Abstract
Blocking hepatic very low density lipoprotein (VLDL) secretion via genetic or pharmacologic inhibition of microsomal triglyceride (TG) transfer protein (Mttp) causes hepatic steatosis, yet the risks for developing hepatic fibrosis are poorly understood. Here we report that liver-specific Mttp knockout mice (Mttp-LKO) exhibit both steatosis and fibrosis, which is exacerbated by a high trans fat, fructose diet. When crossed into germline Liver fatty acid binding protein null mice (Mttp-LKO, L-Fabp−/−, ie DKO mice) hepatic steatosis was greatly diminished and fibrosis prevented, on both low and high fat diets. The mechanisms underlying protection include reduced long chain fatty acid (FA) uptake, shifts in FA distribution (lipidomic profiling) and metabolic turnover, specifically decreased hepatic 18:2 FA and TG species and a shift in 18:2 FA utilization for oxidation versus incorporation into newly synthesized TG. DKO mice were protected against fasting induced hepatic steatosis (a model of enhanced exogenous FA delivery), yet developed steatosis upon induction of hepatic de novo lipogenesis with fructose feeding. Mttp-LKO mice, on either the L-Fabp null or Apobec1 null background (ie apolipoprotein B100 only) exhibited only subtle increases in endoplasmic reticulum stress, suggesting that an altered unfolded protein response is unlikely to account for the attenuated phenotype in DKO mice. Acute, antisense-mediated L-Fabp knockdown in Mttp-LKO mice also reduced FA uptake, increased oxidation versus incorporation of 18:2 species with complete reversal of hepatic steatosis, but increased hepatic injury and worsened fibrosis.
Conclusion
Perturbing exogenous hepatic FA utilization modulates both hepatic steatosis and fibrosis in the setting of hepatic Mttp deletion, adding new insight into the pathophysiological mechanisms and consequences of defective VLDL secretion.
Keywords: Steatosis, fatty acids, metabolic compartmentalization, lipid droplets, lipidomics
Non-alcoholic fatty liver disease (NAFLD) includes a spectrum of disorders that may progress to steatohepatitis (NASH) with fibrosis and end-stage liver disease1. Excess hepatic triglyceride (TG) accumulation reflects persistent imbalance between exogenous (diet and adipose) free fatty acid (FFA) delivery and uptake, driving TG production2, and the removal of intrahepatic TG which is primarily driven by the export of very low density lipoprotein (VLDL)3. Human studies provide evidence for both increased and decreased hepatic VLDL secretion in NAFLD/NASH4, 5. A subset of patients, however, develop hepatic steatosis and NAFLD driven exclusively by defective VLDL secretion, caused by autosomal mutations in either of two gatekeeper genes involved in VLDL assembly, namely APOB (the defective gene in familial hypobetalipoproteinemia) or microsomal triglyceride transfer protein, MTTP (the defective gene in abetalipoproteinemia). However, even within this subset of patients, the factors predicting progression of hepatic steatosis to fibrosis are poorly understood6.
Because of the accompanying hypolipidemia in patients with blocked VLDL secretion, there is considerable interest in exploiting this defect as a therapeutic strategy for patients with severe hypercholesterolemia7. However, as indicated above, there remain long-term safety concerns with this strategy8–10. Earlier work in mice demonstrated that either pharmacologic inhibition or genetic deletion of Mttp caused simple hepatic steatosis11, 12. Moreover, the hepatic steatosis developing in mice with liver specific Mttp deletion (Mttp-LKO) was not associated with insulin resistance or features of the metabolic syndrome11, 13. While those earlier reports suggested that blocking VLDL secretion might be well tolerated, other reports have suggested that Mttp inhibition in mice induces endoplasmic reticulum (ER) stress14, raising the possibility that Mttp inhibition or deletion may promote or exacerbate liver injury12.
Our prior work identified Liver fatty acid binding protein (L-Fabp, Fabp1) as a genetic modifier of hepatic steatosis in mice15, as evidenced by decreased steatosis in L-Fabp−/− mice following fasting16 or high fat diets17 (notably in the C57BL6/J background18) and also attenuated stellate cell activation and fibrogenesis with diet-induced NASH19. These findings reinforce earlier findings in subjects with NASH, where L-Fabp was identified as a biomarker of disease progression in a proteomic screen20. Here we explore the long-term sequelae of Mttp inhibition, showing that Mttp-LKO mice develop fibrosis when fed a low fat diet, which was exacerbated with diet-induced NASH. We show that the hepatic steatosis and fibrosis in Mttp-LKO mice (on both low and high fat diets) was attenuated when these mice were crossed into the L-Fabp−/− background, through mechanisms that include altered FA uptake and shifts in long chain FA utilization. We further show that acute antisense mediated L-Fabp knockdown in Mttp-LKO mice replicated the metabolic phenotypes with lifelong L-Fabp deletion, yet led to hepatic inflammation and exacerbated fibrosis. These findings suggest that strategies to modify FA utilization offer novel insights into the pathophysiological mechanisms and consequences of defective VLDL secretion.
MATERIALS AND METHODS
Expanded methods descriptions for reagents, mouse genotyping, maintenance and dietary regimens, hepatocyte isolation and metabolic studies are in Supplemental Methods.
Animals
Liver specific Mttp deletor mice (Mttp-LKO) were generated as previously described21 and crossed with germline L-Fabp−/− mice21 to generate L-Fabp−/− Mttpf/f Alb CreTg/+ mice (called DKO mice) and with germline Apobec-1−/− mice22 to generate Apobec-1−/− Mttp- DKO mice. All lines are C57BL/6J congenic, and age- and sex-matched C57BL/6J mice purchased from Jackson Labs (#000664) were used as controls.
Targeted lipidomics
Lipids were extracted from 50 μl of liver homogenate with an internal standard mixture. The extracted free fatty acids were derivatized by amino methyl phenyl pyridium (AMPP) into FA-AMPP derivatives in order to obtain high sensitivity in MS. Measurement of lipids was performed with a Shimadzu 10A HPLC system and a Shimadzu SIL-20AC HT auto-sampler coupled to a Thermo Scientific TSQ Quantum Ultra triple quadrupole mass spectrometer operated in SRM mode under ESI(+). Data processing was conducted with Xcalibur (Thermo).
RESULTS
Deletion of L-Fabp in Mttp-LKO mice decreased steatosis and LD size
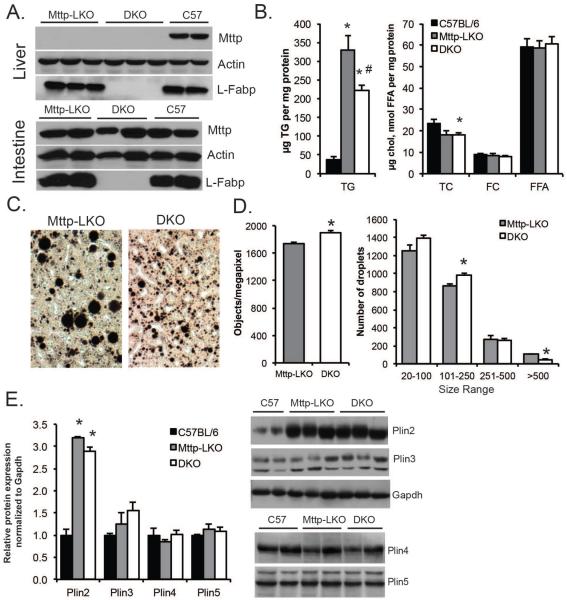
Liver-specific deletion of Mttp was verified in both L-Fabp-sufficient (Mttp-LKO) and L-Fabp deficient mice (Mttp-LKO L-Fabp−/− mice, i.e., DKO mice, Fig 1A). Mttp deletion caused hepatic steatosis by 12 weeks of age in mice fed a low fat, chow diet (Fig. 1B), with accumulation of osmium-staining lipid droplets (Fig. 1C). DKO mice exhibited reduced hepatic steatosis, with a shift to smaller LDs (Fig.1C–D). We examined whether this shift in LD size correlated with a change in expression of specific lipid droplet proteins, but we found only the expression of perilipin 2 to be increased (vs controls), with no difference between Mttp-LKO and DKO genotypes (Fig. 1E).
Figure 1. Liver specific Mttp deletion induces lipid droplet formation.
A. Western blot analysis confirming genotypes, specifically the absence of Mttp in the liver (top panel) but not in the intestinal mucosa (bottom) of Mttp-LKO genotypes. Actin is shown as a loading control. B. Biochemical quantitation of hepatic lipid [TG (left panel); total cholesterol (TC), free cholesterol (FC) and FFA (right panel)] in 12 week, chow fed female mice (n=5-8/genotype). C. Representative images of osmium stained liver tissue from chow fed Mttp-LKO genotypes (400× magnification). D. Morphometric analysis of LD number (left) and size in osmium stained liver tissue from Mttp LKO genotypes. E. Perilipin protein expression (4-5 per genotype) normalized to GAPDH and expressed relative to levels in C57BL/6J mice. Asterisks indicate p<0.05 vs C57BL/6J; # indicates p<0.05 in DKO vs Mttp-LKO.
Liver specific Mttp deletion induces HSC activation and fibrosis
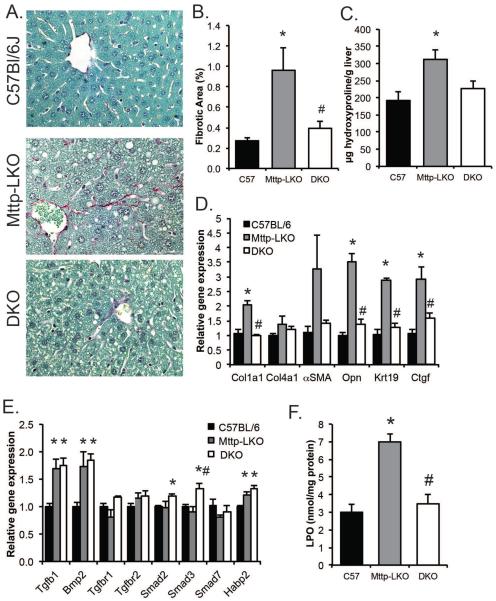
In agreement with earlier studies showing that blocking VLDL secretion in mice does not cause inflammation or provoke an unfolded protein response11, 12, we found no increase in serum transaminases in chow fed Mttp-LKO mice (Supplemental Table 1) yet those mice exhibited both increased fibrosis (Fig. 2A, B) and hydroxyproline content (Fig. 2C) compared to age matched, wild-type (WT) controls. By contrast and despite elevated TG content when compared to controls (Fig 1B), the DKO mice were completely protected against hepatic fibrosis (Fig. 2 A–C). Fibrogenic mRNA expression was also upregulated in livers from Mttp-LKO mice, including Col1a1, αSMA, osteopontin, Ctgf and Krt19 (Fig. 2D), while DKO mice showed no evidence of increased fibrogenesis. These differences in fibrogenesis however, were not generally reflected in the expression pattern of genes related to TGFβ signaling in the liver with increased mRNA abundance of Tgfb1 and Bmp2 in both Mttp-LKO genotypes and only small changes in other mRNAs (Figure 2E). Lipid peroxidation was also increased in Mttp-LKO mice and again this was completely attenuated in DKO mice (Fig. 2F). These data suggest that increased steatosis in Mttp-LKO mice promotes fibrosis and oxidative stress, and that deletion of L-Fabp prevents this, at least in part, by reducing hepatic TG accumulation.
Figure 2. Hepatic fibrosis is increased in Mttp-LKO, but not Mttp-LKO L-Fabp−/− DKO mice.
A. Sirius Red stained liver tissue in 12 week old chow fed mice (400×). B. Quantitation of Sirius Red stained area (n=6/genotype). C. Hydroxyproline content in livers of chow fed male mice, normalized to wet tissue weight (n=3/genotype). D. Hepatic expression of genes related to fibrogenesis and stellate cell activation (n=5/genotype). E. Expression of genes related to Tgfβ signaling in liver of chow fed mice (n=4/genotype). F. Lipoperoxide content in livers of chow fed female mice, normalized to protein concentration (n=9–10/ genotype). For all panels, asterisks indicate p<0.05 vs C57BL/6J control; # indicates p<0.05 in DKO vs Mttp-LKO.
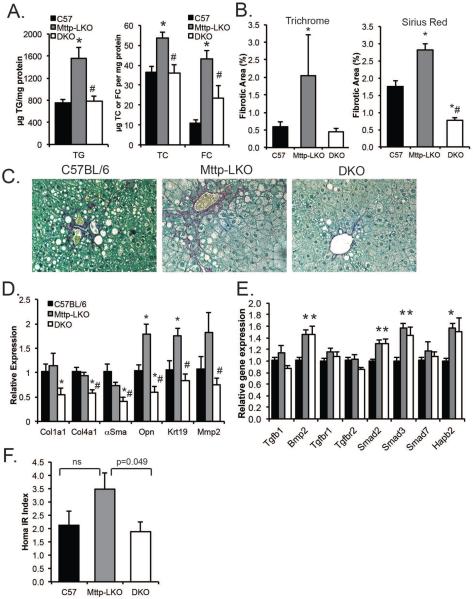
We next examined fibrotic injury in Mttp-LKO mice fed a high trans fat, fructose enriched diet (TFF), a combination shown previously to produce a NASH-like phenotype with hepatocyte ballooning and fibrosis19. Control mice fed a TFF diet for 16 weeks exhibited the expected increase in hepatic TG content (compare Fig.3A to Fig1B) an effect magnified in Mttp-LKO mice, while DKO mice exhibited virtually identical hepatic TG content compared to WT (Fig 3A). In parallel with these changes, hepatic fibrosis was also increased in Mttp-LKO mice, evidenced by both trichrome and sirius red staining (Fig. 3B–C). DKO mice exhibited reduced hepatic fibrosis and reduced fibrogenic mRNA expression compared to Mttp-LKO mice (Fig. 3B–D). These findings (in female mice) were further replicated in male mice (Supplemental fig 1). As noted above in chow fed mice, the differences in fibrosis between Mttp-LKO and DKO mice were not reflected by changes in TGFβ signaling (Figure 3E) pathways. In addition, while the hepatic steatosis accompanying liver specific Mttp deletion has been shown previously not to promote hepatic insulin resistance13, Mttp-LKO mice fed TFF diet showed a non-significant trend to increased HOMA IR versus WT mice with reduced HOMA IR in DKO mice (Fig. 3F). These findings together demonstrate that L-Fabp deletion in the setting of blocked VLDL secretion completely prevents the increased steatosis with high fat feeding and also prevents the development of hepatic fibrosis.
Figure 3. Deletion of L-Fabp reduces steatosis and fibrosis in mice fed high fat fructose (TFF) diet.
A. Hepatic TG (left panel) and cholesterol [right panel, showing total (TC) and free (FC) cholesterol] content in female mice fed TFF diet for 16 weeks (n=4/genotype). B. Quantitation of fibrotic area in liver sections stained with Masson's Trichrome (left) or Sirius Red (right) (n=4/genotype). C. Representative images of Sirius Red staining (400×). D. Expression of fibrogenic genes in livers of TFF fed mice (n= 5-6 mice per genotype). E. Expression of genes related to Tgfβ signaling in livers of TFF fed mice (n=5/genotype). F. HOMA IR index in mice fed TFF diet (n=7–8 per genotype). For all panels, asterisks indicate p<0.05 vs C57BL/6J; # indicates p<0.05 in DKO vs Mttp-LKO.
Hepatic deletion of Mttp produces only subtle increases in ER stress with no exacerbation in apoB100-only background
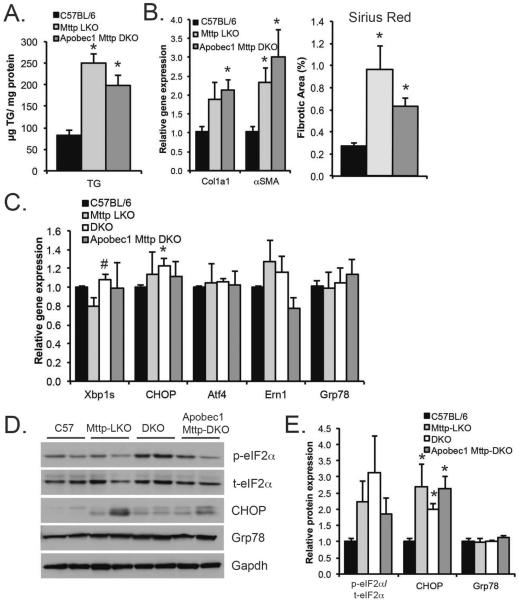
Most studies in which mice were either fed Mttp inhibitors or subjected to short-term, liver-specific Mttp deletion showed no evidence of enhanced ER stress despite increased hepatic steatosis and transaminase elevations11, 12, 14, 23. However, those studies were undertaken in mice where the majority of hepatic apolipoproteinB (apoB) was the edited mRNA form, apoB48, rather than apoB10024. This consideration is relevant since other studies have shown that dysregulation of hepatic apoB100 expression provokes endoplasmic reticulum (ER) stress which contributes to steatotic injury25, 26. In addition, we previously demonstrated that Apobec-1 deletion in intestine-specific Mttp knockout mice exacerbated ER stress particularly with high fat feeding27. Accordingly, we evaluated ER stress in Mttp-LKO mice with a view to understanding the effects of combined deletion of Mttp and Apobec-1 using Apobec-1−/− Mttp-LKO mice in which there is only apoB10024.
Those studies revealed comparable increases in hepatic TG in both Mttp-LKO mice (Fig. 4A) and Apobec-1−/− Mttp-LKO mice. Moreover, we observed no difference in hepatic fibrosis between these mice, assessed by either fibrogenic mRNA expression (Col1a1, αSMA mRNA) or Sirius red stained fibrotic area (Fig. 4B). Similar findings were obtained in Apobec-1−/− Mttp-LKO mice fed TFF diet, with no evidence of enhanced fibrosis compared to Mttp-LKO mice (Supplemental Fig 2). A survey of genes related to ER stress revealed no dramatic increases in expression of ER stress genes in any of the 3 Mttp-LKO genotypes compared to WT (Fig. 4C), with the exception of a slight (albeit statistically significant) increase in CHOP mRNA in L-Fabp Mttp-DKO mice. This subtle increase in mRNA correlated with significantly increased CHOP protein in all Mttp-LKO genotypes (Fig. 4D–E). In addition, there was a trend to increased phosphorylated eIF2α (normalized to total eIF2α) in all 3 Mttp-LKO genotypes compared to WT. These data suggest that activation of the fibrogenic program in Mttp-LKO mice is not driven primarily by changes in hepatic ER stress or by dysregulation of hepatic apoB100, since there were only subtle differences between the Mttp-LKO genotypes.
Figure 4. Genetic modifiers of hepatic lipid metabolism do not increase ER stress in Mttp-LKO mice.
A. Hepatic TG content in 12 week old, chow-fed male mice (n=7-9/genotype. B. Fibrogenic mRNAs (left, n=5–7/genotype) and quantitation of Sirius Red stained fibrotic area (right, n=5/genotype) in male C57, Mttp-LKO, and Apobec1 Mttp DKO mice. C. Hepatic ER stress genes in chow fed mice 4 per genotype. D. Representative Western blots of eIF2α (total and phosphorylated), CHOP, Grp78, and Gapdh proteins. E. Quantitation of protein expression data (n=4/genotype). eIf2α expression is presented as a ratio of phosphorylated to total eIF2α, normalized to C57BL/6J. CHOP and Grp78 expression is normalized to GAPDH, and expressed relative to C57BL/6J. Asterisks indicate p<0.05 versus C57BL/6J; # indicates p<0.05 in DKO vs Mttp-LKO.
Reduced abundance of polyunsaturated FA species in DKO mice
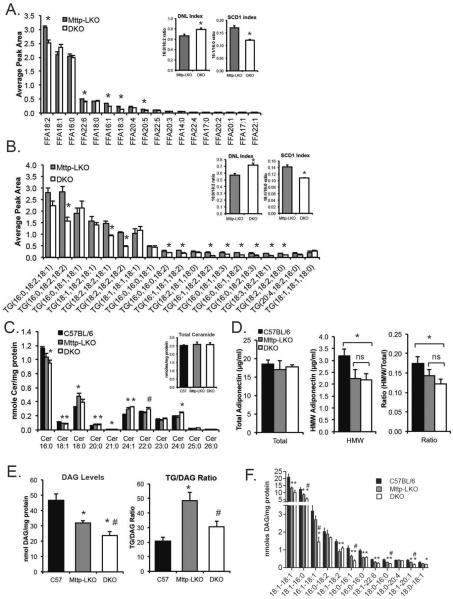
To begin to explore how deletion of L-Fabp attenuates hepatic fibrosis in the setting of reduced hepatic steatosis, we undertook targeted hepatic lipidomic profiling. Total FFA levels were not altered (FFA peak area: Mttp-LKO, 9.4 ± 0.1; DKO, 8.7 ± 0.3, n=4-5), but there were significant differences in specific FFA species by genotype (Fig. 5A). Specifically, DKO mice exhibited decreased content of longer chain FFAs, including 18:2, 18:3, and 22:6. These differences were reflected in both an increased DNL index [a ratio of FA from de novo lipogenic (16:0) and dietary sources (18:2), respectively]28 and a decrease in SCD1 index (16:1/16:0 ratio, a measure of FA saturation29) in DKO mice (Fig. 5A, inset). Total TG mass was reduced in DKO mice (Mttp-LKO, 17.1 ± 1.1 peak area; DKO, 13.3 ± 1.1; n=4-5, p=0.049) as expected, with a specific reduction in TG species containing polyunsaturated fatty acids (i.e. 16:0, 18:2, 18:2), with fewer changes in species containing saturated or mono-unsaturated species (i.e., 16:0, 18:1, 18:1) (Fig. 5B). Again, differences in TG FA species were reflected in altered DNL and SCD1 indices, with changes similar to those observed in FA species (Fig. 5B, inset). Although the expression of genes involved in FA desaturation (SCD, FADS), elongation (Elovl5, Elovl6) and de novo lipogenesis (Fasn) were dramatically reduced in both Mttp-LKO genotypes compared to WT, there were only minor differences between Mttp-LKO and DKO mice, and none consistent with observed differences in FA chain length and saturation (Supplemental fig 3). Together, these data suggest that deletion of L-Fabp affects not only TG accumulation but also influences metabolic compartmentalization of specific FA species.
Figure 5. Altered FA chain length and saturation in TG and FFA species in liver of DKO mice.
A. FFA species in livers of chow fed Mttp-LKO and DKO mice (n=4-5/genotype). Inset panels show DNL index (ratio of 16:0 to 18:2 FA, left panel) and SCD1 index (ratio of 16:1 to 16:0 FA, right), an indicator of FA elongation. Asterisks indicate p<0.05 in DKO vs Mttp-LKO. B. Relative abundance of major TG species in Mttp-LKO and DKO liver (n=4-5/genotype). Minor TG species (representing less than 10% of TG peak area) are not shown, but are included in DNL and SCD1 ratios (inset panels). Asterisks indicate p<0.05 in DKO vs Mttp-LKO. C. Lipidomic analysis of ceramide species in C57BL/6J, Mttp-LKO, and DKO mice fed TFF diet (16 weeks, n=5 per genotype). Total ceramide levels are not different by genotype (inset). D. Levels of total (left) and high molecular weight (middle) adiponectin in the plasma of TFF diet fed mice (n=5-6/genotype). Ratio of HMW to total adiponectin is shown in right panel. E. Left panel: Total DAG levels in C57BL/6J, Mttp-LKO, and DKO mice. Right panel: Relative levels of TG and DAG in TFF-fed mice, expressed as a ratio of TG to DAG. F. DAG species in mice fed TFF diet (5/ genotype). For panels C–F, asterisks indicate p<0.05 vs C57BL/6J control; # indicates p<0.05 in DKO vs Mttp-LKO.
Additional lipidomic profiling in TFF fed mice revealed comparable hepatic ceramide abundance by genotype (Fig 5C, inset) but demonstrated subtle differences in ceramide species, primarily between WT and Mttp-LKO genotypes (Fig. 5C). Plasma ceramide levels were decreased in both Mttp-LKO and DKO mice as expected30 and we found subtle shifts in lysophosphatidylcholine species (Supplemental fig 4). Because adiponectin has been shown to increase hepatic ceramide synthesis31, we measured levels of adiponectin (total and high molecular weight [HMW]) in plasma of TFF fed mice. While total plasma adiponectin levels were unchanged across the groups, we found reduced levels of HMW adiponectin in both Mttp-LKO genotypes compared to controls (Figure 5D). In addition, adiponectin mRNA was reduced in livers of both genotypes of Mttp-LKO mice, with <2-fold changes in mRNA expression of the adiponectin receptors (Supplemental Figure 4C). Thus, although total hepatic ceramide levels do not vary by genotype, minor changes in HMW adiponectin may alter the metabolism of specific ceramide species in both genotypes of Mttp-LKO mice.
Surprisingly, diacylglyceride (DAG) mass was reduced in Mttp-LKO mice and further reduced in DKO mice (Fig. 5E). This decrease was further reflected in reduced abundance of specific DAG species, including 18:1/16:0 and 16:1/18:1 in DKO livers (Fig. 5F). In addition, the findings revealed a disproportionate increase in TG/DAG ratio in Mttp-LKO mice (Fig. 5E, right panel), again suggesting that L-Fabp participates in FA utilization across many complex lipid species, including mediators of fibrogenic injury.
Altered uptake and metabolism of fatty acids in DKO mice in vivo and in vitro
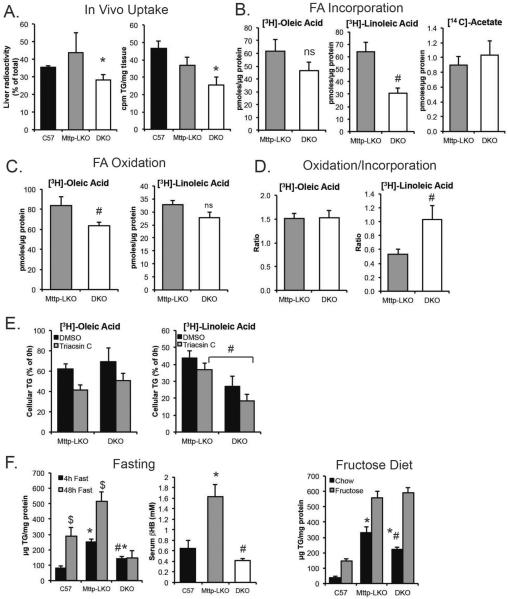
To examine whether the differences in lipidomic profile by genotype could be attributed directly to differences in hepatocyte FA utilization we determined FA uptake both in vivo and also in primary hepatocytes. Hepatic FA uptake at 10' (normalized for liver size and cpm injected) was significantly reduced in DKO mice (Fig. 6A, left panel), with decreased incorporation into TG (Fig. 6A, right panel). Based upon the findings from lipidomic profiling, we examined the metabolic fates of both [3H]-oleic acid (18:1) and [3H]-linoleic acid (18:2) in primary hepatocytes. Uptake and incorporation of each FA into cellular TG was reduced in DKO mice, with a more striking decrease in DKO cells labeled with linoleic acid (Fig. 6B). Similar differences were found using a short (15 minute) labeling period (Supplemental Fig 5). In contrast, de novo lipogenesis was unchanged in DKO mice as inferred from [14C]-acetate labeling (Fig. 6B), suggesting that L-Fabp deletion impairs the utilization of exogenous long chain FA for TG synthesis but not FA synthesized endogenously. We considered the possibility that decreased expression of hepatic FA transporters could potentially explain reduced FA uptake in DKO hepatocytes. We observed a trend to reduced CD36 mRNA in both Mttp-LKO genotypes and no differences in mRNA abundance of other FA transporter mRNAs that would account for decreased FA uptake (Supplemental Figure 6). FA oxidation was also decreased in DKO hepatocytes, suggesting that the reduced FA incorporation into TG does not reflect increased FA oxidation (Fig. 6C). However, when normalized to net FA uptake, the relative FA oxidation/incorporation ratios for linoleic acid indicated disproportionately greater oxidation of 18:2 FA in DKO hepatocytes, suggesting that FA trafficking, specifically 18:2, is preferentially altered with L-Fabp deletion (Fig. 6D).
Figure 6. Altered utilization and metabolism of fatty acids in DKO hepatocytes in vitro and in vivo.
A. In vivo hepatic uptake and utilization of [3H]-oleic acid in male C57BL/6J, Mttp-LKO and DKO mice fed TFF diet for 10 weeks. Left panel shows liver radioactivity, normalized to liver weight and expressed as a percent of total cpm injected. Right panel shows [3H]-oleate incorporated into hepatic triglyceride. (n=5 C57BL/6, 3 Mttp-LKO, 5 DKO). B. Incorporation of [3H]-oleate (left panel), [3H]-linoleate (middle), and [14C]-acetate (right) into cellular TG of hepatocytes isolated from Mttp-LKO and DKO mice. ns, not significant. C. Levels of oxidized FA in media following a 4 hour labeling. D. Ratio of pmoles oxidized to pmoles incorporated into TG in cells labeled with [3H]-oleate or [3H]-linoleate. E. Cellular [3H]-TG levels after a 4h labeling and overnight chase period, in the presence or absence of 20μM Triacsin C (n=4/genotype). F. Left: Hepatic TG content in male mice fasted for 4h (black) or 48h (gray) prior to sacrifice. N= 5-8 mice per genotype for 4h fast; 4-5 animals for 48h fast. Significant differences in TG content between 4h and 48h fast are indicated by $. Middle: Serum β-hydroxybutyrate levels following a 48h fast (n=4-5/genotype). Right: Hepatic TG in female mice fed 60% fructose diet for 3 weeks, n=7-9 female mice/genotype. Liver TG is significantly increased in fructose fed mice compared to chow diet for all genotypes. For all panels, asterisks indicate p<0.05 vs C57BL/6J; # indicates p<0.05 in DKO vs Mttp-LKO.
Based upon the shift in LD morphology and number in DKO mice (Fig.1 C–D), we next examined the rate of FA labeled-TG turnover in primary hepatocytes, in the presence or absence of Triacsin C to block reincorporation of FA32. Turnover of TG was similar in [3H]-oleic acid labeled Mttp-LKO and DKO hepatocytes (Fig. 6E, left panel), but DKO hepatocytes labeled with [3H]-linoleic acid exhibited reduced cellular TG, both with and without Triacsin C (Fig. 6E, right panel) suggesting increased turnover of 18:2 containing TG species in DKO hepatocytes. These findings complement the lipidomic data and together suggest that L-Fabp deletion in Mttp- deficient hepatocytes reduces long chain FA uptake and incorporation, preferentially 18:2 FA. Total lipase activity was reduced in DKO mice (Supplemental Fig 7), diminishing the likelihood that altered lipolytic processing of LDs might account for the increased turnover observed in the labeling experiments.
We further examined hepatic FA utilization in the setting of prolonged fasting, which stimulates in vivo turnover and remodeling of hepatocyte LDs. We found the expected increase in hepatic TG in 48h fasted C57BL/6J mice and observed the same trend in Mttp-LKO mice (Fig. 6F). By contrast, DKO mice showed no increase in hepatic TG after a 48 hour fast, with a corresponding reduction in serum ketones (Fig. 6F, middle). There was no difference in serum FFA by genotype (C57BL/6, 0.35 ± 0.05 mmol/L; Mttp-LKO, 0.28 ± 0.02; DKO, 0.27 ± 0.04), indicating that FFA release from adipose was not defective. Expression of several genes related to FAO and ketogenesis was reduced in DKO livers (Supplemental Fig 8), likely a consequence of reduced FA influx. By contrast, we observed similar increases in hepatic steatosis when Mttp-LKO and DKO mice were fed 60% fructose for 3 weeks as inferred by comparable increases in hepatic TG (Fig. 6F, right panel). Taken together, these data reinforce the conclusion that L-Fabp deletion does not impair FA utilization derived from de novo lipogenesis, but rather alters FA utilization from extrahepatic sources.
Acute knockdown of L-Fabp reduces hepatic steatosis, yet exacerbates injury
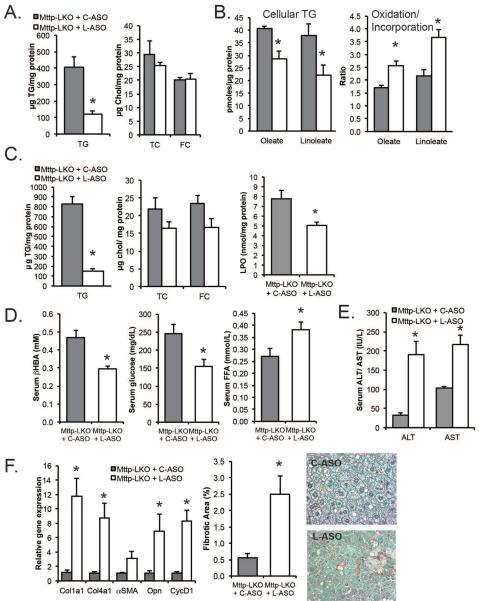
We next asked if short term, second generation antisense (ASO) mediated L-Fabp knockdown (L-ASO) in the setting of blocked VLDL secretion could reverse hepatic steatosis and prevent fibrosis in Mttp-LKO mice. Chow fed Mttp-LKO mice treated for 2 weeks with L-ASO showed decreased hepatic TG (Fig. 7A) compared to mice treated with a scrambled ASO (C-ASO), with >80% reduction in L-Fabp mRNA in whole liver as well as in both isolated hepatocytes and stellate cells (Supplemental Fig 9 A, B). Isolated primary hepatocytes from these groups recapitulated the same reduced FA incorporation and increased oxidation/ incorporation ratio of both oleate and linoleate (Fig 7B, right panel) as seen with lifelong genetic deletion of L-Fabp. L-ASO treated Mttp-LKO mice fed a high fat TFF diet also exhibited reduced hepatic L-Fabp mRNA (Supplemental fig. 9A) together with reduced steatosis (Fig. 7C) and decreased lipid peroxidation, as well as decreased abundance of 4-hydroxynonenal modified proteins by western blotting (Supplemental Fig 10). L-ASO treated TFF fed mice also exhibited reduced ketogenesis, along with decreased serum glucose and increased circulating levels of FFA (Fig 7D).
Figure 7. Acute ASO-mediated knockdown of L-Fabp in Mttp-LKO mice reduced hepatic TG but increased hepatic fibrosis.
A. Hepatic lipid content in chow fed Mttp-LKO mice treated with C-ASO (gray bars) or L-ASO (white bars) for 2 weeks (n=3 per group). B. Incorporation of [3H]-FA into TG in hepatocytes isolated from Mttp-LKO mice treated with C-ASO or L-ASO (left panel, 2–3 independent isolations per genotype). Right panel: Ratio of pmoles FA oxidized to pmoles incorporated into cellular TG ± L-ASO. C. Hepatic lipid (left) and lipoperoxide content (right) in TFF-fed Mttp-LKO mice (n=6-7 per group for hepatic TG, 2–4 for LPO). D. Serum β-hydroxybutyrate, glucose and free fatty acid levels in TFF-fed Mttp-LKO mice treated with C-ASO or L-ASO (n=6-7 per group). E. Serum ALT and AST in TFF-fed Mttp-LKO mice ± L-ASO (n=6-7 per group). F. Relative expression of fibrogenic mRNAs in the ASO-treated Mttp-LKO mice (left) and quantitation of Sirius Red stained fibrotic area. Representative images (400×) are shown on right. Asterisks indicate p<0.05 in L-ASO vs C-ASO treated mice.
However, despite a marked reduction in hepatic TG content, L-ASO treated Mttp-LKO mice exhibited increased injury, with elevated serum ALT and AST (Fig 7E), and induction of mRNAs related to both fibrogenesis and proliferation (Fig. 7F). L-ASO treated mice also showed more extensive fibrosis with increased periportal collagen staining (Fig. 7F). Expression of CHOP mRNA was increased in L-ASO treated Mttp-LKO mice (Supplemental Fig 10), although other markers of ER stress were unchanged. In addition, we found no difference in p62 expression or in the LC3-II to LC3-I ratio between L-ASO and C-ASO injected mice (Supplemental Figure 10), although the expression of unprocessed LC3b was increased by L-ASO treatment. Together these data imply that alterations in cellular autophagy and ER stress are unlikely to mediate the effects noted.
DISCUSSION
The central goals of this study were, first, to evaluate the long-term sequelae of blocking hepatic VLDL secretion in mice, specifically the development of hepatic fibrosis in Mttp-LKO mice, and secondly to test the hypothesis that genetic alterations in FA uptake and utilization pathways (by lifelong deletion of L-Fabp) might mitigate hepatic steatosis in this setting. Our findings demonstrate that Mttp-LKO mice develop hepatic fibrosis even on a low fat diet, a phenotype exaggerated when those mice were fed a TFF diet. L-Fabp deletion imposed on the background of Mttp-LKO mice (ie DKO mice) attenuated both the steatosis and fibrosis phenotypes. We further show that short term, ASO mediated L-Fabp knockdown in Mttp-LKO mice replicated the metabolic phenotypes observed with DKO mice, yet caused worse injury and exacerbated hepatic fibrosis. Several elements of these key observations merit additional consideration.
The current studies extend our understanding of the long-term consequences of blocking hepatic VLDL secretion in mice and provide new insights into the mechanisms underlying the progressive NASH that occurs in a significant proportion of patients with defective VLDL assembly (ABL, FHBL), who are at risk not only for the predictable development of hepatic steatosis, but also for the development of cirrhosis and hepatocellular carcinoma6, 33, 34. Among the distinctive features of the progressive liver injury in those patients are the absence of abdominal obesity, systemic inflammation and insulin resistance, suggesting that the development and progression of NASH in individuals with defective VLDL secretion is driven by different pathways than those in obese, insulin resistant subjects with metabolic syndrome6. The observations in patients with defective VLDL secretion are in line with earlier studies showing normal glucose tolerance and systemic insulin sensitivity in liver-specific Mttp null mice13. Other studies showed that liver-specific transgenic acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) or DGAT2 overexpressing mice exhibited marked steatosis with accumulation of TG as well as lipid intermediates, yet failed to manifest hepatic or systemic insulin resistance, suggesting that hepatic steatosis can be uncoupled from insulin resistance35. In neither of these two models was hepatic inflammation observed but in neither case was the long-term development of hepatic fibrosis examined. Accordingly, a major implication from the current studies is that the hepatic steatosis resulting from blocked VLDL secretion may lead to fibrosis in the absence of either inflammation or insulin resistance. That being said, our preliminary observations suggest that TFF-fed Mttp-LKO mice may display features of altered insulin sensitivity (as inferred by a trend to increased HOMA index), and the extent of hepatic fibrosis was correspondingly worse than that noted in WT mice fed the same fibrogenic diet (Fig 3B–D). While those findings begin to suggest that insulin resistance imposed on the background of blocked VLDL secretion may exacerbate hepatic fibrosis, those suggestions will require more detailed study of hepatic and systemic insulin resistance.
How can we begin to explain the mediators of liver fibrosis in the setting of blocked VLDL secretion? Our targeted lipidomic profiling revealed shifts in hepatic FA distribution among free FA as well as triglyceride and diacylglycerol species, yet hepatic ceramide content was unchanged and DAG content was actually decreased in Mttp-LKO mice and further decreased in DKO mice. In addition we found no changes by genotype in the abundance of hepatic lysophosphatidylcholine (Supplemental Fig 4), another lipid metabolite strongly implicated in the pathogenesis and progression of NASH36. The findings in regard to hepatic ceramide and DAG abundance are at variance with earlier findings that Mttp-LKO mice (using Mx1 Cre induced with pI-pC) exhibit ~30% increases in both hepatic ceramide and DAG13. However, the Mttp-LKO mice in those studies were generated with a different Cre driver (current studies used Albumin Cre) and it is possible that subtle differences in the experimental design (4h versus 12h fasting) might contribute to the variance in the abundance of lipid intermediates13. Other studies showed that siRNA silencing of DGAT2 reduced hepatic steatosis in mice simultaneously treated with an siRNA against Mttp37, suggesting that therapeutic strategies to interrupt metabolic pathways involved in TG assembly may yet offer a viable option to mitigate steatosis in the setting of blocked VLDL secretion. However, enthusiasm for that approach must be tempered by findings that DGAT2 ASO treatment in obese mice improved hepatic steatosis but exacerbated liver fibrosis38.
Another possible consideration as a mediator of the injury with blocked VLDL secretion is hepatic free cholesterol, whose content was increased in Mttp-LKO mice fed a TFF diet (Fig 3A), but not in DKO mice. Previous studies have implicated hepatic free cholesterol content as a key determinant of hepatic fibrogenic injury in mice39,40 and studies have found a relationship between NASH progression in human subjects and hepatic free cholesterol content41. Precedent exists for a plausible relationship between L-Fabp deletion and hepatic cholesterol utilization in the setting of blocked VLDL secretion as evidenced by our earlier findings that increased biliary cholesterol secretion and enhanced gallstone formation in L-Fabp knockout mice was completely prevented in DKO mice21. However, we found no difference in hepatic cholesterol content in L-ASO treated Mttp-LKO mice fed a TFF diet (Fig. 7A), suggesting that alternative mediators may be involved in that setting. Yet another possibility is that changes in hepatic FFA flux play an important role in the lipotoxic injury phenotype observed. The increased serum FFA levels in Mttp-LKO mice treated with L-ASO (Fig. 7D) support the suggestion that increased turnover of hepatic lipid droplets in L-ASO treated mice released FA that was no longer buffered by cytosolic L-Fabp, and might possibly contribute to the lipotoxic injury. We speculate that the absence of changes in serum or hepatic FFA in DKO mice reflects the lifelong duration (versus acute knockdown) of L-Fabp deletion in that setting.
There is a caveat to the conclusion that alterations in hepatic FA utilization by L-Fabp deletion play a dominant role in mitigating both the steatosis and hepatic fibrosis observed following blocked VLDL secretion. In particular, we recognize that DKO mice are germline null for L-Fabp, thus lacking expression in both hepatocytes and small intestinal enterocytes16. Accordingly, alterations or compensatory changes in intestinal lipid metabolism may conceivably play a role in the phenotypes observed. Against this possibility is that intestinal FA uptake and lipid absorption were earlier found to be similar in WT and germline L-Fabp knockout mice, suggesting that fat malabsorption is unlikely to account for the phenotypes observed in DKO mice17. Furthermore, acute, liver-specific knockdown of L-Fabp by ASO treatment recapitulated the phenotype of the germline knockout (Fig. 7). These considerations notwithstanding, we observed that ASO treatment led to a rapid (within two weeks) decline in L-Fabp expression in both hepatocyte and stellate cells (Supplemental fig. 9B). Those observations raise the possibility that cell-specific functions for L-Fabp may need to be considered using cell-type and tissue-specific L-Fabp deletor lines once those animals are developed.
Finally, the current findings permit us to speculate on the challenges for antisense strategies to interrupt hepatic FA utilization as a therapeutic target in the setting of blocked VLDL secretion. We observed no evidence of liver injury in L-ASO treated Mttp-LKO mice fed chow diet, although the duration of ASO treatment (2 weeks) was much shorter than that employed (8–10 weeks) in mice fed the TFF diet. It is also worth noting that ASO mediated knockdown of the lipid droplet protein Pln2 reduced hepatic steatosis in high fat fed mice, and also induced hepatic proliferation and fibrogenic gene expression42. Those data, taken together with earlier findings demonstrating that ASO mediated knockdown of Plin2 reduced hepatic steatosis and improved insulin sensitivity in both diet induced obesity as well as in genetic models of insulin resistance and fatty liver (Lepob mice)43, 44 suggest that further information is needed regarding the mechanisms by which interruption of lipid droplet catabolism impacts pathways of hepatic fibrogenesis.
Supplementary Material
Acknowledgements
We acknowledge the technical contributions of Erick Marigi, Roberto Solis, and Victoria Cooke to this manuscript.
Financial support: Grants (HL-38180, DK-56260 and DDRCC DK-52574, murine models and imaging cores), to NOD; Diabetes Research Center and the Washington University Metabolomics Facility to DSO, supported by P30 DK020579.
List of abbreviations
- L-Fabp
liver fatty acid binding protein
- Mttp
microsomal triglyceride transfer protein
- LKO
liver knock out
- DKO
double knock out
- TG
triglyceride
- DAG
diacylglyceride
- FA
fatty acid
- FFA
free fatty acid
- Chol
cholesterol
- Plin
perilipin
- LD
lipid droplet
- NASH
non-alcoholic steatohepatitis
- NAFLD
non-alcoholic fatty liver disease
- VLDL
very low density lipoprotein
- APOb
apolipoprotein b
- ASO
antisense oligonucleotide
- WT
wild type
- TFF
high trans fat fructose diet
- ABL
abetalipoproteinemia
- FHBP
familial hypobetalipoproteinemia
REFERENCES
- 1.Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol. 2016;22:4079–90. doi: 10.3748/wjg.v22.i16.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1343–6. doi: 10.1053/j.gastro.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M, Sreekumar R, Rasmussen D, et al. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrini E, Mohammed BS, Magkos F, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–31. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Filippo M, Moulin P, Roy P, et al. Homozygous MTTP and APOB mutations may lead to hepatic steatosis and fibrosis despite metabolic differences in congenital hypocholesterolemia. J Hepatol. 2014;61:891–902. doi: 10.1016/j.jhep.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–32. doi: 10.1161/CIRCULATIONAHA.113.001292. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Stanesa M, Hegele RA. Severe hypertriglyceridemia with pancreatitis: thirteen years' treatment with lomitapide. JAMA Intern Med. 2014;174:443–7. doi: 10.1001/jamainternmed.2013.13309. [DOI] [PubMed] [Google Scholar]
- 9.Hashemi N, Odze RD, McGowan MP, et al. Liver histology during Mipomersen therapy for severe hypercholesterolemia. J Clin Lipidol. 2014;8:606–11. doi: 10.1016/j.jacl.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuchel M, Blom DJ, Averna MR. Clinical experience of lomitapide therapy in patients with homozygous familial hypercholesterolaemia. Atheroscler Suppl. 2014;15:33–45. doi: 10.1016/j.atherosclerosissup.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Liao W, Hui TY, Young SG, et al. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J Lipid Res. 2003;44:978–85. doi: 10.1194/jlr.M300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bjorkegren J, Beigneux A, Bergo MO, et al. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J Biol Chem. 2002;277:5476–83. doi: 10.1074/jbc.M108514200. [DOI] [PubMed] [Google Scholar]
- 13.Minehira K, Young SG, Villanueva CJ, et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res. 2008;49:2038–44. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josekutty J, Iqbal J, Iwawaki T, et al. Microsomal triglyceride transfer protein inhibition induces endoplasmic reticulum stress and increases gene transcription via Ire1alpha/cJun to enhance plasma ALT/AST. J Biol Chem. 2013;288:14372–83. doi: 10.1074/jbc.M113.459602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spann NJ, Kang S, Li AC, et al. Coordinate transcriptional repression of liver fatty acid-binding protein and microsomal triglyceride transfer protein blocks hepatic very low density lipoprotein secretion without hepatosteatosis. J Biol Chem. 2006;281:33066–77. doi: 10.1074/jbc.M607148200. [DOI] [PubMed] [Google Scholar]
- 16.Newberry EP, Xie Y, Kennedy S, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:51664–72. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 17.Newberry EP, Xie Y, Kennedy SM, et al. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 2006;44:1191–205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 18.Newberry EP, Kennedy S, Xie Y, et al. Phenotypic divergence in two lines of L-Fabp−/− mice reflects substrain differences and environmental modifiers. Am J Physiol Gastrointest Liver Physiol. 2015;309:G648–61. doi: 10.1152/ajpgi.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen A, Tang Y, Davis V, et al. Liver fatty acid binding protein (L-Fabp) modulates murine stellate cell activation and diet-induced nonalcoholic fatty liver disease. Hepatology. 2013;57:2202–2212. doi: 10.1002/hep.26318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton M, Viker K, Krishnan A, et al. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–84. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Fung HY, Newberry EP, et al. Hepatic Mttp deletion reverses gallstone susceptibility in L-Fabp knockout mice. J Lipid Res. 2014;55:540–8. doi: 10.1194/jlr.M046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanc V, Park E, Schaefer S, et al. Genome-wide identification and functional analysis of Apobec-1-mediated C-to-U RNA editing in mouse small intestine and liver. Genome Biol. 2014;15:R79. doi: 10.1186/gb-2014-15-6-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee RG, Fu W, Graham MJ, et al. Comparison of the pharmacological profiles of murine antisense oligonucleotides targeting apolipoprotein B and microsomal triglyceride transfer protein. J Lipid Res. 2013;54:602–14. doi: 10.1194/jlr.M029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano K, Young SG, Farese RV, Jr, et al. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J Biol Chem. 1996;271:9887–90. doi: 10.1074/jbc.271.17.9887. [DOI] [PubMed] [Google Scholar]
- 25.Su Q, Tsai J, Xu E, et al. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 26.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–32. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Luo J, Kennedy S, et al. Conditional intestinal lipotoxicity in Apobec-1−/− Mttp-IKO mice: a survival advantage for mammalian intestinal apolipoprotein B mRNA editing. J Biol Chem. 2007;282:33043–51. doi: 10.1074/jbc.M705386200. [DOI] [PubMed] [Google Scholar]
- 28.Eissing L, Scherer T, Todter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun. 2013;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JJ, Lambert JE, Hovhannisyan Y, et al. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101:34–43. doi: 10.3945/ajcn.114.092262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal J, Walsh MT, Hammad SM, et al. Microsomal Triglyceride Transfer Protein Transfers and Determines Plasma Concentrations of Ceramide and Sphingomyelin but Not Glycosylceramide. J Biol Chem. 2015;290:25863–75. doi: 10.1074/jbc.M115.659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanninger J, Liebisch G, Eisinger K, et al. Adiponectin isoforms differentially affect gene expression and the lipidome of primary human hepatocytes. Metabolites. 2014;4:394–407. doi: 10.3390/metabo4020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igal RA, Wang P, Coleman RA. Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: evidence for functionally separate pools of acyl-CoA. Biochem J. 1997;324(Pt 2):529–34. doi: 10.1042/bj3240529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cefalu AB, Pirruccello JP, Noto D, et al. A novel APOB mutation identified by exome sequencing cosegregates with steatosis, liver cancer, and hypocholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:2021–5. doi: 10.1161/ATVBAHA.112.301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welty FK. Hypobetalipoproteinemia and abetalipoproteinemia. Curr Opin Lipidol. 2014;25:161–8. doi: 10.1097/MOL.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monetti M, Levin MC, Watt MJ, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 37.Tep S, Mihaila R, Freeman A, et al. Rescue of Mtp siRNA-induced hepatic steatosis by DGAT2 siRNA silencing. J Lipid Res. 2012;53:859–67. doi: 10.1194/jlr.M021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 39.Teratani T, Tomita K, Suzuki T, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152–164. e10. doi: 10.1053/j.gastro.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 40.Mari M, Caballero F, Colell A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–98. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Min HK, Kapoor A, Fuchs M, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–74. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, Boyle S, Varela GM, et al. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol Genomics. 2012;44:1125–31. doi: 10.1152/physiolgenomics.00045.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai Y, Varela GM, Jackson MB, et al. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–54. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 44.Varela GM, Antwi DA, Dhir R, et al. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2008;295:G621–8. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.