Abstract
Purpose
Pathologic fractures occur in 5-10% of pediatric osteosarcoma cases and have historically been considered a contraindication to limb salvage. We purposed to describe the radiographic features of pathologic fracture and examine its impact on local recurrence rates, functional outcomes and overall survival.
Methods
We retrospectively analyzed patients at our institution from 1990-2015 with pathologic fracture at diagnosis or during neoadjuvant chemotherapy. We selected a control group of 50 osteosarcoma patients of similar age and gender without pathologic fracture from 1990-2015. Functional outcomes were scored using Musculoskeletal Tumor Society (MSTS) criteria. Chi square test was used for comparative analysis of groups.
Results
Thirty-six patients with 37 pathologic fractures form the study cohort. Of patients who received surgery, 18/34 patients with fracture underwent amputation, compared to 8/48 in the non-fracture group (p=0.007). Indications for amputation in fracture patients were tumor size (n=7), neurovascular involvement (n=6), and tumor progression during neoadjuvant chemotherapy (n=5). Only one patient (2.9%) in the fracture group who underwent limb salvage suffered local recurrence. Of patients who received neoadjuvant chemotherapy, 25/34 fracture patients showed poor histological response, compared to 24/47 non-fracture patients. (p=0.044) There was no statistically significant difference in overall survival between the two groups (p=0.96). Functional outcomes were significantly lower in fracture patients (median=17.5) than non-fracture patients (median=24) (p=0.023).
Conclusions
Radiographic features of pathologic fractures were highly variable in this population. Limb salvage surgery can be performed without increased risk of local recurrence. Patients with pathologic fracture suffer worse functional outcomes, but show no decrease in overall survival.
Keywords: Pathologic Fracture, Pediatric Osteosarcoma, Functional Outcomes
Introduction
Osteosarcoma (OS) is the most common bone cancer in children and adolescents with about 400 new cases annually in the US.[1] Pathologic fractures occur in 5-10% of cases at the time of diagnosis or during neoadjuvant chemotherapy.[2] Traditionally, pathologic fracture was a contraindication for limb salvage surgery and was thought to be associated with local recurrence.[3] Recent studies have shown pathologic fractures are not a contraindication to limb salvage surgery with local recurrence rates in these patients ranging between 10-25%.[2,4-8] To date, little information is available concerning the types of fractures patients sustain and the functional outcomes of patients with fractures. We investigated the types of pathologic fractures, treatment modulation, local recurrence rates, functional outcomes, and overall survival.
Materials and Methods
Following IRB approval, we retrospectively reviewed medical records and diagnostic imaging of OS patients who were treated at St. Jude Children's Research Hospital with a pathologic fracture either at diagnosis or during neoadjuvant chemotherapy from 1990-2015. Patients who had a fracture either during or after surgery were excluded. Data points collected are summarized in table 1. To compare outcomes, we generated a control group of 50 OS patients without pathologic fractures with similar age and gender characteristics. Data points collected are summarized in table 1.
Table 1.
Patient, Tumor, and Management characteristics of pathologic fracture group and non-pathologic fracture group.
| Characteristic | Patients with fracture | Patients without fracture |
|---|---|---|
| Gender | ||
| Male | 22 | 27 |
| Female | 14 | 23 |
| Age at diagnosis (years) | 12.5 (6-22) | 13 (5-21) |
| Anatomic tumor site | ||
| Distal femur | 14 | 29 |
| Proximal humerus | 14 | 5 |
| Proximal tibia | 2 | 8 |
| Entire femur | 2 | 0 |
| Distal tibia | 1 | 2 |
| Proximal femur | 1 | 2 |
| Ilium | 1 | 2 |
| Radius and ulna | 1 | 0 |
| Mid femur | 1 | 0 |
| Tumor stage | ||
| Metastatic | 19 | 28 |
| Localized | 18 | 22 |
| Biopsy type | 36 | 50 |
| Surgery | 34 | 43 |
| IR | 2 | 7 |
| Histologic subtype | ||
| Conventional | 21 | 47 |
| Telangiectatic | 9 | 0 |
| Small cell | 5 | 0 |
| Mixed | 1 | 2 |
| Chondroblastic | 0 | 1 |
| Neoadjuvant chemotherapy | ||
| IFOS/CBDCA | 6 | 6 |
| Bevacizumab/CDDP/DOX/HDMTX | 6 | 8 |
| CDDP/DOX | 5 | 1 |
| CDDP/DOX/HDMTX | 4 | 16 |
| VCR/DOX/CDDP/IFOS/ETO | 2 | 0 |
| IFOS/ETO/HDMTX/DOX/CDDP | 2 | 9 |
| CTX/CDDP/HDMTX | 2 | 0 |
| IFOS/CBDCA/DOX | 2 | 7 |
| HDMTX | 2 | 1 |
| IFOS/ETO/CDDP/DOX | 1 | 1 |
| IRI/IFOS/DOX | 1 | 0 |
| DOX/IFOS/HDMTX | 1 | 0 |
| Radiation/ETO | 1 | 0 |
| None | 2 | 1 |
| Surgical Management | ||
| Amputation | 18 | 8 |
| Limb salvage | 16 | 40 |
| Percent tumor necrosis | 33 | 47 |
| <90% | 25 | 24 |
| >90% | 9 | 23 |
| Surgical margins | ||
| Negative | 33 | 46 |
| Positive | 1 | 1 |
Abbreviations: IR: interventional radiology; IFOS: ifosfamide; CBDCA: carboplatin; CDDP: cisplatin; DOX: doxorubicin; HDMTX: high dose methotrexate; VCR: vincristine; ETO: etoposide; CTX: cyclophosphamide; IRI: irinotecan.
Disease stage (localized versus metastatic) was determined by chest CT, PET scan (after 2002), and bone scan.
At our institution, protocols have evolved over the 25 years of this study. OS91 used a combination of carboplatin, ifosfamide, doxorubicin, and high-dose methotrexate (HDMTX). [9] OS99 involved carboplatin, ifosfamide, and doxorubicin. [10] OS2008 added a monoclonal antibody, bevacizumab, to a combination of cisplatin, doxorubicin, and HDMTX. [11]
Imaging and radiology reports were used to determine fracture types. Complete fractures were classified to four major types: transverse, spiral, oblique and comminuted. [12] Physeal fractures were classified according to the Salter-Harris classification system. [13]
Tumor necrosis was determined by pathology reports. A tumor showing greater than 90% necrosis was considered to have a good response to neoadjuvant chemotherapy. Tumors showing less than 90% necrosis were considered to have a poor response.
The Musculoskeletal Tumor Society (MSTS) scoring system was used to assess functional outcome for each patient. For upper extremity function, the MSTS used a 0-5 scale for the following variables: pain, function, emotional acceptance, hand position, manual dexterity, and lifting ability. For lower extremity function, the MSTS used a 0-5 scale for the following variables: pain, function, emotional acceptance, supports, walking, and gait. [14]
Patient data was summarized by 2×c contingency tables. The associations between variables were tested by using the exact Person chi-square test and logistic regression model. A Wilcoxon rank-sum test was also performed on the two groups of MSTS scores to determine significance. Kaplan-Meier method was used to estimate overall survival and local recurrence rates.
Results
Patient demographics, Tumor Characteristics, and Tumor Management
From 1990-2015, 36 (22 males, 14 females) OS patients presented with pathologic fracture either at diagnosis or during neoadjuvant chemotherapy. One patient had two different pathologic fractures, one with each of their two primary osteosarcoma sites. Median age was 12.5 years (range, 6-22 years). Table 1 summarizes the patient demographics, tumor characteristics, and management. The most common tumor sites were the distal femur (n=14) and proximal humerus (n=14). Nineteen patients presented with metastatic disease, and 17 presented with localized disease. All except one patient had their primary tumor biopsied (open biopsy, n=34; needle biopsy, n=2). One patient had a positive lung nodule biopsy. The most common chemotherapy regimens used were an ifosfamide/carboplatin regimen and a combination of bevacizumab, cisplatin, doxorubicin, and HDMTX. Thirty-four patients underwent surgery for control of their disease after neoadjuvant chemotherapy. Eighteen underwent amputations; sixteen had limb salvage procedures. Two patients refused any surgical intervention. The majority of patients (n=25, 73%) had poor response to chemotherapy (<90% tumor necrosis). Nine patients (27%) had a good response to chemotherapy (>90% tumor necrosis). Only one patient had positive margins following limb salvage surgery and underwent amputation to obtain local control. This patient was classified as a limb salvage patient for our study and has not had a local recurrence.
Table 1 also summarizes the patient demographics, tumor characteristics and management for the non-fracture group; 50 patients were included (27 males, 23 females). Median age at diagnosis was 13 years (range, 6-21 years). The most common tumor site was distal femur (n=29). Twenty-eight patients presented with metastatic disease. Most patients (n=43) had an open biopsy. Neoadjuvant chemotherapy regimens were similarly highly variable. Forty patients initially had limb salvage surgery and 8 had an amputation. Two patients who had initially undergone a limb salvage had a subsequent amputation. One had an amputation for palliative reasons, and the other due to local recurrence. We classified both these patients at limb salvage patients. Data on final pathology was only available for 47 patients. Twenty-four patients showed a poor response to chemotherapy and 23 showed a good response.
Characteristics of pathologic fractures
Table 2 summarizes the characteristics of pathologic fractures. Twenty- eight fractures (76%) occurred at diagnosis; nine fractures (24%) occurred during neoadjuvant chemotherapy. The most common sites for fracture were the distal femur (n=14) and proximal humerus (n=14). Most fractures occurred through the tumor (n=34), but three fractures occurred near the tumor periphery. Only 13 fractures showed angulation, most commonly medial angulation (n=6). Most fractures showed minimal to partial healing (n=21). Twelve fractures showed no signs of healing while only four showed full healing. Fracture types were highly variable with oblique (n=8) and transverse (n=5) being the most common. Management of their fractures was highly variable. Most patients received either a cast (n=9), a sling (n=8), or a brace (n=7). Other managements included internal fixation (n=3), bed rest (n=3) traction (n=1), surgery (n=1), suspension (n=1), boot (n=1), splint (n=1), and an immobilizer (n=1). One patient had a healed fracture.
Table 2. Characteristics of pathologic fractures.
| Fracture time | |
|---|---|
| Diagnosis | 28 |
| During therapy | 9 |
| Fracture location | |
| Distal femur | 14 |
| Proximal humerus | 14 |
| Mid femur | 3 |
| Proximal tibia | 2 |
| Femoral neck | 1 |
| Proximal femur | 1 |
| Radius and ulna | 1 |
| Distal tibia | 1 |
| Fracture location relative to primary tumor | |
| Through tumor | 34 |
| Tumor periphery | 3 |
| Angulation | |
| Absent | 24 |
| Present | 13 |
| Medial | 6 |
| Posterior | 2 |
| Lateral | 2 |
| Lateral and anterior | 1 |
| Not described | 2 |
| Signs of healing | |
| None | 12 |
| Minimal | 7 |
| Partial | 14 |
| Full | 4 |
| Fracture type | |
| Oblique | 8 |
| Transverse | 5 |
| Impacted and comminuted | 3 |
| Nondisplaced | 3 |
| Transverse and nondisplaced | 2 |
| Transverse and displaced | 2 |
| Spiral | 2 |
| Comminuted | 2 |
| Other | 10 |
| Fracture management | |
| Cast | 9 |
| Sling | 8 |
| Brace | 7 |
| Internal fixation | 3 |
| Other | 6 |
| None | 4 |
Management and Outcomes in Patients with and without Pathologic Fractures
Eighteen patients with fractures underwent amputation due to tumor size (n=7), involvement of neurovascular structures (n=6), and tumor progression on therapy (n=5); 16 underwent limb salvage. Pathologic fracture was not an indication for any of the amputation procedures. Two patients refused surgery. Only one patient (2.9%) had a local recurrence after limb salvage surgery, and underwent no further surgical intervention. She also had metastatic disease and was treated with topotecan and palliative radiation therapy for pain and died of progressive disease one year later. Twenty-two patients (61%) developed distant recurrent disease. The average time off therapy at last follow-up was 3.5 years (range, 0.1-21 years). Overall, 22 (61%) patients were deceased and 14 (39%) were alive.
In the non-fracture group, 40 patients underwent limb salvage surgery and 8 had an amputation; 2 patients did not undergo surgery due to extensive metastatic disease. Of the 48 patients who had surgery, 7 (14.6%) experienced a local recurrence. Thirty-four patients (68%) developed distant recurrent disease. Overall, 27 (54%) patients were deceased, and 23 (46%) patients were alive.
Table 3 shows the functional outcomes for both groups. For the patients with pathologic fractures, the overall average MSTS score was 17.7/30 (median 17.5/30). Patients with pathologic fractures who underwent limb salvage surgery had an average MSTS of 20.8/30. However, for patients with pathologic fractures who had an amputation, the average MSTS was 14.7/30.
Table 3. Association between prognostic factors and fracture status.
| Pathologic Fracture Group N=36 patients with 37 fractures | Non-Fracture Group N=50 | p value | |
|---|---|---|---|
| Age | 12.5 (6-22) | 13 (6-21) | |
| Gender | 0.659 | ||
| Male | 22 | 27 | |
| Female | 14 | 23 | |
| Stage | 0.828 | ||
| Localized | 18 | 22 | |
| Metastatic | 19 | 28 | |
| Surgery | 0.0007 | ||
| Amputation | 18 | 8 | |
| Limb salvage | 16 | 40 | |
| None | 2 | 2 | |
| Necrosis | 0.044 | ||
| >90% | 9 | 23 | |
| <=90% | 25 | 24 | |
| N/A | 3 | 3 | |
| MSTS median (functional outcomes) | 17.5/30 (6-29) | 24/30 (4-30) | 0.023 |
| MSTS median (amputation only) | 13/30 (6-28) | 22.5 (4-29) | 0.080 |
| MSTS median (limb salvage only) | 22/30 (8-29) | 24/30 (6-30) | |
| Overall Survival | 0.96 | ||
| Alive | 14 | 23 | |
| Dead | 22 | 27 |
In the non-fracture group, the overall average MSTS score was 21.3/30 (median 24/30). For non-fracture patients who underwent limb salvage surgery, the average MSTS was 21.7/30. However, for amputation patients, the average MSTS was 19.7/30.
Statistical Results
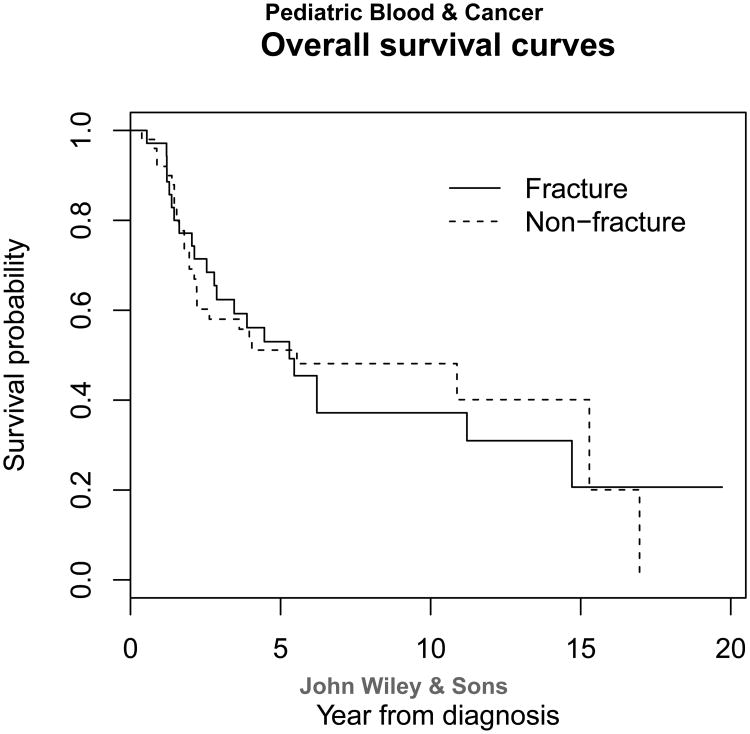
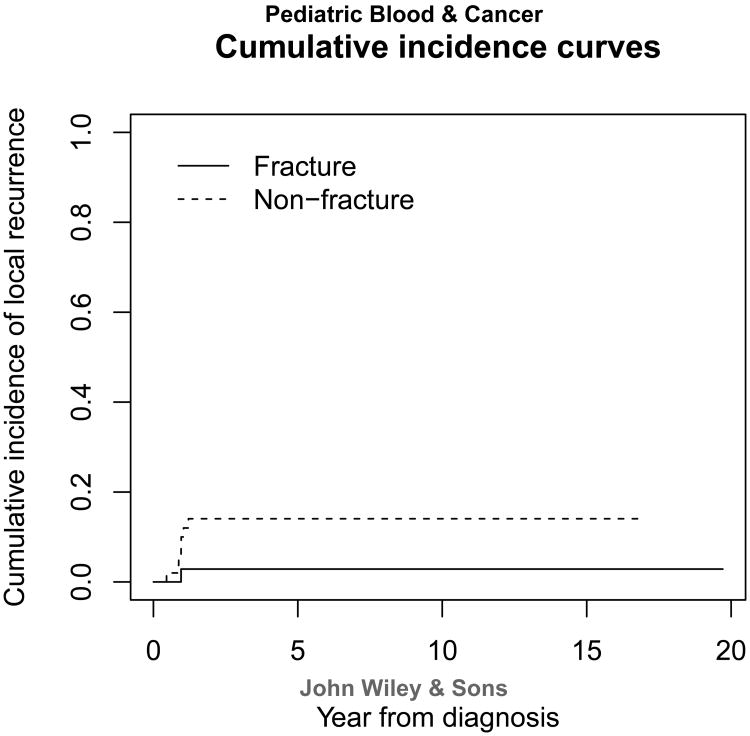
Table 3 shows the association between prognostic factors and fracture status. Surgery type, percent necrosis, and functional outcomes were the only variables reaching statistical significance. More pathologic fracture patients (n=18) underwent amputation when compared with the non-fracture group (n=8; p=0.0007). Pathologic fracture patients had significantly lower median MSTS scores than the non-fracture patients (p=0.023). This indicates that pathologic fracture patients have worse functional outcomes. Because more patients with pathologic fracture had amputations than those without fracture, we compared the functional outcomes of amputees with and without fracture. There was not a statistically significant difference between the two groups. This could be due to our small population size. A significantly greater proportion (p=0.044) of patients with pathologic fracture (73%) showed poor response to neoadjuvant chemotherapy than non-fracture patients (51%). Overall survival distributions (Figure 1) and number of distant recurrences were not statistically significant between the two groups (p=0.96 and 0.410, respectively) Fracture group had a lower cumulative incidence rate of local recurrence (2.9%) than that of non-fracture group (14.6%) even though it was not significant (p=0.131) (Figure 2). Thus the two groups had similar prognosis, and pathologic fracture was not an indicator of poor patient prognosis.
Figure 1.
Figure 2.
Discussion
Pathologic fractures occur in OS patients either spontaneously or as a result of minimal trauma. [15] In our study, the majority of patients (76%) presented at diagnosis of their primary disease with a pathologic fracture. This is consistent with findings in other studies. [2,5,16] To date, little data has been reported on the types of fractures sustained by these patients. We found that type of fracture was highly variable as was fracture healing; 62% of our patients showed evidence of fracture healing. In contrast, Ferguson et al reported 30% of fracture healing in their patients.5 It has been suggested that chemotherapy may assist in the healing of these fractures prior to surgery. [15] Fracture healing has also been associated with more favorable prognosis, including increased overall survival and decreased local recurrence. [17] In our study, of the patients who showed no signs of healing (n=12), 50% were alive at last follow-up. Of the patients who had full fracture healing (n=4), 50% were alive at last follow-up.
The management of pathologic fractures in children with OS has been addressed by several authors. Jackson et al proposed that any type of management should be done after a biopsy and histological diagnosis are obtained. [18] Saraph and Linhart proposed that management of pathologic fractures should take into consideration the pain and comfort of the child, local control of the lesion, stabilization and anatomical alignment of the fracture, fracture union, and functional restoration. [19] Ruggieri et al proposed that fractures should be initially managed by cast or external fixation to avoid microscopic spread of the tumor. [17] The management of pathologic fractures in our study was highly variable. The majority of patients underwent closed management of their fracture, mainly using braces, slings, or casts. Because our result regarding fracture management were so variable, we propose that for our institution in the future we should institute a protocol to investigate fracture management further.
Two prominent studies stated pathologic fracture was significantly associated with an increased local recurrence rate.[2,16] However, multiple recent studies have contradicted their results.[3,4,6-8,20-22,24-25] Our results support these more recent studies. We found that the local recurrence rate among pathologic fracture patients was lower than those without fracture. This could be due to surgeons resecting wider margins in pathologic fracture patients than those without fracture.
While more patients with pathologic fracture underwent amputation, according to detailed pre-operative records, the fracture was not an indication for amputation. Reasons for amputation included localized and metastatic tumor burden, tumor progression, or involvement of neurovascular structures. Statistical analysis showed a significant difference in the number of patients with amputations in the pathologic fracture group compared to the non-fracture group. We attribute this difference not to the pathologic fracture, but due to potentially more aggressive disease in these patients. A statistically significant greater proportion of patients with pathologic fracture (73%) showed a poor response to chemotherapy compared with non-fracture patients (51%). Patients with pathologic fracture in our study appear to have more aggressive local disease, predisposing them to fracture, and demonstrate poor response to chemotherapy, which was an indication for amputation. Even though there was a significant difference found between response to chemotherapy, we found no difference in overall survival between the two groups. In a recent study, Bishop et al report that perhaps histological response to neoadjuvant chemotherapy may not be an accurate long term prognostic marker for patients with osteosarcoma. [26] This could be an explanation for why the fracture group, even though had poorer histological response, did not have worse overall survival.
Chemotherapy regimens varied greatly in and between both groups. This could affect overall survival and outcomes. However, the majority of patients in both groups were grouped into the OS91, OS99, or OS2008 clinical trials. Results from the OS99 trial showed no significant difference in outcomes from the OS91 trial. [27]. Results from the OS2008 trial have still not been published, so at this time we cannot say with certain whether the OS2008 trial showed superior outcomes to the previous trials. Traditional systemic chemotherapy regimens for osteosarcoma have not changed greatly in the last three decades. [28] Overall survival has improved from the 1970s to now, but Hagleitner et al attribute this to improved supportive care regarding toxicity of the chemotherapy agents, not changes in the regimens themselves. [29]. We propose that even though the chemotherapy regiments were varied within and between our study groups, it is possible the chemotherapy regimens do not greatly affect the overall survival in osteosarcoma patients.
Local recurrence rates in pathologic fracture patients range from 10-26% in different studies. [3-6,8,19] We report a much lower rate of local recurrence which occurred in only one patient with pathologic fracture, yielding a rate of 2.9%. For the last three decades, two surgeons have performed most of the limb salvage or amputation procedures at our institution. The high volume of OS cases seen at our institution and the continuity of having the same surgical team caring for the majority of those cases, we believe contributed to the lower local recurrence rate among fracture patients.
Our results support those of other investigators when comparing functional outcomes between pathologic fracture patients and non-fracture patients. [5,6] We found pathologic fracture patients had statistically significant worse functional outcomes compared with non-fracture patients. Prolonged immobilization for management of fracture may lead to greater muscle atrophy which could possibly cause poorer functional outcomes. Further, a wider surgical resection, removing more muscle than if there was no fracture, could contribute to decreased functionality after surgery. This significant difference in functional outcomes could be important knowledge for physical therapists and surgeons planning postoperative rehabilitation programs. While there was no statistically significant difference between the functional outcomes of amputees in both groups, there was a large difference. We are unsure of why there was a larger difference between the amputee groups, and this should be investigated further. The functional outcomes of the limb salvage patients in both groups did not vary as greatly as the amputees
There are mixed results regarding whether pathologic fracture is of prognostic value. Scully et al, Bramer et al, Coley et al, Ferguson et al, Lee et al and Sun et al all have reported a decrease in overall survival in pathologic fracture patients.[2-5,20,22] They attributed this to tumor dissemination. However, other investigators reported no decrease in overall survival in pathologic fracture patients. [7,16,21,23-24] Our results agree with the latter studies. There was no significant difference between the final outcomes in patients with a pathologic fracture and those without. A greater proportion of patients without pathologic fracture were alive at last follow-up than those with fracture, but this was not statistically significant. In addition, there was no significant difference between the number of distant metastases between the two groups. Kim et al reported the presence of a pathologic fracture has no prognostic relevance in patients with localized OS.[24] Xie et al concluded that limb salvage surgery could be used in patients with pathologic fracture without significantly increasing risk of distant metastasis. [7]
Our results must be interpreted recognizing the limitations to this study. First, we had a small population size due to the rarity of pathologic fractures in OS patients. Second, selection bias could be invoked as it was conducted at a single institution with the same surgical team for 30 years. Third, we used a selected control group of 50 OS patients at St. Jude, but a different control group could yield different results. Finally, we are also a large pediatric oncology center with many referral patients. This could yield some referral bias and possibly our patients represent a special group of more difficult osteosarcoma patients.
On the basis of our results, we conclude that pathologic fractures in childhood and adolescent patients with OS are highly variable. Fracture management was highly variable and should be investigated further. The presence of a pathologic fracture did not increase risk of local recurrence or distant metastases. Pathologic fracture, however, did result in lower functional outcomes when compared with non-fracture patients, but did not decrease overall survival.
Acknowledgments
Grant R25CA23944 from the National Cancer Institute.
Abbreviations
- OS
Osteosarcoma
- MSTS
Muscoloskeletal Tumor Society
- CT
Computed tomography
- PET
Positron emission tomography
- HDMTX
High Dose Methotrexate
Footnotes
Conflicts of interest: We report no conflicts of interest for this study.
References
- 1.Ottaviania G, Jaffe N. The Epidemiology of Osteosarcoma. In: Jaffe N, et al., editors. Pediatric and Adolescent Osteosarcoma Cancer Treatment and Research. Vol. 152. 2009. pp. 3–13. [DOI] [PubMed] [Google Scholar]
- 2.Scully SP, Ghert MA, Zurakowsky D, Thompson RC, Gebhardt MC. Pathologic Fracture in Osteosarcoma: Prognostic Importance and Treatment Implications. J Bone Joint Surg Am. 2002 Jan;84-A(1):49–57. [PubMed] [Google Scholar]
- 3.Coley BL, Pool JL. Factors Influencing the Prognosis in Osteogenic Sarcoma. Ann Surg. 1940;112:1114–28. doi: 10.1097/00000658-194012000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramer JAM, Abudu AA, Grimer RJ, Carer SR, Tillman RM. Do Pathological Fractures Influence Survival and Local Recurrence Rate in Bony Sarcomas? European Journal of Cancer. 2007 Jul;43:1944–1951. doi: 10.1016/j.ejca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson PC, McLaughlin CE, Griffin AM, Bell RS, Deheshi BM, Wunder JS. Clinical and Functional Outcomes of Patients with a Pathologic Fracture in High-Grade Osteosarcoma. J Surg Oncol. 2010 Aug;102(2):120–4. doi: 10.1002/jso.21542. [DOI] [PubMed] [Google Scholar]
- 6.Zuo D, Zheng L, Sun W, Hua Y, Cai Z. Pathologic Fracture Does Not Influence Prognosis in Stage IIB Osteosarcoma: a Case-Control Study. World J Surg Oncol. 2013 Jun 24;11:148. doi: 10.1186/1477-7819-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L, Guo W, Li Y, Ji T, Sun X. Pathologic Fracture Does Not Influence Local Recurrence and Survival in High-Grade Extremity Osteosarcoma With Adequate Surgical Margins. J Surg Oncol. 2012 Dec;106(7):820–5. doi: 10.1002/jso.23150. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan M, Govardhan RH, Williams S, Gopal TSR. Limb Salvage Surgery for Pathological Fractures in Osteosarcoma. International Orthopaedics. 2000;24:170–172. doi: 10.1007/s002640000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer WH, Pratt CB, Poquette CA, Rao BN, Parham DM, Marina NM, Pappo AS, Mahmoud HH, Jenkins JJ, Harper J, Neel M, Fletcher BD. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children's Research Hospital OS-91 trial. J Clin Oncol. 2001;19:171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 10.Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ, Quintana J, Luchtman-Jones L, Villarroel M, Santana VM. Frontline treatment of localized osteosarcoma without methotrexate: results of the St Jude Children's Research Hospital OS99 trial. Cancer. 2011;117.12:2770–8. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J, Glass JO, McCarville MB, et al. Assessing vascular effects of adding bevacizumab to neoadjuvant chemotherapy in osteosarcoma using DCE-MRI. Br J Cancer. 2015;113.9:1282–8. doi: 10.1038/bjc.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England S, Sundberg S. Management of Common Pediatric Fractures. Pediatric Clinics of North America. 1996;43:5, 991–1012. doi: 10.1016/s0031-3955(05)70447-8. [DOI] [PubMed] [Google Scholar]
- 13.Brown JH, DeLuca SA. Growth Plate Injuries: salter-Harris Classification. American Family Physician. 1992;46(4):1180–1184. [PubMed] [Google Scholar]
- 14.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A System for the Functional Evaluation of Reconstructive Procedures after Surgical Treatment of Tumors of the Musculoskeletal System. Clin Ortho. 1993;286:241–6. [PubMed] [Google Scholar]
- 15.Jaffe N, Spears R, Eftekhari F, Robertson R, Cangir A, Takaue Y, Carrasco H, Wallace S, Ayala A, Raymond K, Wang YM. Pathologic Fracture in Osteosarcoma: Impact of Chemotherapy on Primary Tumour and Survival. Cancer. 1987;59:701–709. doi: 10.1002/1097-0142(19870215)59:4<701::aid-cncr2820590407>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Abudu A, Sferopoulos K, Tillman R, Carter SR, Grimer RJ. The Surgical Treatment and Outcome of Pathological Fractures in Localised Osteosarcoma. J Bone and Joint Surg. 1996;78B:5:694–698. [PubMed] [Google Scholar]
- 17.Ruggieri P, Mavrogenis A, Casadei R, Errani C, Angelini A, Calabro T, Pala E, Mercuri M. Protocol of Surgical Treatment of Long Bone Pathologic Fractures. Injury. 2010;41:1161–1167. doi: 10.1016/j.injury.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Jackson WFM, Theologic TN, Gibbons CLMH, Matthew S, Kambouroglou G. Early Management of Pathological Fracture in Children. Injury, Int J. 2007;38:194–200. doi: 10.1016/j.injury.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Saraph V, Linhart W. Modern Treatment of Pathological Fractures in Children. Injury, Int J. 2005;36:64–74. doi: 10.1016/j.injury.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Li Y, Zhang J, Li H, Ye Z. Prognostic Value of Pathologic Fracture in Patients with High Grade Localized Osteosarcoma: a Systemic Review and Meta-Analysis of Cohort Studies. J Orthop Res. 2015 Jan;33(1):131–9. doi: 10.1002/jor.22734. [DOI] [PubMed] [Google Scholar]
- 21.Bacci G, Ferrari S, Longhi A, Donati D, Manfrini M, Giacomini S, Briccoli A, Forni C, Galletti S. Nonmetastatic Osteosarcoma of the Extremity with Pathologic Fracture at Presentation. Acta Orthop Scand. 2009;74:4:449–454. doi: 10.1080/00016470310017776. [DOI] [PubMed] [Google Scholar]
- 22.Lee R, Chu W, Leung J, Cheng FW, Li CK. Pathologic Fracture as the Presenting Feature in Pediatric Osteosarcoma. Pediatric Blood Cancer. 2013;60:1118–1121. doi: 10.1002/pbc.24447. [DOI] [PubMed] [Google Scholar]
- 23.Yin K, Liao Q, Zhong D, Ding J, Niu B, Long Q, Ding D. Meta-analysis of Limb Salvage Versus Amputation for Treating High-grade and Localized Osteosarcoma in Patients with Pathological Fracture. Experimental and Therapeutic Medicine. 2012;4:889–894. doi: 10.3892/etm.2012.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim M, Lee S, Lee T, Cho WH, Song WS, Cho SH, Lee JA, Yoo JY, Jung ST, Jeon DG. Prognostic Effect of Pathologic Fracture in Localized Osteosarcoma: A Cohort/Case Controlled study at a Single Institute. J Surg Onc. 2009;100:233–239. doi: 10.1002/jso.21265. [DOI] [PubMed] [Google Scholar]
- 25.Malagelada F, Tarrago LT, Tibrewal S, Ibanez AP, Jeyaseelan L, Alegria IG. Pathological Fracture in Osteosarcoma: Is It Always an Indication for Amputation? Ortop Traumatol Rehabil. 2014;16:1, 67–74. doi: 10.5604/15093492.1097490. [DOI] [PubMed] [Google Scholar]
- 26.Bishop M, Chang Y, Krailo M, Meyers P, Provisor A, Schwartz C, Marina N, Teot L, Gebhardt M, Gorlick R, Janeway K, Chou A. Assessing the Prognostic Signiicance of Histologic Response in Osteosarcoma: A Comparison of Outcomes on CCG-782 and INT0133-A Report From the Children's Oncology Group Bone Tumor Committee. PBC. 2016 doi: 10.1002/pbc.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daw N, Neel M, Rao B, Billups C, Wu J, Jenkins J, Quintana J, Luchtman-Jones L, Villarroel M, Santana V. Frontline Treatment of Localized Osteosarcoma Without Methotrexate: Results of the St. Jude Children's Research Hospital OS99 Trial. Cancer. 2011:2770–2778. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagmay J, Krailo M, Dang H, Kim A, Hawkins D, Beaty O, III, Widemann B, Zwerdling T, Bimgaars L, Langevin A, Grier H, Weigel B, Blaney S, Gorlick R, Janeway K. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children's Cancer Group, Pediatric Oncology Group, and Children's Oncolgy Group: Learning From the Past to Move Forward. J Clin Onc. 2016 doi: 10.1200/JCO.2015.65.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagleitner M, de Bont E, Maroeska D, te Loo WM. Survival Trends and Long-Term Toxicity in Pediatric Patients with Osteosarcoma. Sarcoma. 2012 doi: 10.1155/2012/636405. [DOI] [PMC free article] [PubMed] [Google Scholar]