Abstract
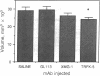
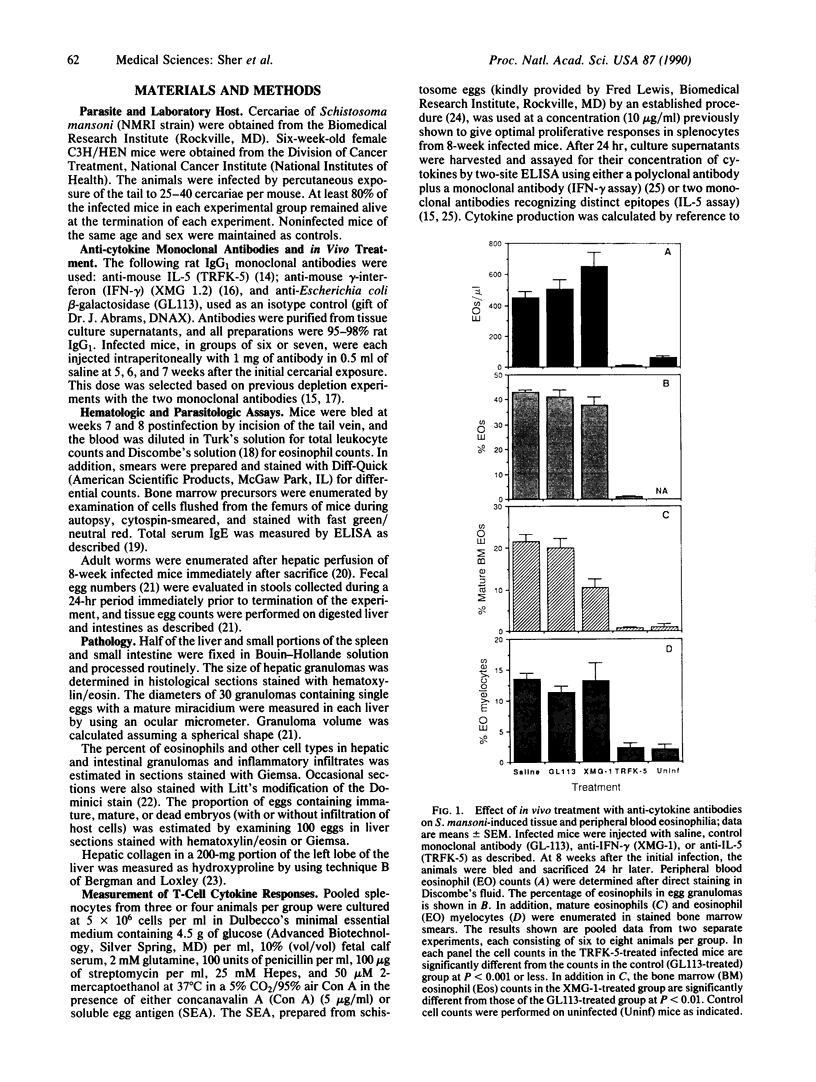
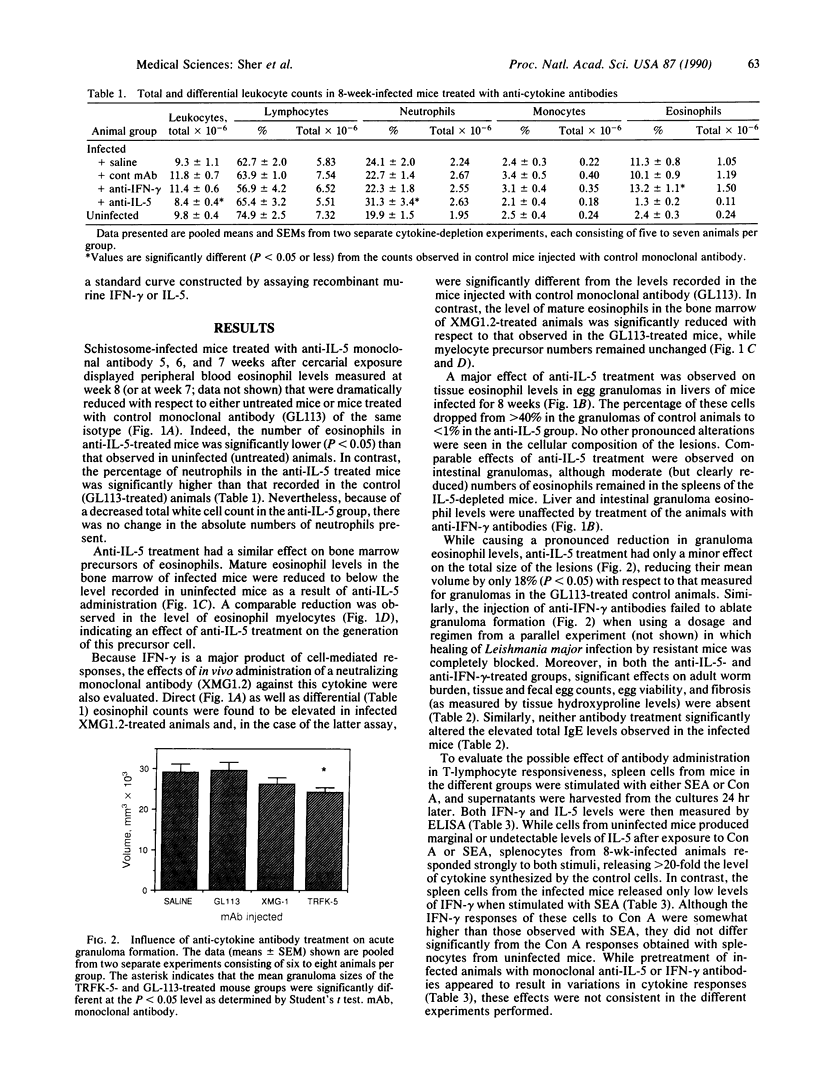
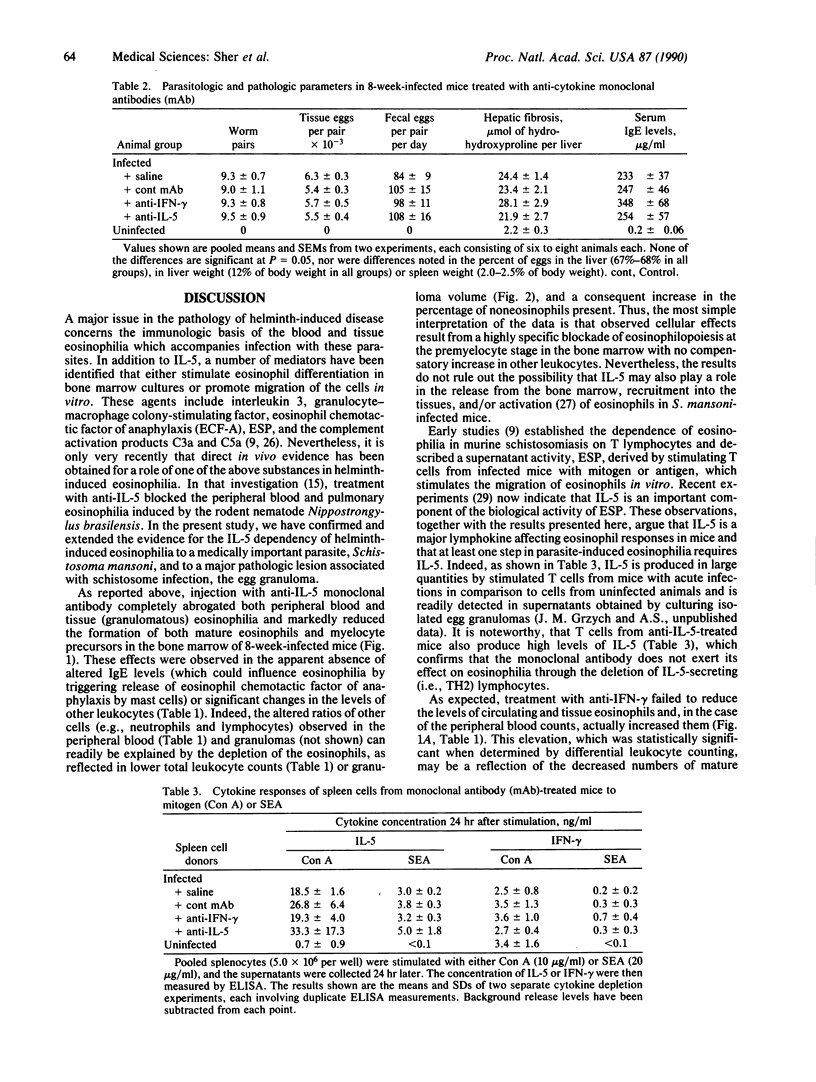
Eosinophils are thought to play a major role in the immunobiology of schistosomiasis. To investigate the immunologic basis of the eosinophil response and directly assess the function of eosinophils in egg-induced pathology, mice infected with Schistosoma mansoni were injected with a monoclonal antibody produced against interleukin 5 (IL-5), a cytokine previously shown to stimulate eosinophil differentiation in vitro. This treatment suppressed the generation of eosinophil myelocyte precursors in the bone marrow and reduced to background levels the numbers of mature eosinophils in the marrow, in circulation, and within acute schistosome egg granulomas. Nevertheless, granulomas in the anti-IL-5-treated/eosinophil-depleted mice at 8 weeks of infection were only marginally smaller than those in animals injected with control monoclonal antibody, and hepatic fibrosis was comparable in the two groups. Additional parameters such as worm burden, egg output, and serum IgE levels were unaltered by the anti-IL-5 treatment. In contrast, infected animals injected with monoclonal antibody against gamma interferon (IFN-gamma) displayed circulating eosinophil levels that were elevated with respect to control mice, possibly because of an enhanced release of mature eosinophils from the marrow, and developed egg granulomas that were indistinguishable in size and cellular composition from those in control animals. Immunologic assays revealed that lymphocytes from acutely infected mice produce large quantities of IL-5 but minimal IFN-gamma when stimulated with either egg antigen or mitogen. Taken together, these results indicate that neither IL-5 nor eosinophils are essential for egg-induced pathology but suggest that lymphocytes that belong to the IL-5-producing TH2 subset predominate during acute infection and may induce granuloma formation by the production of other cytokines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Deb S., Duvall R. H. Granuloma formation in Schistosoma japonicum infected nude mice: the effects of reconstitution with L3T4+ or Lyt2+ splenic cells. Am J Trop Med Hyg. 1989 Jan;40(1):66–71. doi: 10.4269/ajtmh.1989.40.66. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Hudak S., Jackson J., Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989 Jul 21;245(4915):308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Schistosoma mansoni: eosinophilia and the development of lymphocyte blastogenesis in response to soluble egg antigen in inbred mice. Exp Parasitol. 1972 Dec;32(3):520–526. doi: 10.1016/0014-4894(72)90070-7. [DOI] [PubMed] [Google Scholar]
- Duvall R. H., DeWitt W. B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967 Jul;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophil-mediated destruction of Schistosoma mansoni eggs. J Reticuloendothel Soc. 1976 Nov;20(5):359–374. [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. V. Eosinophils in lynph nodes of guinea pigs following primary antigenic stimulation. Am J Pathol. 1963 May;42:529–549. [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg F., Sher A., Gibbons N., Doughty B. L. Eosinophil-enriched inflammatory response to schistosomula in the skin of mice immune to Schistosoma mansoni. Am J Pathol. 1976 Sep;84(3):479–500. [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. L., Grove D. I., Warren K. S. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977 Jan;121(1):41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Role of host granulomatous response in murine schistosomiasis mansoni. eosinophil-mediated destruction of eggs. J Clin Invest. 1980 Dec;66(6):1191–1199. doi: 10.1172/JCI109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., Lammie P. J. Immunopathology of granuloma formation and fibrosis in schistosomiasis. Parasitol Today. 1986 Nov;2(11):296–302. doi: 10.1016/0169-4758(86)90123-7. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). I. Functional characterization of a TRF-producing helper T cell subset and genetic studies on TRF production. J Immunol. 1980 May;124(5):2414–2422. [PubMed] [Google Scholar]
- Von Lichtenberg F., Correa-Oliveira R., Sher A. The fate of challenge schistosomula in the murine anti-schistosome vaccine model. Am J Trop Med Hyg. 1985 Jan;34(1):96–106. doi: 10.4269/ajtmh.1985.34.96. [DOI] [PubMed] [Google Scholar]