Abstract
Background
Hepatic steatosis (HS) is common in persons with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections, but the independent contributions of HCV and HIV to HS is unclear.
Methods
Magnetic resonance imaging and spectroscopy were used to measure visceral adipose tissue (VAT) and liver fat fraction (LFF) [total lipids/(total lipids+water)] in 356 adults: 57 HCV-monoinfected, 70 HIV/HCV-coinfected, 122 HIV-monoinfected, and 107 HIV/HCV-uninfected. Genotype 3 HCV-infected participants were excluded due to the genotype's reported steatogenic effects. For prevalence estimates, HS was defined as LFF≥0.05. We estimated the association of HIV and HCV status with LFF using multivariable linear regression, adjusting for demographic, lifestyle, and metabolic factors including the homeostasis model assessment estimate of insulin resistance (HOMA-IR) and liver fibrosis defined using the AST-to-platelet ratio index (APRI).
Results
The prevalence of HS was highest in the uninfected(33%) and HIV-monoinfected(28%), followed by the HCV-monoinfected(19%) and HIV/HCV-coinfected(11%)(p=0.003 across groups). Compared to uninfected participants, after adjusting for demographic, lifestyle, and metabolic factors, HIV monoinfection, HCV monoinfection and HIV/HCV coinfection were associated with 19%(95%CI:-39%,6%), 38%(95%CI:-55%,-12%) and 42%(95%CI:-59%,-18%) lower LFF, respectively. HCV monoinfection and HIV/HCV coinfection remained strongly associated with lower LFF after further adjusting for APRI, and results were unchanged after excluding subjects with suspected cirrhosis. Among the entire cohort, Hispanic ethnicity, male sex, VAT, and HOMA-IR were independently associated with greater LFF.
Conclusions
Contrary to expectations, HIV/HCV-coinfected and HCV-monoinfected adults had significantly less liver fat than uninfected adults, even after adjusting for demographic, lifestyle, metabolic factors, and hepatic fibrosis. Our findings suggest that non-genotype 3 HCV infection may be protective against HS. The mechanisms by which this occurs and impact of HCV treatment on HS need investigation.
Keywords: HCV, fatty liver disease, NAFLD, MRS, liver
Introduction
Hepatic steatosis (HS) is common in individuals chronically infected with hepatitis C virus (HCV) and is independently associated with increased fibrosis progression (1-5) and development of hepatocellular carcinoma (6-8). The reported prevalence of histologic HS ranges from 35% to 81% in HCV-monoinfected persons and 23% to 72% in HIV/HCV-coinfected persons (5, 9). The high frequency of HS on liver biopsy in patients with HCV, in comparison to the estimated 20%-30% prevalence of HS in the general population (10, 11), suggests that HCV-infected patients have an elevated risk of HS (12).
There are several postulated mechanisms by which HCV may lead to HS, including increased intrahepatic triglyceride accumulation (due to viral inhibition of very-low density lipoprotein assembly and secretion), decreased fatty acid oxidation, and increased de novo lipogenesis (13, 14). Indeed, several studies provide compelling evidence for a direct steatogenic effect of HCV genotype 3 (15-17). However, whether non-genotype 3 HCV affects the risk of HS is unclear, and HS in this group appears to be primarily mediated by metabolic factors (9, 18).
It is difficult to directly compare HS rates from studies conducted among HCV-infected patients to those conducted in HCV-uninfected populations. Variability in the frequency of metabolic comorbidities and other HS risk factors across studies may explain in part the wide range of HS prevalence reported in the literature. In addition, most studies in HCV patients rely on liver biopsy, whereas estimates of HS in the general population are based on noninvasive imaging or serum markers. Only a minority of HCV patients will undergo liver biopsy, and HS is associated with advanced fibrosis in HCV. Therefore, HS may be overestimated in biopsy series if HCV patients with suspected advanced disease are more likely to have a biopsy performed.
Hepatic steatosis is also common in HIV-infected individuals, with a reported prevalence of 13% to 73% depending on the population studied and the imaging technique used for diagnosis (19-25). Like HCV, HIV has been postulated to induce HS, either directly through chronic immune activation, increased lipogenesis, and alterations in insulin signaling, or indirectly via metabolic abnormalities and antiretroviral therapy-induced steatosis(26). Although it has been suggested that HIV independently increases the risk of HS, a report from the Multicenter AIDS Cohort Study found a lower risk of CT-measured HS in HIV-infected compared with HIV-uninfected men (23). However, this study was unable to evaluate the role of HCV infection, and it did not include women.
The objective of our study was to determine whether HIV and HCV infections are independently associated with HS (measured using magnetic resonance spectroscopy [MRS], which is considered the preferred reference standard for non-invasive measurement of liver fat (27)) in a representative cohort of men and women and to identify associated risk factors for HS in this population.
Materials and Methods
Study Design and Patients
Between December 2003 and July 2015, 363 adults with and without HIV and HCV infections underwent MRS to measure liver fat fraction (LFF). One-hundred and thirty nine women were recruited from the Northern California site of the Women's Interagency HIV Study (WIHS) as part of the MRS Steatosis Substudy. Details of WIHS recruitment, characteristics, and study design have been described elsewhere (28). Participants of the Study of Visceral Adiposity, HIV, and HCV: Biologic Mediators of Hepatic Steatosis (VAHH Study), which enrolled men and women without evidence of clinic diabetes and with/without HIV and HCV from clinics and recruitment flyers posted at the San Francisco Veterans Affairs Medical Center, were also included in the study. Of the 224 VAHH and 139 WIHS participants, 356 had MRS data available and were included in the study. Potential WIHS MRS Steatosis Substudy and VAHH participants were excluded if they were pregnant or 3-months post-partum (women only), received medications associated with steatosis including corticosteroids, amiodarone, methotrexate, tamoxifen, and estrogen, were receiving anti-HCV therapy, had detectable hepatitis B surface antigen, had a body mass index (BMI) >35 kg/m2, weighed >300 pounds, had metal objects in their bodies, had evidence of severe renal insufficiency (defined as estimated glomerular filtration rate <30 ml/minute), had evidence of decompensated cirrhosis, or had evidence of chronic liver disease due to primary biliary cirrhosis, hemochromatosis, or autoimmune hepatitis. For this study, 7 participants with genotype 3 HCV were excluded because of the known steatogenic effects of this genotype. There were 10 subjects with unknown genotype (2 HCV-monoinfected and 8 HIV/HCV-coinfected), and they were considered to have non-genotype 3 infection. The study was approved by an Institutional Review Board, and all participants signed informed consent.
Hepatic Steatosis and Adipose Tissue Measurements
From December 2003 through February 2010, MR imaging was conducted on a 1.5 Tesla (T) whole body clinical scanner (General Electric Healthcare, Waukesha, WI). Beginning in March 2010, MR imaging was performed on a 3T whole body scanner (General Electric Healthcare, Waukesha, WI). Data collected from the 1.5T scanner were calibrated to values collected from the 3T scanner using in-house values correcting for field-dependent relaxation times. MRS was acquired from an 8cc voxel similarly to prior reports, with a 128 (1.5T) or 64 (3T) acquisition time series of spectra(29). Spectra were automatically phase, frequency, motion and T2 relaxation time corrected (30-32). Quality was visually confirmed by an MR spectroscopist with over 20 years' experience. We calculated LFF from the corrected MRS measures of CH2 and CH3 lipids and of water as the total lipids / (total lipids + water). The LFF was then multiplied by 100 and expressed as a percent.
Visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) volumes were semi-automatically generated based on either a water suppressed gradient dual echo or an Iterative Decomposition and Echo Asymmetry with Least-squares (IDEAL) estimation sequence (breathheld, slice thickness=10 mm). Slices were located at the discs between lumbar vertebrae L2-3, L3-4, and L4-5. VAT and SAT image masks were manually created by an experienced user, followed by a semi-automatic threshold-based contour-mapping algorithm for total VAT and SAT area. Slices were averaged to generate a total VAT and abdominal SAT volume per patient.
Covariates
HIV infection was defined by documentation of a positive HIV enzyme immunoassay confirmed with western blot, and chronic HCV infection was defined as serum HCV antibody and HCV RNA positive. Alcohol consumption was self-reported and was categorized as: none; light (>0-7 drinks/week); moderate (7-12 drinks/week); or heavy (>12 drinks/week). Additional candidate covariates, including race, ethnicity, history of injection drug use, smoking, and marijuana use were obtained through self-report. Race was categorized as White (including White Hispanic), African American (including African American Hispanic), or other, and ethnicity was categorized as Hispanic or non-Hispanic. Diabetes was defined as any one of the following: ever use of a diabetic medication, a fasting glucose ≥126, or hemoglobin A1c ≥6.5%. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using 8-hour fasting insulin and glucose values. Liver fibrosis was estimated using the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), which incorporates AST and platelets, and the FIB-4 score, which is calculated using the variables age, AST, platelets, and alanine aminotransferase (ALT)(33, 34). Probable cirrhosis was defined as APRI>2 or FIB-4>3.25. For HS prevalence estimates, total lipids / (total lipids + water) ≥ 0.05 was considered HS(29).
Statistical Analysis
We compared demographic and clinical characteristics by disease category (HIV-monoinfected, HCV-monoinfected, HIV/HCV-coinfected, and uninfected controls) using an ANOVA model or Kruskal-Wallis test for continuous variables and the chi-squared test or Fisher's exact test for categorical variables, where appropriate. We compared levels of LFF by disease category using least squared means and their confidence intervals, after adjusting for age, race, ethnicity, and BMI. Because it is possible that MRS might underestimate the LFF in the setting of fibrosis (35), spearman correlations were used to examine the association between APRI and the LFF, stratified by infection category. We used both unadjusted and multivariable adjusted linear regression models to compare differences in LFF by disease category, while controlling for demographic, lifestyle, and metabolic factors, as well as APRI, FIB-4, or suspected cirrhosis. We also performed a sensitivity analysis excluding subjects with suspected cirrhosis. Finally, we performed additional analyses to identify factors associated with LFF stratified in the entire cohort and by HIV and chronic HCV status, adjusting for HIV-related factors and HCV-related factors as appropriate.
Robust regression model with Huber's M-estimation was used to alleviate the impact of influential cases. LFF was found to have a right-skewed distribution and therefore was log-transformed to normalize its distribution. The regression coefficients and their confidence intervals were exponentiated to calculate percentage differences attributable to each risk factor. In models with missing cases, multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 20 repetitions. All analyses were performed using SAS system, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Characteristics of participants
A total of 356 participants were included: 122 HIV-monoinfected, 57 HCV-monoinfected, 70 HIV/HCV-coinfected, and 107 with neither infection. There were several notable demographic and metabolic differences comparing participants by HIV and HCV status (Table 1). The HCV-monoinfected and the HIV/HCV-uninfected groups were predominantly male, whereas the HIV/HCV-coinfected group was majority female. The HCV-infected participants were older and were more likely to have a history of injection drug use. The HCV-monoinfected and the HIV/HCV-uninfected groups were more likely to drink alcohol heavily than the HIV-infected participants. Most of the HIV/HCV-coinfected and the HIV/HCV-uninfected were African American or Hispanic, whereas the majority of the HIV-monoinfected and HCV-monoinfected groups were white. The HIV/HCV-coinfected participants also had the highest HOMA-IR values and were more likely to be diagnosed with diabetes. However, they had on average the lowest BMI, least amount of VAT, and lowest LDL levels. As expected, the HCV-infected participants had the highest APRI and FIB-4 scores and were more likely to have cirrhosis.
Table 1. Demographic and Clinical Characteristics of Study Population by HIV and HCV status.
| Median (IQR) or % | HIV-mono (n=122) |
HCV-mono (n=57) |
HIV/HCV (n=70) |
Uninfected (n=107) |
p-value |
|---|---|---|---|---|---|
| Demographics | |||||
|
| |||||
| Age | 51 (47, 57) | 57 (54, 60) | 56 (51, 59) | 52 (41, 56) | <.001 |
| Male | 53% | 84% | 39% | 73% | <.001 |
| Hispanic | 39% | 21% | 57% | 29% | <.001 |
| Race*: African American | 35% | 37% | 59% | 55% | 0.041 |
| White | 53% | 53% | 29% | 39% | |
| Other | 11% | 11% | 13% | 6% | |
|
| |||||
| Lifestyle | |||||
|
| |||||
| Alcohol: | |||||
| None | 34% | 37% | 44% | 38% | 0.011 |
| Light | 52% | 39% | 47% | 32% | |
| Moderate | 7% | 5% | 21% | 7% | |
| Heavy | 7% | 18% | 4% | 23% | |
| Current smoker | 30% | 44% | 56% | 44% | 0.071 |
| Current marijuana use | 33% | 42% | 43% | 31% | 0.097 |
| Injection drug use, ever | 13% | 63% | 63% | 11% | <.001 |
|
| |||||
| Metabolic | |||||
|
| |||||
| BMI (kg/m2) | 26 (24, 30) | 27 (23, 31) | 24 (22, 29) | 29 (25, 33) | 0.001 |
| Waist Circumference (cm) | 93 (87, 103) | 100 (84, 110) | 88 (82, 101) | 99 (88, 111) | 0.001 |
| VAT (cm3) | 167 (112, 246) | 174 (113, 259) | 114 (90, 164) | 172 (113, 232) | 0.003 |
| Abd SAT (cm3) | 228 (138, 342) | 211 (128, 349) | 211 (125, 294) | 242 (159, 388) | 0.194 |
| Leg fat (kg) | 6.0 (3.5, 9.6) | 6.4 (4.1, 9.9) | 5.6 (3.5, 9.2) | 7.3 (4.7, 10.1) | 0.100 |
| HOMA-IR | 1.85 (0.94, 3.33) | 1.59 (1.09, 3.20) | 3.07 (1.71, 4.61) | 1.64 (0.95, 2.81) | 0.003 |
| Diabetes mellitus | 8.2% | 7% | 27% | 9.3% | 0.0003 |
| LDL (mg/dL) | 105 (86, 126) | 93 (69, 116) | 79 (63, 104) | 102 (87, 127) | <.001 |
| TG (mg/dL) | 123 (85, 187) | 90 (67, 120) | 101 (77, 154) | 94 (68, 146) | 0.001 |
|
| |||||
| Liver-related | |||||
|
| |||||
| ALT (U/L) | 21 (15, 29) | 47 (28, 77) | 34 (25, 54) | 19 (15, 25) | <.001 |
| AST (U/L) | 22 (18, 27) | 44 (27, 66) | 41 (32, 59) | 21 (18, 25) | <.001 |
| Albumin (g/dL) | 4.4 (4.2, 4.6) | 4.2 (4.0, 4.4) | 4.1 (3.5, 4.3) | 4.4 (4.2, 4.6) | <.001 |
| Platelet (109/L) | 224 (191, 260) | 197 (171, 234) | 190 (151, 253) | 218 (187, 254) | 0.001 |
| APRI | 0.30 (0.22, 0.41) | 0.75 (0.37, 1.29) | 0.66 (0.44, 1.10) | 0.31 (0.23, 0.40) | <.001 |
| FIB4 | 1.11 (0.87, 1.53) | 1.72 (1.11, 2.70) | 2.09 (1.21, 3.02) | 1.05 (0.82, 1.50) | <.001 |
| Cirrhosis | 2.5% | 18% | 24% | 0.9% | <.001 |
|
| |||||
| HIV-related | |||||
|
| |||||
| Undetectable HIV RNA | 74% | 54% | 0.006 | ||
| CD4 current (cells/mm3) | 579 (418, 802) | 466 (289, 664) | 0.001 | ||
| CD4 nadir(cells/mm3) | 251 (89, 381) | 211 (146, 298) | 0.666 | ||
| ART use (years) | 7.9 (3.3, 12.5) | 7.4 (3.5, 12.5) | 0.604 | ||
| Current HAART | 78% | 83% | 0.305 | ||
| History of clinical AIDS | 37% | 56% | 0.011 | ||
|
| |||||
| HCV-related | |||||
|
| |||||
| HCV genotype 1 | 76%** | 90%*** | 0.030 | ||
| HCV RNA (IU/mL) | 1,064,968 | 1,776,540 | 0.214 | ||
| Treatment experienced | 20% | 26% | 0.785 | ||
Abbreviations: BMI=body mass index; VAT=visceral adipose tissue; Abd SAT=abdominal subcutaneous adipose tissue; HOMA-IR=homeostatic model assessment of insulin resistance; LDL=low-density lipoprotein cholesterol; TG=triglycerides; APRI=aspartate aminotransferase-to-platelet ratio index; ART=antiretroviral therapy; HAART=highly active antiretroviral therapy; AIDS=acquired immune deficiency syndrome
Categories include Hispanics and non-Hispanics
10 genotype 2, 3 genotype 4, missing for 2 participants
4 genotype 2, 2 genotype 4, missing for 8 participants
Among the 127 HCV-infected participants (with and without HIV), most were infected with genotype 1; 14 had genotype 2 and 5 had genotype 4. The majority of the HIV-infected group was on highly active antiretroviral therapy (HAART) (78% of the HIV-monoinfected and 83% of the HIV/HCV-coinfected). Compared to the HIV/HCV-coinfected group, the HIV-monoinfected group had a higher proportion with undetectable HIV RNA (74% versus 54%, p=0.006), higher CD4 counts (median 579 versus 466, p=0.001), and a lower proportion with a history of clinical AIDS (37% versus 56%, p=0.011)
Hepatic steatosis by HIV and HCV status
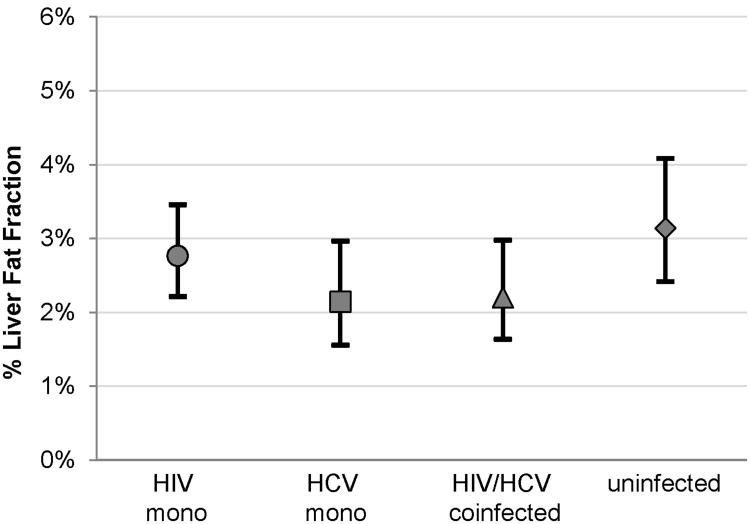
The prevalence of HS was highest in the HIV/HCV-uninfected (33%) and HIV-monoinfected (28%) groups, followed by the HCV-monoinfected (19%) and HIV/HCV-coinfected (11%) groups (p=0.003 across groups). After adjusting for age, race, ethnicity, and BMI, the mean LFF was also highest in the HIV/HCV-uninfected [3.1%; 95% confidence interval (CI) 2.4% to 4.1%], followed by the HIV-monoinfected (2.8%; 95% CI 2.2% to 3.5%), HIV/HCV-coinfected (2.2%; 95% CI 1.6% to 3.0%), and HCV-monoinfected (2.1%; 95% CI 1.6% to 3.0%) groups (Figure 1).
After multivariable adjustment for demographic, lifestyle, and metabolic factors, the HIV-monoinfected participants had 19% lower LFF than the HIV/HCV-uninfected, but the difference was not statistically significant (95% CI -39% to 6%, p=0.12) (Table 2). In contrast, even after adjusting for these factors, HCV-monoinfected and HIV/HCV-coinfected participants had 38% (95% CI -55% to -12%, p=0.006) and 42% (95% CI -59% to -18%, p=0.002) lower LFF, respectively compared to the HIV/HCV-uninfected.
Table 2. Association of HIV and HCV with liver fat fraction, as compared to HIV- and HCV-uninfected controls.
| HIV mono | HCV mono | HIV/HCV | R-square | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| % Estimate (95% CI) | p-value | % Estimate (CI) | p-value | % Estimate (CI) | p-value | ||
| Adjusted for demographics* | -13% (-31%, 10%) | 0.24 | -28% (-46%, -3.7%) | 0.027 | -35% (-51%, -13%) | 0.004 | 8.7% |
| Adjusted for demographics, lifestyle**, & metabolic factors*** | -19% (-39%, 6%) | 0.12 | -38% (-55%, -12%) | 0.006 | -42% (-59%, -18%) | 0.002 | 38% |
| Adjusted for demographics, lifestyle, metabolic factors & APRI | -19% (-39%, 6%) | 0.12 | -48% (-64%, -26%) | <.001 | -54% (-69%, -32%) | <.001 | 39% |
|
| |||||||
| Adjusted for demographics, lifestyle, metabolic factors, excluding cirrhosis | -17% (-37%, 10%) | 0.19 | -36% (-55%, -9%) | 0.012 | -46% (-64%, -20%) | 0.002 | 41% |
Abbreviations: CI=confidence interval; APRI=aspartate aminotransferase-to-platelet ratio index
HIV and HCV disease status, age, gender, race, and Hispanic ethnicity
Current smoking and alcohol use
Visceral adipose tissue (VAT), homeostatic model assessment of insulin resistance (HOMA-IR)
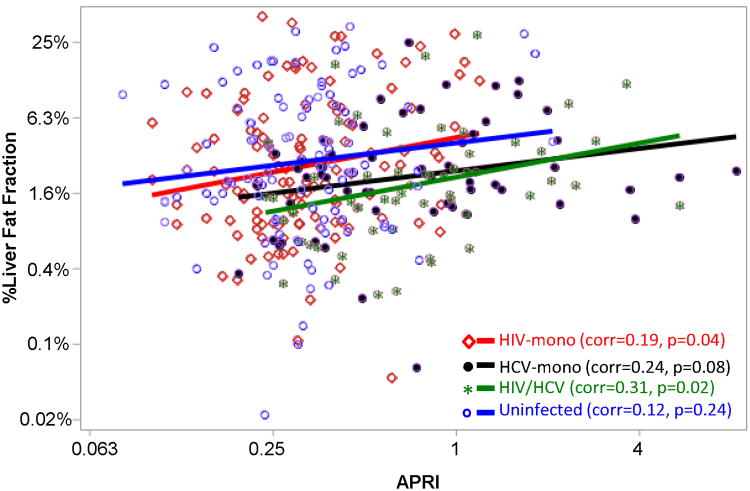
Since MRS might underestimate the LFF in the setting of fibrosis (35), we evaluated the correlation of liver fibrosis (as measured by APRI) with the LFF, stratified by infection category. We found that in each group, increasing LFF was associated with increasing APRI (Figure 2). Notably, even though the HCV-infected participants had the highest APRI values, at any given APRI, the average LFF was lowest in the HCV-infected groups. This is reinforced in our multivariable models— after adjusting for APRI, the association of HCV with lower LFF was strengthened (Table 2). We also performed analyses in which APRI, FIB4, and cirrhosis were tested in the multivariable models, and the results remained the same: HIV monoinfection was associated with lower LFF but this was not statistically significant, while HCV monoinfection and HIV/HCV coinfection were independently associated with significantly lower LFF (APRI results shown in Table 2). Finally, we performed a sensitivity analysis excluding individuals with suspected cirrhosis, and the multivariable results were unchanged (Table 2).
Factors associated with steatosis
Because HIV and HCV infected persons had lower LFF, even after multivariable adjustment, we performed additional analyses to identify factors associated with LFF in the entire cohort and stratified by infection status. Among the entire cohort and after excluding participants with suspected cirrhosis, Hispanic ethnicity, male gender, VAT, and HOMA-IR were each independently associated with higher LFF: 32% higher for Hispanics (95% CI 0% to 74%, p=0.05), 41% higher for men (95% CI 5% to 89%, p=0.024), 83% higher per doubling of VAT (95% CI 58% to 111%, p<0.001), and 34% higher per doubling of HOMA-IR (95% CI 22% to 47%, p<0.001).
Among HIV-infected persons, several factors were associated with higher LFF in unadjusted analysis: male gender, BMI, waist circumference, VAT, abdominal SAT, HOMA-IR, ALT, and albumin (Table 3). By contrast, factors associated with lower LFF in unadjusted analysis included HIV/HCV coinfection, African American race, cigarette smoking, and current marijuana use. On multivariable analysis, VAT remained significantly associated with higher LFF (53% per doubling; 95% CI 31% to 79%, p<0.001), as did HOMA-IR (17% per doubling; 95% CI 7% to 29%, p=0.001), and serum albumin (5% per 0.1 g/dL increase; 95% CI 1% to 8%, p=0.005), while report of current marijuana use was associated with lower LFF (-20%; 95% CI -36% to -1%, p=0.039). Although HIV-related factors, including HIV RNA level, CD4 count, current HAART, history of AIDS, and years on ART, showed little association with LFF on unadjusted analysis, after multivariable adjustment we found that higher HIV RNA was associated with higher LFF (5% increase per doubling of HIV RNA; 95% CI 1% to 10%, p=0.017) (Table 3). Results remained consistent after further adjusting for APRI and after excluding participants with cirrhosis (Supplemental Table 1).
Table 3.
Factors associated with liver fat fraction steatosis among HIV-monoinfected and HIV/HCV-coinfected participants.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
|
|
|
|||
| % estimate (CI) | p-value | % estimate (CI) | p-value | |
| Demographics | ||||
|
| ||||
| HCV | -24.5% (-42.0%, -1.6%) | 0.038 | -7.9% (-31.9%, 24.5%) | 0.59 |
| Age (per decade) | 8.3% (-6.8%, 26.0%) | 0.30 | 1.5% (-11.1%, 16.0%) | 0.82 |
| Male | 38.8% (7.7%, 78.9%) | 0.011 | 17.8% (-11.4%, 56.5%) | 0.23 |
| Hispanic ethnicity | -11.8% (-31.4%, 13.4%) | 0.33 | 21.0% (-6.2%, 56.0%) | 0.14 |
| Race* | ||||
| African American (vs. White) | -37.4% (-51.7%, -18.9%) | <.001 | -7.0% (-28.6%, 21.1%) | 0.59 |
| Other (vs. White) | 21.4% (-17.2%, 77.9%) | 0.32 | 21.5% (-16.0%, 75.8%) | 0.30 |
|
| ||||
| Behavioral factors | ||||
|
| ||||
| Alcohol use | -1.8% (-24.2%, 27.3%) | 0.89 | ||
| Current smoker | -23.6% (-41.1%, -0.9%) | 0.042 | ||
| Current marijuana use | -33.5% (-48.6%, -14.0%) | 0.002 | -20.2% (-35.6%, -1.1%) | 0.039 |
| Injection drug use, ever | -1.8% (-25.5%, 29.4%) | 0.90 | ||
|
| ||||
| Metabolic factors | ||||
|
| ||||
| VAT(per doubling) | 145.0% (104.0%, 194.3%) | <.001 | 53.2% (30.8%, 79.4%) | <.001 |
| Abdominal SAT(per doubling) | 25.6% (3.5%, 52.6%) | 0.021 | ||
| HOMA-IR (per doubling) | 53.8% (35.3%, 74.9%) | <.001 | 17.3% (6.6%, 29.1%) | 0.001 |
|
| ||||
| Liver-related factors | ||||
|
| ||||
| ALT, (per doubling) | 16.7% (2.1%, 33.3%) | 0.023 | 9.6% (-4.4%, 25.6%) | 0.191 |
| Albumin (per 0.1 g/dL) | 4.5% (1.3%, 7.7%) | 0.005 | 4.8% (1.4%, 8.3%) | 0.005 |
| APRI (per doubling) | 10.3% (-2.9%, 25.2%) | 0.132 | ||
|
| ||||
| HIV-related factors | ||||
|
| ||||
| HIV RNA (per doubling) | -1.6% (-6.2%, 3.2%) | 0.498 | 5.2% (0.9%, 9.7%) | 0.017 |
| Current CD4 count (per doubling) | 3.1% (-10.7%, 19.0%) | 0.677 | ||
| Nadir CD4 count (per doubling) | -2.1% (-10.3%, 6.8%) | 0.628 | ||
| Current ART use | 15.7% (-22.0%, 71.6%) | 0.470 | ||
| History of AIDS | 8.1% (-16.4%, 39.6%) | 0.554 | ||
| Years on ART (per doubling) | 2.1% (-8.6%, 13.9%) | 0.717 | ||
Abbreviations: CI=confidence interval; HCV=hepatitis C virus; BMI=body mass index; VAT=visceral adipose tissue; Abd SAT=abdominal subcutaneous adipose tissue; HOMA-IR=homeostatic model assessment of insulin resistance; ART=antiretroviral therapy; AIDS=acquired immune deficiency syndrome; APRI=aspartate aminotransferase-to-platelet ratio index.
Categories include Hispanics and non-Hispanics
Among HCV-infected persons, LFF was primarily mediated by metabolic factors (Table 4). On unadjusted analysis, greater BMI, waist circumference, VAT, abdominal SAT, leg fat, and HOMA-IR were each associated with higher LFF. HCV RNA and history of HCV treatment showed little association with LFF. On unadjusted analysis, greater APRI was associated with higher LFF (18%; 95% CI 6% to 33%, p=0.004), but after multivariable adjustment this association weakened and was no longer statistically significant. On multivariable analysis, the only factors that remained significantly associated with LFF were VAT (49%; 95% CI 26% to 76%, p<0.001) and ALT (18%; 95% CI 3% to 36%, p=0.017). Results were qualitatively similar on sensitivity analysis excluding patients with presumed cirrhosis (Supplemental Table 2).
Table 4.
Factors associated with liver fat fraction among HCV-monoinfected and HIV/HCV-coinfected participants.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
|
|
|
|||
| % estimate (CI) | p-value | % estimate (CI) | p-value | |
| Demographics | ||||
|
| ||||
| HIV/HCV-coinfected vs. HCV monoinfected | -16.1% (-34.7%, 7.8%) | 0.17 | -17.3% (-39.1%, 12.3%) | 0.22 |
| Age (per decade) | 11.1% (-7.2%, 33.1%) | 0.25 | -0.1% (-20.5%, 25.5%) | 0.99 |
| Male | -9.5% (-30.6%, 18.0%) | 0.46 | -12.5% (-40.3%, 28.1%) | 0.49 |
| Hispanic ethnicity | 10.7% (-14.8%, 43.8%) | 0.45 | 27.9% (-12.6%, 87.1%) | 0.21 |
| Race* | ||||
| African American (vs. White) | -7.2% (-27.7%, 19.2%) | 0.56 | 20.8% (-9.5%, 61.1%) | 0.20 |
| Other (vs. White) | 66.7% (14.8%, 142.0%) | 0.007 | 49.7% (-9.5%, 147.6%) | 0.12 |
|
| ||||
| Behavioral factors | ||||
|
| ||||
| Alcohol use | -9.0% (-30.5%, 19.1%) | 0.49 | ||
| Current smoker | -13.7% (-33.7%, 12.2%) | 0.27 | ||
| Current marijuana use | -23.3% (-41.5%, 0.5%) | 0.055 | ||
| Injection drug use, ever | 8.9% (-16.5%, 42.0%) | 0.53 | ||
|
| ||||
| Metabolic factors | ||||
|
| ||||
| VAT(per doubling) | 65.3% (34.8%, 102.8%) | <.001 | 48.8% (25.8%, 75.9%) | <.001 |
| Abdominal SAT(per doubling) | 52.1% (23.7%, 86.9%) | <.001 | ||
| HOMA-IR (per doubling) | 32.5% (15.8%, 51.6%) | <.001 | ||
|
| ||||
| Liver and HCV-related factors | ||||
|
| ||||
| ALT (per doubling) | 18.9% (3.9%, 36.0%) | 0.012 | 18.2% (3.0%, 35.5%) | 0.017 |
| Albumin (per 0.1 g/dL) | -1.8% (-5.0%, 1.5%) | 0.28 | ||
| APRI (per doubling) | 18.3% (5.5%, 32.6%) | 0.004 | ||
| HCV RNA(per doubling) | 0.9% (-4.7%, 6.7%) | 0.76 | 0.6% (-4.5%, 6.0%) | 0.81 |
| History of HCV treatment | 30.0% (-14.5%, 97.5%) | 0.22 | ||
Abbreviations: CI=confidence interval; BMI=body mass index; VAT=visceral adipose tissue; Abd SAT=abdominal subcutaneous adipose tissue; HOMA-IR=homeostatic model assessment of insulin resistance; ART=antiretroviral therapy; AIDS=acquired immune deficiency syndrome; APRI=aspartate aminotransferase-to-platelet ratio index.
Categories include Hispanics and non-Hispanics
Discussion
In this ethnically diverse study of 356 adults with HIV or HCV monoinfection, HIV/HCV coinfection, and neither infection, we found that chronic infection with HIV and/or HCV infection was not associated with greater HS. Rather, HCV-infected participants (with and without HIV) had significantly lower MRS-measured LFF and HIV-monoinfected participants had slightly lower LFF than HIV/HCV-uninfected participants. Our finding of a lower LFF among those with HCV infection was unexpected and persisted despite adjusting for demographic, lifestyle, adipose tissue and metabolic factors. Furthermore, this relationship remained significant after additional adjustment for hepatic fibrosis and after exclusion of participants with suspected cirrhosis, suggesting that HCV impacts liver fat storage independent of fibrosis. Our results challenge the widely held perception that non-genotype 3 HCV is associated with greater HS.
Previous studies have shown a relatively high prevalence of HS among HIV-monoinfected individuals (19-21, 24, 25) but did not include an uninfected comparison group. These estimated prevalence rates vary depending on the modality used to measure HS, with reports of 31% and 54% prevalence using ultrasound, 37% using non-contrast CT, and 40% and 48% using transient elastography with controlled attenuation parameter (19-21, 25, 36). Another study of 62 HIV-monoinfected patients on ART found that 73% had HS on liver biopsy (24). These high HS prevalence estimates have led many to hypothesize that HIV infection is an independent risk factor for HS. Indeed, HIV could lead to HS through a variety of mechanisms, including chronic immune activation, direct interaction with the sterol regulatory element binding protein (SREBP-1), which stimulates lipogenesis, and alterations in insulin signaling(37). Additionally, ART, especially the nucleoside reverse transcriptase inhibitors, have been postulated to cause steatosis via inhibition of mitochondrial polymerase γ, and protease inhibitors by inducing hepatic overexpression of SREBP-1(26, 38), although their effects may be more modest with the use of newer drugs within these classes. Finally, HIV infection and ART are associated with metabolic perturbations that have been strongly linked to HS (39).
Our findings corroborate the results of another study of 719 men in the Multicenter AIDS Cohort Study (MACS) where HIV was not a risk factor for HS, as determined by non-contrast CT, and in fact was associated with a lower prevalence of HS(40). Our study is now the second study with appropriate HIV-uninfected controls that failed to show increased HS attributable to HIV. Unlike in the MACS, we found only a modest reduction in the prevalence of HS in our HIV-monoinfected subjects, perhaps owing to our use of a more sensitive and specific measurement of HS. Nevertheless, we similarly found strong associations of metabolic factors with HS in the setting of HIV. Unexpectedly, on multivariable analysis we found that elevated HIV RNA was associated with greater HS. Several prior studies, primarily in the setting of HIV/HCV coinfection, have not found a relationship between detectable HIV RNA and HS(5, 25). However, HIV viremia despite ART has been associated with increased gut microbial translocation and systemic inflammation, both of which have been implicated in HS pathogenesis(41, 42). Future evaluation of the relationship between HIV-related microbial translocation and HS are warranted.
Hepatic steatosis is common in the setting of HCV and is associated with worsened liver disease progression (1-5). The higher reported prevalence of histologic HS in HCV-infected populations as compared to the general population has resulted in the belief that HS is more prevalent in HCV-infected individuals than in those without HCV (12). However, while genotype 3 HCV can directly cause HS (15-17), HS in the setting of non-genotype 3 HCV infection is primarily mediated by metabolic factors (9, 18). We, too, found a strong association between metabolic factors, particularly VAT, and HS in our entire cohort as well as in the HCV-infected subgroup. Although the prevalence of HS in our HCV-infected group was in the lower range reported in HCV-positive populations (19% in HCV-monoinfected and 11% in HIV/HCV-coinfected), it is important to note the heterogeneity in patient characteristics across these studies. In addition, histologic studies of HS and HCV may overestimate HS if there is selection bias in referral for biopsy since both elevated transaminases and advanced fibrosis are associated with increased HS.
The most surprising finding in our study was the inverse association of HCV with liver fat. To our knowledge, ours is the first study to directly compare HS in a cohort of patients with HIV or HCV monoinfection, HIV/HCV coinfection, and neither infection. A few prospective studies have evaluated risk factors for HS in HIV-infected individuals with and without HCV coinfection, each using different noninvasive imaging modalities to assess HS, and they did not find a difference in HS by HCV status (21, 25, 43). Similarly, when we restricted our analysis to HIV-infected individuals, HCV was not independently associated with HS. However, in the entire cohort analysis, both HCV monoinfection and HIV/HCV coinfection were clearly associated with lower HS.
The reasons for our unexpected finding of lower HS in the HCV-infected groups compared to the HIV/HCV-uninfected participants are unclear. As patients with nonalcoholic steatohepatitis progress to cirrhosis, HS can regress histologically, resulting in so-called “burned-out” cirrhosis (44). Since our study is cross-sectional and the HCV-infected subjects were more likely to have cirrhosis, we considered that this may explain their lower amounts of HS. However, the negative association of HCV with HS persisted even after excluding individuals with suspected cirrhosis. We also considered whether the accuracy of MRS-measured HS may be altered by HCV-related fibrosis or inflammation. We previously demonstrated that MRS and histologic steatosis are strongly associated in 42 HIV/HCV-coinfected men and women, including 20 women who also participated in our current study (45). Others have shown that HCV, hepatic inflammation, and fibrosis do not directly influence the accuracy of MRS in detecting HS (35, 46). However, some have suggested that as fibrosis progresses and hepatocytes are replaced by fibrous tissue, MRS may underestimate the percentage of steatotic hepatocytes (35). We therefore included estimated fibrosis stage in our analysis, and the relationship between HCV and HS did not change. Moreover, HS and liver fibrosis were positively correlated in our cohort. Thus, the higher prevalence of advanced fibrosis and cirrhosis among the HCV-infected individuals in the cohort does not explain the apparent protective effect of HCV against HS.
Although there were differences in the characteristics of the cohort by HIV and HCV disease category, it is notable that the relationship between HCV and HS was not attenuated even after adjusting for demographic, lifestyle, and metabolic factors. It is possible that unmeasured confounders, such as healthier behavior, more frequent physician visits, and more aggressive management of medical comorbidities would preferentially reduce HS in those with known HCV. It is also possible that HCV itself is protective against HS, although the cross-sectional nature of this study limits our ability to demonstrate this. C-reactive protein (CRP) levels are substantially reduced in the setting of chronic HCV, due in part to an attenuated response to interleukin (IL)-6 in the presence of HCV (47, 48). Both hepatic and serum levels of IL-6 are elevated in patients with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (49, 50), suggesting a role of IL-6 in the pathogenesis of HS. It is possible that HCV blunts the hepatic response to IL-6 thereby protecting against HS. Future studies evaluating the inflammatory mechanisms of HS in the setting of HCV will help elucidate these relationships. In addition, prospective studies are needed to clarify the association between HCV and HS risk. This is particularly important because although successful HCV treatment can reduce HS in genotype 3 HCV, HCV cure has not been shown to improve HS in non-genotype 3 HCV (16, 51). As more patients with HCV achieve sustained virologic response with newer HCV antivirals, long-term follow-up of HS in this group may be warranted.
The major limitations of our study include the cross-sectional design and heterogeneity of demographic and clinical characteristics between the infection subgroups. Although we adjusted for many factors in our analysis, there are likely differences by HIV and HCV status in other non-assessed parameters which we could not control for. Therefore, our findings must be interpreted in light of this potential bias. Lack of histology to assess HS is another major limitation. However, it would be infeasible to perform a biopsy study in this cohort, which included HIV-monoinfected and HIV/HCV-uninfected controls without indication for liver biopsy. In addition, our study avoids the selection bias inherent in liver biopsies. Finally, two field strengths were used to acquire MRS liver fat measures. We corrected for this but cannot exclude the possibility of minor remaining field effects on LFF. Study strengths include the use of MRS, the reference standard for non-invasive assessment of HS, extensive characterization of metabolic risk factors and direct measures of visceral adiposity, and the inclusion of HIV- and HCV-uninfected controls.
In summary, we found significantly less HS in HCV-monoinfected and HIV/HCV-coinfected subjects as compared to HIV/HCV-uninfected participants despite adjusting for multiple possible confounders. As expected, visceral adiposity and insulin resistance were strongly associated with greater HS in the cohort. Further research is needed to investigate potential mechanisms by which non-genotype 3 HCV may alter the risk of HS and to evaluate the possible impact of HCV eradication on HS
Supplementary Material
Acknowledgments
JP discloses grant support from Gilead Sciences and Merck and ownership interest in Bristol-Myers Squibb, Johnson and Johnson, Merck, and Abbvie. SN discloses grant support from Gilead Sciences and from Verily Life Sciences.
Financial Support: The Women's Interagency HIV Study (WIHS) is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) (U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). Additional support for the current analysis was provided by the University of California San Francisco Liver Center [P30 DK026743]; by the National Institute of Allergy and Infectious Diseases [K24 AI 108516 (PCT) and R01 AI 087176 (PCT), which is administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center, San Francisco, CA]; by the University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research [P30-AI027763 (JCP)] and by an American College of Gastroenterology Junior Faculty Development Award (JCP). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Footnotes
Conflict of interest: All other authors have nothing to disclose.
References
- 1.Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729–736. doi: 10.1053/jhep.2002.35064. [DOI] [PubMed] [Google Scholar]
- 2.Hu KQ, Kyulo NL, Esrailian E, Thompson K, Chase R, Hillebrand DJ, Runyon BA. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147–154. doi: 10.1016/s0168-8278(03)00479-3. [DOI] [PubMed] [Google Scholar]
- 3.Terrault NA, Im K, Boylan R, Bacchetti P, Kleiner DE, Fontana RJ, Hoofnagle JH, et al. Fibrosis progression in African Americans and Caucasian Americans with chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:1403–1411. doi: 10.1016/j.cgh.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross TJ, Quaglia A, Hughes S, Joshi D, Harrison PM. The impact of hepatic steatosis on the natural history of chronic hepatitis C infection. J Viral Hepat. 2009;16:492–499. doi: 10.1111/j.1365-2893.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 5.Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: a meta-analysis of the risk factors. Hepatology. 52:71–78. doi: 10.1002/hep.23619. [DOI] [PubMed] [Google Scholar]
- 6.Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, et al. alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–1262. doi: 10.1002/hep.26442. [DOI] [PubMed] [Google Scholar]
- 7.Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490–2496. doi: 10.1002/cncr.22701. [DOI] [PubMed] [Google Scholar]
- 8.Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036–3043. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 9.Lonardo A, Loria P, Adinolfi LE, Carulli N, Ruggiero G. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73–80. doi: 10.1111/j.1365-2893.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 10.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 11.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Clement S, Negro F. Hepatitis C virus: the viral way to fatty liver. J Hepatol. 2007;46:985–987. doi: 10.1016/j.jhep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, et al. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089–7103. doi: 10.3748/wjg.v20.i23.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Male PJ, Mentha G, Spahr L, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106–115. doi: 10.1016/s0168-8278(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266–1272. doi: 10.1053/jhep.2002.36370. [DOI] [PubMed] [Google Scholar]
- 17.Jackel-Cram C, Babiuk LA, Liu Q. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: genotype-3a core has a stronger effect than genotype-1b core. J Hepatol. 2007;46:999–1008. doi: 10.1016/j.jhep.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 19.Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, Goodman Z, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D'Amico R, Ligabue G, Fiocchi F, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 21.Li Vecchi V, Soresi M, Giannitrapani L, Di Carlo P, Mazzola G, Colletti P, Terranova A, et al. Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int J Infect Dis. 2012;16:e397–402. doi: 10.1016/j.ijid.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Ryan P, Blanco F, Garcia-Gasco P, Garcia-Merchan J, Vispo E, Barreiro P, Labarga P, et al. Predictors of severe hepatic steatosis using abdominal ultrasound in HIV-infected patients. HIV Med. 2009;10:53–59. doi: 10.1111/j.1468-1293.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 23.Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ, Jr, Witt MD, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109:695–704. doi: 10.1038/ajg.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, Abu-Asab M, et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin Infect Dis. 2015;60:1569–1578. doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macias J, Gonzalez J, Tural C, Ortega-Gonzalez E, Pulido F, Rubio R, Cifuentes C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28:1279–1287. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 26.Lemoine M, Ingiliz P. Liver injury in HIV monoinfected patients: should we turn a blind eye to it? Clin Res Hepatol Gastroenterol. 2012;36:441–447. doi: 10.1016/j.clinre.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 29.Noworolski SM, Lam MM, Merriman RB, Ferrell L, Qayyum A. Liver steatosis: concordance of MR imaging and MR spectroscopic data with histologic grade. Radiology. 2012;264:88–96. doi: 10.1148/radiol.12110673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med. 2001;46:228–239. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 31.Noworolski SM, Tien PC, Merriman R, Vigneron DB, Qayyum A. Respiratory motion-corrected proton magnetic resonance spectroscopy of the liver. Magn Reson Imaging. 2009;27:570–576. doi: 10.1016/j.mri.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilman A, Qayyum A, Nystrom M, Noworolski SM. Liver Fat and Water MR T2 Values at 3T: Dependence Upon Steatosis Level. Intn'l Soc of Mag Res in Med. 2011:734. [Google Scholar]
- 33.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 34.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 35.McPherson S, Jonsson JR, Cowin GJ, O'Rourke P, Clouston AD, Volp A, Horsfall L, et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389–397. doi: 10.1016/j.jhep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Vuille-Lessard E, Lebouche B, Lennox L, Routy JP, Costiniuk CT, Pexos C, Giannakis A, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS. 2016;30:2635–2643. doi: 10.1097/QAD.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 37.Lemoine M, Barbu V, Girard PM, Kim M, Bastard JP, Wendum D, Paye F, et al. Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS. 2006;20:387–395. doi: 10.1097/01.aids.0000206503.01536.11. [DOI] [PubMed] [Google Scholar]
- 38.Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:329–337. doi: 10.1016/s1473-3099(03)00654-6. [DOI] [PubMed] [Google Scholar]
- 39.Pao V, Lee GA, Grunfeld C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep. 2008;10:61–70. doi: 10.1007/s11883-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price J, Post W, Seaberg E, Budoff M, Kinglsey L, Palella F, Witt M, et al. Prevalence of and Risk Factors for Nonalcoholic Fatty Liver Disease in HIV+ and HIV- Men in the Multicenter AIDS Cohort Study. Conference on Retroviruses and Opportunistic Infections; Atlanta, Geoergia. March 3-6, 2013; X-236. [Google Scholar]
- 41.Bastard JP, Soulie C, Fellahi S, Haim-Boukobza S, Simon A, Katlama C, Calvez V, et al. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther. 2012;17:915–919. doi: 10.3851/IMP2093. [DOI] [PubMed] [Google Scholar]
- 42.Reus S, Portilla J, Sanchez-Paya J, Giner L, Frances R, Such J, Boix V, et al. Low-level HIV viremia is associated with microbial translocation and inflammation. J Acquir Immune Defic Syndr. 2013;62:129–134. doi: 10.1097/QAI.0b013e3182745ab0. [DOI] [PubMed] [Google Scholar]
- 43.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–317. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 44.Abdelmalek M, Ludwig J, Lindor KD. Two cases from the spectrum of nonalcoholic steatohepatitis. J Clin Gastroenterol. 1995;20:127–130. doi: 10.1097/00004836-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Ghotb A, Noworolski SM, Madden E, Scherzer R, Qayyum A, Pannell J, Ferrell L, et al. Adipose tissue and metabolic factors associated with steatosis in HIV/HCV coinfection: histology versus magnetic resonance spectroscopy. J Acquir Immune Defic Syndr. 2010;55:228–231. doi: 10.1097/QAI.0b013e3181e1d963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang BK, Yu ES, Lee SS, Lee Y, Kim N, Sirlin CB, Cho EY, et al. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012;47:368–375. doi: 10.1097/RLI.0b013e31824baff3. [DOI] [PubMed] [Google Scholar]
- 47.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah S, Ma Y, Scherzer R, Huhn G, French AL, Plankey M, Peters MG, et al. Association of HIV, hepatitis C virus and liver fibrosis severity with interleukin-6 and C-reactive protein levels. AIDS. 2015;29:1325–1333. doi: 10.1097/QAD.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 50.Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, Torjesen PA, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Castera L, Hezode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, Dhumeaux D. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420–424. doi: 10.1136/gut.2002.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.