trials. (H) Simulated spike count vs. spot diameter for three different OND cells (thin lines); thick line, average curve ± SEM. (I) Half max latency vs. spot diameter, measured for the same cells and simulated PSTHs as in (H). Thin line, individual cells, thick line, average ± SEM (n=3 cells).
These distinct patterns of excitation and inhibition make a prediction about the mechanism of inverted size-latency tuning in the OND RGC. While steady excitation is required to depolarize the cell, rather than firing near the onset of excitation, OND RGC firing is initiated by a release from inhibition. The latency of this release from inhibition decreases with increasing spot size because inhibition becomes smaller and more transient while excitation remains sustained and has less suppression. To test whether the synaptic conductances were sufficient to explain inverted size-latency tuning, we created a leaky integrate-and-fire model of the spiking activity of OND RGCs (Figure 3E–I). Converting measured synaptic currents into conductances (Figure 3E) and feeding them into the model, we simulated membrane voltage dynamics in response to spots of varying sizes (Figure 3F). The only parameters that were chosen per cell were the spiking threshold and an increased time constant following a spike. Fixing these based on the spiking response measured for the same cell at a single spot size (200 μm), we then produced simulated data across the range of spot sizes (Figure 3G–I). The model captured the general shape of the OND RGC’s light response (Figure 3G), and inverted size-latency tuning (Figure 3H). Surround suppression (Figure 3I) seemed somewhat stronger than in experimental results. This may be due to an additional current we discovered arising from stimuli beyond the RGC dendrites (see Figures 5,6). This current was not included in the model directly, with its own driving force, but only via its contribution to the measured excitatory (cation) and inhibitory (chloride) currents.
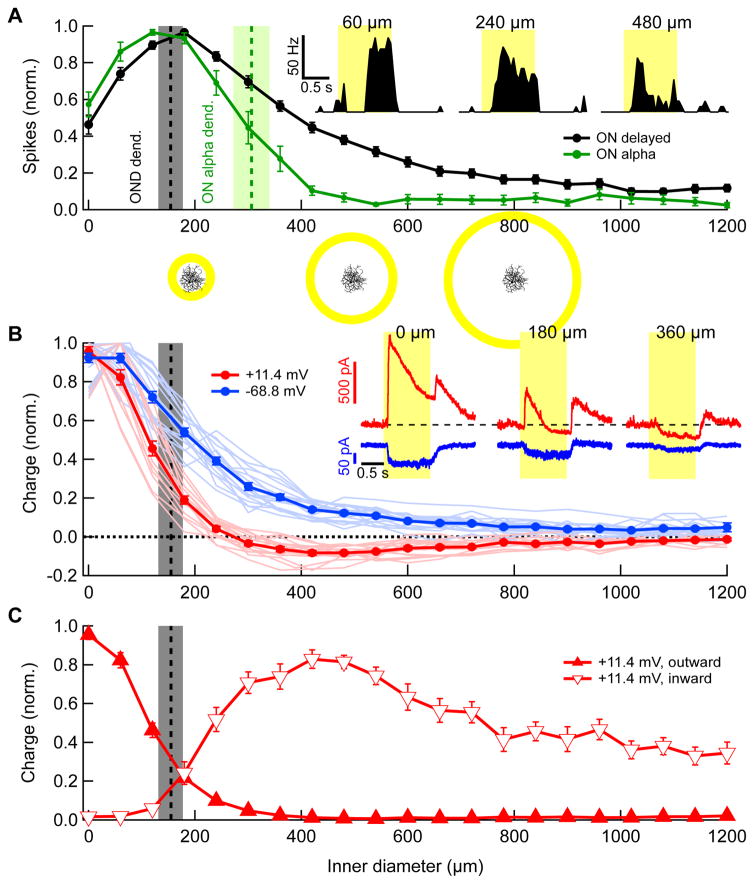
Figure 5. Responses of OND to light annuli with varying diameters.
(A) Spike count during stimulus presentation (normalized by maximum) vs. inner diameter of a bright annulus of constant 60 μm width, shown for OND (black, n = 20 cells) and ON alpha RGCs (green, n = 6). Curves represent mean over cells ± SEM throughout figure. Maximum absolute numbers of spikes were 49 ± 2 and 98 ± 11 in OND and ON alpha RGCs, respectively. Vertical bars in (A, B) are dendritic diameters of OND and ON alpha (mean ± SD, OND cells, n = 18; ON alpha cells, n = 3). Stimulus schematics along with an OND cell are shown to scale below corresponding values on horizontal axis. Inset: PSTH for three different annulus inner diameters, denoted above. (B) Total charge of synaptic currents in OND RGCs vs. inner diameter of annuli, measured at a holding voltage of −68.6 mV (blue) and +11.4 mV (red) and normalized by their maximum. (−68.6 mV, 18 cells, +11.4 mV, 15 cells). Inset: currents vs. time at holding voltages of −68.6 mV and +11.4 mV in an OND cell for three different annulus inner diameters, denoted above (mean traces over 3 trials, colors as in main panel in (B)). (C) Charge caculated and normalized separately, for the outward (open symbols) and inward (closed symbols) components of the currents, at a holding voltage of +11.4 mV.
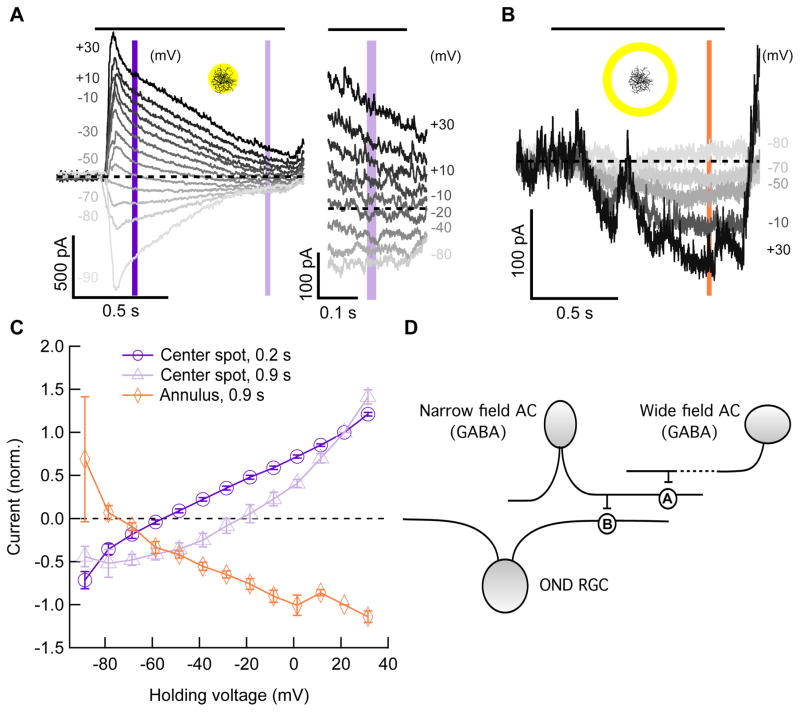
Figure 6. Current-voltage relationships in response to a spot and annulus.
(A) Left, currents vs. time at different holding voltages in an OND RGC, in response to a center spot of light (200 μm, 200 R*/rod/s from dark). Right, same currents as on left panel, zoomed in on last 0.2 s of presentation of spot. Curves for some voltages were omitted for clarity. Horizontal black bar above panel, time of stimulus presentation. Dashed line, zero current. Vertical purple bars denote time points used in panel (C). (B) Currents vs. time at different holding voltages in an OND RGC, in response to a light annulus (400 μm inner diameter, 520 μm outer diameter, 200 R*/rod/s from dark; 7 cells). Vertical orange bar, time point used in panel (C). Insets in (A, B), light stimulus schematics. (C) Current vs. holding voltage curves (mean ± SEM, 7 cells) at time points 0.2 s (dark purple) and 0.9 s (light purple) in response to a center spot of light as in (A), as well as a similar curve taken at time point 0.9 s, in response to a light annulus as in (B). (D) Diagram of model for circuitry to explain the extra-dendritic current (B,C) and the elimination of it by blocking GABAA and GABAB receptors (Figure S5). See text. AC = amacrine cell; ‘A’, synapse containing GABAA, receptors; ‘B’, synapse containing GABAB receptors, providing tonic inhibition onto the OND RGC. See also Figure S5.
Pharmacology of synaptic inputs to the OND RGC
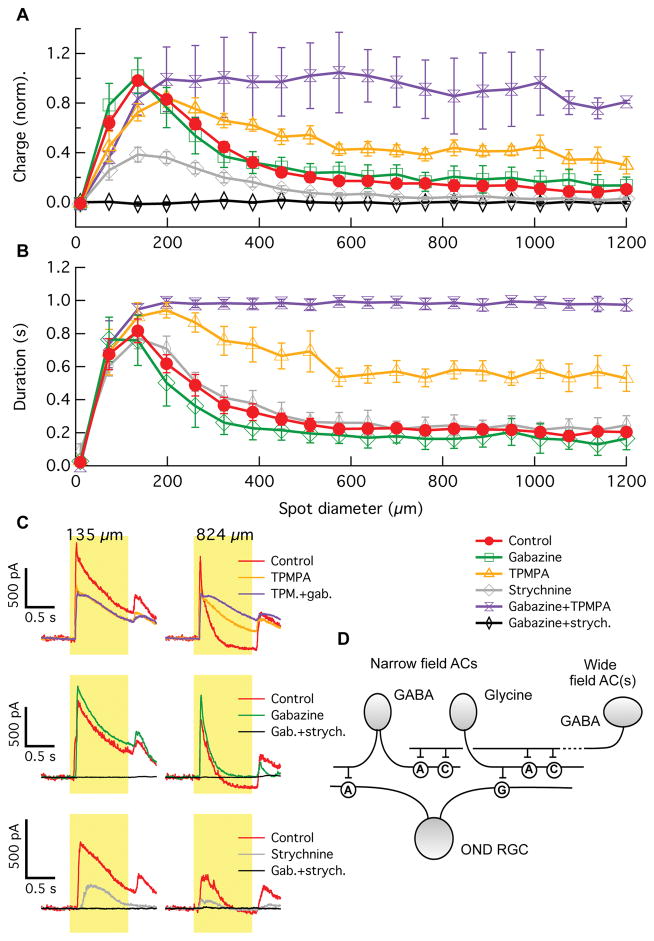
To gain insights into the circuitry upstream of inhibition onto the OND RGC, we repeated our whole-cell receptive field measurements in the presence of different inhibitory receptor blockers. Our pharmacology experiments included gabazine, TPMPA, and/or strychnine to block GABAA, GABAC or glycine receptors, respectively (Figure 4). Two main results came from this set of experiments. First, light induced inhibitory currents are completely dependent on a combination of glycine and GABAA receptors. Blocking both of these receptor types with gabazine + strychnine eliminated inhibitory currents completely (Figure 4A,C). The effects of the two blockers were not additive, however. Gabazine alone did not substantially reduce the total charge of inhibitory currents in OND RGCs, and strychnine alone reduced the currents to about 40% of their control charge. Non-additivity of these blockers suggests an upstream interaction between GABAergic and glycinergic circuits (see model below).
Figure 4. Stimulus size dependence of synaptic inhibitory currents of OND RGCs in the presence of synaptic blockers.
(A) Charge of the inhibitory current in OND RGCs vs. spot diameter, in control condition (red, 8 cells), in the presence of gabazine blocking GABAA receptors (green, 4 cells), TPMPA blocking GABAC receptors (orange, 4 cells), strychnine blocking glycine receptors (grey, 3 cells), TPMPA+gabazine (purple, 3 cells), and strychnine+gabazine (black, 4 cells). (B) Duration of inhibitory current in OND RGCs vs. spot diameter, measured as the time the current was > 25% of its maximum value (see Experimental Procedures). Colors of curves are as in (A). Curves are mean ± SEM over cells in (A, B). (C) Traces of inhibitory current vs. time in OND cells, measured for two different spot sizes denoted above. Colors of traces are as in (A). Each trace is an average over three trials. (D) Diagram of circuit model to explain results in (A–C). AC = amacrine cell; neurotransmitters produced by ACs appear next to somas. ‘A’, ‘C’ and ‘G’ are synapses containing GABAA, GABAc, and glycine receptors, respectively. See also Figure S4.
Second, the strong surround suppression of the inhibitory currents that we found to be critical for inverted size-latency tuning (Figure 3) relies on a combination of GABAA and GABAC receptors. Both the total charge (Figure 4A,C) and the duration (Figure 4B,C) of inhibition was increased in the presence of TPMPA and increased more in the presence of TPMPA + gabazine. Again, the effects of TPMPA and gabazine were non-additive, suggesting an upstream interaction. Our results are consistent with a simplified model of the inhibitory circuit upstream of OND RGCs shown in Figure 4D. In this model, narrow-field glycinergic and GABAergic amacrine cells provide inhibition onto the dendrites of the OND RGC. Wide-field GABAergic amacrine cells in turn inhibit the narrow-field amacrine cells via GABAA and GABAC receptors. While the diagram in Figure 4D is the simplest circuit consistent with our data, other interactions are possible. The amacrine cells shown are not necessarily single types, and they may interact with each other either directly or through the bipolar cells providing excitatory input to them.
Similar experiments measuring the pharmacology of excitatory currents with the same set of inhibitory receptor blockers yielded two main results (Figure S4). First, surround suppression of excitatory currents is predominantly provided by GABAergic inhibition onto GABAC receptors, presumably located on bipolar cell terminals. We also observed an apparent strengthening of the surround of excitatory currents when blocking either GABAA receptors alone or GABAA and glycine receptors. Later experiments suggested that the reduction of current by these blockers was at least in part due to a different source than glutamate from bipolar cells (see Figures 5,6). Second, we found that presynaptic inhibition shapes the kinetics of the excitatory currents, contributing to their slow rise by eliminating an early transient component. This inhibition is provided by GABAA and glycine receptors. In summary, interactions between GABAA, GABAC, and glycine inhibition shape the spatial and temporal properties of the excitatory and inhibitory currents onto OND RGCs, leading to the unusual properties of the currents and, ultimately, to inverted size-latency tuning in the spike responses of the RGC.
The OND RGC can be stimulated by light falling outside its dendrites
Surround suppression of spiking responses was weak for the OND RGC; only ON alpha RGCs had a similarly weak surround among the ON RGCs we tested (Figure 2A). Weak surround suppression could merely be the result of weak inhibition onto both the RGC and its presynaptic bipolar cells for large spots. However, the discrepancy between the small dendritic field of OND RGCs and the observed size of the receptive field center (Fig 2A), motivated us to further test the response of OND RGCs to light stimuli beyond the RGC dendrites.
To test for activation by distant stimuli, we presented annuli of fixed width and varying diameters while measuring the spike responses of both OND RGCs and ON alpha RGCs (Figure 5A). The response curves where compared with the dendritic diameter for each of the cell types (Figure 5A, vertical bars). While ON alpha RGC responses declined sharply for annuli outside the dendritic field, OND RGCs responded to stimuli far outside their dendrites. Spikes elicited by annuli outside the dendritic field were apparent in the OND RGC, but not the ON alpha (Figure 5A). Despite the fact that ON alpha RGCs fire twice as many spikes as OND RGCs at their receptive field maxima, a 480 μm inner diameter annulus elicited 20 ± 2 spikes in OND RGCs (n = 20) and 7 ± 3 spikes in ON alpha RGCs (n = 6). Thus the seemingly similar weak surround suppression observed for both the ON alpha RGC and the OND RGC using spots (Figure 2A) may be due, in part, to different mechanisms, as revealed by the annuli stimulus. ON alpha RGCs have weak suppression but no activation beyond their dendrites, while OND RGCs receive extra-dendritic activation.
Extra-dendritic activation via disinhibition
What synaptic mechanism accounts for extra-dendritic responses in the OND RGC? Many studies have shown excitation in RGCs to be co-extensive with their dendrites [27–29]. This is an intuitive result since the receptive fields of bipolar cells, which provide excitation to RGCs, are typically much smaller (~45 μm) [30,31] than those of RGCs (~250 μm) [12]. Again we turned to voltage-clamp measurements to determine the source of the extra-dendritic activation, clamping the membrane voltage at either 11.4 mV (near the non-specific cation reversal potential) or −68.6 mV (near the chloride reversal potential), in an attempt to isolate inhibitory and excitatory currents respectively (Figure 5B). Consistent with the decreased inhibition for large spots (Figure 3A), annuli beyond the dendritic field of the OND RGC elicited no outward inhibitory currents, but, surprisingly, we measured inward currents at both the chloride and non-specific cation reversal potentials in response to annuli well outside the RGC dendrites (Figure 5B,C). This suggested that perhaps a circuit distinct from those active within the dendritic field of the OND RGC provides activation beyond the dendritic field.
To explore the identity of these currents further, we recorded light responses from OND RGCs for both a spot and an annulus while holding the membrane potential at a range of values (Figure 6). Using a small spot and a large annulus allowed us to measure the current-voltage relationships of the synaptic conductances elicited by light stimuli either within or outside the dendritic field of the RGC. A spot covering mostly the area of the dendrites (200 μm) elicited a response family as seen in Figure 6A. Consistent with the currents recorded at only two different potentials (Figure 5B), the early part of the response (at 200 ms) was dominated by an inhibitory chloride current (reversing at −55.8 ± 2 mV, n = 7, Figure 6C) while the late part of the response included a substantial excitatory component and thus had a reversal potential between the chloride and non-specific cation reversal potentials (−22.7 ± 5 mV, n = 6, Figure 6C).
The light response elicited by annuli (400 μm inner diameter) outside the dendritic field had only a late, sustained component (Figure 6B), and this current had a very different voltage-dependence from those elicited by the spot (Figure 6C, orange). Surprisingly, this current became more negative (inward) with increasing holding voltage, and had an apparent reversal at −72.8 ± 2 mV (n = 7). The fact that the size of this current depended on holding potential excludes gap junctions as its source, as gap-junctional currents are characterized by flat current-voltage relationships. The ‘inverted’ current-voltage relationship could be explained by disinhibition, the removal of a tonic inhibitory conductance by stimuli outside the RGC dendrites. However, the current reversed at a potential too negative for it to be carried by chloride. Therefore we hypothesized that the tonic inhibition removed by extra-dendritic stimuli may at least in part be carried by a potassium conductance, which would account for its negatively shifted reversal potential. This would be the case if potassium channels controlled by metabotropic GABAB receptors mediate this current. While voltage gated potassium channels were blocked in our recordings by cesium ions present in the recording pipette, the inward rectifying potassium channels associated with GABAB receptors are unaffected by internal cesium block [32].
Again we turned to pharmacology experiments to reveal the circuit elements leading to this extra-dendritic disinhibition. While TPMPA (GABAC receptor antagonist) had no effect on the disinhibitory current, gabazine (GABAA antagonist) eliminated it completely (Figure S5). Tetrodotoxin also had no effect on the disinhibitory current (data not shown), suggesting that neither spiking amacrine cells, nor sodium channels in other cells in the retina participate in this circuit. While the extra-dendritic disinhibitory current was eliminated by gabazine, the inhibitory chloride current elicited by stimuli within the dendritic field of the RGC was unchanged in amplitude (Figure S5A). Consistent with the hypothesis that metabotropic activation of a potassium current was involved in the extra-dendritic current, the GABAB agonist, baclofen, eliminated the extra-dendritic current while leaving (chloride-mediated) inhibition within the dendritic field intact (Figure S5).
The diagram in Figure 6D summarizes our conclusions regarding the extra-dendritic current. OND RGCs may receive a tonic inhibitory conductance from a GABAergic amacrine cell, via GABAB receptors. The amacrine cell is in turn inhibited, via GABAA receptors, by a wide-field (non-spiking) GABAergic amacrine cell that is activated by distant light stimulation. In summary, extra-dendritic activation in the OND RGC (Figure 5A), which contributes to its receptive field center being larger than its dendritic field and its weak surround suppression (Figure 2A), may rely on release from tonic inhibition via a serial inhibitory circuit.
Discussion
An RGC type with unusual receptive field characteristics
The long history of research on the light responses of RGCs spanning over seven decades and numerous species has uncovered many different types of receptive fields. To our knowledge, the OND RGC represents a receptive field structure that has not been reported in any of the previous literature. Two of its key functional attributes, inverted size-latency tuning and extra-dendritic activation, are unique among reported RGC receptive fields and among those we have measured. The unusual firing pattern of the OND RGC – in particular its long delay (Figure 1) – provides a reliable measure for functional targeting in wild-type mice, which can easily be further verified by testing for inverted size-latency tuning (Figure 2B). This targeting scheme should enable the OND RGC to be studied systematically across multiple labs.
New functional roles for inhibition in retinal circuits
Two aspects of the role of inhibition in the non-canonical circuitry of the OND RGC are of general importance in sensory and systems neuroscience. The timing of the currents causes the RGC to spike upon release from inhibition, controlling the cell’s unusual response latency pattern (Figures 2,3). A combination of distinct inhibitory pathways gives rise to these special characteristics in the synaptic inputs (Figure 4, Figure S4). The ON direction-selective RGC in rabbit has a similar release from inhibition mechanism to control speed dependence [33]. Glycinergic inhibition has also been shown to play a key role in delaying the onset of spiking in rabbit local edge detector RGCs. [34].
Second, we show a role for disinhibition in activating the RGC with stimuli far beyond its dendritic field (Figures 5,6). Disinhibition of a tonic glycinergic current onto the RGC is known to play an important role in the responses of the two OFF alpha RGC types [5,35]. Disinhibition in the OND RGC does not reverse at the reversal potential for chloride (Figure 6C) and instead is likely carried by a tonic GABAB-receptor-mediated potassium conductance (Figure 5A). Unlike the disinhibitory currents measured in OFF alpha cells, the current we measured was selectively activated for stimuli beyond the dendrites of the RGC (Figures 5B, 6B,C).
Our pharmacology results (Figures 4, S4, S5) constrain models of the circuits upstream of the OND RGC that shape the excitatory, inhibitory, and disinhibitory currents we measured in the RGC. Generally, these results support previous studies of the different receptors mediating direct inhibition onto RGCs, serial inhibition between amacrine cells, and surround inhibition onto bipolar cell terminals [34,36,37]. In particular, our results support the role of GABAC receptors in lateral inhibition in retinal interneurons (onto both amacrine and bipolar cells), but not directly onto RGCs. GABAC receptors limit the spatial extent of inhibitory currents into the OND RGC and control their decay in time. We found multiple roles for GABAA and glycine receptors in the OND RGC circuit, consistent with previous reports of their function in other retinal circuits [38]. Our finding that GABAB receptors provide tonic inhibition that is suppressed by extra-dendritic activation in OND RGCs represents a new role for GABAB receptors in a retinal circuit.
A large dynamic range latency code
RGC response latency can depend on a variety of features of the visual stimulus, and spike latency codes have been implicated in encoding contrast and spatial structure [26,39,40]. Latency shifts at steady luminance due to parameters like contrast, that have been linked to temporal coding, are on the order of several tens of milliseconds [26,40]. Remarkably, the OND RGC response latency shifts by more than 300 ms with spot size (Figure 3B).
While the kinetics of many parts of retinal circuits, including phototransduction, accelerate with increasing luminance, the long latency of response of OND RGCs and the stimulus size latency tuning are robust to a change in luminance (Figure S3). The OND RGC may thus encode spatial information temporally. The role of the latency shift in the encoding strategy of the OND RGC remains unclear, and future studies with a wide range of stimuli may uncover how multiple stimulus features interact to control response latency.
A possible role for the OND RGC in encoding global image focus
What could be the function of the OND RGC? A canonical center-surround organization decorrelates responses of neighboring RGCs and enables them to report properties of the visual scene locally (i.e. occurring only in the receptive field center) [41]. An exception is the M1 ipRGC, which exhibits no surround suppression [14] and encodes global luminance [42], rather than local information. RGCs usually also respond quickly, with latencies comparable to the minimal time delay imposed by phototransduction.
By contrast, OND RGCs respond to rather large stimuli and respond slowly. We suggest that spatial localization and speed are traded off in the OND RGC for achieving high sensitivity, via integration over time and space, in detecting a property of the visual field that is not highly localized and varies slowly. We further speculate that this property may be the degree of image focus detected by the retina.
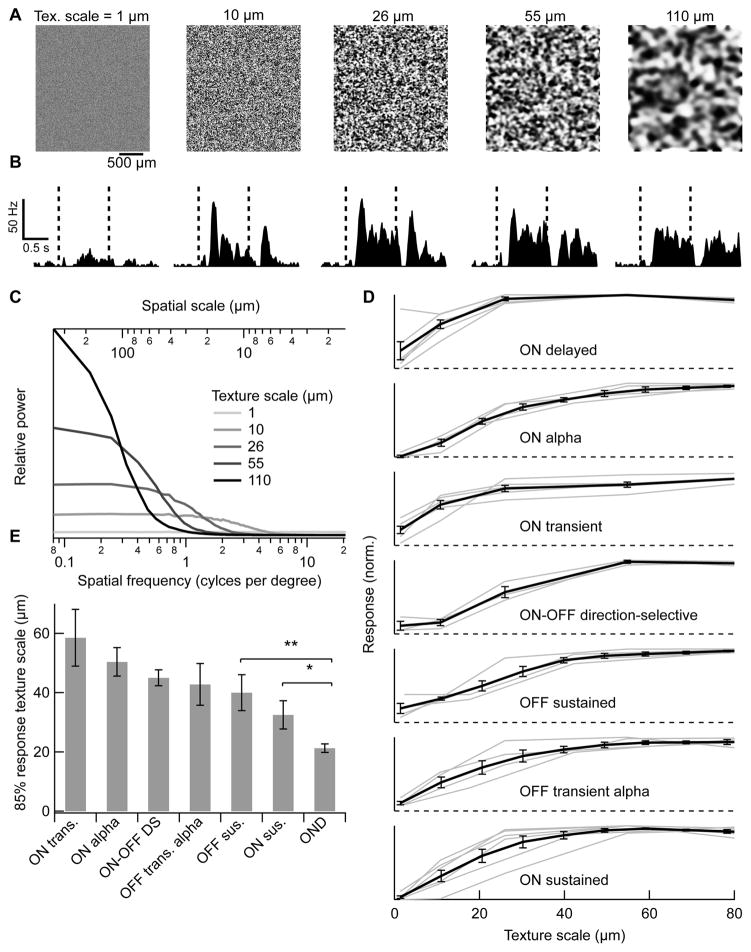
Defocusing an image changes its spatial frequency content, attenuating high frequencies [43]. We measured RGC responses to full-field spatial patterns with equal distributions of positive and negative contrasts, but varying degrees of blur and hence varying spatial frequency content (Figure 7A,C, [31], see Experimental Procedures). The mean intensity in each texture was equal to that of the background presented before and after (1000 R*/rod/s). OND RGCs fired at either the onset or offset of texture patterns, or at both times (Figure 7B). Note that the presence of texture onset and offset responses does not imply a light OFF response since there was no net change in overall light intensity between texture and background. Because we were interested in the dependence of RGC responses on spatial blur and not on local variations in contrast across textures, we quantified the response by summing spikes at onset and offset and averaging across 5 different texture patterns at each spatial scale. Among RGCs we measured, only some cell types, including OND RGCs, responded well to the texture stimulus (> 10 Hz, Fig 7D), while the rest responded poorly or not at all (38 of 70 cells). Moreover, OND RGCs were sensitive to the highest spatial frequencies; the texture scale at which the OND RGC reached 85% of its maximal response (21.3 ± 1.4 μm, n = 6) was the smallest among RGC types we measured (Figure 7E). Thus the OND RGC is exquisitely sensitive to local contrast in a full-field image. The limiting frequency is consistent with both the resolution of single bipolar cells [30,31] and the spatial frequency limit of mouse vision [44].
Figure 7. Response of OND to random textures with varying degrees of blur.
(A) Full field textures, made with the same random seed and varying Gaussian blur. Denoted above each texture is the cutoff spatial scale (‘texture scale’, see text and Experimental Procedures). (B) PSTHs measured in an OND RGC in response to each corresponding spatial scale as in (A). Each PSTH was taken over 15–20 trials in response to different images having the same spatial scale. A texture was presented during the time between red dashed lines, and a uniform background having the same mean luminance (1000 R*/rod/s) was presented before and afterwards. (C) Spatial frequency content in textures. Relative power vs. spatial frequency (bottom horizontal axis) or spatial scale = (spatial frequency)−1 (top axis). Curves are empirically measured averages over 500 texture patterns at each blur size. (D) Spike count during both stimulus onset and offset vs. texture scale, normalized by its maximum. Curves represent the average ± SEM over cells within each group. Numbers of cells: OND, n = 6, ON alpha, n = 4, ON-OFF direction-selective, n = 3, ON transient, n = 5, OFF sustained, n = 4, OFF transient alpha, n = 4, ON sustained, n = 6. (E) Smallest texture scale for which the response reached 85% of its maximum for individual cells in (D) (average ± SEM within each group of cells). This value was the smallest for OND RGCs: relative to ON sustained RGCs, p < 0.05, and to all other groups, p < 0.01, one-tailed unpaired t-test.
OND RGC’s sensitivity to fine spatial scales is consistent with a model of nonlinear subunits for the receptive field of an ON RGC, with the onset to offset response ratio depending on the alignment of the bright and dark patches of the texture with receptive field subunits [31]. Mechanistic connections between this texture sensitivity and the other receptive field properties described in Results remains to be explored in future experiments and modeling. The firing of OND RGCs is not selective solely for fine textures, and this one RGC type alone probably does not carry the full representation of image focus. Nonetheless, we think that it is a good candidate for a role within the focus detection circuit, given the above considerations.
Although its cellular substrate is yet to be discovered [45], an image focus signal exists within the mouse retina. During development, this signal controls emmetropization, the process matching the dimensions of the eye with its refractive power, in species including mouse and human [46,47]. Normal emmetropization depends on the ON pathway of the retina [48]; disruption of this process results in disorders of refractive index, like myopia. A signal of image focus may also have use in the adult animal, in visual accommodation, which brings the retinal image into focus. Although the well known lens accommodation (change in the lens shape) may be absent in the mouse [47], the pupillary near reflex, adjusting the depth-of-focus to the target distance [49–51] may well be present [52].
Future work identifying genetic markers of the OND RGC and tracing its projections in the brain will be needed in order to establish its role in reporting image focus or in other functions.
EXPERIMENTAL PROCEDURES
Electrophysiology experiments were performed on retinas from wild-type mice presented with visual stimuli as described previously [16,22]. Methodological details, including data and image analysis and modeling can be found in Supplementary Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by a Research to Prevent Blindness Career Development Award to G.W.S., by the National Institutes of Health / Director’s Fund and National Eye Institute grant DEY026770A to G.W.S., by a Karl Kirchgessner Foundation Vision Research Award to G.W.S., and by a Knights Templar Eye Foundation career starter grant to A.M. We deeply thank Susan L. Wohlgenant for technical support, Fred M. Rieke, Jon Cafaro, William N. Grimes, Michael B. Manookin, and Petri Ala-Laurila for reviewing earlier versions of this manuscript and providing critical feedback, and the Schwartz laboratory members for helpful discussions. The authors declare no conflict of interest.
Footnotes
AUTHOR CONTRIBUTIONS
Author contributions: A.M. and G.W.S. designed research, performed research, analyzed data and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 3.Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012;31:407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukasiewicz DP. Synaptic mechanisms that shape visual signaling at the inner retina. Prog Brain Res. 2004;147:205–218. doi: 10.1016/S0079-6123(04)47016-2. [DOI] [PubMed] [Google Scholar]
- 5.Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- 6.Hoggarth A, McLaughlin AJ, Ronellenfitch K, Trenholm S, Vasandani R, Sethuramanujam S, Schwab D, Briggman KL, Awatramani GB. Specific wiring of distinct amacrine cells in the directionally selective retinal circuit permits independent coding of direction and size. Neuron. 2015;86:276–291. doi: 10.1016/j.neuron.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc Natl Acad Sci. 2012;109:E2391–E2398. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ölveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- 9.Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM, Huberman AD. Genetic Dissection of Retinal Inputs to Brainstem Nuclei Controlling Image Stabilization. J Neurosci. 2013;33:17797–17813. doi: 10.1523/JNEUROSCI.2778-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollisch T, Meister M. Eye Smarter than Scientists Believed: Neural Computations in Circuits of the Retina. Neuron. 2010;65:150–164. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanes JR, Masland RH. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu Rev Neurosci. 2015;38:221–246. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- 12.Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016;529:345–50. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim T, Soto F, Kerschensteiner D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. Elife. 2015;4:6807. doi: 10.7554/eLife.08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Stafford BK, Godin AL, King WM, Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol. 2014;592:1619–1636. doi: 10.1113/jphysiol.2013.262782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nath A, Schwartz GW. Cardinal Orientation Selectivity Is Represented by Two Distinct Ganglion Cell Types in Mouse Retina. J Neurosci. 2016;36:3208–3221. doi: 10.1523/JNEUROSCI.4554-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sümbül U, Song S, McCulloch K, Becker M, Lin B, Sanes JR, Masland RH, Seung HS. A genetic and computational approach to structurally classify neuronal types. Nat Commun. 2014;5:3512. doi: 10.1038/ncomms4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Völgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivyer B, Vaney DI. Dendritic morphology and tracer-coupling pattern of physiologically identified transient uniformity detector ganglion cells in rabbit retina. Vis Neurosci. 2010;27:159–170. doi: 10.1017/S0952523810000234. [DOI] [PubMed] [Google Scholar]
- 20.Tien NW, Pearson JT, Heller CR, Demas J, Kerschensteiner D. Genetically Identified Suppressed-by-Contrast Retinal Ganglion Cells Reliably Signal Self-Generated Visual Stimuli. J Neurosci. 2015;35:10815–10820. doi: 10.1523/JNEUROSCI.1521-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Zhang Y, Chen M, Zhou ZJ. Segregated Glycine-Glutamate Co-transmission from vGluT3 Amacrine Cells to Contrast-Suppressed and Contrast-Enhanced Retinal Circuits. Neuron. 2016;90:27–34. doi: 10.1016/j.neuron.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby J, Zhu Y, DeVries SH, Schwartz GW. An Amacrine Cell Circuit for Signaling Steady Illumination in the Retina. Cell Rep. 2015;13:2663–2670. doi: 10.1016/j.celrep.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–174. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- 24.Masri RA, Percival KA, Koizumi A, Martin PR, Grunert U. Connectivity between the OFF bipolar type DB3a and six types of ganglion cell in the marmoset retina. J Comp Neurol. 2016;524:1839–1858. doi: 10.1002/cne.23925. [DOI] [PubMed] [Google Scholar]
- 25.Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K, Roska B. Ambient Illumination Toggles a Neuronal Circuit Switch in the Retina and Visual Perception at Cone Threshold. Neuron. 2013;78:325–338. doi: 10.1016/j.neuron.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Gollisch T, Meister M. Rapid Neural Coding in the Retina with Relative Spike Latencies. Science (80-) 2008;319:1108–1111. doi: 10.1126/science.1149639. [DOI] [PubMed] [Google Scholar]
- 27.Koch C, Poggio T, Torre V. Retinal ganglion cells: a functional interpretation of dendritic morphology. Philos Trans R Soc B Biol Sci. 1982;298:227–263. doi: 10.1098/rstb.1982.0084. [DOI] [PubMed] [Google Scholar]
- 28.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 29.Freed MA, Smith RG, Sterling P. Computational model of the on-alpha ganglion cell receptive field based on bipolar cell circuitry. Proc Natl Acad Sci. 1992;89:236–240. doi: 10.1073/pnas.89.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524:879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, Wong RO, Rieke F. The spatial structure of a nonlinear receptive field. Nat Neurosci. 2012;15:1572–1580. doi: 10.1038/nn.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hille B. Ion channels of excitable membranes. 3 2001. [Google Scholar]
- 33.Sivyer B, Van Wyk M, Vaney DI, Taylor WR. Synaptic inputs and timing underlying the velocity tuning of direction-selective ganglion cells in rabbit retina. J Physiol. 2010;588:3243–3253. doi: 10.1113/jphysiol.2010.192716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkataramani S, Van Wyk M, Buldyrev I, Sivyer B, Vaney DI, Taylor WR. Distinct Roles for Inhibition in Spatial and Temporal Tuning of Local Edge Detectors in the Rabbit Retina. PLoS One. 2014;9:e88560. doi: 10.1371/journal.pone.0088560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition Combines with Excitation to Extend the Operating Range of the OFF Visual Pathway in Daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol. 2010;103:25–37. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci. 2011;28:95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C, McCall MA. Receptor targets of amacrine cells. Vis Neurosci. 2012;29:11–29. doi: 10.1017/S0952523812000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gütig R, Gollisch T, Sompolinsky H, Meister M. Computing Complex Visual Features with Retinal Spike Times. PLoS One. 2013;8:e53063. doi: 10.1371/journal.pone.0053063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bölinger D, Gollisch T. Closed-Loop Measurements of Iso-Response Stimuli Reveal Dynamic Nonlinear Stimulus Integration in the Retina. Neuron. 2012;73:333–346. doi: 10.1016/j.neuron.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 41.Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berson DM, Dunn Fa, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 43.Burge J, Geisler WS. Optimal defocus estimation in individual natural images. Proc Natl Acad Sci U S A. 2011;108:16849–16854. doi: 10.1073/pnas.1108491108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umino Y, Solessio E, Barlow RB. Speed, Spatial, and Temporal Tuning of Rod and Cone Vision in Mouse. J Neurosci. 2008;28:189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardue MT, Stone RA, Iuvone PM. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013;114:96–105. doi: 10.1016/j.exer.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daw NW. Visual Development. Boston, MA: Springer US; 2014. Visually Induced Myopia and Emmetropization BT - Visual Development; pp. 217–229. [Google Scholar]
- 47.Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015;137:79–83. doi: 10.1016/j.exer.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufman PL, Alm A, Adler FH. Adler’s Physiology of the Eye: Clinical Application. Mosby; 2003. [Google Scholar]
- 50.Beatty J, Lucero-Wagoner B. Handb Psychophysiol. 2. 2000. The pupillary system; pp. 142–162. [Google Scholar]
- 51.Bando T, Takagi M, Toda H, Yoshizawa T. Functional roles of the lateral suprasylvian cortex in ocular near response in the cat. Neurosci Res. 1992;15:162–178. doi: 10.1016/0168-0102(92)90002-t. [DOI] [PubMed] [Google Scholar]
- 52.Pinto LH, Enroth-Cugell C. Tests of the mouse visual system. Mamm Genome. 2000;11:531–536. doi: 10.1007/s003350010102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.