Abstract
Background
Glioblastoma multiforme (GBM) is associated with a poor prognosis and patients rely heavily on family caregivers for physical and emotional support. The capability and mental health of family caregivers may influence their ability to provide care and affect patient outcomes. We aimed to investigate whether caregivers’ anxiety, depressive symptoms, burden and mastery influenced survival in a sample of patients newly diagnosed with GBM.
Methods
Baseline data from caregiver-patient dyads participating in a NIH funded longitudinal study were used. Cox regression analyses were performed to determine whether caregiver anxiety (Profile of Mood States-Anxiety), depressive symptoms (Center for Epidemiologic Studies-Depression), burden (Caregiver Reaction Assessment), and feelings of mastery (Mastery Scale) predicted GBM patient survival time after controlling for known covariates (patient age, performance status, type of surgery, and postsurgical treatment).
Results
In total, 88 caregiver-patient dyads were included. Median overall survival for the sample was 14.5 months (range 0–88 months). After controlling for covariates, caregiver mastery was predictive of patient survival. With each unit increase in mastery, there was a 16.1% risk reduction of patient death (95% confidence interval: 0.771–0.913, P<0.001).
Conclusions
Our results are among the first to explore the impact of family caregiving for GBM patients’ outcomes. If these results are supported in other studies, providing neuro-oncology caregivers with more structured support and guidance in clinical practice have the potential to improve caregivers’ feelings of mastery, influencing patients’ wellbeing for the better.
Keywords: Glioblastoma, brain tumor, caregivers, mental health, mastery, survival
Condensed abstract
We investigated whether caregivers’ anxiety, depressive symptoms, burden and mastery influenced survival in a sample of patients newly diagnosed with glioblastoma. After controlling for known covariates, caregiver mastery was predictive of patient survival (hazard ratio 0.839; 95% CI: 0.771–0.913).
Introduction
Glioblastoma Multiforme (GBM; World Health Organization (WHO) grade IV) is the most common primary malignant brain tumor in adults.1 This rapidly progressive form of cancer is typically associated with a poor prognosis; median survival is 15 months.2 Standard treatment, surgery followed by temozolomide chemotherapy with concomitant radiotherapy, does not have “curative” intent but rather aims at preventing recurrence and extending survival whilst preserving patients’ quality of life (QOL).3;4 Research has shown that younger age, better performance status, unifocal tumor location, degree of surgical resection, any resection as opposed to biopsy only, and postsurgical treatment (radiotherapy/chemotherapy) are among the most important prognostic factors of survival time after diagnosis.5–7 Moreover, O6-methylguanine-DNA-methyltransferase (MGMT) promotor methylation, which is thought to predict response to chemotherapy,8 is predictive of longer survival following diagnosis of a GBM.9;10
The disease-specific symptom burden that accompanies GBM often includes highly debilitating sequelae such as paresis, sensory loss, visual-perceptual deficits, cognitive deficits, and seizures.(11) Moreover, changes in personality and behavior,12;13 fatigue14 and depression15 occur frequently. GBM patients typically come to rely on their family caregivers (e.g. spouses, adult children, or close friends) for physical and emotional support. Consequently, many family caregivers experience considerable burden and emotional distress.16–18 Caregivers’ level of mastery, which can be defined as the feeling of being in control of the care situation, can influence the amount of distress perceived by family caregivers.19;20 Indeed, mastery has been shown to have a significant effect on the amount of distress reported by the family caregiver as a result of providing care in neuro-oncology,21 and in other caregiving populations.20;22
Caregivers’ emotional health has long been hypothesized to influence the quality of care delivered to patients with chronic illness in the home and patient outcomes, yet little data exists to support this hypothesis. One study suggested that unmet needs for symptom management, financial support or community support in caregivers who provided end-of-life care, appeared to influence the quality of care delivered to the patient (e.g. professional healthcare, dignity-conserving care, individualized care, and family relationships).23 In family caregivers taking care of a person with dementia, decreased burden has been associated with delayed institutionalization of the patient,24 and less psychological distress in caregivers appears to predicts better survival in dementia patients.25 In community-dwelling elderly care recipients, similar effects of caregiver burden on patient survival have been reported.26
Cancer caregiving differs from dementia caregiving as the disease presents with a more abrupt onset, and has a more variable disease course.27 Therefore, caregivers’ emotional health also likely follow a different trajectory, thus affecting patient outcomes differently. Among cancer patients receiving chemotherapy, caregiver distress appears to be negatively associated with patients’ problems in self-care and activities of daily life.28 Furthermore, higher levels of depressive symptoms and burden in family caregivers have been associated with a decline in functional status of breast cancer patients.29 Inversely, there are indications that lower levels of caregiver distress are associated with better physical health of patients with advanced cancer.30 However, it remains unclear whether caregiver emotional health may influence cancer patient survival. This is especially relevant in oncological populations with high caregiver burden and poor disease trajectories, such as GBM.
The neurological and cognitive symptoms that GBM patients experience represent unique challenges to their family caregivers.31 To our knowledge, there have been no studies focusing on how emotional health of family caregivers influences GBM patient outcomes. The purpose of this study was to examine the impact of several key indicators of caregiver distress (i.e., anxiety, depressive symptoms, caregiver burden and mastery) at the time of GBM diagnosis on patient survival after controlling for known covariates (age, extent of resection, postsurgical treatment, and functional status). Obtaining knowledge on whether caregivers’ emotional health and distress influences patient survival could provide leads to intervene and improve both caregiver and patient wellbeing.
Materials and methods
Participants
Caregiver/patient dyads were recruited to participate in a large, longitudinal study of biobehavioral interactions in neuro-oncology caregivers (NCI RO1-CA118711). Recruitment procedures are described in more detail elsewhere.32 Dyads were recruited within three months of the patient’s initial diagnosis from a NCI designated cancer center. Caregivers’ eligibility criteria were: 1) ≥ 21 years of age; 2) able to read and speak English; 3) identified by patients as the primary, non-professional, non-paid person who provided the majority of emotional, financial and/or physical support; and 4) not currently a primary caregiver for anyone else other than children below 21 years of age. Patient eligibility criteria were: 1) ≥ 21 years old, 2) diagnosed (verified via pathology) with a primary malignant brain tumor within three months, and 3) able to read and speak English. Both members of the dyad had to consent to participate for the other to be included. A total of 228 dyads were approached; 164 agreed to participate. The most common reasons for non-consent (N=64) were “lack of interest” (52%) and “feeling overwhelmed” (33%). For the present analyses, only patients with WHO grade IV GBM and their caregivers were included (54% of the sample, N=88). Participants were recruited between October 2005 and March 2012.
Procedure and outcome measures
Assessments took place within three months of the patient’s diagnosis (baseline), and at 4-, 8-, and 12-months. The baseline caregiver assessment was used as a potential predictor of patients’ time to death. This was done because shortly after diagnosis, caregivers’ emotional health is not as heavily influenced by the individual patient’s disease trajectory – which can vary greatly from patient to patient despite the uniform GBM diagnosis. Clinical data were obtained from the patient’s medical records and verified with the neuroradiologist or neuro-oncologist if questions arose. Caregiver data were obtained by a trained research assistant who conducted structured interviews in person or via telephone to ensure completeness. Care recipient functional status, along with the other covariates, was obtained via in person interviews and medical record review. The institutional review board approved the study protocol and all participants provided written, informed consent.
Outcome measures
Caregivers’ depressive symptoms were assessed with the shortened 10-item version of the Center for Epidemiologic Studies-Depression Scale (α=0.88; N=88).33;34 The participant’s experience of depressive symptoms is rated on a 4-point scale. Scores range between 0 and 30, with higher scores indicating higher levels of depression. Based on trajectory modelling analyses in the same longitudinal study, the cut-off score for being at risk for major clinical depression was set at ≥ 8.16
Anxiety was measured using the shortened version of the Profile of Mood States-Anxiety questionnaire (α=0.92; N=86).35 The caregiver’s experience of feeling ‘on edge’, ‘nervous’, and ‘tense’ are evaluated on a 5-point scale, with higher scores indicating more anxiety.
Three constructs of caregiver burden were measured by the Caregiver Reaction Assessment36: the impact of providing care on caregivers’ self-esteem (α=0.84; N=31), on feelings of abandonment (α=0.82; N=36), and on disruptions in caregivers’ schedules (α=0.76; N=82). A higher score on the self-esteem scale indicates better self-esteem, whereas higher scores on the two other scales indicate greater caregiver burden. The original study protocol did not include the items making up the self-esteem and abandonment scales – these were added after participant recruitment had started because it was thought these could add valuable information on caregiver burden. As a result there were increased missing values.
Caregivers’ feelings of mastery were assessed with the Mastery Scale (N=83).37 On a scale ranging from 1 (strongly disagree) to 4 (strongly agree), caregivers’ perception of control over the care situation was evaluated. Eight items were used to calculate the total mastery score (α=0.62). Higher scores indicate higher levels of perceived control.
Patients’ date of diagnosis was defined as the date of the surgery (N=88). Date of death was collected by medical record review and/or by checking obituaries through October 2015 (N=88). Five patients first received surgery at a different hospital, hence the exact date of diagnosis was missing. Here, the date of study entry (<3 months after diagnosis) was used to calculate survival time.
Patient’s performance status was measured with the Karnofsky Performance Status (KPS) scale38 as detailed in the clinical notes (N=85). If available, data on other factors known to influence survival such as MGMT methylation status were obtained through medical record review (N=36). These data were not collected routinely in the clinic during the first years of the study (2005–2012), yielding many missing values.
Patients’ symptom severity was assessed using 28-items of the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT; N=66).39 Symptom severity is scored on a scale from 0 (not present) to 10 (as bad as you can imagine). An average score was calculated per patient to represent overall symptom severity (α=0.89). Missing values were also due to the questions being added to the protocol after participant recruitment had started, as it was thought this would provide valuable information on symptom severity.
Statistical analyses
All statistical analyses were performed with SPSS version 22.0. Descriptive statistics were used to report sociodemographic and clinical characteristics. To compare caregivers with and without assessment of caregiver burden (self-esteem and abandonment scales) in terms of age, gender, educational level (years of education), and relationship with the patient (spouse or other), T-tests and Chi Square tests were performed. Multiple imputation techniques (5 rounds) were used if less than 10% of data for a given predictor or covariate were missing and missingness was determined to be at random (anxiety; caregiver burden due to schedule disruptions; caregiver mastery; KPS). Univariate Cox regression analyses were performed to examine the contribution of caregivers’ depressive symptoms, anxiety, burden (three subscales), and mastery on the survival time of GBM patients. Significant associations at P<0.10 between caregiver measures and patient survival were used to determine inclusion in a multivariable Cox regression analysis. Here, other variables known to affect survival were included in the model: patients’ age, KPS,38 postsurgical treatment (surgery only versus postsurgical chemotherapy versus postsurgical chemo- and radiotherapy), and extent of resection (biopsy only versus resection). Symptom severity and MGMT status were not added as covariates due to the large percentage of missing values; data on MGMT were not routinely collected clinically during the first years of the study. Instead, T-tests and Chi square tests were performed to check whether symptom severity and MGMT status were related to patient’s survival at 12 months (yes/no). For ease of interpretability, significant predictors of patient survival were divided into tertiles and multivariable Cox regression analyses including the aforementioned covariates were performed again. To illustrate possible effects of data imputation, survival plots were generated for significant predictors of patient survival based on both the non-imputed data and the pooled imputed data. To explore if demographic caregiver variables (age, gender, educational level (years of education), relationship with patient) (spouse or other) were associated with the level of mastery, ANOVAs and Chi square tests were performed.
Results
Participant characteristics
In total, 88 caregiver-patient dyads were included in the analyses (see Table 1). The majority of patients were men (64%) and the majority of caregivers were women (74%). Almost 74% of dyads were in a spousal relationship. Consistent with standard treatment protocol,(2) nearly 85% of patients received both postoperative chemotherapy and radiotherapy. Two cases were censored: one person was still alive at the time of analyses (88 months since diagnosis), and one other did not have a recorded date of death. Median overall survival for the sample was 14.5 months (range 0–88 months; interquartile range 14.75). Approximately half (46%) of the patients were still alive 12 months after the initial diagnosis. Table 2 displays disease-specific symptoms as reported by patients from baseline to 12 months. Although data were incomplete, there was no relationship between patients’ baseline symptom severity (N=66) or MGMT methylation status (N=36) and survival at 12 months (all P>0.05). Caregivers with and without data on caregiver burden (self-esteem and abandonment) were comparable in terms of age, gender, educational level, and relationship with the patient (all P>0.05).
Table 1.
Participant characteristics.
| Participants (N=88)* | ||
|---|---|---|
| Age in years (caregiver) M (SD), range (N=88) | 54.3 (10.8), 21–77 | |
| Sex (caregiver) N(%) (N=88) | Male | N=23 (26.1%) |
| Female | N=65 (73.9%) | |
| Education (caregiver) N(%) (N=87) | High school graduate | N=61 (69.3%) |
| Relationship with the patient N(%) (N=88) | Spouse or significant other | N=65 (73.9%) |
| Parent | N=3 (3.4%) | |
| Daughter or son | N=14 (15.9%) | |
| Sibling | N=2 (2.3%) | |
| Other relative | N=1 (1.1%) | |
| Friend | N=3 (3.4%) | |
| Depressive symptoms (caregiver) M (SD), range (N=88) | 10.2 (7.0), 0–24 | |
| Anxiety (caregiver) M (SD), range (N=86) | 9.1 (2.9), 3–15 | |
| Burden (caregiver) M (SD), range | Abandonment (N=36) | 27.3 (3.2), 18–33 |
| Self-esteem (N=31) | 8.9 (3.8), 5–21 | |
| Schedule disruptions (N=82) | 15.8 (4.6), 6–24 | |
| Mastery (caregiver) M (SD), range (N=83) | 23.6 (2.9), 15–31 | |
| Age in years (patient) M (SD), range (N=88) |
60.6 (9.5), 37–86 | |
| Sex (patient) N(%) | Male | N=56 (63.6%) |
| Female | N=32 (36.4%) | |
| Karnofsky Performance Status Median, range (N=85) | 80 (50–100) | |
| Average number of symptoms (MDASI-BT) at baseline M (SD), range (N=66) |
1.9 (1.8), 0–8 | |
| Tumor location N(%) (N=85) | Frontal | N=12 (13.6%) |
| Temporal | N=26 (29.5%) | |
| Parietal | N=4 (4.5%) | |
| Occipital | N=5 (5.7%) | |
| Middle | N=7 (7.9%) | |
| Mixed | N=30 (34.1%) | |
| Posterior | N=1 (1.1%) | |
| EGFRvIII status (N=72) | Positive | N=49 (55.7%) |
| Negative | N=23 (26.1%) | |
| MGMT methylation status (N=36) | Methylated | N=20 (22.7%) |
| Not methylated | N=15 (28.4%) | |
| Inconclusive | N=1 (1.1%) | |
| Number of lesions N(%) (N=85) | Single lesion | N=70 (79.5%) |
| Multiple lesions | N=15 (17.0%) | |
| Neurosurgical intervention N(%) (N=88) | Biopsy | N=27 (30.7%) |
| Resection | N=58 (65.9%) | |
| Craniotomy, unspecified | N=3 (3.4%) | |
| Corticosteroids at discharge N(%) (N=86) | Yes | N=67 (76.1%) |
| No | N=19 (21.6%) | |
| Number of medications at discharge M (SD), range (N=86) | 6.2 (3.4), 1–16 | |
| Postoperative treatment (N=81) | Surgery only | N=2 (2.2%) |
| Surgery and radiotherapy | N=4 (4.5%) | |
| Surgery, radiotherapy and chemotherapy |
N=75 (84.3%) | |
| Deceased within 12 months (N=88) | Yes | N=41 (46.1%) |
| No | N=47 (52.8%) | |
All percentages displayed relative to total sample (N=88).
Table 2.
Patient’s self-reported disease-specific symptoms over time.
| Disease-specific symptoms (MDASI) M(sd), range |
Baseline N=66 | 4 months N=48 | 8 months N=37 | 12 months N=29 |
|---|---|---|---|---|
| Pain | 1.52 (2.85), 0–10 | 1.38 (2.38), 0–8 | 1.57 (2.90), 0–10 | 1.38 (2.32), 0–8 |
| Fatigue | 3.35 (3.15), 0–10 | 3.25 (3.11), 0–10 | 3.14 (2.77), 0–9 | 3.83 (3.24), 0–9 |
| Nausea | 0.74 (1.69), 0–8 | 0.73 (2.12), 0–10 | 0.62 (1.69), 0–8 | 0.71 (1.96), 0–8* |
| Disturbed sleep | 3.02 (3.32), 0–10 | 1.23 (2.28), 0–8 | 1.73 (2.84), 0–9 | 1.93 (2.67), 0–8 |
| Distress | 2.41 (3.21), 0–10 | 1.96 (2.58), 0–10 | 2.11 (2.87), 0–10 | 2.86 (3.38), 0–10 |
| Shortness of breath | 0.64 (1.89), 0–10 | 0.62 (1.41), 0–5 | 0.51 (1.39), 0–7 | 0.64 (1.83), 0–8* |
| Remembering | 2.62 (2.83), 0–10 | 2.56 (2.69), 0–10 | 2.24 (2.55), 0–8 | 3.03 (2.91), 0–9 |
| Lack of appetite | 1.28 (2.41), 0–9* | 2.17 (3.32), 0–10 | 0.70 (1.70), 0–8 | 1.38 (2.72), 0–9 |
| Drowsiness | 2.58 (3.14), 0–10 | 2.40 (2.70), 0–9 | 2.32 (2.89), 0–10 | 3.31 (3.25), 0–10 |
| Dry mouth | 1.50 (2.27), 0–10 | 1.44 (2.41), 0–10 | 1.95 (2.88), 0–10 | 1.97 (3.09), 0–10 |
| Sad | 2.35 (3.12), 0–10 | 1.48 (2.39), 0–10 | 2.05 (3.06), 0–10 | 2.38 (3.09), 0–9 |
| Vomiting | 0.17 (1.02), 0–8 | 0 (0), 0–0 | 0.03 (0.16), 0–1 | 0.29 (1.51), 0–8* |
| Numbness or tingling | 0.77 (1.57), 0–6 | 0.46 (1.30), 0–6 | 0.27 (0.99), 0–5 | 0.83 (2.47), 0–10 |
| Weakness | 2.02 (2.99), 0–10 | 2.00 (2.68), 0–10 | 2.05 (2.76), 0–10 | 2.55 (2.93), 0–9 |
| Understanding | 1.68 (2.82), 0–10 | 1.69 (2.60), 0–9 | 1.54 (2.60), 0–10 | 1.61 (2.53), 0–9* |
| Speaking | 1.35 (2.53), 0–10 | 0.96 (1.91), 0–8 | 1.27 (2.61), 0–10 | 1.48 (2.38), 0–9 |
| Seizures | 0.03 (0.25), 0–2 | 0.23 (1.33), 0–9 | 0.46 (1.98), 0–10 | 0.31 (1.67), 0–9 |
| Concentrating | 2.11 (2.61), 0–10 | 2.33 (2.55), 0–10 | 1.86 (2.61), 0–10 | 2.66 (3.00), 0–10 |
| Vision | 1.27 (2.43), 0–10 | 1.02 (2.16), 0–8 | 0.97 (1.92), 0–8 | 1.28 (2.70), 0–9 |
| Appearance | 1.00 (2.42), 0–10 | 2.17 (3.32), 0–10 | 0.51 (1.74), 0–9 | 0.52 (2.05), 0–10 |
| Bowel pattern | 0.92 (2.19), 0–10 | 1.08 (2.52), 0–10 | 0.97 (2.06), 0–9 | 0.54 (1.75), 0–9* |
| Irritability | 2.48 (2.91), 0–10 | 2.19 (2.57), 0–10 | 2.46 (3.18), 0–10 | 2.17 (3.11), 0–9 |
| General activity | 3.88 (3.34), 0–10 | 3.04 (2.86), 0–10 | 2.54 (3.10), 0–10 | 1.89 (2.82), 0–8* |
| Mood | 2.64 (3.15), 0–10 | 2.44 (2.78), 0–10 | 2.38 (3.23), 0–10 | 1.64 (2.66), 0–8* |
| Work | 3.62 (3.51), 0–10 | 3.40 (3.14), 0–10 | 3.03 (3.41), 0–10* | 2.11 (2.95), 0–10* |
| Relationships | 1.58 (2.61), 0–10 | 1.94 (2.67), 0–9 | 1.70 (2.78), 0–10 | 0.86 (2.03), 0–8* |
| Walking | 2.11 (3.05), 0–10 | 2.44 (3.05), 0–9 | 2.19 (3.08), 0–10 | 1.46 (2.62), 0–8* |
| Enjoyment of life | 2.80 (3.27), 0–10 | 2.92 (3.42), 0–10 | 2.51 (3.44), 0–10 | 1.96 (2.97), 0–9* |
one case missing
Caregiver distress, burden and mastery and patient survival
Univariate Cox regression revealed a significant association between self-esteem (caregiver burden) and patient survival, with a 7.9% increase in the probability of dying sooner (hazard) with each unit increase in caregiver self-esteem (95% CI=0.99–1.18; based on 31 cases, see Table 3). Caregiver mastery was also associated with patient survival, with a 14% reduction in the probability of dying sooner (hazard) with each unit increase in mastery (95% CI=0.79–0.93; based on 88 cases). When known covariates were used in multivariable models (adjusting for the patient’s functional status (KPS), postsurgical treatment, type of surgery, and age), only the association between caregiver mastery and patient survival remained statistically significant. Each unit increase in caregiver mastery was associated with a 16.1% decreased hazard of patient death (95% CI=0.77–0.91; based on 88 cases).
Table 3.
Associations between caregivers’ emotional health and GBM patients’ probability of dying sooner (hazard).
| Caregiver measure N=88 |
Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | Likelihood ratio P | Hazard ratio (95% CI) | Likelihood ratio P | |
| Caregiver burden: self-esteemb |
1.079 (0.989–1.177) | 0.088* | 1.064 (0.955–1.185) | 0.262 |
| Caregiver burden: abandonmentc |
0.976 (0.879–1.084) | 0.646 | ||
| Caregiver burden: schedule disruptions |
1.015 (0.967–1.067) | 0.544 | ||
| Anxiety | 1.022 (0.946–1.103) | 0.585 | ||
| Depression | 1.007 (0.976–1.040) | 0.646 | ||
| Mastery | 0.860 (0.793–0.932) | <0.001* | 0.839 (0.771–0.913) | <0.001* |
Adjusted for KPS, postsurgical treatment, type of surgery, patient age;
Unadjusted analyses based on 31 cases, adjusted analyses based on 31 cases;
Unadjusted analyses based on 36 cases.
p<0.10
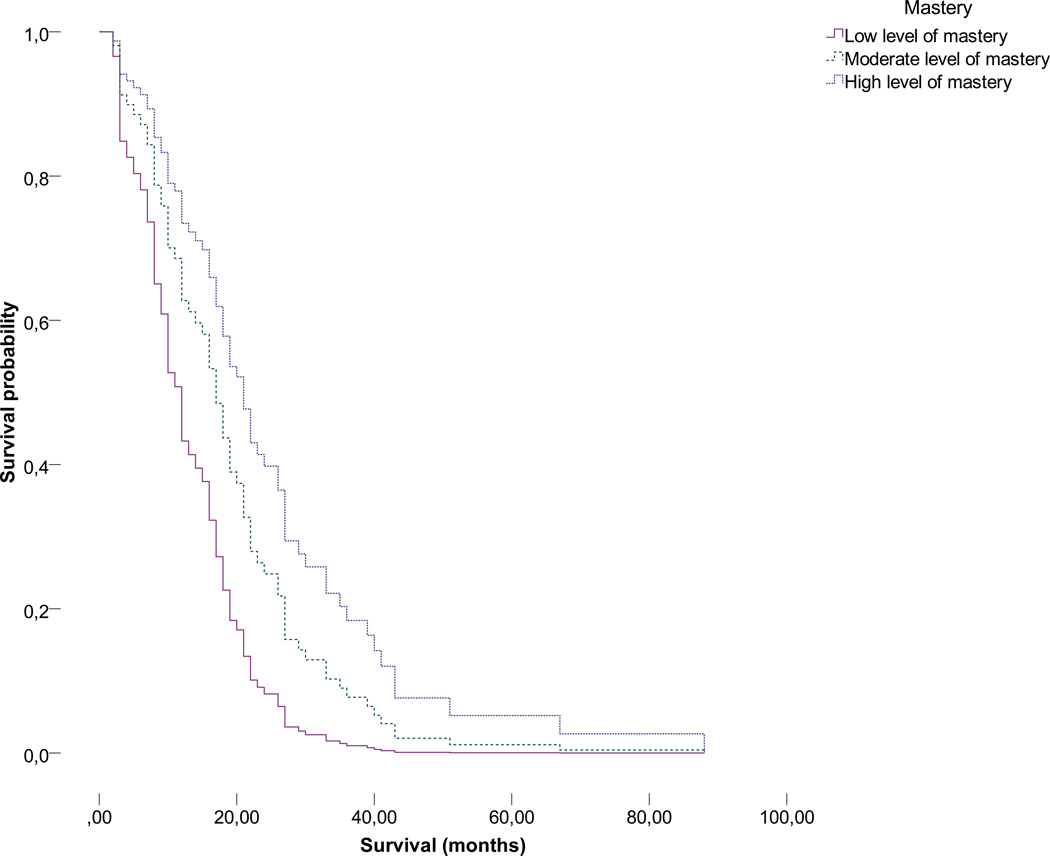
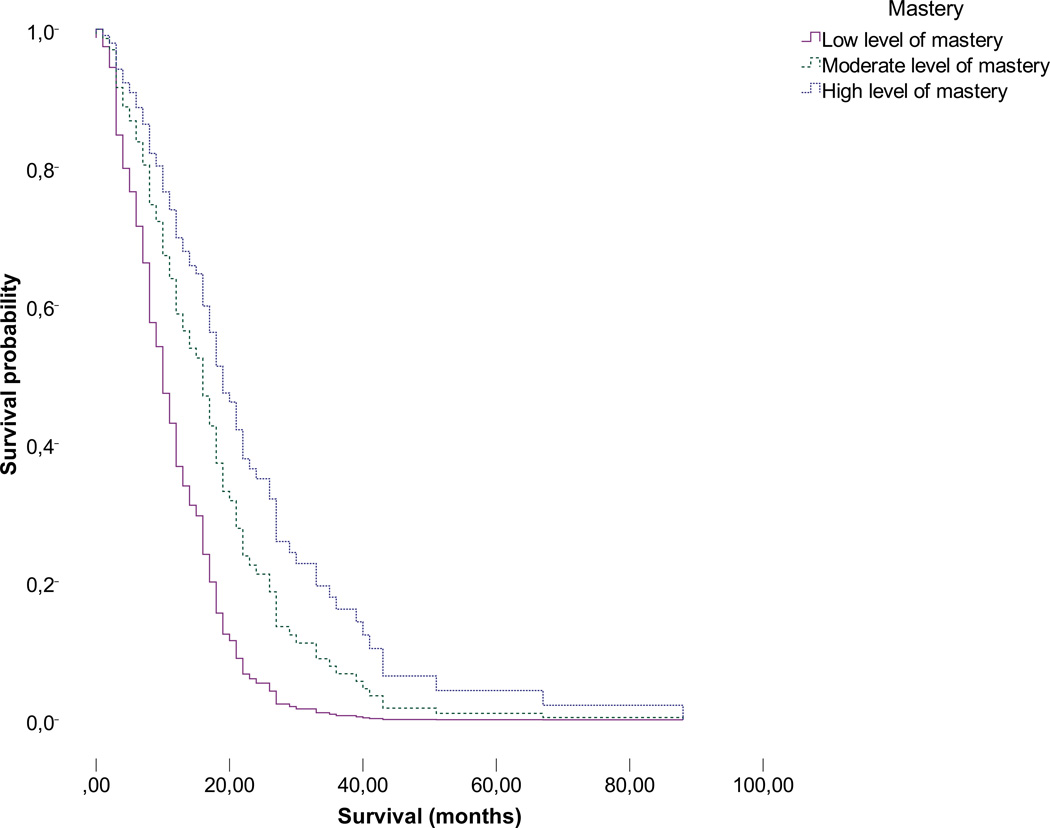
Caregiver mastery was divided into tertiles (low, moderate or high level of mastery) and plotted against patient survival probability, again adjusted for the patient’s functional status (KPS), postsurgical treatment, type of surgery, and age, in Figures 1a (non-imputed data, N=73) and 1b (non-imputed data plus five rounds of imputation; pooled data, N=513). Moderate to high versus lower levels of caregiver mastery both appear to be significantly associated with an extended patient survival time (moderate versus low: Hazard=0.523, 95% CI=0.30–0.90, P=0.020; high versus low: Hazard=0.359, 0.20–0.65, P=0.001, based on 88 cases). No differences between groups of caregivers with low moderate or high levels of mastery and age, gender, educational level or relationship with the patient were found (all P>0.05). Table 4 displays patients’ treatment variables and survival in relation to caregiver mastery.
Figure 1.
a. Association between mastery in tertiles and GBM patient survival (non-imputed data, N=73).
b. Association between mastery in tertiles and GBM patient survival (non-imputed data plus five rounds of imputation; pooled data, N=513).
Table 4.
Caregiver mastery in tertiles and patients’ treatment and survival.
| Low mastery | Moderate mastery |
High mastery | P-value | ||
|---|---|---|---|---|---|
| Patient survival (months) M (sd) | 12.50 (9.66) | 18.54 (12.04) | 23.54 (19.23) | 0.022* | |
| Patient survival (tertiles) N (%) |
Short | 13 (14.8%) | 7 (8.0%) | 6 (6.8%) | 0.069 |
| Moderate | 6 (6.8%) | 12 (13.6%) | 10 (11.4%) | ||
| Long | 5 (5.7%) | 10 (11.4%) | 13 (14.8%) | ||
| Patient initial treatment |
Surgery or biopsy only |
0 (0%) | 1 (1.1%) | 1 (1.1%) | 0.753 |
| Surgery and radiotherapy |
2 (2.3%) | 3 (3.4%) | 1 (1.1%) | ||
| Surgery, radiotherapy, chemotherapy |
24 (27.3%) | 24 (27.3%) | 24 (27.3%) | ||
| Patient additional treatment |
Re-resection | 2 (2.3%) | 4 (4.5%) | 6 (6.8%) | 0.367 |
| Chemotherapy (change or additional) |
3 (3.4%) | 2 (2.3%) | 3 (3.4%) | ||
| Radiotherapy (gamma knife) | 0 (0%) | 3 (3.4%) | 3 (3.4%) | ||
| Bevacizumab +/− CPT | 8 (9.1%) | 6 (6.8%) | 4 (4.5%) | ||
| Patient experimental treatment |
RTOG-0825: Bevacizumab vs placebo |
3 (3.4%) | 1 (1.1%) | 3 (3.4%) | 0.802 |
| Veliparib (ABT-888) | 0 (0%) | 1 (1.1%) | 1 (1.1%) | ||
| NABTC 07-01: VEGF-trap | 0 (0%) | 1 (1.1%) | 2 (2.3%) | ||
| Vaccine trial (unspecified) | 0 (0%) | 0 (0%) | 1 (1.1%) | ||
| Novocure Optune (treatment) | 1 (1.1%) | 1 (1.1%) | 1 (1.1%) | ||
| Novocure Optune (care as usual) | 1 (1.1%) | 1 (1.1%) | 0 (0%) | ||
Based on non-imputed data. All percentages shown are relative to the total sample (N=88).
P<0.05.
Abbreviations: RTOG: Radiation therapy oncology group; G-CSF: Granulocyte-colony stimulating factor; NABTC: North America brain tumor consortium; VEGF: vascular endothelial growth factor.
No statistically significant associations were found between the two other caregiver burden scales (abandonment and schedule disruptions), caregiver depression or caregiver anxiety and GBM patient survival (P>0.10).
Discussion
This study was a first attempt to examine the relationships between caregivers’ emotional health and length of patient survival in neuro-oncology. We report on newly diagnosed GBM patients and their caregivers specifically, as these patients are a relatively uniform group. Most patients receive the same postsurgical treatment, and survival times generally range from one to two years post diagnosis.2 Formal correction for MGMT methylation status, an important predictor for response to chemotherapy,8 was not possible due to missing data. However, corrected for other variables known to influence GBM patient survival, such as functional status (KPS), age, postsurgical treatment, and extent of resection, significant effects of caregiver mastery on the probability of the patient dying sooner remained. Higher feelings of mastery were associated with a 16.1% risk reduction in time to patient death.
Previous studies have suggested relationships between caregivers’ and cancer patients’ mental health40;41 and less often, between caregivers’ and patients’ physical functioning.41;42 The notion that the emotional health of family caregivers could influence cancer patients’ physical functioning and even survival, has been largely an assumption to date. A study in breast cancer patients (N=100) hinted in this direction as distress within the marital relationship influenced patients’ recovery and symptom severity,43 and a small number of other studies have found that caregiver distress can influence cancer patients’ physical functioning.28–30 However, another recent, large study in patients with advanced cancer (N=484) failed to demonstrate any specific effects of caregivers’ mental health or self-efficacy (a construct similar to mastery) on patients’ physical health.41 It should be noted that the studies described above did not include patient survival as an outcome. Regardless, it is possible that the neuro-oncology specific situation, with its unique neurological and cognitive symptom pattern, rapid onset and decline in patients’ health, differs significantly from the general cancer patient population. Inconsistencies between what was found in this analysis and reported in other studies underscore the need for further work in this area.
Given that caregivers’ feelings of mastery may influence the length of time to death in GBM patients, it is vital to determine what constitutes mastery, and through which mechanisms this might influence patients’ physical health. Although the present study can neither confirm nor deny this, it seems plausible that caregivers who experience a high level of mastery may react more quickly to patients’ physical needs, e.g. through contacting health care professionals early in case of a medical emergency, thus improving survival. Moreover, better mastery in caregivers could facilitate communication within the dyad, leading to better mutual support and through this mechanism, influence patient health. However, this remains highly speculative as literature in this area is sparse. We checked whether low/moderate/high levels of mastery were related to caregiver age, gender, educational level and the relationship with the patient, and found no statistically significant differences. In other patient populations, caregiver mastery has been associated with patient gender (better mastery when patient is female)44 and caregiver physical functioning (worse mastery with worse physical functioning).45 In neuro-oncology, the patient’s problem behavior is correlated with mastery.21 Mastery is likely heavily interlinked with other determinants of caregivers’ emotional health, as low levels of mastery are known to influence depressive symptoms in neuro-oncology caregivers.21 It was therefore unexpected that caregiver depression, anxiety and burden were not significantly associated with GBM patient survival in our analyses.
The remarkably strong effects of mastery on patients’ survival time found in this study could, in part, be explained by aspects of the patients’ baseline functioning not covered by the covariates chosen for this analysis. We found no difference in symptom severity between patients who survived shorter or longer than 12 months, making this less likely an issue. Moreover, there may be unknown subgroups of patients within our sample as MGMT methylation status is known to influence the effectiveness of chemotherapy in GBM.8 Due to the recruitment period we were unable to obtain reliable data on biomarkers for the majority of the sample, but in 36 patients MGMT status was not related to patient survival at 12 months. Still, this remains a limitation of the study. Finally, the results with regard to caregiver burden should be interpreted with caution, as we had many missing values and the regression models are based on only 31 and 36 cases for the self-esteem and abandonment scales respectively – less than half of our total sample. Indeed, univariate analysis yielded counterintuitive results for caregiver self-esteem, that were significant on a P<0.10 level despite 1 (unity) being in the confidence interval. More complete data on caregiver burden might yield different results. It seems unlikely that incomplete assessments occurred as a result of burden, as we used baseline caregiver assessments only. Comparisons between completers and non-completers of caregiver burden scales revealed no statistically significant difference in demographic characteristics. Efforts are ongoing to replicate the study with more complete data on MGMT methylation status, to confirm or deny the present study results.
Regardless of these limitations, the present study is the first to examine the potential impact of the emotional health of neuro-oncology family caregivers on patients’ physical health outcomes. A randomized controlled trial showed that feelings of mastery among caregivers of high-grade glioma patients could be improved over a period of 8 months.46 Other, still ongoing intervention studies aimed at improving family caregivers’ emotional health such as nurse and web-based efforts in SmartCare (R01NR013170; PIs Sherwood and Donovan) and Hold on, for each other,47 have included mastery and aspects of caregiver burden as outcome measures. Evaluating the outcome of these intervention studies on caregivers’ emotional health and time of patient survival could help to explain some of these findings. Providing neuro-oncology caregivers with more structured support and guidance in clinical practice might be enough to empower them and lower their levels of distress, thus influencing patients’ health for the better.
Acknowledgments
Funding: National Institutes of Health, National Cancer Institute (R01CA118711). The first author was supported by a Niels Stensen Fellowship.
Footnotes
Conflict of interest: No conflict of interest exists for any author.
Author contributions: All authors have contributed to the conception and design, or analysis and interpretation of data, and drafting the article or revising it. All authors have approved of the final version and agree to be accountable for all aspects of the work.
Reference List
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology. 2015;17:iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, Van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Dirven L, Aaronson N, Heimans J, Taphoorn M. Health-related quality of life in high-grade glioma patients. Chinese Journal of Cancer. 2014;33:40–45. doi: 10.5732/cjc.013.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricard D, Idbaih A, Ucray F, Lahutte M, Hoang-Xuan K, Delattre J. Primary brain tumours in adults. The Lancet. 2012;379:1984–1996. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez de Eulate-Beramendi S, Alvarez-Vega M, Balbin M, Sanchez-Pitiot A, Vallina-Alvarez A, Martino-Gonzalez J. Prognostic factors and survival study in high-grade glioma in the elderly. British journal of neurosurgery. 2016 doi: 10.3109/02688697.2016.1139049. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Fekete B, Werlenius K, Orndal C, Rydenhag B. Prognostic factors for glioblastoma patients - a clinical population-based study. Acta Neurologica Scandinavica. 2015 doi: 10.1111/ane.12481. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? Journal of neurosurgery. 2016;124:977–988. doi: 10.3171/2015.5.JNS142087. [DOI] [PubMed] [Google Scholar]
- 8.Felsberg J, Rapp M, Loeser S, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clinical Cancer Research. 2009;15:6683–6693. doi: 10.1158/1078-0432.CCR-08-2801. [DOI] [PubMed] [Google Scholar]
- 9.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. Journal of Clinical Oncology. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 10.Gorlia T, Van den Bent MJ, Hegi M, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. The Lancet Oncology. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 11.Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. American journal of physical medicine & rehabilitation. 2001;80:346. doi: 10.1097/00002060-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lucas MR. Psychosocial implications for the patient with a high-grade glioma. Journal of Neuroscience Nursing. 2010;42:104–108. doi: 10.1097/jnn.0b013e3181ce5a34. [DOI] [PubMed] [Google Scholar]
- 13.Sterckx W, Coolbrandt A, Dierckx de Casterle B, et al. The impact of a high-grade glioma on everyday life: A systematic review from the patients and caregivers perspective. European Journal of Oncology Nursing. 2013;17:107–117. doi: 10.1016/j.ejon.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Peters K, West M, Hornsby W, et al. Impact of health-related quality of life and fatigue on survival of recurrent high-grade glioma patients. Journal of Neuro-Oncology. 2014;120:499–506. doi: 10.1007/s11060-014-1574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. Journal of the National Cancer Institute. 2011;103:61–76. doi: 10.1093/jnci/djq458. [DOI] [PubMed] [Google Scholar]
- 16.Choi CW, Stone RA, Kim KH, et al. Group-based trajectory modeling of caregiver psychological distress over time. Annals of Behavioral Medicine. 2012;44:73–84. doi: 10.1007/s12160-012-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell B, Collins A, Dowling A, et al. Predicting distress among people who care for patients living longer with high-grade malignant glioma. Supportive Care in Cancer. 2015;24:43–51. doi: 10.1007/s00520-015-2739-0. [DOI] [PubMed] [Google Scholar]
- 18.Trad W, Koh E, Daher M, et al. Screening for psychological distress in adult primary brain tumor patients and caregivers: considerations for cancer care coordination. Frontiers in Oncology. 2015 doi: 10.3389/fonc.2015.00203. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaugler J, Hanna N, Linder J, et al. Cancer caregiving and subjective stress: a multi-site, multi-dimensional analysis. Psycho-Oncology. 2005;14:771–785. doi: 10.1002/pon.916. [DOI] [PubMed] [Google Scholar]
- 20.Mausbach B, Patterson T, von Kanel R, et al. The attenuating effect of personal mastery on the relations between stress and Alzheimer caregiver health: a five-year longitudinal analysis. Aging & mental health. 2007;11:637–644. doi: 10.1080/13607860701787043. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood PR, Given BA, Given CW, et al. The influence of caregiver mastery on depressive symptoms. Journal of Nursing scholarship. 2007;39:249–255. doi: 10.1111/j.1547-5069.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- 22.Bookwala J, Schulz R. The role of neuroticism and mastery in spousal caregivers' assessment of and response to a contextual stressor. The Journals of Gerontology Series B. Psychological Sciences and Social Sciences. 1998;53:155–164. doi: 10.1093/geronb/53b.3.p155. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Kim Y, Kim S, et al. Impact of caregivers' unmet needs for supportive care on quality of terminal cancer care delivered and caregiver's workforce performance. Supportive Care in Cancer. 2010;18:699–706. doi: 10.1007/s00520-009-0668-5. [DOI] [PubMed] [Google Scholar]
- 24.Torti F, Gwyther L, Reed S, Friedman J, Schulman K. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Disease & Associated Disorders. 2004;18:99–109. doi: 10.1097/01.wad.0000126902.37908.b2. [DOI] [PubMed] [Google Scholar]
- 25.Brodaty M, McGilchrist C, Harris L, Peters K. Time until institutionalization and death in patients with dementia: role of caregiver training and risk factors. Archives of neurology. 1993;50:643–650. doi: 10.1001/archneur.1993.00540060073021. [DOI] [PubMed] [Google Scholar]
- 26.Kuzuya M, Enoki H, Hasegawa J, et al. Impact of Caregiver Burden on Adverse Health Outcomes in Community-Dwelling Dependent Older Care Recipients. The American Journal of Geriatric Psychiatry. 2011;19:382–391. doi: 10.1097/JGP.0b013e3181e9b98d. [DOI] [PubMed] [Google Scholar]
- 27.Hebert R, Schulz R. Caregiving at the end of life. Journal of palliative medicine. 2006;9:1174–1187. doi: 10.1089/jpm.2006.9.1174. [DOI] [PubMed] [Google Scholar]
- 28.Vrettos I, Kamposioras K, Kontodimopoulos N, et al. Comparing health-related quality of life of cancer patients under chemotherapy and of their caregivers. the Scientific World Journal. 2012;135283 doi: 10.1100/2012/135283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunfeld E, Coyle D, Whelan T, et al. Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. Canadian Medical Association Journal. 2004;170:1795–1801. doi: 10.1503/cmaj.1031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadhwa D, Burman D, Swami N, Rodin G, Lo C, Zimmermann C. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psycho-Oncology. 2013;22:403–410. doi: 10.1002/pon.2104. [DOI] [PubMed] [Google Scholar]
- 31.McConigley R, Halkett G, Lobb E, Nowak AK. Caring for someone with high-grade glioma: a time of rapid change for caregivers. Palliative Medicine. 2010;24:473–479. doi: 10.1177/0269216309360118. [DOI] [PubMed] [Google Scholar]
- 32.Newberry A, Sherwood P, Hricik A, et al. Understanding recruitment and retention in neurological research. The Journal of neuroscience nursing: journal of the American Association of Neuroscience Nurses. 2010;42:47. doi: 10.1097/jnn.0b013e3181c1fdd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 34.Andresen E, Malmgren J, Carter W, Patrick D. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American Journal of Preventive Medicine. 1994;10:77–84. [PubMed] [Google Scholar]
- 35.Usala P, Hertzog C. Measurement of affective states in adult: Evaluation of an adjective rating scale instrument. Research on Aging. 1989;11:403–426. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]
- 36.Given C, Given B, Stommel M, Collins C, King S, Franklin S. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Research in nursing & health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 37.Pearlin LI, Schooler C. The structure of coping. Journal of health and social behavior. 1978:2–21. [PubMed] [Google Scholar]
- 38.Schag C, Coscarelli R, Heinrich L, Ganz P. Karnofsky performance status revisited: reliability, validity, and guidelines. Journal of Clinical Oncology. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong TS, Mendoza T, Gring I, et al. Validation of the MD Anderson symptom inventory brain tumor module (MDASI-BT) Journal of Neuro-Oncology. 2006;80:27–35. doi: 10.1007/s11060-006-9135-z. [DOI] [PubMed] [Google Scholar]
- 40.Hagedoorn M, Sanderman R, Bolks H, Tuinstra J, Coyne J. Distress in couples coping with cancer: a meta-analysis and critical review of role and gender effects. Psychological bulletin. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Kershaw T, Ellis K, Yoon H, Schafenacker A, Katapodi M, Northouse L. The interdependence of advanced cancer patients' and their family caregivers' mental health, physical health, and self-efficacy over time. Annals of Behavioral Medicine. 2015;49:901–911. doi: 10.1007/s12160-015-9743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: differences between curative and palliative cancer treatment settings. Journal of pain and symptom management. 1999;17:418–428. doi: 10.1016/s0885-3924(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Schuler T. Marital quality and survivorship: slowed recovery for breast cancer patients in distressed relationships. Cancer. 2009;115:217–228. doi: 10.1002/cncr.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaugler J, Linder J, Given C, Kataria R, Tucker G, Regine W. Family cancer caregiving and negative outcomes: the direct and mediational effects of psychosocial resources. Journal of family nursing. 2009;15:417–444. doi: 10.1177/1074840709347111. [DOI] [PubMed] [Google Scholar]
- 45.Cantwell J, Muldoon O, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Research in developmental disabilities. 2014;35:2215–2223. doi: 10.1016/j.ridd.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Boele FW, Hoeben W, Hilverda K, et al. Enhancing quality of life and mastery of informal caregivers of high-grade glioma patients: a randomized controlled trial. J. Neurooncol. 2013;111:303–311. doi: 10.1007/s11060-012-1012-3. [DOI] [PubMed] [Google Scholar]
- 47.Kohle N, Drossaert C, Schreurs K, Hagedoorn M, Verdonck-de Leeuw I, Bohlmeijer E. A web-based self-help intervention for partners of cancer patients based on Acceptance and Commitment Therapy: a protocol of a randomized controlled trial. BMC Public Health. 2015;15:303. doi: 10.1186/s12889-015-1656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]