Abstract
Background and Purpose
To study internal and external generalizability of temporal dose-response relationships for xerostomia after intensity-modulated radiotherapy (IMRT) for head and neck cancer, and to investigate potential amendments of the QUANTEC guidelines.
Material and Methods
Objective xerostomia was assessed in 121 patients (nCohort1=55; nCohort2=66) treated to 70Gy@2Gy in 2006–2015. Univariate and multivariate analyses (UVA, MVA with 1000 bootstrap populations) were conducted in Cohort1, and generalizability of the best-performing MVA model was investigated in Cohort2 (performance: AUC, p-values, and Hosmer-Lemeshow p-values (pHL)). Ultimately and for clinical guidance, minimum mean dose thresholds to the contralateral and the ipsilateral parotid glands (Dmeancontra, Dmeanipsi) were estimated from the generated dose-response curves.
Results
The observed xerostomia rate was 38%/47% (3 months) and 19%/23% (11–12 months) in Cohort1/Cohort2. Risk of xerostomia at 3 months increased for higher Dmeancontra and Dmeanipsi (Cohort1: 0.17•Dmeancontra+0.11•Dmeanipsi−8.13; AUC=0.90±0.05; p=0.0002±0.002; pHL=0.22±0.23; Cohort2: AUC=0.81; p<0.0001; pHL=0.27). The identified minimum Dmeancontra thresholds were lower than in the QUANTEC guidelines (Cohort1/Cohort2: Dmeancontra=12/19 Gy; Dmeancontra, Dmeanipsi=16, 25/20, 26 Gy).
Conclusions
Increased Dmeancontra and Dmeanipsi explain short-term xerostomia following IMRT. Our results also suggest decreasing Dmeancontra to below 20 Gy, while keeping Dmeanipsi to around 25 Gy. Long-term xerostomia was less frequent, and no dose-response relationship was established for this follow-up time.
Keywords: Radiotherapy, Xerostomia, IMRT, Head and neck, dose response, QUANTEC
Introduction
Radiotherapy (RT) is the standard of care for the majority of individuals diagnosed with head and neck cancer (HNC) either within a primary setting with/without chemotherapy, or in an adjuvant setting following surgery [1]. Given an estimated five-year relative survival for localized HNC of around 80% [2], minimizing RT-induced oral complications is essential. Severe hyposalivation (xerostomia) results, in particular, from loss of stimulated saliva and is the most commonly reported RT-induced oral complication, and leads to dental caries, oral infections, pain, reduced mastication and swallowing ability, and speech difficulties [3]. Intensity-modulated RT (IMRT) as a primary treatment for HNC has proven superior over preceding two- or three-dimensional conformal RT techniques in terms of significantly reducing the number of patients suffering from moderate to severe xerostomia up to two years after completed treatment in two randomized controlled trials [4, 5]. Even after IMRT, however, patients may experience xerostomia to a degree that still compromises their quality of life [6].
Stimulated saliva is primarily derived from the parotid glands, and these are, thus, considered the key organs for salivary function [7]. In the salivary gland-specific Quantitative Evaluation of Normal Tissue Effects in the Clinic (QUANTEC) summary it was suggested that xerostomia would be reduced if either the mean dose to the contralateral parotid gland (Dmeancontra) would be kept below 20 Gy, or if neither Dmeancontra, nor Dmean to the ipsilateral parotid gland (Dmeanipsi) would exceed 25 Gy [8]. The one-gland guideline has thereafter proven useful to prevent xerostomia after 3DCRT/IMRT [9, 10], and to some extent after IMRT [11]. Wider use of either guideline following IMRT remains unsettled. Furthermore, it has been suggested that the evolution of RT-induced xerostomia is described by distinct temporal phases with time-specific etiologies [12], and a recovery between around three to twelve months after completed RT has been observed [9, 10, 13, 14].
In this work, we hypothesized that the dose-response relationship for xerostomia depends on underlying temporal-specific patterns. Objectively measured xerostomia data, and dose information were taken from two cohorts including patients treated with IMRT for HNC. Five potentially predisposing variables were addressed, and the study was furthermore performed in a training-test design to explore generalizability within and across cohorts. The ultimate goal was to investigate to what extent both QUANTEC guidelines apply to xerostomia, and to explore at what Dmean threshold(s) xerostomia starts to evolve.
Materials and Methods
Study design and participants
This retrospective Institutionally Review Board approved study included prospectively collected data for patients previously treated with primary IMRT for HNC to the pharynx and the neck at the Memorial Sloan Kettering Cancer Center (MSKCC), or at the British Columbia Cancer Agency-Vancouver Cancer Centre (BCCA) from March 2006 to May 2012 (BCCA) or to March 2015 (MSKCC) [9, 15]. Saliva collection is standard practice at MSKCC, whereas informed consent was received from all BCCA patients. Further inclusion criteria for the current study were: A minimum of three whole-mouth stimulated flow measurements (one WMSFM >1g/5mins assessed pre-IMRT to exclude potential predisposition of baseline xerostomia, and at least two WMSFM assessed within 24 months post-IMRT), and a reasonably high RT prescription dose (≥50.4 Gy).
In total, 55 MSKCC patients and 66 BCCA patients fulfilled the inclusion criteria. These patients had been planned and treated based on Computed Tomography (CT) imaging, and the median prescribed dose to the primary tumor was 70.0 (range: 50.4–70.2) Gy delivered in 1.8 Gy or 2.0 Gy daily fractions (Eclipse, Varian, Palo Alto, CA, US). The average ± standard deviation (SD) age at initiation of IMRT was 57±10 years in the MSKCC, and 56±13 years in the BCCA cohort. The predominant primary tumor site in the MSKCC cohort was tonsil (22%), followed by base of tongue (19%), nasopharynx (14%), and oral tongue (13%). The corresponding figure in the BCCA cohort was nasopharynx (22%), base of tongue (17%), tonsil (17%), and tumors of unknown primary (15%).
Stimulated whole mouth saliva flow measurements, and xerostomia definition
Patients refrained from consuming food, or drinking typically for at least one hour prior to WMSFM. The WMSFM were assessed over a five-minute period, and were triggered by administration of a citrate solution to both sides of the tongue every 30 seconds during a twominute period (MSKCC), or by chewing on a paraffin block (BCCA). Saliva was collected in a pre-weighed plastic cup, and patients were asked to spit into the cup after the triggering procedure. Xerostomia was defined as moderate to severe (≥Grade 4) according to the LENT SOMA tables [16] i.e. WMSFM ≤25% post-relative to pre-RT. The median time to WMSFM after completion of IMRT was 11 (range: 3–24) months in the MSKCC cohort. In the BCCA cohort WMSFM was conducted for all 66 patients at both 3 and 12 months after completion of IMRT.
Follow-up groups
Following previous findings on xerostomia fading in the range 3–12 months after RT [9, 10, 13, 14], we stratified patients in one short, and one long follow-up group. In the BCCA cohort, a straightforward split was possible given complete WMSFM data at both 3 (short follow-up) and 12 (long follow-up) months with an observed xerostomia rate of 47% (n=31), and 23% (n=15), respectively. The corresponding split, with a reasonable number of patients in both follow-up groups for the MSKCC cohort was less than 6 months (short follow-up; n=55) and 6–24 months (long follow-up; n=53) after completion of IMRT, and the median follow-up time was 3 (range: 1–5) months, and 11 (range: 6–24) months with an observed xerostomia rate of 38% (n=15), and 19% (n=10), respectively. Within the short follow-up group, six patients had a follow-up time of five months, and within the long follow-up group only three patients had a follow-up time≥18 months, and four a follow-up time between six and seven months.
Modeling approach
1. Candidate predictors (MSKCC cohort only)
Initially, candidate predictors in the MSKCC cohort were identified for each follow-up group by comparing the distribution of the available variables between patients with and without xerostomia using a Mann-Whitney U test with significance denoted at the two-sided 5% level. The contralateral and ipsilateral parotid glands were defined as the gland with the lower and the higher Dmean, respectively (population median of Dmeanipsi−Dmeancontra: 7.1 Gy across both cohorts and follow-up groups).
2. Variable selection, internal generalizability, and model performance (MSKCC cohort only)
All analyses were performed separately for the short and the long follow-up group. Each candidate predictor identified in 1. was investigated using the following logistic regression-based function (FLogreg)
| (Eq.1) |
Candidatex denotes a candidate predictor x, βCandidatex the related logistic regression coefficient, and β0 the intercept. Univariate logistic regression analysis (UVA) with bootstrap resampling (1000 sample populations with replacement; each population having the same size as the original dataset) was applied to evaluate internal generalizability of model parameters [17]. Predictive ability on UVA was suggested by a two-sided p-value ≤0.05 (assessed as the average p-value over the 1000 bootstrap populations). If multiple variables were suggested, these were subject to multivariate logistic regression analysis (MVA). An analogous bootstrap resampling approach as used in the UVA was applied also in the MVA, and an MVA model was considered a candidate model if it was selected in ≥10% (in at least 100 of the 1000 possible models). A backward-forward stepwise selection was applied in the MVA with the objective of minimizing the Akaike Information Criterion [18]. The performance of all estimated UVA and MVA models was assessed by the area under the receiver-operating characteristics curve (AUC), Spearman’s rank correlation coefficients (Rs), and p-values. Goodness-of-fit of the estimated relative to the observed rate of xerostomia was evaluated using the Hosmer-Lemeshow test (HL; 10 degrees of freedom) [19], and good agreement was considered if pHL was ≥0.05. The AUC, Rs, p-values, and pHL in the MSKCC cohort are all reported as the average ± SD over the 1000 populations.
3. External generalizability (MSKCC models applied to BCCA cohort)
After the final model(s) with the highest AUC had been identified in the MSKCC cohort (cf. 2. above), this model was explored in the BCCA cohort i.e. the logistic regression coefficients (including the intercept) estimated for the MSKCC cohort were applied to the corresponding variables in the BCCA cohort. Also here, model performance was assessed using AUC, Rs and p-values, and goodness-of-fit of the estimated relative to the observed rate of xerostomia was evaluated by the HL test (good agreement: pHL≥0.05). Since no re-fitting process was performed for the BCCA cohort, the AUC, Rs p-value, and pHL are each represented by one value within this cohort. All analyses were conducted in MATLAB v.R2016a.
Estimation of minimum Dmean thresholds
In the dose-response curves, which were all based on the variable-specific regression coefficients from the MSKCC cohort, the minimum Dmean threshold at which xerostomia was observed to evolve was identified. At this threshold the Dmean value, the estimated rate, and the relative risk (RR) were assessed. The RR and 95% confidence intervals (95%CIs) were calculated according to the following expressions:
| (Eq.2) |
| (Eq.3) |
NXero and NNon-xero are the number of patients experiencing, and not experiencing xerostomia, respectively; ≥Threshold, and <Threshold refer to if the Dmean was equal/larger or lower than that of the Dmean threshold, respectively, and SE is the standard error. Similarly, the estimated rate, the RR and its 95%CI for the two QUANTEC guidelines were assessed and compared to those of the identified Dmean thresholds.
Results
Identified candidate predictors
Five candidate predictors were identified for short-term xerostomia: Dmeancontra, Dmeanipsi, WMSFM pre-RT, and concurrent chemotherapy (all being higher/more frequent among patients with xerostomia; Table 1). Also, primary tumor site was a candidate predictor with tumors of unknown primary being present only among the xerostomia patients together with a slight increase in the number of patients treated for tonsil and nasopharyngeal cancer. For long-term xerostomia only Dmeancontra, and Dmeanipsi were candidate predictors. Neither of these candidate predictors was highly correlated with one another (Pearson’s correlation coefficient, |Pr|<0.68 [20]). The median |Pr| in the short follow-up group was 0.24 (range: 0.15–0.59) with the highest correlation observed between Dmeancontra, and Dmeanipsi, while |Pr| was 0.53 between Dmeancontra, and Dmeanipsi in the long follow-up group. The distribution of ΔWMSFM, Dmeancontra, and Dmeanipsi is demonstrated for each follow-up group and cohort in Figure S1.
Table 1.
Comparison of patient characteristics within the MSKCC and the BCCA cohort stratified by follow-up time, and by xerostomia status.
| Follow-up: Short | Follow-up: Short | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSKCC | BCCA | MSKCC | BCCA | |||||||||
| Xerostomia (n=15) | No Xerostomia (n=40) | Xerostomia (n=31) | No Xerostomia (n=35) | Xerostomia (n=10) | No Xerostomia (n=43) | Xerostomia (n=15) | No Xerostomia (n=51) | |||||
| Mean±SD | Mean±SD | p | Mean±SD | Mean±SD | p | Mean±SD | Mean±SD | p | Mean±SD | Mean±SD | p | |
| Age [y] | 56±7 | 57±10 | 0.89 | 56±12 | 56±13 | 0.85 | 53±9 | 58±10 | 0.20 | 61±11 | 55±13 | 0.21 |
| Dmeancontra [Gy] | 24.9±12.1 | 15.3±8.2 | 0.002* | 25.7±8.2 | 18.3±8.1 | 0.004* | 24.6±4.8 | 17.4±10.7 | 0.003* | 24.4±8.3 | 21.0±9.0 | 0.23 |
| Dmeanipsi [Gy] | 43.8±20.3 | 25.1±12.6 | <0.0001* | 42.1±12.4 | 27.4±13.1 | <0.0001* | 39.8±13.8 | 29.4±17.3 | 0.02* | 41.6±11.9 | 32.2±14.8 | 0.02* |
| WMSFM pre-RT [g/5 min] | 3.5±1.5 | 2.4±0.8 | 0.01* | 8.6±4.1 | 8.6±4.1 | 0.92 | 3.4±1.4 | 2.6±1.2 | 0.06 | 10.0±3.9 | 8.2±4.0 | 0.051 |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| Chemotherapy | 14 (93) | 25 (63) | 0.02* | 19 (61) | 15 (43) | 0.14 | 9 (90) | 26 (60) | 0.22 | 7 (47) | 27 (53) | 0.68 |
| N/A | 1 (7) | 4 (10) | - | - | - | 6 (14) | - | - | ||||
| Gender | 0.08 | 0.39 | 0.55 | 0.22 | ||||||||
| Female | 5 (33) | 5 (13) | 12 (39) | 10 (29) | 3 (30) | 9 (21) | 7 (47) | 15 (29) | ||||
| Male | 10 (67) | 34 (87) | 19 (61) | 25 (71) | 7 (70) | 34 (79) | 8 (53) | 36 (71) | ||||
| Histology | 0.36 | - | - | 0.18 | - | - | ||||||
| Myxofibrosarcoma | 1 (7) | - | - | - | - | 2 (5) | - | - | ||||
| NPC | 3 (20) | 5 (13) | - | - | 4 (40) | 5 (12) | - | - | ||||
| Papillary thyroid carcinoma | - | 1 (3) | - | - | - | - | - | - | ||||
| Salivary duct carcinoma | - | 1 (3) | - | - | - | 1 (2) | - | - | ||||
| SCC | 11 (73) | 33 (83) | - | - | 6 (60) | 35 (81) | - | - | ||||
| Involved neck RT | 11 (73) | 30 (75) | 0.79 | - | - | 7 (70) | 31 (72) | 0.82 | - | - | ||
| N-stage | 0.60 | - | - | - | 0.18 | - | - | - | ||||
| 0 | - | 10 (25) | - | - | 1 (10) | 11 (26) | - | - | ||||
| 1 | 7 (47) | 7 (18) | - | - | 1 (10) | 10 (23) | - | - | ||||
| 2 | 7 (47) | 20 (50) | - | - | 7 (70) | 20 (47) | - | - | ||||
| 3 | - | 1 (3) | - | - | - | 1 (2) | - | - | ||||
| N/A | 1 (7) | 1 (3) | - | - | 1 (10) | 1 (2) | - | - | ||||
| Surgery | 1 (7) | 12 (30) | 0.06 | 3 (10) | 7 (20) | 0.25 | 3 (30) | 11 (26) | 1.00 | 4 (27) | 6 (12) | 0.16 |
| N/A | 1 (7) | 4 (10) | - | - | - | 6 (14) | - | - | ||||
| T-stage | 0.17 | - | - | - | 0.63 | - | - | - | ||||
| 1 | 4 (27) | 15 (38) | - | - | 2 (20) | 18 (42) | - | - | ||||
| 2 | 4 (27) | 13 (33) | - | - | 5 (50) | 12 (28) | - | - | ||||
| 3 | 1 (7) | 4 (10) | - | - | 1 (10) | 4 (9) | - | - | ||||
| 4 | 2 (13) | 7 (18) | - | - | 1 (10) | 6 (14) | - | - | ||||
| N/A | 4 (27) | 1 (3) | - | - | 1 (10) | 3 (7) | - | - | ||||
| Tumor site | 0.03* | 0.81 | 0.19 | 0.24 | ||||||||
| Base of tongue | 2 (13) | 7 (18) | 4 (13) | 7 (20) | 2 (20) | 8 (19) | 1 (7) | 10 (20) | ||||
| Buccal mucosa | - | 2 (5) | - | - | 1 (10) | 1 (2) | - | - | ||||
| Floor of mouth | - | 1 (3) | - | - | - | 1 (2) | - | - | ||||
| Glottic | - | 4 (10) | - | - | - | 4 (9) | - | |||||
| Hypopharynx | - | - | - | 1 (3) | - | - | - | 1 (2) | ||||
| Larynx | - | 2 (5) | - | - | 1 (10) | 1 (2) | - | - | ||||
| Max sinus | - | 1 (3) | - | - | - | 1 (2) | - | - | ||||
| Nasal cavity | - | 1 (3) | - | 2 (6) | - | 1 (2) | - | 2 (4) | ||||
| Nasopharynx | 3 (20) | 5 (13) | 15 (48) | 10 (29) | 4 (40) | 5 (12) | 7 (47) | 18 (35) | ||||
| Oral cavity | - | - | 2 (6) | - | - | - | - | 2 (4) | ||||
| Oral tongue | 2 (13) | 5 (13) | - | - | 2 (20) | 5 (12) | - | - | ||||
| Oropharynx | - | - | 1 (3) | - | - | - | - | - | ||||
| Parotid | 1 (7) | 1 (3) | - | 1 (3) | - | 3 (7) | - | 1 (2) | ||||
| Submandibular gland | - | 1 (3) | - | - | - | 1 (2) | - | - | ||||
| Retromolar trigone | - | 1 (3) | - | - | - | - | - | - | ||||
| Thyroid | - | 1 (3) | - | 2 (6) | - | - | 1 (7) | 1 (2) | ||||
| Tonsil | 4 (27) | 8 (20) | 6 (19) | 5 (14) | - | 10 (43) | 3 (20) | 8 (16) | ||||
| Unkown primary | 3 (20) | - | 3 (10) | 7 (20) | - | 2 (5) | 3 (20) | 7 (14) | ||||
Note: The p-values come from a Mann-Whitney U test, and refer to the comparison between patients with and without xerostomia for the respective cohort and follow-up time. Only the variables denoted with * in the MSKCC cohort were subject to the outlined modeling approach.
The mean dose to both parotid glands predict short-term xerostomia
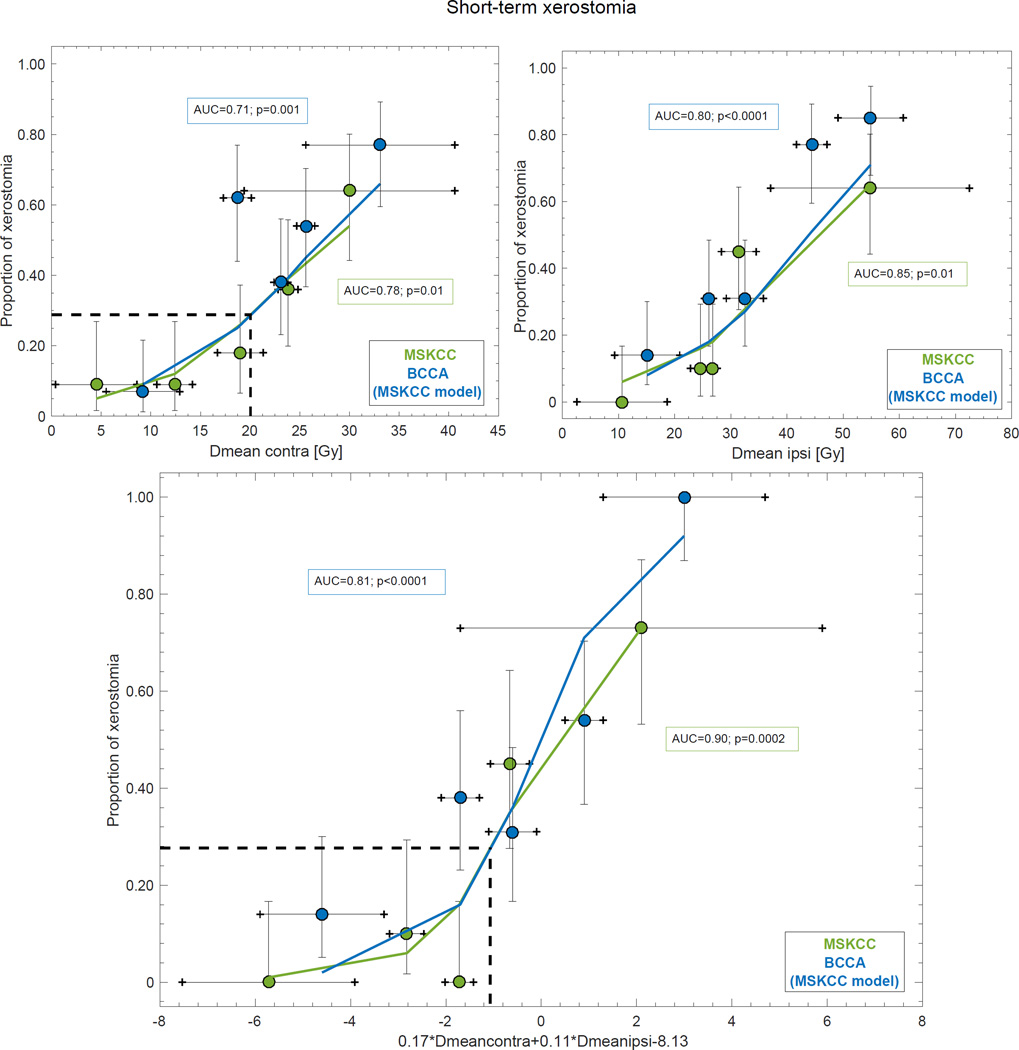
Within the internal generalizability, four of the five identified candidate predictors were found to explain short-term xerostomia in the MSKCC cohort: Dmeancontra (p=0.01±0.07) Dmeanipsi (p=0.01±0.04), use of concurrent chemotherapy (p=0.04±0.05), and WMSFM pre-RT (p=0.02±0.08) with the two dose-related variables presenting with the overall highest discriminative ability (Dmeancontra: AUC=0.78±0.07; Dmeanipsi: AUC=0.85±0.06; Table 2). The most frequently selected MVA model (Figure S2) with the highest AUC suggested that the combined contribution from Dmeancontra and Dmeanipsi best explained the dose-response relationship for xerostomia (AUC=0.90±0.05; Rs=0.66±0.12; p=0.0002±0.002), and pHL>0.05 (pHL=0.22±0.23) suggested good agreement between the estimated and the observed rate (Table 2). The difference between the most and the least risky quintile for the observed rate using this MVA model was 73%, while it was 55% for Dmeancontra and 64% for Dmeanipsi (Figure 1). Neither of the two identified candidate predictors in the long follow-up group explained longterm xerostomia (Dmeancontra: p=0.18±0.21; Dmeanipsi: p=0.25±0.27), and, consequently, no MVA models were generated for this follow-up group, nor was this follow-up group included for the analyses outlined below.
Table 2.
Variable selection results and model performance for the two follow-up times in the MSKCC cohort based on the candidate predictors in this cohort (cf. Table 1).
| MSKCC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up: Short | Follow-up: Long | ||||||||||
| Model | AUC | Rs | p | β | pHL | Model | AUC | Rs | p | β | pHL |
| Dmeancontra* | 0.78 (0.07) | 0.46 (0.12) | 0.01 (0.07) | 0.14 | 0.51 (0.23) | Dmeancontra | 0.80 (0.07) | 0.28 (0.18) | 0.18 (0.21) | 0.07 | 0.39 (0.19) |
| Dmeanipsi* | 0.85 (0.06) | 0.50 (0.13) | 0.01 (0.04) | 0.09 | 0.24 (0.11) | Dmeanipsi | 0.73 (0.09) | 0.24 (0.16) | 0.25 (0.27) | 0.03 | 0.43 (0.10) |
| WMSFM Pre-RT* | 0.71 (0.09) | 0.45 (0.13) | 0.02 (0.08) | 0.84 | 0.51 (0.12) | ||||||
| Tumor site | 0.69 (0.08) | 0.28 (0.13) | 0.13 (0.20) | 0.14 | 0.46 (0.13) | ||||||
| Chemotherapy* | 0.65 (0.04) | 0.33 (0.07) | 0.04 (0.05) | 135 | 0.19 (0.07) | ||||||
| Dmeancontra+Dmeanipsi** | 0.90 (0.05) | 0.66 (0.12) | 0.0002 (0.002) | 0.17/0.11 | 0.22 (0.23) | ||||||
| WMSFM Pre-RT+Dmeanipsi | 0.85 (0.06) | 0.64 (0.12) | 0.001 (0.01) | 0.85/0.09 | 0.45 (0.24) | ||||||
Note: The AUC, Rs, p, and pHL are given as the mean ± standard deviation (SD) over the 1000 bootstrap samples.
Qualified for MVA,
Most frequently selected model (38%).
Figure 1.
The UVA models for Dmeancontra (upper left), and Dmeanipsi (upper right), and the MVA model for Dmeancontra and Dmeanipsi (lower panel) for the MSKCC cohort (green) and as applied to the BCCA cohort (blue). Note: Solid lines denote the estimated xerostomia rate, the quintiles represent the observed xerostomia rate (x-axis: mean ± SD; y-axis: mean and 68% exact binomial confidence intervals), the dashed black lines are the estimated xerostomia rate at the QUANTEC guidelines, and the AUC and p-values (MSKCC: average over the 1000 resamples) are inserted next to the corresponding curve.
Dose-response relationships for short-term xerostomia are generalizable across cohorts
Only the MSKCC model parameters for Dmeancontra, and Dmeanipsi explained short-term xerostomia in the BCCA cohort (Dmeancontra: p=0.001; Dmeanipsi: p<0.0001; Table 3) with discriminative abilities of a comparable magnitude as in the MSKCC cohort (Dmeancontra: AUC=0.71; Dmeanipsi: AUC=0.80). Similarly, but less pronounced compared to the MSKCC cohort, the AUC increased for the MVA model that included both Dmeancontra and Dmeanipsi (AUC=0.81) as opposed to the corresponding UVA models. In general, the AUC values were slightly lower than those of the MSKCC cohort. The difference between the most and the least risky quintile for the observed rate stratified according to this MVA model in the BCCA cohort was 86%, while the corresponding difference for the two UVA models was 70% (Figure 1).
Table 3.
External validation of the final models suggested for the MSKCC cohort as applied to the BCCA cohort.
| BCCA | |||||
|---|---|---|---|---|---|
| Follow-up: Short | |||||
| Model | AUC | Rs | p | β | pHL |
| Dmeancontra | 0.71 | 0.40 | 0.001 | 0.14 | 0.48 |
| Dmeanipsi | 0.80 | 0.53 | <0.0001 | 0.09 | 0.67 |
| WMSFM Pre-RT | 0.50 | 0.01 | 0.96 | −0.003 | 0.22 |
| Chemotherapy | 0.59 | 0.18 | 0.14 | 0.75 | 0.22 |
| Dmeancontra+Dmeanipsi* | 0.81 | 0.53 | <0.0001 | 0.17/0.11 | 0.27 |
The MVA model including WMSFM Pre-RT+Dmeanipsi was not included since WMSFM Pre-RT presented with p>0.05 on UVA.
Identified mean dose thresholds for short-term xerostomia are lower than in the QUANTEC guidelines
The identified minimum threshold for Dmeancontra was 12.4 Gy/18.7 Gy in the MSKCC/BCCA cohort with related estimated xerostomia rates of 12%/25%, while the estimated rate of the one-gland QUANTEC guideline was 29% in both cohorts. In the MSKCC cohort, the RR was slightly lower for these thresholds compared to that of the QUANTEC guideline but the 95%CI was narrower for the former (1.44 (95%CI: 1.04–2.00) vs. 2.29 (95%CI: 1.40–3.74)). In the BCCA cohort, the 95%CI of the RR for the Dmeancontra threshold was reasonable, whereas that of the QUANTEC guideline was not (1.47 (95%CI: 1.06–2.00) vs. 1.38 (95%CI: 0.93–2.04)). Studying both glands, the Dmeancontra threshold was again considerably lower than that of the two-gland QUANTEC guideline in both cohorts (MSKCC/BCCA: 15.5 Gy/19.7 Gy), while the Dmeanipsi threshold was in a similar range (MSKCC/BCCA: 25.0 Gy/25.9 Gy) with corresponding estimated xerostomia rates of 6%/16% in the MSKCC/BCCA cohort compared to 27% for the QUANTEC guideline in both cohorts. The RR for the identified thresholds of the two glands was slightly lower than that of the two-gland QUANTEC guideline but presented with narrower 95%CIs (MSKCC: 1.65 (95%CI: 1.28–2.14) vs. 3.62 (95%CI: 1.97–6.66); BCCA: 1.47 (95%CI: 1.06–2.03) vs. 1.81 (95%CI: 1.18–2.77)). Incorporating the identified minimum Dmeancontra, or Dmeancontra and Dmeanipsi thresholds into (Eq.1), the corresponding expression for the FLogereg function (note: all regression coefficients come from the MSKCC models) was:
Discussion
Addressing both internal and external generalizability, our results indicate that objectively measured severe hyposalivation (xerostomia) after primary IMRT for HNC is in particular observed at a median of three months after completed treatment, and that the mean dose to both the contralateral and the ipsilateral parotid gland (Dmeancontra and Dmeanipsi) increases the risk of developing xerostomia within this follow-up time. Furthermore, fewer patients experienced long-term compared to short-term xerostomia (MSKCC: n=10 vs. 15; BCCA: n=15 vs. 31), and this could explain why no dose-response relationship was established for long-term xerostomia. A similar pattern has previously been observed in several studies including various assessment methods and RT techniques [9, 10, 13, 14]. This indicates an overall recovery of xerostomia, but the exact temporal recovery between the shorter and the longer follow-up time investigated here remains unresolved.
The salivary gland-specific QUANTEC summary advocated that keeping Dmeancontra below 20 Gy, or keeping both Dmeancontra and Dmeanipsi below 25 Gy should be the objective in HNC RT in order to minimize RT-induced xerostomia [8]. In one of the few dose-response focused studies on xerostomia following IMRT published thereafter, Lee et al [12] found that higher Dmeancontra, Dmeanipsi, and advanced age increased the risk of moderate to severe patient-reported xerostomia three months after completed treatment with a similar AUC as observed in this study (AUC=0.86), and that xerostomia at one year was explained by increased Dmeancontra, Dmeanipsi, low educational level, smoking, and decreased T-stage. Lee et al, however, neither addressed inter-variable correlation, nor conducted UVA. Also, educational level and T-stage were each represented by four presumably highly correlated sub variables, and the logistic regression coefficients for T-stage were negative. It is, thus, not surprising that their results, in particular for long-term xerostomia, differ from ours. In another study that to some extent addressed highly correlated variables, and conducted UVA, Dmeancontra and baseline xerostomia were found to predict moderate to severe patient-reported xerostomia six months after IMRT [21] with an AUC of 0.68. The AUC of our UVA model for Dmeancontra in the MSKCC cohort was considerably higher (AUC=0.78±0.07), while in a comparable range in the BCCA cohort (AUC=0.71). Baseline xerostomia rate was an individual predictor for short-term xerostomia in the MSKCC cohort but was not on MVA. In addition, our xerostomia definition was normalized to that of pre-IMRT and by such baseline xerostomia was directly incorporated in all analyses. In both of our cohorts, the AUC for the final MVA models including Dmeancontra and Dmeanipsi was higher (MSKCC: AUC=0.90±0.05; BCCA: AUC=0.81) than that of the corresponding UVA models. Therefore, focusing on Dmean to both individual glands should be the goal in order to inhibit the development of xerostomia after IMRT. Although we recognize potential differences between our cohorts and that of Beetz et al, it is yet somewhat surprising to note that while the majority (86%) of their patients received bilateral neck irradiation [21], and since contralateral sparing techniques is assumed to result in higher Dmeanipsi and increased risk of xerostomia compared to ipsilateral sparing techniques [5, 14], Dmeanipsi was not present in their final MVA model.
Even though the administration of chemotherapy, gender, and WMSFM pre-RT were significantly different between the two included cohorts (Table S1), our final MVA model did not indicate that any of these variables predicted xerostomia. Furthermore, the rate of xerostomia was nine and four percentage points lower in the MSKCC cohort than in the BCCA cohort for short- and long-term xerostomia, respectively, and we do acknowledge that these differences could to some extent also be explained by the slightly different follow-up time splits across the two cohorts, and/or that gustatory stimulation was performed using a citrate solution in the MSKCC cohort and by chewing on a paraffin block in the BCCA cohort. All these aspects together with the MSKCC patients presenting with significantly lower Dmeancontra could explain the observed differences in discriminative ability of the UVA models for Dmeancontra across the two cohorts (MSKCC/BCCA: AUC=0.78/0.71), but the AUC for the BCCA cohort was yet within the standard deviation of the AUC for the MSKCC cohort (0.07). We acknowledge that all potentially predisposing variables could not be accounted for. Furthermore, intra-gland response variability was not addressed, and these aspects may be important forthcoming topics to increase understanding of the dose-response relationship for xerostomia following IMRT [6, 22–24]. This together with the discussion above may justify the overall slightly lower AUC values being observed for the BCCA cohort. In a subsequent effort we performed predictive modeling within the BCCA cohort based on the candidate predictors identified for this cohort (* for BCCA in Table 1). Interestingly, the generated models for short-term xerostomia presented with coefficients and a discriminative ability of a similar magnitude as that of applying the MSKCC model parameters in the BCCA cohort, and the most frequently selected MVA model with the highest discriminative ability included also here Dmeancontra and Dmeanipsi (Table S2). For long-term xerostomia, the only initial candidate predictor (Dmeanipsi) did not describe the observed rate of xerostomia. This suggests that the models identified for short-term xerostomia in the MSKCC cohort are generalizable in a cohort of patients presenting with somewhat differently distributed patient characteristics but treated with a similar RT technique.
Beetz et al [22] previously found that the AUC of their 3DCRT-developed MVA model (age, baseline xerostomia, and the average of Dmeancontra and Dmeanipsi; all with positive regression coefficients) to predict patient-reported xerostomia six months after treatment dropped from 0.82 to 0.66 when applied to an IMRT cohort of a similar size while an AUC of 0.77 was expected taking into account case-mix differences. The observed lack of generalizability across the two RT techniques could be due to both Dmeancontra and Dmeanipsi being significantly lower in the IMRT arm than in the 3DCRT arm resulting in lower rates of xerostomia [5]. However, Beetz et al [21] did not observe a drop in xerostomia rates after IMRT compared to after 3DCRT (51% vs. 52%) potentially since a significantly higher fraction of the patients in the IMRT arm received bilateral neck irradiation, but they did find that both Dmeancontra and Dmeanipsi were significantly different (higher/lower not indicated) across the two RT techniques, and were further less correlated in the IMRT-compared to in the 3DCRT cohort. It was, thus, concluded that Dmeancontra and Dmeanipsi should be included as separate candidate predictors [22]. To this end, and considering that no dose-response focused study has included Dmeancontra and Dmeanipsi as separate candidate predictors, the generalizability of our MVA model for xerostomia following other RT techniques than IMRT thus remains unclear.
For short-term xerostomia in our cohorts, both QUANTEC guidelines applied to the MSKCC cohort, whereas only the two-gland guideline applied to the BCCA cohort as judged by the fairly narrow 95% CIs of the RR. To the best of our knowledge, the applicability of the QUANTEC guidelines following IMRT have only been studied by Lee et al where the one-gland guideline was examined [13]. At their three months follow-up, the negative predicted value (NPV) i.e., the avoidance rate of moderate to severe patient-reported xerostomia when the one-gland QUANTEC guideline was fulfilled was 83%. In our data, the NPV of the one-gland guideline was of a similar magnitude (MSKCC/BCCA: 81%/79%), while slightly higher for the two-gland guideline (MSKCC/BCCA: 86%/84%). Furthermore, the estimated rate at the one-gland guideline was 18% in [13], and thus considerably lower than that estimated in our study (one/two-gland: 29%/27%). More importantly, however, our results indicated that short-term xerostomia after IMRT starts evolving at much lower Dmeancontra values than suggested by QUANTEC. Therefore, if focusing on either contralateral- or ipsilateral parotid gland sparing, we suggest decreasing Dmeancontra to below 20 Gy, and keeping Dmeanipsi according to the recommended QUANTEC level of around 25 Gy. Reducing Dmeancontra to as low as 12.4 Gy, as suggested by our lowest Dmeancontra threshold in the MSKCC cohort, may become clinically impractical as any sparing procedure should foremost not deteriorate the objective of delivering the prescribed tumor dose.
Supplementary Material
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62:400–422. doi: 10.3322/caac.21157. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175–183. doi: 10.1016/S1470-2045(06)70580-0. [DOI] [PubMed] [Google Scholar]
- 4.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 5.Nutting CM, Morden KP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vissink A, van Luijk P, Langdendijk JA, Coppes RP. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Dis. 2015;21:1–10. doi: 10.1111/odi.12222. [DOI] [PubMed] [Google Scholar]
- 7.Dawes C, Wood CM. The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch Oral Biol. 1973;18:337–342. doi: 10.1016/0003-9969(73)90156-8. [DOI] [PubMed] [Google Scholar]
- 8.Deasy JO, Moiseenko V, Marks L, Clifford Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(Suppl 3):58–63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moiseenko V, Wu J, Hovan A, Saleh Z, et al. Treatment planning constraints to avoid xerostomia in head-and-neck radiotherapy: an independent test of the QUANTEC criteria using a prospectively collected dataset. Int J Radiat Oncol Biol Phys. 2012;82:1108–1114. doi: 10.1016/j.ijrobp.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beetz I, Steenbakkers RJHM, Chouvalova O, et al. The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol. 2014;53:597–604. doi: 10.3109/0284186X.2013.831186. [DOI] [PubMed] [Google Scholar]
- 11.Coppes RP, Zeilstra LJ, Kampinga HH, Konings AW. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor antagonists. Br J Cancer. 2001;85:1055–1063. doi: 10.1054/bjoc.2001.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee TF, Chao PJ, Ting HM, et al. Using multivariate regression model with least absolute shrinkage and selection operator (LASSO) to predict the incidence of xerostomia after intensity-modulated radiotherapy for head and neck cancer. PLOS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0089700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TF, Fang FM. Quantitative analysis of normal tissue effects in the clinic (QUANTEC) guideline validation using quality of life questionnaire datasets for parotid gland constraints to avoid causing xerostomia during head-and-neck radiotherapy. Radiother Oncol. 2013;106:352–358. doi: 10.1016/j.radonc.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Miah AB, Gulliford SL, Morden J, et al. Recovery of salivary function: Contralateral parotid-sparing intensity-modulated radiotherapy versus bilateral superficial lobe parotid-sparing intensity-modulated radiotherapy. Clin Oncol. 2016 Mar 17; doi: 10.1016/j.clon.2016.02.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark H, Hovan A, Moiseenko V, Thomas S, Wu J, Reinsberg S. Regional radiation dose susceptibility within the parotid gland. Med Phys. 2015;42:2064–2071. doi: 10.1118/1.4915077. [DOI] [PubMed] [Google Scholar]
- 16.LENT SOMA tables. Radiother Oncol. 1995;35:17–60. [PubMed] [Google Scholar]
- 17.Deasy JO, Chao KS, Markman J. Uncertainties in model-based outcome predictions for treatment planning. Int J Radiat Oncol Biol Phys. 2001;51:1389–1399. doi: 10.1016/s0360-3016(01)02659-1. [DOI] [PubMed] [Google Scholar]
- 18.Collett D. Modeling binary data. 2nd. NY: Chapman & Hall; 2002. [Google Scholar]
- 19.Hosmer DW, Lemeshow S. A goodness-of-fit test for the multiple logistic regression model. Commun in Stats. 1980;10:1043–1069. [Google Scholar]
- 20.Taylor R. Interpretation of the correlation coefficient. A Basic Review. J Diag Med Sonog. 1990;1:35–39. [Google Scholar]
- 21.Beetz I, Schilstra C, van der Schaaf A, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: The role of dosimetric and clinical factors. Radiother Oncol. 2012;105:101–106. doi: 10.1016/j.radonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Beetz I, Schilstra C, van Luijk P, et al. External validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapy. Radiother Oncol. 2012;105:94–100. doi: 10.1016/j.radonc.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 23.van Luijk P, Pringle S, Deasy JO, et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Cancer. 2015;7:1–8. doi: 10.1126/scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buettner F, Miah AB, Gulliford SL, et al. Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol. 2012;103:82–87. doi: 10.1016/j.radonc.2012.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.