Abstract
Tinnitus is defined as a phantom sound (ringing in the ears), and can significantly reduce the quality of life for those who suffer its effects. Ten to fifteen percent of the general adult population report symptoms of tinnitus with 1-2% reporting that tinnitus negatively impacts their quality of life. Noise exposure is the most common cause of tinnitus and the military environment presents many challenging high-noise situations. Military noise levels can be so intense that standard hearing protection is not adequate. Recent studies suggest a role for inhibitory neurotransmitter dysfunction in response to noise-induced peripheral deafferentation as a key element in the pathology of tinnitus. The auditory thalamus, or medial geniculate body (MGB), is an obligate auditory brain center in a unique position to gate the percept of sound as it projects to auditory cortex and to limbic structures. Both areas are thought to be involved in those individuals most impacted by tinnitus. For MGB, opposing hypotheses have posited either a tinnitus-related pathologic decrease or pathologic increase in GABAergic inhibition. In sensory thalamus, GABA mediates fast synaptic inhibition via synaptic GABAA receptors (GABAARs) as well as a persistent tonic inhibition via high-affinity extrasynaptic GABAARs and slow synaptic inhibition via GABABRs. Down-regulation of inhibitory neurotransmission, related to partial peripheral deafferentation, is consistently presented as partially underpinning neuronal hyperactivity seen in animal models of tinnitus. This maladaptive plasticity/Gain Control Theory of tinnitus pathology (see Auerbach et al., 2014; Richardson et al., 2012) is characterized by reduced inhibition associated with increased spontaneous and abnormal neuronal activity, including bursting and increased synchrony throughout much of the central auditory pathway. A competing hypothesis suggests that maladaptive oscillations between the MGB and auditory cortex, thalamocortical dysrhythmia, predicts tinnitus pathology (De Ridder et al., 2015). These unusual oscillations/rhythms reflect net increased tonic inhibition in a subset of thalamocortical projection neurons resulting in abnormal bursting. Hyperpolarizing deinactivation of t-type Ca2+ channels switches thalamocortical projection neurons into burst mode. Thalamocortical dysrhythmia originating in sensory thalamus has been postulated to underpin neuropathies including tinnitus and chronic pain. Here we review the relationship between noise-induced tinnitus and altered inhibition in the MGB.
Keywords: Tinnitus, Auditory Thalamus, Medial Geniculate Body, GABAA Receptor, Thalamocortical Dysrhythmia
1.0 Tinnitus as a significant health problem
Tinnitus is defined as a phantom sound (ringing in the ears), and can significantly affect the quality of life for those experiencing it (Norena, 2011; Tyler et al., 1993). Tinnitus affects an estimated 10–15% of the general adult population with 1.6% to 0.5 % rating tinnitus between severely annoying and profoundly impacting their quality of life (Axelsson et al., 1989; Baguley et al., 2013; Nondahl, 2002; Shargorodsky et al., 2010). The most common cause of tinnitus is high-level noise exposure (Axelsson et al., 1989; Brusis, 1993; Humes et al., 2006; Kaltenbach, 2011; Nondahl et al., 2009; Salmivalli, 1977; Taylor et al., 1966). Helfer et al. (2005) found that soldiers deployed to battle zones were 52.5 times more likely to suffer auditory damage than non-deployed soldiers. The American Tinnitus Association (ATA) reports that 60% of all cases of auditory injury, including tinnitus, within the Iraq and Afghanistan veteran population were the result of a blast-induced mild traumatic brain injury. A Department of Defense study of Iraq service veterans found that 43% of those veterans seen one month after a blast exposure continued to report tinnitus. Noise-induced hearing loss has dramatically increased across the military such that impaired hearing acuity (hearing loss and tinnitus) is the second most common VA disability award, exceeding $1.28 billion per year (ATA). For those disabled by tinnitus, the personal sequelae may include: depression, anxiety, sleep disturbances, inability to concentrate, fatigue, and sometimes suicide (Henry et al., 2013; Roberts et al., 2013; Roberts et al., 2010). Invariably, individuals disturbed by their tinnitus are those whose attention is bound to the sensation in their head. The magnitude of tinnitus distress may relate to attention fixed on this phantom auditory percept (Jacobson et al., 1996; see below and Roberts et al., 2013). Brain circuits that are critically involved in the control of attention, arousal and learning, project to sensory structures including the auditory cortex and auditory thalamus (medial geniculate body, MGB) and associated structures such at the thalamic reticular nucleus (Figure 1) (Metherate, 2011; Motts et al., 2010; Zikopoulos et al., 2006; Zikopoulos et al., 2012). A main goal of the research supported by the military is to identify neural changes associated with loud-sound-induced tinnitus. These changes can then be selectively targeted by drugs that ameliorate the attentional aspects of tinnitus. Scientific advances in this area would benefit the general population as well as military personnel and veterans who experience emotional duress from their tinnitus.
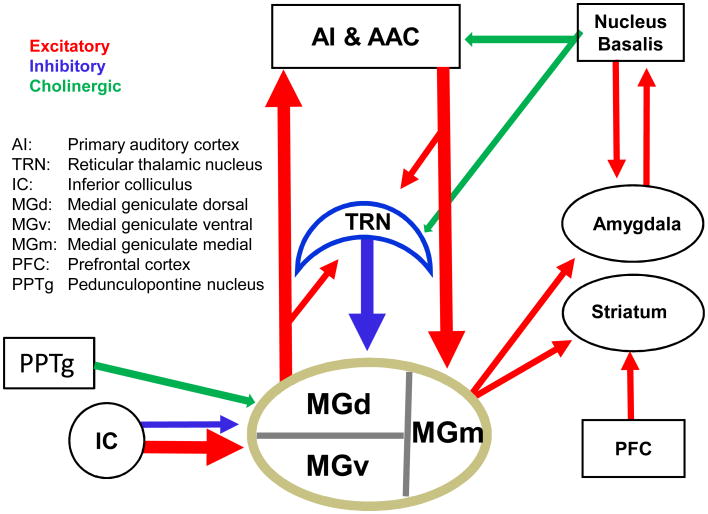
Figure 1. Major connection of the Medial Geniculate Body (MGB).
Ascending projections from the Inferior Colliculus (IC) and descending projections from auditory cortex (AI, AAC) and reticular thalamic nucleus (TRN) include glutamatergic and GABAergic components. Connections to the amygdala, striatum and the TRN are likely important in the tinnitus network.
An emergent hypothesis supported by recent studies suggests that altered balance between excitatory and inhibitory neurotransmission within central nervous system circuits may underpin chronic human neuropathies including tinnitus and chronic pain (Kaltenbach, 2011; Leaver et al., 2011; Norena, 2011; Rauschecker et al., 2015; Roberts et al., 2010; Sedley et al., 2015). The MGB is an obligate auditory brain center well-positioned to gate the percept of sound as it travels to the auditory cortex and to limbic structures. Recent functional models of tinnitus pathology suggest that individuals most affected by tinnitus demonstrate abnormal function in limbic and thalamocortical circuits (Leaver et al., 2011; Rauschecker et al., 2010; Winer et al., 1999). These reviews by Rauschecker and colleagues strongly implicate the MGB and its ascending and descending connections as key components of the tinnitus network (Leaver et al., 2011; Rauschecker et al., 2010; Shinonaga et al., 1994).
2.0 Auditory Thalamus (MGB)
The MGB transforms the ascending sensory code while gating the relative salience of sensory signals, in part, through adjustments in thalamocortical rhythmicity (Cope et al., 2005; Goard et al., 2009; Hughes S.W., 2008; Wafford et al., 2009). The MGB receives lemniscal and extralemniscal ascending inputs as well as inputs from the brainstem, thalamic reticular nucleus, limbic structures and descending inputs from auditory and nonauditory cortices (Figure 1) (Bajo et al., 1995; Lee et al., 2008a; Lee et al., 2008b; Lee et al., 2008c; Rouiller et al., 1990; Rouiller et al., 1991; Winer et al., 1987; Winer et al., 1999). The ventral division (MGv) is believed to be primarily auditory/lemniscal receiving tonotopically-aligned primary ascending excitatory and inhibitory projections from inferior colliculus (Peruzzi et al., 1997; Saint Marie et al., 1997a). The dorsal (MGd) and medial (MGm) divisions of the MGB show poor tonotopic order and are considered non-lemniscal (see Bartlett, 2013), receiving inputs from the non-tonotopically organized regions of the inferior colliculus (Calford et al., 1983) as well as driver-like input from the auditory cortex (Llano et al., 2008). The major output of the MGB is to auditory cortex (Winer et al., 1999). Whereas the ventral division projects to primary auditory cortex (Aitkin et al., 1972), projections from the dorsal division terminate in nonprimary/noncore areas of auditory cortex (Winer et al., 1999). The connectivity of the MGm may be particularly relevant for tinnitus. Unlike the ventral and dorsal divisions, the medial division receives substantial somatosensory input (Bordi et al., 1994b; Khorevin, 1980) and sends direct projections to the ventral striatum and amygdala (Bordi et al., 1994a; Bordi et al., 1994b; LeDoux et al., 1985; LeDoux et al., 1991; Winer et al., 2007; Woodson et al., 2000) as well as to layer 1 of the primary and nonprimary regions of the auditory cortex (Huang et al., 2000; LeDoux et al., 1985; Mitani et al., 1987). Layer 1 projections have been speculated to play a role in binding specific thalamocortical sensory information with contextual arousal-related signals, thus promoting gamma-range oscillation which is potentially relevant for theories of tinnitus (section 3.0) (Llinas et al., 2002).
The thalamic reticular nucleus is likely an important structure relevant to tinnitus and thalamic gating mechanisms. The thalamic reticular nucleus is a poorly understood structure which contains GABAergic neurons that project to dorsal thalamic neurons. The TRN receives input from a wide variety of sources, including branches of thalamocortical axons, branches from corticothalamic axons, as well as projections from the basal forebrain, amygdala, prefrontal cortex and cholinergic brainstem fibers (Asanuma et al., 1990; Bickford et al., 1994; Pare et al., 1988; Pita-Almenar et al., 2014; Zikopoulos et al., 2006; Zikopoulos et al., 2012). Though widely speculated to be important for the production of oscillatory phenomena important for sleep and paroxysmal states (Destexhe et al., 1994; McCormick et al., 2001), and for modulating attention (Crick, 1984; McAlonan et al., 2000; McAlonan et al., 2006; Wimmer et al., 2015), the role of the thalamic reticular nucleus in pathological sensory processing states such as tinnitus is not yet understood.
2.1 Limbic system – thalamus interactions
In addition to direct projections to the striatum and amygdala mentioned above, the MGB projects indirectly to both structures via auditory cortex (LeDoux et al., 1991; Romanski et al., 1993; Shinonaga et al., 1994). MGB connections to the amygdala have been studied extensively for their role in fear conditioning (LeDoux et al., 1991; Romanski et al., 1993), thus providing a substrate for conditioned negative emotional responses to sound, or sound percepts. Pathological increases in activity in this pathway may mediate the negative valence associated with sound in a subset of tinnitus sufferers and perhaps may contribute to comorbid problems experienced by tinnitus sufferers including depression and anxiety. In addition, the direct pathway from the thalamus to striatum provides a direct interface with frontostriatal pathways, which have been implicated in a gating role in both the negative affective components as well as the abnormal perceptual components of tinnitus (Rauschecker et al., 2015). Of potential relevance to gating hypotheses related to the thalamus and tinnitus, neurons in the MGm have physiological properties which are distinct from those in the ventral and dorsal divisions. Many medial division neurons do not display rebound bursting, which is found in most, thalamocortical neurons of the MGv and MGd (Smith et al., 2006), and is felt to be a core component of the thalamocortical dysrhythmia hypothesis described below. This finding suggests that MGm neurons, if they are involved in producing tinnitus, may not do so via burst mechanisms. In addition, MGm neurons have local collaterals, which are not seen in ventral or dorsal divisions of the MGB (Smith et al., 2006). The latter finding provides a potential substrate for the synchronization of activity across regions of the MGm, and therefore the synchronization of outputs to the cortex, to striatum or amygdala. Thus, abnormalities in local connectivity in the MGm induced by deafferentation may produce abnormal synchrony in thalamocortical output, which has also been speculated to be a substrate for tinnitus (Eggermont et al., 2015). Such putative changes in local connectivity in MGm neurons after peripheral deafferentation have not yet been examined experimentally.
2.2 Understanding thalamic attentional gating: a key to understanding tinnitus pathology
Clinically, it is well known that the behavioral phenotype of individuals suffering from tinnitus includes an attentional component (Newman et al., 1997; Rossiter et al., 2006). Tinnitus sufferers report difficulty concentrating and the inability to direct their attention away from their tinnitus (Searchfield et al., 2007). However, when distracted by an engaging task or attention-demanding task, tinnitus is generally less obtrusive (Roberts et al., 2013). To this end, behavioral approaches such as tinnitus retraining therapy and similar methods used to treat chronic pain have focused on directing attention away from the adverse sensory percept (Cuny et al., 2004; Jastreboff et al., 2006; Rainville, 2013; Searchfield et al., 2007). Recently there has been an upsurge of interest on the role of attention in tinnitus (Engineer et al., 2013; Roberts et al., 2013). As indicated in these excellent reviews, the primary focus has been on higher-order forebrain/cortical systems. The gating functions of MGB, although thought to be involved in tinnitus, have not been thoroughly explored (Engineer et al., 2013; Leaver et al., 2011; Rauschecker et al., 2010; Schofield et al., 2011). Roberts and colleagues (2013) developed a detailed model whereby learned representations of one's acoustic environment “serve as a template for filtering and predicting sensory state”. It is likely that what is stored in one's auditory memory represents the history of the organism's environmental exposure to sound. Templates derived from memory, or predictions about upcoming stimuli, may be continually compared to current ongoing acoustic input (De Ridder et al., 2014; Roberts et al., 2013; Searchfield et al., 2007). A similar memory comparator has been detailed for “novel signals” impinging on hippocampal systems (Lisman et al., 2005).
Phantom sounds may produce a perceptual mismatch between expected environmental acoustic events and ongoing sensation, which results in activation of attentional pathways including the basal forebrain and the brainstem reticular pedunculopontine tegmental nucleus (PPTg). Activation of the PPTg in pathways that are maladapted because of sound exposure-related partial peripheral deafferentation could trigger abnormal thalamocortical oscillations (Edeline, 2003;Motts et al., 2010).
2.3 Top-down modulation
Dysregulation of top-down modulatory systems has also been speculated to be involved in the generation of tinnitus and hyperacusis (De Ridder et al., 2014; Song et al., 2015); a hypothesis that is supported by recent behavioral data (Araneda et al., 2015; Heeren et al., 2014). In the absence of bottom-up information, stored representations of sound may be accessed elsewhere, such as via the parahippocampal gyrus, producing abnormal acoustic percepts (De Ridder et al., 2015). Top-down projections are ubiquitous in sensory systems, and the thalamus is a key target of cortical modulation. Synapses from the cortex onto thalamocortical neurons outnumber ascending projections to the thalamus by at least 3:1 (Erisir et al., 1997; Van Horn et al., 2000), and can strongly modulate thalamic output (Groh et al., 2008; Reichova et al., 2004; Theyel et al., 2010). Multiple corticothalamic pathways are candidates for being involved in tinnitus. The prefrontal cortex has been shown to project directly to the thalamic reticular nucleus, including the auditory sectors (Zikopoulos et al., 2006). This finding, combined with evidence for structural and functional abnormalities in the prefrontal cortex of tinnitus patients (Leaver et al., 2011; Mirz et al., 2000; Schlee et al., 2009), led some authors to speculate that weakened projections from the prefrontal cortex to the auditory thalamic reticular nucleus, which sends GABAergic projections to the MGB, may limit the normal inhibitory control exerted over auditory thalamocortical signal transmission, thus increasing “auditory gain” (Lanting et al., 2014; Leaver et al., 2011; Rauschecker et al., 2010). This Gain Control Theory assumes that a decrease in GABAergic inhibition would be found in the thalamus of tinnitus sufferers, a supposition for which the data are mixed (Sametsky et al., 2015; Su et al., 2012, see below). Additional pathways from sites known to show tinnitus-related changes and that project to thalamus may also be involved. For example, there is a large and heterogeneous pathway emanating from the auditory cortex onto MGB neurons, part of which sends branches to the thalamic reticular nucleus (Kimura et al., 2005; Llano et al., 2008; Llano et al., 2009; Ojima, 1994; Rouiller et al., 2004) and there are direct projections from the amygdala to neurons in the auditory sector of the thalamic reticular nucleus (Zikopoulos et al., 2012). Both of these pathways involve the thalamic reticular nucleus, and therefore both have the potential to alter the balance of excitation and inhibition in the MGB, but their specific contributions in tinnitus have not yet been specifically explored. Other descending pathways may also be involved in tinnitus pathology. For example, it has been hypothesized that projections from the anterior cingulate to noise-canceling pathways emanating from the tectum, such as the tectal longitudinal column (De Ridder et al., 2015; Saldana et al., 2007), or that peripheral pathways emanating from the superior olive, may be involved in tinnitus (Attias et al., 1996; Knudson et al., 2014; Riga et al., 2015). According to these theories, inadequate noise cancellation may lead to hyperactivation of ascending auditory pathways, resulting in tinnitus.
Alterations in the structure and function of the auditory top-down pathways have not yet been studied in animal models of tinnitus. However, it should be noted that in both somatosensory and visual systems, considerable plastic expansion in top-down feedback pathways occurs after deafferentation (Djavadian et al., 2001; Fukuda et al., 1984; Garcia del Cano et al., 2002; Jung et al., 2002; reviewed in Lesicko et al., 2016). If there is a deafferentation-induced expansion of the layer 6-derived corticothalamic pathway, for example, this expansion could lead to enhancement or broadening of inhibition derived from the thalamic reticular nucleus. This in turn could lead to a relative enhancement of GABAergic tone, setting the stage for low-frequency oscillations and subsequent thalamocortical dysrhythmia. In the MGB, this would most likely occur outside of the medial division given the diminished bursting capacity of these neurons (Smith et al. 2006).
3.0 GABAA Receptors
To better understand how peripheral damage/deafferentation-related compensatory changes in inhibition could underpin the pathology of tinnitus, it is necessary to understand unique features of the GABAA receptor (GABAAR) in sensory thalamus. Two major types of GABAA receptors (GABAARs) exist in MGB: 1) a fast, synaptic, ligand-gated, Cl− ion fluxing “wild type” receptor formed by coassembly of 2α12β2γ2 subunits and 2) a high affinity non-desensitizing, extrasynaptic GABAARs where a δ subunit replaces the γ2 subunit (Quirk, 1995; Wisden et al., 1992). GABAAR constructs containing δ subunits differ pharmacologically and functionally from “wild type” synaptic GABAARs, showing increased receptor affinity for GABA and decreased rates of desensitization, which contribute to the persistent (tonic) inhibitory postsynaptic current observed for thalamic neurons (Figure 2) (Saxena et al., 1994; Sur et al., 1999). Extrasynaptic GABAAR constructs are found in high concentration in thalamus, where they may carry as much as 90% of the total inhibitory current in visual thalamus (Cope et al., 2005). Activation of extrasynaptic GABAARs changes the discharge properties of MGB neurons from tonic to burst mode altering normal physiologic functions, implicitly suggesting involvement in conditions such as epilepsy, chronic pain and tinnitus (Cope et al., 2005; Walker et al., 2008). Inhibitory neurotransmission is essential for accurate processing of acoustic information. It shapes input/output functions, alters frequency tuning, and sharpens temporal response accuracy while gating neurotransmission to auditory cortex (AI) (Bartlett, 2013; Edeline, 2011; Llano et al., 2000; Richardson et al., 2012; Wang et al., 2008). In vivo and in vitro electrophysiological findings suggest distinct properties of inhibitory neurotransmission in the MGB relative to other ascending auditory structures, such as inferior colliculus (IC) (Cai et al., 2015; Cai et al., 2013; Richardson et al., 2011). The rat MGB contains relatively few GABAergic interneurons, but there are substantial GABAergic inhibitory projections from inferior colliculus and from the thalamic reticular nucleus (Bartlett et al., 2000; Peruzzi et al., 1997; Saint Marie et al., 1997b; Winer et al., 1988; Winer et al., 1996a; Winer et al., 1996b). MGB neurons have been shown to be exquisitely sensitive to GABA application relative to IC neurons, showing increased suppression of acoustically evoked unit responses in vivo and larger hyperpolarizing responses of MGB neurons in vitro relative to those published for IC (Cai et al., 2013). When the magnitude of tonic GABAAR gaboxadol (GBX) evoked currents recorded in vitro were compared between MGB and IC neurons, MGB dose-response was significantly shifted to the left of IC dose-response, showing GBX to be significantly more potent in activating tonic currents in MGB units than in IC (Cai et al., 2013). Spectroscopy showed significantly higher ambient GABA levels in MGB relative to other central auditory structures, and GABA levels were higher in the dorsal division of the MGB compared to the ventral division of MGB (Cai et al., 2013).
Figure 2. Patch-clamp slice recording from an MGB neuron.
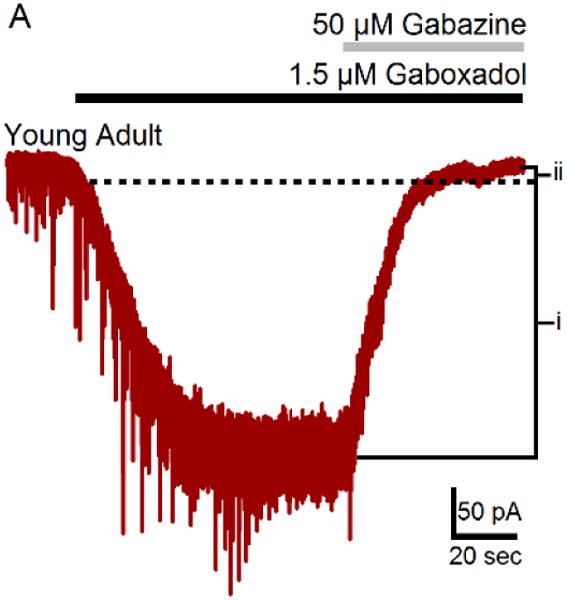
Exemplar shows gaboxadol evoked GABAAR-mediated tonic inhibitory current from a ventral MGB neuron during application of 1.5 μm gaboxadol and subsequent blockade of synaptic and high-affinity GABAARs with 50 μm gabazine. (Modified from Richardson et al., 2011).
3.1 Down-regulation of inhibitory function
has been suggested to underpin tinnitus pathology in dorsal cochlear nucleus (DCN) (Pilati et al., 2012a; Pilati et al., 2012b; Wang et al., 2009), inferior colliculus (IC) (Bauer et al., 2008; Dong et al., 2009), and AI (Brozoski et al., 2002; Llano et al., 2012; Norena et al., 2003; Roberts et al., 2012; Yang et al., 2007; Yang et al., 2011) where tinnitus is accompanied by increased spontaneous activity, bursting, enhanced sound-evoked responses, and reduction of neurochemical markers of inhibitory neurotransmission (Eggermont et al., 2004; Gold et al., 2014; Middleton et al., 2011). This down-regulation of inhibitory function is thought to reflect altered homeostatic plasticity compensating for the loss of peripheral excitatory drive supporting the “Gain Control Theory of Tinnitus” (Brozoski et al., 2002; Norena, 2011; Richardson et al., 2012; Shore, 2008). In a recent human study, Sedley et al. (2015) used magnetic resonance spectroscopy to assess relative GABA levels in the auditory cortex of patients with and without unilateral tinnitus. They found a tinnitus-related reduction in GABA levels in the right auditory cortex of patients, regardless of tinnitus laterality.
3.2 Increased tonic inhibition
has also been suggested to underpin tinnitus pathology in a subset of thalamocortical circuits resulting in tinnitus-related abnormal oscillations between thalamus and auditory cortex (De Ridder et al., 2015; Llinas et al., 2005). Llinas et al. (2005) postulated that excessive inhibition in thalamus could be the origin of a pathologically increased hyperpolarization of thalamic neurons that results in deinactivation of T-type Ca2+ channels. The thalamocortical dysrhythmia model suggests that elevated inhibition acting at extrasynaptic GABAARs results in focal burst activity associated with abnormal low-frequency oscillations in thalamus. This ectopic disinhibition resulting in fast oscillatory activity in AI, can correlate with the percept of tinnitus (Llinas et al., 2006; Sametsky et al., 2015). GABAergic inhibition is thus critically involved in determining higher frequency thalamocortical oscillations as well as slow-wave sleep (Llinas et al., 2006). Thalamocortical dysrhythmia originating in sensory thalamus has been postulated to underpin neurologic disorders including tinnitus and chronic pain. The de-inactivation of T-type Ca2+ channels leads to rhythmic bursting in thalamic neurons in the delta or theta range. When this focal slowing reaches the cortex, lack of normal thalamocortical input to inhibitory interneurons leads to the production of focal gamma oscillations in adjacent areas of the auditory cortex, referred to as the “edge effect” (Llinas et al., 1999). This leads to the false percept of tinnitus or pain, in somatosensory areas. This theory predicts that tinnitus should be associated with focal cortical gamma oscillations and that these gamma oscillations should be coupled to abnormal theta or delta activity.
An assumption here is that sensory deafferentation, which is nearly universally seen in tinnitus, leads to hyperpolarization and spontaneous bursting in thalamic neurons. One concern is the lack of data establishing whether auditory deafferentation leads to hyperpolarization of thalamic neurons in vivo. This is a prerequisite for the thalamocortical dysrhythmia model, and was noted in vitro after acute direct application of salicylate (Su et al., 2012). Given that: 1) there is prominent ascending inhibition in the auditory tectothalamic pathway (Llano et al., 2014; Peruzzi et al., 1997; Venkataraman et al., 2013; Winer et al., 1996b) and 2) there is downregulation of GABAergic inhibition in the IC and increases in spontaneous activity of IC neurons after deafferentation (Argence et al., 2006; Bauer et al., 2008; Butt et al., 2016; Chen et al., 1995). It is not clear that simple peripheral deafferentation leads to a decrease or an increase in excitatory drive to the thalamus and subsequent hyperpolarization and abnormal bursting of MGB neurons (see 4.0 and Figure 5). Therefore, if MGB neurons are involved in abnormal bursting during tinnitus, the substrate of hyperpolarization may emanate from an intrinsic change in the nature/sensitivity of GABAARs located on MGB neurons or from sources of inhibition outside of the tectothalamic pathway, such as the thalamic reticular nucleus, zona incerta or intrinsic inhibitory interneurons.
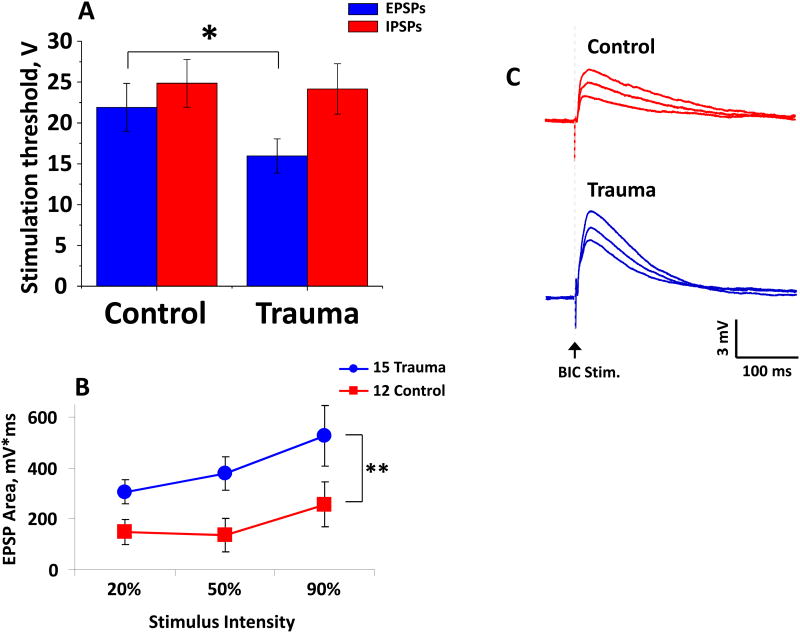
Figure 5. Stimulation of IC Inputs Reflects Increased Excitability in MGB.
Stimulation of the brachium of the inferior colliculus (BIC), which contains both excitatory and inhibitory projections to MGB, finds a significant tinnitus-related decrease in electrical stimulation threshold needed to evoke excitatory but not inhibitory synaptic potentials (A). There were significant tinnitus-related increases in the EPSP area under the curve at all stimulus strengths tested (B). Exemplars of BIC stimulation show evoked EPSPs from MGB neurons in slices from control and sound-exposed.
3.3 Data supporting either hypothesis
Human studies have pointed to both structural and functional abnormalities in the thalamus of patients with tinnitus (Landgrebe et al., 2009; Lanting et al., 2009; Muhlau et al., 2006). The thalamocortical dysrhythmia hypothesis makes predictions that can be measured in subjects with tinnitus. Given the limitations of what is feasible in humans (i.e., human thalamic recordings are only available in exceptional cases for tinnitus patients), the approach most closely approximating an electrophysiological test of the hypothesis would come from magneto-encephalographic recordings in patients with tinnitus. It is predicted that the presence of tinnitus would be associated with an increase in gamma activity over the auditory cortex, and that this increase would be coupled to pathological slowing in the theta or delta range in nearby sites. The data in this regard from human subjects with tinnitus are mixed. Multiple studies have shown increases in auditory cortical gamma activity in patients with tinnitus (Ashton et al., 2007; Llinas et al., 2005; van der Loo et al., 2009; Weisz et al., 2007), and in some cases have shown that this activity is either correlated with tinnitus loudness (van der Loo et al., 2009) or disappears in the presence of masking (Llinas et al., 2005). Other studies have shown predicted increases in slow-wave activity (either delta or theta) over the auditory cortex (Adjamian et al., 2012; Weisz et al., 2005). Likewise, this delta activity is sensitive to masking (Kahlbrock et al., 2008). Finally, a few (De Ridder et al., 2011; Weisz et al., 2007), but not all (Adjamian et al., 2012; Ashton et al., 2007; Kahlbrock et al., 2008), studies have shown an increase in slow wave as well as a coupled increase in gamma activity, as predicted by the model. Given the importance of the thalamus in generating delta-range oscillations (Steriade et al., 1993), the data showing changes in delta activity point to thalamocortical correlates of tinnitus. The lack of full agreement among studies may very well reflect the difficulties in conducting such studies in humans. Tinnitus is likely heterogeneous in its central mechanisms, and even when controlling for clinical factors (such as age and hearing loss), there may be other factors that make it difficult to find underlying pathophysiological signatures, particularly if they only exist in a subset of patients.
Several studies have more directly examined the thalamocortical dysrhythmia hypothesis in patients and animal models of chronic deafferentation-related pain, often seen as an analog of deafferentation-related tinnitus (De Ridder et al., 2011). Despite the fact that there are several studies in humans and animals that have pursued this hypothesis directly, the data supporting the hypothesis remain mixed. For example, multiple studies in human patients with chronic pain have revealed abnormal spontaneous and evoked burst patterns of firing in the thalamus, reminiscent of t-type Ca2+ channel-mediated bursts (Lee et al., 2005; Lenz et al., 1989; Rinaldi et al., 1991), though not all studies found bursting to be more prevalent in pain compared to other patient populations (Radhakrishnan et al., 1999). In addition, similar to what has been observed in humans with tinnitus, magneto-encephalography studies have found focal increases in slow-wave (delta and theta) activity in the somatosensory cortex of chronic pain patients, though the data supporting gamma oscillations in chronic pain are more sparse (Ray et al., 2009; Sarnthein et al., 2006; Schulman et al., 2005; Walton et al., 2010b). The animal literature is also inconsistent with respect to the role of thalamic bursting in chronic pain. Several studies have suggested that bursting in thalamic neurons is, in fact, associated with diminished pain perception (Cheong et al., 2008; Huh et al., 2012; Kim et al., 2003), while others have found increases in thalamic bursting in chronic pain models (Hains et al., 2006; Iwata et al., 2011). The disparate findings may be explained by the variety of different types of pain models that are studied in both animal and human studies, whether the pain is phantom pain vs. allodynia-related pain, and the physiological conditions underlying the measurement of thalamic activity.
It is also possible that multiple mechanisms, all involving GABAergic inhibition, may be altered by deafferentation. GABA in the auditory thalamus is derived from three main sources: the thalamic reticular nucleus, intrinsic interneurons (though the contribution is small in rodents, Winer et al., 1988), the zona incerta (Bartho et al., 2002), in addition to ascending input from the colliculus (Llano et al., 2014; Peruzzi et al., 1997; Venkataraman et al., 2013; Winer et al., 1996b). There are at least three inhibitory receptor types: synaptic GABAA, extrasynaptic GABAA and GABAB receptors. Each of these GABAergic pathways receives different inputs and therefore is likely activated (and disrupted) under different behavioral states. For example, the thalamic reticular inputs may be dominant during states of low arousal or during attentional tasks, while ascending GABAergic inputs and GABAergic interneurons may be activated in any scenario when sound is present. Therefore each of these pathways is likely to be differentially modulated by deafferentation, leading to a mixture of findings in the literature.
4.0 Animal data concerning the thalamocortical dysrhythmia hypothesis in tinnitus
Animal studies of tinnitus may permit some uniformity in the approaches to the study of the role of the thalamus in tinnitus, and will permit mechanistic assessment of the role of bursting in the generation of the tinnitus percept. A recent series of experiments examined single unit recordings from sound-exposed animals with behavioral evidence of tinnitus. Eighty days post a sound-exposure consistent with the development of tinnitus in rats (Bauer et al., 1999), auditory brainstem response and MGB unit acoustic thresholds were not statistically different from controls (Kalappa et al., 2014). As described for other brainstem auditory structures, awake MGB spontaneous activity and acoustically driven discharge rates at higher intensities were significantly increased in sound-exposed animals with behavioral evidence of tinnitus (Figure 3A, 3B) (Kalappa et al., 2014). Many single-unit metrics commonly thought of as markers of tinnitus were linearly related to the magnitude of the behavioral assessment of tinnitus (Figure 3C, 3D) (Kalappa et al., 2014). These findings were consistent with an increased thalamocortical output from MGB to auditory cortex but do not distinguish between a Gain Control model and the thalamocortical dysrhythmia hypotheses of tinnitus pathology.
Figure 3. (A) Spontaneous firing rates (SFRs) of MGB units recorded from unexposed control (control animals) and sound-exposed animals with behavioral evidence of tinnitus (tinnitus animals).
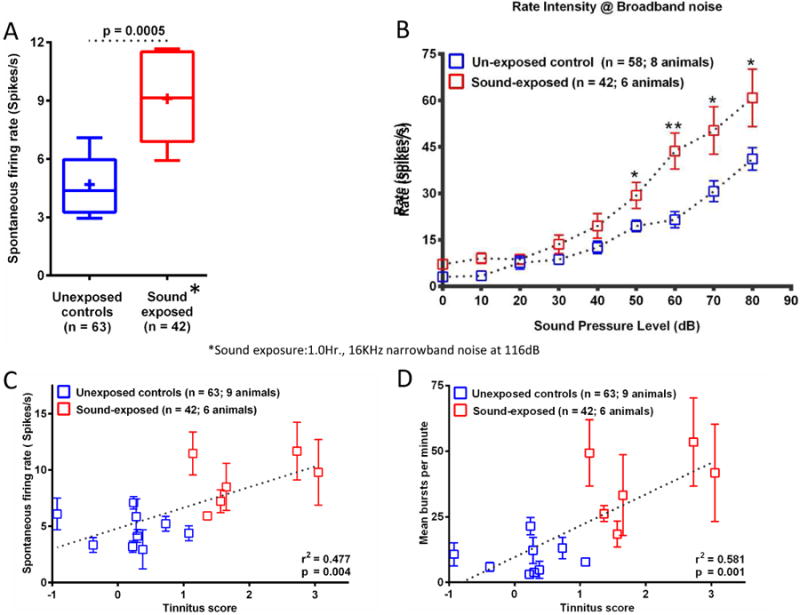
(A) Average SFRs of MGB units recorded from sound-exposed animals (red, 6 animals) were significantly elevated (p = 0.0005) compared to controls. + represents mean SFR. (B) MGB unit group rate-level functions in response to an increasing intensity broadband noise (BBN) stimulus. Single units from unexposed control animals (blue; 8 animals) compared to sound-exposed animals (red; 6 animals). (C&D) Scatter plots of average SFR/rat MGB (C) and average bursts per minute/per rat MGB (D), of MGB units recorded from individual animals (y-axis) exhibited a significant correlation with the animal's tinnitus z-scores (x-axis)(see Kalappa et al., 2014). The higher the z-score the stronger the behavioral evidence of tinnitus. Blue and red squares represent control and sound-exposed animals, respectively. The error bars represent standard error of the mean. See Kalappa et al (2014) for details (adapted from Kalappa et al., 2014).
In a recent series of in vitro studies, Sametsky et al. (2015) addressed two questions related to the role of inhibition in the pathology of tinnitus: 1) How are MGB neurons from sound-exposed animals with behavioral evidence of tinnitus functionally different from unexposed control animals? Membrane properties, spontaneous IPSCs and responses to depolarizing current steps were compared. 2) How are extrasynaptic GABAARs in MGB neurons from sound-exposed animals with behavioral evidence of tinnitus different from extrasynaptic GABAARs from unexposed controls? These studies examined extrasynaptic and synaptic GABAAR currents in MGB neurons in vitro contralateral to a sound exposure in three groups of adult rats: unexposed control (Ctrl), sound-exposed with behavioral evidence of tinnitus, and sound-exposed with no behavioral evidence of tinnitus. Tonic extrasynaptic GABAAR currents were evoked using the selective “super” agonist gaboxadol. These studies found that MGB neurons from sound-exposed rats with behavioral evidence of tinnitus showed significant increases in the number of spikes per burst evoked using suprathreshold injected current steps (see Figure3, Sametsky et al., 2015). No tinnitus-related synaptic changes in spontaneous inhibitory or GABAAR, postsynaptic currents were observed between groups. MGB neurons showed significant tinnitus-related increases (∼20%) in gaboxadol-evoked tonic extrasynaptic GABAAR currents, three months following a tinnitus-inducing sound exposure (Figure 4) (Sametsky et al., 2015). Supporting these findings were increased mRNA levels for the GABAAR δ subunit contralateral to the sound exposure. Taken together, these data show increased GABAAR efficacy in animals with behavioral evidence of tinnitus, supporting the possibility of selectively increased t-type Ca2+ channels activity driving the bursting/oscillations suggestive of thalamocortical dysrhythmia (De Ridder et al., 2015).
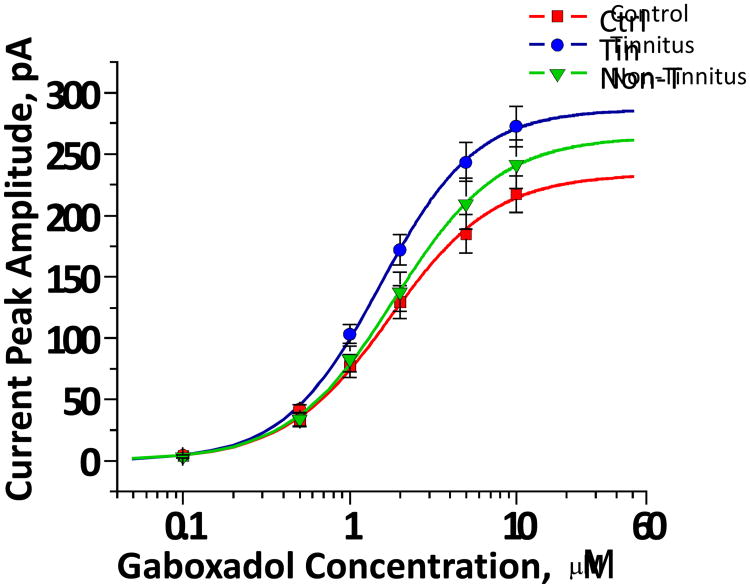
Figure 4. Patch-clamp slice recordings group data from MGB neurons.
Gaboxadol evoked dose-response semi-log plots of tonic GABAA current mean amplitudes in control (Ctrl), sound-exposed non-tinnitus (Non-T), and sound-exposed tinnitus (Tin) rats. Tin rats showed significantly larger responses to increasing GBX doses (0.1 -10 μM GBX (F = 3.15; p = 0.048). Data are presented as mean ± standard error. (detailed in Sametsky et al., 2015).
An additional consideration in the interpretation of these studies is the impact of enhanced tonic GABAergic signaling on driven activity in the thalamus. Because of the strong filtering that occurs at the thalamocortical synapse (Chung et al., 2002; Gil et al., 1999; Stratford et al., 1996), burst-mediated thalamocortical firing, which is necessarily preceded by a period of silence to permit de-inactivation of t-type Ca2+ channels, under certain stimulus paradigms may produce stronger cortical responses than tonic firing (Lisman, 1997; Swadlow et al., 2001; Willis et al., 2015). Thus, an increase in tonic GABAergic hyperpolarization on thalamic neurons may alter the bandwidth and strength of sound-driven activity in the auditory cortex, providing a potential substrate for hyperacusis, commonly seen in patients with tinnitus. Preliminary findings also suggest that an increase in excitatory drive could underpin an upregulation of extrasynaptic GABAAR function (Figure 5). In the same slice preparation described above and in Sametsky et al. (2015), electrical stimulation of IC projection fibers to MGB showed a tinnitus-related decrease in EPSP threshold in MGB neurons but not in IPSP threshold (Figure 5A). MGB neurons from sound-exposed rats showed tinnitus-related increases in the evoked EPSP area under the curve for the stimulus strengths tested (Figure 5B and 5C). Increased excitatory drive would likely result in a compensatory upregulation of extrasynaptic GABAAR function, since tonic GABAARs appear sensitive to activity dependent changes (Cope et al., 2009; Herd et al., 2014; Mody et al., 2004). These findings support maladaptive homeostatic changes at the level of IC that impact auditory thalamus. These data also suggest that increased GABAAR sensitivity could underpin thalamocortical dysrhythmia.
5.0 Hybrid models: Maladaptive plasticity/Gain control vs. thalamocortical dysrhythmia
Gain Control models of thalamic involvement in tinnitus are based on the hypothesis that thalamocortical transmission may be enhanced by a disruption of GABA transmission in the MGB, possibly related to changes in thalamic reticular nucleus function (Leaver et al., 2011; Rauschecker et al., 2010). Although such maladaptive downregulation may lead to an increase in stimulus-driven activation of the auditory cortex, possibly leading to hyperacusis, it is not clear how such a model can lead to the spontaneous auditory cortical activation and percepts associated with tinnitus. In addition, since putative downregulation of GABAergic tone to the MGB would put more thalamocortical cells into a tonic response mode, cortical responses may actually be paradoxically suppressed, for the reasons described above (Chung et al., 2002; Gil et al., 1999; Lisman, 1997; Stratford et al., 1996; Swadlow et al., 2001; Willis et al., 2015). An important consideration is that the mechanisms underlying spontaneous or increased thalamic activity may differ based on thalamic subdivision. For example, the finding that bursting is more common in the dorsal and ventral divisions than the medial division the MGB (Smith et al. 2006) may make these regions more vulnerable to thalamocortical dysrhythmia, In contrast, given the lack of bursting neurons in the MGm and the strong projections of MGm neurons to limbic structures (Bordi et al., 1994a; Bordi et al., 1994b; LeDoux et al., 1985; LeDoux et al., 1991; Winer et al., 2007; Woodson et al., 2000), the Gain Control hypothesis might be better suited when framed around the MGm's processing of the emotional components of sound. Therefore, the pathological mechanisms affecting different parts of the auditory thalamus may differ based on their likelihood of showing burst firing and their underlying connectivity.
With respect to the thalamocortical dysrhythmia hypothesis, although recent findings from human and animals studies support the idea of an increase in bursting and/or oscillations in individuals with tinnitus or in sound-exposure animal models of tinnitus, these changes do not occur in isolation. Maladaptive plasticity at lower levels of the central auditory system may in fact underpin the changes which occur at the level of MGB. Compensatory down-regulation of inhibition at the level of IC would lead to an increase in evoked excitatory input to the MGB and/or an increase in spontaneous firing rates of colliculo-thalamic neurons. The resulting increase in excitatory input to the MGB could lead to a compensatory upregulation of tonic inhibition in the MGB, as has been observed (Sametsky et al., 2015). Although deafferentation disorders including tinnitus and chronic pain may be a reflection of thalamocortical dysrhythmia resulting from hyperpolarization of thalamocortical neurons and resultant initiation of low-threshold Ca2+ spike bursts, the mechanism(s) leading to hyperpolarization have not yet been established (Llinas et al., 1999; Walton et al., 2010a). Simple deafferentation models assume that lack of input from the cochlea will also lead to lack of input to MGB neurons. Most of the evidence points to the opposite conclusion. Auditory deafferentation leads to downregulation of inhibition in the IC and an increase in spontaneous firing rates of IC neurons (Argence et al., 2006; Bauer et al., 2008; Butt et al., 2016; Chen et al., 1995; Dong et al., 2010). Auditory deafferentation also results in increased evoked signals from the IC in tinnitus patients, as assessed using fMRI (Gu et al., 2010; Lanting et al., 2008; Melcher et al., 2000). These findings imply that IC output to the MGB is increased, not decreased, in tinnitus patients. Therefore, an alternative explanation is that the increased excitatory drive from IC to MGB results in a compensatory increase in tonic GABA-mediated hyperpolarization. Sametsky et al. (2015) findings of a tinnitus-related increase in tonic extrasynaptic GABAAR current, in action potentials/evoked bursts, and in upregulated GABAAR δ subunit gene expression, could be a response to the increased excitatory drive seen in Figure 5. Either of these models is compatible with additional mechanisms involving auditory plasticity, for example those that alter the relative timing of excitatory and inhibitory inputs (D'Amour J et al., 2015). These studies suggest that tinnitus-related changes in tonic GABAergic function may be a marker for tinnitus pathology in the MGB. Clearly, more work is needed to establish the details of the impact of peripheral deafferentation on colliculo-thalamic transmission, and to define and separate the impact of tonic GABAergic inhibition and synaptically-mediated GABAergic inhibition in the thalamus.
Highlights.
Auditory thalamus/Medial Geniculate Body is a key component of the tinnitus circuit
Extrasynaptic GABAA receptors can switch thalamocortical neurons to burst mode
Maladaptive increases in MGB inhibition may underpin abnormal bursting
Abnormal bursting may result in oscillations called thalamocortical dysrhythmia
Thalamocortical dysrhythmia may reflect a response to increased excitation from IC
Acknowledgments
This review was supported by Awards from the Office of Naval Research (DMC) and by National Institute on Deafness and Other Communication Disorders Grants DC-000151 (DMC) and DC014765-01, DC013073-02 (DAL). We thank Lynne Ling for her work on the manuscript and Dr. Evgeny Sametsky for collecting data presented in Figure 5.
Abbreviations
- AAC
auditory association cortex
- AI
primary auditory cortex
- Ca2+
calcium
- GABA
g-amino buteric acid
- GBX
gaboxadol
- IC
inferior colliculus
- MGB
medial geniculate body
- MGv
ventral division of MGB
- MGd
dorsal division of MGB
- MGm
medial division of MGB
- PPTg
pedunculopontine tegmental nucleus
- TRN
thalamic reticular nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjamian P, Sereda M, Zobay O, Hall DA, Palmer AR. Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. J Assoc Res Otolaryngol. 2012;13:715–31. doi: 10.1007/s10162-012-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitkin LM, Webster WR. Medial geniculate body of the cat: organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972;35:365–380. doi: 10.1152/jn.1972.35.3.365. [DOI] [PubMed] [Google Scholar]
- Araneda R, De Volder AG, Deggouj N, Philippot P, Heeren A, Lacroix E, Decat M, Rombaux P, Renier L. Altered top-down cognitive control and auditory processing in tinnitus: evidences from auditory and visual spatial stroop. Restorative neurology and neuroscience. 2015;33:67–80. doi: 10.3233/RNN-140433. [DOI] [PubMed] [Google Scholar]
- Argence M, Saez I, Sassu R, Vassias I, Vidal PP, de Waele C. Modulation of inhibitory and excitatory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: an in situ and immunofluorescence study. Neuroscience. 2006;141:1193–207. doi: 10.1016/j.neuroscience.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Porter LL. Light and electron microscopic evidence for a GABAergic projection from the caudal basal forebrain to the thalamic reticular nucleus in rats. J Comp Neurol. 1990;302:159–72. doi: 10.1002/cne.903020112. [DOI] [PubMed] [Google Scholar]
- Ashton H, Reid K, Marsh R, Johnson I, Alter K, Griffiths T. High frequency localised “hot spots” in temporal lobes of patients with intractable tinnitus: a quantitative electroencephalographic (QEEG) study. Neurosci Lett. 2007;426:23–8. doi: 10.1016/j.neulet.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Attias J, Bresloff I, Furman V. The influence of the efferent auditory system on otoacoustic emissions in noise induced tinnitus: clinical relevance. Acta Otolaryngol. 1996;116:534–9. doi: 10.3109/00016489609137885. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, Salvi RJ. Central gain control in tinnitus and hyperacusis. Frontiers in neurology. 2014;5:206. doi: 10.3389/fneur.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson A, Ringdahl A. Tinnitus--a study of its prevalence and characteristics. Br J Audiol. 1989;23:53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382:1600–7. doi: 10.1016/S0140-6736(13)60142-7. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Rouiller EM, Welker E, Clarke S, Villa AE, de Ribaupierre Y, de Ribaupierre F. Morphology and spatial distribution of corticothalamic terminals originating from the cat auditory cortex. Hear Res. 1995;83:161–74. doi: 10.1016/0378-5955(94)00199-z. [DOI] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Bartlett EL. The organization and physiology of the auditory thalamus and its role in processing acoustic features important for speech perception. Brain and language. 2013;126:29–48. doi: 10.1016/j.bandl.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW, Smith PH. Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience. 2000;100:811–28. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121:457–62. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Gunluk AE, Van Horn SC, Sherman SM. GABAergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. J Comp Neurol. 1994;348:481–510. doi: 10.1002/cne.903480402. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ledoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res. 1994a;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ledoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994b;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusis T. Should tinnitus be considered in disability evaluation for acoustic trauma. Hno. 1993;21:55–57. [PubMed] [Google Scholar]
- Butt S, Ashraf F, Porter LA, Zhang H. Sodium salicylate reduces the level of GABAB receptors in the rat's inferior colliculus. Neuroscience. 2016;316:41–52. doi: 10.1016/j.neuroscience.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Cai R, Caspary DM. GABAergic inhibition shapes SAM responses in rat auditory thalamus. Neuroscience. 2015;299:146–55. doi: 10.1016/j.neuroscience.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Kalappa BI, Brozoski TJ, Ling LL, Caspary DM. Is GABA Neurotransmission Enhanced in Auditory Thalamus Relative to Inferior Colliculus? J Neurophysiol. 2013 doi: 10.1152/jn.00556.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983;3:2365–80. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82:158–78. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Cheong E, Lee S, Choi BJ, Sun M, Lee CJ, Shin HS. Tuning thalamic firing modes via simultaneous modulation of T- and L-type Ca2+ channels controls pain sensory gating in the thalamus. J Neurosci. 2008;28:13331–40. doi: 10.1523/JNEUROSCI.3013-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–46. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. Journal of Neuroscience. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–8. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Norena A, El Massioui F, Chery-Croze S. Reduced attention shift in response to auditory changes in subjects with tinnitus. Audiol Neurootol. 2004;9:294–302. doi: 10.1159/000080267. [DOI] [PubMed] [Google Scholar]
- D'Amour J A, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron. 2015;86:514–28. doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Freeman W. The Bayesian brain: phantom percepts resolve sensory uncertainty. Neurosci Biobehav Rev. 2014;44:4–15. doi: 10.1016/j.neubiorev.2012.04.001. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Langguth B, Llinas R. Thalamocortical Dysrhythmia: A Theoretical Update in Tinnitus. Frontiers in neurology. 2015;6:124. doi: 10.3389/fneur.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, van der Loo E, Vanneste S, Gais S, Plazier M, Kovacs S, Sunaert S, Menovsky T, van de Heyning P. Theta-gamma dysrhythmia and auditory phantom perception. Journal of neurosurgery. 2011;114:912–21. doi: 10.3171/2010.11.JNS10335. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Sejnowski TJ, Steriade M. A model of spindle rhythmicity in the isolated thalamic reticular nucleus. J Neurophysiol. 1994;72:803–18. doi: 10.1152/jn.1994.72.2.803. [DOI] [PubMed] [Google Scholar]
- Djavadian RL, Bialoskorska K, Turlejski K. Reorganization of the corticotectal projections introduced by neonatal monocular enucleation in the Monodelphis opossum and the influence of serotoninergic depletion. Neuroscience. 2001;102:911–23. doi: 10.1016/s0306-4522(00)00532-7. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Robertson D. Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience. 2009;159:1164–74. doi: 10.1016/j.neuroscience.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci. 2010;31:1616–28. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Edeline JM. Physiological Properties of Neurons in the Medial Geniculate Body. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. Springer; US: 2011. pp. 251–274. [Google Scholar]
- Edeline JM. The thalamo-cortical auditory receptive fields: regulation by the states of vigilance, learning and the neuromodulatory systems. Exp Brain Res. 2003;153:554–72. doi: 10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in neurosciences. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Tass PA. Maladaptive neural synchrony in tinnitus: origin and restoration. Frontiers in neurology. 2015;6:29. doi: 10.3389/fneur.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Moller AR, Kilgard MP. Directing neural plasticity to understand and treat tinnitus. Hear Res. 2013;295:58–66. doi: 10.1016/j.heares.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proc Natl Acad Sci U S A. 1997;94:1517–20. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Hsiao CF. Bilateral changes in soma size of geniculate relay cells and corticogeniculate cells after neonatal monocular enucleation in rats. Brain Res. 1984;301:13–23. doi: 10.1016/0006-8993(84)90398-6. [DOI] [PubMed] [Google Scholar]
- Garcia del Cano G, Gerrikagoitia I, Martinez-Millan L. Plastic reaction of the rat visual corticocollicular connection after contralateral retinal deafferentiation at the neonatal or adult stage: axonal growth versus reactive synaptogenesis. J Comp Neurol. 2002;446:166–78. doi: 10.1002/cne.10179. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron. 1999;23:385–97. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–9. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JR, Bajo VM. Insult-induced adaptive plasticity of the auditory system. Front Neurosci. 2014;8:110. doi: 10.3389/fnins.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, de Kock CP, Wimmer VC, Sakmann B, Kuner T. Driver or coincidence detector: modal switch of a corticothalamic giant synapse controlled by spontaneous activity and short-term depression. J Neurosci. 2008;28:9652–63. doi: 10.1523/JNEUROSCI.1554-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104:3361–70. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury. J Neurophysiol. 2006;95:3343–52. doi: 10.1152/jn.01009.2005. [DOI] [PubMed] [Google Scholar]
- Heeren A, Maurage P, Perrot H, De Volder A, Renier L, Araneda R, Lacroix E, Decat M, Deggouj N, Philippot P. Tinnitus specifically alters the top-down executive control sub-component of attention: evidence from the Attention Network Task. Behav Brain Res. 2014;269:147–54. doi: 10.1016/j.bbr.2014.04.043. [DOI] [PubMed] [Google Scholar]
- Helfer TM, Jordan NN, Lee RB. Postdeployment hearing loss in U.S. Army soldiers seen at audiology clinics from April 1, 2003, through March 31, 2004. Am J Audiol. 2005;14:161–8. doi: 10.1044/1059-0889(2005/018). [DOI] [PubMed] [Google Scholar]
- Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ. Underlying Mechanisms of Tinnitus: Review and Clinical Implications. American Academy of Audiology. 2013 doi: 10.3766/jaaa.25.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Lambert JJ, Belelli D. The general anaesthetic etomidate inhibits the excitability of mouse thalamocortical relay neurons by modulating multiple modes of GABAA receptor-mediated inhibition. Eur J Neurosci. 2014;40:2487–501. doi: 10.1111/ejn.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427:302–31. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hughes SW, E A, Lorincz ML, Kekesi KA, Juhaszx G, Orban G, Cope DW, Drunelli V. Novel modes of rhythmic burst firing at cognitively-relevant frequencies in thalamocortical neurons. Brain Research. 2008:12–20. doi: 10.1016/j.brainres.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh Y, Bhatt R, Jung D, Shin HS, Cho J. Interactive responses of a thalamic neuron to formalin induced lasting pain in behaving mice. PLoS One. 2012;7:e30699. doi: 10.1371/journal.pone.0030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Joellenbeck LM, Durch JS. Noise and Military Service: Implications for Hearing Loss and Tinnitus. The National Academies Press; 2006. [Google Scholar]
- Iwata M, LeBlanc BW, Kadasi LM, Zerah ML, Cosgrove RG, Saab CY. High-frequency stimulation in the ventral posterolateral thalamus reverses electrophysiologic changes and hyperalgesia in a rat model of peripheral neuropathic pain. Pain. 2011;152:2505–13. doi: 10.1016/j.pain.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Calder JA, Newman CW, Peterson EL, Wharton JA, Ahmad BK. Electrophysiological indices of selective auditory attention in subjects with and without tinnitus. Hear Res. 1996;97:66–74. [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy: a different view on tinnitus. ORL J Otorhinolaryngol Relat Spec. 2006;68:23–9. doi: 10.1159/000090487. discussion 29-30. [DOI] [PubMed] [Google Scholar]
- Jung SC, Shin HC. Reversible changes of presumable synaptic connections between primary somatosensory cortex and ventral posterior lateral thalamus of rats during temporary deafferentation. Neurosci Lett. 2002;331:111–4. doi: 10.1016/s0304-3940(02)00863-7. [DOI] [PubMed] [Google Scholar]
- Kahlbrock N, Weisz N. Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol. 2008;6:4. doi: 10.1186/1741-7007-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG, Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol. 2014;592:5065–78. doi: 10.1113/jphysiol.2014.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA. Tinnitus: Models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorevin VI. Interaction of reactions induced by acoustic and somatosensory stimuli in neurons of the magnocellular portion of the medial geniculate body. Neirofiziologiia = Neurophysiology. 1980;12:368–74. [PubMed] [Google Scholar]
- Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin HS. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302:117–9. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Tamai Y. Topography of projections from the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular nucleus in the rat. Neuroscience. 2005;135:1325–42. doi: 10.1016/j.neuroscience.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Knudson IM, Shera CA, Melcher JR. Increased contralateral suppression of otoacoustic emissions indicates a hyperresponsive medial olivocochlear system in humans with tinnitus and hyperacusis. J Neurophysiol. 2014;112:3197–208. doi: 10.1152/jn.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46:213–8. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Lanting CP, De Kleine E, Bartels H, Van Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol. 2008;128:415–21. doi: 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, Langers DR, van Dijk P. Unilateral tinnitus: changes in connectivity and response lateralization measured with FMRI. PLoS One. 2014;9:e110704. doi: 10.1371/journal.pone.0110704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett. 1991;134:139–44. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: II. Commissural system. J Comp Neurol. 2008a;507:1901–19. doi: 10.1002/cne.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol. 2008b;507:1920–43. doi: 10.1002/cne.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol. 2008c;507:1879–900. doi: 10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (Ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol. 2005;94:1676–87. doi: 10.1152/jn.00343.2005. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res. 1989;496:357–60. doi: 10.1016/0006-8993(89)91088-3. [DOI] [PubMed] [Google Scholar]
- Lesicko AMH, Llano DA. Impact of peripheral hearing loss on top-down auditory processing. Hearing Research. 2016 doi: 10.1016/j.heares.2016.05.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Llano DA, Feng AS. Computational models of temporal processing in the auditory thalamus. Biol Cybern. 2000;83:419–33. doi: 10.1007/s004220000174. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. J Comp Neurol. 2008;507:1209–27. doi: 10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb Cortex. 2009;19:2810–26. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Turner J, Caspary DM. Diminished cortical inhibition in an aging mouse model of chronic tinnitus. J Neurosci. 2012;32:16141–8. doi: 10.1523/JNEUROSCI.2499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Slater BJ, Lesicko AM, Stebbings KA. An auditory colliculothalamocortical brain slice preparation in mouse. J Neurophysiol. 2014;111:197–207. doi: 10.1152/jn.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends in neurosciences. 2005;28:325–33. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci U S A. 2002;99:449–54. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ, Bowman EM. Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. J Neurosci. 2000;20:8897–901. doi: 10.1523/JNEUROSCI.20-23-08897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. Journal of Neuroscience. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annual review of physiology. 2001;63:815–46. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–72. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- Metherate R. Functional connectivity and cholinergic modulation in auditory cortex. Neurosci Biobehav Rev. 2011;35:2058–63. doi: 10.1016/j.neubiorev.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–6. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol Suppl. 2000;543:241–3. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- Mitani A, Itoh K, Mizuno N. Distribution and size of thalamic neurons projecting to layer I of the auditory cortical fields of the cat compared to those projecting to layer IV. J Comp Neurol. 1987;257:105–21. doi: 10.1002/cne.902570108. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–75. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Cholinergic and non-cholinergic projections from the pedunculopontine and laterodorsal tegmental nuclei to the medial geniculate body in Guinea pigs. Frontiers in neuroanatomy. 2010;4:137. doi: 10.3389/fnana.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlau M, Rauschecker JP, Oestreicher E, Gaser C, Rottinger M, Wohlschlager AM, Simon F, Etgen T, Conrad B, Sander D. Structural brain changes in tinnitus. Cereb Cortex. 2006;16:1283–8. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- Newman CW, Wharton JA, Jacobson GP. Self-focused and somatic attention in patients with tinnitus. J Am Acad Audiol. 1997;8:143–149. [PubMed] [Google Scholar]
- Nondahl DM, Shi X, Cruickshanks KJ, Dalton DS, Tweed TS, Wiley TL, Carmichael LL. Notched audiograms and noise exposure history in older adults. Ear Hear. 2009;30:696–703. doi: 10.1097/AUD.0b013e3181b1d418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol. 2002;13:323–331. [PubMed] [Google Scholar]
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev. 2011;35:1089–109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hearing research. 2003;183:137–53. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–63. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y, Parent A, Steriade M. Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci. 1997;17:3766–77. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilati N, Large C, Forsythe ID, Hamann M. Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hearing research. 2012a;283:98–106. doi: 10.1016/j.heares.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilati N, Ison MJ, Barker M, Mulheran M, Large CH, Forsythe ID, Matthias J, Hamann M. Mechanisms contributing to central excitability changes during hearing loss. Proceedings of the National Academy of Sciences of the United States of America. 2012b;109:8292–7. doi: 10.1073/pnas.1116981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita-Almenar JD, Yu D, Lu HC, Beierlein M. Mechanisms underlying desynchronization of cholinergic-evoked thalamic network activity. J Neurosci. 2014;34:14463–74. doi: 10.1523/JNEUROSCI.2321-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of delta-subunit containing GABAA receptors from rat brain. Eur J Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan V, Tsoukatos J, Davis KD, Tasker RR, Lozano AM, Dostrovsky JO. A comparison of the burst activity of lateral thalamic neurons in chronic pain and non-pain patients. Pain. 1999;80:567–75. doi: 10.1016/S0304-3959(98)00248-6. [DOI] [PubMed] [Google Scholar]
- Rainville R. Pain and the emotional response to noxious stimuli. In: Armony J, Vuilleumier P, editors. The Cambridge Handbook of Human Affective Neuroscience. Cambridge University Press; 2013. pp. 223–242. [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–26. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, May ES, Maudoux A, Ploner M. Frontostriatal Gating of Tinnitus and Chronic Pain. Trends in cognitive sciences. 2015;19:567–78. doi: 10.1016/j.tics.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Kringelbach ML, Hansen PC, Pereira EA, Brittain JS, Holland P, Holliday IE, Owen S, Stein J, Aziz T. Abnormal thalamocortical dynamics may be altered by deep brain stimulation: using magnetoencephalography to study phantom limb pain. J Clin Neurosci. 2009;16:32–6. doi: 10.1016/j.jocn.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–97. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS ONE. 2011;6:e16508. doi: 10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Brozoski TJ, Ling LL, Caspary DM. Targeting inhibitory neurotransmission in tinnitus. Brain Res. 2012;1485:77–87. doi: 10.1016/j.brainres.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga M, Katotomichelakis M, Danielides V. The potential role of the medial olivocochlear bundle in the generation of tinnitus: controversies and weaknesses in the existing clinical studies. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015;36:201–8. doi: 10.1097/MAO.0000000000000384. [DOI] [PubMed] [Google Scholar]
- Rinaldi PC, Young RF, Albe-Fessard D, Chodakiewitz J. Spontaneous neuronal hyperactivity in the medial and intralaminar thalamic nuclei of patients with deafferentation pain. Journal of neurosurgery. 1991;74:415–21. doi: 10.3171/jns.1991.74.3.0415. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Bosnyak DJ, Thompson DC. Neural plasticity expressed in central auditory structures with and without tinnitus. Frontiers in systems neuroscience. 2012;6:40. doi: 10.3389/fnsys.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Husain FT, Eggermont JJ. Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev. 2013;37:1754–73. doi: 10.1016/j.neubiorev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Organization of rodent auditory cortex: anterograde transport of PHA-L from MGv to temporal neocortex. Cereb Cortex. 1993;3:499–514. doi: 10.1093/cercor/3.6.499. [DOI] [PubMed] [Google Scholar]
- Rossiter S, Stevens C, Walker G. Tinnitus and its effect on working memory and attention. J Speech Lang Hear Res. 2006;49:150–60. doi: 10.1044/1092-4388(2006/012). [DOI] [PubMed] [Google Scholar]
- Rouiller EM, de Ribaupierre F. Arborization of corticothalamic axons in the auditory thalamus of the cat: a PHA-L tracing study. Neurosci Lett. 1990;108:29–35. doi: 10.1016/0304-3940(90)90701-a. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hear Res. 1991;56:179–90. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Durif C. The dual pattern of corticothalamic projection of the primary auditory cortex in macaque monkey. Neurosci Lett. 2004;358:49–52. doi: 10.1016/j.neulet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Stanforth DA, Jubelier EM. Substrate for rapid feedforward inhibition of the auditory forebrain. Brain Res. 1997a;765:173–6. doi: 10.1016/s0006-8993(97)00654-9. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Stanforth DA, Jubelier EM. Substrate for rapid feedforward inhibition of the auditory forebrain. Brain Res. 1997b;765:173–176. doi: 10.1016/s0006-8993(97)00654-9. [DOI] [PubMed] [Google Scholar]
- Saldana E, Vinuela A, Marshall AF, Fitzpatrick DC, Aparicio MA. The TLC: a novel auditory nucleus of the mammalian brain. J Neurosci. 2007;27:13108–16. doi: 10.1523/JNEUROSCI.1892-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmivalli A. Acoustic trauma in regular army personnel. Clinical audiologic study. Acta oto-laryngologica Suppl. 1977:1–85. [PubMed] [Google Scholar]
- Sametsky EA, Turner JG, Larsen D, Ling L, Caspary DM. Enhanced GABAA-Mediated Tonic Inhibition in Auditory Thalamus of Rats with Behavioral Evidence of Tinnitus. J Neurosci. 2015;35:9369–80. doi: 10.1523/JNEUROSCI.5054-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–86. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Motts SD, Mellott JG. Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex. Hear Res. 2011;279:85–95. doi: 10.1016/j.heares.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JJ, Ramirez RR, Zonenshayn M, Ribary U, Llinas R. Thalamocortical dysrhythmia syndrome: MEG imaging of neuropathic pain. Thalamus & Related Systems. 2005;3:33–39. [Google Scholar]
- Searchfield GD, Morrison-Low J, Wise K. Object identification and attention training for treating tinnitus. Progress in Brain Research. 2007;166:441–460. doi: 10.1016/S0079-6123(07)66043-9. [DOI] [PubMed] [Google Scholar]
- Sedley W, Parikh J, Edden RA, Tait V, Blamire A, Griffiths TD. Human Auditory Cortex Neurochemistry Reflects the Presence and Severity of Tinnitus. J Neurosci. 2015;35:14822–8. doi: 10.1523/JNEUROSCI.2695-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–8. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Direct projections from the non-laminated divisions of the medial geniculate nucleus to the temporal polar cortex and amygdala in the cat. J Comp Neurol. 1994;340:405–26. doi: 10.1002/cne.903400310. [DOI] [PubMed] [Google Scholar]
- Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–93. doi: 10.1016/j.jaci.2008.03.004. quiz 1094-5. [DOI] [PubMed] [Google Scholar]
- Smith PH, Bartlett EL, Kowalkowski A. Unique combination of anatomy and physiology in cells of the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. J Comp Neurol. 2006;496:314–34. doi: 10.1002/cne.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Vanneste S, De Ridder D. Dysfunctional noise cancelling of the rostral anterior cingulate cortex in tinnitus patients. PLoS One. 2015;10:e0123538. doi: 10.1371/journal.pone.0123538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–61. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Su YY, Luo B, Jin Y, Wu SH, Lobarinas E, Salvi RJ, Chen L. Altered neuronal intrinsic properties and reduced synaptic transmission of the rat's medial geniculate body in salicylate-induced tinnitus. PLoS One. 2012;7:e46969. doi: 10.1371/journal.pone.0046969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–8. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Taylor GD, Williams E. Acoustic trauma in the sports hunter. Laryngoscope. 1966;76:863–79. doi: 10.1288/00005537-196605000-00005. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–8. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RS, Gogel SA, Gehringer AK. Tinnitus activities treatment. Progress in brain research. 1993;116:425–434. doi: 10.1016/S0079-6123(07)66041-5. [DOI] [PubMed] [Google Scholar]
- van der Loo E, Gais S, Congedo M, Vanneste S, Plazier M, Menovsky T, Van de Heyning P, De Ridder D. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;4:e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn SC, Erisir A, Sherman SM. Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol. 2000;416:509–20. [PubMed] [Google Scholar]
- Venkataraman Y, Bartlett EL. Postnatal development of synaptic properties of the GABAergic projection from the inferior colliculus to the auditory thalamus. J Neurophysiol. 2013;109:2866–82. doi: 10.1152/jn.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABA(A) receptors. Results Probl Cell Differ. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Walton KD, Llinas RR. Translational Pain Research: From Mouse to Man. Llc.; Boca Raton, FL: 2010a. Central Pain as a Thalamocortical Dysrhythmia: A Thalamic Efference Disconnection? [PubMed] [Google Scholar]
- Walton KD, Dubois M, Llinas RR. Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) type I. Pain. 2010b;150:41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–59. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience. 2008;154:294–303. doi: 10.1016/j.neuroscience.2008.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2:e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–84. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AM, Slater BJ, Gribkova ED, Llano DA. Open-loop organization of thalamic reticular nucleus and dorsal thalamus: a computational model. J Neurophysiol. 2015;114:2353–67. doi: 10.1152/jn.00926.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526:705–9. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Patterns of reciprocity in auditory thalamocortical and corticothalamic connections: study with horseradish peroxidase and autoradiographic methods in the rat medial geniculate body. J Comp Neurol. 1987;257:282–315. doi: 10.1002/cne.902570212. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Anatomy of glutamic acid decarboxylase immunoreactive neurons and axons in the rat medial geniculate body. J Comp Neurol. 1988;278:47–68. doi: 10.1002/cne.902780104. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc Natl Acad Sci USA. 1996a;93:3083–3087. doi: 10.1073/pnas.93.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Lee CC. The distributed auditory cortex. Hear Res. 2007;229:3–13. doi: 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Kelly JB, Larue DT. Neural architecture of the rat medial geniculate body. Hear Res. 1999;130:19–41. doi: 10.1016/s0378-5955(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward prior colliculus to the medial geniculate body. Proc Natl Acad Sci USA. 1996b;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson W, Farb CR, Ledoux JE. Afferents from the auditory thalamus synapse on inhibitory interneurons in the lateral nucleus of the amygdala. Synapse. 2000;38:124–37. doi: 10.1002/1098-2396(200011)38:2<124::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–53. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14974–9. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–61. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci. 2012;32:5338–50. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]