Abstract
Objectives
Obesity is associated with multiple health problems and often originates in childhood. The purpose is to investigate the associations of genetic polymorphisms in genes related to risk-taking behaviors with body mass index (BMI) trajectory over adolescence among Mexican Americans.
Methods
This study included 1,229 Mexican American adolescents who participated in a large population-based cohort study in Houston, Texas. BMI data were obtained at baseline and two follow-ups. The median follow-up time was 59 months. Participants were genotyped for 672 functional and tagging variants in genes involved in the dopamine, serotonin, and cannabinoid pathways.
Results
After adjusting for multiple comparisons, three genetic variants, namely rs933271 and rs4646310 in COMT gene, and rs9567733 in HTR2A gene, were significantly associated with BMI growth over adolescence. Using those three variants, we created an allelic score, and the allelic score was associated with BMI growth over adolescence (P<0.001). With the increase number of variant allele, the rate of BMI growth over adolescence was slower. Finally, we identified another two genetic variants, namely rs17069005 in HTR2A gene and rs3776511 in SLC6A3A gene, were associated with obesity at last follow-up.
Conclusions
The results suggest that genetic variants in selected genes involved in dopamine and serotonin pathways have noticeable effects on BMI over adolescence.
Keywords: risk-taking behaviors, genetic polymorphism, body mass index, adolescence, Mexican Americans
INTRODUCTION
Mexican Americans adolescents (age 11 to 17) were 1.6 times more likely to be overweight or obese than Non-Hispanic white adolescents. Adolescent obesity is highly predictive of adult-onset and severe obesity1, 2. Adult obesity, in turn, increases the risk of chronic diseases. Clearly, understanding factors that drive the development of obesity among Mexican American adolescents will be crucial for developing effective strategies to prevent the expected “outbreak” of obesity-related cancers and cardiovascular disease that is rapidly approaching.
With the rapid increasing of genome wide association studies (GWAS) and genome sequencing studies, obesity related genetic variants have been identified3, 4. However, most of those genetic variants are identified from the non-Hispanic white population. Whether they have the same effects in the Mexican American population remains to be determined. Furthermore, few studies examined these associations in adolescents and even fewer have compared the magnitude of effect during different phases of the life course5–7.
In adolescents, obesity is thought to be a multi-determined phenotype and associated with several risk-taking behaviors, including eating behavior and physical activity8, 9. Thus, examining polymorphisms associated with these risk-taking behaviors may provide new insights into the genetic underpinnings of obesity among adolescents. Studies in the past decade have demonstrated that several genetic pathways, namely dopamine, serotonin, and cannabinoid pathways, are actively involved in the development of risk-taking behaviors10, 11. In our previous analysis using the same study subjects as current study, we found that participants with at least one copy of the minor allele for SNPs in SNAP25 (rs363035 OR=0.53; p=0.005) and CNR1 (rs6454672 OR=0.62; p=0.022) have decreased likelihood of meeting physical activity recommendations12.
To date, there has been no comprehensive study of how genetic variants in genes involved in dopamine, serotonin, and cannabinoid pathways influence BMI growth over adolescence. Instead of looking at BMI at one time point, our study has measured BMIs at multiple times during adolescence. This allows us to apply a longitudinal approach to examine the BMI developmental trajectory and associated genetic variants during adolescence. The knowledge from this study may shed light on the biological pathways involved, as well as insights into the development of obesity to inform the design of interventions.
MATERIALS AND METHODS
Study design and population
Our data were derived from Mexican origin adolescents who were participants enrolled in a prospective cohort study of smoking behavior that began in 2005–06. In 2008–09, 90.6% of the original participants completed a follow-up; in 2010–11, 75.8% of the original participants completed a second follow-up. Participants were drawn from a population-based cohort of Mexican-American households launched in 2001 by the Department of Epidemiology at The University of Texas M. D. Anderson Cancer Center, called the Mano-a-Mano Mexican American Cohort Study (MACS). To be eligible to participate in the MACS, participants had to self-identify as Mexican or Mexican American; thus all parents of our participants are either Mexican or Mexican American. Households were initially recruited into the cohort from predominantly Mexican American neighborhoods in Houston, Texas. A detailed description of the cohort recruitment methodology has been described previously13. A total of 3,000 households with potential age-eligible (adolescents between the ages of 11 and 13 years) participants were identified from the cohort database. Of the first 1,425 potential participants’ parents or legal guardians contacted to assess interest in the study, just over 90% agreed to enroll their child in the study (N=1,328) at baseline. All parents and legal guardians provided informed consent and all minors provided informed assent. The institutional review board at The University of Texas M. D. Anderson Cancer Center approved all aspects of this study.
Data collection
The BMI measurement data included in the current analysis were collected via measurement in the home at baseline and two follow-ups by research staffs. Informed written consent and saliva samples were obtained at baseline, when participants enrolled in the study. A detailed description of the data collection procedures has been published14. Briefly, after consenting into the study, each participant completed a 5-minute personal interview during which basic demographic (gender, age, nativity status (US or Mexico)), acculturation data15, and parental educational attainment were collected. Household socio-economic status (SES) was assessed using parental educational attainment rather than household income because more than 40% of the parents did not report their income, while the majority reported educational attainment16. The adolescent’s subjective view of where he or she lies in the school-based social hierarchy, was assessed using a version of the MacArthur Scale of Subjective Social Status adapted for adolescents17.
SNP selection and genotyping
Saliva samples were obtained in Oragene vials (DNA Genotek, Ottawa, Ontario, Canada). DNA extraction was performed per the manufacturer’s protocol. Candidate genes were identified from literature searches using the following key words: sensation seeking, novelty seeking, risk taking, gambling, smoking, and alcohol use18. This list was cross-referenced with the Gene Ontology Database (http://pid.nci.nih.gov/) and Kegg Pathway to confirm pathway information. Tagging SNPs were selected from the International HapMap Project (Release 21 with NCBI build 36; http://www.hapmap.org). The following selection criteria were used: located in the respective gene or within 10 kb upstream or downstream of the gene ends to cover the regulatory regions; minor allele frequency (MAF) >5% in various ethnic groups; and not already represented by a current tag SNP at a linkage disequilibrium (LD) of r2>0.80. We also targeted SNPs in coding and regulatory regions. Genotyping was conducted following standard procedures19. Genotype calls were made when a genotype yielded a quality score (Gencall value) of 95% or higher. Among these markers, 1.2% of calls were missing (8 of 672). Seventy blind duplicate pairs were included, and the concordance of SNP genotype calls was greater than 99%.
Statistical analyses
Chi-square tests were used to compare socio-demographic characteristics and categorical measures of TAS and subjective social status. First, we assessed the association between each single SNP and BMI trajectory. We used individual growth curve modeling, a technique well suited for longitudinal data20, 21. Individual growth curves in BMI are characterized by their intercept (or level) and slope (rate of change). We modeled the effects of age, age2, sex, acculturation, parental education, trait anxiety, subjective social status and their interactions on the development of BMI using random coefficients linear models. Here, we added SNP genotype (and their interactions with age, age2, sex, acculturation, parental education, TAS, and subjective social status) as fixed effects to a mixed linear model that includes the intercept, age and age2 and as random effects. Model effects of SNP genotype, sex, acculturation, parental education, TAS, subjective social status and their interactions represent effects on the growth curve level. Effects on the rate of change of BMI over adolescence were modeled as interactions with age and age2. The analysis of individual growth curves was implemented using mixed linear models in Proc Mixed of the SAS/Stat software package (Release 9.4, SAS Institute Inc., Cary, NC, USA). For single SNP analysis, we performed bootstrapping analysis to evaluate the chance of false positive associations for the variants studied using P≥0.80 as the cutoff point. This process resulted in the identification of 3 significant SNPs, namely rs933271, rs9567733, and rs4646310. Because a priori we do not know the mode of inheritance, we tested each significant SNP using dominant, recessive, and additive models and selected the most parsimonious. The dominant model was the most parsimonious model for all 3 SNPs. Second, using those 3 SNPs, we created an allelic score. The score ranges from 0 (no variant allele) to 3 (all 3 variant alleles). The allelic score was treated as the categorical variable. We used a similar approach, described above for single SNP analysis, to assess the effect of allelic score on BMI growth trajectory. Linear regression analysis was performed to assess the effect of variant alleles on BMI. Survival analysis was used to estimate the effects of SNPs on obesity at last follow-up. Hazard ratio (HR) and 95% conference interval (95% CI) were calculated. Socio-demographic characteristics and categorical measures of trait anxiety and subjective social status were adjusted in the analysis as appropriate.
RESULTS
Table 1 summarizes selected demographic characteristics and psychosocial risk factors at baseline, 1st and 2nd follow-ups. At baseline in 2005–06, we had 1,229 Mexican American adolescents recruited for the study. The mean BMI was 22.9. The average acculturation score was 3.5 on a five-point scale, with higher scores reflecting higher levels of acculturation. In 2008–09, 1,114 of the original participants (90.6%) completed the 1st follow-up. And in 2010–11, 932 of the original participants (75.8%) completed the 2nd follow-up. There were no significant distribution changes in terms of gender, birth country, parent education, acculturation, subjective social status, and trait anxiety from baseline to 1st and 2nd follow-ups. However, the mean BMI increased from 22.9 at baseline to 25.1 at 1st and 25.6 at 2nd follow-ups (p<0.01).
Table 1.
Characteristics of the study participants
| Variables | Baseline | Follow-up 1 | Follow-up 2 |
|---|---|---|---|
| N=1229 | N=1114 | N=932 | |
| Age | |||
| mean(std) | 11.9(0.84) | 14.3(1.04) | 16.7(1.12) |
| Sex N(%) | |||
| Male | 608(49.47) | 552(49.55) | 452(48.50) |
| Female | 621(50.53) | 562(50.45) | 480(51.50) |
| Birth Country N(%) | |||
| Mexico | 320(26.06) | 290(26.03) | 244(26.18) |
| US | 908(73.94) | 824(73.97) | 688(73.82) |
| Parent Education N(%) | |||
| < High School | 789(65.48) | 715(65.54) | 605(65.98) |
| High School | 209(17.34) | 188(17.23) | 148(16.14) |
| > High School | 207(17.18) | 188(17.23) | 164(17.88) |
| BMI | |||
| mean(std) | 22.9(5.77) | 25.1(6.47) | 25.6(6.59) |
| Acculturation | |||
| mean(std) | 3.5(0.89) | 3.5(0.72) | 3.4(0.71) |
| Years live in the U.S. (Mexico born only) | |||
| mean(std) | 6.8(3.31) | 9.5(3.38) | 11.7(3.37) |
| Subjective social status | |||
| mean(std) | 8.2(1.66) | 7.8(1.49) | 7.9(1.42) |
| trait anxiety | |||
| mean(std) | 38.1(9.87) | 38.4(9.53) | 39.1(9.40) |
Genotyping was completed on a total of 672 SNPs, 8 SNPs were failed on all participants, and 86 SNPs failed the frequency test (MAF<0.05). Thus 578 SNPs were included in the analysis. There were 26 SNPs with P<0.05 based on the best model fit (additive, dominant, or recessive model) (Supplemental Table 1). After controlling for false discovery, we identified 3 SNPs with a statistically significant at 0.80 and prior probability of 0.05. They were rs933271, rs9567733, and rs4646310 (Supplemental Table 2). Both rs933271 and rs4646310 are tagSNPs for Catechol-O-methyltransferase (COMT) gene. COMT gene encodes an important modulator in the catabolism of extra-neural dopamine, which plays an important role in drug reward mechanisms. rs9567733 is a tagSNP for 5-Hydroxytryptamine (Serotonin) Receptor 2A (HTR2A) gene. HTR2A gene encodes one of the receptors for serotonin, and HTR2A is a neurotransmitter that plays an important role in many physiologic processes. As shown in Figure 1a, BMI increased with age. However, individuals with rs933271 G variant allele had statistically significantly slower BMI growth than those without rs933271 G variant allele (P=0.002). Similar trends were also observed for rs9567733 and rs4646310 (Figures 1b and 1c) (P=0.003 and 0.004, respectively).
Figure 1. Individual SNPs and allelic score predicting rates of BMI changes over adolescence.
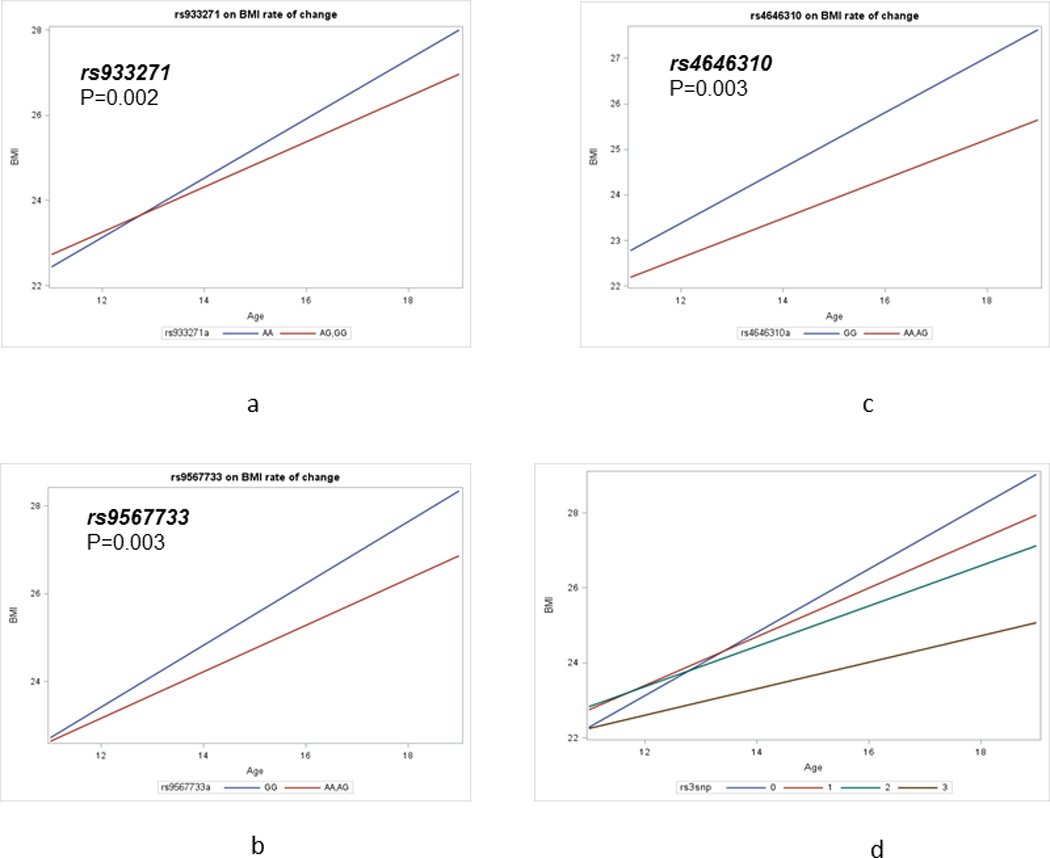
1a) rs933271; 1b) rs9567733; 1c) rs4646310; 1d) allelic score
Using those 3 significant SNPs, we created an allelic score, ranging from 0 (without any variant allele) to 3 (with all 3 variant alleles). After controlling for socio-demographic characteristics, TAS, and subjective social status, we found that the allelic score was statistically significantly associated with slower BMI growth over adolescence (P<0.001) (Figure 1d). With the increase number of variant allele, the rate of BMI growth over adolescence was slower. Compared to those with all 3 variant alleles, those with two, one and no variant alleles had BMI increase of 0.185, 0.289, and 0.491 kg/m2 per year (Table 2). The P for trend was statistically significant (P<0.001). In further stratified analysis by gender, we didn’t observe any evidence for gender difference for allelic score (data not shown).
Table 2.
Growth curve modeling analysis to assess the relationships between the allelic score and BMI growth trajectories over adolescence
| Effect | Estimate | Standard Error | t Value | Pr > |t| | |
|---|---|---|---|---|---|
| Intercept | 22.188 | 0.517 | 42.94 | <0.001 | |
| Age | 0.644 | 0.078 | 8.25 | <0.001 | |
| Age2 | −0.060 | 0.010 | −6.11 | <0.001 | |
| Acculturation | 0.213 | 0.112 | 1.9 | 0.058 | |
| Gender | 0.457 | 0.371 | 1.23 | 0.218 | |
| Subjective social status | −0.038 | 0.043 | −0.9 | 0.367 | |
| Trait anxiety | 0.002 | 0.007 | 0.33 | 0.743 | |
| Number of variant allele | |||||
| 3 | 0 | . | . | . | |
| 2 | 0.795 | 0.551 | 1.44 | 0.149 | |
| 1 | 0.852 | 0.569 | 1.50 | 0.135 | |
| 0 | 0.526 | 0.920 | 0.57 | 0.567 | |
| Number of variant allele × age | |||||
| 3 | 0 | . | . | . | |
| 2 | 0.185 | 0.071 | 2.62 | 0.009 | |
| 1 | 0.289 | 0.073 | 3.96 | <0.001 | |
| 0 | 0.481 | 0.119 | 4.03 | <0.001 | |
Next, we explored the relationship between the SNPs and BMI at the last follow-up. A total of 25 SNPs were found to be associated with BMI at last follow-up (P<0.05). After controlling for false discovery, we identified 3 SNPs with a statistically significant at 0.80 and prior probability of 0.05. They were rs933271, rs9567733, and rs4646310 (Supplemental Figure 1). Interestingly, they were the same SNPs which were associated with BMI trajectories. Compared to those without rs933271 variant alleles, those with rs933271 variant alleles had less BMI at last follow-up, after controlling socio-demographic characteristics, TAS, and subjective social status (26.3 vs 25.5, P=0.003). Similar difference was observed for rs9567733, and rs4646310.
Finally, we investigated whether the SNPs might predict the occurrence of obesity at last follow-up. A total of 36 SNPs were found to be associated with obesity at last follow-up. After controlling for false discovery, we identified 2 SNPs with a statistically significant at 0.80 and prior probability of 0.05. They were rs17069005 and rs3776511 (Supplemental Table 2); the first (rs17069005) is a tag SNP for HTR2A and the second (rs3776511) is a tag SNP for SLC6A3A. Compared to those without rs9567733 variant allele, those with at least one rs9567733 variant allele were associated with 3.81 fold increased risk of obesity (HR=3.81, 95% CI: 1.78, 8.18), after controlling baseline BMI, age at baseline, sex, birth location, years in US, acculturation, parent education, and overweight status of parent. A similar association was observed for rs3776511 (HR=2.98, 95% CI: 1.44, 6.19).
DISCUSSION
In this longitudinal analysis, we investigated the association of selected genetic variants in genes known to be involved in dopamine, serotonin, opioid, and cannabinoid pathways with BMI trajectories over adolescence among Mexican American adolescents. We found a total of 26 SNPs that were associated with BMI growth over adolescence (P<0.05). The significant associations remained for rs933271 and rs4646310 on COMT and rs9567733 on HTR2A genes, even after adjusting for multiple comparisons. Using the 3 SNPs, we created an allelic score, and found that the allelic score was significantly associated with the rate of BMI growth during adolescence (P<0.001). Although we are not the first study to examine an allelic score in relation to BMI growth over adolescence, the SNPs we examined are different from previous studies5–7. To date no studies have investigated relationship between SNPs on genes in the dopamine, serotonin, opioid, and cannabinoid pathways, with BMI trajectories during adolescence. In addition, to date, no studies have investigated allelic scores and BMI trajectory in Mexican American adolescents, who are more likely to be overweight or obese than their Non-Hispanic white counterparts.
The neurotransmitters, serotonin, noradrenaline and dopamine are important in the central nervous system regulation of many physiological processes including energy and glucose homeostasis. In a recent study in Turkey, Araz et al. found that 1359G/A polymorphism in cannabinoid receptor-1 (CNR1) genes was associated with childhood obesity22. In a recent genetic association analysis of 30 genes related to obesity in a European American population, rs912127 in HTR2A was associated with obesity23. This SNP was not included in our analysis, but we found rs9567733, a tag SNP in the promoter region of HTR2A, was significantly associated with BMI trajectory during adolescence (P=0.003). HTR2A encodes one of the receptors for serotonin. HTR2A is a neurotransmitter that plays an important role in many physiologic processes such as sleep, appetite, thermoregulation, pain perception, hormone secretion, and sexual behavior24. How serotonin affects food intake is not fully understood yet. However, evidence suggests that serotonin enhances melanocortin 4 receptors (MC4), which are involved in the control of food intake in the hypothalamus25.
COMT is an important modulator in the catabolism of extra-neural dopamine that plays an important role in drug reward mechanisms. In Danish adults, Kring et al. observed that rs4680 GG-genotype in COMT gene was associated with BMI (OR = 1.08, CI = 1.01–1.16), although the association was not significant after multiple comparison adjustment26. In our study, we did not observe a significant association between rs4680 variant alleles and BMI growth during adolescence. However, we observed two tag SNPs, rs933271 and rs4646310, on COMT gene, which were significantly associated with the BMI trajectory during adolescence. There is evidence in both animal and human studies that dopamine plays a role in obesity through regulation of appetite27, 28.
The allelic score method has been used previously in studying BMI growth during childhood and adolescence3, 5, 7. Using 32 adult obesity-related GWAS SNPs, Warrington et al. created an allelic score and found that the allelic score was associated with BMI growth throughout childhood, explaining 0.58% of the total variance in BMI in females and 0.44% in males5. However, because different SNP sets are used in different studies, it is difficult to compare the results across the studies. A useful extension to the current study would be to create a comprehensive allelic score including SNPs used in other studies (e.g. obesity related GWAS SNPs) as well as ones used in current studies. Such effort would help us further understand the genetic etiology of BMI growth during adolescence.
Interestingly, the three SNPs associated with BMI growth during adolescence are also associated with BMI at last follow-up. In the current study, the age at last follow-up is around 17 years old, which is the transition period from adolescence to adulthood. Guo et al. showed that BMI at age 17 was a predictor of adult obesity at age 35 years old for young males29. If BMI at age 17 is over 75%, 85%, and 95% percentile of the Centers for Disease Control and Prevention BMI-for-age growth chart, the probabilities of adult obesity at age 35 for young males were 0.11, 0.20, and 0.52. The probabilities were even higher for young women at age 3529. Thus, in addition to reported associations with BMI growth trajectory and BMI at 17 years old, these three significant SNPs may also be associated with adult obesity. Such an observation is consistent with the life course epidemiology hypotheses – the determinants of adult susceptibility to obesity begin in early life (childhood and adolescence) and develop over the life course.
The main strength of this study is the prospective design, which allowed us to examine BMI growth over adolescence. The main limitation is the lack of an independent replication population. Thus we must consider out findings preliminary and interpret our results cautiously. The selection of our study population may be biased toward low social economic status since only 17% of the parents of our study subjects have above high school education. This may pose a problem in potential generalizability of the findings to other groups. In addition, we don’t have metabolic biomarkers on the study subjects30. Nevertheless, the results from this study provide the first line of evidence to support the involvement of dopamine and serotonin pathways in adolescent BMI growth trajectory.
In conclusion, we conducted an association analysis in a large Mexican American adolescence population to investigate the effect of known genes involved in dopamine, serotonin, opioid, and cannabinoid pathways on adolescent BMI growth trajectory. Such knowledge, if confirmed, could help identify Mexican American adolescents who are at high risk of developing obesity during their adulthood when they just become adolescents.
Supplementary Material
Values shown are mean and 95% confidence interval.
Acknowledgments
We thank the field staff for their ongoing work with participant recruitment and follow-up. Most importantly, we thank our study participants and their parents for their cooperation and participation, without which this research would not be possible.
Funding Sources
The Mexican American Cohort receives funds collected pursuant to the Comprehensive Tobacco Settlement of 1998 and appropriated by the 76th legislature to The University of Texas MD Anderson Cancer Center and from the Caroline W. Law Fund for Cancer Prevention and the Duncan Family Institute for Risk Assessment and Cancer Prevention. This research is also supported by the National Cancer Institute grants [CA105203 and CA126988]. The funders did not contribute to the design and conduct of the study, the data collection, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest
Authors have declared there are no any competing financial interests in relation to the work described.
REFERENCES
- 1.Nicholson LM, Browning CR. Racial and ethnic disparities in obesity during the transition to adulthood: the contingent and nonlinear impact of neighborhood disadvantage. J Youth Adolesc. 2012;41:53–66. doi: 10.1007/s10964-011-9685-z. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Gortmaker SL, Dietz WH. Risk factors for obesity in young adults: Hispanics, African Americans and Whites in the transition years, age 16–28 years. Biomed Pharmacother. 1994;48:143–156. doi: 10.1016/0753-3322(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez JR, Estevez MN, Giralt PS, et al. Genetic risk profiles for a childhood with severe overweight. Pediatr Obes. 2014;9:272–280. doi: 10.1111/j.2047-6310.2013.00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Albuquerque D, Stice E, Rodriguez-Lopez R, Manco L, Nobrega C. Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Mol Genet Genomics. 2015;290:1191–1221. doi: 10.1007/s00438-015-1015-9. [DOI] [PubMed] [Google Scholar]
- 5.Warrington NM, Wu YY, Pennell CE, et al. Modelling BMI trajectories in children for genetic association studies. PLoS One. 2013;8:e53897. doi: 10.1371/journal.pone.0053897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega-Alonso A, Pietilainen KH, Silventoinen K, Saarni SE, Kaprio J. Genetic and environmental factors influencing BMI development from adolescence to young adulthood. Behav Genet. 2012;42:73–85. doi: 10.1007/s10519-011-9492-z. [DOI] [PubMed] [Google Scholar]
- 7.Mei H, Chen W, Jiang F, et al. Longitudinal replication studies of GWAS risk SNPs influencing body mass index over the course of childhood and adulthood. PLoS One. 2012;7:e31470. doi: 10.1371/journal.pone.0031470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padron A, Galan I, Rodriguez-Artalejo F. Behavioral risk factors and mental health: single and cluster associations in Spanish adolescents. J Dev Behav Pediatr. 2012;33:698–704. doi: 10.1097/DBP.0b013e31826ba9d9. [DOI] [PubMed] [Google Scholar]
- 9.Friedman HL. The health of adolescents: beliefs and behaviour. Soc Sci Med. 1989;29:309–315. doi: 10.1016/0277-9536(89)90279-7. [DOI] [PubMed] [Google Scholar]
- 10.Bell AM. Approaching the genomics of risk-taking behavior. Adv Genet. 2009;68:83–104. doi: 10.1016/S0065-2660(09)68004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson AV, Gabriel KP, Wang J, et al. Sensation-seeking genes and physical activity in youth. Genes Brain Behav. 2013;12:181–188. doi: 10.1111/gbb.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcenas CH, Wilkinson AV, Strom SS, et al. Birthplace, years of residence in the United States, and obesity among Mexican-American adults. Obesity (Silver Spring) 2007;15:1043–1052. doi: 10.1038/oby.2007.537. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson AV, Waters AJ, Vasudevan V, Bondy ML, Prokhorov AV, Spitz MR. Correlates of susceptibility to smoking among Mexican origin youth residing in Houston, Texas: a cross-sectional analysis. BMC Public Health. 2008;8:337. doi: 10.1186/1471-2458-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin G, Gamba RJ. A new measurement of acculturation for Hispanics: The bidimensional acculturation scale for Hispanics (BAS) Hispanic Journal of Behavioral Sciences. 1996;18:297–316. [Google Scholar]
- 16.Morrongiello BA, Lasenby J. Finding the daredevils: development of a Sensation Seeking Scale for children that is relevant to physical risk taking. Accid Anal Prev. 2006;38:1101–1106. doi: 10.1016/j.aap.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Goodman E, Adler NE, Daniels SR, Morrison JA, Slap GB, Dolan LM. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obes Res. 2003;11:1018–1026. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- 18.Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson AV, Bondy ML, Wu X, et al. Cigarette experimentation in Mexican origin youth: psychosocial and genetic determinants. Cancer Epidemiol Biomarkers Prev. 2012;21:228–238. doi: 10.1158/1055-9965.EPI-11-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJ, Snieder H. Growth of left ventricular mass in African American and European American youth. Hypertension. 2002;39:943–951. doi: 10.1161/01.hyp.0000015612.73413.91. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med. 2002;21:3291–3315. doi: 10.1002/sim.1264. [DOI] [PubMed] [Google Scholar]
- 22.Col Araz N, Nacak M, Oguzkan Balci S, et al. Childhood obesity and the role of dopamine D2 receptor and cannabinoid receptor-1 gene polymorphisms. Genet Test Mol Biomarkers. 2012;16:1408–1412. doi: 10.1089/gtmb.2012.0244. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Tiwari HK, Lin WY, et al. Genetic association analysis of 30 genes related to obesity in a European American population. Int J Obes (Lond) 2014;38:724–729. doi: 10.1038/ijo.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton N, Owen MJ. HTR2A: association and expression studies in neuropsychiatric genetics. Ann Med. 2005;37:121–129. doi: 10.1080/07853890510037347. [DOI] [PubMed] [Google Scholar]
- 25.Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kring SI, Werge T, Holst C, et al. Polymorphisms of serotonin receptor 2A and 2C genes and COMT in relation to obesity and type 2 diabetes. PLoS One. 2009;4:e6696. doi: 10.1371/journal.pone.0006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szczypka MS, Kwok K, Brot MD, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 28.Meguid MM, Fetissov SO, Varma M, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 29.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 30.Farook VS, Reddivari L, Chittoor G, et al. Metabolites as novel biomarkers for childhood obesity-related traits in Mexican-American children. Pediatr Obes. 2015;10:320–327. doi: 10.1111/ijpo.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values shown are mean and 95% confidence interval.


